Abstract
Background:
Fragile X-associated tremor/ataxia syndrome (FXTAS), a neurodegenerative disorder caused by FMR1 gene premutations, typically associated with frontal-subcortical type cognitive impairments. High prevalence (~50%) of superimposed Alzheimer’s pathology has been reported in FMR1 premutation carriers, and standardized neuropsychological tests have not yielded any robust discriminators between FXTAS and Alzheimer’s disease (AD) dementia. The similarities/differences in memory processes between FXTAS and early AD remain underexplored.
Methods:
32-channel event-related potentials (ERPs) were obtained from a semantic judgment task in which semantically congruous (50%) and incongruous pairs repeat pseudorandomly. The N400 and late positive component (LPC) of 25 FXTAS patients (Mage = 71.2, MMSE = 26.6) were compared to a matched group of 25 patients with MCI or early AD (1 mild AD dementia, 24 amnestic MCI, of whom 18 later converted to AD; Mage = 73.4, MMSE = 26.4), and 25 healthy elderly.
Results:
Both patient groups showed similar reductions in the N400 repetition effect and N400 congruity effect amplitudes, compared to controls, reflecting abnormal semantic priming and repetition priming. The MCI/AD group, however, had significantly smaller LPC word repetition effects and poorer learning and memory on the CVLT than FXTAS. The LPC and N400 repetition effects both correlated with verbal memory across all subjects, but only the N400 correlated with memory in FXTAS.
Conclusion:
FXTAS patients show relative sparing of the LPC repetition effect, and less disruption of explicit memory than prodromal/early AD. N400 abnormalities in FXTAS appear to account for much of their mild impairments in verbal learning and memory.
Keywords: fragile X premutation, dementia, memory, language, EEG, N400, P600
Introduction
Fragile X-associated tremor/ataxia syndrome (FXTAS) is a neurodegenerative disorder caused by premutation CGG expansion (ranging from 55-200 repeats) of the fragile X mental retardation 1 (FMR1) gene.[1] FXTAS is characterized by intention tremor, cerebellar ataxia, peripheral neuropathy, and cognitive impairments progressing to a mixed “frontal-subcortical” type dementia in up to 50% of older males.[2] Neuropathological examination shows numerous eosinophilic mRNA-containing intranuclear inclusions in neurons and astrocytes of FXTAS brain (Greco et al, 2002, 2006). A recent neuropathological case series of 8 autopsied female premutation carriers (6 with FXTAS) reported Alzheimer disease (AD)-type neuropathology in 4 women (50%) - including one woman without clinical AD symptoms, suggesting a high prevalence of AD-type neuropathology in association with the FMR1 premutation.[3] It is also the case that the predilection sites for the intranuclear inclusions in FXTAS have considerable overlap (e.g., hippocampus) with the distribution of AD pathology.[4-6] Studies employing standardized neuropsychological tests have found comparable deficits in working memory, verbal fluency and language between FXTAS dementia and AD, but somewhat more preserved attention abilities in FXTAS dementia than AD.[2] However, the similarities and differences between semantic memory and episodic memory processes between individuals with FXTAS and AD remain largely unknown.
Event-related potentials (ERPs), comprised primarily of summated excitatory and inhibitory post-synaptic potentials, have an exquisite temporal resolution, which may be useful in differentiating early-stage (relatively automatic) vs. late-stage (controlled) memory processes.[7] In this study, we characterized and compared two well-studied cognitive ERP components relevant to semantic (N400) and episodic/declarative (LPC) memory processes in mild FXTAS and early AD. The late positive component (LPC), sometimes termed the “P600”, has been linked to both memory encoding and recognition processes.[8,9] The LPC repetition effect amplitude correlates with episodic verbal memory ability.[10-12] The N400 amplitude is closely related to semantic processing load and is sensitive to repetition priming and semantic priming.[13,14]
Patients with mild cognitive impairment (MCI) typically convert to AD dementia at a rate of ~15% per year.[15] Our prior studies have demonstrated the sensitivity of N400 and LPC word repetition effects to prodromal AD, i.e. individuals with amnestic MCI who subsequently converted to AD dementia.[16.17] The conversion rate from MCI to dementia appears much higher (~3 to 8-fold) in those with abnormal ERP word repetition effects.[17] Investigation with the same ERP paradigm in FXTAS found significantly reduced N400 repetition effect amplitude, but relatively intact LPC repetition effects.[12] In the present study, we compared a FXTAS patient group to a patient group who predominantly had prodromal AD, matched on global cognitive impairment. We hypothesized that, similar to our prior studies, the FXTAS patient group would show less severe decrements in the LPC repetition effect compared to MCI/early AD, which may reflect less severe rapid forgetting. We also investigated the relationship between the LPC and N400 repetition effects and verbal learning and memory on the CVLT, across all subjects and within each patient group.
Material and Methods
Participants
Patients
Twenty-five patients with mild-or-moderate FXTAS (Mean FXTAS stage = 3.4 ± 1.1, range = 2-5; 2 met DSM-IV-TR criteria for dementia)[18] and 25 patients with amnestic MCI or early AD (18 MCI converters, 6 amnestic MCI who have not yet developed dementia, 1 with probable AD dementia of mild severity) were studied. Informed consent was obtained for each participant following protocols approved by the University of California Davis Institutional Review Board or the University of California San Diego Human Research Protection Program. FXTAS was diagnosed following published criteria.[19] All 24 MCI patients met Peterson criteria for amnestic MCI.[15] The two patient groups were selected, matched (+/− 1 point) on the Mini-Mental State Examination (MMSE) score, with secondary considerations given to gender, age, and educational level (Table 1). The majority of the participants were male (15 MCI/AD, 18 FXTAS). Participants with CNS-active medications for dementia, anxiety, depression, or neuropathic pain were not excluded because these medications have a high prevalence of use in representative samples of FXTAS. Fourteen FXTAS patients were on CNS-active medications (most commonly citalopram, memantine and donepezil). Seven of the MCI/AD patients were on CNS-active medications (e.g., donepezil, sertraline).
