Abstract
Immunotherapy has emerged as a promising approach that can be used in conjunction with conventional chemotherapy and radiotherapy to further improve the survival rate of patients with advanced cancer. We have recently shown in previous studies that chemotherapy and radiation therapy can alter the tumor microenvironment and allow intratumoral vaccination to prime the adaptive immune system leading to the generation of antigen-specific cell-mediated immune responses. Here, we investigated whether intratumoral injection of a foreign immunodominant peptide (GP33) and the adjuvant CpG into tumors following cisplatin chemotherapy could lead to potent antitumor effects and antigen-specific cell-mediated immune responses. We observed that treatment with all three agents produced the most potent antitumor effects compared to pairwise combinations. Moreover, treatment with cisplatin, CpG and GP33 was able to control tumors at a distant site, indicating that our approach is able to induce cross-presentation of the tumor antigen. Treatment with cisplatin, CpG and GP33 also enhanced the generation of GP33-specific and E7-specific CD8+ T cells and decreased the number of MDSCs in tumor loci, a process found to be mediated by the Fas-FasL apoptosis pathway. The treatment regimen presented here represents a universal approach to cancer control.
Keywords: immunotherapy, cisplatin, immunodominant CTL epitope
Introduction
Immunotherapy has emerged as an alternative, innovative therapy for cancer patients that may improve their survival when combined with conventional chemotherapy and/or radiation therapy. Immunotherapies have the ability to activate the adaptive immune system to generate tumor-specific immune responses that can specifically recognize and target tumor cells while sparing normal cells. Furthermore, immunotherapeutic agents, such as adjuvants, can be used to prime the immune system for additional therapeutic administration. Thus, cancer immunotherapy represents a potentially promising approach that can be used in conjunction with conventional chemotherapy and radiotherapy to further improve the survival rate of patients with advanced stage cervical cancer.
It has recently been shown that chemotherapy and/or radiation therapy can transform the tumor microenvironment into a suitable setting for subsequent immunotherapeutic vaccination [1, 2]. We have previously used cisplatin chemotherapy to prime the tumor microenvironment for vaccination with a recombinant protein [1]. This treatment regimen induced potent antitumor effects and antigen-specific cell-mediated immune responses with the initial cisplatin treatment occurring 5 days after tumor challenge [1]. We have also shown that radiation therapy induces the accumulation of immunosuppressive myeloid-derived dendritic cells (MDSCs) in the tumor microenvironment that are rendered susceptible to cell-mediated immune responses elicited by subsequent peptide vaccination [2]. This approach was effective in generating antitumor effects when a peptide was used for intratumoral vaccination following radiation therapy [2]. Thus, these data indicate that chemotherapy and radiation therapy can alter the tumor microenvironment and allow intratumoral vaccination to prime the adaptive immune system leading to the generation of antigen-specific cell-mediated immune responses.
Some immunostimulatory agents can be used to enhance immune responses to vaccination. The toll-like receptor 9 (TLR9) agonist CpG is a commonly used adjuvant that has been shown to have antitumor effects when directly injected into the tumor [3–5]. CpG co-administration has been found to enhance the antitumor effects generated by bortezomib and an agonistic antibody to the TRAIL receptor DR5 (α-DR5) in mice injected with breast cancer cells [5]. CpG also improved the prevention and treatment of spontaneous mammary tumors in Balb/neuT mice treated with bortezomib and α-DR5 [5]. Furthermore, CpG has been shown to block the immunosuppressive activity of MDSCs in tumor-bearing mice [6]. These studies suggest the possible immunostimulatory function of CpG may enhance antigen-specific CD8+ T cell immune responses induced by therapeutic vaccination.
In the current study, we investigated whether intratumoral injection of vaccine consisting of a foreign immunodominant peptide (GP33) and CpG into HPV16 E7-expressing TC-1 tumors following cisplatin chemotherapy could lead to potent antitumor effects and antigen-specific cell-mediated immune responses. GP33 is an immunodominant peptide from the lymphocytic choriomeningitis virus (LCMV) [7] that has been shown to be highly immunogenic. We found that treatment with all three agents produced the most potent antitumor effects. Moreover, treatment with cisplatin, CpG and GP33 was able to control tumors at a distant site, indicating that our approach is able to induce cross-presentation of the tumor antigen. We found that treatment with cisplatin, CpG and GP33 enhanced the generation of GP33-specific and E7-specific CD8+ T cells. Treatment with cisplatin, CpG and GP33 also decreased the number of MDSCs in tumor loci. The results provide insight into a potential method of controlling cancer.
Materials and methods
Experimental animals
Six- to eight-week-old female C57BL/6 mice were obtained from the National Cancer Institute-Frederick Animal Production Area (Frederick, MD). Mice were housed in the oncology animal facility of the Johns Hopkins Hospital (Baltimore, MD). All animal procedures were performed according to approved protocols and in accordance with recommendations for the proper use and care of laboratory animals.
Cells
The TC-1 tumor model was generated by transformation of primary lung epithelial cells from C57BL/6 mice with active Ras together with HPV-16 E6 and E7 oncogenes and the production and maintenance of this cell line has been described previously [8]. Cells were cultured in RPMI-1640 medium containing 10% FBS, 2mM L-glutamine, 10% sodium pyruvate, 10% non-essential amino acid, and 100pg/ml streptomycin in humidified atmosphere of 5% CO2/95% air at 37°C. E7-specific T-cells were generated from the splenocytes of E7 vaccinated mice and were stimulated with irradiated TC-1 cells and interleukin-2 (IL-2; 10 IU) every week. OT-1 T-cells were generated from OT-1 Rag−/− TCR transgenic mice from our lab as previously mentioned [9].
