Abstract
Objectives
To describe the relationship between ankle brachial index (ABI) and the risk for heart failure (HF).
Background
The ABI is a simple, non-invasive measure associated with atherosclerotic cardiovascular disease and death; however, the relationship between ABI and risk of HF is less well characterized.
Methods
Between 1987–1989 in the Atherosclerosis Risk in Communities study an oscillometric device was used to measure blood pressure in a single upper and randomly chosen lower extremity to determine the ABI. Incident HF events were defined by the first hospitalization with an ICD-9 code 428.x through 2008. The risk of HF was assessed across the ABI range using restricted cubic splines and Cox proportional hazards models.
Results
ABI was available in 13,150 participants free from prevalent HF. Over a mean 17.7 years of follow up, 1809 incident HF events occurred. After adjustment for traditional HF risk factors, prevalent CHD, subclinical carotid atherosclerosis, and interim MI, as compared to an ABI of 1.01–1.40, participants with an ABI ≤0.90 were at increased risk for HF (HR 1.40, 95% CI 1.12–1.74), as were participants with an ABI of 0.91–1.00 (HR 1.36, 95%CI 1.17–1.59).
Conclusions
In a middle aged community cohort, an ABI ≤ 1.00 was significantly associated with an increased risk of HF independent of traditional HF risk factors, prevalent CHD, carotid atherosclerosis, and interim MI. Low ABI may not only reflect overt atherosclerosis, but also pathologic processes in the development of HF beyond epicardial atherosclerotic disease and MI alone. A low ABI, as a simple non-invasive measure, may be a risk marker for HF.
Keywords: Ankle brachial index, heart failure, coronary artery disease, myocardial infarction, vascular stiffness
Introduction
The ankle brachial index (ABI) is a simple non-invasive tool for the diagnosis of peripheral arterial disease (PAD) (1). The ABI also carries prognostic information related to all-cause mortality, cardiovascular death (2–9), and non-fatal cardiovascular events, including coronary heart disease (CHD) and stroke (6,10–12). However, the association between ABI and incident heart failure (HF) has been less well characterized (8,13).
In the Cardiovascular Health Study (CHS), an ABI <0.90, as compared to an ABI ≥0.90, was associated with an increased risk for HF in those without prevalent CHD (relative risk 1.61, 95%CI 1.14–2.29), but not in those with prevalent CHD (8). In the Heart Outcomes Prevention Evaluation (HOPE) trial, the incidence of HF was higher in those with clinical evidence of PAD or ABI <0.9 (4.6%) as compared to those with normal ABI (2.6%) (13). However, CHS evaluated an older population and HOPE recruited patients with known cardiovascular disease or several cardiovascular risk factors. The association between ABI and incident HF in a middle aged community population over a long follow up period has not been evaluated. Therefore, we sought to describe HF risk across the spectrum of ABI in the Atherosclerosis Risk in Communities (ARIC) Study.
Methods
Study population
ARIC is an ongoing, prospective observational study of the natural history of cardiovascular risk factors and atherosclerotic diseases. Detailed study rationale, design, and procedures have been previously published (14). The original cohort included 15,792 participants recruited between 1987–1989 using probability sampling of middle aged (45–64 years old) men and women from 4 communities in the United States (Forsyth County, NC; Jackson, MS; suburban Minneapolis, MN; and Washington County, MD). The Jackson field center enrolled an entirely African American cohort. Subsequent follow up visits occurred at 3 year intervals up to 1998, with annual telephone interviews conducted between visits and to the present. Institutional review boards from each site approved the study and informed consent was obtained from all participants.
Ankle brachial index and covariates
ABI was measured as previously described in ARIC (15). Briefly, resting ankle and brachial blood pressures (BP) were measured at visit 1 using an automated oscillometric device (Dinamap 1846 SX). BPs were assessed with the cuff placed just above the ankle with the “artery marker” aligned over the posterior tibial artery of one randomly selected leg and over the brachial artery of the right arm (most commonly). ABI was calculated as the ratio of lower extremity to upper extremity systolic BPs.
Established definitions for hypertension, obesity, diabetes mellitus, prevalent CHD, stroke, and smoking status, as previously described in ARIC were utilized (16). Intermittent lower extremity claudication was identified from participant questionnaires (17). Obesity was defined as a body mass index ≥ 30 kg/m2. Electrocardiographic LVH was determined by Cornell criteria. Estimated glomerular filtration rate (eGFR), hematologic parameters, lipids, and glucose were measured according to standardized protocols with chronic kidney disease (CKD) defined as an eGFR < 60 ml/min/1.73m2 (14,18,19). The presence of carotid atherosclerotic plaque was determined from B-mode ultrasound (20). Incident myocardial infarction (MI) was defined as hospitalized MI. Incident CHD was defined as fatal or non-fatal MI, “silent” MI by serial ECG, and/or coronary revascularization.
Outcome
Prevalent HF at baseline was defined by stage 3 (manifest HF) according to Gothenburg criteria or the use of medications for HF (21). The Gothenburg criterion is a validated scoring system composed of 3 components a) cardiac, b) pulmonary, and c) therapy, in which stage 3 (manifest HF) requires 1 point from each component (22). The primary outcome was incident HF, defined by the first hospitalization with ICD-9 code for HF (428.x) listed at discharge or a death certificate including HF among the listed causes (21). The follow-up period was the time elapsed from the date of visit 1 to the date of HF hospitalization or death, with censoring at the date of last contact for those lost to follow-up, date of non HF death, or December 31, 2008. Deaths were ascertained through annual phone calls to participants or their kin and ongoing surveillance of health department death certificate files.
Statistical Methods
After sequential exclusion of those at baseline with prevalent or missing HF status (n=1039), missing ABI (n=522), race other than African American or Caucasian and African Americans from sites other than Jackson, MS and Forsyth County, NC (n=91), and those missing baseline covariates (n=990), 13,150 participants were included in the final analysis. Following AHA/ACC guidelines, ABI was categorized as ≤0.90, 0.91 – 1.00, 1.01 – ≤1.40, and >1.40 (23). Summary statistics for covariates were calculated as counts (percentages), and medians (interquartile ranges) for categorical and continuous data, respectively. Comparisons were made across categories of ABI using Spearman correlation or Wilcoxon rank sum tests, as appropriate. For each ABI category, incidence rates for HF were calculated (number of events divided by person-time at risk) and Kaplan Meier time to event analysis was performed. The risk of HF was assessed across the spectrum of ABI using Cox proportional hazards models and restricted cubic splines. Covariates included in multivariable models were based upon the ARIC HF risk model (24) as well as carotid atherosclerosis. Interim MI or CHD after baseline were also included as time varying covariates. Interactions by age, sex, race, race by sex, prevalent CHD, hypertension, diabetes mellitus, body mass index (BMI), and smoking (ever) were assessed using Cox proportional hazards models and likelihood ratio tests. The proportion of incident cases of heart failure attributable to an exposure (i.e. ABI ≤ 1.00) was calculated using the “punafcc” package to determine population attributable risk (PAR). Two-sided p values <0.05 were considered significant, except for interactions where p <0.10 was considered significant. Analyses were performed using Stata 11.2–13.0 (Stata Corp., College Station, Texas).
