Abstract
Background
In animal studies, organochlorine pesticide (OCP) exposure alters pubertal development, however, epidemiological data are limited and inconsistent.
Objective
To evaluate the associations of serum OCP concentrations [hexachlorobenzene (HCB), β-hexachlorocylohexane (β-HCH), and p,p′-dichlorodiphenyldichloroethylene (p,p′-DDE)] with male pubertal onset.
Methods
In Chapaevsk, Russia, a town environmentally contaminated with OCPs, 350 8–9 year old boys with measured OCPs were enrolled during 2003–2005 and were followed annually for eight years. We evaluated three measures of pubertal onset: testicular volume (TV) > 3 mL in either testis, or stage 2 or greater for genitalia (G2+), or pubic hair (P2+). We used multivariable interval-censored models to evaluate associations of OCPs (quartiles) with physician-assessed pubertal onset.
Results
In adjusted models, boys with higher HCB concentrations had later mean ages of TV > 3 mL and P2+ (but not G2+). Mean age at attaining TV > 3 mL was delayed 3.6 (95% CI: −2.6, 9.7), 7.9 (95% CI: 1.7, 14.0), and 4.7 months (95% CI: −1.4, 10.9) for HCB Q2, Q3, and Q4, respectively, compared to Q1 (trend p: 0.06). Boys with higher HCB concentrations reached P2+ 0.1 months earlier (95% CI: −5.8, 5.6) for Q2, 4.7 months later (95% CI: −1.0, 10.3) for Q3 and 4.6 months later (95% CI: −1.1, 10.3) for Q4 compared to Q1 (trend p: 0.04). There were no associations of serum β-HCH and p,p′-DDE concentrations with age of pubertal onset.
Conclusion
Higher prepubertal serum HCB concentrations were associated with later age of gonadarche and pubarche.
Keywords: β-HCH; HCB; organochlorine pesticides; male puberty; p,p′-DDE
1. INTRODUCTION
Puberty is a dynamic process, characterized by rapid hormonal, physiologic, and behavioral changes including secondary sexual maturation and acceleration in linear and muscle mass growth (Giedd et al., 2006; Tanner and Whitehouse, 1976). Two hormonally distinct processes are involved in puberty: the maturation of the hypothalamic pituitary gonadal (HPG) system (“gonadarche”) and adrenarche (Havelock et al., 2004; Kronenberg et al., 2008). These two pathways appear to be discretely regulated and activated by different cues (Havelock et al., 2004; Kronenberg et al., 2008). While androgens produced by either the adrenals or the testes can cause virilization of the genitalia and pubic hair, testicular enlargement indicates HPG activation. Pubic hair growth (“pubarche”) is typically associated with adrenarche (Havelock et al., 2004). A number of factors including body composition, diet, and environmental exposures may interfere with gonadarche or pubarche (Golub et al., 2008; Kaplowitz 2008; Mustanski et al., 2004).
Environmental chemicals that may affect male puberty include organochlorine pesticides (OCPs) such as hexachlorobenzene (HCB), β-hexachlorocyclohexane (β-HCH), and 1,1,1,-trichloro-2,2,bis(p-chlorophenyl)ethane (DDT), which were historically used as insecticides and fungicides (Barber et al., 2005; Breivick et al., 1999; Jaga and Dharmani, 2003). HCB and β-HCH can also be unintentional by-products of chlorinated chemicals manufacturing (Courtney 1979; Jung et al., 1997). These lipophilic compounds as well as DDT’s primary metabolite, p,p′-dichlorodiphenyldichloroethylene (p,p′-DDE), are highly stable, environmentally persistent, have long biological half-lives, and bioaccumulate through the food chain (Barber et al., 2005; Breivick et al., 1999; Jaga and Dharmani, 2003; Jung et al., 1997).
In rodents, fetal and peripubertal (post-natal day 21) p,p′-DDE exposure delayed male preputial separation (PPS) (Kelce et al., 1995), a marker of male puberty, and caused other male reproductive abnormalities (Ashby and Lefevre, 2000; Fry and Toone, 1981; Gray et al., 2001; Guillette et al., 1994; Kelce et al., 1995; Quinn et al., 2008). In contrast, in utero or post-natal HCB and β-HCH exposure caused reproductive and developmental abnormalities but did not affect the timing of PPS (Arnold et al., 1985; Courtney 1979; Simon et al., 1979; Van Velsen et al., 1986). Epidemiologic evidence on the associations of OCPs and puberty is sparse and inconsistent. While in a North Carolina prospective study, measures of prenatal and lactational p,p′-DDE exposures were not associated with pubertal stage among boys aged 10–15 years (Gladen et al., 2000), a cross-sectional study of Flemish boys aged 14–15 years found an association of both serum p,p′-DDE and HCB concentrations with earlier pubic hair and genital development (Den Hond et al., 2010). To date, no studies on the association between β-HCH and pubertal development in children have been published. Given the suggestive animal evidence and the limited epidemiologic evidence on the relationship between OCPs and male puberty, we sought to assess this using a study design that improved upon methodological issues identified in prior studies.
Among Russian boys residing in a city with high environmental organochlorine contamination, we previously demonstrated that prepubertal serum dioxin concentrations were associated with a later pubertal onset defined as testicular volume (TV) > 3 mL (Korrick et al., 2011). In contrast, in the same cohort, maternal PCB concentrations measured at boys’ study entry (age 8–9 years) were associated with earlier pubertal onset defined by Tanner genitalia stage 2 (G2) or higher (Humblet et al., 2011). In the present analysis, we examined the association of the boys’ prepubertal serum concentrations of HCB, β-HCH, and p,p′-DDE with their age at pubertal onset.
2. METHODS
2.1. Study Population
The Russian Children’s Study is an ongoing prospective cohort study of 499 boys in Chapaevsk, Russia, enrolled at age 8–9 years in 2003–2005, in a community with high environmental organochlorine contamination (Burns et al., 2012; Lam et al., 2013). A factory complex produced organochlorine compounds, including HCB, HCH and its derivatives (α, β, γ-HCH) but not DDT (Akhmedkanov et al., 2002). Briefly, 623 boys 8–9 years of age were identified from the town-wide health insurance system. Of these, 572 were eligible and 516 (90%) agreed to participate, although 17 boys were subsequently excluded because they were orphans (precluding collection of residential history and other information) (Burns et al., 2012; Lam et al. 2013). OCPs were not measured for the first 144 boys enrolled in the study, and five boys with severe chronic medical conditions that could affect growth were excluded from this analysis, leaving 350 of the original 499 boys with measured OCPs. The study was approved by the Human Studies Institutional Review Boards of the Chapaevsk Medical Association, Harvard School of Public Health, University of Massachusetts Medical School, and Brigham and Women’s Hospital. The parent/guardian gave informed consent and the boys signed assent forms prior to participation.
The parent/guardian completed nurse-administered health and lifestyle questionnaires at entry on the child’s birth, medical, and family history, physical activity, parental occupation and education, residential history, household income, and also completed a Russian Institute of Nutrition food frequency questionnaire to ascertain the child’s dietary information; similar questionnaires were completed at annual study visits.