Peptide, antibodies and reagents
The H2-Db-restricted LCMV GP33-41 peptide (KAVYNFATC), and H2-Db-restricted HPV16 E7aa49-57 peptide (RAHYNIVTF), were synthesized by Beckman Coulter at a purity of ≥70%.
FITC, PE and APC-conjugated anti-mouse CD8a (clone 53.6.7), FITC-conjugated anti – mouse IFN-γ (clone XMG1.2), APC-conjugated anti-mouse CD11b (clone M1/70), PE-conjugated anti-mouse Ly6G (clone 1A8), anti-mouse CD95 (Fas) antibodies, and FITC Annexin V Apoptosis Detection Kit, were purchased from BD Pharmingen (San Diego, CA). PE-conjugated anti-mouse CD95 (Fas, clone 15A7), PE-conjugated anti-mouse Annexin V antibodies were purchased from eBioscience (San Diego, CA). PE-conjugated, HPV16 E7aa49-57 peptide and RAHYNIVTF loaded H2-Db tetramers were obtained from National Institute of Allergy and Infectious Diseases tetramer Facility (Atlanta, GA). Fas/FasL antagonist Kp7-6 was purchased from EMD Chemicals (San Diego, CA).
In vivo tumor treatment experiments and antibody depletion
On day 0, 1×105 TC-1 tumor cells were inoculated subcutaneously into C57BL/6 mice 5 per group, respectively. Three days later, tumor-bearing mice were administrated with cisplatin (5mg/kg body weight) or PBS control intraperitoneally. On day 4, mice were immunized intratumorally with 20μg GP33 peptide, or 10μg CpG, or both. All the treatments were repeated 2 more times at 7-day intervals. Tumor growth was monitored by visual inspection and palpation twice a week, and expressed as volume (length×width2×1/2). Mice were sacrificed when length of subcutaneous tumor reached 20mm.
For systemic antitumor assessment, mice (5 per group) were challenged subcutaneously with 1×105 TC-1 tumor cells on right flanks, 5 days later, 3×104 TC-1 tumor cells were inoculated subcutaneously on the left flanks. Tumor-bearing mice then were either untreated or underwent triple therapy (cisplatin 5mg/kg IP, combined with GP33 20μg and CpG10μg by intratumoral injection) three times at 5 days intervals. In the CD8+ T cell depletion group, 100μg anti-mouse CD8 antibody (clone 2.43) was delivered via intra-peritoneal injection on the day 3, 4, 5 after tumor challenge, then 150 μg/mouse per week to maintain more than 90% depletion of CD8+ T cells.
Preparation of single-cell suspensions from TC-1 Subcutaneous tumor
The tumor tissues were gently dissected from the mice. The solid tumors were then minced into 1- to 2- mm pieces and incubated with serum-free RPMI 1640 medium containing 0.05mg/ml collagenase I, 0.05mg/ml collagenase IV, 0.025mg/ml hyaluronidase IV, 0.25mg/ml DNase I (both from Roche, Indianapolis, IN), 100U/ml penicillin, and 100μg/ml streptomycin and incubated at 37°C for 60 minutes as previously described [10].
Cell surface staining, intracellular cytokine staining and flow cytometry analysis
For tetramer staining, single-cell suspended splenocytes or tumor infiltrating lymphocytes were first stained with purified anti-mouse CD16/32 (Fc block, BD pharmingen, San Diego, CA), and then with anti-mouse FITC-CD8, PE-conjugated HPV16 E7aa49-57 peptide, RAHYNIVTF loaded H2-Db tetramer at 4°C. The cells were stained with 7-AAD prior to flow cytometry analysis to exclude dead cells.
To detect HPV16 E7-specific and LCMV GP33-specific CD8+ T cell responses by IFN-γ intracellular staining, peripheral blood mononuclear cells (PBMCs), single-cell suspended splenocytes or tumor infiltrating lymphocytes were stimulated with either HPV E7aa49-57 or LCMV GP33 peptide (1μg/ml) in the presence of Golgiplug (BD Pharmingen, San Diego, CA) at 37°C overnight. The stimulated cells were then washed once with FACScan buffer and stained with PE-conjugated monoclonal rat anti-mouse CD8a. Cells were subjected to intracellular cytokine staining using the Cytofix/Cytoperm kit according to the manufacturer’s instruction (BD Pharmingen, San Diego, CA). Intracellular IFN-γ was stained with FITC-conjugated rat anti-mouse IFN-γ. Flow cytometry analysis was performed using FACSalibur with CELLQuest software. All analyses were performed with Flowjo software (Tree star).
Isolation of myeloid-derived suppressor cells in tumor-bearing mice
CD11b+ Ly6GHi MDSCs were isolated from splenocyte suspensions from TC-1 tumor-bearing C57BL/6 mice using CD11b+Ly6G isolation kits and LS columns for magnetic separation according to the manufacture’s instructions (Miltenyi Biotec). The purity was no less than 95%.
In vitro MDSC apoptosis evaluation
Purified MDSCs were incubated at 37°C alone or with E7-specific CD8+ T cells or OT-1-specific CD8+ T cells at a 1:1 ratio for up to 24 hours in 96-well plates. Twenty-four hours later, cells were were stained with APC anti-mouse CD11b, PE anti-mouse Ly6G, FITC anti-mouse Annexin V antibody and 7-AAD (FITC Annexin V Apoptosis Detection Kit, BD Pharmingen, San Diego, CA) and examined by flow cytometry. MDSCs were first gated on CD11b+ and Ly6GHi, and then defined as apoptotic by Annexin V+ and 7-AAD-.