Results
Cohort characteristics
Among the 13,150 included ARIC participants, an ABI ≤0.90 was present in 3.9%, while 12.6% had an ABI of 0.91–1.00, and 2.4% of had an ABI >1.40 (Table 1). Those with an ABI ≤1.00 were more likely to be female and African American. There was no significant trend in CHD prevalence across ABI categories, although the frequency of carotid atherosclerosis increased with lower ABI. Traditional HF risk factors, including hypertension, diabetes mellitus, CKD, and smoking were also more common among those with an ABI ≤1.00. Systolic BP, pulse pressure, heart rate, as well as total and LDL cholesterols were higher in those with the lowest ABI. There were no significant trends in BMI, eGFR, or the prevalence of electrocardiographic LVH across ABI categories.
Table 1.
Baseline characteristics of 13,150 ARIC participants free from prevalent heart failure according to category of ankle brachial index.
| Ankle Brachial Index | |||||
|---|---|---|---|---|---|
| ≤ 0.90 N=513 (3.9%) |
0.91–1.00 N=1,656 (12.6%) |
1.01–1.40 N=10,664 (81.1%) |
>1.40 N=317 (2.4%) |
P-trend | |
| ABI | 0.84 (0.79–0.88) | 0.97 (0.94–0.99) | 1.16 (1.09–1.23) | 1.44 (1.42–1.49) | |
| Age, yrs | 56 (50–61) | 54 (49–59) | 54 (49–59) | 56 (50–60) | 0.18 |
| Sex, Female | 354 (69.0) | 1170 (70.7) | 5547 (52.0) | 132 (41.6) | <0.001 |
| African American | 156 (30.4) | 428 (25.9) | 2714 (25.5) | 54 (17.0) | 0.005 |
| CHD | 30 (5.8) | 75 (4.5) | 393 (3.7) | 21 (6.6) | 0.15 |
| Carotid plaque* | 194 (48.5) | 488 (38.8) | 2767 (33.4) | 79 (34) | <0.001 |
| Hypertension | 228 (44.4) | 581 (35.1) | 3317 (31.1) | 93 (29.3) | <0.001 |
| Diabetes mellitus | 74 (14.4) | 187 (11.3) | 1079 (10.2) | 38 (10.0) | 0.025 |
| Obese | 142 (27.7) | 493 (29.8) | 2688 (25.2) | 88 (27.8) | 0.001 |
| CKD | 31 (6.0) | 52 (3.1) | 251 (2.4) | 12 (3.8) | <0.001 |
| Claudication | 26 (5.1) | 12 (0.7) | 51 (0.5) | 2 (0.6) | <0.001 |
| Smoking, ever | 336 (65.5) | 963 (58.2) | 6149 (57.7) | 176 (55.5) | 0.020 |
| Alcohol, current | 263 (51.3) | 923 (55.7) | 6147 (57.6) | 168 (53.0) | 0.059 |
| BMI, kg/m2 | 26.5 (23.6–30.5) | 26.8 (23.8–31.0) | 26.7 (23.9–30.0) | 27.8 (25.3–31.0) | 0.84 |
| SBP, mmHg | 122 (110–136) | 120 (108–133) | 118 (108–130) | 117 (108–127) | <0.001 |
| DBP, mmHg | 71 (64–79) | 72 (65–80) | 73 (66–80) | 72 (65–78) | 0.020 |
| MAP, mmHg | 88 (81–97) | 88 (80–97) | 88 (81–96) | 87 (80–94) | 0.19 |
| PP, mmHg | 50 (41–62) | 47 (39–57) | 44 (38–53) | 45 (37–53) | <0.001 |
| Heart Rate, bpm | 68 (61–75) | 68 (61–75) | 65 (59–72) | 63 (58–70) | <0.001 |
| LVH (Cornell) | 18 (3.5) | 30 (1.8) | 204 (1.9) | 7 (2.2) | 0.37 |
| QRS dur, msec | 94 (88–102) | 95 (89–101) | 96 (90–103) | 99 (92–105) | <0.001 |
| Hgb, g/dL | 13.7 (12.8–14.5) | 13.7 (12.8–14.6) | 13.9 (12.9–14.9) | 14.2 (13.2–15.1) | <0.001 |
| WBC | 6.3 (5.1–7.7) | 6.0 (5.0–7.3) | 5.7 (4.8–6.9) | 5.7 (4.7–7.0) | <0.001 |
| Glucose, mg/dL | 100 (93–109) | 99 (93–107) | 99 (92–107) | 98 (93–107) | 0.12 |
| eGFR | 88 (76–102) | 88 (78–102) | 89 (79–102) | 87 (77–100) | 0.47 |
| LDL-c, mg/dL | 143 (120–172) | 135 (112–162) | 135 (111–160) | 135 (109–164) | 0.001 |
| HDL-c, mg/dL | 50 (39–63) | 51 (40–64) | 49 (39–61) | 46 (38–57) | <0.001 |
| Trig, mg/dL | 117 (82–164) | 108 (79–151) | 107 (77–151) | 116 (81–169) | 0.11 |
Values presented are counts (percentages) or median (interquartile range) for categorical and continuous data, respectively. CHD = coronary heart disease; Obese defined as body mass index (BMI) ≥ 30 kg/m2; CKD = chronic kidney disease, defined as a eGFR <60 ml/min/1.73m2; SBP = systolic blood pressure; DBP = diastolic blood pressure; MAP = mean arterial pressure; PP = pulse pressure; LVH = left ventricular hypertrophy; Hgb = hemoglobin; WBC = white blood cell count; eGFR = estimated glomerular filtration rate (ml/min/1.73m2); LDL-c = low density lipoprotein cholesterol; HDL-c = high density lipoprotein cholesterol; Trig = triglycerides.
Carotid ultrasound to identify atherosclerotic plaque was available in 10,183 participants.