Table 1.
Demographics and behavioral data: Mean (SD).
| MCI/AD | FXTAS |
p value*
(FXT vs. MCI/AD) |
NC |
p value (3-group ANOVA) |
|
|---|---|---|---|---|---|
| N | 25 | 25 | 25 | ||
| Age (years) | 73.4 (7.1) | 71.9 (6.8) | .47 | 69 (10.0) | .16 |
| Sex | 15 M/10 F | 18 M/7 F | .37 | 17 M/8 F | .66 |
| Education (years) | 16.5 (2.5) | 16.2 (3.4) | .78 | 16.7 (2.7) | .84 |
| Handedness | 24 R/1 L | 23 R/2 L | .55 | 24 R/1 L | .77 |
| MMSE | 26.6 (1.7) | 26.4 (2.2) | .91 | 28.4 (2.1) | .001** |
| Task Accuracy (%) | |||||
| Congruous Items | 98.1 (1.7) | 98.8 (1.6) | .21 | 99.5 (0.6) | .004** |
| Incongruous Items | 98.9 (1.4) | 97.9 (4.3) | .58 | 99.8 (0.3) | .047* |
| All Items | 98.6 (1.4) | 98.4 (2.8) | 1.0 | 99.7 (0.4) | .032* |
| CVLT measures | |||||
| Trials 1-5 | 27.2 (10.5) | 36 (12.7) | .034* | 56.5 (11.6) | <.0001**** |
| SD-CR | 5.3 (3.2) | 8.0 (3.8) | .012* | 12.7 (2.0) | <.0001**** |
| LD-CR | 4.5 (3.2) | 8.2 (3.5) | .0004*** | 12.3 (2.4) | <.0001**** |
| Discriminability | 70.5 (17.1) | 84.0 (14.8) | .004** | 93.5 (6.3) | <.0001**** |
P-values are from post hoc tests with Bonferroni correction performed on continuous variables or from chi-square tests performed on categorical variables (i.e., sex, handedness).
p < 0.05,
p < 0.01,
p < 0.001.
CVLT = California Verbal Learning Test, SD = short delay, LD = long delay, CR = cued recall.
Normal Controls
Twenty-five cognitively normal older controls were also included. All were community-dwelling paid volunteers. The normal control (NC) group was selected to be comparable to the patients with respect to age, education, gender and handedness (Table 1). Three controls were taking CNS-active medications.
All participants were given extensive clinical research evaluations, including medical and neurological history, neurological examination, and a neuropsychological test battery (for details, please see Olichney et al. 2006 and Grigsby et al. 2008)[16,20] which included the California Verbal Learning Test (CVLT)[21]. Evaluations were performed either at the M.I.N.D. Institute, the UC Davis Alzheimer’s Disease Center, or at the Shiley-Marcos ADRC at UC San Diego (UCSD).
Experimental Procedure
Participants were fitted with an electrode cap and seated about 100 cm from a computer monitor. Category statements were read aloud, each followed approximately one second later by a visually-presented target word (visual angle ~0.4°). Subjects were instructed to wait three seconds following the appearance of the target word, then say the perceived word aloud and whether it was an exemplar of the defined category or not. Three blocks of ERPs were recorded, each containing 144 trials and lasting slightly over 20 minutes.
Experimental Stimuli
The stimuli were 216 spoken phrases each describing a category (e.g., “a female relative”), and followed by a target word.[10] The semantically congruous target words, making up 50% of the target words, were medium-typicality category exemplars (e.g. “sister” after “a female relative”). The other 50% of the target words were concrete nouns “incongruous” to their category (e.g. “freeze” after “a type of ship”), but matched to the congruous targets for length and frequency of usage.[22]
Subjects were assigned to one of three counterbalanced stimulus lists, each of which contained 36 congruous targets presented once, 36 presented twice, 36 presented three times, and equal numbers of incongruous words presented with the same underlying repetition patterns, for a total of 432 trials. Half of the target stimuli were new words, and half were repeated. Words that were repeated always appeared after the same category statement as on the initial presentation. For words repeated once, the lag between presentations was 0-3 trials (approximately 10-40 s) and for doubly repeated words, lag for both second and third presentations was 10-13 trials (approximately 120 s).
Electrophysiological Recording
Electroencephalogram (EEG) was recorded from tin electrodes embedded in an electrode cap (Electrocap, Eaton, OH) from lateral frontal (F3, F4, F7, F8, FC1, FC2, FP1, FP2), midline (Fz, Cz, Pz, POz), temporal (T5, T6), parietal (P3, P4, CP1, CP2), and occipital sites (O1, O2, PO7, PO8) defined by the international 10-20 system. Additional sites included pairs which approximate Broca’s area (Bl), Wernicke’s area, (Wl) and their right hemisphere homologues (Br, Wr), and an electrode pair 33% of the interaural distance lateral to Cz over the superior temporal lobe (41L, 41R). All scalp electrodes were referenced online to the left mastoid electrode, then re-referenced offline to an average of the left and right mastoids. Electro-oculogram recording from four electrodes (one below and one at the outer canthus of each eye) monitored both vertical and horizontal eye movements. EEG was recorded with a Nicolet SM2000 digital amplifier, using 0.016-100 Hz band pass and a 250 Hz sampling rate. Identical amplifiers, filter settings, electrodes and EEG montages were used at both sites (UCSD and UC Davis).
Data analyses
ERPs to the visual target words were averaged after off-line rejection of trials contaminated by eye movements, blinks, or other artifacts. Trials were most commonly rejected due to eye-blinks.