To measure antibody-induced apoptosis of MDSCs, isolated MDSCs were cultured for 12 hours with anti-mouse Fas agonistic antibody (2μg/ml, BD Pharmingen, San Diego, CA), or isotype mAb. For the in vitro blocking assay, isolated MDSCs were co-cultured with E7-specific CD8+ T cells or OT-1 specific CD8+ T cells at a 1:1 ratio and then incubated with isotype mAb, Fas-Fas L antagonist (Kp7-6, CALBIOCHEM for 24 hours.
Statistical analysis
All data presented in this study are expressed as mean ± SE where indicated. Comparisons between individual data points for intracellular cytokine staining with flow cytometry analysis and tumor treatment were made using the two-tailed student’s t-test by Graph Prism 6.0. In the tumor treatment experiments, the principal outcome of interest was duration until mice were sacrificed (tumor diameters exceeded 20mm). The event time distributions for different mice were compared using the Kaplan–Meier method and the log-rank test by Graph Prism 6.0. All p-values less than 0.05 were considered significant.
Results
Treatment of TC-1 tumor-bearing mice with cisplatin, CpG and GP33 generates potent antitumor effects
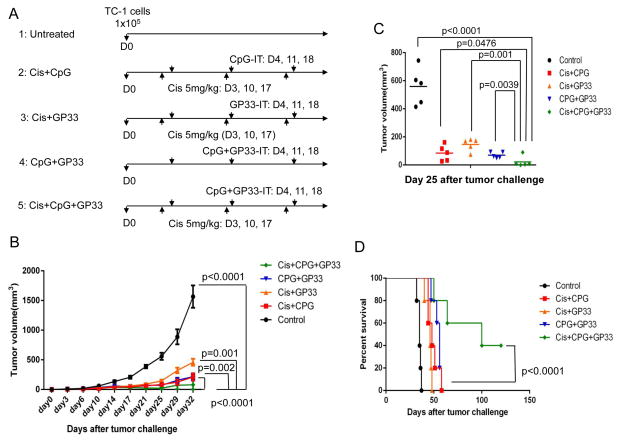
We first used TC-1 tumor-bearing mice to examine the antitumor effects of various combinations of intraperitoneal cisplatin chemotherapy, intratumoral injection of GP33 peptide and intratumoral injection of CpG. C57BL/6 mice were challenged with 1×105 TC-1 tumor cells on day 0, and divided into five treatment groups. Mice in group 1 were untreated, those in group 2 were treated with cisplatin and intratumoral CpG, those in group 3 were treated with cisplatin and intratumoral GP33 peptide, those in group 4 were treated with intratumoral CpG and GP33, and those in group 5 were treated with cisplatin, CpG and GP33 (triple treatment) (Figure 1A). A shown in Figure 1B and C, each treatment regimen significantly reduced tumor volume in TC-1 tumor-bearing mice compared to the untreated mice. The most potent antitumor effects were generated by triple treatment, with cisplatin, CpG and GP33. The survival of tumor-bearing mice was also significantly extended in those that were triple treated compared to all other treatment groups (Figure 1D). These data suggest that combination of cisplatin, CpG and GP33 has significant antitumor effects against TC-1 tumors.
Figure 1. Antitumor effect in TC-1 tumor-bearing mice treated with intratumoral injection of irrelevant peptide combined with cisplatin and CpG.
C57BL/6 mice (5 mice/group) were challenged subcutaneously with TC-1 tumor cells and treated with various combinations of cisplatin, CpG and GP33 peptide. A. Schematic diagram of the experiment. B. Scatter plot of tumor growth kinetics. C. Bar graph depicting quantification of tumor size (mean ± S.E.) on day 25 after tumor challenge. D. Kaplan-Meier survival plot.
Treatment with cisplatin, CpG and GP33 elicits potent systemic antitumor effects
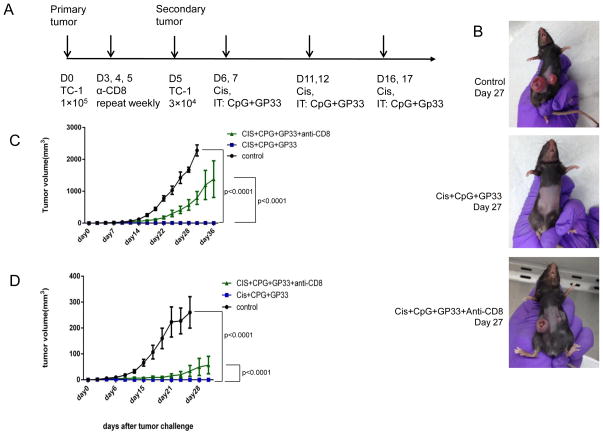
Next, we examined whether triple therapy with cisplatin, CpG and GP33 could generate antitumor effects in a secondary tumor. C57BL/6 mice were challenged subcutaneously with 1×105 TC-1 tumor cells in the right flank on day 0, and then with 3×104 TC-1 cells in the left flank on day 5. A group of mice was then treated with clone 2.43 anti-CD8α antibody on days 3, 4 and 5 and then weekly to deplete CD8+ T cells. Mice were treated as shown in Figure 2A, with cisplatin intraperitoneally and with CpG and GP33 intratumorally in the primary tumor on the right flank. As shown in Figure 2B and C, the volume of the primary tumor was significantly decreased in triple treated mice compared to untreated mice. Furthermore, the volume of the secondary tumor was also significantly decreased in triple treated mice compared to untreated mice (Figure 2B and D). Depletion of CD8 α-expressing cells significantly decreased the therapeutic effect of cisplatin, CpG and GP33 treatment in both the primary and secondary tumors, indicating that CD8+ T cells are important for treatment efficacy (Figure 2C and D). It is important to note that other CD8α-expressing cells such as dendritic cells and a subset of macrophages were also depleted. These cells are likely to also play a role in contributing to the treatment efficacy. These data indicate that the triple treatment generates potent antitumor immune responses capable of acting against tumors at distant sites.
