Summary
Unlike mammals, zebrafish can regenerate a damaged retina. This remarkable regenerative response is mediated by Müller glia (MG) that undergo a reprogramming event that drives their proliferation and the generation of multipotent progenitors for retinal repair. The mechanisms driving MG reprogramming are poorly understood. Here we report that Leptin and Gp130-coupled receptors, acting via a Jak/Stat signaling pathway, stimulate MG reprogramming and progenitor formation in the injured retina. Importantly, we found that ascl1a gene expression, which drives MG reprogramming in fish and mammals, is regulated in a Jak/Stat-dependent manner and requires consensus Stat binding sites for injury-dependent activation. Finally, we identified cytokines that are induced by retinal injury and exhibit a remarkable synergy in their ability to activate Jak/Stat signaling and MG reprogramming in the uninjured retina. Our study not only furthers our understanding of retina regeneration in zebrafish, but also suggests new strategies for awakening retina regeneration in mammals.
Introduction
Because of their robust regenerative powers, zebrafish have become an ideal model system for studying retina regeneration. Following retinal injury, MG reprogram so they acquire progenitor characteristics that allow them to regenerate all major retinal cell types (Bernardos et al., 2007; Fausett and Goldman, 2006; Fimbel et al., 2007; Ramachandran et al., 2010a; Ramachandran, 2010). A key event in MG reprogramming is the activation of ascl1a gene expression (Fausett et al., 2008), which encodes a nodal transcription factor impacting reprogramming genes and signaling cascades that affect almost all aspects of retina regeneration (Lenkowski et al., 2013; Nelson et al., 2013; Nelson et al., 2012; Powell et al., 2012; Ramachandran et al., 2010a; Ramachandran et al., 2011, 2012; Wan, 2012). Importantly, ASCL1 also controls MG reprogramming in the postnatal mouse retina (Pollak et al., 2013).
The mechanisms by which injury signals are conveyed to the MG genome to activate reprogramming genes, like ascl1a, are not well understood. However, one candidate is Stat3, a signal transducer and activator of transcription whose activity and nuclear localization is controlled by growth factors and cytokines (Cao et al., 1996; Grandis et al., 1998; Hirano et al., 2000; Levy and Darnell, 2002; Vogt and Hart, 2011). Importantly, Stat3 expression is induced in the injured retina (Kassen et al., 2007; Kassen et al., 2009; Nelson et al., 2012). However, this expression is detected in all retinal layers and in the inner nuclear layer (INL), quiescent MG as well as proliferating MG express Stat3 (Kassen et al., 2007; Nelson et al., 2012). This expression pattern, along with the assumption that injury-induced Stat3 expression reflects that of activated p-Stat3 (Kassen et al., 2007), has led to models assigning different roles for injury-induced Stat3 in quiescent MG, MG stem cells and MG-derived progenitors (Gorsuch and Hyde, 2013; Nelson et al., 2013; Nelson et al., 2012). However, it remains unknown whether total Stat3 is a true indicator of p-Stat3 in the injured retina, nor is it known if endogenous cytokines acting via Jak/Stat signaling stimulate MG reprogramming and retina regeneration following retinal injury.
Here we report that unlike total Stat3 expression, activated p-Stat3 signaling is restricted to a subset of MG that reprogram and proliferate in response to retinal injury. We show that Jak/Stat signaling directly controls reprogramming genes like ascl1a and identify endogenous cytokines acting via Leptin and Gp130-coupled receptors that stimulate Jak/Stat signaling and progenitor formation in the injured retina. Importantly, we show that Leptin and IL-6-like cytokines are sufficient for stimulating MG proliferation in the uninjured retina and that they exhibit a remarkable synergy in their action. The local release of cytokines by injury responsive MG and their synergistic action may be critical for their effectiveness in stimulating MG reprogramming and progenitor formation.
Results
Stat3 signaling is restricted to MG progenitors in the injured retina
In the injured retina, Stat3 activation is assumed to reflect total Stat3 expression (Kassen et al., 2007; Nelson et al., 2012). To test this idea, we took advantage of an antibody that specifically detects activated, phosphorylated Stat3 (p-Stat3) (Yamashita et al., 2002). Although p-Stat3 stained processes in the outer plexiform and ganglion cell layers in the uninjured retina, there was no labeling of cells in the INL where MG cell bodies reside (Figure S1A). However, following retinal injury with a needle poke, we observed p-Stat3 staining in the INL that was restricted to BrdU+ cells at the injury site (Figures 1A and S1A). We previously demonstrated that essentially all of the proliferating cells in the INL following retinal injury are MG-derived progenitors (Fausett and Goldman, 2006). Furthermore, we show here that although microglia migrate to the injury site, they do not contribute to the proliferating cell population (Fig. S1B). Importantly, we repeated these experiments using a light damage model of photoreceptor death where only about 50% of the MG reprogram and proliferate (Figure S1C and S1D) (Nelson et al., 2012). In this model both quiescent and proliferating MG show increased Stat3 expression and it has been assumed that this reflects activated p-Stat3 expression (Kassen et al., 2007; Nelson et al., 2012); however, we found that p-Stat3 was restricted to the proliferating population of MG (Figures S1D), suggesting Jak/Stat signaling is also restricted to these cells.
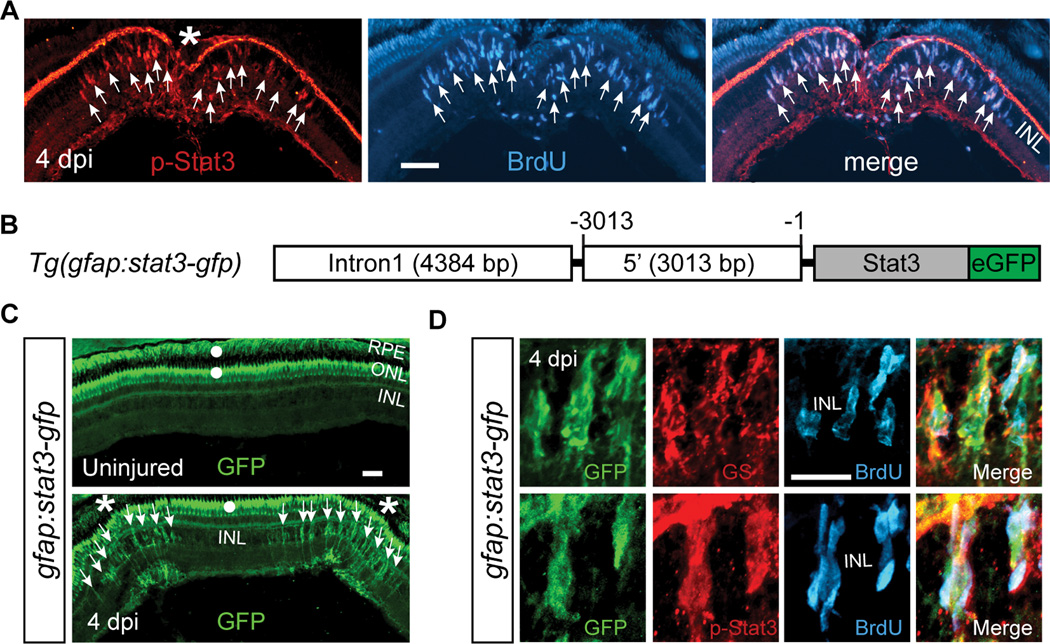
Figure 1. Jak/Stat3 signaling pathway is activated following retinal injury.
(A) Immunofluorescence on retinal sections shows activated p-Stat3 expression in BrdU+ MG-derived progenitors that are localized to the injury site at 4 dpi. (B) Schematic of gfap:stat3-gfp transgene construct shows the fusion gene, stat3-gfp, under control of the gfap promoter regulatory elements. (C) In gfap:stat3-gfp transgenic fish, Stat3-GFP fusion protein expression is undetectable in MG of the uninjured eye, while restricted to MG-derived progenitors at the injury site at 4 dpi. (D) Confocal images showing co-localization of Stat3-GFP with glutamine synthetase (GS)+/p-Stat3+/BrdU+ MG-derived progenitors at 4 dpi. In (A and C) the asterisk marks the injury site (needle poke) and arrows point to MG-derived progenitors. In (C) white dots indicate autofluorescence unique to the green channel (see Figure S2G). Error bars, s.d. Scale bars, 50 µm (A, C) and 20 µm (D). INL, inner nuclear layer; ONL, outer nuclear layer; RPE, retinal pigment epithelium; dpi, days post injury. See also Figures S1and S2.
To further explore Stat3 activation in the injured retina, we created gfap:stat3-gfp transgenic fish, where the MG-specific gfap promoter drives transgene expression (Figure 1B) (Bernardos and Raymond, 2006). During development, the Stat3-GFP fusion protein was restricted to the nervous system (Figure S2A) and in the retina it was localized to glutamine synthetase (GS)+ MG (Figure S2B). In the uninjured adult retina, we detected stat3-gfp mRNA (Figure S2C) that was localized to GS+ MG cell bodies and processes in the INL (Figure S2D), but we were unable to detect any Stat3-GFP protein in this layer (Figure 1C, top panel). The green signal noted in the outer nuclear layer (ONL) and retinal pigment epithelium (RPE) (white dots in Fig. 1C) is autofluorescence unique to the green channel (see Figure S2G). Interestingly, Stat3 mRNA has been reported in neuronal processes where, in response to injury, it is locally translated (Ben-Yaakov et al., 2012). Whether it serves a similar function in MG remains unknown. The detection of stat3-gfp mRNA, but not protein, indicates that the Stat3 may be unstable in the uninjured retina. Remarkably, following retinal injury with a needle poke, stat3-gfp mRNA levels remained unchanged (Figure S2C), but Stat3-GFP protein increased locally at the injury site in cells exhibiting a typical MG morphology (Figure 1C, bottom panel). Importantly, Stat3-GFP specifically accumulates in BrdU+ and p-Stat3+ MG-derived progenitors localized to the injury site (Figures 1D, S2E and S2F).
