Abstract
Recent actions can benefit or disrupt our current actions and the prefrontal cortex (PFC) is thought to play a major role in the regulation of these actions before they occur. The left PFC has been associated with overcoming interference from past events in the context of language production and working memory. The right PFC, and especially the right IFG, has been associated with preparatory inhibition processes. But damage to the right PFC has also been associated with impairment in sustaining actions in motor intentional disorders. Moreover, bilateral dorsolateral PFC has been associated with the ability to maintain task-sets, and improve the performance of current actions based on previous experience. However, potential hemispheric asymmetries in anticipatory regulation of action have not yet been delineated. In the present study, patients with left (n=7) vs. right (n=6) PFC damage due to stroke and 14 aged- and education-matched controls performed a picture naming and a verbal Simon task (participants had to say “right” or “left” depending on the color of the picture while ignoring its position). In both tasks, performance depended on the nature of the preceding trial, but in different ways. In the naming task, performance decreased if previous pictures were from the same rather than from different semantic categories (i.e., semantic interference effect). In the Simon task, performance was better for both compatible (i.e., response matching the position of the stimulus) and incompatible trials when preceded by a trial of the same compatibility (i.e. Gratton effect) relative to sequential trials of different compatibility. Left PFC patients were selectively impaired in picture naming; they had an increased semantic interference effect compared to both right PFC patients and aged-matched controls. Conversely, right PFC patients were selectively impaired in the Simon task compared to controls or left PFC patients; they showed no benefit when sequential trials were compatible (cC vs. iC trials) or a decreased Gratton effect. These results provide evidence for a double dissociation between left and right PFC in the anticipatory regulation of action. Our results are in agreement with a preponderant role of the left PFC in overcoming proactive interference from competing memory representations and provide evidence that the right PFC, plays a role in sustaining goal-directed actions consistent with clinical data in right PFC patients with motor intentional disorders.
Keywords: Proactive control, Prefrontal cortex, Hemispheric differences, Semantic Interference effect, Gratton effect
Introduction
Our ability to anticipate and regulate our actions is crucial to navigating through our everyday lives. The anticipatory regulation of action, or anticipatory control processes, is distinguished from online control processes wherein we control our actions during execution or “on-line” (e.g. Ridderinkhof et al., 2011). Anticipatory control processes can enhance or diminish online control processes depending on the context of the action performance or the recent history of the action. For instance, if you barely succeed in braking to stop at a red traffic light that you noticed a little late, your anticipation to subsequent traffic lights will be enhanced. However, if you encounter several green lights in a row, your attention may start to drift away from the color of subsequent traffic lights, until you reach a red light. These sequential behavioral adjustments are a reflection of anticipatory regulations of action and have been extensively studied experimentally (e.g., see review by Egner, 2007). However, whether or not different aspects of the anticipatory regulation of action are supported by different brain regions remains to be clarified. The goal of the present study is to shed light on this issue.
Terminology of proactive control processes
The anticipatory regulation of action control can be subdivided into distinct processes. Interference- (or conflict) driven adjustments in cognitive control, memory effects of stimulus-response associations, as well as repetition expectancy may contribute to sequential behavioral adjustments (Egner, 2007). The adjustments following the late detection of the red traffic light are an example of inteference-driven behavioral adjustments and have been described as being reactive in nature (Ridderinkhof et al., 2011). Indeed, seeing the red light at the last moment and having to hit the brakes faster or harder than usual represents a difficult or “high interference” situation. This reaction to unexpected difficulty will engender enhanced control in subsequent, similar situations (modeled within the conflict-monitoring framework of Botvinick et al., 2001). In contrast, memory effects of stimulus-response associations or repetition expectancy (Gratton et al., 1992) would occur in anticipation of a future event and would be described as being prospective in nature. For example, if you generally run into busy traffic at a particular intersection, you might increase your attention as you approach that point. Thus, here it is the memory of an event and the associated appropriate behavior that drives proactive control. As in the driving example, such repetition (e.g. a series of green lights) may trade off with interference-driven behavioral adjustments. In this study, we focus on these interference- and repetition-driven aspects of proactive control processes.
These different categories of behavioral adjustments have been mainly studied using tasks in which the compatibility in stimulus-response (S-R) associations are manipulated somewhat arbitrarily, such as in the Simon task (Simon, 1969), the Eriksen flanker task (Eriksen and Eriksen, 1974) and the Stroop task (Stroop, 1935). In these tasks, on some of the trials (i.e., incompatible trials) a prepotent response has to be inhibited for the correct (i.e., rule-based) response to be produced, while on other trials (i.e., compatible trials) the prepotent response matches the rule-based response. Within-trial compatibility effects are characterized by worse performance due to a need for increased on-line cognitive control demands for incompatible as opposed to compatible trials (Ridderinkhof et al., 2011). These tasks offer the possibility to look at sequential behavioral adjustments by looking at the Gratton effect (Gratton et al., 1992), which shows that the size of the compatibility effect on a given trial n is a function of whether or not the S-R association on the previous trial (i.e., trial n-1) was compatible. The compatibility effect at trial n will be increased if trial n-1 was compatible compared to situations in which trial n-1 was incompatible.
The anticipatory regulation of action has also been studied in the context of working memory and language production, and similar proactive control processes have been argued to be involved in both types of cognitive functions (Jonides and Nee, 2006, Kan and Thompson-Schill, 2004). In both cases, the activation of a given representation in memory can either interfere with or facilitate the execution of the current action and this can be manipulated in specific tasks (e.g. the recent probe task in working memory, Monsell, 1978; the blocked cyclic picture naming paradigm in language production, Damian et al., 2001). If the representation already activated in memory is close to (e.g., in the same response-set or from the same semantic category), performance will be impaired. Symmetrically, if the representation already activated in memory matches the one to be accessed at present time, performance will be facilitated. In language production, lexical selection is one of the processes which is sensitive to the activation levels of previously accessed representations. Lexical selection accesses the mental representation of a word from a given concept during speech and is thought to be a competitive process: When we select a given word (e.g. “mosquito”), words of the same semantic category are also activated (e.g. “bee”, “fly”, “bug”, etc.). Competition takes place so that the accurate target can be selected and produced. This competition notion is supported by the occurrence of errors occurring in our everyday life (i.e. semantic errors) and by several experimental paradigms eliciting the so-called “semantic interference effect” (e.g. Lupker, 1979, Kroll and Stewart, 1994, Damian et al., 2001, Howard et al., 2006).
Thus in various cognitive domains, proactive control has been found to create both interference and facilitation effects on behavior. These have been linked to various processes among which reactive enhancement of cognitive control after difficult trials, and repetition expectancy facilitating stimulus-response associations if repeated from one trial onto the next. However, whether the same brain substrate or substrates are associated with these different types of proactive control mechanisms is unclear.
Brain correlates of proactive control processes
The prefrontal cortex (PFC) is thought to play a major role in the anticipatory regulation of action across different cognitive domains (Fuster, in Stuss and Knight, 2013; Thompson-Schill et al., 2005). The left inferior frontal gyrus (LIFG) has been associated with resolving interference caused by previous trials on the processing of current ones in language production (e.g., Schnur et al., 2006, 2009, Thompson-Schill et al., 1997, 1998) and in working memory tasks (Jonides and Nee, 2006; Thompson-Schill et al., 2002; Hamilton and Martin, 2005). The mid-ventro-lateral PFC, a sub-part of the LIFG, has also been associated with facilitatory effects of proactive control using a word version of the recent probe task (Badre and Wagner, 2005) suggesting the same brain substrate may be involved in both inhibitory and facilitatory aspects of proactive control.
Other regions in the PFC have been associated with control over non-linguistic motor responses. The right PFC has been implicated in stopping a prepotent response, e.g. the Stop-Signal and Go-No Go tasks (e.g. Aron et al., 2004), in maintaining inhibitory control (Kramer et al, 2013), and in modifying a current motor plan in target reaching tasks (e.g. Mutha et al., 2014; Schaefer et al., 2012). Although most studies have focused on the reactive inhibitory process, some have also addressed the aspect of proactive control (see Aron, 2011 for a review). Recent evidence suggests the same inhibitory control network, comprised of the right inferior frontal cortex, the pre-supplementary motor area (preSMA) and the sub-thalamic nucleus (STN), that is associated with reactive stopping is also active when preparing to stop (e.g. Chikazoe et al., 2009; Jahfari et al., 2010; Swann et al., 2012). This would imply a role of the right PFC in proactive inhibitory control
Interestingly, right PFC damage can lead to different types of impairment. In agreement with the response inhibition interpretation, Aron et al. (2003) showed that right IFG damage seems to be particularly detrimental to response inhibition in comparison to damage in other parts of the right PFC. However, impairment in sustaining actions that are dependent on directed attention, i.e. motor impersistence, can also be observed acutely (Heilman, 2004, Kertesz et al., 1985) when damage occurs in the right hemisphere and especially in the right frontal cortex (Kim et al., 2013). This would argue for a role of the right PFC in facilitatory aspects of proactive control. We note that the precise brain region(s) involved in this facilitatory aspect of proactive control has not been delineated. Thus, it is possible that different regions of the right PFC may be involved in facilitatory vs. inhibitory proactive control mechanisms.