2.2. Physical Examination and Pubertal Assessment
At study enrollment and annual visits thereafter, a standardized physical examination was conducted. Body mass index (BMI; kg/m2) was calculated from measured height and weight. A single investigator (O.S.) performed annual pubertal assessments for up to eight follow-up visits according to a written protocol and without knowledge of the boys’ OCP serum concentrations. TV was measured using a Prader orchidometer. Pubertal assessment for genitalia and pubic hair was based on a scale of 1 (immature) to 5 (sexually mature) by visual inspection according to established criteria (Tanner and Whitehouse, 1976). Pubertal onset was defined as stage 2 or higher for genitalia (G2+), or pubic hair growth (P2+), or TV > 3 mL for either testis.
2.3. Organochlorine Exposure Assessment
Fasting blood samples were collected from eligible boys at study entry and then centrifuged, aliquoted, and stored at −35°C until shipment on dry ice to the U.S. Centers for Disease Control and Prevention, Atlanta, GA for analysis. The samples, including method blanks and quality control samples, were spiked with 13C12-labeled pesticides, extracted by C18 solid-phase extraction (SPE) followed by a multi-column automated cleanup and enrichment procedure using either large-volume (Turner et al., 1997) or small-volume SPE (Sjodin et al., 2004). Samples were analyzed with high-resolution mass spectrometry in selective ion monitoring mode (Barr et al., 2003). Total serum lipid content was determined from enzymatic measurements of total cholesterol and triglycerides (Phillips et al., 1989). Analytical coefficients of variation (CVs) for individual OCPs in QA/QC samples ranged between 10 and 15%. All OCP concentrations were above the limit of detection and were expressed as both a wet-weight (pg/g serum) and lipid-normalized (ng/g lipid).
2.4. Statistical Analysis
Interval-censored survival analyses, both unadjusted and adjusted for potential confounders, were used to evaluate associations between boys’ serum OCP concentrations (wet-weight concentrations categorized into quartiles with total serum lipids included as a covariate) and age of pubertal onset; each quartile was compared to the lowest quartile and tests for trend were performed by modeling OCP quartiles as an ordinal variable. We assumed a normal distribution for age at pubertal onset. Maximum likelihood estimates of regression parameters were obtained with the SAS LIFEREG procedure. The interval-censored approach assumed onset occurred in the interval between study visits (interval-censored), already occurred (left-censored), or had not yet occurred (right-censored). We calculated the overall mean age of onset for each pubertal measure. Additionally, we calculated the mean age of onset for each OCP quartile and pubertal measure for a given set of model-specific covariates set at the mean or reference levels.
Covariates considered in the models included potential determinants of pubertal onset obtained at enrollment (Table 1): boys’ birth weight, gestational age, nutrition (total caloric intake, percent calories from fat, protein, and carbohydrates), blood lead levels (BLLs), BMI and height z-scores (defined according to WHO child growth standards) (de Onis et al., 2007), maternal age at menarche, maternal age at son’s birth, breastfeeding duration, parity, monthly household income, parental education, and household smoking during pregnancy. We first developed a core model by evaluating the associations of each covariate with pubertal onset and retaining those with a p < 0.20. Covariates meeting this criterion were then included into a full model; backwards selection (likelihood ratio test) was then used to exclude the covariates with p > 0.10. To check for confounding, covariates were added individually back into the final model and retained if they resulted in a ≥ 10% change in the OCP coefficient estimates obtained from the trend test. Each onset model (G2+, TV > 3 mL, P2+) was allowed to have unique model covariates. Statistical significance was defined as p ≤ 0.05. All statistical analyses were conducted using SAS statistical software, version 9.2 (SAS Institute Inc., Cary, North Carolina).
Table 1.
Characteristics of participants in the Russian Children’s Study with serum organochlorine pesticide measurements at study entry (ages 8–9 years)
| Characteristic | Total boys (n=350) |
|---|---|
| Child Characteristics | Mean ± SD or N (%) |
| Growth Measurements | |
| Height (cm) | 129.0 ± 6 |
| Weight (kg) | 26.6 ± 5.4 |
| Body Mass Index (BMI) | 15.9 ± 2.3 |
| WHO Height z-score | 0.12 ± 1.0 |
| WHO BMI z-score | −0.17 ± 1.3 |
| Birth and Neonatal History | |
| Birth Weight (kg) | 3.3 ± 0.5 |
| Gestational Age (wks) | 39.0 ± 1.8 |
| Preterm birth (gestational age <37 wks) | 33 (9) |
| Macronutrients | |
| Total calories (calories) | 2695.7 ± 931.0 |
| % carbohydrates | 54.3 ± 6.6 |
| % fat | 34.2 ± 5.9 |
| % protein | 11.6 ± 1.6 |
| Other Characteristics | |
| High blood lead levels (>5μg/dL) | 86 (25) |
| Parental and Residential Characteristics | |
| Any household smoking during pregnancy | 58 (17) |
| Maternal age at son’s birth (<25 yrs) | 222 (63) |
| Maternal age at menarche (yrs) | 13 ± 1.3 |
| Maximum Parental Education | |
| High School or Less | 29 (8) |
| Jr College/Technical School | 198 (57) |
| University/Post-Graduate Training | 121 (35) |
| Household Income, US $ per month | |
| <175$ | 107 (31) |
| 175–250$ | 88 (25) |
| >250$ | 154 (44) |
Percentages may not total 100% due to rounding.
Missing: Birth weight, n=1; Any household smoking during pregnancy, n=5; Maternal age at birth, n=3; Maternal age at menarche, n=26; Maximum parental education, n=2; Household income, n=1; Macronutrients, n=3
Since height and BMI at enrollment may be on the causal pathway between OCPs and pubertal onset, we excluded these covariates in the primary analysis but conducted sensitivity analyses by adding them to final models. Because maternal age at menarche was missing for 8% of boys (n=26) and was a predictor for only G2+ and TV > 3 mL, we conducted sensitivity analyses comparing models with and without maternal age at menarche for these two measures. We also conducted sensitivity analyses further adjusting for reported daily physical activity at baseline in final models, given some evidence, although more in females, that intense exercise (e.g., gymnastics, ballet) may delay puberty (Georgopoulos et al., 1999; Warren 1980; Warren and Perlroth, 2001). Finally, we performed an alternative analysis using quartiles of lipid-normalized serum OCP concentrations (wet-weight levels divided by lipid concentrations) for comparison to our analysis with wet-weight serum OCPs.