The N400 congruity effect amplitude (ERPs to new incongruous vs. new congruous words) was analyzed using split-plot ANOVAs with the between-subjects factor of Group and two within-subjects factors: Congruity and Electrode (28 scalp sites) between 300-550 ms. For the N400 word repetition effect (ERPs to new vs. old incongruous words), mean amplitudes between 300-550 ms post target-word onset were submitted to split-plot ANOVAs with the between-subjects factor of group and two within-subjects factors: Repetition (New/Old) and Electrode. The LPC word repetition effect (ERPs to new vs. old congruous words) was analyzed using split-plot ANOVAs of mean amplitude with the between-subjects factor of Group and three within-subjects factors: Repetition (New/Old), Latency (300-550 ms/550-800 ms) and Electrode. All ERP repetition and congruity effects were first analyzed in 3-group ANOVAs (with NC group included) and significant group differences were then followed-up with 2-group ANOVAs (FXTAS vs. MCI/AD) to determine if there were any significant differences between the two patient groups. Two-tailed p-values of ≤ 0.05 were considered significant.
ANOVAs were also performed on the CVLT data, and two-tailed p-values of ≤ 0.05 were considered significant for the overall effect of group. Bonferroni correction for multiple comparisons was applied to post hoc tests which compared 2 of the 3 subject groups to each other (e.g. FXTAS vs. MCI/AD).
Forward-step linear regressions were performed to test which ERP word repetition effect amplitudes (LPC repetition effect and/or N400 repetition effect amplitude) best predicted each of the main verbal learning and memory scores on the CVLT (all measures in Table 1, plus Free Recall scores at short delay and long delay). The ERP measures, age and sex were allowed to enter the model if p-values ≤ 0.05, and subsequently removed if p > 0.10.
Results
Behavioral results
Accuracy on the category decision task was near ceiling (≥ 98%) in all 3 groups for both congruous items and incongruous items (Table 1). There was a significant main effect of group for accuracy (p = 0.03) and post-hoc t-tests showed lower accuracy in FXTAS than controls (p = 0.04), but no significant difference between controls and MCI/AD (p = 0.12), or between MCI/AD and FXTAS (p = 1.0).
The group data for the main CVLT measures of verbal learning and memory performance are summarized in Table 1. We found significant group differences on all these measures, with impaired performance in both patient groups. The MCI/AD group performed even poorer than the FXTAS group. It should be noted that the FXTAS group had particularly severe impairments (approximately 2 standard deviations (SD) below the NC group) in acquisition (Trials 1-5 on list A) and short delay recall. However, they showed no decrement in cued recall from short delay (mean = 8.0) to long delay (mean =8.2) (20 minutes later), unlike the MCI/AD group (see Table 1).
For the control group, 28.7% of EEG trials were rejected due to artifacts. The two patient groups had comparable, but significantly higher than controls, rejection rates (FXTAS = 48.8%, MCI/AD = 48.3%, p = 0.99; patients vs controls: ps < 0.001).
New words- N400 congruity effect
Figure 1 shows ERPs elicited by new congruous target and incongruous target words in the controls, FXTAS and MCI/AD groups. The congruity effect (the difference between ERPs to the new congruous and new incongruous words) had a right temporal maximum, which began at about 300 ms post-stimulus and became smaller after ~550 ms in all three groups. The congruity effect appeared largest in the NC group.
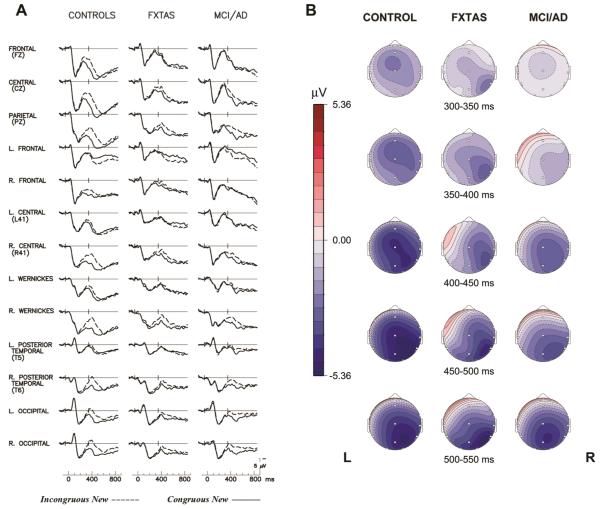
Figure 1.
The N400 Congruity Effect. (A) Grand average ERPs to congruous new (solid line) and incongruous new target words (dashed line) in 3 groups. The NC group’s N400 peak to incongruous new words is labelled ‘N4’ in the right Wernicke's (Wr) channel tracing. (B) Scalp topography maps of N400 congruity effect (voltage difference to new incongruous– congruous words) in 3 groups. L= left, R= right.
A 3-group ANOVA found a significant Group × Congruity interaction for the N400 congruity effect (F = 5.64, p = 0.005). There was also a significant Group × Electrode interaction (F = 2.69, ε = 0.22, p = 0.01) indicating group differences in the ERP scalp topographies. The NC group’s N400 congruity effect was largest over the right Wernicke’s area electrode, but was also evident at anterior electrodes, whereas the patient groups showed more posteriorly restricted tempo-occipital congruity effects (which could be due to smaller amplitudes overall, as Urbach & Kutas 2002 pointed out)[23] (see Figure 1 and Figure 2A for comparison at channel Wr). For N400 amplitude, there was a significant main effect of Congruity (F = 24.2, p < 0.0001), but no main effect of Group (F = 2.25, p = 0.11).
Figure 2.