Incidence Rates
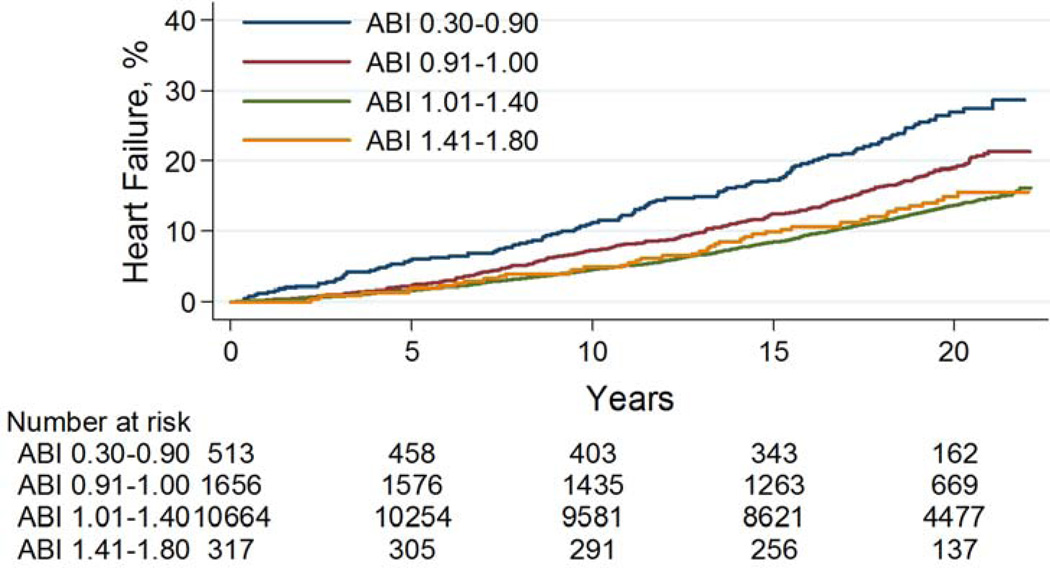
Over a mean 17.7 years of follow up, 1809 incident HF events occurred and there was a gradient in frequency according to ABI such that 23%, 18%, 13%, and 14% of participants with an ABI ≤0.90, 0.91–1.00, 1.01–1.40, and >1.40 developed HF, respectively (Table 2). As compared to an ABI of 1.01–1.40, ABIs ≤ 0.90 or 0.91–1.00 were both significantly associated with a higher rate of incident HF (log rank p<0.0001), while an ABI >1.40 was not (log rank p=0.62) (Figure 1A).
Table 2.
Risk of incident heart failure according to ankle brachial index in the ARIC study, 1987–2008.
| Ankle Brachial Index Category | ||||
|---|---|---|---|---|
| ≤ 0.90 | 0.91–1.00 | 1.01–1.40 | >1.40 | |
| Heart Failure, n (%) | 119 (23.2) | 294 (17.8) | 1352 (12.7) | 44 (13.9) |
| *Incidence rate (95%CI) | 1.48 (1.24–1.77)† | 1.03 (0.92–1.15)† | 0.71 (0.67–0.75) | 0.77 (0.57–1.03)‡ |
| Model 1 | 2.16 (1.79–2.61) | 1.46 (1.29–1.66) | Ref | 1.08 (0.80–1.46) |
| Model 2 | 1.59 (1.32–1.93) | 1.42 (1.25–1.61) | Ref | 0.92 (0.68–1.25) |
| Model 2a | 1.40 (1.12–1.75) | 1.36 (1.16–1.58) | Ref | 0.90 (0.62–1.30) |
| Model 3 | 1.55 (1.28–1.88) | 1.43 (1.26–1.63) | Ref | 0.94 (0.70–1.27) |
| Model 3a | 1.40 (1.12–1.74) | 1.36 (1.17–1.59) | Ref | 0.95 (0.66–1.38) |
| Model 4 | 1.52 (1.26–1.84) | 1.41 (1.24–1.60) | Ref | 0.92 (0.68–1.24) |
| Model 4a | 1.38 (1.10–1.72) | 1.35 (1.16–1.57) | Ref | 0.90 (0.62–1.30) |
| Model 5 | 1.57 (1.22–2.01) | 1.43 (1.22–1.69) | Ref | 0.79 (0.52–1.18) |
| Model 5a | 1.35 (1.01–1.81) | 1.38 (1.14–1.68) | Ref | 0.87 (0.54–1.40) |
Incidence rate = number per 100 person years.
Reference ABI 1.01–1.40:
log rank p < 0.0001.
log rank p = 0.62.
Values from models are hazard ratios (95% confidence intervals)
Model 1 = unadjusted.
Model 2 = adjusted for age, sex, race, center, prevalent coronary heart disease, anti-hypertensive medication use, systolic blood pressure, diabetes mellitus, body mass index, smoking status, and heart rate.
Model 2a = model 2 + carotid atherosclerosis (n=10,183)
Model 3 = model 2 + interim myocardial infarction as a time varying covariate.
Model 3a = model 3 + carotid atherosclerosis (n=10,183)
Model 4 = model 2 + interim coronary heart disease as a time varying covariate.
Model 4a = model 4 + carotid atherosclerosis (n=10,183)
Model 5 = model 2 after excluding participants with prevalent coronary heart disease or interim myocardial infarction (n=11,558).
Model 5a = model 5 + carotid atherosclerosis (n=9,007).
