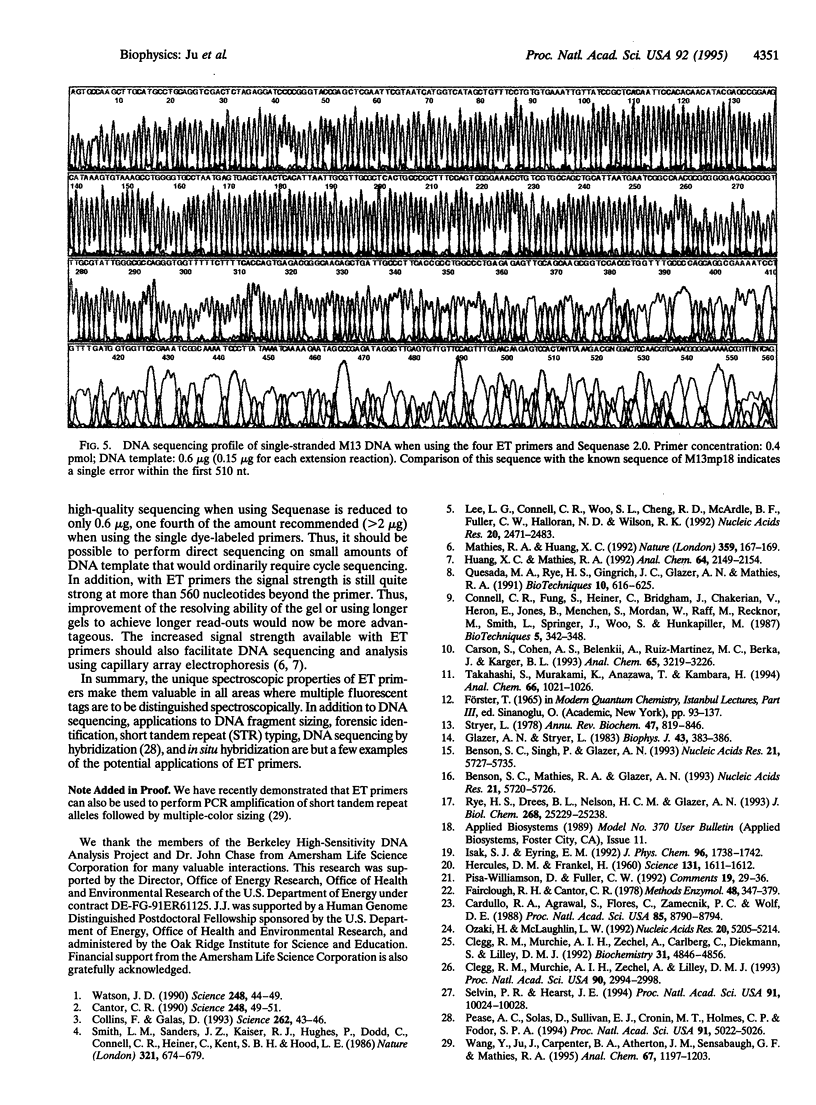
Abstract
Fluorescent dye-labeled DNA primers have been developed that exploit fluorescence energy transfer (ET) to optimize the absorption and emission properties of the label. These primers carry a fluorescein derivative at the 5' end as a common donor and other fluorescein and rhodamine derivatives attached to a modified thymidine residue within the primer sequence as acceptors. Adjustment of the donor-acceptor spacing through the placement of the modified thymidine in the primer sequence allowed generation of four primers, all having strong absorption at a common excitation wavelength (488 nm) and fluorescence emission maxima of 525, 555, 580, and 605 nm. The ET efficiency of these primers ranges from 65% to 97%, and they exhibit similar electrophoretic mobilities by gel electrophoresis. With argon-ion laser excitation, the fluorescence of the ET primers and of the DNA sequencing fragments generated with ET primers is 2- to 6-fold greater than that of the corresponding primers or fragments labeled with single dyes. The higher fluorescence intensity of the ET primers allows DNA sequencing with one-fourth of the DNA template typically required when using T7 DNA polymerase. With single-stranded M13mp18 DNA as the template, a typical sequencing reaction with ET primers on a commercial sequencer provided DNA sequences with 99.8% accuracy in the first 500 bases. ET primers should be generally useful in the development of other multiplex DNA sequencing and analysis methods.