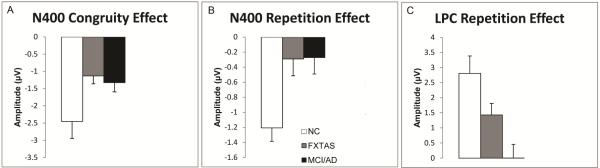
Mean amplitudes and standard errors (SE) for: (A). N400 congruity effect amplitude (300-550 ms, at right Wernicke's (Wr) electrode); (B). N400 repetition effect amplitude (300-550 ms at electrode T6) and (C) LPC repetition effect amplitude (550-800 ms, at electrode Pz).
Follow-up 2-group ANOVAs comparing the N400 congruity effect between the MCI/AD and FXTAS groups found no significant Congruity × Group interaction (F = 1.24, p = 0.27, see also Figure 2A), no main effect of group (F = 0.08, p = 0.77), or any other significant interactions involving group (ps ≥ 0.49).
N400 repetition effect for incongruous words
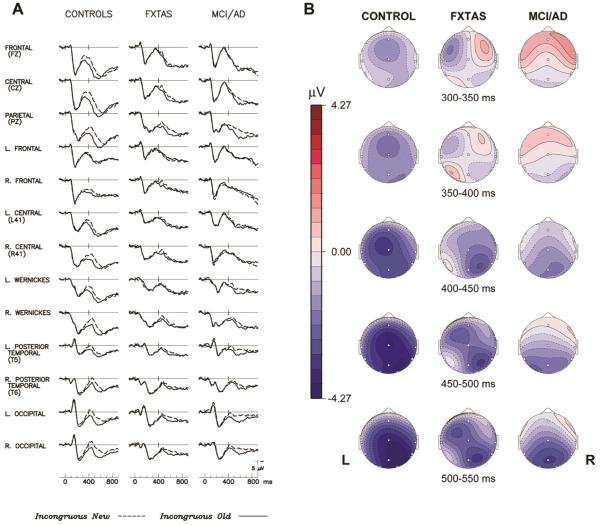
The grand-average ERPs and topography maps in Figure 3 illustrate the N400 repetition effect (the difference between ERPs to initial and repeated presentations of incongruous target words). In normal controls, there was a smaller N400 elicited by repeated incongruous words compared to their initial presentation. This N400 repetition effect had a right > left bi-temporal distribution (Figure 3B) similar to the N400 congruity effect. The N400 repetition effect was smaller and less wide spread in both the FXTAS and MCI/AD groups. While all 3 groups had a right posterior maximum during the later portion of the N400 repetition effect (see 400-550 ms maps in Figure 3B), NC subjects also showed a fronto-central distribution from 300-400 ms, which resembles the FN400 potential, sometimes attributed to familiarity-related memory processes.[8] A 3-group ANOVA of the N400 repetition effect revealed a significant main effect of Repetition (F = 24.26, p < 0.0001), as well as a Group × Repetition interaction (F = 12.49, p < 0.0001) that warranted follow-up 2-group comparisons. A significant Repetition × Electrode interaction was observed (F = 2.70, p = 0.03), indicating the predominantly posterior distribution of the N400 repetition effect observed in all subject groups (Figures 3A and 3B). However, there were no significant Group × Electrode (F = 0.45, ε = 0.17, p = 0.64), nor Group × Electrode × Repetition interactions (F = 1.42, ε = 0.29, p = 0.23). In the ANOVA comparing the two patient groups’ N400 repetition effect, there was no significant Group × Repetition interaction (F = 0.00, p = 0.96) (Figures 2B and 3A), nor main effect of Group (F = 0.00, p = 0.99).
Figure 3.
The N400 repetition effect. (A). Grand average ERPs to initial (dashed line) and repeated (solid line) presentations of incongruous target words in 3 groups; (B). Scalp topography maps of N400 word repetition effect (voltage difference to new–old incongruous words) in 3 groups. L= left, R= right.
Congruous words- late positive component (LPC) repetition effect
The grand-average ERPs in Figure 4A show that an LPC repetition effect, with larger positive voltage to new than old congruous words, was present in both the normal and FXTAS groups. This effect was larger in the midline channels (see Fz, Cz and Pz in Figure 4A), started at about 300 ms, peaked at 500-600 ms, and persisted past 800 ms after stimulus onset. In contrast, there was no reliable LPC repetition effect in the MCI/AD group. The LPC repetition effect amplitude in FXTAS group was intermediate to that of the control and MCI/AD groups. The scalp topography (Figure 4B) depicts the significantly smaller LPC repetition effect in MCI/AD compared to the FXTAS group.
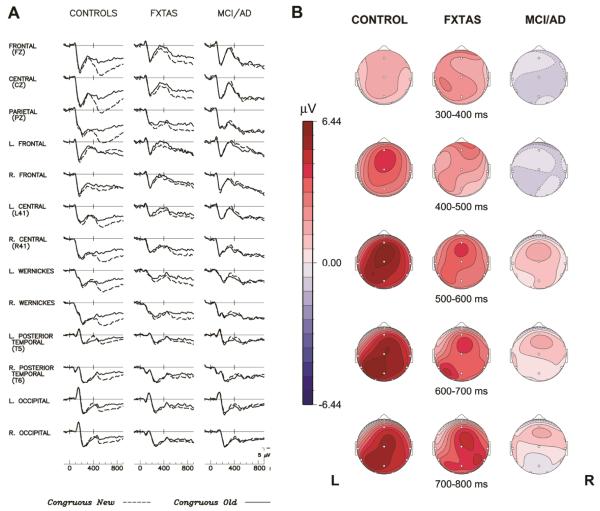
Figure 4.
The LPC repetition effect: (A). Grand average ERPs to initial (dashed line) and repeated (solid line) presentations of congruous target words in 3 groups. The LPC to new congruous words is illustrated/labelled in the FXTAS grand average at channel Pz. (B). Scalp topography maps of the LPC word repetition effect (new–old congruous words) in FXTAS (left) vs. MCI/early AD (right).