3. RESULTS
3.1. OCP concentrations and demographic characteristics
The median (25th, 75th percentiles) concentrations for wet-weight serum HCB, β-HCH, and p,p′-DDE were 754 (522, 1159), 814 (560, 1294), and 1408 (904, 2324) pg/g serum, respectively. The median (25th, 75th percentiles) concentrations for lipid-normalized serum HCB, β-HCH, and p,p′-DDE were 159 (107, 247), 168 (114, 272), and 287 (189, 492) ng/g lipid, respectively. At study entry, the boys were generally within age-expected ranges for stature and weight (mean WHO height and BMI Z-score=0.12 and −0.17, respectively) (Table 1). Boys with and without OCP measurements (n=350 vs. 144) did not differ significantly by height and BMI z-scores, weight, and birth characteristics (Lam et al., 2013). However a greater percentage of boys with OCP measurements were in the highest household income (44% vs. 26%) and parental education categories (35% vs. 27%) than boys without OCP measurements.
3.2. Pubertal onset characteristics
The retention rate was 72% after 8 years and 71% had at least 8 examinations by age 16–17 years. The overall estimated mean age (95% CI) of pubertal onset for G2+, TV > 3 mL, and P2+ was 9.5 (9.3, 9.7), 10.3 (10.1, 10.5), and 12.0 years (11.8, 12.2), respectively. By the 16–17 year study visit, 92%, 89%, and 78% had attained pubertal onset defined by G2+, TV > 3 mL, and P2+, respectively.
3.3. Determinants of pubertal onset
In unadjusted analyses, on average, boys with high baseline BLLs (≥ 5μg/dL) had significantly later pubertal onset, with onset occurring 10.1 months (95% CI: 4.3, 15.8) for G2+, 8.7 months (95% CI: 3.5, 14.0) for TV > 3 mL, and 6.4 months (95% CI: 1.6, 11.3) for P2+ later, compared to boys with low BLLs. Baseline dietary nutritional intake was also significantly associated with G2+, which occurred a mean of 1.6 months earlier (95% CI: −3.0, −0.2) for each additional 500 calories consumed per day. Greater percent calories from dietary fat were associated with earlier G2+, TV > 3 mL and P2+. Additionally, for every 500 grams increase in birth weight, G2+ and TV > 3 mL occurred 5 to 5.5 months earlier on average.
WHO height z-scores and BMI at study entry were significant predictors for all pubertal onset measures. Boys who were classified as thin or very thin (de Onis et al., 2007) reached G2+, TV > 3 mL, and P2+ a mean of 12.3 months (95% CI: 6.6, 18.1), 7.7 months (95% CI: 2.4, 13.1), and 5.8 months (95% CI: 0.9, 10.7) later, respectively, compared to those classified with a normal BMI. Higher height z-scores were also associated with significantly earlier pubertal onset, ranging from 3.7 to 7.7 months earlier for each 1 SD increase.
3.4. Association of OCPs with gonadarche (TV > 3 mL and G2+)
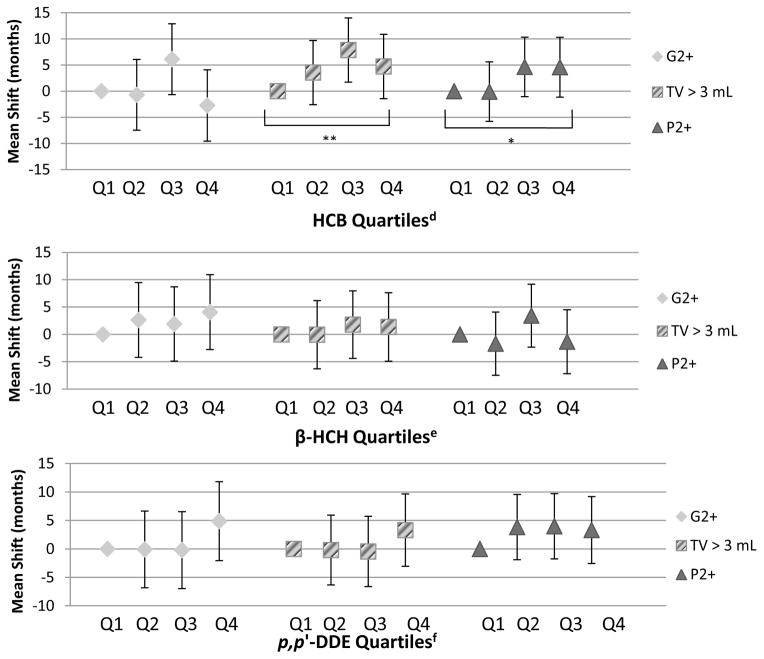
In adjusted models, boys with higher HCB concentrations attained TV > 3 mL later compared to Q1 (trend p: 0.06, Table 2, Figure 1). The estimated mean age of pubertal onset was 9.8 years (95% CI: 9.4, 10.2) for TV > 3 mL for the lowest HCB quartile and 10.2 years (95% CI: 9.8, 10.6) for the highest HCB quartile. While we observed no difference in age at onset for Q2 or Q3 of p,p′-DDE compared to Q1, we observed a suggestive though non-significant later mean age of TV > 3 mL and G2+ for Q4 vs. Q1 (Table 2). No association was found for β-HCH with TV > 3 mL, nor did we observe associations of G2+ with HCB or β-HCH.
Table 2.