Figure 2. Local intratumoral treatment triggered systemic antitumor effect.
C57BL/6 mice (5 mice/group) were challenged sequentially with TC-1 tumor cells on day 0 (right flank) and day 5 (left flank), and then were either untreated or treated with cisplatin combined with intratumoral CpG and GP33 peptide injection in the primary tumor. In the CD8+ T cell depletion group, mice were treated as above, with the addition of 100μg anti-mouse CD8 antibody (clone 2.43) injected intraperitoneally on days 3, 4, and 5 after tumor challenge and then 150μg weekly A. Schematic diagram of the treatment regimen. B. Representative photograph of tumor size on day 27 after primary tumor challenge. C. Scatter plot of primary tumor growth kinetics. D: Scatter plot of secondary tumor growth kinetics.
Treatment with cisplatin, CpG and GP33 enhances systemic GP33-specific CD8+ T cell responses in TC-1 tumor-bearing mice
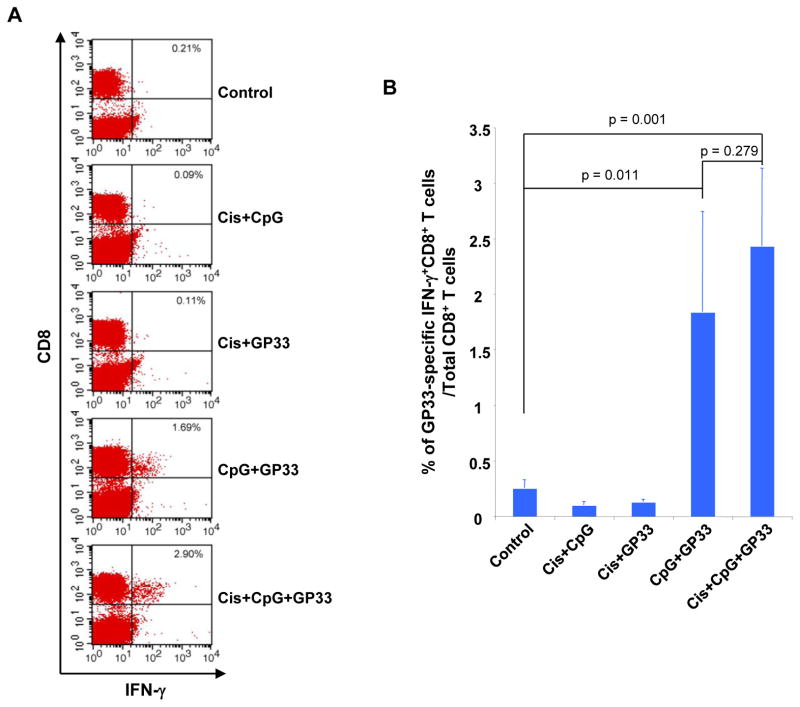
We then examined the effect of triple treatment specifically on systemic CD8+ T cell immune responses. TC-1 tumor-bearing mice were treated with various combinations of cisplatin, CpG and GP33 peptide as shown in Figure 1A. Peripheral blood mononuclear cells (PBMCs) of all populations were analyzed 25 days after tumor challenge after the last antigen delivery for the presence of IFN-γ secreting GP33-specific CD8+ T cells. As shown in Figure 3A and B, tumor-bearing mice treated with CpG and GP33 or cisplatin, CpG and GP33 generated significantly greater IFN-γ+ GP33-specific CD8+ T cells systemically compared to untreated control mice. These data indicate that triple treatment with cisplatin, CpG and GP33 is capable of generating potent antigen-specific CD8+ T cell responses in TC-1 tumor-bearing mice.
Figure 3. Systemic immune response to LCMV-GP33 peptide.
C57BL/6 mice (5 mice/group) were challenged subcutaneously with TC-1 tumor cells and subsequently treated with various combinations of cisplatin, CpG and GP33 peptide as shown. The PBMCs were analyzed 1 week after the last antigen delivery. The presence of GP33-specific CD8+ T cells in PBMCs was analyzed using intracellular cytokine staining for IFN-γ and CD8+ staining followed by flow cytometry analysis. A. Representative flow cytometry contour plot depicting the frequency of IFN-γ-secreting CD8+ T cells among PBMCs after being pulsed with GP33 peptide. B. Bar graph depicting the number of IFN-γ+ CD8+ T cells among PBMCs (mean ±S.E).