In the 3-group ANOVA of LPC repetition effect, the Group × Repetition interaction was highly significant (F = 6.48, p = 0.003), indicating larger mean LPC repetition effect amplitudes in controls (2.81 ± 2.9 μV) than in FXTAS (1.43 ± 1.9), with minimal LPC repetition effect amplitudes in MCI/AD (0.0016 ± 2.28). There was a significant main effect of Repetition (F = 17.52, p < 0.0001), but no main effect of Group (F = 2.1, p = 0.13) on the LPC amplitude.
The 2-group follow-up ANOVA comparing the two patient groups revealed a significant Group × Repetition interaction for the LPC amplitude (F = 5.28, p = 0.026), confirming that individuals with FXTAS have larger LPC repetition effect than MCI/AD patients. The main effect of Repetition was marginal (F = 3.42, p = 0.07) within these patient groups. There was no significant main effect of Group (F = 1.54, p = 0.22 on LPC amplitude), nor any other significant interaction involving Group.
Regression analyses of ERPs and CVLT performance
Across all 3 groups, both the LPC repetition effect amplitude at Pz and N400 repetition effect amplitude at T6 entered as significant predictors in all 6 forward-step linear regression models of the CVLT measures (Table 2). For 5 of these models, the LPC repetition effect entered first, except for the model of CVLT short delay cued recall, in which the N400 repetition effect entered first (see Table 2 for details). Age and sex were insignificant (i.e., did not enter) in any of these models.
Table 2.
Linear regression models of CVLT measures, all subjects combined.
| Dependent Variable |
Predictors (repetition effect) (mean amplitude) |
B | SE | β | p |
|---|---|---|---|---|---|
| Trials 1-5 | LPC N400 |
3.01 −4.59 |
0.61 1.49 |
.49 −.31 |
< .00001 .003 |
|
|
|||||
| Short Delay Free Recall |
LPC N400 |
0.77 −1.15 |
0.19 0.45 |
.44 −.27 |
< .0001 .013 |
|
|
|||||
| Short Delay Cued Recall |
N400 LPC |
−1.37 0.56 |
0.42 0.17 |
−.35 .35 |
.002 .002 |
|
|
|||||
| Long Delay Free Recall |
LPC N400 |
0.66 −1.32 |
0.19 0.47 |
.37 −.31 |
.001 .006 |
|
|
|||||
| Long Delay Cued Recall |
LPC N400 |
0.61 −1.10 |
0.18 0.44 |
.37 −.28 |
.001 .014 |
|
|
|||||
| Discriminability | LPC N400 |
2.04 −3.45 |
0.70 1.70 |
.34 −.23 |
.005 .047 |
B : unstandardized coefficient; SE: standard error; β = standardized coefficient.
Linear regression models with the same parameters were also constructed for each patient group. Within the MCI/AD group, the LPC repetition effect amplitude entered as the only significant predictor of scores on the CVLT List A trials 1-5 (β = 0.73, p < 0.0001), and on both the Short and Long Delay Free Recall measures (β = 0.44, p = .036 and β = 0.42, p = .046, respectively). In contrast, for the FXTAS group, only the N400 repetition effect amplitude significantly predicted performance on the CVLT (all 6 measures, 0.62 < βs < −0.47, ps < 0.025).
Sensitivity and specificity of ERP repetition effects
By applying the 16th percentile in normal elderly as cutoff amplitudes for the N400 (−0.56 μV at T6) and LPC (0.49 μV at Pz) repetition effects (Figure 2B and 2C), we found the sensitivity of the LPC repetition effect cutoff was 64% in MCI/AD and 32% in FXTAS, with 84% of controls correctly identified (i.e. 84% specificity). The N400 repetition effect cutoff had a 56% sensitivity to both MCI/AD and FXTAS, with 84% of controls correctly classified. Among the MCI/AD patients, 84% had either an abnormal/reduced N400 or LPC repetition effect, as was also observed in 60% of the FXTAS patients.
Discussion
In this ERP study of language and memory processes, both the FXTAS and MCI/early AD patient groups showed similar, marked reductions in the N400 component, with decreased amplitudes in the N400 repetition effect as well as the N400 congruity effect compared to normal controls. The N400 component, strongly correlated with semantic processing load,[13,24] is thought to reflect semantic/conceptual activation or other implicit memory processes.[10,14] The FN400 prominent at mid-frontal scalp sites has been linked to the familiarity process of recognition memory.[8,25,26] Previous studies have consistently found N400 effect abnormalities (both amplitude and latency) in mild AD,[27] which may relate to early involvement (atrophy, neurofibrillary tangles) of the anterior temporal lobe [4,28] and the well-known semantic deficits in AD.[29] FXTAS intranuclear inclusions are also abundant in lateral and inferior temporal cortical neurons,[5] and thus both disorders share these predilection sites. Glutamatergic signaling abnormalities may also be important in both these disorders [30,31] and contribute to the loss of the normal N400 repetition effect, which has been shown to be significantly disrupted by the NMDA receptor antagonist ketamine in a study with intracranial electrodes implanted in human hippocampus.[32]
In contrast to the N400 results, FXTAS patients demonstrated relatively spared LPC word repetition effects compared to the MCI/Early AD patient group with comparable global cognitive impairment. We believe this is related to the relative sparing of episodic memory and memory-related neural circuits in most patients with mild-to-moderate FXTAS.[12] Despite the relatively high concentration of intranuclear inclusions in the hippocampus of FXTAS brains, the connectivity of the medial temporal lobe and neocortex may be more preserved in FXTAS than in early AD, which has been described as a severe hippocampal disconnection syndrome.[6,33] Depth recording studies have found putative P600 generators in the hippocampus, parahippocampal gyrus (PHG), cingulate cortex and several regions of multi-modal association neocortex.[34] By adapting our word repetition paradigm for functional magnetic resonance imaging (fMRI), we have identified congruous word repetition effects (New > Old BOLD response) in left PHG, hippocampus, middle and superior temporal gyri (MTG and STG), inferior and middle frontal gyri (IFG and MFG), inferior parietal lobule (IPL), insula, bilateral fusiform, and cingulate cortex [35]. As mentioned earlier, the LPC repetition effect is highly sensitive to early AD [16] and decreased amplitude is associated with poorer outcome in amnestic MCI.[17]
The more pervasive loss of ERP word repetition effects (both LPC and N400) in very early AD is consistent with a more generalized loss of synaptic plasticity and memory processes than in FXTAS, or in chronic amnesia due to Korsakoff’s syndrome or circumscribed medial temporal lobe lesions.[10] Multiple basic research studies have demonstrated that altered synaptic function and synaptic plasticity occur very early in AD pathogenesis models.[37,38].
