Abstract
Background
Extracellular histones exert part of their prothrombotic activity through the stimulation of blood cells. Besides platelets, histones can bind to red blood cells (RBCs), which are important contributors to thrombogenesis, but little is known about the functional consequences of this interaction.
Objectives
To evaluate the effect of histones on the procoagulant potential of human RBCs with particular regard to the expression of surface phosphatidylserine (PS).
Methods
PS exposure on human RBCs treated with a natural mixture of histones or recombinant individual histones was evaluated by FITC-Annexin-V binding and measured by flow cytometry. Calcium influx in RBCs loaded with the calcium-sensitive fluorophore Fluo-4 AM was assessed by flow cytometry. The procoagulant potential of histone-treated RBCs was evaluated by a purified prothrombinase assay and a one-stage plasma recalcification clotting test.
Results
Natural histones induced PS exposure on RBCs in a dose-dependent manner and neutralization or cleavage of histones by heparin or activated protein C, respectively, abolished PS externalization. Histone H4 was mainly responsible for the stimulating activity of histones, while the other subtypes were almost ineffective. Similarly, natural histones and H4 induced influx of calcium into RBCs while the other individual histones did not. Histone-induced exposure of PS on RBCs translated into increased prothrombinase complex-mediated prothrombin activation and accelerated fibrin formation in plasma.
Conclusions
Histones induce RBCs to express a procoagulant phenotype through the externalization of PS. This finding provides new insight into the prothrombotic activity of extracellular histones.
Keywords: Histones, erythrocytes, phosphatidylserine, blood coagulation, inflammation
Introduction
Translocation of histones from the cell nucleus into the extracellular space consequent to cell death or inflammatory cell activation is a deleterious event associated with clotting activation and the development of venous, arterial and microvascular thrombosis [1-3]. Several mechanisms have been described to explain the prothrombotic activity of these cationic proteins. Histones derange the haemostatic balance toward excessive coagulation by interfering with the protein C pathway [4] and promoting prothrombin activation [5]. Because the direct prothrombin activation is quite slow, the pathophysiological relevance of the latter study has not been established; moreover, histones interact with several blood cells involved in the thrombotic process. While a considerable amount of data is available about the stimulating effect of histones on platelets and the underlying mechanisms [6,7], little is known about the influence of histones on erythrocyte functions. Despite commonly regarded as innocent bystanders, red blood cells (RBCs) are active contributors to the thrombogenic process. RBCs are abundant cellular components of venous thrombi and can initiate thrombus development at the site of endothelial damage in the presence of blood flow reduction [8]. Furthermore, RBCs can support prothrombin activation through the exposure of surface phosphatidylserine (PS) [9]. Old papers reported that histones increase the adhesiveness of RBCs [10], and more recently histones were shown to bind directly to RBCs “in vitro” [11]. In this report we investigated the effect of histones on the procoagulant properties of human RBCs, mainly focusing on the exposure of phosphatidylserine.
Materials and Methods
Venous peripheral blood was drawn from healthy donors under Institutional Review Board approval of the Oklahoma Medical Research Foundation and collected in sodium citrate-containing tubes. Blood was centrifuged at 180xg for 10 minutes, platelet-rich plasma and buffy coat were discarded and the RBCs-containing pellet was washed with Hepes-NaCl buffer (50 mM Hepes, 150 mM NaCl, pH 7.4). Afterwards, RBCs were passed through a 1:1 (w/w) mixture of α-cellulose and microcrystalline cellulose (Sigmacell type 50) (both from Sigma-Aldrich Chemical Co., St Louis, MO, USA) packed in a chromatography column to remove contaminating leukocytes and platelets [12], and washed again. For PS exposure analysis, 5×105 μL-1 RBCs were suspended in buffer containing 1 mM calcium chloride and incubated for 10 minutes at 37°C with the indicated concentrations of a mixture of histones from calf thymus (Sigma) or recombinant histone subtypes (New England Biolabs, Ipswich, MA, USA). Then, RBCs were diluted 200-fold in Hepes-buffer containing 0.1% bovine serum albumin, FITC-conjugated annexin-V (1:100 final dilution, Life Technologies, Grand Island, NY, USA) and 2.5 mM calcium chloride, and incubated in the dark for 10 minutes at room temperature (RT). Annexin-V binding to RBCs was evaluated by flow cytometry on a BD FACSCalibur (Becton Dickinson, San Jose, CA, USA). Calcium chloride in the FITC-annexin-V reaction mixture was in some cases replaced by EDTA (2 mM) to impede the binding of annexin to PS. Where indicated, histones were pre-incubated with heparin (1 IU mL-1, APP Pharmaceuticals, Schaumburg, IL, USA) for 10 minutes at RT, or with activated protein C (APC, 6 μg mL-1, Xigris,™ Eli