The above data suggested Jak/Stat3 signaling regulates progenitor formation in the injured retina. Indeed, Jak/Stat signaling inhibitors, JSI-124 and P6 (Blaskovich et al., 2003; Pedranzini et al., 2006) suppressed the generation of MG-derived progenitors (Figures 2A and 2B). Furthermore, by inhibiting Jak/Stat from either 0–2 dpi or 2–4 dpi, we found that Jak/Stat signaling impacts both progenitor formation, which is just beginning at 2 dpi (Fausett and Goldman, 2006) and their later expansion (Figures S3A–S3D). Importantly, Jak inhibitors did not stimulate apoptosis (Figures S3E and S3F).
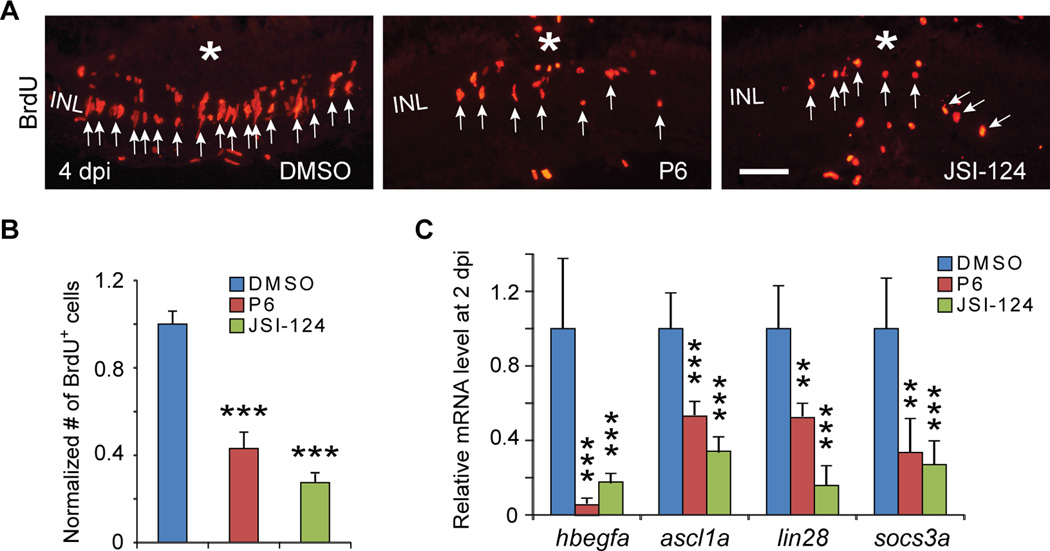
Figure 2. Jak/Stat3 signaling pathway is necessary for retina regeneration.
(A) BrdU immunofluorescence shows Jak inhibitors, P6 and JSI-124, suppress progenitor formation at 4 dpi. (B) Quantification of BrdU+ progenitors in (A); ***P<0.001, n=4. (C) qPCR showing Jak inhibitors, P6 and JSI-124, inhibit reprogramming gene induction at 2 dpi; **P<0.01, ***P<0.001; n=4, 4, and 5 for DMSO, P6 and JSI-124, respectively. In (A) the asterisk marks the injury site (needle poke) and arrows point to MG-derived progenitors. Error bars, s.d. Scale bars, 20 µm (A). INL, inner nuclear layer; dpi, days post injury. See also Figure S3.
We next investigated if Jak/Stat signaling was necessary for injury-dependent Stat3-GFP stabilization. For this analysis we treated gfap:stat3-gfp transgenic fish with JSI-124 and assayed GFP immunofluorescence in the injured retina. Consistent with the idea that Stat3-GFP stabilization reflects activated p-Stat3 expression, JSI-124 treatment dramatically reduced Stat3-GFP expression in the injured retina (Figures S3G and S3H). Whether Jak-mediated stabilization is a direct consequence of Stat3 phosphorylation or phosphorylation of other proteins remains unknown.
Finally, because socs3 gene expression is often used as a sensitive read-out of Stat3 activation (Liang et al., 2012), we characterized its expression in the injured retina and observed injury-dependent socs3a and socs3b induction within 3 hrs post retinal injury (hpi) (Figure S3I). This induction is before the first detection of p-Stat3 at 2 dpi (Figure S1A) and likely reflects the increased sensitivity of PCR over immunofluorescence. Regardless, our data suggests that Stat signaling is activated specifically in MG at the injury site.
Jak/Stat signaling regulates MG reprogramming
The above data suggests Jak/Stat signaling regulates the generation of MG-derived progenitors. However, prior to progenitor formation, MG reprogram their genome to acquire properties of a retinal stem cell (Fausett et al., 2008; Kassen et al., 2007; Nagashima et al., 2013; Ramachandran et al., 2010a; Ramachandran et al., 2011, 2012). This reprogramming is characterized by the very rapid activation of genes like hbegfa, ascl1a, lin28, stat3 and socs3a (Figure S3G) (Fausett et al., 2008; Kassen et al., 2007; Ramachandran et al., 2010a; Wan, 2012). To investigate if Jak/Stat signaling also regulated the expression of reprogramming genes, we injured retinas with and without Jak/Stat inhibition and assayed reprogramming gene expression 2 days later when reprogrammed MG are mostly quiescent (Fausett and Goldman, 2006). These experiments showed that injury-dependent induction of reprogramming genes was suppressed by Jak/Stat inhibitors (Figure 2C).
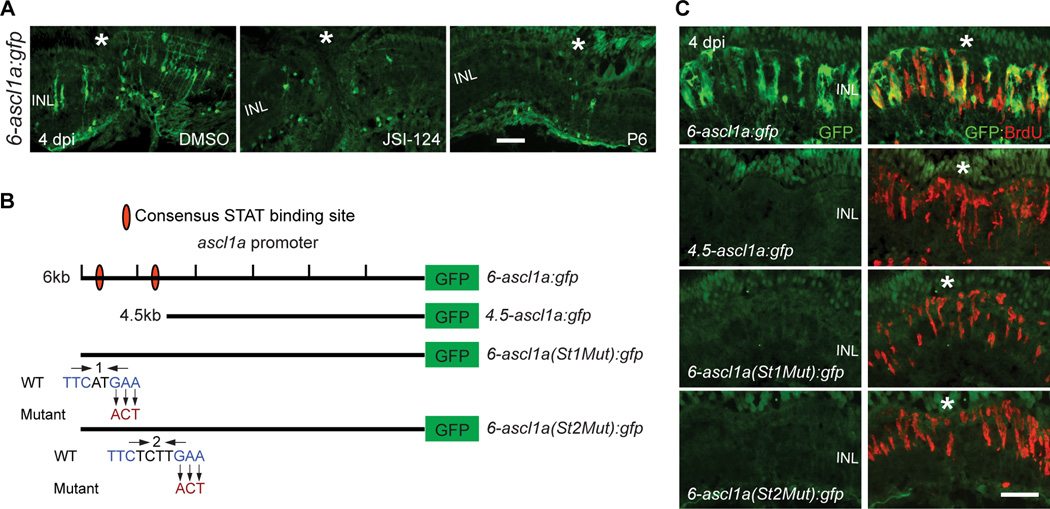
To determine if Jak/Stat signaling directly impinged on reprogramming genes, we focused on the ascl1a promoter since Ascl1a drives MG reprogramming in both zebrafish and mice (Fausett et al., 2008; Pollak et al., 2013; Ramachandran et al., 2010a; Ramachandran et al., 2011). For this analysis we took advantage of 6-ascl1a:gfp fish that harbor a 6 kb ascl1a promoter fragment that restricts GFP expression to reprogrammed MG and MG-derived progenitors (Wan, 2012). Jak/Stat inhibitors suppressed injury-dependent GFP expression in these fish (Figure 3A). A variety of 5’ promoter deletions allowed us to narrow-in on a distal 1.5 kb promoter fragment that was necessary for injury-dependent ascl1a promoter activation (Figures 3B, 3C, S4A and S4B). Within this fragment, we identified two consensus Stat3 binding sites (Ehret et al., 2001), whose mutation prevented injury-dependent ascl1a promoter activation (Figured 3B, 3C, S4C and S4D). BrdU-labeling indicated a normal injury response in these fish (Figures 3C, S4C and S4D). Thus, Jak/Stat signaling stimulates MG reprogramming by activating genes necessary for this process.
Figure 3. Jak/Stat3 signaling mediates injury-dependent induction of the reprogramming gene, ascl1a.