As hypothesized, we found strong correlations between LPC repetition effect amplitude and verbal learning and memory abilities. This is consistent with our past studies in normal subjects, chronic amnesia, and MCI. Consistent with prior studies, the present study found that the N400 was the strongest predictor of the CVLT short delay cued recall,[39] while the LPC repetition effect was the strongest predictor of all the long delay measures on the CVLT.[10,11] It should be noted that the regression analyses done within FXTAS found that the N400 repetition effect amplitude correlated significantly with all 6 measures of verbal learning and memory abilities on the CVLT. In a ERP substudy of the first placebo-controlled, double-blind, randomized clinical trial conducted in FXTAS, we have recently reported that one year of memantine treatment benefited individuals with FXTAS in the N400 repetition effect and recall memory performance, which were positively correlated with each other.[40] Taken together, these findings suggest that the neural circuits which generate and modulate the N400 may be responsible for their deficits in verbal learning and memory, and sensitive to modest improvement in verbal memory produced by memantine. Future work appears warranted to see if N400 abnormalities might be a useful biomarker for tracking FXTAS neuropathology of response to treatment designed to improve their learning and memory.
We found impaired verbal learning and memory on the CVLT in FXTAS patients, but these impairments are generally milder than prodromal/early AD. A prior study of FXTAS dementia and AD [2] did not find intergroup differences in working memory, but did not analyze any measures of episodic memory. We are not aware of any publications comparing CVLT performance in FXTAS to AD or MCI, and report here that FXTAS patients have milder deficits in verbal learning and memory, compared to prodromal AD patients of comparable global deficits. Thus, these CVLT measures, like the ERP LPC repetition effect, both show promise in potentially differentiating the learning and memory impairment due to FXTAS versus early AD.
In conclusion, we found the MCI stage of AD had a more generalized loss of ERP word repetition effects than mild FXTAS. This may be related to the multiple mechanisms through which amyloid species, both soluble and insoluble, have been shown to disrupt synaptic plasticity.[41-43] Although the LPC repetition effect was less affected in FXTAS, both patient groups had very similar N400 abnormalities. FXTAS is also a disorder with synaptic dysfunction, but via a different mechanism. Fragile X Mental Retardation Protein (FMRP) is an mRNA binding protein concentrated in synaptic terminals, where it regulates the translation/expression of numerous synaptic proteins, including amyloid precursor protein (APP).[44] Low levels of FMRP are thought to cause important increases in mGluR-mediated long-term depression (LTD), as was firstly identified in FMR1 knock-out mice.[45,46] Mouse models of the fragile X premutation have found reduced induction of long-term potentiation (LTP) and increases in both NMDA and mGluR-mediated LTD.[47] These effects are large 10-20 minutes post-conditioning, and then became smaller and insignificant by 40-50 minutes.[47] Hippocampal neurons from mice with the fragile X premutation show a clustered burst firing pattern and imbalanced excitatory–inhibitory (i.e., glutamate-GABA) neurotransmission [48]. Further studies with cognitive ERP/EEG and brain-imaging modalities are needed to determine the macroscopic effects of the synaptic dysfunction in both FXTAS and AD, and elucidate their impact on memory, language and other cognitive processes.
Future advances in our understanding of memory in neurodegenerative disorders will likely require us to move beyond lesion models of memory loss. Both AD and FXTAS have diffuse neuropathological lesions, and can be well-conceptuated as disorders of synaptic function. In the case of FXTAS, the MTL-cortical connectivity appears to be relatively spared, at least as assessed by the LPC repetition effect, compared to prodromal AD. We provide evidence here that dysfunction of the N400 generators, thought to reside mainly in temporal neocortex, makes an important contribution to the verbal learning and memory deficits in patients with FXTAS. In contrast, the memory deficits in amnestic MCI appear to be primarily due to dysfunction in the MTL-neocortical circuits which modulate the LPC repetition effect. Finally, it should be noted that the LPC and N400 repetition effects had independent and significant effects on verbal learning and memory when analyzed across the whole sample of 75 elderly persons. These components appear to represent independent, complementary memory functions, with the LPC being more strongly predictive of most longer delayed measures (dependent on memory storage and later retrieval), while the N400 has more substantial effects on learning/acquisition and short delayed recall, and was more sensitive to the memory dysfunction in FXTAS.
Acknowledgements
This study was supported by NIH grants P30 AG10129, UL1DE019583, RL1AG032115, RO1 AG018442, RO1 AG008313, P50AG05131 and the State of California Alzheimer’s Disease Program.
Footnotes
Conflict of Interest:
Dr. Randi Hagerman has received funding from Roche, Novartis, Forest, Seaside Therapeutics, and Curemark for clinical trials in fragile X syndrome or autism. She has consulted with Novartis regarding clinical trials in fragile X syndrome. Dr. Olichney receives support from Genentech for a clinical drug trial and has served as a consultant for Lundbeck Pharmaceuticals.
