Abstract
Objectives
The knowledge of the basic principles of lymphatic function, still remains, to a large degree, rudimentary and will require significant research efforts. Recent studies of the physiology of the mesenteric lymphatic vessels (MLVs) suggested the presence of an endothelium-derived relaxing factor (EDRF) other than nitric oxide. In this study we tested the hypothesis that lymphatic endothelium-derived histamine relaxes MLVs.
Methods
We measured and analyzed parameters of lymphatic contractility in isolated and pressurized rat mesenteric lymphatic vessels under control conditions and after pharmacological blockade of nitric oxide by Nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME, 100 μM) or/and histamine production by α-methyl-DL-histidine dihydrochloride (α-MHD, 10 μM). Effectiveness of α-MHD was confirmed immunohistochemically. We also used immunohistochemical labeling and western blot analysis of the histamine-producing enzyme, histidine decarboxylase (HDC). Additionally we blocked HDC protein expression in MLVs by transient transfection with vivo-morpholino oligos.
Results
We found that only combined pharmacological blockade of nitric oxide and histamine production completely eliminates flow-dependent relaxation of lymphatic vessels, thus confirming a role for histamine as an EDRF in MLVs. We also confirmed the presence of histidine decarboxylase and histamine inside lymphatic endothelial cells.
Conclusions
Our study supports a role for histamine as an EDRF in MLVs.
Keywords: mesenteric lymphatic vessels, lymphatic endothelium, histamine, EDRF
INTRODUCTION
The lymphatic system provides necessary routes for lymph flow in the majority of body systems and is tightly involved in the maintenance of fluid and macromolecule homeostasis, as well as transport of newly absorbed dietary lipids and immune cells. In contrast to advancements in the biology of the blood vasculature and other systems of the body, knowledge of the basic principles of lymphatic function, especially related to the mechanisms of lymph flow, still remains, to a large degree, rudimentary and will require significant research efforts.
Studies in the last two decades demonstrated the importance of nitric oxide (NO) for regulation of lymphatic contractility as a crucial driving force maintaining effective lymph flow [5,20,24,25,33,36,43]. Considered as a classical endothelium-derived relaxing factor (EDRF) for blood vasculature [17], NO plays an important role as an EDRF in lymphatic vessels through the inhibitory/relaxing effect of its increased basal level during the periods of increased imposed/passive flow [20,24], while oscillatory phasic fluctuations of NO at conditions of absence/minimal diastolic flow support an effective diastolic filling in the largest lymphatic trunk of the body, the thoracic duct [24]. Pump-conduit duality of lymphatic contractile behavior [20,40] can be explained by short-term partial diastolic NO-driven lymphatic relaxation at low levels of basal NO [24], and by long-term permanent NO-driven relaxation at higher levels of basal NO [20]. However regional heterogeneity of lymphatic contractile behavior [18], complicated patterns of NO release in vivo during combined effects of increased stretch and shear [5] and differences in lymphatic contractility in various species [19] have led to conclusions concerning existence of controversy in current literature data on the functional importance of the NO molecule in regulating the active lymph pump [43]. With current limits in our knowledge of mechanisms regulating lymphatic contractility and tone in various lymphatic networks in different species, such pseudo-controversy appears to be an understandable consequence of limitations of our knowledge on lymphatic function.
In contrast to the extensive literature on the predominant but not exclusive role of NO in the regulation of tone in the blood vasculature, there are only sparse literature reports indicating the potential existence of other endothelium-dependent mechanisms regulating lymphatic contractility and tone in a shear-dependent manner linked to measured changes in flow inside of lymphatic vessels [23,24,33,44]. At the same time, in our past experiments, we found that endothelial NO blockade, either by Nω-Methyl-L-arginine acetate (L-NMMA) [20] or by Nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME) [35], was not able to completely eliminate all endothelium-dependent relaxation induced by increases in imposed flow in rat mesenteric lymphatic vessels (MLVs). Our recent studies [35] directly suggested the existence of a yet undiscovered, shear-dependent, but NO-independent regulatory mechanism in rat MLVs. Later, in experiments with immunohistochemical characterization of the phenotype of the mast cells located in close proximity to MLVs [9], we identified the presence of a signal for the histamine-producing enzyme histidine decarboxylase (HDC), not only in mast cells but also in the walls of lymphatic vessels. This intriguing finding, at that moment, together with literature evidence of the role of histamine as an EDRF in some blood vasculature compartments [2,12,16,27,28] and the potential of high but still physiological doses of externally-added histamine to relax lymphatic vessels [38], moved us towards the idea of evaluating the role of histamine as another potential lymphatic endothelium-derived relaxing factor in MLVs.
MATERIALS AND METHODS
For the current studies, we used Fischer-344 male rats, 9-mo old, representing adulthood [9]. All animal procedures for the current studies were reviewed and approved by our Institutional Animal Care and Use Committee and were in accordance with federal and local regulations.
Pharmacological blockade of nitric oxide and histamine production in isolated mesenteric lymphatic vessels
To isolate MLVs, rats were anesthetized with a solution containing a combination of Fentanyl/Droperidol (0.3ml/kg IM) and Diazepam (2.5mg/kg IM). A 4-cm long midline abdominal incision was made through the skin, underlying fascia and muscle layers. A small loop of intestine, 6–7 cm in length was exteriorized through the incision. A section of the mesentery containing lymphatic vessels was positioned in a dissection chamber within the field of view of a dissecting microscope and continuously suffused with standard Dulbecco’s phosphate-buffered saline (Invitrogen Corp., Carlsbad, CA, USA, Cat. # 14040-133). Suitable mesenteric lymphatic vessels were identified and cleared of all surrounding tissue. Sections of mesenteric lymphatic vessels 1–1.5 cm in length were carefully dissected and used for experiments. After isolating mesenteric lymphatic vessels, the rat was euthanized with pentobarbital (120 mg/kg body weight IC). When pressurized to a transmural pressure of 5 cm H2O, the outer diastolic diameters of the MLV segments averaged 136±8 μm.