(A) GFP immunofluorescence in 6-ascl1a:gfp fish shows that Jak inhibitors, P6 and JSI-124, applied at the time of retinal injury, inhibit injury-dependent transgene induction. (B) Diagram of ascl1a promoter constructs used to generate transgenic lines. (C) GFP immunofluorescence shows that a distal 1.5 kb fragment of the ascl1a promoter is required for injury-dependent transgene expression and that both consensus Stat3 sites located in this promoter fragment are necessary for this expression. BrdU+ cells indicate the injury site and a normal regenerative response. The asterisk marks the injury site (needle poke). Scale bar, 50 µm. INL, inner nuclear layer; dpi, days post injury. See also Figure S4.
IL-6 family cytokines drive MG progenitor formation
Because cytokines are often increased in response to tissue damage and stimulate Jak/Stat signaling, we were interested in identifying those that may activate Jak/Stat signaling following retinal injury. A clue to their nature comes from the observation that CNTF can stimulate a small amount of MG proliferation in the uninjured retina (Faillace et al., 2002; Kassen et al., 2009). However, a cntf gene remains unidentified in the zebrafish genome, making Cntf an unlikely candidate for mediating injury-dependent MG reprogramming and retina regeneration.
CNTF is a member of a family of IL-6-like cytokines whose receptors share the common signaling subunit Gp130 (Hirano et al., 2000). Consistent with a role for Gp130-coupled receptors in regulating MG reprogramming, we detected a low basal expression of gp130 mRNA in the uninjured retina that was increased in MG-derived progenitors following injury (Figures 4A, 4B, and S5A). Interestingly, a variety of IL-6 family member genes are induced in MG-derived progenitors shortly after injury; including m17 (also referred to as lif), clcf1, crlf1a, il-11a, il-11b and their related receptors (Figures 4A, 4B, S5A and S5B). This local expression in MG-derived progenitors may indicate that IL-6 family members act in both an autocrine and paracrine fashion to stimulate MG reprogramming and progenitor formation.
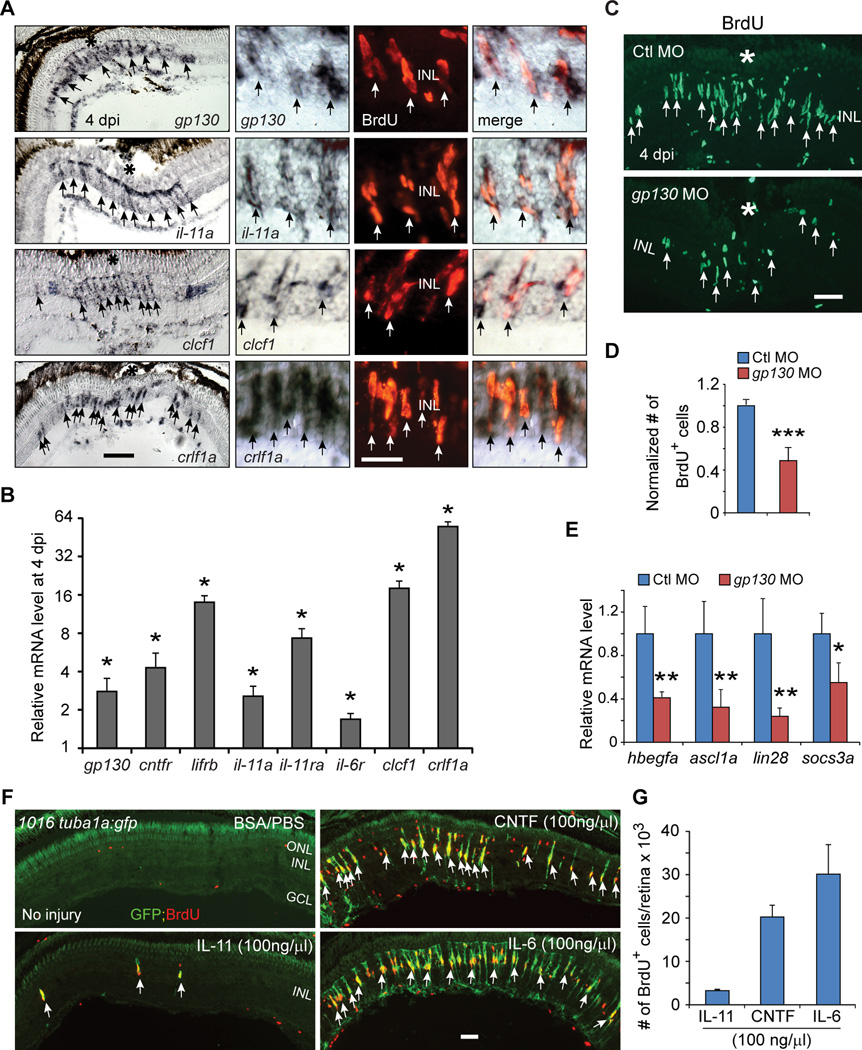
Figure 4. IL-6 family cytokines signaling through Gp130 are necessary and sufficient for retina regeneration.
(A) In situ hybridization and immunofluorescence shows that gp130, il-11a, crlf1a and clcf1 are expressed in BrdU+ MG-derived progenitors localized to the injury site. (B) qPCR quantifies il-6 family gene induction in MG-derived progenitors (FACS purified from 1016tuba1a:gfp fish retinas at 4 dpi) relative to MG from uninjured retina (FACS purified from uninjured gfap:gfp fish retinas); *P<0.05, n=3. (C, D) Gp130 knockdown inhibits the generation of BrdU+ MG-derived progenitors at 4 dpi. Control (Ctl) or gp130-targeting MOs were electroporated into the retina at the time of injury and fish received an i.p. injection of BrdU 3h before sacrifice on 4 dpi. ***P<0.001, n=4. (E) qPCR showing Gp130 knockdown inhibits injury-dependent induction of reprogramming genes at 2 dpi; *P<0.05, **P<0.01, n=4. (F) Intravitreal injection of recombinant mammalian IL-6-like cytokines into the uninjured eye of 1016tuba1a:gfp fish stimulates GFP expression and BrdU incorporation in MG throughout the retina’s inner nuclear layer. Intravitrial injection of PBS/BSA did not stimulate GFP expression or BrdU incorporation. The green fluorescence above the ONL in the top left-hand panel is autofluorescence unique to green channel (See Figure S7K). (G) Quantification BrdU+ cells following intravitreal injection of recombinant mammalian IL-6-like cytokines, n=3. Error bars, s.d. In (A, C), the asterisks mark the injury site (needle poke). Arrows point to MG-derived progenitors (A, C, F). Scale bars, 20 µm (A, C); 50 µm (F). ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer; dpi, days post injury. Primers are listed in Table S1. See also Figures S5 and S6.
To directly test if IL-6 family cytokines regulated injury-dependent MG reprogramming, we knocked down their common signaling component (Gp130) using a lissamine-tagged morpholino-modified antisense oligonucleotide (MO) whose effectiveness was confirmed in zebrafish embryos overexpressing a Gp130-GFP fusion protein (Figures S5C–S5E). In the adult retina, Gp130 knockdown reduced the generation of BrdU+ progenitors assayed at 4 dpi (Figures 4C and 4D) and inhibited injury-dependent induction of reprogramming genes hbegfa, ascl1a, lin28 and socs3a assayed at 2 dpi (Figure 4E), a time when most reprogrammed MG have not yet begun to divide (Fausett and Goldman, 2006). Together, these data suggest that IL-6 family cytokines are increased in the injured retina and may contribute to MG reprogramming and proliferation.
We next investigated if IL-6 family cytokines were able to stimulate MG reprogramming in the uninjured retina. For this analysis, recombinant mammalian IL-6, IL-11 or CNTF was intravitreally injected once daily for 3 days into the uninjured eye of 1016 tuba1a:gfp transgenic fish. On the 4th day, fish received an IP injection of BrdU 3 hrs before sacrifice. In these fish, GFP reports MG reprogramming and BrdU reports MG proliferation (Fausett and Goldman, 2006; Fausett et al., 2008; Ramachandran et al., 2010a; Ramachandran et al., 2011, 2012; Wan, 2012). Using this strategy, we previously showed that HB-EGF can stimulate MG reprogramming and proliferation in the uninjured retina (Wan, 2012). However, concerns were raised suggesting that using a needle to puncture the cornea and that injection of a 2 µl volume might cause retinal injury and result in a MG response independent of HB-EGF (Nelson et al., 2013). Therefore, we felt it was important to first address these concerns prior to testing the cytokines described above.
It was suggested that access to the intravitreal space by cutting the cornea with a sapphire blade would cause less trauma than a needle puncture and that delivery of fluid volumes below 2 µl would also result in less intraocular pressure (Nelson et al., 2013); so we compared these variables. When an incision was made with a sapphire blade, we delivered HB-EGF or vehicle with a blunt 33 gauge needle as suggested (Nelson et al., 2013). We found that delivery of 0.5–2 µl volumes of HB-EGF, regardless of the delivery method, stimulated MG proliferation to a similar extent (Figures S6A–S6D). Importantly, intravitreal injection of vehicle caused no MG proliferation. Because the vehicle control (Figure S6C) and certain cytokines (see below) do not stimulate MG proliferation using these methods, any noted effects on MG proliferation are significant. However, the reason HB-EGF did not stimulate MG proliferation in a previous study is puzzling (Nelson et al., 2013). Perhaps the location of HB-EGF delivery influenced results. To test this possibility, we compared HB-EGF delivery above and below the lens. Interestingly, only when HB-EGF was delivered below the lens did we observe a robust proliferative response (Figures S6E and S6F). These studies demonstrate that injection of drugs beneath the lens using either a sapphire blade or a beveled needle to gain access to the intravitreal space, along with injection volumes ranging from 0.5–2 µl, are appropriate for investigating the effects of substances on MG proliferation.