References
- 1.Hagerman PJ, Hagerman RJ. Advances in clinical and molecular understanding of the FMR1premutation and fragile X-associated tremor/ataxia syndrome. Lancet Neurol. 2013;12:786–798. doi: 10.1016/S1474-4422(13)70125-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seritan AL, Nguyen DV, Farias ST, Hinton L, Grigsby J, Bourgeois JA, Hagerman RJ. Dementia in fragile X-associated tremor/ataxia syndrome (FXTAS): comparison with Alzheimer’s disease. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1138–1144. doi: 10.1002/ajmg.b.30732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tassone F, Greco CM, Hunsaker MR, Seritan AL, Berman RF, Gane LW, Jacquemont S, Basuta K, Jin LW, Hagerman PJ, Hagerman RJ. Neuropathological, clinical and molecular pathology in female fragile X premutation carriers with and without FXTAS. Genes Brain Behav. 2012;11:577–585. doi: 10.1111/j.1601-183X.2012.00779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 5.Greco CM, Berman RF, Martin RM, Tassone F, Schwartz PH, Chang A, Trapp BD, Iwahashi C, Brunberg J, Grigsby J, Hessl D, Becker EJ, Papazian J, Leehey MA, Hagerman RJ, Hagerman PJ. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) Brain. 2006;129:243–255. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]
- 6.Zhou J, Grecius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, Kramer JH, Weiner M, Miller BL, Seeley WW. Divergent network connecivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain. 2010;133:1352–1367. doi: 10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nunez PL, Srinivasan R. Electric fields of the brain: the neurophysics of EEG. 2nd Oxford University Press; New York (NY): 2006. pp. 163–166. [Google Scholar]
- 8.Rugg MD, Curran T. Event-related potentials and recognition memory. Trends Cogn Sci. 2007;11:251–257. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Paller KA, Kutas M. Brain potentials during memory retrieval provide neurophysiological support for the distinction between conscious recollection and priming. J Cogn Neurosci. 1992;4:375–391. doi: 10.1162/jocn.1992.4.4.375. [DOI] [PubMed] [Google Scholar]
- 10.Olichney JM, Van Petten C, Paller KA, Salmon DP, Iragui VJ, Kutas M. Word repetition in amnesia. Electrophysiological measures of impaired and spared memory. Brain. 2000;123:1948–1963. doi: 10.1093/brain/123.9.1948. [DOI] [PubMed] [Google Scholar]
- 11.Olichney JM, Morris SK, Ochoa DP, Salmon DP, Thal LJ, Kutas M, Iragui VJ. Abnormal verbal event related potentials in mild cognitive impairment and incipient Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2002;74:377–384. doi: 10.1136/jnnp.73.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olichney JM, Chan S, Wong LM, Schneider A, Seritan A, Niese A, Yang JC, Laird K, Teichholtz S, Khan S, Tassone F, Hagerman R. Abnormal N400 word repetition effects in fragile X-associated tremor/ataxia syndrome. Brain. 2010;133:1438–1450. doi: 10.1093/brain/awq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chwilla DJ, Brown CM, Hagoort P. The N400 as a function of the level of processing. Psychophysiology. 1995;32:274–85. doi: 10.1111/j.1469-8986.1995.tb02956.x. [DOI] [PubMed] [Google Scholar]
- 14.Kutas M, Federmeier KD. Thirty years and counting: finding memory in the N400 component of event–related brain potential (ERP) Annu Rev Psychol. 2011;62:621–47. doi: 10.1146/annurev.psych.093008.131123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 16.Olichney JM, Iragui VJ, Salmon DP, Riggins BR, Morris SK, Kutas M. Absent event-related potential (ERP) word repetition effects in mild Alzheimer's disease. Clin Neurophysiol. 2006;117:1319–30. doi: 10.1016/j.clinph.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olichney JM, Taylor JR, Gatherwright J, Salmon DP, Bressler AJ, Kutas M, Iragui-Madoz VJ. Patients with MCI and N400 or P600 abnormalities are at very high risk for conversion to dementia. Neurology. 2008;70:1763–70. doi: 10.1212/01.wnl.0000281689.28759.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Revised Fourth American Psychiatric Association; Washington DC: 2000. [Google Scholar]
- 19.Jacquemont S, Hagerman RJ, Leehey M, Grigsby J, Zhang L, Brunberg JA, Greco C, Des Portes V, Jardini T, Levine R, Berry-Kravis E, Brown WT, Schaeffer S, Kissel J, Tassone F, Hagerman PJ. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am J Hum Genet. 2003;72:869–78. doi: 10.1086/374321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grigsby J, Brega AG, Engle K, Leehey MA, Hagerman RJ, Tassone F, Hessl D, Hagerman PJ, Cogswell JB, Bennett RE, Cook K, Hall DA, Bounds LS, Paulich MJ, Reynolds A. Cognitive profile of fragile X premutation carriers with and without fragile X-associated tremor/ataxia syndrome. Neuropsychology. 2008;22:48–60. doi: 10.1037/0894-4105.22.1.48. [DOI] [PubMed] [Google Scholar]
- 21.Delis DC, Kramer JH, Kaplan E, Ober B. The California Verbal Learning Test. The Psychological Corporation; New York: 1987. [Google Scholar]
- 22.Francis WN, Kucera H. Frequency analysis of English usage: lexicon and grammar. Houghton Mifflin; Boston: 1982. [Google Scholar]
- 23.Urbach TP, Kutas M. The intractability of scaling scalp distributions to infer neuroelectric sources. Psychophysiology. 2002;39:791–808. doi: 10.1111/1469-8986.3960791. [DOI] [PubMed] [Google Scholar]
- 24.Kutas M, Hillyard S. Brain potentials during reading reflect word expectancy and semantic association. Nature. 1984;307:161–3. doi: 10.1038/307161a0. [DOI] [PubMed] [Google Scholar]