In addition, bilateral PFC, and especially the dorso-lateral PFC (DLPFC), has been shown to play an important role in maintaining task-sets, i.e. associations between visual cues and actions (for reviews see Miller and Cohen, 2001; Sakai, 2008). The maintenance of such task-sets helps reduce possible interference from previous items but also facilitates the processing of repeating trial features. This would be in agreement with the idea that different parts of the lateral PFC play different roles in facilitatory vs. inhibitory aspects of proactive control.
In summary, it is not clear whether hemispheric asymmetries in the anticipatory regulation of different domains of action exist. Both inhibitory and facilitatory aspects of proactive control have been associated with left and right PFC activations and whether or not interference- and repetition-driven aspects of proactive control processes are associated with overlapping brain regions is uncertain.
Present study
Here, we attempt to dissociate the involvement of left and right PFC in two tasks that engage these different types of proactive control. We tested patients with damage to either the right or left PFC and age-, gender-, and education-matched controls as they performed a picture naming task and a verbal version of the Simon task (Proctor and Vu, 2002; Wühr, 2006). In both tasks, difficulty varied depending on the nature of the preceding trial enabling the assessment of proactive control mechanisms. The naming task was a blocked cyclic picture naming task: pictures were presented within a semantically-homogeneous or heterogeneous context (Damian et al., 2001). Performance was predicted to decrease if previous pictures were from the same rather than different semantic categories (i.e., the semantic interference effect) because increased interference-driven proactive control is present in homogeneous blocks where the competition between semantically-related alternatives is greater than in heterogeneous blocks. In the Simon task, performance on compatible trials (i.e., responses match the position of the stimulus) was expected to be better than on incompatible trials (i.e. responses do not match the position of the stimulus), which is the Simon effect. Moreover, performance was expected to improve when trial types would repeat, i.e. when compatible trials followed compatible trials (cC) and when incompatible trials followed incompatible trials (iI) as compared with when trial types would alternate, i.e. incompatible-compatible (iC) and compatible-incompatible (cI), respectively (i.e. the Gratton effect, Gratton et al., 1992). This effect is hypothesized to reflect both facilitatory and inhibitory aspects of proactive control. Here we focused particularly on repetition-driven facilitatory proactive control.
We expected to replicate previous studies showing an increased semantic interference effect in left PFC patients vs. controls (Schnur et al., 2006) but also aimed to extend these findings to the comparison with right PFC patients. Indeed, we predicted left PFC patients would show a greater semantic interference effect than right PFC patients, supporting a role for the left PFC in overcoming interference caused by response history as previously shown for both language and working memory tasks (e.g., Schnur et al., 2006, 2009; Thompson-Schill et al., 1997, 1998; Jonides and Nee, 2006; Thompson-Schill et al., 2002; Hamilton and Martin, 2005). In contrast, we predicted right PFC patients could be impaired in both facilitatory and inhibitory aspects of proactive control as observed in the Simon task. Indeed, the right hemisphere and more specifically the right frontal cortex has been associated with sustaining goal-oriented actions and the rIFG has been associated with inhibitory proactive control. We predicted disruption of facilitatory aspects of proactive control to be particularly evident in the Simon task as actions (saying “right” or “left”) can be repeated from one trial to the next in this task but not or less so in the picture naming task (see methods).
Methods
All analyses were performed on data previously reported in Riès et al. (2013), with the addition of 2 new aged-matched control participants. Here we report those methods that differed from the previous study. That previous study clearly differed from the present study in that it was focused on EEG components associated with monitoring processes. The effects of the experimental manipulations (semantic interference in the Naming task and Simon and Gratton effect in the Simon task) were not reported there.
2.1. Participants
All patients had left or right PFC lesions due to stroke in the left or right precentral branch of the middle cerebral artery, which provides the major blood supply to the PFC. All were chronic stroke patients and were tested on the neuropsychological tests (mentioned below) and for the current study at least 6 months post-stroke. Lesions were delineated onto the MRICRO templates by a neurologist (RTK) using input from T1, T2 and Flair scans acquired at least 6 months post-stroke on a Siemens Allegra 3.0 Tesla MRI scanner for 13 of the 17 participants included in the analyses, on a MRI-Philips Edge scanner (for 2 of the participants), and on CT-Siemens scanners (Definition or Sensation, for the 2 remaining patients). Of the 17 patients tested, 4 left frontal patients (2 males) could not perform the experimental tasks adequately due to marked aphasia: they either did not understand the instructions properly or their error rate on the experimental tasks was over 40% (mean score on Sequential Commands1: 71/80, SD = 7.79, individual scores: 64, 65, 75, and 80; mean score for Spontaneous Speech: 17/20, SD = 2.16, individual scores: 18, 17, 19, and 14, respectively; thus the one patient who had a good comprehension score of 80 had a poor production score of 14). The data of these 4 left PFC patients were excluded from the analysis. The remaining 7 left PFC patients had a mean Spontaneous Speech score of 18.86/20 (SD = 0.69), reflecting overall good production abilities despite some articulation problems (two patients had a score of 18 reflecting a lack of detail in the picture description or in answering one of the questions). The mean Sequential Command score was of 75.75/80 (SD = 8.81 based on the 6 patients for which we had the Sequential Command score. The last patient had an overall Comprehension score of 9.8/10 reflecting good comprehension abilities). We note that 4 out of the 6 patients had a perfect score of 80, only one had a relatively low score of 59.5 and the other had a score of 75. The patient with the low comprehension score asked to be reminded of the Simon task rule regularly at the breaks but was nevertheless able to perform both tasks correctly. Thus the language production deficits of the left PFC patients included in the analysis were overall mild in nature allowing the patients to perform the tasks adequately. All right PFC patients were included in the analyses as none of them showed impairment preventing them to perform the tasks adequately. None of the right PFC patients had evidence of neglect or extinction based on neurological examination. For two of the right PFC patients, language assessment was performed despite no apparent language problems. Their spontaneous speech and sequential command scores were perfect (20/20 and 80/80 respectively). Lesion overlaps of the 13 patients included in the analyses is presented in Figure 1 and Table 1 provides information about total lesion volume, percent damage in and outside the PFC by patient. Left PFC patients’ lesions were centered in both the inferior frontal gyrus and the middle frontal gyrus. Right PFC patients’ lesions were centered in the middle frontal gyrus. The two groups did not differ in terms of lesion volume (mean lesion volume right PFC patients: 79 cc, σ = 66 cc; mean lesion volume left PFC patients: 104 cc, σ = 63 cc; t(10.521)=0.67, p = .516).
Figure 1.
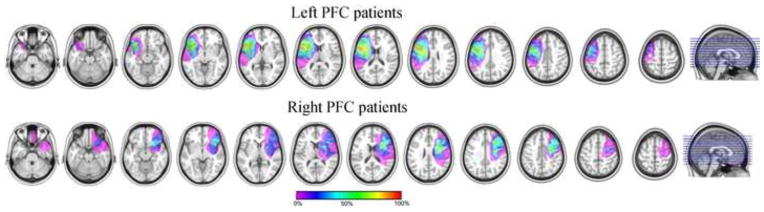
Lesion overlapping of the 7 left (top) and 6 right (middle) PFC patients included in the analyses. Left PFC patients’ lesions are centered in both the inferior frontal gyrus and the middle frontal gyrus. Right PFC patients’ lesions are centered in the middle frontal gyrus. The color bar shows the regions with least (pink) to maximum (red) overlap in the patients within each group: Areas colored in pink were damaged in only 1 of the patients, whereas areas colored in red were damaged in all patients whose lesion reconstructions were added in the overlaps.
Table 1.
Percent damage in the PFC and outside the PFC (to the insula, basal ganglia or temporal lobe) derived from manual delimitation onto the MRICRO templates by a neurologist (RTK), and total lesion volume per patient included in the study.
| % Damage in PFC | % Damage outside the PFC | Total lesion volume | |||
|---|---|---|---|---|---|
| Insula | Basal Ganglia | Temporal lobe | |||
| P1 (right PFC) | 95.10% | 4.90% | 0.00% | 0.00% | 64.60 cc |
| P3 (right PFC) | 96.98% | 0.00% | 3.02% | 0.00% | 53.52 cc |
| P7 (right PFC) | 100.00% | 0.00% | 0.00% | 0.00% | 32.22 cc |
| P11 (right PFC) | 79.31% | 5.70% | 4.57% | 10.42% | 207.34 cc |
| P15 (right PFC) | 100.00% | 0.00% | 0.00% | 0.00% | 29.89 cc |
| P23 (right PFC) | 30.81% | 12.79% | 11.53% | 44.87% | 88.08 cc |
| P4 (left PFC) | 82.85% | 9.45% | 2.47% | 5.23% | 134.02 cc |
| P5 (left PFC) | 81.46% | 7.00% | 8.40% | 3.14% | 72.53 cc |
| P6 (left PFC) | 78.68% | 6.42% | 6.63% | 8.27% | 187.29 cc |
| P9 (left PFC) | 69.35% | 4.46% | 0.52% | 25.67% | 96.98 cc |
| P10 (left PFC) | 44.87% | 6.99% | 3.83% | 44.31% | 167.00 cc |
| P14 (left PFC) | 70.20% | 10.89% | 0.25% | 18.66% | 59.96 cc |
| P21 (left PFC) | 100.00% | 0.00% | 0.00% | 0.00% | 7.34 cc |
| Average | 79.20% | 5.28% | 3.17% | 12.35% | 92.36 cc |
| Standard Deviation | 21.57% | 4.33% | 3.74% | 16.37% | 63.26 cc |
The data of 14 controls (8 females; mean age: 66, SD = 8.79 years old) matched in age, gender and education to the remaining 13 patients (8 females; mean age: 63, SD = 11.47 years old, t(22.49)<1) were collected. Patients had on average 16.5 years of education (SD = 2.85) and controls had on average 16.4 years of education (SD = 1.74; t(19.57)<1).