We next investigated if IL-6 family members that are induced during retina regeneration are sufficient to stimulate MG proliferation in the uninjured retina. For these studies, we used CNTF as a positive control since it was known to stimulate a small amount of MG proliferation (Faillace et al., 2002; Kassen et al., 2009). Interestingly, both CNTF and IL-6 stimulated MG proliferation, while IL-11 was barely effective (Figures 4F and 4G). The green fluorescence noted above the ONL in the top left-hand panel of Fig. 4F is autofluorescence unique to the green channel (Figure S7K). Notable in the CNTF-treated retina was BrdU+/GFP− cells that, based on their location in the ONL, likely represent proliferating rod progenitors (Stenkamp, 2011).
Although CNTF and IL-6 stimulated MG proliferation in the uninjured retina, a zebrafish cntf gene remains unidentified and il-6 mRNA was undetectable in the injured retina (Figure S5A). However, MG and MG-derived progenitors do express RNAs encoding receptors for these IL-6 family cytokines (Figures 4B, S5A and S5B). Furthermore, genes encoding the alternative Cntf receptor ligand, Clcf1/Crlf1a (Elson et al., 2000), are highly induced in the injured retina (Figures 4A, 4B and S5A). Therefore, Clcf1/Crlf1a may be responsible for Cntf receptor activation in the injured retina. Although il-6 mRNA was not detected in the injured retina, it is possible that IL-6 is present in blood and released from damaged vessels following retinal puncture. This IL-6 may then act via MG-resident IL-6 receptors to stimulate MG reprogramming and proliferation. However, this possibility remains untested. The stimulation of MG proliferation by CNTF and IL-6 in the uninjured retina is consistent with the idea that MG are capable of responding to injury signals mediated by IL-6 family members.
Leptin signaling stimulates MG reprogramming and proliferation
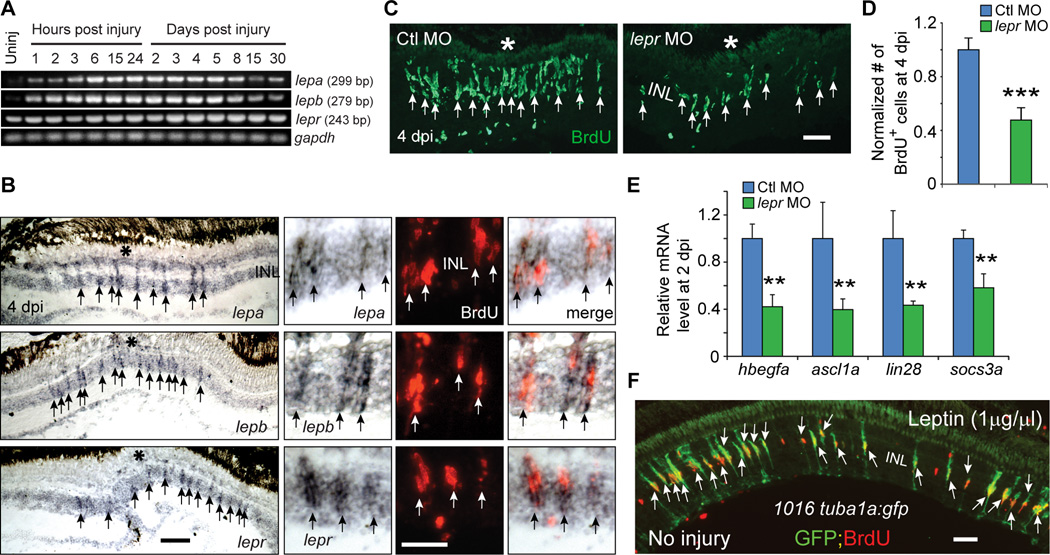
Although IL-11 had little effect on MG proliferation in the uninjured retina (Figures 4F and 4G), we were intrigued by the robust induction of il-11 mRNA following retinal injury (Figures 4A, 4B and S5A) and wondered if it may synergize with other cytokines. Because Gp130 knockdown incompletely suppressed MG reprogramming in the injured retina (Figures 4C–4E), we suspected that IL-11 may synergize with cytokines acting independent of Gp130. A search for cytokines that met this criteria identified the mammalian Leptin homologues lepa and lepb, that are increased within 1 hpi and restricted to MG-derived progenitors at 4 dpi (Figures 5A, 5B and S7A). Although lepa and lepb mRNAs are rapidly induced following injury, they only begin to return to pre-injury levels around 8 dpi. It is not clear why these mRNAs persist for so long or even if the protein is still being expressed; however, this expression may reflect an incompletely recovered retina.
Figure 5. Leptin signaling is necessary and sufficient for retina regeneration.
(A) RT-PCR analysis of mRNAs (from whole retina) encoding Leptin and Leptin receptor at various times after retinal injury. (B) In situ hybridization and BrdU immunofluorescence shows lepa, lepb and lepr RNAs are increased in BrdU+ MG-derived progenitors at the injury site. Asterisk marks the injury site (needle poke). (C, D) MO-mediated knockdown of the Leptin receptor inhibits the generation of BrdU+ MG-derived progenitors at 4 dpi; ***P<0.001, n=4. (E) qPCR shows Leptin receptor knockdown suppresses injury-dependent induction of reprogramming genes at 2 dpi; **P<0.01, n=4. (F) Intravitreal injection of recombinant human Leptin into the uninjured eye of 1016tuba1a:gfp fish stimulates GFP expression and BrdU incorporation in MG throughout the retina’s INL. Error bars, s.d. In (B, C,) arrows point to MG-derived progenitors. Scale bars, 20 µm (B, C); 50 µm (E). INL, inner nuclear layer. Primers are listed in Table S1. See also Figures S7.
Importantly, lepr (leptin receptor) RNA is detected in MG-derived progenitors (Figures 5B and S7A) and Leptin receptor knockdown using a previously verified lepr-targeting MO (Liu et al., 2012), decreased progenitor formation and suppressed the injury-dependent induction of reprogramming genes (Figures 5C–5E). Surprisingly, the effects of Gp130 and Leptin receptor knockdown were not additive (Figures S7B–S7D) and this was also reflected in Stat3-GFP expression (Figures S7E and S7F). This suggests that both Leptin and Gp130 signaling pathways must be stimulated in the same cell in order to achieve sufficient Stat3 activation for MG to reprogram and proliferate. Consistent with this idea we found that almost all BrdU+ progenitors express both gp130 and lepr mRNAs (Figure S7G), suggesting MG-derived progenitors are a relatively homogenous population.
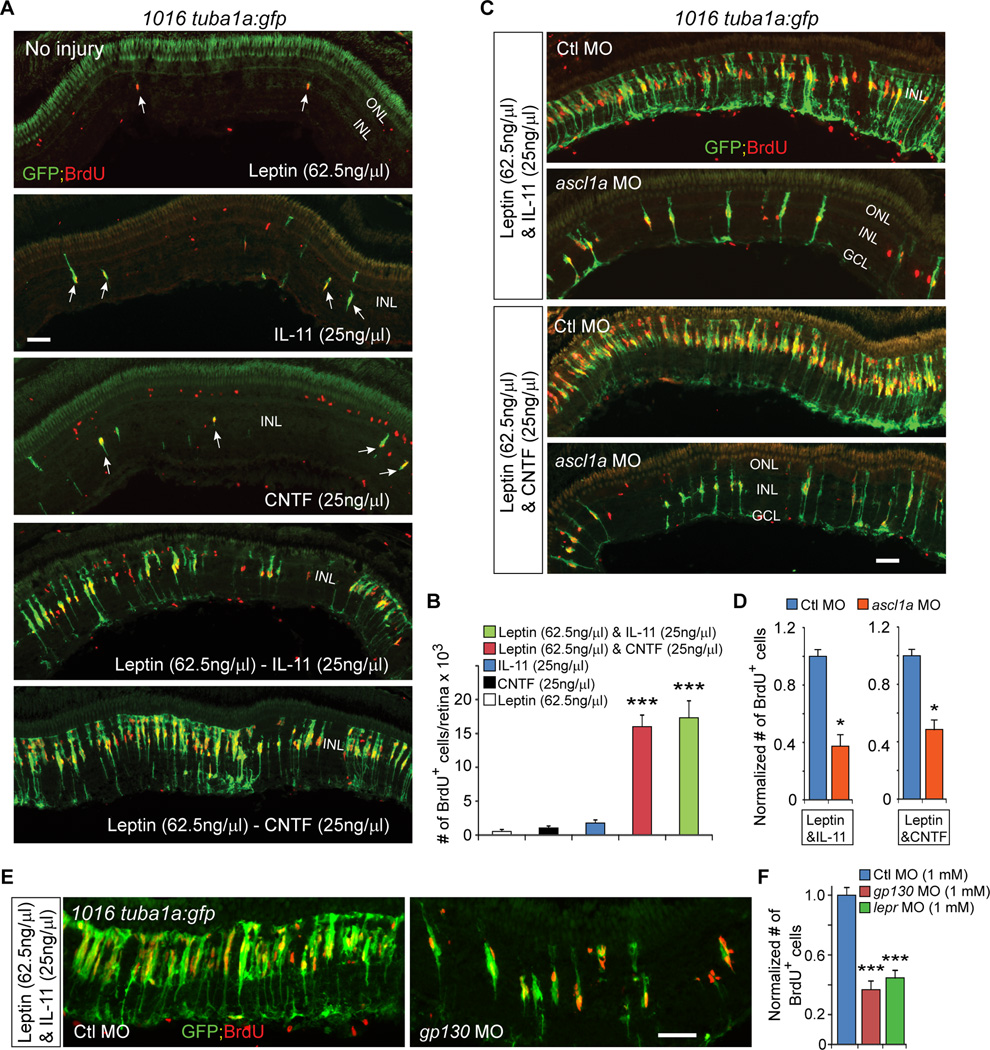
We next investigated if Leptin could stimulate MG reprogramming and proliferation in the uninjured retina. For these experiments Leptin (1 µg/µl) was intravitreally injected once daily for 3 days into the uninjured eye of 1016 tuba1a:gfp transgenic fish. On the 4th day, fish received an IP injection of BrdU 3 hrs before sacrifice. Interestingly, Leptin stimulated a remarkable amount of MG reprogramming (GFP expression) and proliferation (BrdU incorporation) (Figure 5F). However, these effects required a high dose (1 µg/µl) and when lower amounts were used, a very meager response was noted (Figures 6A, 6B, S7H and S7I).