Adjusted Mean Shifts in Age at Pubertal Onset (Months, 95% CIs) by Quartiles of Wet-Weight Serum OCP Concentrations Among 350 Russian Boys
| G2+ (n=346)a | TV > 3 mL (n=346)b | P2+ (n=344)c | ||||
|---|---|---|---|---|---|---|
| Mean Shift (months) and 95% CI | p-value | Mean Shift (months) and 95% CI | p-value | Mean Shift (months) and 95% CI | p-value | |
| HCBd | ||||||
| Q1 | REF | REF | REF | |||
| Q2 | −0.68 (−7.45, 6.08) | 0.84 | 3.56 (−2.57, 9.70) | 0.25 | −0.08 (−5.77, 5.60) | 0.82 |
| Q3 | 6.11 (−0.66, 12.88) | 0.08 | 7.87 (1.73, 14.02) | 0.01 | 4.65 (−1.04, 10.34) | 0.11 |
| Q4 | −2.72 (−9.55, 4.10) | 0.43 | 4.73 (−1.42, 10.88) | 0.13 | 4.59 (−1.13, 10.31) | 0.12 |
| p for trend | 0.93 | 0.06 | 0.04 | |||
| β-HCHe | ||||||
| Q1 | REF | REF | REF | |||
| Q2 | 2.64 (−4.20, 9.47) | 0.45 | −0.05 (−6.29, 6.19) | 0.99 | −1.69 (−7.47, 4.09) | 0.57 |
| Q3 | 1.91 (−4.88, 8.69) | 0.58 | 1.79 (−4.38, 7.96) | 0.57 | 3.43 (−2.33, 9.19) | 0.24 |
| Q4 | 4.07 (−2.78, 10.92) | 0.24 | 1.35 (−4.91, 7.62) | 0.67 | −1.33 (−7.18, 4.51) | 0.65 |
| p for trend | 0.30 | 0.56 | 0.91 | |||
| p,p′-DDEf | ||||||
| Q1 | REF | REF | REF | |||
| Q2 | −0.08 (−6.82, 6.66) | 0.98 | −0.19 (−6.32, 5.95) | 0.95 | 3.85 (−1.88, 9.58) | 0.19 |
| Q3 | −0.20 (−6.96, 6.55) | 0.95 | −0.44 (−6.61, 5.73) | 0.89 | 3.99 (−1.74, 9.72) | 0.17 |
| Q4 | 4.89 (−2.05, 11.83) | 0.17 | 3.31 (−3.05, 9.66) | 0.31 | 3.33 (−2.55, 9.21) | 0.27 |
| p for trend | 0.20 | 0.36 | 0.28 | |||
G2+ model adjusted for baseline covariates: boys’ total serum lipids, birth weight, macronutrients (total caloric intake, percent calories from dietary carbohydrates, fat, and protein), blood lead levels; missing birth weight (n=1), macronutrients (n=3)
TV > 3 mL model adjusted for baseline covariates: boys’ total serum lipids, birth weight, macronutrients (total caloric intake, percent calories from dietary carbohydrates, fat, and protein), blood lead levels; missing birth weight (n=1), macronutrients (n=3)
P2+ model adjusted for baseline covariates: boys’ total serum lipids, macronutrients (total caloric intake, percent calories from dietary carbohydrates, fat, and protein), blood lead levels, maternal age at birth, household income; missing macronutrients (n=3), household income (n=1), maternal age at birth (n=2)
HCB wet-weight quartiles (Q1–Q4, pg/g serum): Q1: 169–516; Q2: 517–751; Q3: 752–1,156; Q4: 1,157–15,482
β-HCH wet-weight quartiles (Q1–Q4, pg/g serum): Q1: 209–567; Q2: 568–814; Q3:815–1,294 Q4: 1,295–13,732
p,p′-DDE wet-weight quartiles (Q1–Q4, pg/g serum): Q1: 261–907; Q2: 908–1,406; Q3: 1,407–2,327; Q4: 2,328–41,301
Figure 1. Adjusted Mean Shifts in Age at Pubertal Onset (Months, 95% CIs) by Quartiles of Wet-Weight Serum OCP Concentrations Among 350 Russian Boysa,b,c.
*p ≤ 0.05
**p ≤ 0.10
Shift in months is relative to Q1 (reference)
aG2+ model adjusted for baseline covariates: boys’ total serum lipids, birth weight, macronutrients (total caloric intake, percent calories from dietary carbohydrates, fat, and protein), blood lead levels; missing birth weight (n=1), macronutrients (n=3)
bTV > 3 mL model adjusted for baseline covariates: boys’ total serum lipids, birth weight, macronutrients (total caloric intake, percent calories from dietary carbohydrates, fat, and protein), blood lead levels; missing birth weight (n=1), macronutrients (n=3)
cP2+ model adjusted for baseline covariates: boys’ total serum lipids, macronutrients (total caloric intake, percent calories from dietary carbohydrates, fat, and protein), blood lead levels, maternal age at birth, household income; missing macronutrients (n=3), household income (n=1), maternal age at birth (n=2)
dHCB wet-weight quartiles (Q1–Q4, pg/g serum): Q1: 169–516; Q2: 517–751; Q3: 752–1,156; Q4: 1,157–15,482
eβ-HCH wet-weight quartiles (Q1–Q4, pg/g serum): Q1: 209–567; Q2: 568–814; Q3:815–1,294 Q4: 1,295–13,732
fp,p′-DDE wet-weight quartiles (Q1–Q4, pg/g serum): Q1: 261–907; Q2: 908–1,406; Q3: 1,407–2,327; Q4: 2,328–41,301
3.5. Association of OCPs with pubarche (P2+)
There was no difference in mean age of reaching P2+ for Q2 vs. Q1 of HCB, but at higher mean concentrations of HCB, P2+ was attained later in adjusted models (trend p: 0.04, Table 2, Figure 1). The estimated mean age of P2+ ranged from 11.7 years (95% CI: 11.3, 12.1) for the lowest HCB quartile to 12.1 years (95% CI: 11.7, 12.5) for the highest HCB quartile. We found no association between β-HCH or p,p′-DDE and P2+.
3.6. Sensitivity analyses
Given the complex interrelationship of height and BMI with pubertal onset, in which both may be related to pubertal onset and/or the causal pathway between serum OCP concentrations and onset, we performed sensitivity analyses further adjusting for height z-scores and BMI. After adjustment, the effect estimates for the association of serum OCPs with pubertal onset were attenuated but dose-response patterns across quartiles were similar to primary models without BMI and height z-scores (Table 3). Further adjustment for maternal age at menarche in 324 boys also demonstrated slight attenuation in HCB effect estimates, but overall similar dose-response patterns to the primary models without maternal age at menarche (Supplemental Table 1). Results were essentially unchanged in sensitivity analyses adjusting for physical activity at enrollment (data not shown).
Table 3.
Adjusted Mean Shifts in Age at Pubertal Onset (Months, 95% CIs) by Quartiles of Wet-Weight Serum OCPs Additionally Adjusted for Baseline BMI Categories and Height Z-scores in 350 Russian Boys
| G2+ (n=346)a | TV > 3 mL (n=346)b | P2+ (n=344)c | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean Shift (months) and 95% CI | p-value | Mean Shift (months) and 95% CI | p-value | Mean Shift (months) and 95% CI | p-value | |
| HCBd | ||||||
| Q1 | REF | REF | REF | |||
| Q2 | −0.40 (−6.77, 5.98) | 0.90 | 3.66 (−2.28, 9.60) | 0.23 | −0.30 (−5.89, 5.30) | 0.92 |
| Q3 | 4.38 (−2.24, 11.00) | 0.19 | 6.65 (0.46, 12.83) | 0.04 | 2.95 (−2.86, 8.76) | 0.21 |
| Q4 | −4.34 (−10.98, 2.29) | 0.20 | 3.42 (−2.74, 9.58) | 0.28 | 2.82 (−3.03, 8.67) | 0.34 |
| p for trend | 0.54 | 0.21 | 0.23 | |||
| β-HCHe | ||||||
| Q1 | REF | REF | REF | |||
| Q2 | 4.04 (−2.50, 10.59) | 0.23 | 0.20 (−5.92, 6.33) | 0.95 | −2.01 (−7.76, 3.73) | 0.49 |