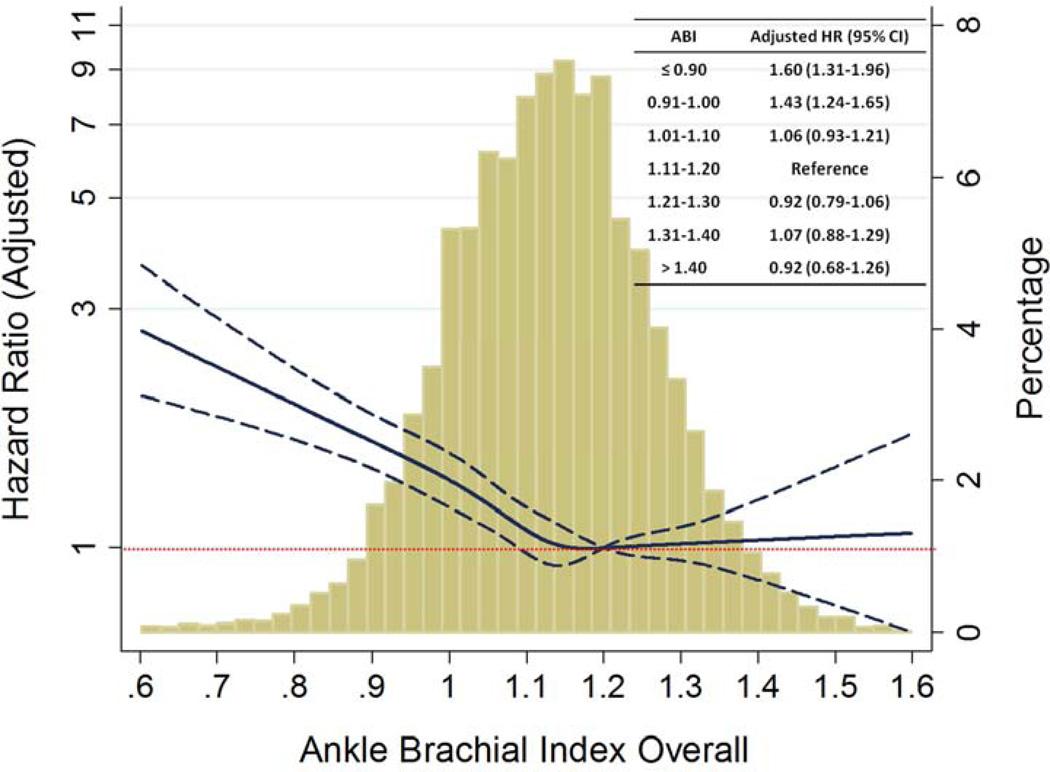
Figure 1. Incident heart failure according to categories of ankle brachial index in the ARIC study.
A: Cumulative incidence plot for heart failure according to ankle brachial index.
B: Risk of incident heart failure according to ABI as a continuous variable in the ARIC study.
ABI of 1.2 was reference value for restricted cubic spline analysis. Solid blue line indicates hazard ratio, with dashed lines indicating 95% confidence interval.
Hazard ratio adjusted for age, sex, race, center, coronary heart disease, anti-hypertensive medication use, systolic blood pressure, diabetes mellitus, body mass index, smoking status, and heart rate. Inset table shows adjusted hazard ratios for categories of ABI as compared to an ABI of 1.11–1.20.
Risk for Heart Failure
In unadjusted Cox proportional hazards analysis, an ABI ≤0.90 was significantly associated with more than twice the risk of incident HF as compared to an ABI of 1.01–1.40 (Table 2). Those with an ABI 0.91–1.00 were also at significantly increased risk of HF; however, an ABI >1.40 was not associated with an increased risk of HF. In multivariate analysis, adjusted for traditional HF risk factors,(24) including prevalent CHD, the risk of incident HF remained significant, with a 59% and 42% increased risk of HF, in those with ABI ≤ 0.90 and ABI 0.91–1.00, respectively (Table 2, Model 2).
Role of atherosclerosis and myocardial infarction
As a low ABI can be a marker of atherosclerosis, we also adjusted for the presence of subclinical carotid atherosclerotic plaque and found that both an ABI ≤0.90 and ABI of 0.91–1.00 remained significantly associated with 40% and 36% increased risks for incident HF, respectively, as compared with an ABI of 1.01–1.40 (Table 2, Model 2a). In addition, as MI is a strong risk factor for the development of HF, we adjusted for interim MI as a time varying covariate and found that an ABI ≤0.90 remained significantly associated with an increased risk for incident HF (HR 1.40, 95%CI 1.12–1.74), compared with an ABI of 1.01–1.40 (Table 2, Model 3a). Similarly, an ABI of 0.91–1.00 was significantly associated with an increased risk of incident HF (HR 1.36, 95%CI 1.17–1.59). We performed additional sensitivity analyses to corroborate that the relationship between low ABI and incident HF was independent of carotid plaque and overt atherosclerotic epicardial CHD. With 1) inclusion of the presence of carotid plaque as well as interim CHD as a time varying covariate (Table 2, Model 4a) or 2) exclusion of participants with prevalent CHD and those that developed a MI in follow-up (Table 2, Model 5a), ABIs <1.00 remained significantly associated with an increased risk of HF, as compared to an ABI of 1.01–1.40.
When examined as a continuous variable in restricted cubic spline analysis, there was a significant relationship between lower ABI and incident HF (Figure 1B). The gradient of risk for HF, even when adjusted for conventional HF risk factors, appeared to extend above an ABI of 1.00, when compared to a reference ABI value of 1.20. We therefore repeated analyses with further subdivision of the ABI 1.00–1.40 category into 4 groups by increments of 0.10. An ABI of 1.11–1.20 was most common and has been previously utilized by the ABI Collaborative group as a reference range (6). We compared the risk of incident HF across the spectrum of ABI against an ABI of 1.11–1.20 and again found a significant association with incident HF only at an ABI ≤1.00 (Figure 1B inset).
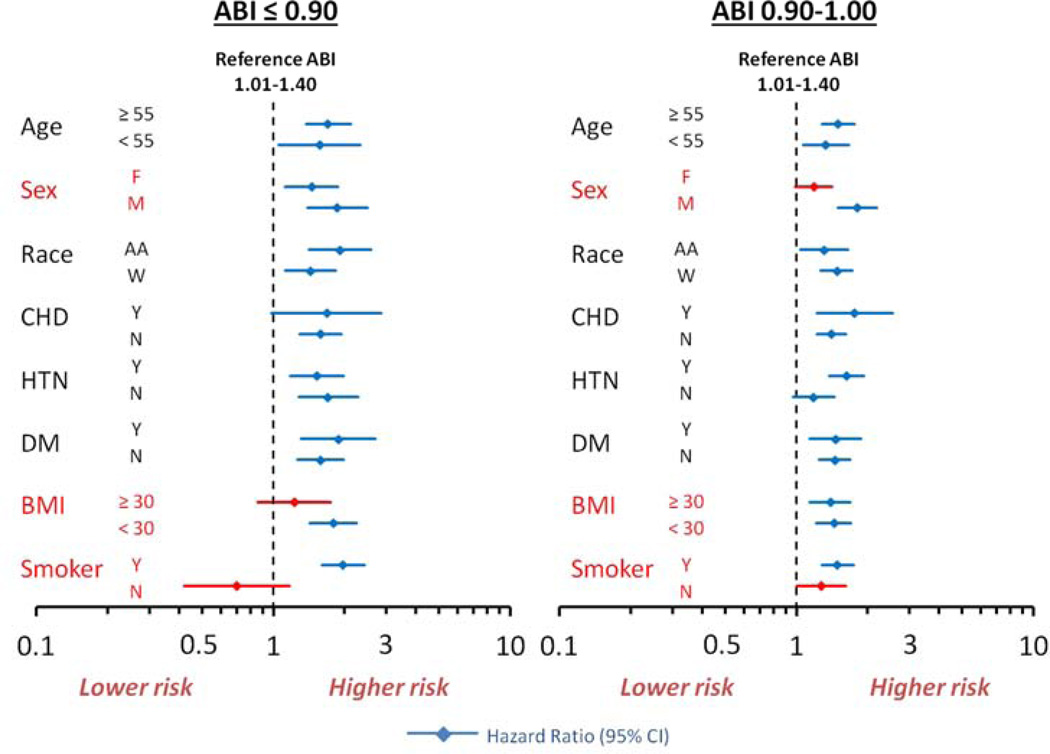
Subgroup Analyses
The relationship between ABI and incident HF was also assessed within subgroups of participants with or without risk factors for HF. There were significant interactions by sex, obesity, and smoking, but not by age, race, prevalent CHD, hypertension, or diabetes mellitus (Figure 2). Among men, the risk for incident HF was significantly elevated at an ABI ≤ 1.00, while for women, this risk was only significant for an ABI ≤ 0.90. Among those with obesity, an ABI ≤ 0.90 was not statistically associated with an increased risk of HF, while among non-obese participants an ABI ≤ 0.90 was associated with an increased risk for incident HF. Similarly, among ever smokers, an ABI ≤ 0.90 was significantly associated with an increased risk of HF, while this was not the case among never smokers (Figure 2). Never smokers with an ABI ≤0.90 were more commonly female, obese, with less CHD, and a trend towards less frequent diabetes mellitus. They were also younger, with slightly higher ABI, and HDL-c, and lower LDL-c, suggesting they were a healthier population with fewer HF risk factors as compared to ever smokers with a low ABI (data not shown).