Once excised, the isolated MLVs segments were transferred to standard 35-mm petri dishes (one vessel/dish) completely filled with ~37°C DMEM/F-12 solution (Invitrogen Corp., Carlsbad, CA, USA, cat.# 11039) supplemented with antibiotic mixture (Invitrogen Corp., Carlsbad, CA, USA, cat.# 15140) to achieve a concentration of 100 units of Penicillin and 100 μg of streptomycin per ml of DMEM/F12 and cultured overnight with unaffected lymphatic contractility [19]. Care was taken to ensure that the vessel segment was completely submerged under the medium in the dish. MLVs were separated into two groups – control and α-methyl-DL-histidine dihydrochloride (α-MHD)-treated groups. On each experimental day, we used both control and α-MHD-treated MLVs obtained from the same animal. α-MHD (Sigma-Aldrich, St. Louis, MO, USA, catalog # M8628) was used at 10 μM overnight (18–20 hours) to block histidine decarboxylase. While α-MHD is confirmed as an effective HDC blocker [15] and various pharmacological inhibitors are able to block HDC activity within a maximum of 1–2 hours, depending on tissue type [29,42], it has been demonstrated that depletion of intracellular reserves of histamine after induced HDC blockade may take up to 4–6 hours [6,39,46]. Thus, to avoid action of pre-formed and stored histamine, we used overnight pharmacological blockade of HDC for functional studies and for subsequent verifications of the effectiveness of α-MHD for the selected purpose (see below).
After overnight culture, control or α-MHD-treated MLV segments were transferred to an isolated vessel chamber (modified Living Systems Instrumentation single vessel chamber model CH/1) filled with pre-warmed (38°C) DMEM/F-12 at pH 7.36. The isolated MLV segments were cannulated and tied onto two carefully matched glass pipettes (100–110 μm). The inflow and outflow pipettes were connected to independently adjustable pressure reservoirs filled with DMEM/F12. All pre-experimental equilibration procedures were performed as described earlier [20,24]. During all experiments, MLV segments were constantly superfused with pre-warmed (38°C) DMEM/F-12, and the isolated vessel chamber was constantly warmed to maintain a temperature of 38±0.1°C in the DMEM/F-12 surrounding the vessel. A CCD video camera, monitor, Windows-operated computer equipped with a National Instruments data acquisition card NI PCI-1410 and DVD/HDD recorder were used to observe and record the lymphatic segments and to track their diameter continuously in all experiments.
At the beginning of every experiment, we evaluated the contractile responses of MLV segments at a transmural pressure of 5 cm H2O for 5 minutes. We chose this transmural pressure because we have shown that the isolated MLVs display maximal active pumping at 5 cm H2O [18,20,21,35]. To determine the imposed flow-induced responses of MLVs, vessel segments were exposed to sets of imposed flow gradients, which were generated using previously established techniques [18,20,21,35]. Because MLVs are very sensitive to changes in transmural pressure, it is important to maintain a constant net transmural pressure at any given imposed flow gradient. As described before, this was accomplished by raising the pressure on the inflow end of the isolated vessel segment and lowering the pressure on the outflow end of the isolated vessel segment by identical amounts so as to create an axial intraluminal pressure gradient (imposed flow gradient) of 1, 3 and 5 cm H2O in MLV segments for 5 minutes at each gradient subsequently without altering the effective transmural pressure in the vessel. These imposed flow gradients have been recently verified to be consistent with those that exist in vivo in rat MLVs [13]. The measurements of the lymphatic contractile activity were performed while superfusing with DMEM/F-12 for control vessels; for α-MHD-treated MLV segments - DMEM/F12 was supplemented with 10 μM α-MHD.
After completion of the imposed flow range in DMEM/F-12 (control) or in DMEM/F-12 with α-MHD (α-MHD-treated group), the solutions in the chamber were additionally supplemented with the nitric oxide synthase (NOS) inhibitor L-NAME (Sigma-Aldrich, St. Louis, MO, USA, catalog #N5751) at 100 μM [1,33,41,45]. The effectiveness of NOS blockade in rat lymphatic vessels induced by application of L-NAME at this concentration has been demonstrated by us in numerous previous reports [5,22,24,35]. The above transmural pressure and imposed flow ranges were repeated in the presence of L-NAME in vessels from control and α-MHD-treated groups. At the end of each experiment, the passive (relaxed) diameter was measured at each pressure after the vessels were exposed to a nominally calcium-free, EDTA-supplemented physiological saline solution (PSS) (in mM: 145.00 NaCl, 4.7 KCl, 1.17 MgSO4, 1.2 NaH2PO4, 5.0 Dextrose, 2,0 Sodium Pyruvate, 3.0 EDTA, 3.0 MOPS) for 15 minutes.
Data Analysis and Statistics for Isolated Vessel Experiments
Lymphatic diameters were tracked continuously during experiments using “Vessel Track” software developed previously [11]. We used cardiac pump analogies to define systole and diastole in reference to the lymphatic contractile cycle [3,18], and the end-diastolic and end-systolic points in the diameter tracings were recorded for each 5-minute interval for the transmural pressure of 5 cm H2O and for imposed flow gradients of 1, 3 and 5 cm H2O. From the lymphatic end-diastolic and end-systolic diameters (EDD and ESD), the following lymph pump parameters were calculated: lymphatic tone index (the difference between the passive lymphatic diameter in Ca-free PSS and end-diastolic diameter, expressed as a percentage of the passive lymphatic diameter in Ca-free PSS), contraction amplitude (the difference between the diastolic and systolic diameters), contraction frequency, ejection fraction (EF, the fraction of end-diastolic volume ejected during the single lymphatic contraction, calculated using formula EF=(EDD2−ESD2)/EDD2 [3], and fractional pump flow (FPF, an index of lymph pump flow, calculated as the ejection fraction times the contraction frequency). To compare the changes in diameters during the lymphatic contractile cycle, the diastolic and systolic diameters were normalized to the passive lymphatic diameters in Ca-free PSS at the corresponding transmural pressure because of the anatomical variations between lymphatic vessels. To investigate solely comparative roles of nitric oxide and histamine in flow-dependent adaptive reactions of the MLVs, we normalized all data in each data set to values of corresponding parameters at zero flow conditions. Statistical differences were determined by ANOVA, regression analysis and Students t-test (JMP software version 5.0.1.2. for Windows, SAS Institute Inc., Cary, NC, USA) and considered significant at P ≤ 0.05. Only one vessel segment was used from one animal for control and one - for α-MHD-treated group. We used data from 7 animals for the functional analysis.
