Abstract
Background
Pre-transfusion washing of red blood cells (RBCs) stored for longer duration may have theoretical advantages but few data exist to support this practice. In many hospital settings, use of a point-of-care cell washer could conceivably be used to quickly wash allogeneic RBCs prior to transfusion. The purpose of this preliminary study was to compare a point-of-care device with a common blood-bank device for washing longer stored RBCs.
Study Design and Methods
Forty RBC units stored for 40-42 days were randomized to washing with the Terumo Cobe 2991 device (FDA-cleared for washing stored RBCs) or the Haemonetics Cell Saver Elite (FDA-cleared point-of-care device for processing/washing fresh autologous shed whole blood). Supernatant and unit RBCs from unwashed (baseline) and washed blood were assayed for potassium, lactate, intracellular ATP, percent RBC recovery, cell-free hemoglobin, RBC-microparticles, and RBCs were examined for susceptibility to hemolysis by physical stress.
Results
Both devices recovered a high percentage of RBCs and efficiently removed extracelluar potassium. Washing with the Elite resulted insignificant increases in cell-free Hb, % hemolysis, and RBC microparticle production whereas washing with the Cobe 2991 did not (fold Δ = 2.1 vs 1.0, 4.6 vs 1.2, 2.0 vs 1.1, respectively; P<0.05). Hemolysis induced by physical stress was not altered by washing.
Conclusion
Although point-of-care washing of longer stored RBCs is appealing, these preliminary data suggest that transfusion of washed, longer-stored units could result in potentially greater exposure to plasma free Hb. More data are needed before this practice can be routinely recommended.
Keywords: storage lesion, cell salvage, washing, red blood cell, erythrocyte, transfusion, hemoglobin, microparticle
Introduction
Red blood cell (RBC) transfusion is one of the most common medical procedures. FDA regulations permit storage of RBCs in additive solution for up to 42 days under controlled conditions. Stored RBCs develop numerous biochemical and morphological abnormalities, often referred to as the “storage lesion”.1 A complex process of cellular injury underlies such irregularities with banking and often manifests as RBC hemolysis within the unit, thus promoting increases in supernatant potassium and free hemoglobin (Hb) concentrations.1 In addition, RBC-derived microparticles are formed as a result of storage-induced blebbing, shedding, and fragmentation.2 Exposure of patients to plasma free hemoglobin and RBC micropaticles may contribute to adverse events associated with transfusion.2-6 For example, plasma free Hb concentrations in septic patients were reported to be an independent predictor of mortality.7
There is growing enthusiasm for the prospect of improving stored RBC quality and removing injurious by-products of RBC storage by washing units prior to transfusion.8-10 In a recent prospective clinical trial, cardiac surgical patients who were transfused washed RBCs (vs unwashed) exhibited less systemic inflammation and a trend toward decreased mortality (2 vs 6 deaths),10 suggesting a potential advantage for erythrocyte washing prior to transfusion. Theoretical benefits of washing can be divided into those affecting the RBC directly and those affecting the supernatant. Washing longer stored banked RBCs may selectively remove RBCs that are more fragile and prone to hemolysis or sequestration. For example, Masalunga et al observed a reduction in osmotic fragility of allogeneic RBCs after washing,11 indicating that fragile cells may be removed during washing. Washing before transfusion may also provide value by eliminating toxic agents that accumulate within the supernatant in direct proportion to banking duration (e.g. potassium, cell-free Hb, RBC-microparticles). Both Hb and RBC-derived microparticles are avid scavengers of nitric oxide (NO),2,12 and the loss of NO and its derivatives may result in impaired perfusion of organs, leading to or exacerbating organ injury associated with transfusion of stored blood.2,4,6,13
The theoretical advantages cited above, however, may be offset by putative injury caused by washing. While longer-stored RBCs (vs short-term storage) could benefit most from being washed, vulnerable cells in these units are presumably more fragile and hence susceptible to damage mediated by the actual wash procedure. For example, Harm et al observed that washing increases the mechanical fragility of RBCs,14 which could lead to increases in in vivo plasma free hemoglobin and bilirubin; this theory is consistent with a study of the effects of washed blood in neonates.11 Therefore, it is also plausible that washed RBCs are more susceptible than unwashed RBCs to hemolytic injury upon stressors encountered in circulation.
Therefore, we undertook a preliminary study of washing longer stored (40-42 day) RBCs with either a point-of-care cell washer or another, commonly used device, to determine and compare the effects of washing on free hemoglobin, RBC-microparticles, and the vulnerability of washed RBCs to mechanical stress.