Full text
PDF
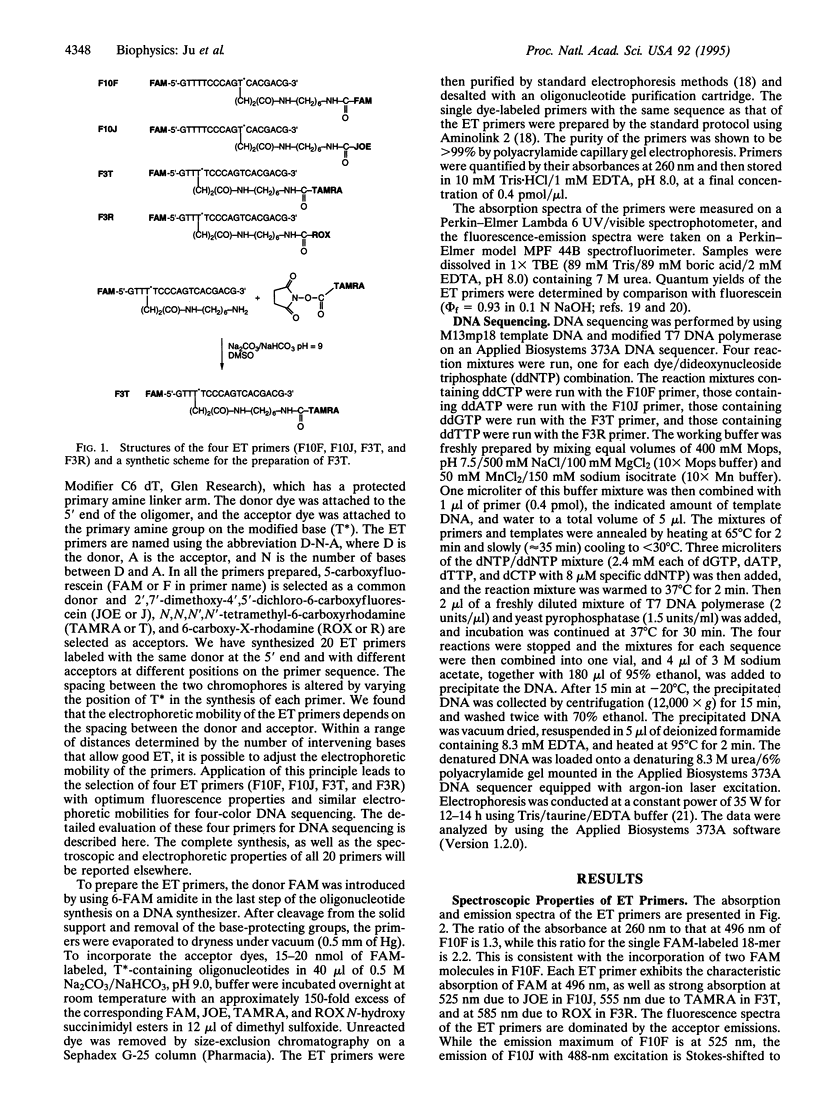
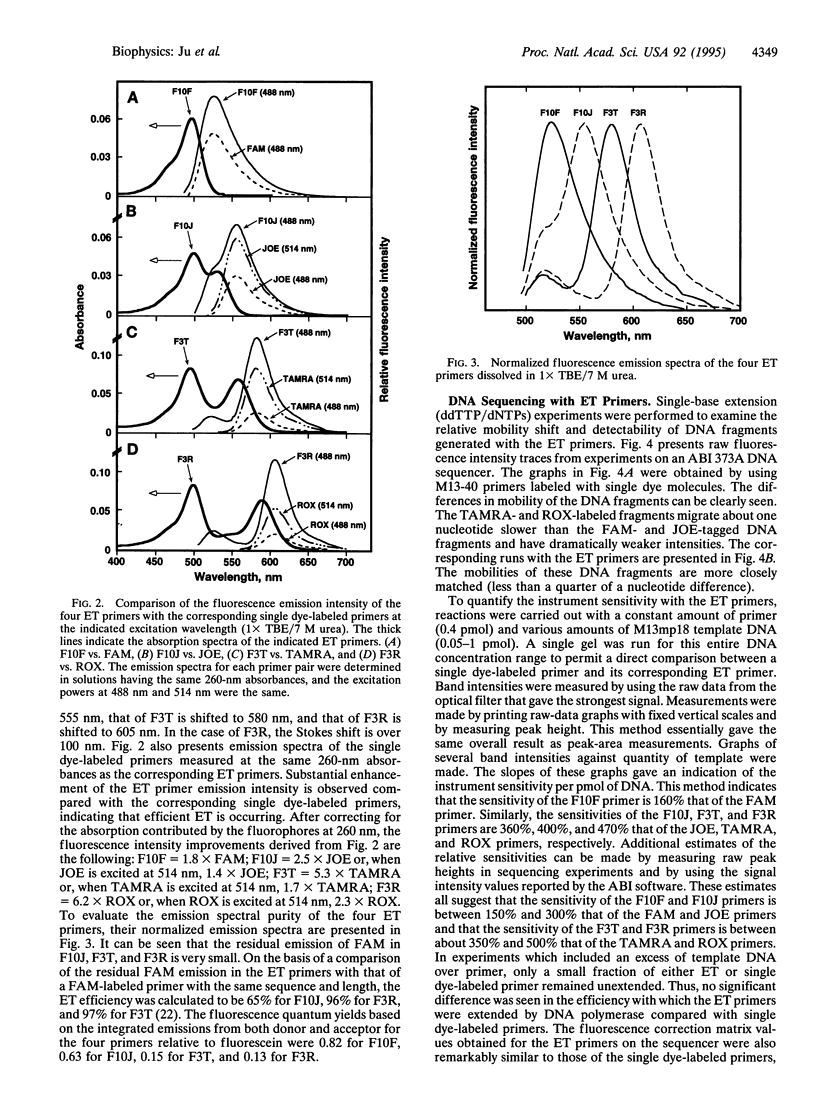
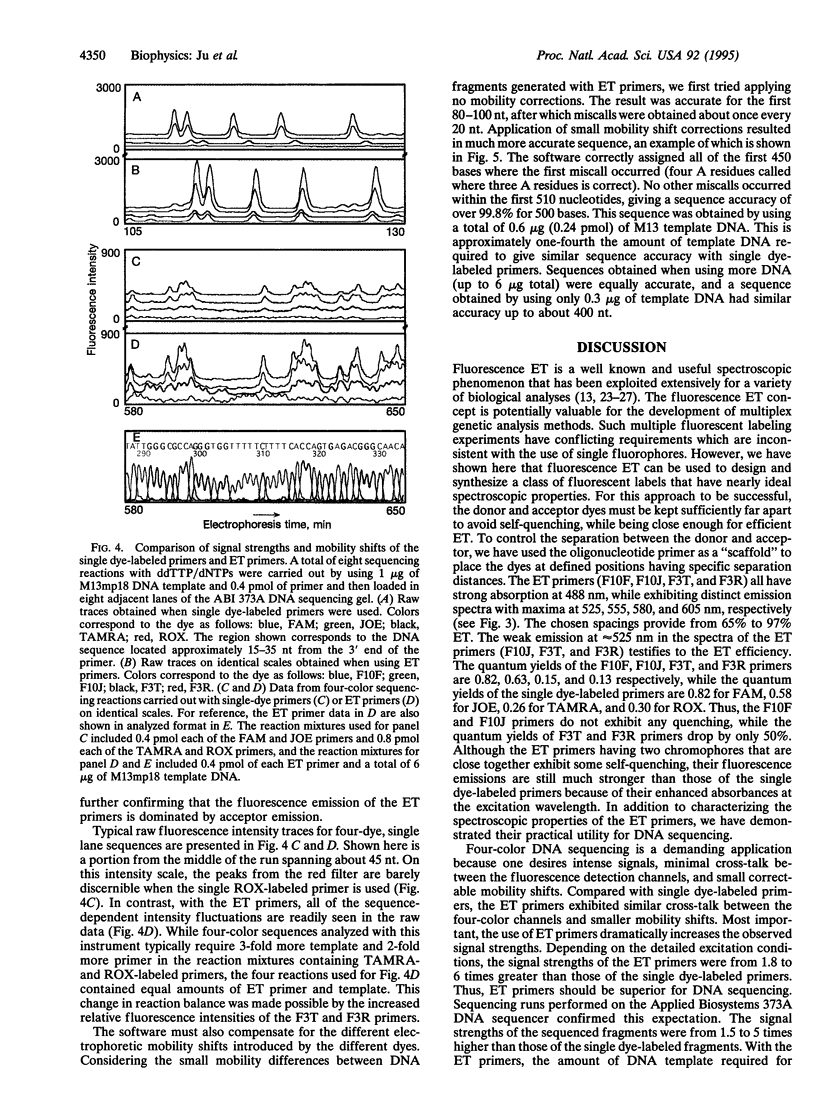
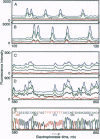
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson S. C., Mathies R. A., Glazer A. N. Heterodimeric DNA-binding dyes designed for energy transfer: stability and applications of the DNA complexes. Nucleic Acids Res. 1993 Dec 11;21(24):5720–5726. doi: 10.1093/nar/21.24.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson S. C., Singh P., Glazer A. N. Heterodimeric DNA-binding dyes designed for energy transfer: synthesis and spectroscopic properties. Nucleic Acids Res. 1993 Dec 11;21(24):5727–5735. doi: 10.1093/nar/21.24.5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor C. R. Orchestrating the Human Genome Project. Science. 1990 Apr 6;248(4951):49–51. doi: 10.1126/science.2181666. [DOI] [PubMed] [Google Scholar]
- Cardullo R. A., Agrawal S., Flores C., Zamecnik P. C., Wolf D. E. Detection of nucleic acid hybridization by nonradiative fluorescence resonance energy transfer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8790–8794. doi: 10.1073/pnas.85.23.8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson S., Cohen A. S., Belenkii A., Ruiz-Martinez M. C., Berka J., Karger B. L. DNA sequencing by capillary electrophoresis: use of a two-laser-two-window intensified diode array detection system. Anal Chem. 1993 Nov 15;65(22):3219–3226. doi: 10.1021/ac00070a009. [DOI] [PubMed] [Google Scholar]