Immunohistochemical analysis of HDC and histamine
For immunohistochemical labeling, the isolated MLV segments were cannulated on glass pipettes in a custom-made single vessel chamber, fixed for 15 min at room temperature in 4% paraformaldehyde, and washed with 0.05% Tween-20 in phosphate-buffered saline (PBST). The vessels were blocked for 1 hour at room temperature in 5% normal goat serum (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA, catalog # 005-000-121) in PBST, washed three times for 15 min each in PBST, and then incubated for overnight at 4°C in PBST containing primary antibodies against HDC (Abcam, Cambridge, MA, USA, catalog # ab37291, 2.6 μg/ml). The next day, after washing three times for 15 min each with PBST, the vessels were stained for CD31 (PECAM-1) (to visualize endothelial cell junctions; clone TLD-3A12, IgG1 isotype, BD Pharmingen/BD Biosciences, San Jose, CA, USA, catalog # 555025; 2 μg/ml) for 1 hr. After washing three times for 15 min each with PBST, the vessels were incubated 1 hr in PBST containing goat anti-mouse IgG1 (catalog # A21121) Alexa Fluor 488 and anti-rabbit IgG (catalog # A21245) Alexa Fluor 647 conjugate (both from Invitrogen Corp., Carlsbad, CA, USA). After further washing in PBST, the vessels were mounted on glass slides under coverslips using ProLong Gold antifade reagent (Invitrogen Corp., Carlsbad, CA, USA, cat. # P36934) and imaged using an Olympus DP72 fluorescence camera and Olympus CKX71 fluorescence microscope equipped with filters suitable for AlexaFluor 488 and 647 imaging (Olympus America Inc., Center Valley, PA, USA). The same parameters of image capturing were utilized for all vessels. To clearly visualize the organization of the cells composing the MLV, some fluorescently-labeled MLVs were imaged on an Olympus Fluoview300 confocal microscope using a PLAPON 60X oil-immersion objective (1.42 N.A.) (Olympus America Inc., Center Valley, PA, USA). The two fluorescent channels were captured sequentially using 488 nm and 633 nm lasers for excitation with 2x Kalman frame averaging. The confocal aperture diameter was set to one airy disk and approximately 60–90 Z-sections were captured per region (0.3 μm Z-step sizes). Confocal images are displayed as an average intensity projection of 5 sections in the middle of the vessel using NIH ImageJ software. The same approach was used for confocal imaging of MLVs in the set of experiments with vivo-morpholino transfections (below).
For histamine detection the protocol was the same as described above for HDC with the following modifications: the isolated vessels underwent the additional fixation in 100% methanol for 20 min on ice. For washings we used 0.3% PBS-TritonX100 (Sigma-Aldrich, St. Louis, MO, USA, catalog # T8787). Finally, vessels were stained with primary antibody against histamine (Abcam, Cambridge, MA, USA, catalog # ab43870, diluted 1/500) together with CD31 staining. The negative controls for all experiments were produced using similar procedures except that the samples were not incubated with the primary antibodies but with immunoglobulins of the same host species. The corresponding negative controls were scanned at the same instrument settings as the unknowns for valid comparison of relative fluorescence intensities.
To verify the effectiveness of pharmacological blockade of HDC by α-MHD, we used MLV segments cultured overnight using the same protocol as control and α-MHD-treated MLVs cultured for functional analysis described above. Then next day these MLVs underwent immunohistochemical labeling for histamine and CD31.
Western blot analysis of HDC
7–10 isolated MLVs obtained from one animal were pooled as one sample and homogenized in RIPA buffer (0.5M Tris pH 7.4, 150mM NaCl, 1% NP-40, 1% Sodium Deoxycholate, 0.1% SDS and 1X protease inhibitor). Total protein concentration was determined by Micro BCA Protein Assay Kit (Thermo Fischer Scientific Inc., Rockford, IL, USA, catalog # 23235). Sample proteins were separated under reducing conditions via SDS-PAGE on a Criterion XT 4–12% Bis-Tris gel (Bio-Rad Laboratories, Hercules, CA, USA, catalog # 345-0125) and then electrotransferred to nitrocellulose membrane. After transfer, the blots were blocked for 2 hrs with 5% non–fat dry milk in 0.05% Tween 20 in 1X tris buffered saline (TTBS) followed by incubation for 2 hrs in blocking solution containing primary antibodies against HDC (Abcam, Cambridge, MA, USA, catalog # ab37291, 2.6 μg/ml) or β-actin (as reference protein according to [8]) (Sigma-Aldrich Corp., St. Louis, MO, USA, catalog # A1978, 5 μg/ml). The blots were washed with TTBS and incubated with goat anti-mouse IgG or anti-rabbit IgG HRP conjugate (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA, catalog # 715-035-151 or catalog # 805-035-180) for 1 hr. After further washing with TTBS, the bands were detected via chemiluminescence (SuperSignal West Dura Extended Duration Substrate, Thermo Fischer Scientific Inc., Rockford, IL, USA, catalog # 34076) and imaged using a LAS-4000 imager (FujiFilm, Tokyo, Japan).