Materials and Methods
Red Blood Cell Acquisition, Randomization, and Washing
Red blood cell units stored for 40-42 days were acquired from the American Red Cross. We studied a total of 40 red cell units over the course of 4 different days. Ten units per day were randomized 1:1 to each washing device using a computer-generated randomization sequence (5 units washed with each device per day).
Standardized Washing Methods
Washing of RBCs was performed according to standard procedures recommended by the manufacturer of each device. A trained medical technologist from Duke University Hospital Transfusion Service (co-author AEH) was responsible for washing all 40 study units.
Haemonetics Cell Saver Elite Autotransfusion System
Each unit was washed with 1,500 mL of 0.9% NaCl per the manufacturer's guidelines using the default program, which includes a standard centrifugation speed of 5,650 RPM (2,034 g-force) and 225-mL bowl. There was no bowl size for the Elite that would have allowed us to only wash “full” bowls, therefore, use of the 225 ml bowl resulted in washing each unit with one full bowl wash and one partial bowl wash. Duke University owns 10 Elite devices, and all regularly undergo quality control checks. We chose this point of care washing device since it is readily available at Duke University Medical Center. In addition, this device is a commonly used cell-saver machine that could conceivably be used in the operating room or intensive care unit for point of care washing of allogeneic RBCs.
Terumo Cobe 2991 Cell Processor
Each unit was washed with 1,000 mL of 0.9% NaCl per the manufacturer's guidelines and Duke Transfusion Service's protocol for washing a single unit of allogeneic RBCs, which includes a standard centrifugation speed of 3,000 RPM (1245 g-force). Duke Transfusion Service owns three Cobe 2991 devices, and all undergo regular quality control checks.
For all assays (below) samples from each RBC unit were collected immediately prior to and after washing.
Determination of Unit Cell Free Hemoglobin and % Hemolysis
Cell free hemoglobin was measured in the unit supernatant and percent hemolysis calculated as an index of RBC injury using standard spectrophotometric techniques (BMG Labtech). One hundred microliters of blood was centrifuged at 1000g for 2 min at room temperature. The optical density of 25uL supernatant was determined and free hemoglobin was calculated via the Harboe method from absorbance output at 380, 415, and 450 wavelengths.15 The hematocrit was measured by a standard blood gas analyzer. In order to appropriately account for the extent of hemolysed RBCs,16 the percent hemolysis was calculated as:
The total unit amount of free hemoglobin was normalized to the cell free volume for each unit and calculated as:
Cell free unit volume was calculated from an individual unit's hematocrit and total unit volume. Total unit volume was calculated by determining the total mass of each unit and subtracting from this the known mass of the storage bag and tubing; and dividing this net unit mass by an estimated specific gravity for that unit. An estimated specific gravity was predicted based on reported literature17-19 and the following formula:
where SGRBC stands for specific gravity of red blood cells, , and SGSUSP for the specific gravity of the suspending medium.
Percent RBC recovery was estimated as an index of RBCs recovered following wash procedures, and was calculated as: % RBC recovery = RBC Unit Volume Pre-Wash/RBC Unit Volume Post-Wash * 100.
Red Blood Cell Microparticle Assessment
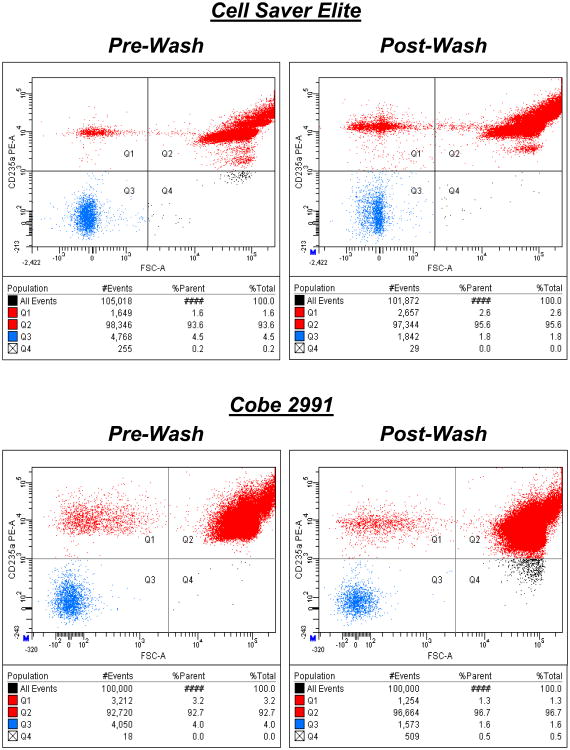
Aliquots from each red cell unit were sampled directly, (i.e. not subjected to additional washing prior to the assay). Red cell suspensions in phosphate-buffered saline (PBS) were labelled with anti-CD235a (anti-glycophorin A, specific to RBCs and RBC-derived microparticles) antibody conjugated to a fluorescent label for flow cytometry as previously described.3 Results from a typical 100,000-event analysis for a pre- and post-wash sample are shown in Figure 1 A and B as the forward scatter (FSC, reflecting size of the cell or particle) vs. the CD235a-positive signal. Two CD235a-positive populations are seen, with distinct size ranges: RBC-derived microparticles in the upper left (lower FSC) and RBCs in the upper right quadrants. The cluster of smaller-sized objects corresponds to microparticles.