Figure 6. Leptin synergizes with IL-11 and CNTF to stimulate MG reprogramming and proliferation in the uninjured retina.
(A) GFP and BrdU immunofluorescence shows Leptin synergizes with IL-11 and CNTF to stimulate GFP expression and MG proliferation in the uninjured retina 1016tuba1a:gfp fish, while Leptin, IL-11 or CNTF alone had little effect. Arrows point to MG-derived progenitors in top 3 panels. (B) Quantification of the effects of cytokines on MG proliferation when delivered individually or in combination to the uninjured retina; ***P<0.001 (combination vs individual), n=4 per group. (C, D) MO-mediated Ascl1a knockdown inhibits the synergistic effects of Leptin/IL-11 or Leptin/CNTF on GFP induction and MG proliferation; *P<0.05, n=3. (E, F) knockdown of Gp130 or Lepr inhibits the synergistic effects of Leptin/IL-11 on proliferation; ***P<0.001, n=4. Error bars, s.d. Scale bars, 50 µm (A, C, E). ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer.
Leptin and IL-6 family cytokines synergize with each other to stimulate MG reprogramming and proliferation
The relative ineffectiveness of Leptin and IL-11 in stimulating MG reprogramming at low doses (Leptin at 62.5 ng/µl and IL-11 at 25 ng/µl; Figure 6A) prompted us to investigate if they would exhibit synergy in their action. For these experiments uninjured retinas of 1016 tuba1a:gfp transgenic fish received daily intravitreal injections of Leptin (62.5 ng/µl) and IL-11 (25 ng/µl) as described above. This combination of cytokine injection resulted in a remarkable synergy that stimulated widespread MG reprogramming (GFP expression) and proliferation (BrdU incorporation) (Figures 6A and 6B). However, when lower amounts of Leptin and IL-11 were used, only small responses were noted (Figure S7J). The synergy noted between Leptin and IL-11 may reflect a general feature of Leptin and IL-6 family cytokines since Leptin also exhibited synergy with CNTF (Figures 6A and 6B). Importantly, knockdown of Gp130 or Lepr suppressed the synergistic effect of Leptin/IL-11 on MG reprogramming and proliferation (Figures 6E and 6F), suggesting they are acting via their cognate receptors. Furthermore, like injury-dependent MG reprogramming (Fausett et al., 2008; Ramachandran et al., 2010a; Ramachandran et al., 2011), cytokine-mediated reprogramming in the uninjured retina was dependent on Ascl1a expression (Figures 6C and 6D).
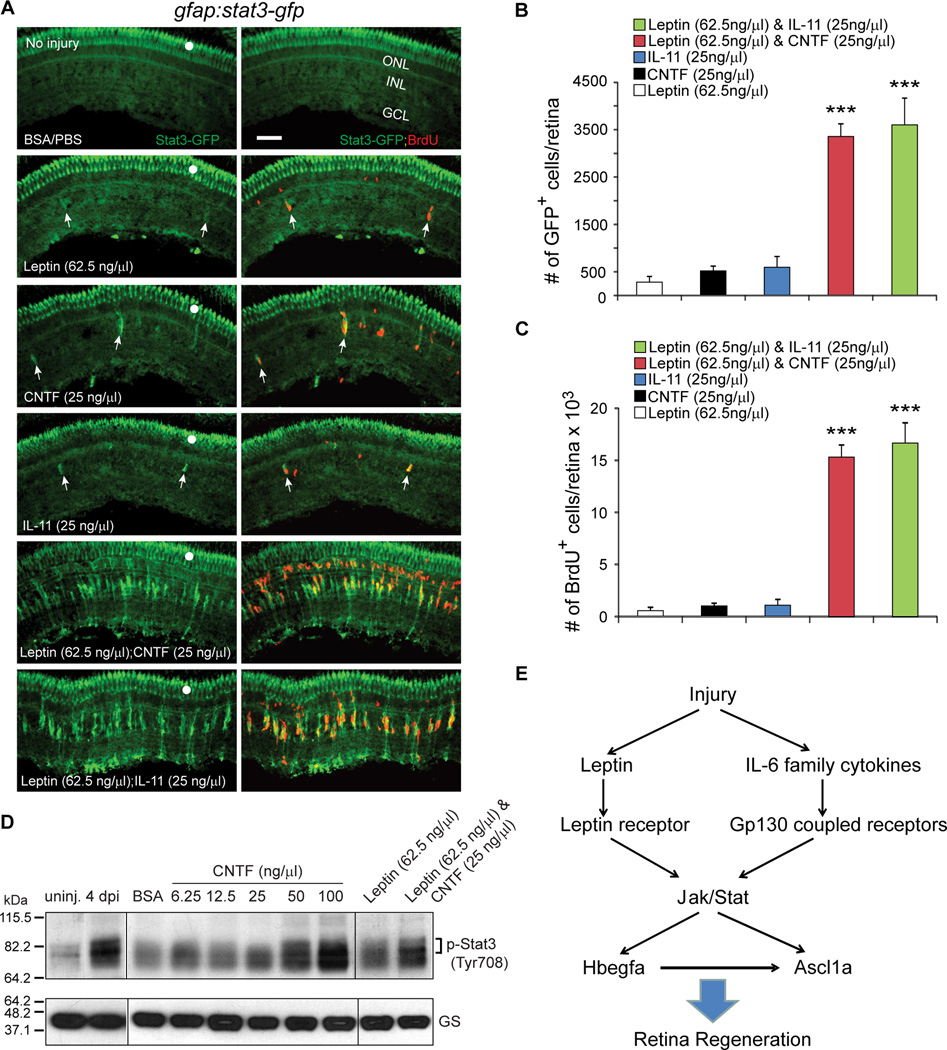
We next investigated if the synergistic actions of Leptin and IL-6 family cytokines on MG reprogramming and proliferation was reflected in Jak/Stat signaling. For this analysis uninjured gfap:stat3-gfp transgenic fish that report Stat3 activation (Figures 1 and S2), received an intravitreal injection of Leptin, CNTF, and IL-11 individually or in combination. As expected, the synergistic action of cytokines was reflected in both Stat3 activation (GFP) and progenitor formation (BrdU) (Figures 7A–7C). We further confirmed this synergy by Western blot analysis of endogenous p-Stat3 in whole retinal extracts from uninjured eyes that received intravitreal injections of increasing amounts of CNTF or Leptin with and without CNTF co-injection (Figure 7D). Together these data indicate that cytokines in the injured retina synergize with each other to stimulate MG reprogramming and proliferation by activating a Jak/Stat signaling cascade and that these cytokines are sufficient for driving MG proliferation in the uninjured retina.
Figure 7. Leptin synergizes with IL-11 and CNTF to stimulate Jak/Stat3 signaling in MG-derived progenitors.
(A) Intravitreal injection of Leptin/CNTF, or Leptin/IL-11 into the eye of gfap:stat3-gfp fish stimulates Stat3-GFP expression and BrdU incorporation throughout the uninjured retina’s INL, while Leptin, CNTF or IL-11 alone had little effect. Note that GFP reports activated p-Stat3 expression (Figure 1; Figure S2). White dots indicate autofluorescence unique to the green channel (see Figure S7K). The arrows point to MG-derived progenitors in the top 4 panels. (B) Quantification of Stat3-GFP+ cells in (A); ***P<0.001 (combination vs individual), n=4. (C) Quantification of BrdU+ cells in (A); ***P<0.001 (combination vs individual), n=4. (D) Western blot shows that retinal injury or intravitreal injection of cytokines into uninjured eye increases p-Stat3 expression. GS serves as loading control. (E) A model showing Leptin and IL-6 family cytokines synergize to stimulate MG reprogramming and retina regeneration via a Jak/Stat3 signaling pathway, which is essential for activating reprogramming genes like hbegf and ascl1a. Error bars, s.d. Scale bars, 50 µm. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer.