Figure 2. Risk of incident heart failure according to ABI and risk factors for heart failure.
Reference ABI for hazard ratios = 1.01–1.40. CHD = coronary heart disease, HTN = hypertension, DM = diabetes mellitus, BMI = body mass index. Hazard ratios adjusted for age, sex, race, center, coronary heart disease, anti-hypertensive medication use, systolic blood pressure, diabetes mellitus, body mass index, smoking status, and heart rate. Significant interactions are shown in red: sex (p <0.001), BMI (p = 0.001), and smoker (p <0.001).
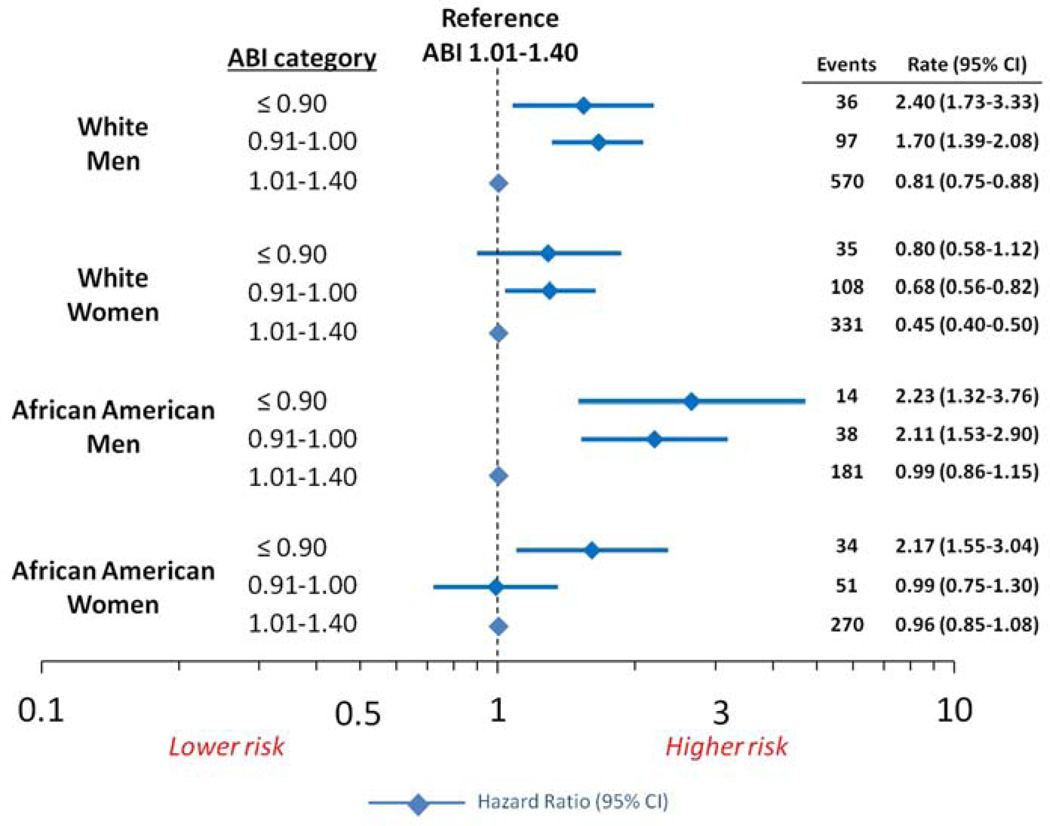
Female gender and African-American race were more common among those with the lowest ABI, therefore we evaluated for effect modification on the relationship between ABI and incident HF by sex and race together (p-interaction = 0.059) (Figure 3). Regardless of race, an ABI ≤1.00 in men was significantly associated with an increased risk for HF, as compared to those with an ABI between 1.01–1.40. Similarly, in Caucasian women, an ABI ≤1.00 was associated with an elevated risk for HF. However, in African American women, an ABI ≤0.90 conferred an increased risk for HF, while an ABI 0.91–1.00 did not. Notably, the HF risk factor profile in African American women with an ABI between 0.90–1.00 was more similar to those with an ABI of 1.00–1.40, than it was to those with an ABI ≤ 0.90 (data not shown).
Figure 3. Forest Plot of risk of incident heart failure along with incidence rates per 100 person years according to ankle brachial index stratified by race and gender in the ARIC study.
Within each race gender category, the reference ABI for hazard ratios was 1.01–1.40. Hazard ratios adjusted for age, center, coronary heart disease, anti-hypertensive medication use, systolic blood pressure, diabetes mellitus, body mass index, smoking status, and heart rate. Interaction for race×sex, p = 0.059. Rate is per 100 person years.
Population attributable risk
The multivariate adjusted (model 2a) PAR associated with an ABI ≤ 1.00 was 6.0% (95% CI 3.8–8.2%), while the population risk attributable to a history of CAD was 7.8% (95% CI 7.2–8.5%). Hypertension and diabetes mellitus accounted for an estimated 15% and 14% of heart failure events, respectively (data not shown).