2.2. Material and Design
The same 252 stimuli were used in both tasks (i.e., line-drawings of common objects or animals colored in green or purple presented on the left or on the right of the fixation cross, 216 were used as experimental items and 36 were used as practice items). They were issued from 6 semantic categories (e.g. animals, vehicles) and each member (e.g. cat) was represented by 7 different items (e.g. 7 different cats: 6 for the experiment in itself and one for the familiarization to the picture names). In the blocked cyclic naming task as generally used, the same group of pictures (e.g. the animals if the block is homogeneous) are repeated for several cycles (5 or 6 most often; e.g. Damian et al., 2001; Maess et al., 2002). The interference effect emerges only after the first cycle, that is only after all the pictures of this group have been named once. Repeating the same pictures several times may cause the retrieval of lexical representations to be less and less necessary as the items are repeated. In our study, we used different pictures to represent the same member of a category (e.g. different types of cats) to increase the need for lexical retrieval and therefore also the need to solve competition caused by previous memory representations in each trial. We were confident this design would give us a reliable semantic interference effect based on the evidence that the semantic interference effect extends to new items (Belke, Meyer and Damian, 2005). The stimuli were line drawings of common objects or animals selected from published collections (Snodgrass and Vanderwart, 1980; Bonin et al., 2003), the Internet, or constructed by us. Their name agreement was tested on a set of 10 controls whose data were not included in the experiment but whose mean age was not significantly different from that of the patients tested here [t(18.06) = 1.42, p = 0.17; mean name agreement: 91.25%, SD = 8%]. Within each experimental run, the order in which the items were presented was mixed pseudorandomly using the software MIX (Casteren and Davis, 2006) such that consecutive items were phonologically unrelated (i.e., two pictures in a row never had the same initial phoneme) and items sharing the same name would be at least 3 trials away from one-another. Moreover, the same stimulus color and the stimulus-response compatibility (described below) could not be repeated more than 4 times in a row. Thus, in the Simon task, there were never more than four identical expected responses in a row. Half of the trials were compatible (i.e., the response to be made matched the position of the stimulus on the screen) and the other half were incompatible (i.e., the response to be made did not match the position of the stimulus on the screen). As the analysis of the Gratton effect was conducted post-hoc we did not initially control for the number of trials in each sequential compatibility condition (compatible-compatible, cC, incompatible-incompatible, iI, compatible-incompatible cI, incompatible-compatible, iC). The mean proportions of each of these trial types were as follows: cC=21% (σ = 1.7%), iI=21% (σ = 1.6%), cI=29% (σ = 1.6%), iC=29% (σ = 1.5%). There were significantly more trials in which there was an alternation between compatibility-types (cI and iC) than trials in which there was a repetition between compatibility-types (cC and iI) (F(1,26)=231.6, p <.001). Importantly here, this did not differ from one participant group to another (F(2,24) = 1.7, p =.204): this imbalance in trial types was present in similar proportions in right PFC, left PFC patients, and aged-matched controls. Thus, we believe the differences we observed between groups did not depend on this aspect of the experimental design.
2.3. Procedure
The procedure was the same as described in Riès et al. (2013). Here we repeat the relevant aspects for the current study. Participants were tested in a sound-attenuated dimly-lit environment. They were seated comfortably 148 cm from a computer screen on which the stimuli were displayed. The experiment was controlled by the Eprime 2.0 Professional software (Psychology Software Tools, Inc., Pittsburgh, PA), which allows on-line recording of the participants’ verbal responses.
A trial consisted of the following events: (1) a fixation point (“plus“ sign presented at the center of the screen) for 500 ms; (2) a picture for 2000 ms (3) a blank screen for 2000 ms. The following trial started automatically. Participants performed two tasks in separate blocks: a picture naming task and a verbal Simon task. In the Naming task, participants were asked to name the picture by saying the name of the picture preceded by the possessive determiner “my” (e.g. “my cat”). In the Simon task, participants were asked to say “my right” or “my left” depending on the color of the picture while ignoring the side to which the picture was presented. Thus interference is greater for a picture presented on the left of the fixation cross when the response to be given is “my right” and vise-versa. The stimulus-response association rule (i.e., saying “my right” for a green picture and “my left” for a purple picture or vise-versa) and the order in which the tasks were performed were counterbalanced across participants. The possessive determiner was added to reduce variability in vocal onsets and because we also recorded EMG activity of three facial articulators for unrelated purposes.
Vocal-onsets were used as the response-onset measure. Each task was split into 4 blocks of 108 trials each, with two pauses equally spaced within each block. Participants performed all 4 blocks of one task before the 4 blocks of the other task. Altogether, the participant saw the same item 4 times corresponding to the 4 possible color/side configurations: green on the left, green on the right, purple on the right and purple on the left. The type of configuration seen per task and per type of block was counterbalanced across participants.
The participants were asked to give their response verbally as fast and as accurately as possible. The task order was counterbalanced and before each task, participants performed a short practice. Before the naming task, the practice served as familiarization with the picture names. We wanted to avoid visual habituation to the experimental stimuli and thus used a set of 36 randomly-presented pictures for practice consisting of a seventh exemplar of each member of each category used in the experiment. Importantly, these practice items had the same names as the experimental stimuli. During practice, the experimenter made verbal corrections when an incorrect or unexpected response was produced. The experimental session lasted 1 to 2 hours.
2.4. Data pre-processing and Analysis
The accuracy of the responses and the verbal reaction times were measured offline using the software CheckVocal (Protopapas, 2007). Trials were excluded from the analysis if the participant did not respond (which represented 44% of all errors), or produced any kind of verbal error: partial or complete production of incorrect words (17%), omission of the pronoun “my” (3%), verbal dysfluencies (e.g., stuttering, utterance repairs: 4%) and hesitations (e.g., if the experimenter perceived the production of the possessive pronoun to be abnormally lengthened or separated from the production of the noun by a pause: 29%).
All statistical analysis were performed using R 3.1.1 (R Core Team, 2012) using the packages “lme4” to compute the mixed effect models (Bates et al., 2014a and b) and “car” to compute analysis of deviance tables for the fixed effects of the mixed effect models (Fox and Weisberg, 2014). We analyzed all data using generalized linear (for reaction times) and logistic (for accuracy rates) mixed-effects models (Baayen, Davidson, and Bates, 2008; Jaeger, 2008), which rely on single-trial data rather then on averages over participants or items, and are also free from the assumptions of homogenous variance and sphericity that are inherent to the more classic ANOVA (Pinheiro & Bates, 2000). The individual reaction times (RTs) were log-transformed to reduce skewness and approach a normal distribution. Visual inspection of residual plots did not reveal any obvious deviations from homogeneity of variance or normality. The analyses were performed on log-transformed RTs and accuracy rates separately for each task. In both tasks, we tested for fixed effects of Group (control, left PFC, and right PFC patients) as a between-subject factor and stimulus position (i.e., left or right of the fixation cross) as a within-subject factor. In the Naming task, in addition to Group and Stimulus Position, we tested for fixed effects of Semantic Context (homogeneous vs. heterogeneous) and Repetition (from 2 to 6) as within-subject factors and all possible interactions between these predictors. In the Simon task, in addition to Group and Stimulus Position, we tested for fixed effects of Compatibility (incompatible vs. compatible), and Compatibility at trial n-1 and all possible interactions between these predictors. As random effects, we had intercepts for participants and picture name, as well as by-subject random slopes for within-subject factors. P-values were obtained using type-III analysis-of-deviance tables (given the presence of significant interactions between the factors of interest) providing Wald chi-square tests for the fixed effects in the generalized linear mixed-effects models. For all models, we report Wald χ2-values and p-values from the analysis of deviance tables as well as raw β estimates, 95% confidence intervals around these β estimates, standard errors, t-values for reaction times, and Wald z and associated p-values for accuracy rates. We report these values for all significant effects in the text and for all of the elements of the planned comparisons in 4 additional tables in the supplementary materials (Tables S1 to S4). In the Naming task, the behavioral pattern in response to the first presentation of stimuli can differ from what is observed in following repetitions. Indeed, the semantic interference effect can be absent or even reversed in the first presentation (e.g., Rahman and Melinger, 2007, Damian et al., 2001, Belke, Meyer, and Damian, 2005). Therefore, it is common practice to exclude the first occurrence of each stimulus on each block from the analysis of the semantic interference effect (e.g., Ewald et al., 2012). For consistency, we also removed these trials from the analysis of the Simon task.