| Q3 | 0.42 (−6.39, 7.23) | 0.90 | −0.33 (−6.71, 6.05) | 0.92 | 0.94 (−5.09, 6.97) | 0.76 |
| Q4 | 2.06 (−4.77, 8.96) | 0.55 | −1.24 (−7.71, 5.23) | 0.71 | −4.74 (−10.93, 1.45) | 0.13 |
| p for trend | 0.78 | 0.69 | 0.24 | |||
| p,p′-DDEf | ||||||
| Q1 | REF | REF | REF | |||
| Q2 | 0.84 (−5.96, 7.64) | 0.81 | −0.68 (−6.99, 5.63) | 0.83 | 2.85 (−3.06, 8.76) | 0.34 |
| Q3 | −0.08 (−6.83, 6.66) | 0.98 | −1.68 (−7.99, 4.62) | 0.60 | 3.08 (−2.80, 8.97) | 0.30 |
| Q4 | 1.29 (−5.86, 8.43) | 0.72 | 0.60 (−7.30, 6.10) | 0.86 | 0.10 (−6.11, 6.31) | 0.97 |
| p for trend | 0.81 | 0.79 | 0.98 | |||
G2+ model adjusted for baseline covariates: boys’ total serum lipids, birth weight, macronutrients (total caloric intake, percent calories from dietary carbohydrates, fat, and protein), blood lead levels, WHO BMI z-score, WHO height z-score; missing birth weight (n=1), macronutrients (n=3)
TV > 3 mL model adjusted for baseline covariates: boys’ total serum lipids, birth weight, macronutrients (total caloric intake, percent calories from dietary carbohydrates, fat, and protein), blood lead levels, WHO BMI z-score, WHO height z-score; missing birth weight (n=1), macronutrients (n=3)
P2+ model adjusted for baseline covariates: boys’ total serum lipids, macronutrients (total caloric intake, percent calories from dietary carbohydrates, fat, and protein), blood lead levels, maternal age at birth, household income, WHO BMI z-score, WHO height z-score; missing macronutrients (n=3), household income (n=1), maternal age at birth (n=2)
HCB wet-weight quartiles (Q1–Q4, pg/g serum): Q1: 169–516; Q2: 517–751; Q3: 752–1,156; Q4: 1,157–15,482
β-HCH wet-weight quartiles (Q1–Q4, pg/g serum): Q1: 209–567; Q2: 568–814; Q3:815–1,294 Q4: 1,295–13,732
p,p′-DDE wet-weight quartiles (Q1–Q4, pg/g serum): Q1: 261–907; Q2: 908–1,406; Q3: 1,407–2,327; Q4: 2,328–41,301
Alternative analyses based on lipid-normalized serum OCPs demonstrated stronger associations of HCB with TV > 3 mL and P2+ than models with wet-weight serum OCPs adjusted for serum lipids (Supplemental Tables 2–4, Supplemental Figures 1–2). The suggestive association of later G2+ with the highest p,p′-DDE quartile was stronger with lipid-normalized measures.
4. DISCUSSION
In our prospective cohort study, we found a relationship between higher prepubertal serum HCB concentrations and later pubertal onset delineated by TV > 3 mL and P2+. We also identified several predictors of male pubertal onset that were consistent with prior studies, including birth weight, caloric intake, and high BLLs (Adair 2001; Denham et al., 2005; Karaolis-Danckert et al., 2009; Ong et al., 2009; Selevan et al., 2003; Wang et al., 2012; Williams et al., 2010; Wu et al., 2003).
The predicted mean age of 12.0 years for pubarche (P2+) in our Russian cohort was consistent with other cross-sectional studies (Herman-Giddens et al., 2001; Juul et al., 2006; Sun et al., 2002), but the predicted mean age of onset of genital development (G2+) (9.5 years) was slightly younger than other pediatric cohorts (Table 4). In comparison to a Danish cohort using the same definition of TV onset (> 3 mL) (Sorensen et al., 2010), the boys in our cohort on average reached onset at 10.3 years, which is more than 1 year younger than the Danish boys. Our findings however were comparable to the mean age of TV onset (≥ 3 mL) in U.S. boys (Herman-Giddens et al., 2012).
Table 4.
Published Mean Ages (years) of Attaining Male Pubertal Onset
| Study/Authors | Study Type | Birth Year(s) | Span of Study | Country | Subjects | Age Range | G2+ | P2+ |
|---|---|---|---|---|---|---|---|---|
| Russian Children’s Study | L | 1994–1997 | 2003/2005–2013 | Russia | 350 white | 8–17 | 9.5 | 12.0 |
| Susman et al. (2010) | L | 1991 | 2000–2006 | US | 364 white | 9.5–15.5 | 10.4 | 11.5 |
| Papadimitriou et al. (2011) | CS | 1991–2001 | 2007–2009 | Greece | 932 white | 8–16 | 12.3 | 11.6 |
| Herman-Giddens et al. (2012) | CS | 1989–2004 | 2005–2010 | US | 2070 white | 6–16 | 10.1 | 11.5 |
| Sorensen et al. (2010) | CS/L | 1986–2002 | 2006–2008 | Denmark | 704 white | 5.8–19.8 | 11.59 | 12.38 |
| Facchini et al. (2008) | CS | 1984–1997 | 2002–2004 | Kazakhstan | 588 white | 7–18 | 9.7* | 12.5* |
| Castellino et al. (2005) | CS | 1985–1995 | 1998–2001 | Italy | 1858 white | 6–13 | 11.1 | 11.3 |
| Juul et al. (2006) | CS | 1972–1987 | 1991–1993 | Denmark | 826 white | 6–19 | 11.8 | 11.9 |
| Sorensen et al. (2010) | CS | 1971–1987 | 1991–1993 | Denmark | 824 white | 5.8–19.8 | 11.83 | 11.89 |
| Herman-Giddens et al. (2001) | CS | 1969–1986 | 1988–1994 | US | 536 white | 8–19 | 10.1* | 12.0* |
| Sun et al. (2002) | CS | 1969–1986 | 1988–1994 | US | 537 white | 8–19 | 10.0* | 12.0* |
| Roche et al. (1995) | L | 1969–1983 | 1985–1993 | US | 78 white | 9.5–16 | 11.3 | 11.3 |
| Willers et al. (1996) | CS | 1967–1978 | 1984–1986 | Germany | 8685 white | 8–17 | 10.8* | 11.5* |
| Mul et al. (2001) | CS | 1944–1989 | 1965–1997 | Netherlands | 4019 white | 8–21 | 11.5* | 12.0* |
Reported ages are median
L: Longitudinal; CS: Cross-sectional
Sorted by Approximate Birth Year(s)
Our primary finding was an association of serum HCB concentrations with later onset defined by P2+ as well as TV > 3 mL. The observed effects on TV > 3mL and P2+ may be mediated through the aryl hydrocarbon receptor (AhR) for which HCB is a weak agonist (Hahn et al., 1989). AhR signaling has been shown to inhibit androgen production and androgen receptor binding in animals (Hahn et al., 1989; Ralph et al., 2003). Given that HCB is lipophilic and present in fat and androgen-producing endocrine glands (Foster et al., 1993), and pubic hair growth in humans typically results from rising levels of adrenal or testicular androgens (Kronenberg et al., 2008), we speculate that HCB may impair either androgen metabolism or receptor binding, which could affect sexual hair follicular development (Randall 2008). The regulation of adrenarche is poorly understood but it is possible that HCB could perturb maturation of the zona reticularis of the adrenals, the sex steroid producing zone (Havelock et al., 2004; Zawatski and Lee, 2013). Measurement of adrenal androgens in this cohort will help elucidate the mechanism.