Lilly, Indianapolis, IN, USA) for 1 hour at 37°C. For the evaluation of calcium influx, RBCs were incubated with the calcium-sensitive fluorophore Fluo-4 AM (3 μM, Life Technologies) for 30 minutes at 37°C in the dark, washed, treated with histones and then analyzed by flow cytometry. The ability of histone-stimulated RBCs to support prothrombin activation by the prothrombinase complex was evaluated under purified conditions by incubating histone-treated RBCs with human FXa (2 nM,), FVa (0.2 nM), prothrombin (1.4 μM) (all from Haematologic Technologies, Essex Junction, VT, USA) and calcium chloride (2 mM) for 3 minutes at 37°C. The reaction was terminated with EDTA and thrombin activity in the supernatant was kinetically evaluated at 405 nm by cleavage of the chromogenic substrate Spectrozyme TH (American Diagnostica, Stamford, CT, USA). To evaluate the procoagulant properties of RBCs in plasma, histone-stimulated RBCs were assayed in a recalcification clotting test performed in the absence of any other exogenous phospholipid source in a STart 4 Hemostasis Analyzer (Diagnostica Stago, Parsippany, NJ, USA). Data are expressed as mean ± SEM, and statistical analysis was performed with paired t-test. A value of p<0.05 was considered statistically significant.
Results and Discussion
Isolated RBCs were incubated with buffer, natural histones (20 μg mL-1), or calcium ionophore A23187 (4 μM, Sigma) as positive control, in a calcium-containing medium, labeled with FITC-Annexin-V and then subjected to flow cytometry. Fig. 1A shows representative flow cytometer dot plots. Upon incubation with histones, RBCs exhibited a slight increase in the mean forward scatter values (FSC-Height), which is compatible with the formation of small aggregates of RBCs induced by histones [10]. Most importantly, histones generated a subpopulation of FITC-annexin-V-positive RBCs which amounted to about 14% (Fig. 1B). Similar results were obtained when staining PS with Alexa Fluor 488-Annexin-V (Life Technologies). Omitting calcium or replacing it with EDTA in the FITC-annexin-V reaction mixture (Fig. 1A and 1B) abolished the staining of RBCs, confirming that annexin-V bound specifically to externalized PS. Histones induced PS exposure on RBCs in a dose-dependent manner (Fig. 1C), and the active concentrations fell within the range which is considered pathophysiologically relevant in several inflammatory and vascular diseases [3]. Unfractionated heparin and APC have the known ability to block several histone functions [3,6]. Neutralization with heparin or cleavage with APC completely prevented histone-induced PS exposure on RBCs (Fig. 1D), extending the previously reported inhibitory properties of these drugs and suggesting that the observed effect was not due to possible contaminants present in the histone preparation. To identify which histone(s) was primarily responsible for PS exposure, 10 μg mL-1 of each histone subtype in the recombinant form was separately incubated with RBCs. Fig. 2A depicts representative dot plots of FITC-annexin-V labelled RBCs showing the different behavior of the various histones. Histone H4 induced significant exposure of PS on RBCs, yielding a subpopulation of annexin-V-positive cells amounting to 31.7 ± 6.4% (Fig. 2B), while all the other histones exhibited limited to negligible activity at the concentration employed. The presence of histone subfractions with different potency is consistent with the observation that a comparable amount of the natural histone mixture is less efficient than H4 alone (compare Fig. 1C to Fig. 2B). One of the recognized mechanisms responsible for the translocation of PS to the outer leaflet of the RBC membrane is the influx of calcium with consequent activation of the scramblase enzyme [13]. Histones are known to bind to and penetrate through the membrane of some cell types [14] and, in so doing, they can induce the formation of pores which alter the permeability of the cell plasma membrane [3,7,15]. We therefore evaluated the influx of calcium into RBCs pre-loaded with the calcium-sensitive fluorophore Fluo-4 AM and exposed to histones by flow cytometry. Influx of calcium occurred upon treatment with 20 μg mL-1 natural histones (25.7 ± 5.8% cells exhibited increased fluorescence intensity) and 10 μg mL-1 H4 (43.8 ± 8.7%). Again the other histone subtypes at the same concentrations were almost ineffective, which is compatible with the relative potency of individual histones in stimulating PS exposure. As PS is of paramount importance for the activity of coagulation factors, we evaluated the ability of histone-stimulated RBCs to support prothrombin activation by the prothrombinase complex. Under purified conditions, RBCs stimulated by natural histones efficiently supported thrombin generation producing a concentration-dependent increase over control cells (Fig. 3A). RBCs treated with histones were assayed for their ability to sustain coagulation in plasma in a one-stage recalcification clotting test; also in this case, RBCs stimulated by 40 μg mL-1 natural histones significantly accelerated clotting of plasma with respect to untreated cells (Fig. 3B), confirming that upon stimulation with histones RBCs acquire a procoagulant phenotype.