Transient transfection with vivo-morpholino oligos in mesenteric lymphatic vessels
The transient transfection to knockdown HDC was performed using vivo-morpholino oligos from GeneTools LLC (Philomath, OR, USA), according to the manufacturer’s protocol [available from manufacturer by request]. Briefly, the isolated MLVs were cannulated on glass pipettes in a custom-made single vessel chamber in a pressurized condition. The vessels were treated with vivo-morpholino against HDC (5′-GCTCCATCATCTTTCTTGACTTGGC-3′) or standard control (scrambled) morpholino (5′-CCTCTTACCTCAgTTACAATTTATA-3′) at 15 μM concentration in DMEM/F-12 supplemented with antibiotic mixture (as described above for functional studies) for 24 hours in a 37°C 10% CO2 incubator. Control non-treated MLVs were also cultured overnight. The next day all MLVs underwent the immunohistochemical analysis for HDC as described above (without CD31 labeling). For all experiments with immunohistochemical labeling of HDC and histamine, western blot analyses and experiments with vivo-morpholino transfections, we used MLVs from other animals than those which were used for functional studies, due to the necessity of collecting a sufficient number of undamaged MLVs in comparatively short window of dissection time (up to 1.5–2 hours/animal).
RESULTS
Effect of combined pharmacological blockade of nitric oxide and histamine production on flow-dependent relaxation of mesenteric lymphatic vessels
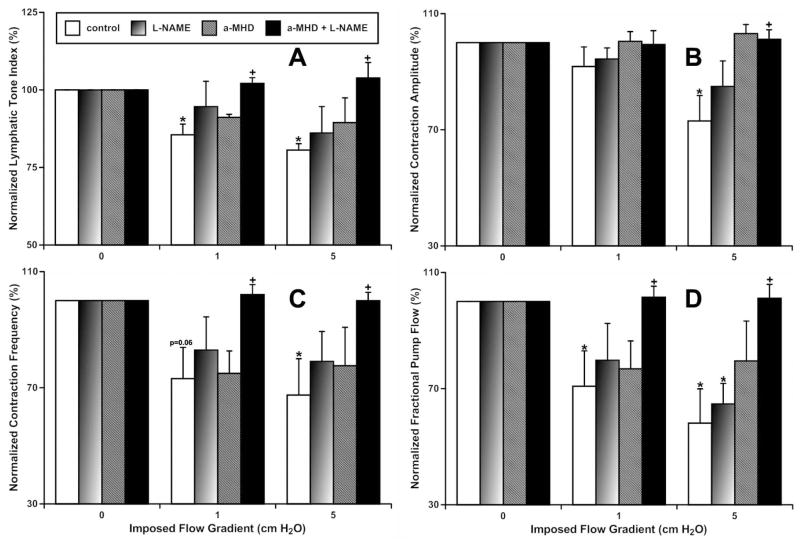
In this study, we compared parameters of the contractile activity in MLVs that underwent overnight blockade of HDC with 10 μM α-MHD (n=6) and time-matched controls (n=7). Then, after subsequent acute (15 minutes) blockade of nitric oxide synthases with 100 μM L-NAME, these parameters were determined again in the same MLV segments (Fig. 1). Control MLVs, after overnight culture, demonstrated contractile activity parameters similar to those described previously for acute and overnight culture experiments [18–20,35]. In particular, these vessels demonstrated significant relaxation (20% decrease in lymphatic tone) and contractile inhibition (27%, 33% and 42% decrease in contraction amplitude, contraction frequency and fractional pump flow, respectively) when an axial imposed flow gradient of 5 cm H2O was utilized, compared to no flow conditions (Fig. 1). (Here and below we will discuss the most profound changes of lymphatic contractility we observed at an axial imposed flow gradient of 5 cm H2O. A similar direction of changes was observed at an axial imposed flow gradient of 1 cm H2O, while some of them were not significant as has been observed at higher flow (Fig. 1).) Blockade of NO production in control MLVs did not eliminate completely such flow-dependent relaxation and contractile inhibition (Fig. 1). Similarly, overnight blockade of the HDC also was not able to eliminate completely such flow-dependent relaxation and contractile inhibition (Fig. 1). During existence of the axial imposed flow gradient of 5 cm H2O, we observed statistically significant differences only between contractile parameters of control MLVs and contractile parameters of those MLVs which underwent overnight HDC blockade with additional NO production blockade. Therefore only combined elimination of both histamine and NO was able to prevent the relaxation and contractile inhibition of MLV developed during existence of an axial imposed flow gradient of 5 cm H2O (Fig. 1.). We want to emphasize here that at an axial imposed flow gradient of 5 cm H2O the contractile parameters in the NO-only- or in the histamine-only-blocked MLVs were reduced by flow in lesser degree than in control but not significantly different from the contractile parameters of control vessels at this high flow. This may create the false impression that these blockades had no effect on contractility of MLVs. However, these even small changes in the contractile parameters of the NO-only- and the histamine-only-blocked MLVs eliminated the significant differences between these parameters at no flow and at 5 cm H2O flow conditions. By strict statistical definition, the latter means that in the NO-only- and histamine-only-blocked vessels, the majority of the flow-induced effects are already gone, and each of these blockades affected vessels by themselves. However only after complete elimination of the effects of both NO and histamine we statistically significantly removed the effects of imposed flow compared to control MLVs at an axial imposed flow gradient of 5 cm H2O (Fig. 1).
Figure 1.
Effect of combined pharmacological blockade of nitric oxide and histamine production on flow-dependent relaxation of mesenteric lymphatic vessels.
A – lymphatic tone index; B – contraction amplitude; C- contraction frequency; D – fractional pump flow.
Control – control MLVs after overnight culture;
L-NAME – same control vessels after subsequent acute pharmacological blockade of NO production by the nitric oxide synthase non-specific blocker, L-NAME (100 μM);
a-MHD – MLVs after overnight pharmacological blockade of histamine production by HDC specific blocker, α-MHD in 10 μM;
a-MHD + L-NAME – same α-MHD-treated vessels after subsequent acute pharmacological blockade of NO production by nitric oxide synthases non-specific blocker, L-NAME in 100 μM.
* indicates significant differences (P < 0.05) between lymphatic contractile parameters within each treatment group at no-flow and at any imposed flow condition.