The observed association with TV > 3 mL, but not G2+, may reflect the more reliable and precise measurement of TV, which is measured with a standardized orchidometer, compared to the more subjective Tanner genitalia staging (Ankarberg-Lindgren et al., 2004; Biro et al., 1995; Euling et al., 2008; Largo and Prader, 1983; Sorensen et al., 2012; Zachmann et al., 1974). Alternatively, differential effects on the regulation of gonadarche by the HPG system could account for our findings (Kronenberg et al., 2008). Kisspeptins, a family of peptides found in the hypothalamus, stimulates pulsatile secretion of gonadotropin releasing hormone (GnRH) to induce puberty (Zawatski and Lee, 2013). GnRH stimulates luteinizing hormone (LH) and follicle stimulating hormone (FSH) secretion from the pituitary. LH, in turn, stimulates the Leydig cells to produce testosterone, resulting in masculinization of the genitalia (Kronenberg et al., 2008; Zawatski and Lee, 2013). In the Sertoli cells, FSH in combination with testosterone promotes maturation of the seminiferous tubules and spermatogenesis, which contributes to puberty-associated increases in testicular size (Kronenberg et al., 2008; Zawatski and Lee, 2013). We found no association of timing of G2+ with prepubertal OCP measurements, but did find a delay in testicular growth. In the early phases of puberty, much of the increase in testicular size reflects growth of the seminiferous tubules, therefore we speculate that HCB may differentially affect maturation of the seminiferous tubules but not the interstitial Leydig cells. The lack of disruption with Leydig cell androgen production would result in normal onset of genitalia staging, but the delay in Sertoli cell proliferation and spermatogenesis would manifest as a delay in testicular enlargement (Kronenberg et al., 2008; Zawatski and Lee, 2013). Our plans to measure reproductive hormones in this cohort will help elucidate the pathways affected by the pesticide concentrations.
As OCPs are lipophilic and the optimal approach to account for lipids in analyses of these measures is uncertain (Li et al., 2013; Schisterman et al., 2005), we conducted alternative analyses comparing lipid-normalized measures with wet-weight measures adjusted for total serum lipids. Though lipid-normalized measures led to stronger dose-response associations compared to wet-weight measures adjusted for serum lipids, our interpretations of the main findings were unchanged. Based on simulation studies by Schisterman et al. (2005) comparing lipid-normalized vs. wet-weight models adjusted for serum lipids and uncertainty regarding how consistently OCPs are partitioned in the lipid fraction of serum, we believe that the use of wet-weight measures is most appropriate and has minimal bias. Additionally as it is not yet clear which approach is appropriate for analyses of pubertal onset and because our findings for wet-weight and lipid-normalized OCP concentrations were similar, we believe that potential bias related to the approach used for lipid-normalized concentrations is unlikely to have impacted our findings.
A limitation of our study is having a single OCP measurement at enrollment during the prepubertal window, thus we were unable to assess ongoing exposures. HCB concentrations among the boys in this cohort were much higher than other pediatric populations in the U.S. (Lam et al., 2013; Patterson et al., 2009) (median HCB in Russia vs. U.S: 158 ng/g lipid vs. 13.4 ng/g lipid). Thus, while the lowest quartile serves as the reference category in our analyses, some boys in this lowest quartile still had relatively high HCB concentrations compared to U.S. boys. This may affect our ability to generalize to populations with lower concentrations. In contrast, perhaps because p,p′-DDE was never manufactured in Chapaevsk, p,p′-DDE concentrations in our cohort were comparable to background levels in the U.S. and other countries (Lam et al., 2013; Patterson et al., 2009). While we did not observe a difference in age of pubertal onset between Q2 and Q3 of p,p′-DDE compared to Q1, there was suggestive evidence of a later onset (especially for G2+) in the most exposed (Q4) quartile, consistent with a potential threshold dose-response.
Strengths of our cohort study include a prospective design, a fairly large sample size, a wide range of HCB, β-HCH, and p,p′-DDE serum concentrations obtained in a prepubertal developmental window, and the use of three established pubertal markers assessed by a single physician, eliminating inter-examiner variability (Carlsen et al., 2000). Finally, retention in this cohort is high and there is no detectable loss to follow-up bias by demographic factors.
5. CONCLUSION
We found that higher prepubertal serum HCB concentrations were associated with later onset of pubic hair development and testicular growth. Our future plans to assess reproductive hormones in this cohort will enable us to better understand how HCB is delaying pubertal onset. Further follow-up of this cohort will provide the opportunity to assess the relationship between OCPs and sexual maturity. Disruptions in pubertal development can have long-lasting effects on adult behavior and reproductive health as observed in both human and animal studies. Therefore, identifying environmental agents that affect pubertal onset and maturation is of public health importance.
Supplementary Material
Highlights.
Higher prepubertal serum HCB levels were associated with later male pubertal onset.
Associations were observed at high HCB serum levels relative to other populations.
No significant associations noted with p,p′-DDE or β-HCH and male pubertal onset.
Acknowledgments
Funding Source: This work was funded by the U.S. Environmental Protection Agency (grant R82943701), the National Institute of Environmental Health Sciences (grant R01 ES014370, P30 ES000002, and R03 ES017117), and the intramural program of the National Cancer Institute, National Institutes of Health. TL was supported by the National Institute for Occupational Safety and Health training grant T42-OH008416-09.
Abbreviations
- β-HCH
β-hexachlorocylohexane
- BLL
blood lead level
- BMI
body mass index
- G
Genitalia Tanner stage
- HCB
Hexachlorobenzene
- DDT
1,1,1,-trichloro-2,2,bis(p-chlorophenyl)ethane
- EDC
Endocrine disrupting chemical
- OCP
Organochlorine pesticide
- P
Pubic hair Tanner stage
- p,p′-DDE
p,p′-dichlorodiphenyldichloroethylene
- PPS
Preputial separation
- TV
Testicular volume measured with orchidometer
Footnotes
Financial Disclosure Statement: TL is employed by Quintiles (Cambridge, MA). LMA is employed by Environmental Health and Engineering, Inc. (Needham, MA). DGP Jr is employed by Axys Analytical Solutions (Sidney, BC, Canada), EnviroSolutions Consulting Inc. (Auburn, GA, USA), and Exponent, Inc. (Maynard, MA, USA). The other authors declare they have no actual or potential competing financial interests to disclose.
Conflict of Interest Statement: The opinions expressed in this article are those of the authors and do not necessarily reflect the official opinion of the Centers for Disease Control and Prevention. The authors have no conflicts of interest to disclose.