Figure 1. Effect of natural histones on the exposure of phosphatidylserine (PS) on red blood cells (RBCs).
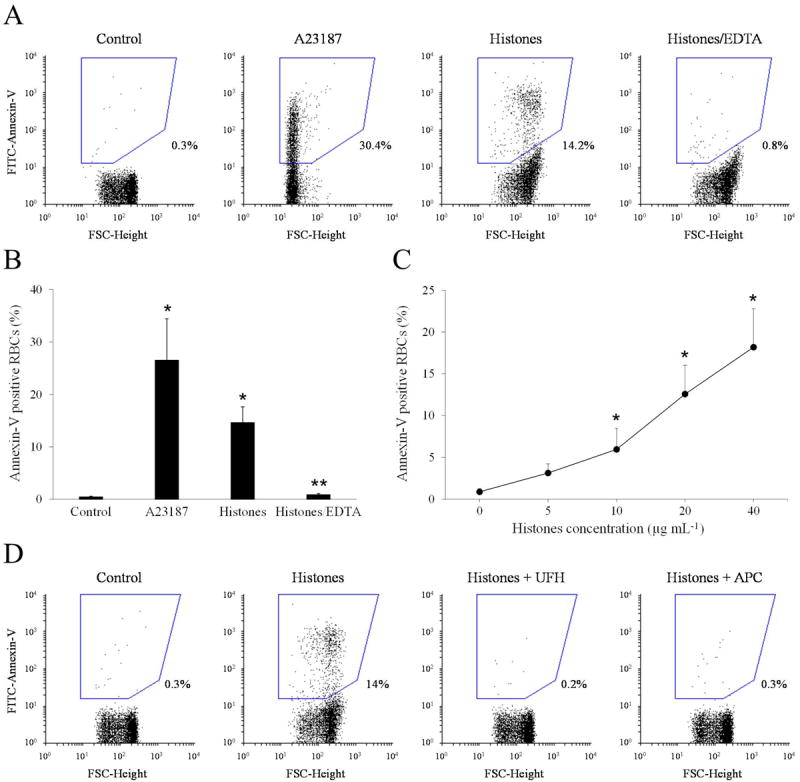
A) Typical dot plot analysis of FITC-annexin-V binding to RBCs upon treatment with buffer, ionophore A23187 (4 μM) or calf thymus histones (20 μg mL-1); histone-stimulated RBCs were stained with FITC-Annexin-V either in the presence or in the absence of calcium, a condition achieved by chelation with EDTA (Histones/EDTA); the percentage of PS-expressing RBCs is indicated next to the drawn region. B) Percentage of PS-expressing RBCs upon stimulation with buffer, A23187 or histones (plus or minus EDTA). Results are the mean ± SEM, n=5; *, p<0.01 vs control; **, p<0.01 vs histones by paired t-test. C) PS exposure induced by increasing concentrations of calf thymus histones on RBCs; results are the mean ± SEM of 3 independent experiments; *, p<0.05 versus control by paired t-test. D) Representative dot plot analysis of FITC-annexin-V binding to RBCs incubated with histones (20 μg mL-1) pretreated with heparin (1 IU mL-1) or APC (6 μg mL-1).
Figure 2. Effect of individual histones on the exposure of PS on RBCs.