- Clegg R. M., Murchie A. I., Zechel A., Carlberg C., Diekmann S., Lilley D. M. Fluorescence resonance energy transfer analysis of the structure of the four-way DNA junction. Biochemistry. 1992 May 26;31(20):4846–4856. doi: 10.1021/bi00135a016. [DOI] [PubMed] [Google Scholar]
- Clegg R. M., Murchie A. I., Zechel A., Lilley D. M. Observing the helical geometry of double-stranded DNA in solution by fluorescence resonance energy transfer. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2994–2998. doi: 10.1073/pnas.90.7.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F., Galas D. A new five-year plan for the U.S. Human Genome Project. Science. 1993 Oct 1;262(5130):43–46. doi: 10.1126/science.8211127. [DOI] [PubMed] [Google Scholar]
- Fairclough R. H., Cantor C. R. The use of singlet-singlet energy transfer to study macromolecular assemblies. Methods Enzymol. 1978;48:347–379. doi: 10.1016/s0076-6879(78)48019-x. [DOI] [PubMed] [Google Scholar]
- Glazer A. N., Stryer L. Fluorescent tandem phycobiliprotein conjugates. Emission wavelength shifting by energy transfer. Biophys J. 1983 Sep;43(3):383–386. doi: 10.1016/S0006-3495(83)84361-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotley D. C., Ball D. E., Owen R. W., Williamson R. C., Cooper M. J. Evaluation and surgical correction of esophagitis after partial gastrectomy. Surgery. 1992 Jan;111(1):29–36. [PubMed] [Google Scholar]