+ indicates significant differences (P < 0.05) between lymphatic contractile parameters in any treatment groups and control within any given imposed flow condition.
Verification of effectiveness of pharmacological blockade of histamine production
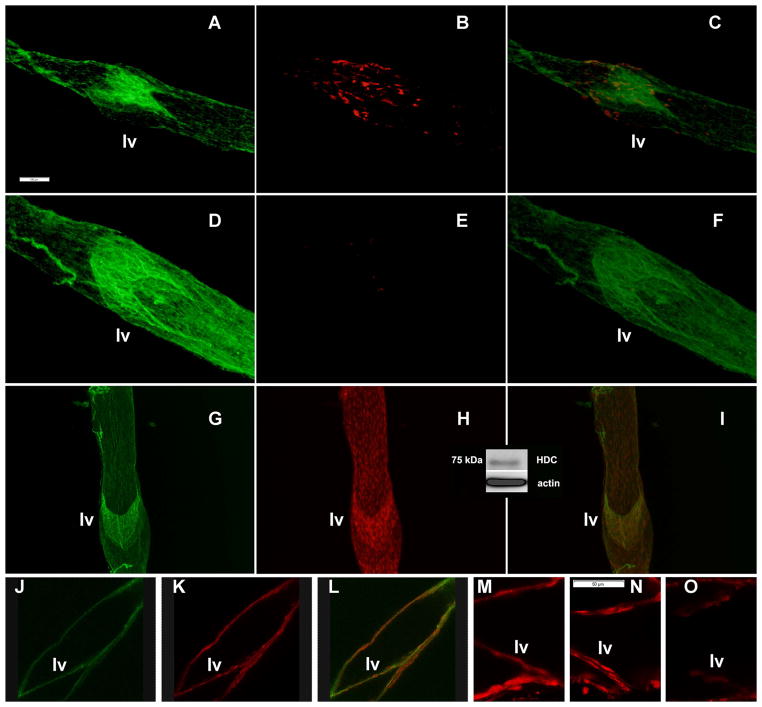
In the next set of experiments, we performed overnight culture of mesenteric vessels in control conditions and under overnight blockade of HDC by α-MHD, as it was performed for functional studies with subsequent immunohistochemical labeling for histamine to confirm the effectiveness of α-MHD application. We used 9 control and 11 α-MHD-treated MLVs obtained from 4 animals. We found profound depletion of the histamine signal after overnight blockade of HDC, compared to MLVs cultured in control conditions. Representative images of control MLVs are presented in Figure 2, A–C, and images of α-MHD-treated MLV are presented in Figure 2, D–F.
Figure 2.
Representative epifluorescence (Panels A–I) and confocal fluorescence (Panels J–O) images of rat isolated mesenteric lymphatic vessels. CD31 (PECAM-1) is stained in green to indicate lymphatic endothelial cell junctions and therefore to delineate location of lymphatic endothelial cells and lymphatic valves (lv).
A–C: Histamine (stained in red) in control MLVs cultured overnight.
D–F: Histamine (stained in red) in α-MHD-treated MLVs cultured overnight.
G–I: Axial view of MLVs stained for histidine decarboxylase (stained in red).
J–L: Cross sectional view of MLVs stained for histidine decarboxylase (stained in red).
M–O: Cross sectional view of MLVs stained for histidine decarboxylase (stained in red). M - presence of HDC signal inside lymphatic valves in control MLVs; N - scrambled morpholino constructs were not able to change HDC signal; O - specific HDC-targeted morpholino constructs were able to deplete HDC signal.
Merged images (Panels C, F, I and L) demonstrate localization of histamine or histidine decarboxylase inside the lymphatic endothelial cells.
Insert (between Panels H and I) demonstrates representative western blot analysis of histidine decarboxylase in MLVs.
Scale bar on Panel A represents 100 μm and applies to images on Panels A–L, scale bar on Panel M represents 50 μm and applies to images on Panels M–O.
Presence of histidine decarboxylase in lymphatic endothelial cells
To localize the presence of histidine decarboxylase in MLVs and particularly inside the lymphatic endothelial cells, we performed fluorescence imaging of the MLVs. We used various approaches for such experiments. Initially we performed epifluorescence imaging of the MLVs using immunohistochemical labeling of these lymph vessels by antibodies to CD31 (PECAM-1) to visualize lymphatic endothelial cell junctions and therefore to delineate the position of the lymphatic endothelium. At the same time we used antibodies to HDC to detect and localize this enzyme within MLVs. For epifluorescence imaging, we used 24 MLVs obtained from 6 animals. We found HDC signal within MLVs corresponds to the pattern of signal for lymphatic endothelial cells (representative images shown in Fig. 2, G, H). Merged images demonstrated that HDC signal exists within lymphatic endothelial cells (Fig. 2, I). We confirmed the findings using confocal imaging of 28 MLVs obtained from 7 animals. Fig. 2, J, K demonstrate representative images for the pattern of signal for lymphatic endothelial cells and HDC, while Fig. 2, L shows a merged image demonstrating that the HDC signal is located inside endothelial cells. We want to specifically direct attention to the parts of the images which show the lymphatic valve (lv on Fig. 2), consisting of only endothelial cells. In addition we performed western blot analysis of HDC protein in MLVs, for which we used 5 pooled MLV samples obtained from 5 animals. Representative results demonstrate presence of HDC in MLVs shown as an insert on Fig. 2 between panels H and I.
Blocking of histidine decarboxylase protein expression by transient transfection with vivo-morpholino oligos in mesenteric lymphatic vessels
Finally, we reconfirmed the presence of HDC inside lymphatic endothelial cells by specific targeting of HDC protein expression by transient transfection with vivo-morpholino oligos applied to MLVs. We performed these experiments with subsequent confocal imaging of MLVs with specific focus on lymphatic valve zones, which consist of only lymphatic endothelial cells. We used 20 control MLVs obtained from 5 animals. Images demonstrated the presence of HDC signal inside lymphatic valves (representative image shown in Fig. 2, M). Scrambled morpholino constructs were not able to change the HDC signal (9 MLVs from 4 animals, representative image shown in Fig. 2, N), while specific HDC-targeted morpholino constructs were able to deplete the HDC signal visibly (11 MLVs from 4 animals, representative image shown in Fig. 2, O), re-confirming the presence of HDC protein within lymphatic valvular endothelial cells.