Figure 1. RBC Microparticles and the Influence of Washing.
Results from typical analyses for pre- and post-wash samples are shown as the forward scatter (FSC, reflecting size of cells or particles) vs. the CD235a-positive signal. Two CD235a positive populations are seen, with distinct size ranges: RBC-derived microparticles in the upper left (lower FSC) and RBCs in the upper right quadrants. The cluster of smaller-sized objects corresponds to microparticles. 100,000 CD235a-positive events were counted in each analysis.
Induction of Mechanical Stress
The purpose of this assay was to determine the mechanical fragility of unwashed and washed RBCs, and was performed using methodology similar to that of Harm and colleagues.14 Ten glass beads of ∼1000nm diameter (Sigma) were placed into a microcentrifuge tube containing 100uL of a 10% hematocrit RBC suspension (PBS as suspending medium) and vigorously shaken (GeneMate Vortex Mixer, maximum setting) at room temperature for 1 minute in a customized foam adapter. Free Hb was measured before and after mechanical stress in the manner noted above to quantify the change in % hemolysis as a result of mechanical stress.
Induction of Pore Filtration Stress
We also subjected the RBCs to another physical stress (pore filtration) based on the possibility that RBCs can hemolyze after passage through micropores. This methodology was optimized in our laboratory. RBCs were suspended at 10% hematocrit in PBS within a 3cc syringe, and pumped through filters containing 5 μm diameter pores (Millipore; Isopore Membrane Filters) at a pump rate of 100μL/hr. Cell free hemoglobin was measured before and after filtration in the manner noted above in order to quantify the change in % hemolysis as a result of pore filtration stress.
Intracellular ATP and other secondary parameters
Intracellular ATP was measured to examine the impact of washing on intracellular biochemical properties. ATP was determined by luciferin-luciferase procedures using an ATP bioluminescence assay kit (Sigma FLAA Kit) and tube luminometer (20/20n Promega Glomax) on Days 1, 2, & 3 (total N=15 per wash device). RBCs were suspended in PBS at 10% haematocrit, lysed with water and diluted in PBS (600×). Relative light units produced from this media were measured immediately and converted to ATP by use of an ATP standard curve that was created for each study session. Cell free Hb was measured in each sample as described above in order to normalize ATP to Hb concentration (μmol ATP/g Hb).
Oxygen saturation and hematocrit were measured by blood gas analysis (Siemens Rapidpoint 405 analyzer). Glucose was measured in half of the red cell units (total N=10 per wash device) on Days 3 & 4 by the same analyzer. Potassium and Lactate were measured in the supernatant by the clinical laboratory at Duke University Medical Center via standard procedures. If a value was reported out of range, either the corresponding minimum/maximum value for that instrument was inputted.
Statistics
RBC units were randomized (1:1) using a computer-generated randomization sequence. All values are reported as means ± S.E.M. Statistical comparison was limited to primary endpoints (e.g. hemolysis, cell-free Hb) and key secondary endpoints. Comparison of endpoint values at specific time points between red cell washing devices were made with unpaired t-tests, and the values within each washing (pre wash vs post wash) condition with paired t-tests. Significance was set at P<0.05. Given the preliminary nature of this study and the lack of preliminary data in this area no sample size/power calculation was performed. Randomization (1:1) of 40 units was estimated to be a reasonable sample size for this preliminary study.
Results
Four different Cell Saver Elite machines and two different Cobe 2991 machines were utilized over the course of investigation. Additive solution within RBC units was either AS-1 (11 for the Cell Saver Elite, 12 for the Cobe 2991) or AS-3 (9 for the Cell Saver Elite, 8 for the Cobe 2991). Blood types allocated between devices were similar (Cell Saver Elite = 15AB+, 4B+, 1A+; Cobe 2991 = 15AB+, 5 B+). All 40 units were washed 40-42 days after collection. The duration from sample collection to start of endpoint analysis was similar between study arms (mean ∼3.5hours post washing).