- Hercules D. M., Frankel H. Colloidal Silica as a Standard for Measuring Absolute Fluorescence Yield. Science. 1960 May 27;131(3413):1611–1612. doi: 10.1126/science.131.3413.1611. [DOI] [PubMed] [Google Scholar]
- Huang X. C., Quesada M. A., Mathies R. A. DNA sequencing using capillary array electrophoresis. Anal Chem. 1992 Sep 15;64(18):2149–2154. doi: 10.1021/ac00042a021. [DOI] [PubMed] [Google Scholar]
- Lee L. G., Connell C. R., Woo S. L., Cheng R. D., McArdle B. F., Fuller C. W., Halloran N. D., Wilson R. K. DNA sequencing with dye-labeled terminators and T7 DNA polymerase: effect of dyes and dNTPs on incorporation of dye-terminators and probability analysis of termination fragments. Nucleic Acids Res. 1992 May 25;20(10):2471–2483. doi: 10.1093/nar/20.10.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H., McLaughlin L. W. The estimation of distances between specific backbone-labeled sites in DNA using fluorescence resonance energy transfer. Nucleic Acids Res. 1992 Oct 11;20(19):5205–5214. doi: 10.1093/nar/20.19.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease A. C., Solas D., Sullivan E. J., Cronin M. T., Holmes C. P., Fodor S. P. Light-generated oligonucleotide arrays for rapid DNA sequence analysis. Proc Natl Acad Sci U S A. 1994 May 24;91(11):5022–5026. doi: 10.1073/pnas.91.11.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada M. A., Rye H. S., Gingrich J. C., Glazer A. N., Mathies R. A. High-sensitivity DNA detection with a laser-excited confocal fluorescence gel scanner. Biotechniques. 1991 May;10(5):616–625. [PubMed] [Google Scholar]