Discussion
Our studies identified Leptin and IL-6 family cytokines induced in the injured retina that regulate MG reprogramming and the generation of MG-derived progenitors via a Jak/Stat3 signaling pathway (Figure 7E). We show that this signaling is important for driving MG to acquire progenitor characteristics by stimulating the expression of reprogramming genes, like ascl1a. By treating uninjured retinas with combinations of Leptin and IL-6 family members we found that these cytokines were sufficient to stimulate MG reprogramming and proliferation and that they acted in a synergistic fashion. Finally, the endogenous expression of these cytokines by injury-responsive MG suggests that MG themselves may be contributing to their own reprogramming and proliferation.
The expression of cytokines by injury-responsive MG provides a convenient mechanism for amplifying a local signal that may initiate the injury response. The cytokine-activated Jak/Stat signaling pathway is well poised to serve as an early responder to injury since Stat proteins can rapidly transduce information from membrane receptors to the nucleus in the absence of new protein synthesis (Hirano et al., 2000). Indeed, we found that this pathway contributes to the activation of a variety of reprogramming genes including ascl1a, lin28, hbegfa and socs3 that are induced within hours following retinal injury and well before MG cell division that begins around 2 dpi (Fausett and Goldman, 2006). Furthermore, selectively inhibiting Jak/Stat signaling at 2–4 dpi, we were able to show that this pathway is not only important for MG reprogramming, but also for progenitor proliferation and amplification.
It was previously suggested that increased Stat3 expression in MG of the injured retina reflects activated p-Stat3 (Kassen et al., 2007). Importantly, this expression was identified in both quiescent and proliferating MG, and two types of proliferating MG were proposed: primary responders and secondary responders (Gorsuch and Hyde, 2013; Kassen et al., 2007; Nelson et al., 2012). How 3 types of MG could harbor activated Stat3, yet yield different phenotypes has remained an enigma. Our data provides clarity to these issues by specifically assaying for activated p-Stat3 expression in the injured retina. We found that regardless of the type of injury (mechanical injury to all retinal layers or light-induced injury that is restricted to photoreceptors), activated p-Stat3 is only detected in proliferating MG-derived progenitors. Thus, injury-dependent induction of Stat3 does not reflect activated p-Stat3 as previously proposed (Kassen et al., 2007; Nelson et al., 2012).
The high correlation of p-Stat3 expression with progenitor proliferation suggests that it does not distinguish primary from secondary responding MG and that this distinction between progenitors may simply represent a temporal sequence of events controlled by similar mechanisms. The idea that Stat3 differentially affected primary and secondary MG was based on the observation that morpholino-mediated Stat3 knockdown only partially reduced progenitor formation/proliferation in the injured retina (Nelson et al., 2012). We suggest that this may reflect either incomplete Stat3 knockdown allowing residual p-Stat3 signaling or the action of other Stat proteins that collaborate with Stat3 in mediating an injury response.
The restricted expression of activated p-Stat3 to MG-derived progenitors at the injury site suggests that cytokines and/or their receptors exhibit a similar spatial restriction. Since MG reprogramming and proliferation can be induced by cytokines intravitreally injected into eye without retinal injury, these cytokine receptors probably preexist on MG at low levels. The injury dependent induction of cytokines and their receptors in reprogrammed MG may further amplify their responsiveness to these factors.
The source of cytokines and other factors contributing to MG reprogramming may be dying cells, invading microglia, vasculature and MG themselves. Previous studies have suggested that phagocytosis of dying cells by MG and the release of TNFα from dying cells may represent the initial signals triggering MG reprogramming and proliferation (Bailey et al., 2010; Nelson et al., 2013). However, an effect of invading microglia or MG themselves has not been ruled out. Our data are consistent with the idea that MG at the injury site are an important source of cytokines that regulate their own reprogramming and proliferation.
We observed a remarkable synergy in the ability of Leptin and IL-6 family members to stimulate MG proliferation in the uninjured retina. This synergy is reflected in Jak/Stat3 signaling and although the mechanism underlying this synergy remains unexplained it may result from the effect these cytokines have on additional signaling molecules that are coupled to the Jak/Stat3 signaling pathway. Importantly, this synergy may be necessary for stimulating MG reprogramming and retina regeneration in mammals.
We used mammalian Leptin and IL-6 family members to stimulate MG reprogramming and proliferation in the uninjured zebrafish retina. These cytokines exhibit limited (~20–30%) identity with their zebrafish counterparts and this likely contributes to the relatively high concentrations needed to elicit a response (Table S2). Nonetheless, zebrafish and mammalian Leptin and IL-6 family members share a characteristic cytokine fold and other sequence elements that determine their receptor binding specificity (Gorissen et al., 2009; Huising et al., 2006; Prokop et al., 2012; Varela et al., 2012). More important is a consideration of the Leptin and IL-6 family receptors where amino acid identity between fish and mammals ranges from 20% for the IL-6R to 53% for the CntfR. However, this overall homology hides the fact that domains of high conservation exist and that structural conservation may be as important as amino acid identity. Indeed, the cytokine binding domain of these receptors from zebrafish and mammals share Ig superfamily, fibronectin type-III, and WSXWS domains that are organized in a similar fashion (Huising et al., 2006; Prokop et al., 2012; Varghese et al., 2002). Importantly, and consistent with the idea that these structures allow mammalian cytokines to act in a receptor specific fashion in zebrafish, we found that knockdown of LepR or Gp130 inhibits the action of mammalian cytokines acting through these receptor components.
It is interesting that IL-6 family cytokines and p-Stat3 signaling stimulate MG to reprogram and generate progenitors in the zebrafish retina, while in birds and mammals these signaling molecules appear to act on MG to stimulate a gliotic response that functions to protect the retina from damage (Fischer et al., 2004a; Fischer et al., 2004b; Peterson et al., 2000; Rhee et al., 2013; Xue et al., 2011). In fish, Jak/Stat signaling may collaborate with other signaling pathways to stimulate retina regeneration while in mammals these additional pathways may not be regulated in a similar fashion. In addition, the downstream targets of these pathways may differ in fish and mammals. The identification of these signaling pathways and an understanding of their mechanisms of action in both fish and mammals may suggest strategies for switching MG from a gliotic to a regenerative response when confronted with a damaged retina. Our data suggests that Jak/Stat signaling may be one component of the regenerative response in fish. In the accompanying paper by Wan et al., we report on additional signaling pathways that collaborate with Jak/Stat signaling in the injured fish retina. We speculate that this collaboration is a key element underlying retina regeneration in fish.
Experimental Procedures
Animals and retinal injury
The animals used in this study were treated in accordance with the guidelines of the University Committee on Use and Care of Animals at the University of Michigan. Zebrafish were kept at 26–28 °C on a 14h/10h light/dark cycle. 1016tuba1a:gfp, ascl1a:gfp and gfap:gfp transgenic fish have been previously described (Fausett and Goldman, 2006; Kassen et al., 2007; Wan, 2012). Fish were anesthetized in tricaine methane sulfonate before injection or injury. Retinal lesions were performed as previously described (Fausett and Goldman, 2006; Ramachandran et al., 2010a). Photoreceptor damage by UV light was performed as previously described (Bernardos et al., 2007). Fish were exposed to UV light for 30 min and then returned to their home tanks. Adult zebrafish of similar age and size were randomly allocated to experimental groups.
Florescence-activated cell sorting (FACS)
GFP+ MG from gfap:gfp retinas and GFP+ MG-derived progenitors from 1016 tuba1a:gfp retinas at 4 dpi were isolated on a BC Biosciences FACSViDa 3 laser high speed cell sorter as previously described (Ramachandran et al., 2010a).
Plasmid construction and generation of transgenic lines
The Tol2 transposon system was used to generate transgenic lines using the Tol2 vector pTAL200R150G (Urasaki et al., 2006). gfap regulatory elements (Bernardos and Raymond, 2006) were amplified from zebrafish genomic DNA, and stat3 coding sequence was amplified from cDNA and cloned into a Tol2 vector. A distal 1.5 kb promoter of ascl1a was deleted from ascl1a:gfp (Wan, 2012) using restriction enzymes. PCR-mediated site-directed mutagenesis was done as previously described (Ramachandran et al., 2012) to generate 6-ascl1a(St1Mut):gfp and 6-ascl1a(St2Mut):gfp constructs. To generate DIG (Roche) labeled in situ hybridization probes, gp130, clcf1, crlf1a, lepa and lepb were cloned into pCS2, while cntfr and lifrb were cloned into pBSSK. PCR products using T3 and T7 primers were used as template to generate DIG labeled in situ hybridization probes for lepr, il-11a, il-11ra and il-6r.
RT-PCR and qPCR
Total RNA was isolated from retinas using TRIzol (Invitrogen). Oligo(dT) and Superscript II reverse transcriptase (Invitrogen) were used to generate cDNA. PCR reactions used Taq polymerase and gene-specific primers (Table S1). qPCR was carried out in triplicate with Absolute SYBR Green Fluorescein Master Mix (Thermo Scientific) on an iCycler real-time PCR detection system (BioRad). The ΔΔCt method was used to determine relative expression of mRNAs in control and injured retinas and normalized to gapdh mRNA levels. Primers sequences are list in Table S1.