Influence of Washing on Basic Characteristics of Longer-Stored RBCs
RBC washing parameters are presented in Table 1. The Cell Saver Elite produced a lower hematocrit and greater cell-free volume after washing compared to the Cobe 2991. This was due to the fact that the Elite device cannot wash the entire RBC unit in one 225 ml bowl, and necessitated a wash cycle for a full bowl and a wash cycle for a partial bowl. Glucose, lactate, and potassium were all decreased as a result of washing. Washing with the Cell Saver Elite increased HbO2 saturation (89±2 to 98±1) but this was not observed with the Cobe 2991 (92±2 to 92±2). Intracellular ATP content was generally low,20 and was unaffected by the washing process from either machine. Percentage of RBC recovery was slightly lower with the Cell Saver Elite but this difference was not statistically significant (p=0.18).
Table 1. RBC Unit Parameters and Impact of Washing.
| Wash Device |
Condition | RBC Recovery (%) |
HCT (%) |
Unit Volume (mL) |
Cell Free Volume (mL) |
Wash Time (seconds) |
Glucose (mg/dL) |
Lactate (mg/dL) |
Potassium (mmol/L) |
ATP (μmol/g Hb) |
Hb Sat (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell Saver Elite (n=20) | Pre-Wash | -- | 65.3±0.9 | 300±6 | 104±3 | -- | 555±26 | 24±1 | 51±1 | 2.74±0.19 | 89±2 |
| Post-Wash | 85±1 | 37.2±0.9* | 449±3* | 282±5* | 730±47 | 66±5* | 5±1* | 1±1* | 2.63±0.18 | 98±1* | |
| Cobe 2991 (n=20) | Pre-Wash | -- | 61.4±1.0† | 288±4 | 111±3 | -- | 579±26 | 24±2 | 50±1 | 2.65±0.11 | 92±2 |
| Post-Wash | 88±2 | 65.8±1.1† | 237±7*† | 82±4*† | 801±45 | 40±3*† | 10±1*† | 2±1*† | 2.57±0.11 | 92±2† |
P < 0.05, Pre-Wash vs Post-Wash within a device.
P < 0.05, Comparison between two devices (eg Pre Elite vs Pre Cobe, or Post Elite vs Post Cobe). Glucose, Lactate, and Potassium results are expressed in concentration units, and thus do not reflect total unit exposure since they are not corrected for cell free volume as was done for the primary endpoint (cell-free hemoglobin, Figure 2a).
Hemolysis in Response to Washing 40-42 Day Stored RBCs
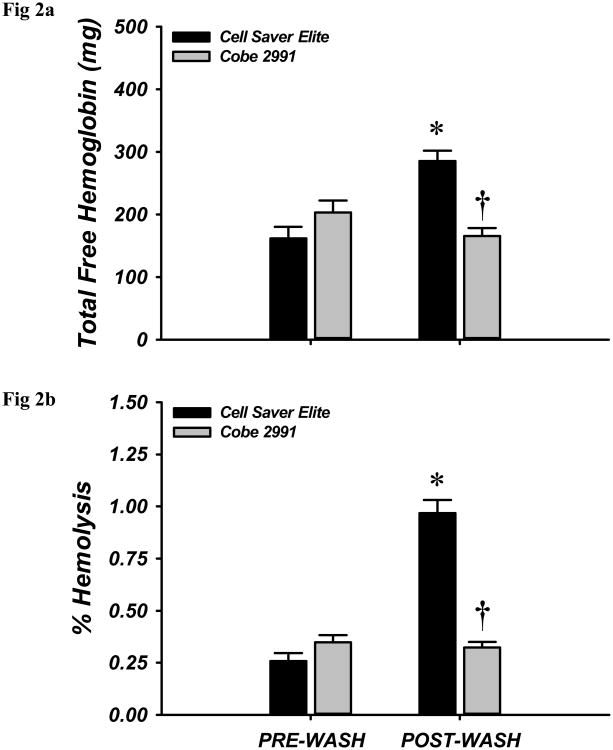
In order to estimate a patient's potential cell-free Hb exposure with transfusion of a washed RBC unit, we expressed cell-free Hb as the total number of grams in a washed unit and as the cell-free Hb concentration. As shown in Figure 2A-B (showing mean pre-wash and mean post-wash values), the Cell Saver Elite increased the total cell-free Hb and % hemolysis substantially. When calculated as the mean post-to-pre ratio values of the individual units, 2.1- and 2.5-fold increases were seen in the total Hb mass and total cell-free Hb concentrations, respectively). The Cobe 2991 did not significantly increase cell-free Hb or % hemolysis (1.0- and 1.2 fold changes, respectively). Cell-free Hb concentration (NOT total cell-free hemoglobin) decreased significantly after washing by the Cell Saver Elite (161.6 + 20.5 mg/dL pre vs. 101.3 + 6.1 mg/dL post-wash; p=0.009), but did not change significantly in units washed by the Cobe 2991 (187.1 + 18.7 mg/dL pre- vs. 213.1 + 19.7 mg/dL post-wash; n.s.).