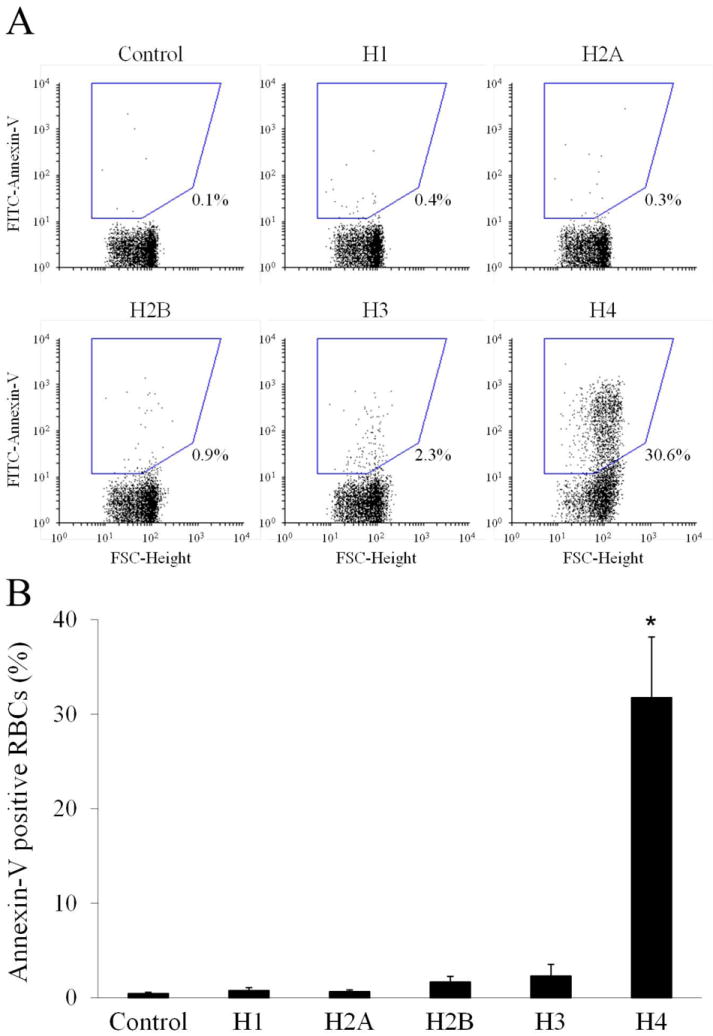
A) Representative dot plot analysis showing FITC-annexin-V binding to RBCs treated with 10 μg mL-1 individual recombinant histones. B) Summary of PS externalization on RBCs induced by 10 μg mL-1 recombinant histone subtypes; mean ± SEM, n=3; *, p<0.01 versus control by paired t-test.
Figure 3. Effect of natural histones on the expression of a procoagulant phenotype in RBCs.
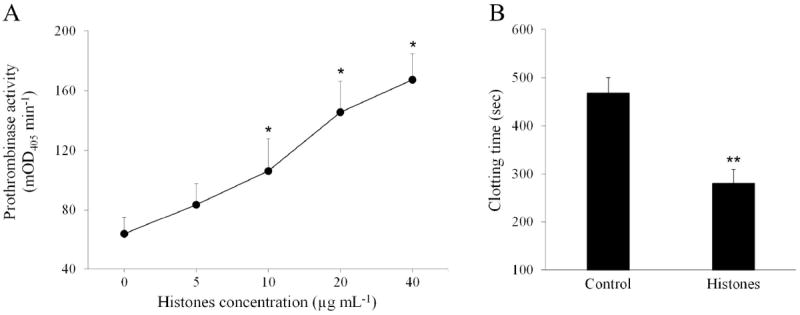
A) Prothrombinase activity of RBCs treated with increasing concentrations of calf thymus histones evaluated by chromogenic assay and expressed as mOD405 min-1 (mean ± SEM, n=3). *, p<0.05 versus control by paired t-test. B) Recalcification clotting test in plasma enriched with untreated (control) RBCs or cells stimulated by histones (40 μg mL-1). Results are the mean ± SEM, n=4; **, p<0.01 by paired t-test.
Histones released by activated neutrophils in the form of extracellular traps (NETs) have been abundantly detected in sections of animal and human thrombi [1,2,16,17], and in particular they have been found to decorate the RBC-rich (“red”) regions of venous thrombi [1]. We propose that upon recruitment of RBCs on the surface of NETs [16], histones, and in particular H4, could stimulate RBCs to expose PS on the outer surface and, in so doing, favor thrombus development by promoting coagulation and fibrin deposition. This novel procoagulant mechanism adds to those previously described for histones and contributes to broaden our understanding of their pathophysiological role in thrombotic diseases.
Acknowledgments
The authors wish to thank G.L. Dale and P. Friese (Department of Medicine, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA) for their help with erythrocyte isolation and flow cytometry setup. This work was supported by a grant from the National Institutes of Health, grant no. UM1 HL120877 (CTE). F.S. was recipient of a fellowship from the University of Bari “Aldo Moro”, Bari, Italy.