Inhibitors and recombinant protein
The Jak inhibitor, P6 (EMD Chemicals Inc.) was used at 10 µM and JSI-124 (Indofine Chemical Company Inc.) was used at 1 µM. Inhibitors were delivered at the time of retinal injury or were injected intravitreally at the indicated time. Recombinant human Leptin (gift of Amylin Pharmaceuticals Inc.), recombinant rat CNTF, recombinant human IL-6 and recombinant mouse IL-11 (R&D Systems) were reconstituted in PBS with 0.1% BSA, and 0.5–2 µl was injected intravitreally at the indicated concentration (See Table S2 for dissociation constants and estimates of intravitreal concentrations). Intravitreal injection was done through the front of the eye by first making a small incision with either a double-edge sapphire blade (World Precision Instruments, Inc.) or a 30 gauge beveled needle attached to a Hamilton syringe. If a sapphire blade was used to make the incision, a Hamilton syringe equipped with a blunt 33 gauge needle was used to deliver molecules behind the lens. If a Hamilton syringe equipped with a 30 gauge beveled needle was used to make an incision, recombinant molecules were delivered through this needle. Similar results were obtained regardless of the method used for intravitreal injection. Recombinant proteins were injected once daily for 3 days, and 4 days after the first injection fish received an intraperitoneal injection of BrdU 3 h prior to sacrifice. Experimenters remained blind to the material injected into the vitreous until after data analysis.
Morpholino (MO) electroporation
Lissamine-tagged MOs (Gene Tools, LLC) were introduced at the time of injury using a Hamilton syringe. MO delivery to cells was accomplished by electroporation as previously described (Fausett et al., 2008). The control, ascl1a MO and lepr MO (5’-TGAAGACAGACATCATTTCACTTGC-3’) have been previously described (Fausett et al., 2008; Liu et al., 2012). The gp130 MO is: 5'-ACAGCCAATGATGTGAAGTGTCCAT-3'. The amount of control MO was used to match the highest amount of experimental MO for each experiment.
BrdU labeling and in situ hybridization
BrdU labeling was accomplished by injecting 20 µl of BrdU (20 mM) intraperitoneally 3 h prior to sacrifice. Fish were overdosed with tricaine methane sulfonate and eyes were dissected, enucleated, fixed and sectioned as previously described (Fausett and Goldman, 2006). In situ hybridization was performed on retinal sections with digoxigenin-labeled cRNA probes (DIG RNA labeling kit, Roche Diagnostics). Immunoflourescence protocols and antibodies were as previously described (Ramachandran et al., 2011).
Immunofluorescence and Western Blots
Anti-GFP and 4C4 immunofluorescence were as previously described (Craig et al., 2010; Fausett and Goldman, 2006). p-Stat3 immunoflourescence was performed using mouse anti-phospho-zebrafish Stat3 (Tyr708) antibody (MBL) at 1:100 dilution. For p-Stat3 epitope retrieval, the slides were boiled in 10 mM citrate buffer (pH 6) for 40 min. For BrdU immunoflourescence, sections were treated with 2N HCl at 37 °C for 20 min, rinsed in 0.1 sodium borate (pH 8.5) for 10 min and then processed using standard procedures (Fausett and Goldman, 2006). SDS-PAGE and Western blots were carried out using standard protocols. Mouse anti-phospho-zebrafish Stat3 (Tyr708) antibody (MBL) was used at 1:1000 dilution, and mouse anti-Glutamine Synthetase (GS) antibody (Millipore) was used at 1:3000 dilution.
TUNEL assay
An in situ Cell Death Detection Kit fluorescein (Roche Applied Science) was used to detect apoptotic cells according to the manufacturer’s protocol. Oubain-treated eye served as a positive control.
Microscopy and Statistical Analysis
Slides were examined with a Zeiss Axiophot, Observer.Z1 microscope or an Olympus FluoView FV1000 confocal imaging system. Cell counts were determined by counting fluorescently labeled BrdU+ or GFP+ cells in retinal sections visualized using fluorescent microscopy. All experiments were done in triplicate or more and repeated at least twice. Experimenters were blind to the animal treatments until after data analysis. ANOVA with Bonferroni/Dunn post hoc t-test was used for multiple comparisons and a two-tailed unpaired Student’s t test for single comparison (experimental vs control group). When n=3, the Mann-Whitney test was employed for single comparison between control and experimental group.
Supplementary Material
Acknowledgements
This research was supported by NEI grant RO1 EY 018132 from the NIH, a Research to Prevent Blindness Innovative Ophthalmic Research Award and a gift from the Marjorie and Maxwell Jospey Foundation. We thank Hasan Korkaya (University of Michigan) for recombinant IL-6; Peter Hitchcock (University of Michigan) for 4C4 antibody; the University of Michigan Flow Cytometry Core for cell sorting; Amylin Pharmaceutical/BMS for the generous gift of recombinant Leptin; Peter Macpherson for statistics; Randall Karr and Joshua Kirk for fish care and the Goldman lab for helpful comments and suggestions during the course of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- Figure S1: Injury-dependent activation of the Jak/Stat3 signaling pathway. Related to Figure 1.
- Figure S2: Stat3-GFP expression in developing and adult gfap:stat3-gfp fish. Related to Figure 1.
- Figure S3: Jak/Stat signaling regulates proliferation of MG-derived progenitors and Stat3-GFP expression. Related to Figure 2.
- Figure S4: Jak/Stat3 signaling mediates injury-dependent activation of the ascl1a promoter. Related to Figure 3.
- Figure S5: Genes encoding IL-6 family cytokines are expressed in MG-derived progenitors upon retinal injury. Related to Figure 4.
- Figure S6: HB-EGF stimulates MG reprogramming and proliferation in the uninjured retina. Related to Figure 4.
- Figure S7: Leptin signaling regulates the generation of MG-derived progenitors. Related to Figure 5.
- Table S1: Primers sequences for PCR. Related to Experimental Procedures.
- Table S2. Kd, ED50 and intravitreal concentration of recombinant mammalian cytokines. Related to Experimental Procedures.
References
- Bailey TJ, Fossum SL, Fimbel SM, Montgomery JE, Hyde DR. The inhibitor of phagocytosis, O-phospho-L-serine, suppresses Muller glia proliferation and cone cell regeneration in the light-damaged zebrafish retina. Exp Eye Res. 2010;91:601–612. doi: 10.1016/j.exer.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yaakov K, Dagan SY, Segal-Ruder Y, Shalem O, Vuppalanchi D, Willis DE, Yudin D, Rishal I, Rother F, Bader M, et al. Axonal transcription factors signal retrogradely in lesioned peripheral nerve. Embo J. 2012;31:1350–1363. doi: 10.1038/emboj.2011.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J Neurosci. 2007;27:7028–7040. doi: 10.1523/JNEUROSCI.1624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardos RL, Raymond PA. GFAP transgenic zebrafish. Gene Expr Patterns. 2006;6:1007–1013. doi: 10.1016/j.modgep.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Blaskovich MA, Sun J, Cantor A, Turkson J, Jove R, Sebti SM. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res. 2003;63:1270–1279. [PubMed] [Google Scholar]
- Cao X, Tay A, Guy GR, Tan YH. Activation and association of Stat3 with Src in v-Src-transformed cell lines. Mol Cell Biol. 1996;16:1595–1603. doi: 10.1128/mcb.16.4.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig SE, Thummel R, Ahmed H, Vasta GR, Hyde DR, Hitchcock PF. The zebrafish galectin Drgal1–l2 is expressed by proliferating Muller glia and photoreceptor progenitors and regulates the regeneration of rod photoreceptors. Invest Ophthalmol Vis Sci. 2010;51:3244–3252. doi: 10.1167/iovs.09-4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret GB, Reichenbach P, Schindler U, Horvath CM, Fritz S, Nabholz M, Bucher P. DNA binding specificity of different STAT proteins. Comparison of in vitro specificity with natural target sites. J Biol Chem. 2001;276:6675–6688. doi: 10.1074/jbc.M001748200. [DOI] [PubMed] [Google Scholar]
- Elson GC, Lelievre E, Guillet C, Chevalier S, Plun-Favreau H, Froger J, Suard I, de Coignac AB, Delneste Y, Bonnefoy JY, et al. CLF associates with CLC to form a functional heteromeric ligand for the CNTF receptor complex. Nat Neurosci. 2000;3:867–872. doi: 10.1038/78765. [DOI] [PubMed] [Google Scholar]
- Faillace MP, Julian D, Korenbrot JI. Mitotic activation of proliferative cells in the inner nuclear layer of the mature fish retina: regulatory signals and molecular markers. J Comp Neurol. 2002;451:127–141. doi: 10.1002/cne.10333. [DOI] [PubMed] [Google Scholar]