Figure 2. Influence of Washing Red Blood Cells on Total Unit Cell Free Hemoglobin and Percent Hemolysis.
Red blood cells of late duration storage were exposed to either the Cell Saver Elite or Cobe 2991 washing device. Neither the total free hemoglobin in the unit (A) nor the % hemolysis (B) were different in pre-wash conditions. Washing by the Cell Saver Elite, but not the Cobe 2991, produced significant elevations in total free hemoglobin and % hemolysis. Furthermore, post-wash effects were significantly different between devices. * P <0.05 vs within wash device; † P <0.05 vs Cell Saver Elite.
Impact of Washing on RBC-Derived Microparticle Formation
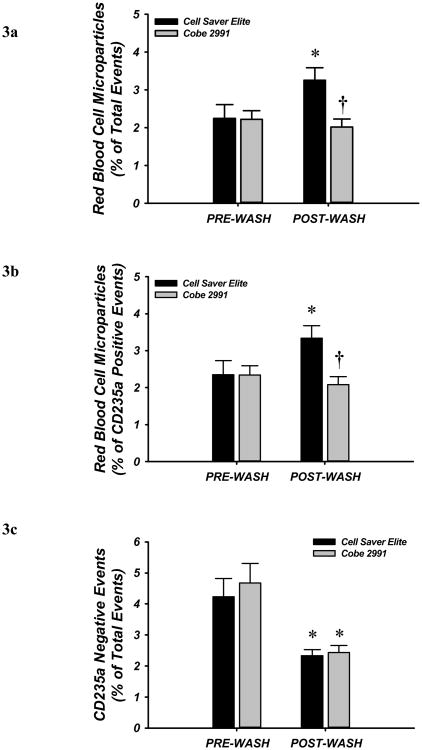
To determine whether the washing process increases fragmentation of red cells, we measured unit contents for RBC-derived microparticles by flow cytometry before and after washing. RBC-derived (CD235a-positive) microparticles were significantly increased from 2.2 to 3.3% (as a percentage of the 100,000 events enumerated), and from 2.4 to 3.3% (of all CD235a positive events) following wash with the Cell Saver Elite. The high concordance between % of total events and the % of CD235a-positive events indicates that platelets or non-RBC-derived microparticles are rare in these units (i.e,. demonstrates effective leukofiltration and platelet removal from the original units), and therefore serves an internal quality control. By contrast, no difference in microparticle frequency was seen after washing by the Cobe 2991 machine (Fig. 3A-B). Non-CD235a positive events (i.e. non-red cells or fragments of leukocyte or platelet origin) were significantly reduced after washing with both devices (Figure 3C).
Figure 3. Red Blood Cell (RBC)-derived Microparticles and the Influence of Washing.
RBC microparticles were determined by flow cytometry. Microparticles per total 100,000 events and specific RBC mircoparticles evidenced via CD235a labeling were greater after washing with the Cell Saver Elite, but not different after washing with Cobe 2991. CD235a negative events (lower panel) were significantly reduced after washing with both machines. . * P <0.05 vs within wash device; †P <0.05 vs Cell Saver Elite.
Induction of Hemolysis by Agitation and Pore Filtration Stress
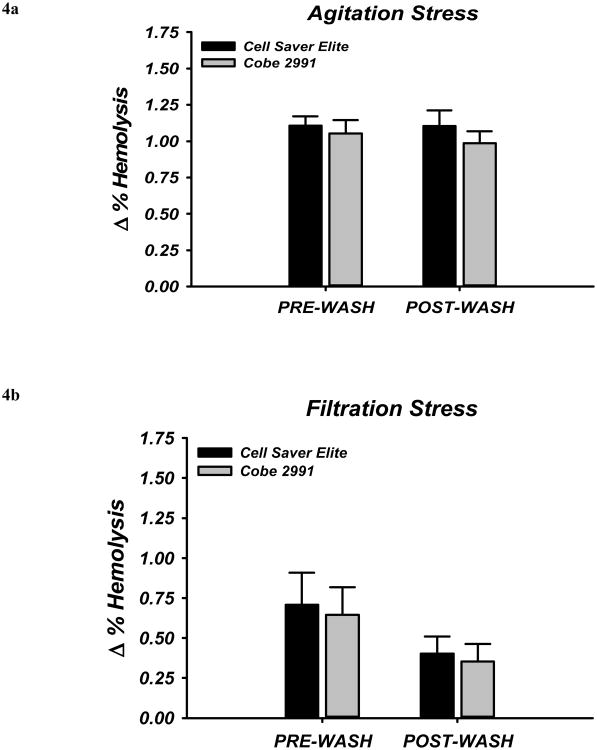
To investigate whether washing of RBCs in the last few days of storage affects cell fragility, we examined the susceptibility of cells to hemolysis following exposure to two different types of physical stress. Mechanical stress from shaking the RBCs with glass beads caused an increase in % hemolysis in all samples (pre- as well as post-wash) and this did not differ with washing from either device (Figure 4A). Hemolysis induced by pore filtration yielded similar results (Figure 4B).