Footnotes
F. Semeraro and C. T. Ammollo designed and performed the experiments, analyzed the data, and wrote the manuscript; N. L. Esmon and C. T. Esmon contributed helpful comments, assisted in manuscript preparation and oversaw the project.
Disclosure of Conflicts of Interest
C. T. Esmon holds a patent on extracellular histones as biomarkers for prognosis and molecular targets for therapy (US Patent No. 8,716,218 B2).
All other authors state that they have no conflict of interest.
References
- 1.Brill A, Fuchs TA, Savchenko AS, Thomas GM, Martinod K, De Meyer SF, Bhandari AA, Wagner DD. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. 2012;10:136–44. doi: 10.1111/j.1538-7836.2011.04544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Longstaff C, Varjú I, Sótonyi P, Szabó L, Krumrey M, Hoell A, Bóta A, Varga Z, Komorowicz E, Kolev K. Mechanical stability and fibrinolytic resistance of clots containing fibrin, DNA, and histones. J Biol Chem. 2013;288:6946–56. doi: 10.1074/jbc.M112.404301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–21. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ammollo CT, Semeraro F, Xu J, Esmon NL, Esmon CT. Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J Thromb Haemost. 2011;9:1795–803. doi: 10.1111/j.1538-7836.2011.04422.x. [DOI] [PubMed] [Google Scholar]
- 5.Barranco-Medina S, Pozzi N, Vogt AD, Di Cera E. Histone H4 promotes prothrombin autoactivation. J Biol Chem. 2013;288:35749–57. doi: 10.1074/jbc.M113.509786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Semeraro F, Ammollo CT, Morrissey JH, Dale GL, Friese P, Esmon NL, Esmon CT. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood. 2011;118:1952–61. doi: 10.1182/blood-2011-03-343061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuchs TA, Bhandari AA, Wagner DD. Histones induce rapid and profound thrombocytopenia in mice. Blood. 2011;118:3708–14. doi: 10.1182/blood-2011-01-332676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu FT, Armstrong JK, Tripette J, Meiselman HJ, Cloutier G. A local increase in red blood cell aggregation can trigger deep vein thrombosis: evidence based on quantitative cellular ultrasound imaging. J Thromb Haemost. 2011;9:481–8. doi: 10.1111/j.1538-7836.2010.04164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whelihan MF, Zachary V, Orfeo T, Mann KG. Prothrombin activation in blood coagulation: the erythrocyte contribution to thrombin generation. Blood. 2012;120:3837–45. doi: 10.1182/blood-2012-05-427856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ponder E. Effect of basic proteins on the adhesiveness of red cells. Nature. 1966;209:307–8. doi: 10.1038/209307a0. [DOI] [PubMed] [Google Scholar]
- 11.Rosenbluh J, Hariton-Gazal E, Dagan A, Rottem S, Graessmann A, Loyter AJ. Translocation of histone proteins across lipid bilayers and Mycoplasma membranes. J Mol Biol. 2005;345:387–400. doi: 10.1016/j.jmb.2004.10.046. [DOI] [PubMed] [Google Scholar]
- 12.Beutler E, West C, Blume KG. The removal of leukocytes and platelets from whole blood. J Lab Clin Med. 1976;88:328–33. [PubMed] [Google Scholar]
- 13.Woon LA, Holland JW, Kable EP, Roufogalis BD. Ca2+ sensitivity of phospholipid scrambling in human red cell ghosts. Cell Calcium. 1999;25:313–20. doi: 10.1054/ceca.1999.0029. [DOI] [PubMed] [Google Scholar]
- 14.Hariton-Gazal E, Rosenbluh J, Graessmann A, Gilon C, Loyter A. Direct translocation of histone molecules across cell membranes. J Cell Sci. 2003;116:4577–86. doi: 10.1242/jcs.00757. [DOI] [PubMed] [Google Scholar]
- 15.Kleine TJ, Lewis PN, Lewis SA. Histone-induced damage of a mammalian epithelium: the role of protein and membrane structure. Am J Physiol. 1997;273:C1925–36. doi: 10.1152/ajpcell.1997.273.6.C1925. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880–5. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oklu R, Albadawi H, Watkins MT, Monestier M, Sillesen M, Wicky S. Detection of extracellular genomic DNA scaffold in human thrombus: implications for the use of deoxyribonuclease enzymes in thrombolysis. J Vasc Interv Radiol. 2012;23:712–8. doi: 10.1016/j.jvir.2012.01.072. [DOI] [PubMed] [Google Scholar]