DISCUSSION
In this study we, for the first time, demonstrated histamine as an additional endothelium-derived relaxing factor in MLVs. The detection of HDC protein in these cells suggests the ability of these cells to synthesize histamine locally within the vascular wall. HDC is the only enzyme responsible for the formation of histamine in mammalian tissues and thus represents the fundamental regulatory entity for histamine production [26,34]. In this study, we not only detected the HDC protein by immunohistochemical labeling and western blot analysis, but also reconfirmed its presence in lymphatic endothelium by specific vivo-morpholino silencing. We also verified the presence of histamine within lymphatic vessels (which in many cases [as in Fig. 2B] was concentrated more by within lymphatic valves. The reason for this should be determined in follow up studies, but could be reasonably linked to the more complicated flow patterns in the areas of lymphatic valves [4,10]. Combined, our findings significantly expand knowledge of the biology of lymphatic vessels by identifying histamine as an additional lymphatic EDRF that together with NO contributes to the fine tuning of lymphatic contractility. Due to the lack of knowledge, in our opinion it is too premature to discuss in detail the timing and the mechanisms of the potential interaction between NO and histamine in providing the relaxation of MLVs. However the potential of high but still physiological doses of externally-added histamine to relax lymphatic vessels has been demonstrated recently [32,38]. Such findings [32,38] appear to be highly supportive of the idea that NO and histamine realize their relaxatory effects on lymphatic muscle through the same molecular targets. As lower doses of externally-added histamine increase frequency and amplitude of lymphatic contractions [14,38] while higher doses of externally-added histamine relax lymphatic vessels [32,38]. We propose that the effects of endothelium-dependent release of internal histamine corresponds to its relaxatory effects observed in other studies [32,38]. We believe that further in depth investigations are necessary to understand the mechanisms of combined effects of NO and histamine in lymphatic vessels.
In light of the new findings presented in this report, some recent findings of our and other groups may be considered more in depth, broadening the general understanding of physiology of the mesenteric lymph flow. As an example of such, a recent report linking the increase in intestinal fat intake with the subsequent increase in histamine concentration in mesenteric lymph initially explained these findings solely by activation of rat intestinal mucosal mast cells [30]. However, during increases in fat absorption, the related rise of lymph viscosity will be a factor that will increase wall shear stress in MLVs [37]. Such events will induce endothelium-derived release of histamine in these lymph vessels as an additional factor that provides its own impact on the increase in histamine concentration in lymph observed in this report [30].
The presence of HDC in lymphatic endothelium suggests not only regulation of lymphatic function directly by histamine. For example, a previous study suggested that histamine may also act as an intracellular signaling molecule specifically in the regulation of the synthesis of lipid mediators by altering cytochrome P450 activity [7]. This could then influence synthesis of other regulators of lymphatic contractility. Further studies are needed to investigate the signaling pathways by which the locally synthesized histamine regulates lymphatic function. In addition, our discovery of the role of histamine as an EDRF in mesenteric lymphatic vessels considered together with well-known roles of histamine in mediating inflammatory and allergic responses provide new important directions to investigate links between lymphatic function, lymph flow, lymph content, inflammation in the gut and immune response. In particular, our current findings of permanent flow-dependent release of histamine by mesenteric lymphatic endothelial cells explain the observed higher density of mast cells in close proximity to MLVs [9]. While released in MLVs, histamine is able to diffuse outside of the lymphatic vessel and to support permanent chemotaxis of mast cells [31] towards this source of histamine, i.e. in the direction of the MLVs. Future studies on the ability of lymphatic vessels to release histamine due to inflammatory activation will improve our understanding of the formation of tissue edema and the initiation of immune responses.
Perspectives.
In this study we provide the first demonstration of a functional role of histamine as an endothelial-derived relaxing factor in mesenteric lymphatic vessels. In addition, using immunohistochemical labeling, western blotting and vivo-morpholino-based transient transfection, we confirmed the presence of the histamine-producing enzyme, histidine decarboxylase, in endothelial cells of mesenteric lymphatic vessels. Our findings provide the basis for new significant advancements in lymphatic physiology, by providing the foundation for better explanations of previous findings and providing new important directions for investigations of the functional interactions between lymphatic function, lymph flow, lymph content, inflammation in the gut and immune response.
Acknowledgments
Funding: This work was supported in parts by the National Institutes of Health (NIH RO1 grants AG-030578 and HL-094269) and by the Texas A&M University Health Science Center College of Medicine and Department of Medical Physiology.
The authors thank Anna Webb and Texas A&M University Health Science Center Microscopy Imaging Facility for help for the confocal imaging studies.