Results
3.1. Picture naming task
3.1.1. Reaction times
There was a main effect of Semantic Context on reaction times (Wald χ2(1) = 32.74, p < .001): RTs were longer in semantically homogeneous versus heterogeneous blocks (βraw = 2.84 × 10−2, CI= [1.87 × 10−2 3.81 × 10−2], SE= 4.97× 10−3, t=5.72; HOM: mean RT = 839 ms, σ = 102 ms; HET: mean RT = 790 ms, σ = 97 ms). This semantic interference effect was larger with increasing repetitions (Wald χ2(4) = 17.31, p = .002, βraw = 1.66 × 10−2, CI= [8.31 × 10−3 2.48 × 10−2], SE= 4.21× 10−3, t=3.93, see Figure 2 A and Table 2). There was also an interaction between Group and Stimulus Position (Wald χ2(2) = 7.24, p =.027), which was due to the comparison between left and right PFC patients (βraw = −7.64 × 10−3, CI= [−1.37 × 10−2 −1.53 × 10−3], SE= 3.11× 10−3, t=−2.45): Patients were slower to respond to stimuli presented contra-laterally to their lesion side (Right PFC patients: mean RT for right stimuli = 853 ms, σ = 58 ms, mean RT for left stimuli = 866 ms, σ = 56 ms; Left PFC patients: mean RT for right stimuli = 812 ms, σ = 120 ms, mean RT for left stimuli = 802 ms, σ = 117 ms see Figure 3 A and Table 2). None of the other relevant comparisons reached statistical significance (see Table S1 for statistical details).
Figure 2.
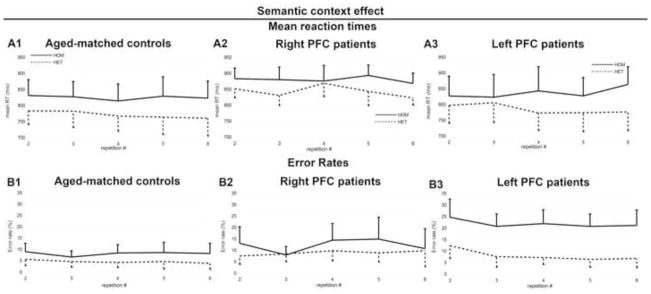
Semantic context effect in the Naming task on reaction times (A1 to A3) and error rates (B1 to B3) for aged-matched controls (A1 and B1), right PFC patients (A2 and B2), and left PFC patients (A3 and B3). Values for homogeneous blocks (HOM) are depicted by the solid lines and values for heterogeneous blocks (HET) are depicted by the dotted lines. Mean values for repetitions 2 to 6 are presented. Standard deviations are represented by the horizontal lines (only positive values are presented for the homogeneous condition and only negative values are presented for the heterogeneous condition for visual clarity).
Table 2.
Mean reaction times (in ms) and error rates (in %) in the Naming task per group, semantic condition, repetition number, and side of stimulus presentation. Standard deviations are in bracket.
| REACTION TIMES | ||||||||
|---|---|---|---|---|---|---|---|---|
| Repetition # | 2 | 3 | 4 | 5 | 6 | average | ||
| Aged-matched controls | HOM | Left stimuli | 842 (110) | 822 (82) | 817 (108) | 833 (138) | 810 (111) | 825 (106) |
| Right stimuli | 819 (93) | 831 (110) | 809 (109) | 824 (111) | 835 (106) | 823 (100) | ||
|
| ||||||||
| HET | Left stimuli | 779 (90) | 778 (102) | 771 (88) | 762 (113) | 766 (117) | 771 (98) | |
| Right stimuli | 786 (85) | 786 (103) | 763 (109) | 765 (108) | 755 (107) | 771 (99) | ||
|
| ||||||||
| Right PFC patients | HOM | Left stimuli | 878 (68) | 880 (72) | 889 (102) | 909 (63) | 868 (79) | 884 (70) |
| Right stimuli | 888 (72) | 880 (91) | 861 (100) | 877 (87) | 869 (62) | 874 (67) | ||
|
| ||||||||
| HET | Left stimuli | 838 (68) | 846 (70) | 895 (98) | 857 (91) | 818 (31) | 851 (59) | |
| Right stimuli | 862 (54) | 814 (71) | 841 (72) | 831 (107) | 828 (67) | 835 (64) | ||
|
| ||||||||
| Left PFC patients | HOM | Left stimuli | 831 (128) | 811 (145) | 844 (142) | 823 (95) | 862 (135) | 833 (124) |
| Right stimuli | 819 (125) | 833 (146) | 840 (166) | 830 (139) | 865 (97) | 838 (132) | ||
|
| ||||||||
| HET | Left stimuli | 792 (128) | 791 (122) | 759 (103) | 773 (130) | 775 (118) | 778 (118) | |
| Right stimuli | 801 (100) | 818 (128) | 786 (119) | 774 (113) | 777 (121) | 791 (114) | ||
|
| ||||||||
| ERROR RATES | ||||||||
| Repetition # | 2 | 3 | 4 | 5 | 6 | average | ||
|
| ||||||||
| Aged-matched controls | HOM | Left stimuli | 9 (7) | 6 (7) | 6 (6) | 4 (5) | 8 (7) | 8 (7) |
| Right stimuli | 4 (5) | 8 (7) | 3 (3) | 7 (9) | 4 (6) | 9 (8) | ||
|
| ||||||||
| HET | Left stimuli | 9 (8) | 6 (5) | 7 (5) | 5 (5) | 8 (7) | 4 (5) | |
| Right stimuli | 4 (3) | 10 (11) | 6 (9) | 10 (9) | 4 (5) | 5 (6) | ||
|
| ||||||||
| Right PFC patients | HOM | Left stimuli | 13 (8) | 6 (2) | 13 (7) | 14 (10) | 14 (10) | 12 (8) |
| Right stimuli | 13 (8) | 9 (5) | 16 (8) | 16 (10) | 7 (6) | 12 (8) | ||
|
| ||||||||
| HET | Left stimuli | 11 (9) | 6 (5) | 10 (10) | 11 (9) | 12 (17) | 10 (10) | |
| Right stimuli | 4 (3) | 10 (8) | 9 (9) | 6 (6) | 7 (11) | 7 (8) | ||
|
| ||||||||
| Left PFC patients | HOM | Left stimuli | 21 (17) | 21 (13) | 25 (14) | 17 (10) | 23 (18) | 22 (14) |
| Right stimuli | 29 (15) | 20 (9) | 18 (9) | 24 (11) | 19 (7) | 22 (11) | ||
|
| ||||||||
| HET | Left stimuli | 9 (8) | 8 (10) | 10 (6) | 6 (7) | 9 (11) | 8 (8) | |
| Right stimuli | 16 (13) | 7 (9) | 5 (5) | 6 (7) | 5 (4) | 8 (9) | ||
Figure 3.
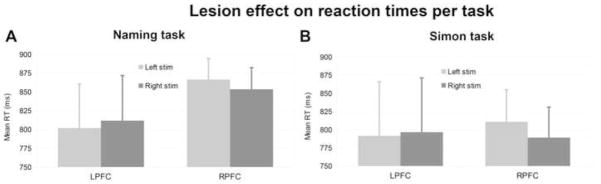
Lesion effect on reaction times per task (A: Naming task, B: Simon task). Light gray bars are for left presented stimuli and dark gray bars are for right presented stimuli. Standard deviations are represented by the horizontal lines.
3.1.2. Accuracy rates
There was a main effect of Semantic Context on error rates (Wald χ2(1) = 40.21, p < .001): Overall, participants were less accurate in semantically homogeneous versus heterogeneous blocks (βraw = −4.62 × 10−1, CI= [−6.05 × 10−1 −3.19 × 10−1], SE= 7.28 × 10−2, Wald z=−6.34, p < .001, Figure 2 B and Table 1). The semantic interference effect did not interact with Repetition on accuracy rates and there was no interaction with Stimulus Position (see Table S2 for statistical details). There was a main effect of Group (Wald χ2(2) = 8.52, p = .014), which was significant in both the comparison between controls and right PFC (Controls were more accurate than Right PFC patients: βraw =5.95 × 10−1, CI= [1.71 × 10−1 1.02], SE= 2.16 × 10−1, Wald z=2.75, p =.006) and in the comparison between left and right PFC (Left PFC patients were less accurate than right PFC patients: βraw = −4.83 × 10−1, CI= [−9.69 × 10−1 2.52 × 10−3], SE= 2.48 × 10−1, Wald z=−1.95, p =.051). Critically, there was an interaction between Group and Semantic Context (Wald χ2(2) = 5.99, p =.050), which was due to the the comparison between left and right PFC patients reflecting a larger semantic interference effect for the left than the right PFC patients (βraw = −2.44 × 10−1, CI= [−4.40 × 10−1 −4.84 × 10−2], SE= 9.98 × 10−2, Wald z=−2.45, p =.015: Left PFC patients: HOM mean error rate = 22%, σ = 9%; HET mean error rate = 8%, σ = 5%, Right PFC patients: HOM mean error rate = 12%, σ = 4%; HET mean error rate = 9%, σ = 7%; Controls: HOM: mean error rate = 8%, σ = 5%; HET: mean error rate = 5%, σ = 3%). There was no difference in the size of the semantic interference effect between controls and right PFC patients (βraw = 7.68 × 10−2, CI= [−1.02 × 10−1 2.56 × 10−1], SE= 9.12 × 10−2, Wald z=0.84, p =.400). There was no interaction between Group and Stimulus Position nor any other significant effects in the other comparisons under analysis (statistical details in Table S2).