Submission Declaration: This manuscript has not been published previously nor is it under consideration for publication elsewhere. This manuscript is approved by all authors. If accepted, it will not be published elsewhere including electronically in the same form, in English or in any other language, without the written consent of the copyright-holder.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adair LS. Size at birth predicts age at menarche. Pediatrics. 2001;107:e59–e65. doi: 10.1542/peds.107.4.e59. [DOI] [PubMed] [Google Scholar]
- Akhmedkanov A, Revich B, Adibi JJ, Zeilert V, Masten SA, Patterson DG, Jr, et al. Characterization of dioxin exposure in residents of Chapaevsk, Russia. J Expo Anal Environ Epidemiol. 2002;12:409–417. doi: 10.1038/sj.jea.7500243. [DOI] [PubMed] [Google Scholar]
- Ankarberg-Lindgren C, Norjavaara E. Changes of diurnal rhythm and levels of total and free testosterone secretion from pre to late puberty in boys: testis size of 3 mL is a transition stage to puberty. Eur J Endocrinol. 2004;151:747–757. doi: 10.1530/eje.0.1510747. [DOI] [PubMed] [Google Scholar]
- Arnold DL, Moodie CA, Charbonneau SM, Grice HC, McGuire PF, Bryce FR, et al. Long-term toxicity of hexachlorobenzene in the rat and the effect of dietary Vitamin A. Food Chem Toxicol. 1985;23:779–793. doi: 10.1016/0278-6915(85)90278-9. [DOI] [PubMed] [Google Scholar]
- Ashby J, Lefevre PA. The peripubertal male rat assay as an alternative to the Hershberger castrated male rat assay for the detection of anti-androgens, oestrogens, and metabolic modulators. J Appl Toxicol. 2000;20:35–47. doi: 10.1002/(sici)1099-1263(200001/02)20:1<35::aid-jat633>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Barber JL, Sweetman AJ, van Wijk D, Jones KC. Hexachlorobenzene in the global environment: emissions, levels, distributions, trends, and processes. Sci Total Environ. 2005;349:1–44. doi: 10.1016/j.scitotenv.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Barr JR, Maggio VL, Barr DB, Turner WE, Sjodin A, Sundau CD, et al. New high-resolution mass spectrometric approach for the measurement of polychlorinated biphenyls and organochlorine pesticides in human serum. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;794:137–148. doi: 10.1016/s1570-0232(03)00451-3. [DOI] [PubMed] [Google Scholar]
- Biro FM, Lucky AW, Huster GA, Morrison JA. Pubertal staging in boys. J Pediatr. 1995;127:100–102. doi: 10.1016/s0022-3476(95)70265-2. [DOI] [PubMed] [Google Scholar]
- Breivick K, Pacyna JM, Munch J. Use of α, β, γ-hexachlorocyclohexane in Europe, 1970–1996. Sci Total Environ. 1999;239:151–163. doi: 10.1016/s0048-9697(99)00291-0. [DOI] [PubMed] [Google Scholar]
- Burns JS, Williams PL, Sergeyev O, Korrick SA, Lee MM, Revich B, et al. Serum concentrations of organochlorine pesticides and growth among Russian boys. Environ Health Perspect. 2012;120:303–308. doi: 10.1289/ehp.1103743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen E, Andersen AG, Buchreitz L, Jorgensen N, Magnus O, Matulevicuus V, et al. Inter-observer variation in the results of the clinical andrological examination including estimation of testicular size. Int J Androl. 2000;23:248–253. doi: 10.1046/j.1365-2605.2000.00240.x. [DOI] [PubMed] [Google Scholar]
- Castellino N, Bellone S, Rapa A, Vercellotti A, Binotti M, Petri A, et al. Puberty onset in Northern Italy: a random sample of 3597 Italian children. J Endocrinol Invest. 2005;28:589–594. doi: 10.1007/BF03347256. [DOI] [PubMed] [Google Scholar]
- Courtney KD. Hexachlorobenzene (HCB): a review. Environ Res. 1979;20:225–266. doi: 10.1016/0013-9351(79)90001-x. [DOI] [PubMed] [Google Scholar]
- de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Hond E, Dhooge W, Bruckers L, Schoeters G, Nelen V, van de Mieroop E, et al. Internal exposure to pollutants and sexual maturation in Flemish adolescents. J Expo Sci Environ Epidemiol. 2010;21:224–233. doi: 10.1038/jes.2010.2. [DOI] [PubMed] [Google Scholar]
- Denham M, Schell LM, Deane G, Gallo MV, Ravenscroft J, DeCaprio AP, et al. Relationship of lead, mercury, mirex, dichlorodiphenyldichloroethylene, hexachlorobenzene, and polychlorinated biphenyls to timing of menarche among Akwesasne Mohawk girls. Pediatrics. 2005;115:e127–e134. doi: 10.1542/peds.2004-1161. [DOI] [PubMed] [Google Scholar]
- Euling SY, Herman-Giddens ME, Lee PA, Selevan SG, Juul A, Sorensen TI, et al. Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings. Pediatrics. 2008;121:S172–S191. doi: 10.1542/peds.2007-1813D. [DOI] [PubMed] [Google Scholar]
- Facchini F, Fiori G, Bedogni G, Galletti L, Ismagulov O, Ismagulova A, et al. Puberty in modernizing Kazakhstan: a comparison of rural and urban children. Ann Hum Biol. 2008;35:50–64. doi: 10.1080/03014460701784567. [DOI] [PubMed] [Google Scholar]
- Foster WG, Pentick JA, McMahon A, Lecavalier PR. Body distribution and endocrine toxicity of hexachlorobenzene (HCB) in the female rat. J Appl Toxicol. 1993;13:79–83. doi: 10.1002/jat.2550130203. [DOI] [PubMed] [Google Scholar]
- Fry M, Toone CK. DDT-induced feminization of gull embryos. Science. 1981;213:922–924. doi: 10.1126/science.7256288. [DOI] [PubMed] [Google Scholar]
- Georgopoulos N, Markou K, Theodoropoulou A, Paraskevopoulou P, Varaki L, Kazantzi Z, et al. Growth and pubertal development in elite female rhythmic gymnasts. J Clin Endocrinol Metab. 1999;84:4525–4530. doi: 10.1210/jcem.84.12.6177. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Lenroot R, Greenstein D, Wallace GL, Ordaz S, et al. Puberty-related influences on brain development. Mol Cell Endocrinol. 2006;254–255:154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Gladen BC, Ragan B, Rogan WJ. Pubertal growth and development and prenatal and lactational exposure to polychlorinated biphenyls and dichlorodiphenyl dichloroethene. J Pediatr. 2000;136:490–496. doi: 10.1016/s0022-3476(00)90012-x. [DOI] [PubMed] [Google Scholar]
- Golub MS, Collman GW, Foster PMD, Kimmel CA, Rajpert-DeMeyts E, Reiter EO, et al. Public health implications of altered puberty timing. Pediatrics. 2008;121:S218–S230. doi: 10.1542/peds.2007-1813G. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby J, Furr J, Wolf CJ, Lambright C, Parks L, et al. Effects of environmental antiandrogens on reproductive development in experimental animals. Hum Reprod Update. 2001;7:248–264. doi: 10.1093/humupd/7.3.248. [DOI] [PubMed] [Google Scholar]