- 25.Addante RJ, Ranganath C, Yonelinas AP. Examining ERP correlates of recognition memory: evidence of accurate source recognition without recollection. NeuroImage. 2012;62:439–50. doi: 10.1016/j.neuroimage.2012.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu SS, Rugg MD. Dissociation of the electrophysiological correlates of familiarity strength and item repetition. Brain Res. 2010;1320:74–84. doi: 10.1016/j.brainres.2009.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olichney JM, Hillert DG. Clinical applications of cognitive event-relatd potentials in Alzheimer’s disease. Phys Med Rehabil Clin N Am. 2004;15:205–33. doi: 10.1016/s1047-9651(03)00103-7. [DOI] [PubMed] [Google Scholar]
- 28.McCarthy G, Nobre AC, Bentin S, Spencer DD. Language-related field potentials in the anterior medial temporal lobe: I. Intracranial distribution and neural generators. J Neurosci. 1995;15:1080–9. doi: 10.1523/JNEUROSCI.15-02-01080.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butters N, Granholm E, Salmon DP, Grant I, Wolfe J. Episodic and semantic memory: a comparison of amnesic and demented pateints. J Clin Exp Neuropsychol. 1987;9:479–97. doi: 10.1080/01688638708410764. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Koscielska KA, Cao Z, Hulsizer S, Grace N, Mitchell G, Nacey C, Githinji J, McGee J, Garcia-Arocena D, Hagerman RJ, Nolta J, Pessah IN, Hagerman PJ. Signaling defects in iPSC-derived fragile X premutation neurons. Hum Mol Genet. 2012;21:3795–805. doi: 10.1093/hmg/dds207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reisberg B, Doody R, Stöffler A, Schmitt F, Ferris S, Möbius HJ. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med. 2003;14:1333–41. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 32.Grunwald T, Beck H, Lehnertz K, Blümcke I, Pezer N, Kurthen M, Fernández G, Van Roost D, Heinze HJ, Kutas M, Elger CE. Evidence relating human verbal memory to hippocampal N-methyl-D-aspartate receptors. Proc Natl Acad Sci U S A. 1999;96:12085–9. doi: 10.1073/pnas.96.21.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hyman BT, Van Hoesen G, Damasio A, Barnes CL. Alzheimer’s disease: cell specific pathology isolates the hippocampal formation. Science. 1984;224:1168–70. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- 34.Halgren E, Baudena P, Heit G, Clarke JM, Marinkovic K, Clarke M. Spatio-temporal stages in face and word processing 1 Depth-recorded potentials in the human occipital, temporal and parietal lobes. J Physiol Paris. 1994;88:1–50. doi: 10.1016/0928-4257(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 35.Olichney JM, Taylor JR, Hillert DG, Chan SH, Salmon DP, Gatherwright J, Iragui VJ, Kutas M. fMRI congruous word repetition effects reflect memory variability in normal elderly. Neurobiol Aging. 2010;31:1975–90. doi: 10.1016/j.neurobiolaging.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sestieri C, Capotosto P, Tosoni A, Luca Romani G, Corbetta M. Interference with episodic memory retrieval following transcranial stimulation of the inferior but not the superior parietal lobule. Neuropsychologia. 2013;51:900–6. doi: 10.1016/j.neuropsychologia.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 37.Mucke L, Selkoe DJ. Neurotoxicity of amyloid β-protein: synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2012;2:a006338. doi: 10.1101/cshperspect.a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klein WL. Synaptic targeting by A beta oligomers (ADDLS) as a basis for memory loss in early Alzheimer’s disease. Alzheimers Dement. 2006;2:43–55. doi: 10.1016/j.jalz.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Helmstaedter C, Kurthen M, Linke DB, Elger CE. Patterns of language dominance in focal left and right hemishpere epilepsies: relation to MRI findings, EEG, sex and age at onset of epilepsy. Brain Cogn. 1997;33:135–50. doi: 10.1006/brcg.1997.0888. [DOI] [PubMed] [Google Scholar]
- 40.Yang JC, Niu YQ, Simon C, Chen L, Seritan A, Schneider A, Torabi Moghaddam S, Hagerman PJ, Hagerman RJ, Olichney JM. Memantine Effects on Verbal Memory in FXTAS: a Double-blind ERP Study. Neuropsychopharmacology. doi: 10.1038/npp.2014.122. doi:10.1038/npp.2014.122. Accepted article preview online May 29, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–42. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–7. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 43.Walsh DM, Klyubin I, Fadeeva JV, Rowan MJ, Selkoe DJ. Amyloid-beta oligomers: their production, toxicity and therapeutic inhibition. Biochem Soc Trans. 2002;30:552–7. doi: 10.1042/bst0300552. [DOI] [PubMed] [Google Scholar]
- 44.Westmark CJ, Malter JS. FMRP mediates mGluR5-dependent translation of amyloid precursor protein. PLoS Biol. 2007;5:e52. doi: 10.1371/journal.pbio.0050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci USA. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hunsaker MR, Kim K, Willemsen R, Berman RF. CGG trinucleotide repeat length modulates neural plasticity and spatiotemporal processing in a mouse model of the fragile X premutation. Hippocampus. 2012;22:2260–75. doi: 10.1002/hipo.22043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao Z, Hulsizer S, Tassone F, Tang HT, Hagerman RJ, Rogawski MA, Hagerman PJ, Pessah IN. Clustered burst firing in FMR1 premutation hippocampal neurons: amelioration with allopregnanolone. Hum Mol Genet. 2012;21:2923–35. doi: 10.1093/hmg/dds118. [DOI] [PMC free article] [PubMed] [Google Scholar]