Discussion
In a community cohort of middle-aged Americans with up to 22 years of follow up, we found that a low ABI (≤ 1.00) from a single randomly chosen lower extremity was significantly associated with an increased risk for the development of HF. As compared to an ABI of 1.01–1.40, an ABI ≤0.90 was associated with a 40% increased risk (HR 1.40, 95%CI 1.12–1.74), while an ABI of 0.91–1.00 was also significantly associated with an increased risk of HF (HR 1.36, 95%CI 1.17–1.59). The relationship between low ABI and incident HF was independent of traditional HF risk factors, as well as prevalent CHD, subclinical carotid atherosclerosis, and interim MI or CHD. These findings demonstrate that among middle-aged Americans a low ABI is independently associated with an increased risk for HF.
Few studies have evaluated the association between ABI and incident HF (8,13). Most literature involving ABI focuses on atherosclerotic cardiovascular disease as the ABI is a simple non-invasive tool for the diagnosis of PAD among symptomatic patients and it has previously been suggested that an ABI ≤ 0.90 is a marker of generalized atherosclerosis (1,6–12,25). Therefore, it is presumed that a low ABI reflects co-existent atherosclerosis in the epicardial coronary arteries, risk for MI, and subsequent HF. This is supported by the HOPE trial, which enrolled participants with known cardiovascular disease or those at high CV risk, and demonstrated that a low ABI was associated with an increased rate of HF (13). Furthermore, small single center studies of symptomatic PAD patients referred for ABI measurement demonstrated that approximately 1/3 of patients with an ABI <0.90 have concomitant left ventricular dysfunction (26,27). While these patients with symptomatic PAD likely had subclinical or unrecognized atherosclerotic CHD contributing to LV dysfunction, the relationship between ABI and LVEF may also be independent of prevalent CHD (27).
The Cardiovascular Health Study demonstrated that an ABI < 0.90 was independently associated with an increased risk of HF among participants without prevalent CHD (8). It could be argued that these people had subclinical CHD (28) and were at high risk for the progression of CAD and subsequent HF, particularly as no adjustment was made for interim MI. However, we found that the relationship between low ABI and HF was independent of baseline CHD, subclinical carotid atherosclerosis, and interim MI or CHD. Additionally, there was no significant interaction between ABI and prevalent CHD on incident HF, indicating that even among participants without CHD a low ABI conferred an increased risk for the development of HF. Together, these findings suggest that a low ABI may not only reflect overt atherosclerosis, but also pathologic processes in the development of HF beyond epicardial atherosclerotic disease and MI alone.
Mechanistically, a low ABI may be a marker for abnormal vascular stiffness and atherosclerotic microvascular dysfunction which may contribute to the pathogenesis of HF even in the absence of clinically apparent epicardial CHD (29). In this study, pulse pressure, which is a validated measure of vascular stiffness (30), was widest among those with low ABI. Additionally, there was a significant interaction according to smoking status, such that the significant association with an increased risk of HF with ABI ≤ 1.0 was observed only in smokers. While smoking is a risk factor for atherosclerotic disease, smoking also increases arterial stiffness even in the absence of obstructive atherosclerotic disease (31,32). Vascular stiffness raises afterload resulting in increased myocardial wall stress and oxygen consumption, while a widened pulse pressure may impair coronary blood flow, all of which may contribute to ischemia even in the absence of evident obstructive epicardial coronary artery disease (33). Moreover, in the Multi Ethnic Study of Atherosclerosis (MESA), a low ABI was associated with non-compliant small vessels, which in turn, has been associated with an increased risk for adverse cardiovascular events, including HF (34,35). Low ABI and peripheral arterial disease have also been associated with functional decline and exercise intolerance, which may also increase the risk for the development of HF (36–39). However, further studies are needed to clarify the pathophysiologic mechanisms relating low ABI to HF risk.
The prognostic relationship between an ABI ≤ 1.00 and incident HF is non-trivial as approximately 1 of 6 participants in ARIC had a low ABI, which is comparable to other population based studies (range 13–18%) (6,11,40). Furthermore, we found that the risk for HF attributable to an ABI ≤ 1.00 was approximately 6.0%, which is lower than that of hypertension or diabetes, but similar to that of CAD (7.8%). Moreover, participants with lower ABI were more commonly female and African American (41–43); however, the prevalence and incidence of atherosclerotic cardiovascular disease tends to be lower in women (44,45). The pathogenesis of HF in women and African Americans may be less related to epicardial CAD and more attributable to endothelial and metabolic derangements leading to microvascular dysfunction and possibly heart failure with preserved ejection fraction (HFpEF) (44,46–49). Moreover, African Americans, despite a higher prevalence of atherosclerotic risk factors, may have a lower prevalence of CHD and perhaps a higher prevalence of HFpEF (50,51).
In this analysis, race and sex together modified the relationship between ABI and incident HF, such that among African American women, only an ABI ≤0.90 was associated with an elevated risk. However, this does not mitigate the risk of HF in African American women with an ABI ≥0.90 as it has been previously demonstrated in ARIC that the rate of incident HF in African American women was greater than that of Caucasian women (21). Similarly, men were at greater risk for HF than women across all ABI categories and, regardless of race, in men the risk of incident HF was significantly elevated with an ABI ≤1.00. These findings are congruent with previous reports that African-Americans and men are at greater risk for incident HF than Caucasians and women, respectively (21,46).
While we evaluated a large bi-racial community cohort over a long follow-up, limitations should be noted. ABI was calculated from oscillometric, rather than currently recommended Doppler BP; although the oscillometric device has been previously validated (8,13,42). Ankle BP was measured in one randomly chosen extremity which may have led to underestimation of the prevalence of low ABI. Incident HF was determined from hospitalization and death certificate ICD-9 codes, although these HF codes have been previously demonstrated to have high validity in ARIC (21,52). The observed HF incidence rates may not be directly applicable to a contemporary time period due to temporal changes in the management of cardiovascular disease and its risk factors. We did not find an increased risk of HF among participants with an ABI >1.40, although the relatively low numbers of participants with high ABI (2.4%) and incident HF may limit statistical power in this group. Finally, future studies are needed to clarify the pathophysiologic mechanisms and clinical implications of low ABI to HF risk.
Conclusions
In a community based bi-racial cohort of middle aged Americans, an ABI ≤ 1.00 was significantly associated with an increased risk of HF independent of traditional HF risk factors, prevalent CHD, carotid atherosclerosis, and interim MI. Low ABI may not only reflect overt atherosclerosis, but also pathologic processes in the development of HF beyond epicardial atherosclerotic disease and MI alone. A low ABI, as a simple non-invasive measure, may be a risk marker for HF.