- Rye H. S., Drees B. L., Nelson H. C., Glazer A. N. Stable fluorescent dye-DNA complexes in high sensitivity detection of protein-DNA interactions. Application to heat shock transcription factor. J Biol Chem. 1993 Nov 25;268(33):25229–25238. [PubMed] [Google Scholar]
- Selvin P. R., Hearst J. E. Luminescence energy transfer using a terbium chelate: improvements on fluorescence energy transfer. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10024–10028. doi: 10.1073/pnas.91.21.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. M., Sanders J. Z., Kaiser R. J., Hughes P., Dodd C., Connell C. R., Heiner C., Kent S. B., Hood L. E. Fluorescence detection in automated DNA sequence analysis. Nature. 1986 Jun 12;321(6071):674–679. doi: 10.1038/321674a0. [DOI] [PubMed] [Google Scholar]
- Stryer L. Fluorescence energy transfer as a spectroscopic ruler. Annu Rev Biochem. 1978;47:819–846. doi: 10.1146/annurev.bi.47.070178.004131. [DOI] [PubMed] [Google Scholar]
- Wang Y., Ju J., Carpenter B. A., Atherton J. M., Sensabaugh G. F., Mathies R. A. Rapid sizing of short tandem repeat alleles using capillary array electrophoresis and energy-transfer fluorescent primers. Anal Chem. 1995 Apr 1;67(7):1197–1203. doi: 10.1021/ac00103a010. [DOI] [PubMed] [Google Scholar]
- Watson J. D. The human genome project: past, present, and future. Science. 1990 Apr 6;248(4951):44–49. doi: 10.1126/science.2181665. [DOI] [PubMed] [Google Scholar]