Treatment with cisplatin, CpG and GP33 enhances local CD8+ T cell responses in TC-1 tumor-bearing mice
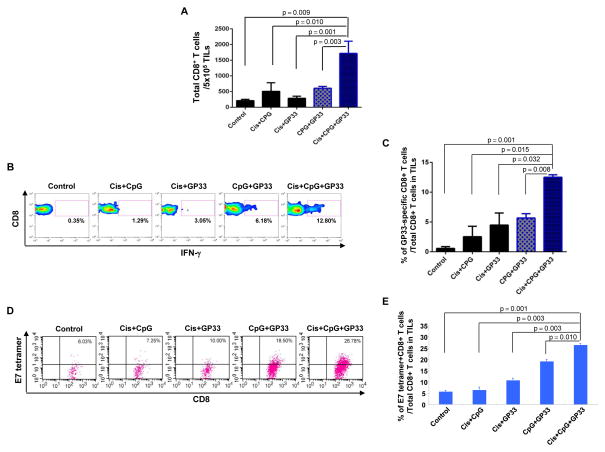
In order to study the local immune responses generated by triple treatment, TC-1 tumor-bearing mice were treated with various combinations of cisplatin, CpG and GP33 peptide as shown in Figure 1A. 30 days after the initial tumor challenge, tumor-infiltrating lymphocytes (TILs) were harvested for analysis. The presence of GP33-specific CD8+ T cells was characterized intracellular cytokine staining for IFN-γ and CD8 staining and analyzed by flow cytometry. As shown in Figure 4A–C, tumor-bearing mice treated with cisplatin, CpG and GP33 generated a significantly higher percentage of IFN-γ+ GP33-specific CD8+ T cells among TILs compared to all other treatment groups. Furthermore, triple treated tumor-bearing mice generated the highest number of E7-specific CD8+ T cells among TILs compared to all other treatment groups (Figure 4D and E). It is important to note that TILs collected from within the tumor microenvironment are in an activated state where the T cell receptors are being internalized. Thus, the staining appears as a gradient based on the various levels of TCR internalization. This appearance differs from the staining pattern usually observed in assessment of antigen specific T cells in the systemic circulation, where a distinct population would indicate their presence. Taken together, these data suggest that triple treatment can lead to cross-presentation of the tumor antigen, E7, resulting in enhanced tumor antigen-specific CD8+ T cell-mediated immune responses in the tumor loci.
Figure 4. Local immune response to GP33 peptide and tumor antigen (HPV E7).
C57BL/6 mice (3 mice/group) were challenged subcutaneously with TC-1 tumor cells and treated with various combinations of cisplatin, CpG and GP33 peptide as indicated. 12 days after the last antigen delivery, tumor-infiltrating lymphocytes were harvested to analyze the immune cell subsets. A. Representative flow cytometry analysis depicting the frequency of GP33-specific IFN-γ-secreting CD8+ T cells among tumor infiltrating CD8+ T cells. B. Representative flow cytometry analysis depicting the absolute number of E7-tetramer binding CD8+ T cells in 5×105 single-cell suspension prepared from tumors. C. Bar graph quantification of the absolute number of total CD8+ T cells in 5×105 single-cell suspension prepared from tumors (mean ± S.E.). D. Bar graph quantification of the percentage of GP33-specific IFN-γ-secreting CD8+ T cells among tumor infiltrating CD8+ T cells (mean ± S.E.). E. Bar graph depicting the number of H2-Db tetramer+ CD8+ T cells in 5×105 tumor infiltrating cells (mean ± S.E.).
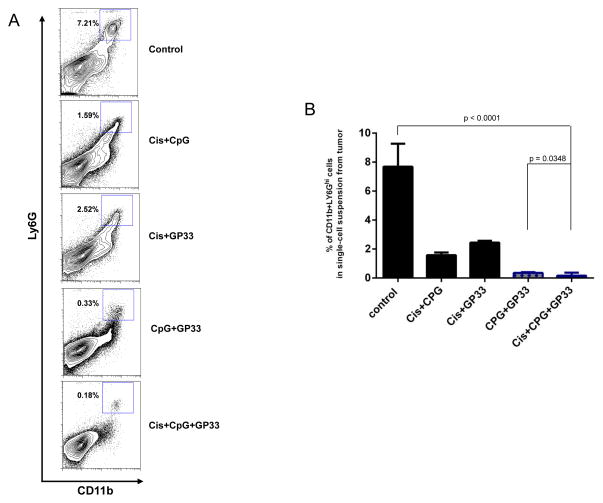
Intratumoral GP33 peptide injection combined with cisplatin and CpG reduces the myeloid derived suppressor cell population in the tumor microenvironment
We analyzed the tumor infiltrating-lymphocytes of TC-1 tumor-bearing mice treated with various combinations of cisplatin, CpG andGP33 by harvesting tumors between 30 and 35 days after tumor challenge to examine the MDSC populations. Figure 5A shows representative flow cytometry analysis of the CD11b+ Ly6GHi MDSCs in the tumor loci of mice in each treatment group. We found that mice treated with cisplatin, CpG and GP33 had significantly reduced populations of CD11b+ Ly6GHi MDSCs in tumor loci compared to all other treatment groups (Figure 5B). These data suggest that treatment with cisplatin, CpG and GP33 elicits antitumor effects at least in part by reducing the population of MDSCs in the tumor microenvironment.
Figure 5. Intratumoral GP33 peptide injection combined with cisplatin and CpG reduced the myeloid derived suppressor cell population in the tumor microenvironment.
C57BL/6 mice were challenged subcutaneously with TC-1 tumor cells and treated with various combinations of cisplatin, CPG and GP33 peptide. At 30–35 days after tumor challenge, when the tumor diameters exceeded 10mm, tumor infiltrating cells were harvested to analyze the CD11b+ Ly6Ghi MDSCs. A. Representative contour plots and B. bar graph depicting the frequency of CD11b+ Ly6Ghi MDSC in single-cell suspension prepared from tumor.