Figure 4. Stress-Induced Hemolysis and the Influence of Washing.
Susceptibility to hemolysis in response to physical stress was determined by (A) agitation stress or (B) filtration stress. Induction of hemolysis by agitation stress was not different before or after washing (A), and this was independent of the specific washing device. Similarly, hemolysis by pore filtration stress was not statistically different between groups prior to washing but was non-significantly reduced by about 30% in each condition.
Discussion
We observed that washing longer (40-42 day) stored RBCs with the Cell Saver Elite and Cobe 2991 devices produces generally similar results with respect removal of glucose, potassium, and lactate, and hemolysis induced by agitation (glass bead) or pore filtration stress. However, in this preliminary study washing with the point-of-care-compatible Cell Saver Elite appears to result in more hemolysis and total cell-free hemoglobin (Hb), as well as RBC-derived microparticle formation. These changes were not evident in units washed with the Cobe 2991. Both devices were similar with respect to removal of non-RBC cells and cell fragments (Figure 3C).
Our results should be of interest to investigators and clinicians who already wash or are considering washing longer-stored RBCs prior to transfusion. It is possible that longer stored RBCs may benefit more from washing immediately prior to transfusion if this process lowers the concentration of potentially toxic compounds (eg free Hb, microparticles) while selectively removing fragile RBCs that are more prone to hemolysis after transfusion. On the other hand, the washing process itself may be more likely to injure the membranes of RBCs in older units, which could result in more in vitro hemolysis with greater exposure to cell-free hemoglobin; moreover, injured but intact RBCs in the washed product may be more susceptible to hemolysis in vivo after transfusion, leading to an even greater exposure to free hemoglobin. This possibility is consistent with results from a study of neonates undergoing –extracorporeal membrane oxygenation that compared groups receiving saline-washed RBCs (n = 31 neonates) vs. unwashed RBCs (n = 29 neonates).11 Peak plasma free hemoglobin and total bilirubin concentrations were statistically significantly higher in the washed-RBC group after transfusion despite no difference in pretransfusion in vivo Hb nor difference in unit Hb.
Our findings, especially those related to cell free hemoglobin (Figure 2) and RBC microparticle production (Figures 3A, B), suggest that based on available data the Cobe 2991 may be a more appropriate candidate to wash longer-stored RBCs. That being said, we do not believe that use of a non-portable transfusion service based device (such as the Cobe 2991) is a pragmatic solution at most centers for washing stored RBCs immediately prior to transfusion. A point-of-care device (e.g. Cell Saver Elite) already used for collecting shed blood in many high blood loss surgeries, would allow for more timely and efficient washing of RBCs. It would also obviate the need, if using a Transfusion Service based device, to wash RBC units ahead of time, some or all of which may not need to be transfused and potentially wasted. Any adverse (or beneficial) effects of washing might vary depending on the time interval between RBC-unit washing and subsequent transfusion as suggested by the study of O'Leary et al.21.
We can only speculate on causes for the differences observed between the two washing devices. One possibility is that washing with the Elite causes slightly more cell damage as suggested by numerically lower RBC recovery (Table 1), and more hemolysis (Figure 2) and microparticle formation (Figure 3). This could be due to the fact that the Cell Saver Elite, which is FDA cleared for washing fresh shed whole blood but not stored blood, exposes cells to higher g-force (2,034) compared with the Cobe 2991 (g-force 1245), which is FDA cleared for washing stored RBCs. This higher g-force might result in more - stress and disruption of the 40-42 day stored RBC membranes. The Cell Save Elite may be more appropriate for washing shed whole blood since it is designed to process “fresh” whole blood that might withstand a higher g-force without injury. It is possible that the programming for the Cell Saver Elite could be modified to allow for a mode with “gentler”/lower g-force specifically for washing stored RBCs.