3.2. Simon task
3.2.1. Reaction times
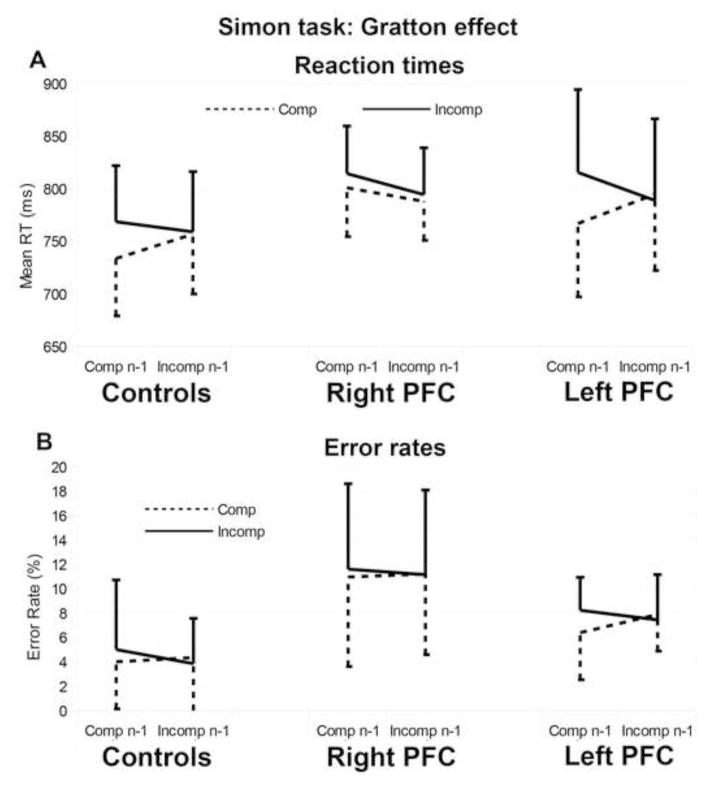
There was a main effect of compatibility at trial n (Wald χ2(1) = 39.31, p < .001): Participants were faster on compatible trials than on incompatible trials (βraw = −2.15 × 10−2, CI= [−2.83 × 10−2 −1.48 × 10−2], SE= 3.44 × 10−3, t=−6.27: mean RT for incompatible trials = 784 ms, σ = 116 ms; mean RT for compatible trials = 767 ms, σ = 112 ms), thus revealing a within-trial Simon effect. There was also an interaction between compatibility at trial n and compatibility at the preceding trial (i.e., trial n-1) (Wald χ2(1) = 28.62, p < .001): the Simon effect at trial n was larger if trial n-1 was compatible than if trial n-1 was incompatible (βraw = 2.20 × 10−2, CI= [1.39 × 10−2 3.00 × 10−2], SE= 4.11× 10−3, t=5.35), thus revealing a Gratton effect (Table 3 and Figure 4A). There was no main effect of Group but, critically, there was a triple interaction between Group, Compatibility at trial n and Compatibility at trial n-1 (Wald χ2(2) = 5.83, p =.054), which was due to the comparison between left and right PFC patients (βraw = 1.41 × 10−2, CI= [1.80 × 10−3 2.64 × 10−2], SE= 6.28× 10−3, t=2.25). This interaction can be explained by the observation that right PFC patients showed an abnormal Gratton effect compared to left PFC patients. As can be seen on Figure 4A, left PFC patients and controls exhibited a Gratton effect for both compatible and incompatible trials, whereas right PFC patients exhibited a Gratton effect for only incompatible trials (Table 3). There was no triple interaction in the comparison between the Gratton effect in controls vs. left PFC patients (βraw = 1.34 × 10−3, CI= [−8.64 × 10−3 1.13 × 10−2], SE= 5.09× 10−3, t=0.26).
Table 3.
Mean reaction times (in ms) and error rates (in %) in the Simon task per group, compatibility at trial n (Comp and Incomp), compatibility at trial n-1 (Comp n-1 and Incomp n-1), and side of stimulus presentation. Standard deviations are in bracket.
| REACTION TIMES | Average over side of stimulus presentation | |||||
|---|---|---|---|---|---|---|
| Comp | Incomp | Comp | Incomp | |||
| Aged-matched controls | Comp n-1 | Left stimuli | 737 (106) | 774 (111) | 734 (109) | 769 (107) |
| Right stimuli | 732 (115) | 763 (105) | ||||
| Incomp n-1 | Left stimuli | 771 (110) | 759 (116) | 757 (114) | 759 (114) | |
| Right stimuli | 743 (120) | 759 (113) | ||||
|
| ||||||
| Right PFC patients | Comp n-1 | Left stimuli | 797 (93) | 831 (95) | 801 (93) | 788 (74) |
| Right stimuli | 807 (107) | 799 (89) | ||||
| Incomp n-1 | Left stimuli | 798 (82) | 816 (95) | 814 (91) | 794 (89) | |
| Right stimuli | 782 (73) | 778 (91) | ||||
|
| ||||||
| Left PFC patients | Comp n-1 | Left stimuli | 774 (144) | 802 (149) | 767 (139) | 795 (145) |
| Right stimuli | 760 (135) | 830 (169) | ||||
| Incomp n-1 | Left stimuli | 801 (154) | 780 (148) | 816 (158) | 789 (155) | |
| Right stimuli | 789 (137) | 796 (161) | ||||
|
| ||||||
| ERROR RATES | Average over side of stimulus presentation | |||||
| Comp | Incomp | Comp | Incomp | |||
|
| ||||||
| Aged-matched controls | Comp n-1 | Left stimuli | 4 (5) | 5 (5) | 4 (4) | 5 (6) |
| Right stimuli | 4 (4) | 5 (7) | ||||
| Incomp n-1 | Left stimuli | 5 (5) | 3 (4) | 4 (5) | 4 (4) | |
| Right stimuli | 3 (4) | 4 (5) | ||||
|
| ||||||
| Right PFC patients | Comp n-1 | Left stimuli | 11 (15) | 11 (11) | 11 (15) | 11 (13) |
| Right stimuli | 11 (15) | 11 (15) | ||||
| Incomp n-1 | Left stimuli | 15 (17) | 11 (12) | 12 (14) | 11 (14) | |
| Right stimuli | 9 (11) | 12 (18) | ||||
|
| ||||||
| Left PFC patients | Comp n-1 | Left stimuli | 9 (9) | 7 (5) | 6 (8) | 8 (6) |
| Right stimuli | 4 (6) | 8 (7) | ||||
| Incomp n-1 | Left stimuli | 8 (6) | 6 (7) | 8 (5) | 7 (7) | |
| Right stimuli | 8 (6) | 9 (9) | ||||
Figure 4.
Gratton effect in the Simon task on reaction times (A) and error rates (B) per participant group. The compatibility at trial n is coded by the color of the bars: Mean values for compatible trials n are depicted by the solid lines and mean values for the incompatible trials are coded by the dotted lines. Compatibility at trial n-1 is represented on the x-axis and mean RT and mean error rates are represented on the y-axis. Standard deviations are represented by the horizontal lines (only positive values are presented for the incompatible condition and only negative values are presented for the compatible condition for visual clarity).
Finally, there was a significant triple interaction between Compatibility at trial n, Group, and Stimulus Position (Wald χ2(2) = 8.70, p =.013), which was due to the comparison between left and right PFC patients (βraw = 1.17 × 10−2, CI= [3.03 × 10−3 2.03 × 10−2], SE= 4.42× 10−3, t=2.65): the within trial Simon effect was more pronounced when the stimulus was presented contra-laterally to the lesion side (Figure S2 and Table 2). We note there was no significant interaction between Group and Stimulus Position. The averages across subjects seemed to indicate a similar trend as in the Naming task especially in right PFC patients: slower RTs to stimuli presented contralateral to the lesion side (Figure 3B) but the absence of overall interaction was probably due to the absence of the effect of Stimulus Position in left PFC patients.
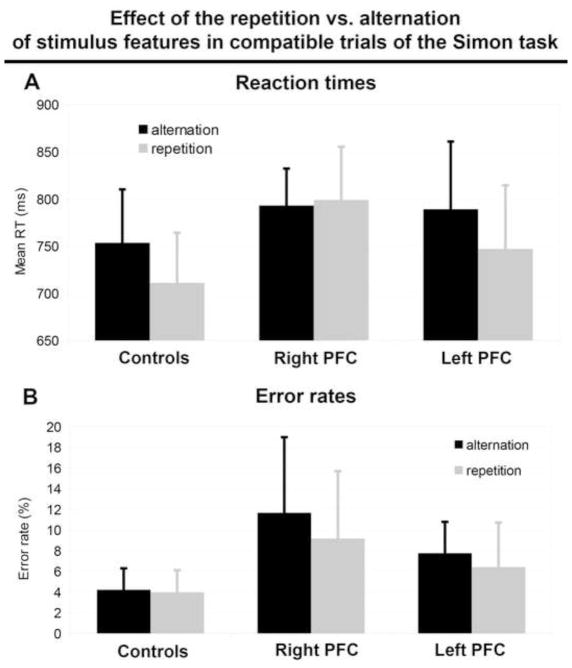
In order to better understand the abnormal Gratton effect in right PFC patients, we conducted an additional analysis on compatible trials alone as it was on these trials that performance was different in right PFC patients than in the other groups. We tested whether the repetition of stimulus features (color and position) had a different effect depending on the group by running an additional mixed-effect model with picture name and subject (and its interaction with Repetition of Stimulus Features) as random effects and Group, Repetition of Stimulus Features, and their interaction as fixed effects. There was a main effect of the repetition of stimulus features (Wald χ2(1) = 26.40, p <.001), where participants were faster to respond when the features repeated vs. alternated from one stimulus onto the next (βraw = 1.70 × 10−2, CI= [8.47× 10−3 25.62× 10−3], SE= 4.375× 10−3, t[26.070]=3.897, p < .001). Critically, there was an interaction between Group and the repetition of stimulus features (Wald χ2(2) = 8.48, p =.014), which was visible mainly in the comparison between left and right PFC patients (βraw = 1.19 × 10−2, CI= [5.70× 10−3 31.89× 10−3], SE= 6.68× 10−3, t=2.81). The effect was smaller in the comparison between controls and left PFC patients (βraw = 1.07 × 10−2, CI= [0.09× 10−3 21.28× 10−3], SE= 5.404× 10−3, t=1.978). We then computed post-hoc paired t-tests to test for an effect of the repetition of stimulus features per group: In controls, there was an expected benefit on performance when the stimulus at trial n shared the same features as the stimulus at trial n-1: RTs were faster for repetitions (mean RT = 711 ms, σ = 106 ms) than for alternations of stimulus features (mean RT = 754 ms, σ = 113 ms; t(13) = 6.69, bonferroni-corrected p-value < .001). This was also the case in left PFC patients (t(6) = 4.26, bonferroni-corrected p-value = .016; mean RT in repetitions = 747 ms, σ = 135 ms; mean RT in alternations = 789 ms, σ = 144 ms) but not in right PFC patients (t(5) < 1; mean RT in repetitions = 799 ms, σ = 113 ms; mean RT in alternations = 793, σ = 78 ms) (Figure 5 A).