Acknowledgements
The authors thank the staff and participants of the ARIC study for their important contributions.
Financial Support: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Support was also provided by the National Heart, Lung, and Blood Institute training grant (T32 HL094301-02). There are no relationships with industry related to this manuscript.
Abbreviations
- ABI
ankle brachial index
- PAD
peripheral arterial disease
- CHD
coronary heart disease
- HF
heart failure
- CHS
Cardiovascular Health Study
- HOPE
Heart Outcomes Prevention Evaluation
- ARIC
Atherosclerosis Risk in Communities
- BP
Blood pressure
- CKD
chronic kidney disease
- MI
myocardial infarction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carter SA. Clinical measurement of systolic pressures in limbs with arterial occlusive disease. JAMA. 1969;207:1869–1874. [PubMed] [Google Scholar]
- 2.McKenna M, Wolfson S, Kuller L. The ratio of ankle and arm arterial pressure as an independent predictor of mortality. Atherosclerosis. 1991;87:119–128. doi: 10.1016/0021-9150(91)90014-t. [DOI] [PubMed] [Google Scholar]
- 3.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 4.Vogt MT, Cauley JA, Newman AB, Kuller LH, Hulley SB. Decreased ankle/arm blood pressure index and mortality in elderly women. JAMA. 1993;270:465–469. [PubMed] [Google Scholar]
- 5.Feringa HH, Bax JJ, van Waning VH, et al. The long-term prognostic value of the resting and postexercise ankle-brachial index. Arch Intern Med. 2006;166:529–535. doi: 10.1001/archinte.166.5.529. [DOI] [PubMed] [Google Scholar]
- 6.Fowkes FG, Murray GD, Butcher I, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leng GC, Fowkes FG, Lee AJ, Dunbar J, Housley E, Ruckley CV. Use of ankle brachial pressure index to predict cardiovascular events and death: a cohort study. BMJ. 1996;313:1440–1444. doi: 10.1136/bmj.313.7070.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newman AB, Shemanski L, Manolio TA, et al. Ankle-arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group. Arterioscler Thromb Vasc Biol. 1999;19:538–545. doi: 10.1161/01.atv.19.3.538. [DOI] [PubMed] [Google Scholar]
- 9.Aronow WS, Ahmed MI, Ekundayo OJ, Allman RM, Ahmed A. A propensity-matched study of the association of peripheral arterial disease with cardiovascular outcomes in community-dwelling older adults. Am J Cardiol. 2009;103:130–135. doi: 10.1016/j.amjcard.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weatherley BD, Nelson JJ, Heiss G, et al. The association of the ankle-brachial index with incident coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) study, 1987–2001. BMC Cardiovasc Disord. 2007;7:3. doi: 10.1186/1471-2261-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Criqui MH, McClelland RL, McDermott MM, et al. The ankle-brachial index and incident cardiovascular events in the MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2010;56:1506–1512. doi: 10.1016/j.jacc.2010.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng ZJ, Sharrett AR, Chambless LE, et al. Associations of ankle-brachial index with clinical coronary heart disease, stroke and preclinical carotid and popliteal atherosclerosis: the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 1997;131:115–125. doi: 10.1016/s0021-9150(97)06089-9. [DOI] [PubMed] [Google Scholar]
- 13.Ostergren J, Sleight P, Dagenais G, et al. Impact of ramipril in patients with evidence of clinical or subclinical peripheral arterial disease. Eur Heart J. 2004;25:17–24. doi: 10.1016/j.ehj.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 14.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 15.Tsai AW, Folsom AR, Rosamond WD, Jones DW. Ankle-brachial index and 7-year ischemic stroke incidence: the ARIC study. Stroke. 2001;32:1721–1724. doi: 10.1161/01.str.32.8.1721. [DOI] [PubMed] [Google Scholar]
- 16.Folsom AR, Yamagishi K, Hozawa A, Chambless LE. Absolute and attributable risks of heart failure incidence in relation to optimal risk factors. Circ Heart Fail. 2009;2:11–17. doi: 10.1161/CIRCHEARTFAILURE.108.794933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rose GA. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull World Health Organ. 1962;27:645–658. [PMC free article] [PubMed] [Google Scholar]
- 18.Kottgen A, Russell SD, Loehr LR, et al. Reduced kidney function as a risk factor for incident heart failure: the atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol. 2007;18:1307–1315. doi: 10.1681/ASN.2006101159. [DOI] [PubMed] [Google Scholar]
- 19.Folsom AR, Peacock JM, Boerwinkle E. Variation in PCSK9, low LDL cholesterol, and risk of peripheral arterial disease. Atherosclerosis. 2009;202:211–215. doi: 10.1016/j.atherosclerosis.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li R, Duncan BB, Metcalf PA, et al. B-mode-detected carotid artery plaque in a general population. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Stroke. 1994;25:2377–2383. doi: 10.1161/01.str.25.12.2377. [DOI] [PubMed] [Google Scholar]
- 21.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study) Am J Cardiol. 2008;101:1016–1022. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 22.Wilhelmsen L, Eriksson H, Svardsudd K, Caidahl K. Improving the detection and diagnosis of congestive heart failure. Eur Heart J. 1989;10(Suppl C):13–18. doi: 10.1093/eurheartj/10.suppl_c.13. [DOI] [PubMed] [Google Scholar]
- 23.Rooke TW, Hirsch AT, Misra S, et al. 2011 ACCF/AHA Focused Update of the Guideline for the Management of Patients With Peripheral Artery Disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58:2020–2045. doi: 10.1016/j.jacc.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agarwal SK, Chambless LE, Ballantyne CM, et al. Prediction of incident heart failure in general practice: the Atherosclerosis Risk in Communities (ARIC) Study. Circ Heart Fail. 2012;5:422–429. doi: 10.1161/CIRCHEARTFAILURE.111.964841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDermott MM, Liu K, Criqui MH, et al. Ankle-brachial index and subclinical cardiac and carotid disease: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2005;162:33–41. doi: 10.1093/aje/kwi167. [DOI] [PubMed] [Google Scholar]
- 26.Ward RP, Goonewardena SN, Lammertin G, Lang RM. Comparison of the frequency of abnormal cardiac findings by echocardiography in patients with and without peripheral arterial disease. Am J Cardiol. 2007;99:499–503. doi: 10.1016/j.amjcard.2006.09.102. [DOI] [PubMed] [Google Scholar]