Treatment with cisplatin followed by CpG and GP33 reduced the number of MDSCs in tumor loci
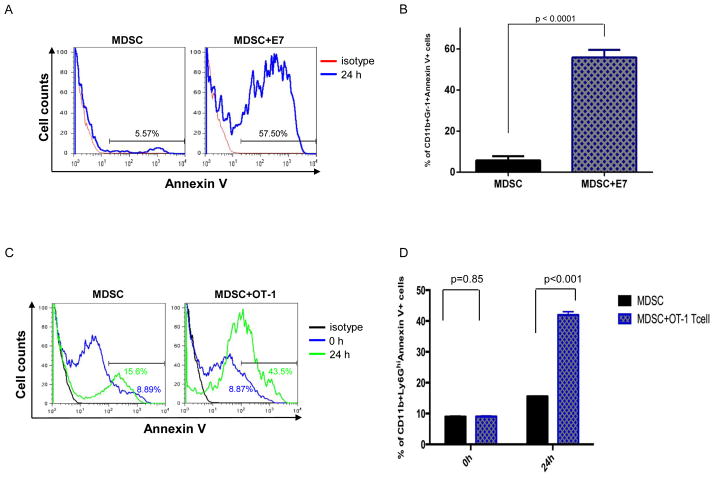
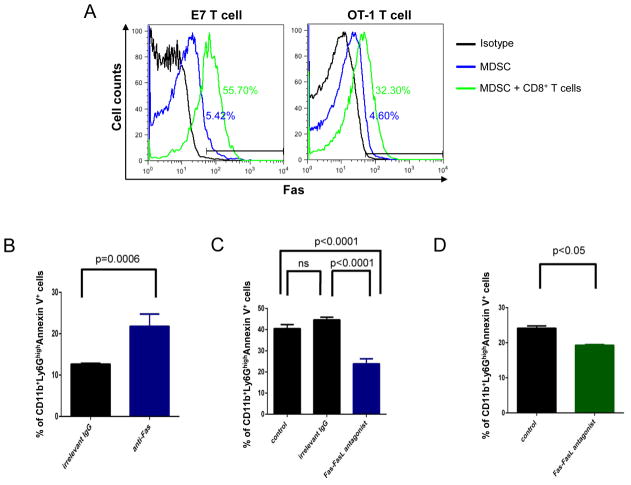
Previously, it has been shown that MDSCs express Fas and will undergo Fas-FasL-mediated apoptosis if it encounters a T cell expressing FasL [11]. Therefore, we characterized the effect of activated E7-specific CD8+ T cells and OT-1 T cells on MDSCs in terms of apoptotic activity and found that MDSCs incubated with E7-specific CD8+ T cells and OT-1 T cells had significantly higher expression of annexin V compared to those incubated with medium control, indicating that activated T cells are able to induce apoptosis of MDSCs (Figure 6). Additionally, we observed that MDSCs co-cultured with E7-specific CD8+ T cells and OT-1 T cells combined with Fas-FasL antagonist Kp7-6 significantly decreased the frequency of apoptosis compared to control (Figure 7). Taken together, these data suggest that activated CD8+ T cells induce apoptosis in MDSCs through a Fas-FasL-dependent pathway, indicating that the MDSC killing may occur via a non-antigen-specific pathway.
Figure 6. Activated CD8+ T cells directly induced apoptosis of MDSCs.
Purified splenic CD11b+ Ly6GHi MDSCs incubated with E7-specific T cells, OT-1 T cells or medium control for up to 24 hours. A. Representative flow cytometry histograms depicting the frequency of CD11b+ Ly6GHi Annexin V+ MDSC co-cultured with E7-specific T cells. B. Bar graph quantification of the data A (mean ± S.E.). C. Representative flow cytometry histograms depicting the frequency of CD11b+ Ly6GHi Annexin V+ MDSCs co-cultured with OT-1 T cells. D. Bar graph quantification of the data C (mean ± S.E.).
Figure 7. MDSCs underwent apoptosis via Fas dependent pathway.
A. Expression of Fas in MDSCs in the presence and absence of E7-specific T cells or OT-1 T cells. B. Purified splenic Ly6GHi MDSCs were incubated for 12 hours with Fas agonist antibody or irrelevant control IgG, and the CD11b + Ly6GHi cells were analyzed for the frequency of apoptosis via Annexin V+ and 7-AAD-. C. MDSCs were co-cultured with E7-specific CD8+ T cells for 24 hours, and combined with either irrelevant control IgG or Fas-FasL antagonist, Kp7-6. The CD11b+ Ly6GHi cells were analyzed the frequency of apoptosis via Annexin V+ and 7-AAD-. D. MDSCs were co-cultured with OT-1 specific CD8+ T cells for 24 hours, with or without Fas-FasL antagonist Kp7-6, and the CD11b+ Ly6GHi cells were analyzed for the frequency of apoptosis via Annexin V+ and 7-AAD.
Discussion
In the present study, we have shown that treatment with cisplatin followed by intratumoral injection of a potent adjuvant, CpG, and a foreign immunodominant peptide, GP33, generates potent antigen-specific cell-mediated immune responses and antitumor effects. Of note, treatment with cisplatin, CpG and CP33 was able to control tumors at distant sites. Furthermore, our treatment induced a reduction of MDSCs in the tumor loci thereby reducing immunosuppression in the tumor microenvironment. We predict that the GP33 peptide vaccination induces the generation of memory CD8+ T cells, which have been shown to perform immunologic helper functions [12, 13]. Taken together, the combination of chemotherapy, adjuvant and immunodominant peptide presented here represents a systemic approach to tumor control.