Figure 5.
Effect of the repetition vs. alternation of stimulus features in compatible trials of the Simon task on mean reaction times (A) and mean error rates (B) for aged-matched controls, right PFC patients and left PFC patients. Black bars indicate values for trials in which there is a complete alternation of stimulus features in comparison to the preceding trial and gray bars indicate values for trials in which there is a complete repetition of stimulus features in comparison to the preceding trial.
3.2.2. Accuracy rates
Error rates in the Simon task were overall relatively low and the effects we reported on reaction times were not observed on accuracy rates (see Table 2 and S4).
The analysis of deviance table only revealed an interaction between Compatibility at trial n and Stimulus position (Wald χ2(1) = 7.69, p =.006). However, the βraw was small and the confidence intervals crossed zero indicating this effect was very small (βraw = −0.09, CI= [−.21 0.04], SE= 0.06, z =−1.36, p=.174). There was no interaction between Compatibility at trial n-1 and at trial n, no effect of Group nor any interaction between Group and any of the other factors under analysis (see Table S4 for statistical details).
When compatible trials were analyzed separately, there was no main effect of the repetition of stimulus features on accuracy rates (Wald χ2(1) = 1.09, p =.297), indicating participants were not more accurate when the features repeated vs. alternated from one stimulus to the next (βraw = −0.17, CI= [−.46 .12], SE= 0.15, z =−1.135, p =.257). There also was no interaction between Group and the repetition of stimulus features (Wald χ2(2) = .49, p =.783, both ts < 1 in the output of the mixed effect model, Figure 5B).
4. Discussion
We observed a double dissociation between left and right PFC patients using two different measures of proactive control. Left PFC patients were selectively impaired on the Naming task, and also showed an increased semantic interference effect compared to both right PFC patients and aged-matched controls. Conversely, right PFC patients were selectively impaired on the Simon task; for compatible trials, they did not show any benefit of the repetition of stimulus features in comparison to both left PFC patients and aged-matched controls. Thus, our results suggest the left and right PFC play differential roles in the anticipatory regulation of action.
Role of the left PFC in overcoming interference from previous trials
Our results replicate the observations made by Schnur et al. (2006) using a similar paradigm: left PFC patients showed a greater semantic interference effect compared to aged-matched controls on error rates. Our results also extend previous studies by comparing the performance of left PFC patients to that of right PFC patients on the same task. We show that the increased semantic interference effect was specifically observed in left vs. right PFC patients. Thus, the left but not the right PFC seems to play a central role in overcoming interference caused by semantically-related representations. Similarly, the left but not the right inferior frontal gyrus (IFG) has been described as playing a central role in overcoming proactive interference in working memory tasks (for a review see Jonides and Nee, 2006). A question that can be raised is whether or not the specific involvement of the left vs. the right IFG is linked to the linguistic nature of the stimuli. Jonides and Nee (2006) report a mixed conclusion as some studies show a greater involvement of the left inferior cortex in overcoming proactive interference caused by linguistic versus non-linguistic stimuli (e.g., Mecklinger et al., 2003), but others fail to report such differences (Brandon et al., 2003, see also Brandon et al., 2004). Interestingly, none of the reviewed studies reported a greater involvement of the right vs. left IFG when stimuli were non-linguistic and at best, activations were bilateral.
In the present study, we used both a linguistic and a non-linguistic task to assess proactive control. The Gratton effect observed in the Simon task provides two measures of proactive control. Below, we focus on the facilitatory aspect, whereby performance is better for a compatible trial n if it follows a compatible trial n-1 than if it follows an incompatible trial n-1. However, previous brain imaging studies have mainly focused on another aspect of the Gratton effect - the strengthening of proactive control which occurs after an incompatible trial n-1 and leads to a reduction of the compatibility effect at trial n. These studies have shown that the medial frontal cortex, and especially the anterior cingulate cortex (ACC) and SMA, and not the left PFC, mediates an increase in proactive control following incompatible trials (e.g., Botvinick et al., 1999; Dosenbach et al., 2006; Kerns et al., 2004, Kerns, 2006, Horga et al.; 2011; Nachev et al., 2005). This region was intact in the population we tested and accordingly, all groups showed a benefit on performance in incompatible trials n when trial n-1 was incompatible. Thus, it appears that the type of proactive control that is up-regulated after an incompatible trial in the Simon task is of a different nature than that tested using working memory tasks (as reviewed by Jonides and Nee, 2006) or language production tasks manipulating semantic interference (e.g. the blocked cyclic picture naming paradigm used here and for example in Schnur et al., 2006, 2009). We argue that a dissociation of these two types of proactive control mechanisms may be due to differences in the level of interference in the two tasks used here. Indeed, according to the classification of compatibility effects by Kornblum et al.,(1990), interference in the Simon task occurs at the level of responses: the irrelevant stimulus dimension (i.e., the position of the stimulus) can directly activate the inappropriate response and there will then be a competition between the correct rule-based response and the incorrect response. In contrast, in the picture naming task the interference is more likely to occur at the level of the stimuli as it is due to prior knowledge linked to the organization of our semantic representations. Such an interpretation is supported by fMRI evidence directly contrasting these two types of conflict using the Stroop task (Milham et al., 2001), which suggests that the ACC and right PFC are associated with situations of response conflict whereas the left PFC is associated with situations of conflict at non-response levels. Our results are in agreement with such a distinction and further provide evidence for a causal role of the left PFC in the resolution of proactive interference at the level of abstract memory representations.
Role of the right PFC in facilitatory aspects of anticipatory regulations of action
Our results revealed a novel finding in the field of proactive control: right PFC patients had an abnormal Gratton effect in the Simon task. This was caused by an abnormal pattern on compatible trials: in these trials, right PFC patients did not show a benefit of having just performed a compatible trial whereas left PFC patients and aged-matched controls did. More particularly, right PFC patients did not benefit from the serial repetition of trial features (i.e., position and color) whereas the other groups did.
As reviewed in the introduction, the role of the right PFC, and right inferior frontal cortex in particular, in cognitive control has been linked mainly to response inhibition using tasks in which participants have to stop a prepotent response as the stop-signal paradigm (Aron et al., 2003 and Aron, 2011 for a review), or modify a current motor plan in target reaching tasks (e.g., Mutha et al., 2014; Schaefer et al., 2012). Although it could be argued the compatibility effect in the Simon task may be due to an increased need for inhibition of the incorrect response, right PFC patients in our study did not show an abnormal behavior following incompatible trials. This result seems at odds with the neuropsychological results reported in Aron et al. (2003). This may be due to 2 main differences between Aron et al.’s (2003) study and ours. First, the task we used is different. Aron et al. (2003) used a stop-signal task in which participants have to stop an already programed response. The nature of inhibitory control could be different and the need for inhibitory control may be stronger in this task compared to ours. This is suggested by a study using repetitive transcranial magnetic stimulation (rTMS, Chambers et al., 2007) and combining a Flanker task with a stop-signal task. The results show altered inhibitory control only for conditions of high-response competition: After rTMS of the right IFG, stop-signal inhibition was impaired only for incompatible trials. However, on go-trials the compatibility effect was not significantly increased after rTMS of the right IFG. The second reason may be linked to lesion location. In our study, the right PFC patients’ lesions overlapped maximally in the middle frontal gyrus (MFG) and not in the IFG, which was interpreted as the critical region for response inhibition in Aron et al. (2003, but see Krämer et al., 2013). Thus, it is possible that different regions of the right PFC may be involved in different aspects of proactive control. Supporting evidence for distinct proactive control mechanisms within the lateral PFC comes from the dissociation that has been made between DLPFC and inferior ventrolateral PFC. As mentioned in the introduction, bilateral DLPFC has been shown to play an important role in maintaining task-sets (Miller and Cohen, 2001; Sakai, 2008). The maintenance of such task-sets helps reduce possible interference from previous items but also facilitates the processing of repeating trial features. Indeed, our data suggests the right PFC is necessary for another type of proactive control which facilitates the reiteration of an action when trial features are identical from one trial to the next.