- 27.Rizvi S, Kamran H, Salciccioli L, Saiful F, Lafferty J, Lazar JM. Relation of the ankle brachial index to left ventricular ejection fraction. Am J Cardiol. 2010;105:129–132. doi: 10.1016/j.amjcard.2009.08.664. [DOI] [PubMed] [Google Scholar]
- 28.Aboyans V, McClelland RL, Allison MA, et al. Lower extremity peripheral artery disease in the absence of traditional risk factors. The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2011;214:169–173. doi: 10.1016/j.atherosclerosis.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheung N, Sharrett AR, Klein R, et al. Aortic distensibility and retinal arteriolar narrowing: the multi-ethnic study of atherosclerosis. Hypertension. 2007;50:617–622. doi: 10.1161/HYPERTENSIONAHA.107.091926. [DOI] [PubMed] [Google Scholar]
- 30.Chae CU, Pfeffer MA, Glynn RJ, Mitchell GF, Taylor JO, Hennekens CH. Increased pulse pressure and risk of heart failure in the elderly. JAMA. 1999;281:634–639. doi: 10.1001/jama.281.7.634. [DOI] [PubMed] [Google Scholar]
- 31.Tomiyama H, Hashimoto H, Tanaka H, et al. Continuous smoking and progression of arterial stiffening: a prospective study. J Am Coll Cardiol. 2010;55:1979–1987. doi: 10.1016/j.jacc.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 32.Mancini M, Di Donato O, Saldalamacchia G, Liuzzi R, Rivellese A, Salvatore M. Contrast-enhanced ultrasound evaluation of peripheral microcirculation in diabetic patients: effects of cigarette smoking. Radiol Med. 2013;118:206–214. doi: 10.1007/s11547-012-0830-x. [DOI] [PubMed] [Google Scholar]
- 33.Vaccarino V, Holford TR, Krumholz HM. Pulse pressure and risk for myocardial infarction and heart failure in the elderly. J Am Coll Cardiol. 2000;36:130–138. doi: 10.1016/s0735-1097(00)00687-2. [DOI] [PubMed] [Google Scholar]
- 34.Wilkins JT, McDermott MM, Liu K, Chan C, Criqui MH, Lloyd-Jones DM. Associations of noninvasive measures of arterial compliance and ankle-brachial index: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Hypertens. 2012;25:535–541. doi: 10.1038/ajh.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duprez DA, Jacobs DR, Jr, Lutsey PL, et al. Association of small artery elasticity with incident cardiovascular disease in older adults: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2011;174:528–536. doi: 10.1093/aje/kwr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDermott MM, Ferrucci L, Liu K, et al. Women with peripheral arterial disease experience faster functional decline than men with peripheral arterial disease. J Am Coll Cardiol. 2011;57:707–714. doi: 10.1016/j.jacc.2010.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDermott MM, Liu K, Ferrucci L, et al. Decline in functional performance predicts later increased mobility loss and mortality in peripheral arterial disease. J Am Coll Cardiol. 2011;57:962–970. doi: 10.1016/j.jacc.2010.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kraigher-Krainer E, Lyass A, Massaro JM, et al. Association of physical activity and heart failure with preserved vs. reduced ejection fraction in the elderly: the Framingham Heart Study. Eur J Heart Fail. 2013 doi: 10.1093/eurjhf/hft025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu G, Jousilahti P, Antikainen R, Katzmarzyk PT, Tuomilehto J. Joint effects of physical activity, body mass index, waist circumference, and waist-to-hip ratio on the risk of heart failure. Circulation. 2010;121:237–244. doi: 10.1161/CIRCULATIONAHA.109.887893. [DOI] [PubMed] [Google Scholar]
- 40.Cimminiello C, Kownator S, Wautrecht JC, et al. The PANDORA study: peripheral arterial disease in patients with non-high cardiovascular risk. Intern Emerg Med. 2011;6:509–519. doi: 10.1007/s11739-011-0511-0. [DOI] [PubMed] [Google Scholar]
- 41.Aboyans V, Criqui MH, McClelland RL, et al. Intrinsic contribution of gender and ethnicity to normal ankle-brachial index values: the Multi-Ethnic Study of Atherosclerosis (MESA) J Vasc Surg. 2007;45:319–327. doi: 10.1016/j.jvs.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 42.Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890–2909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 43.Criqui MH, Vargas V, Denenberg JO, et al. Ethnicity and peripheral arterial disease: the San Diego Population Study. Circulation. 2005;112:2703–2707. doi: 10.1161/CIRCULATIONAHA.105.546507. [DOI] [PubMed] [Google Scholar]
- 44.Dunlay SM, Weston SA, Jacobsen SJ, Roger VL. Risk factors for heart failure: a population-based case-control study. Am J Med. 2009;122:1023–1028. doi: 10.1016/j.amjmed.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Safford MM, Brown TM, Muntner PM, et al. Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308:1768–1774. doi: 10.1001/jama.2012.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168:2138–2145. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shah S. Review: Heart failure with preserved ejection fraction in African Americans. Ethn Dis. 2012;22:432–438. [PubMed] [Google Scholar]
- 48.Gardner AW, Montgomery PS, Blevins SM, Parker DE. Gender and ethnic differences in arterial compliance in patients with intermittent claudication. J Vasc Surg. 2010;51:610–615. doi: 10.1016/j.jvs.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richey Sharrett A, Coady SA, Folsom AR, Couper DJ, Heiss G. Smoking and diabetes differ in their associations with subclinical atherosclerosis and coronary heart disease-the ARIC Study. Atherosclerosis. 2004;172:143–149. doi: 10.1016/j.atherosclerosis.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 50.Kamath SA, Drazner MH, Wynne J, Fonarow GC, Yancy CW. Characteristics and outcomes in African American patients with decompensated heart failure. Arch Intern Med. 2008;168:1152–1158. doi: 10.1001/archinte.168.11.1152. [DOI] [PubMed] [Google Scholar]
- 51.Gupta DK, Shah AM, Castagno D, et al. Heart Failure With Preserved Ejection Fraction in African Americans: The ARIC (Atherosclerosis Risk in Communities) Study. JACC: Heart Failure. 2013;1:8. doi: 10.1016/j.jchf.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Avery CL, Mills KT, Chambless LE, et al. Long-term association between self-reported signs and symptoms and heart failure hospitalizations: the Atherosclerosis Risk In Communities (ARIC) Study. Eur J Heart Fail. 2010;12:232–238. doi: 10.1093/eurjhf/hfp203. [DOI] [PMC free article] [PubMed] [Google Scholar]