Here, we use GP33, an immunodominant peptide from the lymphocytic choriomeningitis virus (LCMV) to stimulate the adaptive immune response. However, the success of GP33 in this approach opens the opportunity to use other immunodominant peptides. For future translational applications, our current approach would be modified to include a panel of peptides specific for multiple MHC alleles, covering the majority of the population. Additionally, the current approach could be expanded to include CD4+ T cell specific epitope to enhance immune responses and antitumor effects, including the generation of memory T cells. It is possible that other irrelevant antigens may be used in a similar fashion. Previously, we have used the PADRE (Pan HLA-DR reactive epitope) peptide in a similar treatment regimen and found that treatment with cisplatin, CpG and PADRE control tumors through the generation of antigen-specific CD4+ T cells (Wu et al, personal communication). Of note, PADRE can potentially be used in humans because it is an epitope specific for many different HLA-DR alleles.
A major advantage of the current treatment approach is that it leads to systemic antitumor effects and is capable of controlling distant tumors. As shown in Figure 2A, mice were challenged with TC-1 tumor cells in the right and left flanks, but only treated in the right flank. We found that treatment with cisplatin followed by CpG and GP33 injected into the right flank tumor effectively controlled the tumor in the left flank. This suggests that this treatment approach could be effective in generating systemic antitumor effects against distant metastases. Because we have shown that the use of a local treatment in an accessible solid tumor can illicit a system response that can prevent the growth of other the same type of tumor in the body, this treatment may be applicable in preventing the metastases of other accessible solid tumors such as in colon and best cancer. Although future studies on other tumor models would be needed to confirm this, the current study suggests the possibility of irrelevant antigen treatment for the general control of tumor growth because the treatment is not specific to the viral origin of the tumor.
Origin non-specific treatment may offer a universal strategy for controlling solid tumor cancers, including those of non-viral origin, The first round of treatment may induce priming of the T cells in the tumor microenvironment resulting in the generation of GP33-specific CD8+ T cells (Figure 3). Following the subsequent rounds of treatment, tumor cells are coated with GP33 antigen, rendering them susceptible to killing by GP33-specific CD8+ T cells. Furthermore, the release of tumor antigen leads to the cross-priming and the generation of tumor antigen-specific CD8+ T cells, which are likewise able to kill tumor cells (Figure 4). MDSCs are also killed by the antigen-specific CD8+ T cells generated by this treatment (Figure 7). Due to this mechanism, it is not necessary to know immunodominant tumor antigens, making the approach potentially widely applicable. We believe the treatment would be useful because the strategy is not emphasizing the viral origin of the tumor model, but rather, focuses on triggering an immune response using unrelated antigen. Theoretically, a cancer of unknown origin with an unidentified antigen may be treated with a local injection of the treatment that will stimulate an immune response to the tumor, and prevent metastasis by stimulating a systemic response. Future tests of this strategy for breast cancer and prostate cancer are suggested to determine the efficacy of this treatment in solid tumor of non-viral origin.
We recognize the need for future studies of local and systemic treatment and responses in more developed tumors and in different types of cancers. In the current study, cisplatin chemotherapy is administered to prepare the tumor microenvironment for treatment three days after tumor challenge. Our previous study found that cisplatin chemotherapy was an effective initial modulating agent for the tumor microenvironment when administered five days after tumor challenge, suggesting the possibility of this strategy’s efficacy on longer-established tumors.[1] Future studies would be useful in determining the efficacy of treatment for tumors that have been established for a greater than five days.
Additionally, it is important to point out that our study demonstrates efficacy of the current treatment for a specific type of solid tumor. The current approach is most applicable to accessible tumors, such as head and neck, skin and cervical tumors. However, since the therapy used in this study includes intratumor vaccination with antigenic peptide, it may be limited in the case of inaccessible tumors, an issue that needs to be addressed in order to further extend this concept for clinical translation. The administration of local vaccination would not be possible for liquid or inaccessible malignancies such as those of lung cancer, and the systemic administration of the vaccine may produce a different immune response. The efficacy of experimental treatments on other types of solid and non-solid tumors, particularly those where the stromal microenvironment is overwhelmed by disease should be studied in the future. The efficacy of cisplatin followed by vaccination with an irrelevant antigen administered systemically or locally against a variety of other cancer models should be studied to determine if this strategy will work to control non-solid tumors or other types of cancer. In such studies, the limitations of the current treatment may be resolved by modifying the delivered peptide to include tumor homing or tumor environment targeting aptamers. For example, CD13 ligand has been shown to be capable of delivering the antigenic peptide to tumor loci [14, 15] and elicit an antigen-specific antitumor response [1]. Additionally, we have shown that mesothelin and NKG2D can be used as tumor-homing modules in chimeric proteins, delivering the therapeutic proteins to tumor loci [16, 17]. It is also important to note that cisplatin may not be the optimal chemotherapeutic drug in certain cancers. Therefore, on top of evaluating the use of our treatment approach in other cancers, further investigations should also adopt other chemotherapies in different tumor systems. Because our studies suggest the possibility of an effective systemic immune due to the control of the second tumor after vaccination, we propose additional future studies comparing systemic and local responses of both systemically and locally administered treatments.
Previously, we’ve showed that radiation treatment followed by intratumoral antigenic peptide vaccination could lead to a reduction in population of suppressive myeloid cells in the tumor and enhance antigen-specific CD8+ T cell antitumor effects [2]. Thus, we believe that radiation treatment may be applied in conjunction with our current approach and achieve a more potent immune response and better tumor control. Though future studies still need to be done to confirm this.