Evidence for a preponderant role of the right over the left PFC in the maintenance of goal directed actions comes from clinical observations of motor impersistence which is described as an impairment in sustaining actions (Heilman, 2004) that are dependent on directed attention (Kertesz et al., 1985). This symptom is part of a class of disorders referred to as motor intentional disorders (Heilman, 1991) and is generally observed acutely after right hemisphere damage (Kertesz et al., 1985). Patients with frontal or subcortical lesions have been found to be significantly more likely to have motor impersistence than patients with posterior lesions (Kim et al., 2013). The role of the right hemisphere in general has been framed in the context of the intentional dominance theory as mediating the preparation for initiation of goal-oriented actions (Heilman et al., 1985). Though generally not presented in the literature as a form of proactive control, the preparation for initiation of goal-oriented actions seems to fit perfectly well in the context of the anticipatory regulation of action. Though the patients we tested were not acute, their failure to benefit from stimulus feature repetition may be evidence for a lasting more subtle deficit in the ability to maintain goal directed actions. Our results are in agreement with the idea that the right PFC plays a major role in sustaining goal-directed actions from one trial to the next and further suggest deficits in this function following right PFC damage persist chronically. This interpretation is supported by both neuropsychological (Eslinger et al., 1999) and transcranial magnetic stimulation evidence (Neubert et al., 2010). Thus, there is a converging body of evidence supporting our interpretation of the causal role of the right PFC in facilitatory repetition-driven aspects of the anticipatory regulation of action.
Conclusion
In sum, our results shed light on the differential roles played by the left and right PFC in proactive control. The left PFC is associated with overcoming stimulus-based proactive interference whereas the right PFC is associated with maintaining goal-directed actions and facilitatory repetition-driven aspects of proactive control. The prefrontal dependent hemispheric dissociation we observed is in agreement with a preponderant role of the left inferior frontal cortex in overcoming proactive interference from competing memory representations and provides evidence that the right PFC has a specific role in sustaining goal-directed actions.
Supplementary Material
Figure S1: Gratton effect by stimulus position in the Simon task on reaction times per participant group. The compatibility at trial n is coded by the color of the bars: Mean values for compatible trials n are depicted by the solid lines and mean values for the incompatible trials are coded by the dotted lines. Compatibility at trial n-1 is represented on the x-axis and mean RT and mean error rates are represented on the y-axis. Standard deviations are represented by the horizontal lines (only positive values are presented for the incompatible condition and only negative values are presented for the compatible condition for visual clarity).
Figure S2: Within-trial Simon effect by stimulus position on mean reaction times per participant group. Values for incompatible trials are depicted by the dark gray bars and values for compatible trials are depicted by the light gray bars. Standard deviations are represented by the horizontal lines. In the patient groups, the Simon effect tended to be more pronounced when the stimulus was presented contra-laterally to the lesion side.
Highlights.
-
2
We investigate two measures of proactive control in chronic frontal stroke patients.
-
3
All patients and controls performed a picture naming and a verbal Simon task.
-
4
Left and right PFC patients are differentially impaired in proactive control.
-
5
The left PFC helps to overcome proactive interference from memory representations.
-
6
The right PFC helps sustain goal-directed actions from one trial to the next.
Acknowledgments
This research was supported by a post-doctoral grant from the Fyssen Foundation and by the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health under Award Number F32DC013245 to S.K.R., a grant to KYH from the Clinical Sciences Research and Development Service (101BX007080) of the Veterans Affairs Office of Research and Development, Individual Department of Veterans Affairs Clinical Sciences Research and Development Merit Awards to K.Y.H. and N.F.D., NINDSH Grant 2R37NS21135 and the Nielsen Corporation to R.T.K.. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would like to thank Donatella Scabini, Jessica Black, Jenna Keller, and Jennifer Hogan for patient delineation and neuropsychological testing, Clay Clayworth for lesion reconstruction and Lee Stapp for technical assistance. Finally, we are very thankful to the research volunteers who took part in this study.
Footnotes
All left frontal patients were examined on at least 2 subtests of the Western Aphasia Battery (WAB; Kertesz, 1982), measuring spontaneous speech (assessing general conversational speech production abilities; maximum score of 20) and comprehension of sequential commands (assessing general speech comprehension skills; maximum score of 80). We note the score of one patient on Sequential Commands was not available; we only had the overall WAB comprehension score (grouping 3 comprehension subtests including the Sequential Commands).
Conflict of Interest statement
We declare that the research reported here was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aron AR. From Reactive to Proactive and Selective Control: Developing a Richer Model for Stopping Inappropriate Responses. Biol Psychiatry. 2011;69:e55–e68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Baayen HR, Davidson DJ, Bates DM. Mixed effects modeling with crossed random effects for subjects and items. J Mem Lang. 2008;59:390–412. [Google Scholar]
- Badre D, Wagner AD. Frontal Lobe Mechanisms that Resolve Proactive Interference. Cereb Cortex. 2005;15:2003–2012. doi: 10.1093/cercor/bhi075. [DOI] [PubMed] [Google Scholar]
- Bartlett MS. Properties of sufficiency and statistical tests. Proceedings of the Royal Society of London Series A. 1937;160:268–282. [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1–7. 2014a <URL: http://CRAN.R-project.org/package=lme4>.
- Bates D, Maechler M, Bolker BM, Walker S. lme4: Linear mixed-effects models using Eigen and S4. Journal of Statistical Software. 2014b ArXiv e-print; submitted to. <URL: http://arxiv.org/abs/1406.5823>.
- Belke E, Meyer AS, Damian MF. Refractory effects in picture naming as assessed in a semantic blocking paradigm. Q J Exp Psychol Sect A. 2005;58:667–692. doi: 10.1080/02724980443000142. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- van Casteren M, Davis MH. Mix, a program for pseudorandomization. Behav Res Methods. 2006;38:584–589. doi: 10.3758/bf03193889. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Bellgrove MA, Gould IC, English T, Garavan H, McNaught E, Kamke M, Mattingley JB. Dissociable Mechanisms of Cognitive Control in Prefrontal and Premotor Cortex. J Neurophysiol. 2007;98:3638–3647. doi: 10.1152/jn.00685.2007. [DOI] [PubMed] [Google Scholar]
- Chikazoe J, Jimura K, Hirose S, Yamashita K, Miyashita Y, Konishi S. Preparation to Inhibit a Response Complements Response Inhibition during Performance of a Stop-Signal Task. J Neurosci. 2009;29:15870–15877. doi: 10.1523/JNEUROSCI.3645-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damian MF, Vigliocco G, Levelt WJM. Effects of semantic context in the naming of pictures and words. Cognition. 2001;81:B77–B86. doi: 10.1016/s0010-0277(01)00135-4. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural Mechanisms of Selective Visual Attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A Core System for the Implementation of Task Sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T. Congruency sequence effects and cognitive control. Cogn Affect Behav Neurosci. 2007;7:380–390. doi: 10.3758/cabn.7.4.380. [DOI] [PubMed] [Google Scholar]
- Egner T, Delano M, Hirsch J. Separate conflict-specific cognitive control mechanisms in the human brain. NeuroImage. 2007;35:940–948. doi: 10.1016/j.neuroimage.2006.11.061. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys. 1974;16:143–149. [Google Scholar]
- Eslinger PJ, Biddle K, Pennington B, Page RB. Cognitive and behavioral development up to 4 years after early right frontal lobe lesion. Dev Neuropsychol. 1999;15:157–191. [Google Scholar]
- Ewald A, Aristei S, Nolte G, Abdel Rahman R. Brain oscillations and funtional connectivity during overt language production. Frontiers in Psychology. 2012;3 doi: 10.3389/fpsyg.2012.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J, Weisberg S. An {R} Companion to Applied Regression. 2. Thousand Oaks CA: Sage; 2011. URL: http://socserv.socsci.mcmaster.ca/jfox/Books/Companion. [Google Scholar]
- Gratton G, Coles MG, Donchin E. Optimizing the use of information: strategic control of activation of responses. J Exp Psychol Gen. 1992;121:480–506. doi: 10.1037//0096-3445.121.4.480. [DOI] [PubMed] [Google Scholar]
- Hamilton AC, Martin RC. Dissociations among tasks involving inhibition: A single- case study. Cogn Affect Behav Neurosci. 2005;5:1–13. doi: 10.3758/cabn.5.1.1. [DOI] [PubMed] [Google Scholar]
- Heilman KM. Intentional neglect. Front Biosci J Virtual Libr. 2004;9:694–705. doi: 10.2741/1261. [DOI] [PubMed] [Google Scholar]
- Horga G, Maia TV, Wang P, Wang Z, Marsh R, Peterson BS. Adaptation to Conflict via Context-Driven Anticipatory Signals in the Dorsomedial Prefrontal Cortex. J Neurosci Off J Soc Neurosci. 2011;31:16208–16216. doi: 10.1523/JNEUROSCI.2783-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D, Nickels L, Coltheart M, Cole-Virtue J. Cumulative semantic inhibition in picture naming: experimental and computational studies. Cognition. 2006;100:464–482. doi: 10.1016/j.cognition.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Jaeger TF. Categorical data analysis: Away from ANOVAs (transformation or not) and towards logit mixed models. J Mem Lang. 2008;59:434–446. doi: 10.1016/j.jml.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahfari S, Stinear CM, Claffey M, Verbruggen F, Aron AR. Responding with Restraint: What Are the Neurocognitive Mechanisms? J Cogn Neurosci. 2010;22:1479–1492. doi: 10.1162/jocn.2009.21307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J, Nee DE. Brain mechanisms of proactive interference in working memory. Neuroscience. 2006;139:181–193. doi: 10.1016/j.neuroscience.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Kan IP, Thompson-Schill SL. Selection from perceptual and conceptual representations. Cogn Affect Behav Neurosci. 2004;4:466–482. doi: 10.3758/cabn.4.4.466. [DOI] [PubMed] [Google Scholar]
- Kerns JG. Anterior cingulate and prefrontal cortex activity in an FMRI study of trial-to-trial adjustments on the Simon task. NeuroImage. 2006;33:399–405. doi: 10.1016/j.neuroimage.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS. Anterior Cingulate Conflict Monitoring and Adjustments in Control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kertesz A. Western Aphasia Battery. New York: Grune and Stratton; 1982. [Google Scholar]
- Kertesz A, Nicholson I, Cancelliere A, Kassa K, Black SE. Motor impersistence: a right-hemisphere syndrome. Neurology. 1985;35:662–666. doi: 10.1212/wnl.35.5.662. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Lee B, Jo MK, Jung K, You H, Lee BH, Cho HJ, Sung SM, Jung DS, Heilman KM, et al. Directional and spatial motor intentional disorders in patients with right versus left hemisphere strokes. Neuropsychology. 2013;27:428–437. doi: 10.1037/a0032824. [DOI] [PubMed] [Google Scholar]
- Kornblum S, Hasbroucq T, Osman A. Dimensional overlap: cognitive basis for stimulus-response compatibility--a model and taxonomy. Psychol Rev. 1990;97:253–270. doi: 10.1037/0033-295x.97.2.253. [DOI] [PubMed] [Google Scholar]
- Krämer UM, Solbakk AK, Funderud I, Løvstad M, Endestad T, Knight RT. The role of the lateral prefrontal cortex in inhibitory motor control. Cortex J Devoted Study Nerv Syst Behav. 2013;49:837–849. doi: 10.1016/j.cortex.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll JF, Stewart E. Category Interference in Translation and Picture Naming: Evidence for Asymmetric Connections Between Bilingual Memory Representations. J Mem Lang. 1994;33:149–174. [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB. Tests for random and fixed effects for linear mixed effect models (lmer objects of lme4 package) 2014 http://cran.r-project.org/web/packages/lmerTest/index.html.