There are other aspects of the wash cycle that could account for our findings, however, wash duration was similar for both devices (approx 11 minutes total per unit) and the volume of normal saline used was similar (1 L for Cobe 2991 vs. 1.5 L for the Cell Saver Elite). Partly as a consequence of this difference in saline volume used, washing with the bedside-compatible Elite lowered free Hb concentration more than after washing with the Cobe 2991, but the total mass of free Hb was modestly higher in the washed-RBC product from the Elite. In addition, there was no bowl size for the Elite that would have allowed us to only wash “full” bowls, therefore, use of the 225 ml bowl resulted in washing each unit with one full bowl wash and one partial bowl wash. We did not perform separate measurements on the partial bowl wash so we do not know whether this had adverse effects on the quality of the product. Additional studies are needed to ascertain what aspects of the washing process are relevant when washing stored RBCs. Furthermore, while less of an issue in most surgical patients, administration of multiple units of the more diluted Elite washed RBCs could increase the risk of transfusion associated circulatory overload (TACO) in anemic patients who require red cells but are euvolemic.
Of note, our observations on the effects of washing on RBC fragility are not consistent with those of Harm and colleagues who observed an increase in RBC fragility following washing with the Cobe 2991 device.14 We did not observe any increase in fragility due to washing (Figure 3) based on either our mechanical or pore filtration stress assays. This could be due to a difference in methodology or timing given that metal beads and an hour of continuous agitation were utilized in their study vs our briefer stimulus of 1 minute with glass beads. For the filtration stress assay we chose filters containing 5μm diameter micropores in an effort to closely mimic capillary diameter, thus providing some semblance of true physiology. Incorporating the present observations with those made previously11,14,21, it is conceivable that red cell fragility is not greatly influenced (or possibly reduced11) immediately post washing but increases significantly over time.11
Our study has several strengths including randomization of a relatively large number (n=40) of RBC units of similar storage duration (all between 40-42 days). In addition, a Transfusion Service Technologist (co-author AEH) was responsible for washing of all units with both devices. Furthermore, by randomizing (1:1) 10 units on each of the 4 study days we minimized potential confounding that might have occurred if we only washed with one device on the different study days.
Limitations of our study include that it was not practical to add additional arms to study other washing devices. Therefore, we do not know how other point-of-care devices perform with regard to washing longer-stored RBCs. In addition, our results may not be generalizable to washing of RBCs that have been stored for shorter periods of time. Moreover, we washed RBCs with each device according to the manufacturer's recommended procedure (spin rate, wash duration, volume of saline), therefore, it is possible that modifications of one or more of these variables could yield different results. As noted above washing with the Elite device necessitated use of a partial bowl for part of each RBC unit, therefore, this may have had an adverse effect on the wash quality. Finally, our study was not a clinical trial, therefore we cannot comment on the comparative clinical effectiveness of these two washing devices.
In summary, although point-of-care washing of longer stored RBCs is theoretically advantageous, our preliminary data suggest that transfusion of point-of-care washed units could result in potentially greater exposure to plasma free hemoglobin. Transfusion of this washed blood could be deleterious, especially if multiple units are administered. More data are needed before washing of stored RBCs can be routinely recommended for transfusion in patients.
Acknowledgments
We would like to thank Duke University Transfusion Service for their assistance with this study.
Sources of Support: This research was supported in part by National Institutes of Health awards T32-HL007057 (supporting BSK), and R21-HL113943 (TJM). This study was funded in part by Haemonetics Inc.
Footnotes
Reprints will not be available from the author.
Conflict of Interest: Dr. Bennett-Guerrero has attended 2 Advisory Board Meetings for Haemonetics Inc.
Contributor Information
Elliott Bennett-Guerrero, Duke Clinical Research Institute, Duke University, Durham NC.
Brett S. Kirby, Department of Medicine – Division of Hematology, Department of Medicine – Division of Pulmonary, Allergy, and Critical Care Medicine, Duke University Medical Center, Durham, NC 27710 USA.
Hongmei Zhu, Department of Medicine – Division of Pulmonary, Allergy, and Critical Care Medicine, Duke University Medical Center, Durham, NC 27710 USA.
Annadele E. Herman, Department of Pathology, Duke University Medical Center, Durham, NC 27710 USA
Nicholas Bandarenko, Department of Pathology, Duke University Medical Center, Durham, NC 27710 USA.