Abbreviations list
- NO
nitric oxide
- EDRF
endothelium-derived relaxing factor
- L-NMMA
Nω-Methyl-L-arginine acetate
- L-NAME
Nω-Nitro-L-arginine methyl ester hydrochloride
- MLVs
mesenteric lymphatic vessels
- HDC
histidine decarboxylase
- α-MHD
α-methyl-DL-histidine dihydrochloride
- NOS
nitric oxide synthases
- EDD
end-diastolic diameter
- ESD
end-systolic diameter
- EF
ejection fraction
- FPF
fractional pump flow
- PSS
physiological saline solution
- PBST
phosphate-buffered saline
- DMEM
Dulbecco’s modified Eagle’s medium
References
- 1.Arenas IA, Xu Y, Davidge ST. Age-associated impairment in vasorelaxation to fluid shear stress in the female vasculature is improved by TNF-{alpha} antagonism. Am J Physiol Heart Circ Physiol. 2006;290:H1259–1263. doi: 10.1152/ajpheart.00990.2005. [DOI] [PubMed] [Google Scholar]
- 2.Benedito S, Prieto D, Nielsen PJ, Nyborg NC. Histamine induces endothelium-dependent relaxation of bovine retinal arteries. Investigative ophthalmology & visual science. 1991;32:32–38. [PubMed] [Google Scholar]
- 3.Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am J Physiol. 1989;257:H2059–2069. doi: 10.1152/ajpheart.1989.257.6.H2059. [DOI] [PubMed] [Google Scholar]
- 4.Bohlen HG, Gasheva OY, Zawieja DC. Nitric oxide formation by lymphatic bulb and valves is a major regulatory component of lymphatic pumping. Am J Physiol Heart Circ Physiol. 2011;301:H1897–1906. doi: 10.1152/ajpheart.00260.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohlen HG, Wang W, Gashev A, Gasheva O, Zawieja D. Phasic contractions of rat mesenteric lymphatics increase basal and phasic nitric oxide generation in vivo. Am J Physiol Heart Circ Physiol. 2009;297:H1319–1328. doi: 10.1152/ajpheart.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouclier M, Jung MJ, Gerhart F. alpha-Fluoromethyl histidine. Inhibition of histidine decarboxylase in pylorus ligated rat. Biochemical pharmacology. 1983;32:1553–1556. doi: 10.1016/0006-2952(83)90326-x. [DOI] [PubMed] [Google Scholar]
- 7.Brandes LJ, LaBella FS, Glavin GB, Paraskevas F, Saxena SP, McNicol A, Gerrard JM. Histamine as an intracellular messenger. Biochemical pharmacology. 1990;40:1677–1681. doi: 10.1016/0006-2952(90)90341-h. [DOI] [PubMed] [Google Scholar]
- 8.Bridenbaugh EA, Nizamutdinova IT, Jupiter D, Nagai T, Thangaswamy S, Chatterjee V, Gashev AA. Lymphatic muscle cells in rat mesenteric lymphatic vessels of various ages. Lymphat Res Biol. 2013;11:35–42. doi: 10.1089/lrb.2012.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatterjee V, Gashev AA. Aging-associated shifts in functional status of mast cells located by adult and aged mesenteric lymphatic vessels. Am J Physiol Heart Circ Physiol. 2012;303:H693–702. doi: 10.1152/ajpheart.00378.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis MJ, Rahbar E, Gashev AA, Zawieja DC, Moore JE., Jr Determinants of valve gating in collecting lymphatic vessels from rat mesentery. Am J Physiol Heart Circ Physiol. 2011;301:H48–60. doi: 10.1152/ajpheart.00133.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis MJ, Zawieja DC, Gashev AA. Automated measurement of diameter and contraction waves of cannulated lymphatic microvessels. Lymphat Res Biol. 2006;4:3–10. doi: 10.1089/lrb.2006.4.3. [DOI] [PubMed] [Google Scholar]
- 12.DeForrest JM, Hollis TM. Shear stress and aortic histamine synthesis. The American journal of physiology. 1978;234:H701–705. doi: 10.1152/ajpheart.1978.234.6.H701. [DOI] [PubMed] [Google Scholar]
- 13.Dixon JB, Greiner ST, Gashev AA, Cote GL, Moore JE, Zawieja DC. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation. 2006;13:597–610. doi: 10.1080/10739680600893909. [DOI] [PubMed] [Google Scholar]
- 14.Fox JLR, von der Weid P-Y. Effects of histamine on the contractile and electrical activity in isolated lymphatic vessels of the guinea-pig mesentery. Br J Pharmacol. 2002;136:1210–1218. doi: 10.1038/sj.bjp.0704820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francis H, DeMorrow S, Venter J, Onori P, White M, Gaudio E, Francis T, Greene JF, Jr, Tran S, Meininger CJ, Alpini G. Inhibition of histidine decarboxylase ablates the autocrine tumorigenic effects of histamine in human cholangiocarcinoma. Gut. 2012;61:753–764. doi: 10.1136/gutjnl-2011-300007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 1989;3:2007–2018. [PubMed] [Google Scholar]
- 17.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 18.Gashev AA, Davis MJ, Delp MD, Zawieja DC. Regional variations of contractile activity in isolated rat lymphatics. Microcirculation. 2004;11:477–492. doi: 10.1080/10739680490476033. [DOI] [PubMed] [Google Scholar]
- 19.Gashev AA, Davis MJ, Gasheva OY, Nepiushchikh ZV, Wang W, Dougherty P, Kelly KA, Cai S, Von Der Weid PY, Muthuchamy M, Meininger CJ, Zawieja DC. Methods for lymphatic vessel culture and gene transfection. Microcirculation. 2009;16:615–628. doi: 10.1080/10739680903120778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gashev AA, Davis MJ, Zawieja DC. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol. 2002;540:1023–1037. doi: 10.1113/jphysiol.2001.016642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gashev AA, Delp MD, Zawieja DC. Inhibition of active lymph pump by simulated microgravity in rats. Am J Physiol Heart Circ Physiol. 2006;290:H2295–2308. doi: 10.1152/ajpheart.00260.2005. [DOI] [PubMed] [Google Scholar]
- 22.Gashev AA, Zawieja DC. Hydrodynamic regulation of lymphatic transport and the impact of aging. Pathophysiology. 2010;17:277–287. doi: 10.1016/j.pathophys.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gasheva OY, Gashev AA, Zawieja DC. Imposed flow-dependent inhibition in rat thoracic duct is not dependent on K channel blockade. FASEB J. 2007;21:A485. [Google Scholar]