- Fausett BV, Goldman D. A role for alpha1 tubulin-expressing Muller glia in regeneration of the injured zebrafish retina. J Neurosci. 2006;26:6303–6313. doi: 10.1523/JNEUROSCI.0332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausett BV, Gumerson JD, Goldman D. The proneural basic helix-loop-helix gene ascl1a is required for retina regeneration. J Neurosci. 2008;28:1109–1117. doi: 10.1523/JNEUROSCI.4853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fimbel SM, Montgomery JE, Burket CT, Hyde DR. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J Neurosci. 2007;27:1712–1724. doi: 10.1523/JNEUROSCI.5317-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Omar G, Eubanks J, McGuire CR, Dierks BD, Reh TA. Different aspects of gliosis in retinal Muller glia can be induced by CNTF, insulin, and FGF2 in the absence of damage. Mol Vis. 2004a;10:973–986. [PubMed] [Google Scholar]
- Fischer AJ, Schmidt M, Omar G, Reh TA. BMP4 and CNTF are neuroprotective and suppress damage-induced proliferation of Muller glia in the retina. Mol Cell Neurosci. 2004b;27:531–542. doi: 10.1016/j.mcn.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Gorissen M, Bernier NJ, Nabuurs SB, Flik G, Huising MO. Two divergent leptin paralogues in zebrafish (Danio rerio) that originate early in teleostean evolution. J Endocrinol. 2009;201:329–339. doi: 10.1677/JOE-09-0034. [DOI] [PubMed] [Google Scholar]
- Gorsuch RA, Hyde DR. Regulation of Muller glial dependent neuronal regeneration in the damaged adult zebrafish retina. Exp Eye Res. 2013 doi: 10.1016/j.exer.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandis JR, Drenning SD, Chakraborty A, Zhou MY, Zeng Q, Pitt AS, Tweardy DJ. Requirement of Stat3 but not Stat1 activation for epidermal growth factor receptor- mediated cell growth In vitro. J Clin Invest. 1998;102:1385–1392. doi: 10.1172/JCI3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548–2556. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- Huising MO, Kruiswijk CP, Flik G. Phylogeny and evolution of class-I helical cytokines. The J Endocrinol. 2006;189:1–25. doi: 10.1677/joe.1.06591. [DOI] [PubMed] [Google Scholar]
- Kassen SC, Ramanan V, Montgomery JE, C TB, Liu CG, Vihtelic TS, Hyde DR. Time course analysis of gene expression during light-induced photoreceptor cell death and regeneration in albino zebrafish. Dev Neurobiol. 2007;67:1009–1031. doi: 10.1002/dneu.20362. [DOI] [PubMed] [Google Scholar]
- Kassen SC, Thummel R, Campochiaro LA, Harding MJ, Bennett NA, Hyde DR. CNTF induces photoreceptor neuroprotection and Muller glial cell proliferation through two different signaling pathways in the adult zebrafish retina. Exp Eye Res. 2009;88:1051–1064. doi: 10.1016/j.exer.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Lenkowski JR, Qin Z, Sifuentes CJ, Thummel R, Soto CM, Moens CB, Raymond PA. Retinal regeneration in adult zebrafish requires regulation of TGFbeta signaling. Glia. 2013;61:1687–1697. doi: 10.1002/glia.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DE, Darnell JE., Jr Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- Liang J, Wang D, Renaud G, Wolfsberg TG, Wilson AF, Burgess SM. The stat3/socs3a pathway is a key regulator of hair cell regeneration in zebrafish. J Neurosci. 2012;32:10662–10673. doi: 10.1523/JNEUROSCI.5785-10.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Dalman M, Chen Y, Akhter M, Brahmandam S, Patel Y, Lowe J, Thakkar M, Gregory AV, Phelps D, et al. Knockdown of leptin A expression dramatically alters zebrafish development. Gen Comp Endocrinol. 2012;178:562–572. doi: 10.1016/j.ygcen.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima M, Barthel LK, Raymond PA. A self-renewing division of zebrafish Muller glial cells generates neuronal progenitors that require N-cadherin to regenerate retinal neurons. Development. 2013;140:4510–4521. doi: 10.1242/dev.090738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Ackerman KM, O'Hayer P, Bailey TJ, Gorsuch RA, Hyde DR. Tumor necrosis factor-alpha is produced by dying retinal neurons and is required for Muller glia proliferation during zebrafish retinal regeneration. J Neurosci. 2013;33:6524–6539. doi: 10.1523/JNEUROSCI.3838-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Gorsuch RA, Bailey TJ, Ackerman KM, Kassen SC, Hyde DR. Stat3 defines three populations of Muller glia and is required for initiating maximal muller glia proliferation in the regenerating zebrafish retina. J Comp Neurol. 2012;520:4294–4311. doi: 10.1002/cne.23213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedranzini L, Dechow T, Berishaj M, Comenzo R, Zhou P, Azare J, Bornmann W, Bromberg J. Pyridone 6, a pan-Janus-activated kinase inhibitor, induces growth inhibition of multiple myeloma cells. Cancer Res. 2006;66:9714–9721. doi: 10.1158/0008-5472.CAN-05-4280. [DOI] [PubMed] [Google Scholar]
- Peterson WM, Wang Q, Tzekova R, Wiegand SJ. Ciliary neurotrophic factor and stress stimuli activate the Jak-STAT pathway in retinal neurons and glia. J Neurosci. 2000;20:4081–4090. doi: 10.1523/JNEUROSCI.20-11-04081.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak J, Wilken MS, Ueki Y, Cox KE, Sullivan JM, Taylor RJ, Levine EM, Reh TA. ASCL1 reprograms mouse Muller glia into neurogenic retinal progenitors. Development. 2013;140:2619–2631. doi: 10.1242/dev.091355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell C, Elsaeidi F, Goldman D. Injury-dependent Muller glia and ganglion cell reprogramming during tissue regeneration requires Apobec2a and Apobec2b. J Neurosci. 2012;32:1096–1109. doi: 10.1523/JNEUROSCI.5603-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop JW, Duff RJ, Ball HC, Copeland DL, Londraville RL. Leptin and leptin receptor: analysis of a structure to function relationship in interaction and evolution from humans to fish. Peptides. 2012;38:326–336. doi: 10.1016/j.peptides.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Fausett BV, Goldman D. Ascl1a regulates Muller glia dedifferentiation and retinal regeneration through a Lin-28-dependent, let-7 microRNA signalling pathway. Nat Cell Biol. 2010a;12:1101–1107. doi: 10.1038/ncb2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Reifler A, Parent JM, Goldman D. Conditional gene expression and lineage tracing of tuba1a expressing cells during zebrafish development and retina regeneration. J Comp Neurol. 2010;518:4196–4212. doi: 10.1002/cne.22448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Zhao XF, Goldman D. Ascl1a/Dkk/{beta}-catenin signaling pathway is necessary and glycogen synthase kinase-3{beta} inhibition is sufficient for zebrafish retina regeneration. Proc Natl Acad Sci U S A. 2011;108:15858–15863. doi: 10.1073/pnas.1107220108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Zhao XF, Goldman D. Insm1a-mediated gene repression is essential for the formation and differentiation of Muller glia-derived progenitors in the injured retina. Nat Cell Biol. 2012;14:1013–1023. doi: 10.1038/ncb2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee KD, Nusinowitz S, Chao K, Yu F, Bok D, Yang XJ. CNTF-mediated protection of photoreceptors requires initial activation of the cytokine receptor gp130 in Muller glial cells. Proc Natl Acad Sci U S A. 2013;110:E4520–E4529. doi: 10.1073/pnas.1303604110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenkamp DL. The rod photoreceptor lineage of teleost fish. Prog Retin Eye Res. 2011;30:395–404. doi: 10.1016/j.preteyeres.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urasaki A, Morvan G, Kawakami K. Functional dissection of the Tol2 transposable element identified the minimal cis-sequence and a highly repetitive sequence in the subterminal region essential for transposition. Genetics. 2006;174:639–649. doi: 10.1534/genetics.106.060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela M, Dios S, Novoa B, Figueras A. Characterisation, expression and ontogeny of interleukin-6 and its receptors in zebrafish (Danio rerio) Dev Comp Immunol. 2012;37:97–106. doi: 10.1016/j.dci.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Varghese JN, Moritz RL, Lou MZ, Van Donkelaar A, Ji H, Ivancic N, Branson KM, Hall NE, Simpson RJ. Structure of the extracellular domains of the human interleukin-6 receptor alpha -chain. Proc Natl Acad Sci U S A. 2002;99:15959–15964. doi: 10.1073/pnas.232432399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt PK, Hart JR. PI3K and STAT3: a new alliance. Cancer Discov. 2011;1:481–486. doi: 10.1158/2159-8290.CD-11-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Ramachandran R, Goldman D. HB-EGF is necessary and sufficient for Müller glia dedifferentiation and retina regeneration. Dev Cell. 2012;22:334–347. doi: 10.1016/j.devcel.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W, Cojocaru RI, Dudley VJ, Brooks M, Swaroop A, Sarthy VP. Ciliary neurotrophic factor induces genes associated with inflammation and gliosis in the retina: a gene profiling study of flow-sorted, Muller cells. PLoS One. 2011;6:e20326. doi: 10.1371/journal.pone.0020326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita S, Miyagi C, Carmany-Rampey A, Shimizu T, Fujii R, Schier AF, Hirano T. Stat3 Controls Cell Movements during Zebrafish Gastrulation. Dev Cell. 2002;2:363–375. doi: 10.1016/s1534-5807(02)00126-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.