Timothy J. McMahon, Department of Medicine – Division of Pulmonary, Allergy, and Critical Care Medicine, Duke University Medical Center, Durham, NC 27710 USA
References
- 1.Bennett-Guerrero E, Veldman TH, Doctor A, et al. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci U S A. 2007;104:17063–8. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donadee C, Raat NJ, Kanias T, et al. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–76. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu H, Zennadi R, Xu BX, et al. Impaired adenosine-5′-triphosphate release from red blood cells promotes their adhesion to endothelial cells: a mechanism of hypoxemia after transfusion. Crit Care Med. 2011;39:2478–86. doi: 10.1097/CCM.0b013e318225754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reynolds JD, Bennett KM, Cina AJ, et al. S-nitrosylation therapy to improve oxygen delivery of banked blood. Proc Natl Acad Sci U S A. 2013;110:11529–34. doi: 10.1073/pnas.1306489110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belizaire RM, Prakash PS, Richter JR, et al. Microparticles from stored red blood cells activate neutrophils and cause lung injury after hemorrhage and resuscitation. Journal of the American College of Surgeons. 2012;214:648–55. doi: 10.1016/j.jamcollsurg.2011.12.032. discussion 56-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reynolds JD, Ahearn GS, Angelo M, Zhang J, Cobb F, Stamler JS. S-nitrosohemoglobin deficiency: a mechanism for loss of physiological activity in banked blood. Proc Natl Acad Sci U S A. 2007;104:17058–62. doi: 10.1073/pnas.0707958104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janz DR, Bastarache JA, Peterson JF, et al. Association between cell-free hemoglobin, acetaminophen, and mortality in patients with sepsis: an observational study. Crit Care Med. 2013;41:784–90. doi: 10.1097/CCM.0b013e3182741a54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blumberg N, Heal JM, Gettings KF, et al. An association between decreased cardiopulmonary complications (transfusion-related acute lung injury and transfusion-associated circulatory overload) and implementation of universal leukoreduction of blood transfusions. Transfusion. 2010;50:2738–44. doi: 10.1111/j.1537-2995.2010.02748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blumberg N, Heal JM, Rowe JM. A randomized trial of washed red blood cell and platelet transfusions in adult acute leukemia [ISRCTN76536440] BMC Blood Disord. 2004;4:6. doi: 10.1186/1471-2326-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cholette JM, Henrichs KF, Alfieris GM, et al. Washing red blood cells and platelets transfused in cardiac surgery reduces postoperative inflammation and number of transfusions: results of a prospective, randomized, controlled clinical trial. Pediatr Crit Care Med. 2012;13:290–9. doi: 10.1097/PCC.0b013e31822f173c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masalunga C, Cruz M, Porter B, Roseff S, Chui B, Mainali E. Increased hemolysis from saline pre-washing RBCs or centrifugal pumps in neonatal ECMO. J Perinatol. 2007;27:380–4. doi: 10.1038/sj.jp.7211748. [DOI] [PubMed] [Google Scholar]
- 12.Stapley R, Owusu BY, Brandon A, et al. Erythrocyte storage increases rates of NO and nitrite scavenging: implications for transfusion-related toxicity. Biochem J. 2012;446:499–508. doi: 10.1042/BJ20120675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–39. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 14.Harm SK, Raval JS, Cramer J, Waters JH, Yazer MH. Haemolysis and sublethal injury of RBCs after routine blood bank manipulations. Transfus Med. 2012;22:181–5. doi: 10.1111/j.1365-3148.2011.01127.x. [DOI] [PubMed] [Google Scholar]
- 15.Malinauskas RA. Plasma hemoglobin measurement techniques for the in vitro evaluation of blood damage caused by medical devices. Artif Organs. 1997;21:1255–67. doi: 10.1111/j.1525-1594.1997.tb00486.x. [DOI] [PubMed] [Google Scholar]
- 16.Sowemimo-Coker SO. Red blood cell hemolysis during processing. Transfus Med Rev. 2002;16:46–60. doi: 10.1053/tmrv.2002.29404. [DOI] [PubMed] [Google Scholar]
- 17.Horlocker TT, Wedel DJ. Density, specific gravity, and baricity of spinal anesthetic solutions at body temperature. Anesthesia and analgesia. 1993;76:1015–8. doi: 10.1213/00000539-199305000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Trudnowski RJ, Rico RC. Specific gravity of blood and plasma at 4 and 37 degrees C. Clinical chemistry. 1974;20:615–6. [PubMed] [Google Scholar]
- 19.Reznikoff P. A Method for the Determination of the Specific Gravity of Red Blood Cells. J Exp Med. 1923;38:441–4. doi: 10.1084/jem.38.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salzer U, Zhu R, Luten M, et al. Vesicles generated during storage of red cells are rich in the lipid raft marker stomatin. Transfusion. 2008;48:451–62. doi: 10.1111/j.1537-2995.2007.01549.x. [DOI] [PubMed] [Google Scholar]
- 21.O'Leary MF, Szklarski P, Klein TM, Young PP. Hemolysis of red blood cells after cell washing with different automated technologies: clinical implications in a neonatal cardiac surgery population. Transfusion. 2011;51:955–60. doi: 10.1111/j.1537-2995.2010.02935.x. [DOI] [PubMed] [Google Scholar]