- 24.Gasheva OY, Zawieja DC, Gashev AA. Contraction-initiated NO-dependent lymphatic relaxation: a self-regulatory mechanism in rat thoracic duct. J Physiol. 2006;575:821–832. doi: 10.1113/jphysiol.2006.115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagendoorn J, Padera TP, Kashiwagi S, Isaka N, Noda F, Lin MI, Huang PL, Sessa WC, Fukumura D, Jain RK. Endothelial Nitric Oxide Synthase Regulates Microlymphatic Flow via Collecting Lymphatics. Circ Res. 2004 doi: 10.1161/01.RES.0000135549.72828.24. 01.RES.0000135549.0000172828.0000135524. [DOI] [PubMed] [Google Scholar]
- 26.Hocker M, Zhang Z, Koh TJ, Wang TC. The regulation of histidine decarboxylase gene expression. Yale J Biol Med. 1996;69:21–33. [PMC free article] [PubMed] [Google Scholar]
- 27.Hollis TM, Ferrone RA. Effects of shearing stress on aortic histamine synthesis. Exp Mol Pathol. 1974;20:1–10. doi: 10.1016/0014-4800(74)90038-0. [DOI] [PubMed] [Google Scholar]
- 28.Hollis TM, Rosen LA. Histidine decarboxylase activity of bovine aortic endothelium and intima-media. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine. 1972;141:978–981. doi: 10.3181/00379727-141-36914. [DOI] [PubMed] [Google Scholar]
- 29.Ishizuka T, Murakami M, Yamatodani A. Involvement of central histaminergic systems in modafinil-induced but not methylphenidate-induced increases in locomotor activity in rats. Eur J Pharmacol. 2008;578:209–215. doi: 10.1016/j.ejphar.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Ji Y, Sakata Y, Yang Q, Li X, Xu M, Yoder S, Langhans W, Tso P. Activation of rat intestinal mucosal mast cells by fat absorption. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1292–1300. doi: 10.1152/ajpgi.00011.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keles N, Yavuz Arican R, Coskun M, Elpek GO. Histamine induces the neuronal hypertrophy and increases the mast cell density in gastrointestinal tract. Exp Toxicol Pathol. 2012;64:713–716. doi: 10.1016/j.etp.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Kurtz KM, Souza-Smith FM, Breslin JW. Involvement of NO/sGC, but not ROCK, in histamine-induced collecting lymphatic relaxation. FASEB J. 2013;27:681.612. [Google Scholar]
- 33.Mizuno R, Koller A, Kaley G. Regulation of the vasomotor activity of lymph microvessels by nitric oxide and prostaglandins. Am J Physiol. 1998;274:R790–796. doi: 10.1152/ajpregu.1998.274.3.R790. [DOI] [PubMed] [Google Scholar]
- 34.Moya-Garcia AA, Medina MA, Sanchez-Jimenez F. Mammalian histidine decarboxylase: from structure to function. BioEssays: news and reviews in molecular, cellular and developmental biology. 2005;27:57–63. doi: 10.1002/bies.20174. [DOI] [PubMed] [Google Scholar]
- 35.Nagai T, Bridenbaugh EA, Gashev AA. Aging-associated alterations in contractility of rat mesenteric lymphatic vessels. Microcirculation. 2011;18:463–473. doi: 10.1111/j.1549-8719.2011.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohhashi T, Takahashi N. Acetylcholine-induced release of endothelium-derived relaxing factor from lymphatic endothelial cells. Am J Physiol. 1991;260:H1172–1178. doi: 10.1152/ajpheart.1991.260.4.H1172. [DOI] [PubMed] [Google Scholar]
- 37.Orlov RS, Borisova RP, Bubnova NA, Gashev AA, Erofeev NP, Lobov GI, Pan’kova MN, Petunov SG. The lymphatic vessels: their tonus, motility and regulation. [In Russian] Fiziol Zh SSSR Im I M Sechenova. 1991;77:140–149. [PubMed] [Google Scholar]
- 38.Petunov SG, Egorova AA, Orlov RS, Nikitina ER. Effect of histamine on spontaneous contractions of mesenteric lymphatic vessels and lymph nodes of white rats: endothelium-dependent responses. Doklady biological sciences: proceedings of the Academy of Sciences of the USSR, Biological sciences sections / translated from Russian. 2010;432:176–180. doi: 10.1134/S0012496610030038. [DOI] [PubMed] [Google Scholar]
- 39.Puebla L, Arilla E. alpha-Fluoromethylhistidine influences somatostatin content, binding and inhibition of adenylyl cyclase activity in the rat frontoparietal cortex. Regulatory Peptides. 1995;59:111–120. doi: 10.1016/0167-0115(95)00080-u. [DOI] [PubMed] [Google Scholar]
- 40.Quick CM, Venugopal AM, Gashev AA, Zawieja DC, Stewart RH. Intrinsic pump-conduit behavior of lymphangions. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1510–1518. doi: 10.1152/ajpregu.00258.2006. [DOI] [PubMed] [Google Scholar]
- 41.Rees DD, Palmer RM, Schulz R, Hodson HF, Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990;101:746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saxena SP, McNicol A, Brandes LJ, Becker AB, Gerrard JM. A role for intracellular histamine in collagen-induced platelet aggregation. Blood. 1990;75:407–414. [PubMed] [Google Scholar]
- 43.Scallan JP, Davis MJ. Genetic removal of basal nitric oxide enhances contractile activity in isolated murine collecting lymphatic vessels. J Physiol. 2013;591:2139–2156. doi: 10.1113/jphysiol.2012.250662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsunemoto H, Ikomi F, Ohhashi T. Flow-mediated release of nitric oxide from lymphatic endothelial cells of pressurized canine thoracic duct. Jpn J Physiol. 2003;53:157–163. doi: 10.2170/jjphysiol.53.157. [DOI] [PubMed] [Google Scholar]
- 45.Watanabe S, Yashiro Y, Mizuno R, Ohhashi T. Involvement of NO and EDHF in flow-induced vasodilation in isolated hamster cremasteric arterioles. J Vasc Res. 2005;42:137–147. doi: 10.1159/000083652. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe T, Yamatodani A, Maeyama K, Wada H. Pharmacology of alpha-fluoromethylhistidine, a specific inhibitor of histidine decarboxylase. Trends in pharmacological sciences. 1990;11:363–367. doi: 10.1016/0165-6147(90)90181-7. [DOI] [PubMed] [Google Scholar]