In summary, we have successfully developed a strategy to control tumors using cisplatin treatment followed by intratumoral injection of CpG and GP33 peptide. This strategy may serve as a platform technology for the treatment of a variety of cancers, potentially including metastatic cancer. Additional studies will be needed to further enhance the stimulated immune responses and to extend the approach to inaccessible tumors.
Acknowledgments
We thank Dr. Shiwen Peng for helpful discussions and critical review of this manuscript including the organization and editing of the figures. We also thank Jessica Jeang and Benjamin Yang for preparation of the manuscript. This research is supported by NCI SPORE in Cervical Cancer 2P50CA098252, 2R01CA114425-06, and R01CA142691 grants.
Footnotes
Conflict of interest statement: The authors declare no conflicts of interest exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kang TH, Mao CP, Lee SY, Chen A, Lee JH, Kim TW, et al. Chemotherapy acts as an adjuvant to convert the tumor microenvironment into a highly permissive state for vaccination-induced antitumor immunity. Cancer Res. 2013;73:2493–504. doi: 10.1158/0008-5472.CAN-12-4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu CY, Yang LH, Yang HY, Knoff J, Peng S, Lin YH, et al. Enhanced Cancer Radiotherapy through Immunosuppressive Stromal Cell Destruction in Tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:644–57. doi: 10.1158/1078-0432.CCR-13-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carpentier AF, Chen L, Maltonti F, Delattre JY. Oligodeoxynucleotides containing CpG motifs can induce rejection of a neuroblastoma in mice. Cancer Res. 1999;59:5429–32. [PubMed] [Google Scholar]
- 4.Meng Y, Carpentier AF, Chen L, Boisserie G, Simon JM, Mazeron JJ, et al. Successful combination of local CpG-ODN and radiotherapy in malignant glioma. International journal of cancer Journal international du cancer. 2005;116:992–7. doi: 10.1002/ijc.21131. [DOI] [PubMed] [Google Scholar]
- 5.Lee S, Yagita H, Sayers TJ, Celis E. Optimized combination therapy using bortezomib, TRAIL and TLR agonists in established breast tumors. Cancer immunology, immunotherapy : CII. 2010;59:1073–81. doi: 10.1007/s00262-010-0834-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zoglmeier C, Bauer H, Norenberg D, Wedekind G, Bittner P, Sandholzer N, et al. CpG blocks immunosuppression by myeloid-derived suppressor cells in tumor-bearing mice. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:1765–75. doi: 10.1158/1078-0432.CCR-10-2672. [DOI] [PubMed] [Google Scholar]
- 7.Hudrisier D, Oldstone MB, Gairin JE. The signal sequence of lymphocytic choriomeningitis virus contains an immunodominant cytotoxic T cell epitope that is restricted by both H-2D(b) and H-2K(b) molecules. Virology. 1997;234:62–73. doi: 10.1006/viro.1997.8627. [DOI] [PubMed] [Google Scholar]
- 8.Lin KY, Guarnieri FG, Staveley-O’Carroll KF, Levitsky HI, August JT, Pardoll DM, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–6. [PubMed] [Google Scholar]
- 9.Peng S, Monie A, Kang TH, Hung CF, Roden R, Wu TC. Efficient delivery of DNA vaccines using human papillomavirus pseudovirions. Gene therapy. 2010;17:1453–64. doi: 10.1038/gt.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng S, Lyford-Pike S, Akpeng B, Wu A, Hung CF, Hannaman D, et al. Low-dose cyclophosphamide administered as daily or single dose enhances the antitumor effects of a therapeutic HPV vaccine. Cancer immunology, immunotherapy : CII. 2013;62:171–82. doi: 10.1007/s00262-012-1322-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinha P, Chornoguz O, Clements VK, Artemenko KA, Zubarev RA, Ostrand-Rosenberg S. Myeloid-derived suppressor cells express the death receptor Fas and apoptose in response to T cell-expressed FasL. Blood. 2011;117:5381–90. doi: 10.1182/blood-2010-11-321752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura Y, Watchmaker P, Urban J, Sheridan B, Giermasz A, Nishimura F, et al. Helper function of memory CD8+ T cells: heterologous CD8+ T cells support the induction of therapeutic cancer immunity. Cancer Res. 2007;67:10012–8. doi: 10.1158/0008-5472.CAN-07-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frentsch M, Stark R, Matzmohr N, Meier S, Durlanik S, Schulz AR, et al. CD40L expression permits CD8+ T cells to execute immunologic helper functions. Blood. 2013;122:405–12. doi: 10.1182/blood-2013-02-483586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279:377–80. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 15.Ellerby HM, Arap W, Ellerby LM, Kain R, Andrusiak R, Rio GD, et al. Anti-cancer activity of targeted pro-apoptotic peptides. Nature medicine. 1999;5:1032–8. doi: 10.1038/12469. [DOI] [PubMed] [Google Scholar]
- 16.Kang TH, Ma B, Wang C, Wu TC, Hung CF. Targeted coating with antigenic peptide renders tumor cells susceptible to CD8(+) T cell-mediated killing. Molecular therapy : the journal of the American Society of Gene Therapy. 2013;21:542–53. doi: 10.1038/mt.2012.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang TH, Knoff J, Yang B, Tsai YC, He L, Hung CF, et al. Control of spontaneous ovarian tumors by CD8+ T cells through NKG2D-targeted delivery of antigenic peptide. Cell & bioscience. 2013;3:48. doi: 10.1186/2045-3701-3-48. [DOI] [PMC free article] [PubMed] [Google Scholar]