- Lupker SJ. The semantic nature of response competition in the picture-word interference task. Mem Cognit. 1979;7:485–495. [Google Scholar]
- Mecklinger A, Weber K, Gunter TC, Engle RW. Dissociable brain mechanisms for inhibitory control: effects of interference content and working memory capacity. Cogn Brain Res. 2003;18:26–38. doi: 10.1016/j.cogbrainres.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Webb A, Barad V, Cohen NJ, Wszalek T, Kramer AF. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Brain Res Cogn Brain Res. 2001;12:467–473. doi: 10.1016/s0926-6410(01)00076-3. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An Integrative Theory of Prefrontal Cortex Function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Monsell S. Recency, immediate recognition memory, and reaction time. Cognit Psychol. 1978;10:465–501. [Google Scholar]
- Mutha PK, Stapp LH, Sainburg RL, Haaland KY. Frontal and Parietal cortex contributions to action modification. Cortex. 2014;57:38–50. doi: 10.1016/j.cortex.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P, Rees G, Parton A, Kennard C, Husain M. Volition and Conflict in Human Medial Frontal Cortex. Curr Biol. 2005;15:122–128. doi: 10.1016/j.cub.2005.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert FX, Mars RB, Buch ER, Olivier E, Rushworth MFS. Cortical and subcortical interactions during action reprogramming and their related white matter pathways. Proc Natl Acad Sci U S A. 2010;107:13240–13245. doi: 10.1073/pnas.1000674107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM. Mixed-Effects Models in S and S-PLUS. Springer; New York: 2000. [Google Scholar]
- Proctor RW, Vu KPL. Mixing location-irrelevant and location-relevant trials: Influence of stimulus mode on spatial compatibility effects. Mem Cognit. 2002;30:281–293. doi: 10.3758/bf03195289. [DOI] [PubMed] [Google Scholar]
- Protopapas A. Check Vocal: A program to facilitate checking the accuracy and response time of vocal responses from DMDX. Behav Res Methods. 2007;39:859–862. doi: 10.3758/bf03192979. [DOI] [PubMed] [Google Scholar]
- R CoreTeam. R: A Language and Environment For Statistical Computing. Vienna: R Foundation for Statistical Computing; 2012. Available at: http://www.R-project.org/ [Google Scholar]
- Rahman RA, Melinger A. When bees hamper the production of honey: Lexical interference from associates in speech production. J Exp Psychol Learn Mem Cogn. 2007;33:604–614. doi: 10.1037/0278-7393.33.3.604. [DOI] [PubMed] [Google Scholar]
- Rahman RA, Melinger A. Semantic context effects in language production: A swinging lexical network proposal and a review. Lang Cogn Process. 2009;24:713–734. [Google Scholar]
- Richard Ridderinkhof K, Forstmann BU, Wylie SA, Burle B, van den Wildenberg WPM. Neurocognitive mechanisms of action control: resisting the call of the Sirens. Wiley Interdiscip Rev Cogn Sci. 2011;2:174–192. doi: 10.1002/wcs.99. [DOI] [PubMed] [Google Scholar]
- Riès SK, Xie K, Haaland KY, Dronkers NF, Knight RT. Role of the lateral prefrontal cortex in speech monitoring. Front Hum Neurosci. 2013;7:703. doi: 10.3389/fnhum.2013.00703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K. Task Set and Prefrontal Cortex. Annu Rev Neurosci. 2008;31:219–245. doi: 10.1146/annurev.neuro.31.060407.125642. [DOI] [PubMed] [Google Scholar]
- Schnur TT, Schwartz MF, Brecher A, Hodgson C. Semantic interference during blocked-cyclic naming: Evidence from aphasia. J Mem Lang. 2006;54:199–227. [Google Scholar]
- Schnur TT, Schwartz MF, Kimberg DY, Hirshorn E, Coslett HB, Thompson-Schill SL. Localizing interference during naming: convergent neuroimaging and neuropsychological evidence for the function of Broca’s area. Proc Natl Acad Sci U S A. 2009;106:322–327. doi: 10.1073/pnas.0805874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JR. Reactions toward the source of stimulation. J Exp Psychol. 1969;81:174–176. doi: 10.1037/h0027448. [DOI] [PubMed] [Google Scholar]
- Stürmer B, Leuthold H. Control over response priming in visuomotor processing: a lateralized event-related potential study. Exp Brain Res. 2003;153:35–44. doi: 10.1007/s00221-003-1579-1. [DOI] [PubMed] [Google Scholar]
- Stürmer B, Leuthold H, Soetens E, Schröter H, Sommer W. Control over location-based response activation in the Simon task: behavioral and electrophysiological evidence. J Exp Psychol Hum Percept Perform. 2002;28:1345–1363. doi: 10.1037//0096-1523.28.6.1345. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Knight RT. Principles of Frontal Lobe Function. Oxford University Press; 2013. [Google Scholar]
- Swann NC, Cai W, Conner CR, Pieters TA, Claffey MP, George JS, Aron AR, Tandon N. Roles for the pre-supplementary motor area and the right inferior frontal gyrus in stopping action: electrophysiological responses and functional and structural connectivity. NeuroImage. 2012;59:2860–2870. doi: 10.1016/j.neuroimage.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci U S A. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, Swick D, Farah MJ, D’Esposito M, Kan IP, Knight RT. Verb generation in patients with focal frontal lesions: a neuropsychological test of neuroimaging findings. Proc Natl Acad Sci U S A. 1998;95:15855–15860. doi: 10.1073/pnas.95.26.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, Jonides J, Marshuetz C, Smith EE, D’Esposito M, Kan IP, Knight RT, Swick D. Effects of frontal lobe damage on interference effects in working memory. Cogn Affect Behav Neurosci. 2002;2:109–120. doi: 10.3758/cabn.2.2.109. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Bedny M, Goldberg RF. The frontal lobes and the regulation of mental activity. Curr Opin Neurobiol. 2005;15:219–224. doi: 10.1016/j.conb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Wühr P. The Simon effect in vocal responses. Acta Psychol (Amst) 2006;121:210–226. doi: 10.1016/j.actpsy.2004.12.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Gratton effect by stimulus position in the Simon task on reaction times per participant group. The compatibility at trial n is coded by the color of the bars: Mean values for compatible trials n are depicted by the solid lines and mean values for the incompatible trials are coded by the dotted lines. Compatibility at trial n-1 is represented on the x-axis and mean RT and mean error rates are represented on the y-axis. Standard deviations are represented by the horizontal lines (only positive values are presented for the incompatible condition and only negative values are presented for the compatible condition for visual clarity).
Figure S2: Within-trial Simon effect by stimulus position on mean reaction times per participant group. Values for incompatible trials are depicted by the dark gray bars and values for compatible trials are depicted by the light gray bars. Standard deviations are represented by the horizontal lines. In the patient groups, the Simon effect tended to be more pronounced when the stimulus was presented contra-laterally to the lesion side.