- Guillette LJ, Jr, Gross TS, Masson GR, Matter JM, Percival HF, Woodward AR. Developmental abnormalities of the gonad and abnormal sex hormone concentrations in juvenile alligators from contaminated and control lakes in Florida. Environ Health Perspect. 1994;102:680–688. doi: 10.1289/ehp.94102680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn ME, Goldstein JA, Linko P, Gasiewicz TA. Interaction of hexachlorobenzene with the receptor for 2,3,7,8 – tetrachlorodibenzo-p-dioxin in vitro and in vivo. Arch Biochem Biophys. 1989;270:344–355. doi: 10.1016/0003-9861(89)90037-4. [DOI] [PubMed] [Google Scholar]
- Havelock JC, Auchus RJ, Rainey WE. The rise in adrenal androgen biosynthesis: adrenarche. Semin Reprod Med. 2004;22:337–347. doi: 10.1055/s-2004-861550. [DOI] [PubMed] [Google Scholar]
- Herman-Giddens ME, Wang L, Koch G. Secondary sexual characteristics in boys: estimates from the national health and nutrition examination survey III, 1988–1994. Arch Pediatr Adolesc Med. 2001;155:1022–1028. doi: 10.1001/archpedi.155.9.1022. [DOI] [PubMed] [Google Scholar]
- Herman-Giddens ME, Steffes J, Harris D, Slora E, Hussey M, Dowshen SA, et al. Secondary sexual characteristics in boys: data from the Pediatric Research in Office Settings Network. Pediatrics. 2012;130:e1058–e1068. doi: 10.1542/peds.2011-3291. [DOI] [PubMed] [Google Scholar]
- Humblet O, Williams PL, Korrick SA, Sergeyev O, Emond C, Birnbaum LS, et al. Dioxin and polychlorinated biphenyl concentrations in mother’s serum and the timing of pubertal onset in sons. Epidemiology. 2011;22:827–835. doi: 10.1097/EDE.0b013e318230b0d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaga K, Dharmani C. Global surveillance of DDT and DDE levels in human tissues. Int J Occup Med Environ Health. 2003;16:7–20. [PubMed] [Google Scholar]
- Jung D, Becher H, Edler L, Flesch0Janys D, Gurn P, Konietzko J, et al. Elimination of β-Hexachlorocyclohexane in occupationally exposed persons. J Toxicol Environ Health. 1997;51:23–34. doi: 10.1080/00984109708984009. [DOI] [PubMed] [Google Scholar]
- Juul A, Teilmann G, Scheike T, Hertel NT, Holm K, Laursen EM, et al. Pubertal development in Danish children: comparison of recent European and US data. Int J Androl. 2006;29:247–255. doi: 10.1111/j.1365-2605.2005.00556.x. [DOI] [PubMed] [Google Scholar]
- Kaplowitz PB. Link between body fat and the timing of puberty. Pediatrics. 2008;121:S208–S217. doi: 10.1542/peds.2007-1813F. [DOI] [PubMed] [Google Scholar]
- Karaolis-Danckert N, Buyken AE, Sonntag A, Kroke A. Birth and early life influences on the timing of puberty onset: results from the DONALD (Dortmund Nutritional and Anthropometric Longitudinally Designed) Study. Am J Clin Nutr. 2009;90:1559–1565. doi: 10.3945/ajcn.2009.28259. [DOI] [PubMed] [Google Scholar]
- Kelce WR, Stone CR, Laws SC, Gray LE, Kemppainen JA, Wilson EM. Persistent DDT metabolite p, p′-DDE is a potent androgen receptor antagonist. Nature. 1995;375:581–585. doi: 10.1038/375581a0. [DOI] [PubMed] [Google Scholar]
- Korrick S, Lee MM, Williams PL, Sergeyev O, Burns JS, Patterson DG, et al. Dioxin exposure and age of pubertal onset among Russian boys. Environ Health Perspect. 2011;119:1339–1344. doi: 10.1289/ehp.1003102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg HM, Melmed S, Polonsky KS, Larsen PR. Williams Textbook of Endocrinology. 11. Saunders, Elsevier Inc; Philadelphia: 2008. [Google Scholar]
- Lam T, Williams PL, Burns JS, Sergeyev O, Korrick SA, Lee MM, et al. Predictors of serum chlorinated pesticide concentrations among prepubertal Russian boys. Environ Health Perspect. 2013;121:1372–1377. doi: 10.1289/ehp.1306480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Largo RH, Prader A. Pubertal development in Swiss boys. Helv Paediatr Acta. 1983;38:211–228. [PubMed] [Google Scholar]
- Li D, Longnecker MP, Dunson DB. Lipid adjustment for chemical exposures accounting for concomitant variables. Epidemiology. 2013;24:921–928. doi: 10.1097/EDE.0b013e3182a671e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mul D, Frederiks AM, van Buuren S, Oostdijk W, Verloove-Vanhorick SP, Wit JM. Pubertal development in the Netherlands 1965–1997. Pediatr Res. 2001;50:479–486. doi: 10.1203/00006450-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Mustanski BS, Viken RJ, Kaprio J, Pulkkinen L, Rose RJ. Genetic and environmental influences on pubertal development: longitudinal data from Finnish twins at ages 11 and 14. Dev Psychol. 2004;40:1188–1198. doi: 10.1037/0012-1649.40.6.1188. [DOI] [PubMed] [Google Scholar]
- Ong KK, Emmett P, Northstone K, Golding J, Rogers I, Ness AR, et al. Infancy weight gain predicts childhood body fat and age at menarche in girls. J Clin Endocrinol Metab. 2009;94:1527–1532. doi: 10.1210/jc.2008-2489. [DOI] [PubMed] [Google Scholar]
- Papadimitriou A, Douros K, Kleanthous K, Papadimitriou DT, Attilakos A, Fretzayas A. Pubertal maturation of contemporary Greek boys: no evidence of a secular trend. J Adolesc Health. 2011;49:434–436. doi: 10.1016/j.jadohealth.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Patterson DG, Jr, Wong LY, Turner WE, Caudill SP, Dipietro ES, McClure PC, et al. Levels in the U.S. population of those persistent organic pollutants (2003–2004) included in the Stockholm Convention or in other long range trans-boundary air pollution agreements. Environ Sci Technol. 2009;43:1211–1218. doi: 10.1021/es801966w. [DOI] [PubMed] [Google Scholar]
- Phillips DL, Pirkle JL, Burse VW, Bernert JT, Jr, Henderson LO, Needham LL. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol. 1989;18:495–500. doi: 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- Quinn MJ, Jr, Summitt CL, Ottinger MA. Consequences of in ovo exposure to p, p′-DDE on reproductive development and function in Japanese quail. Horm Behav. 2008;53:249–253. doi: 10.1016/j.yhbeh.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Ralph JL, Orgebin-Crist MC, Lareyre JJ, Nelson CC. Disruption of androgen regulation in the prostate by the environmental contaminant Hexachlorobenzene. Environ Health Perspect. 2003;111:461–466. doi: 10.1289/ehp.5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall VA. Androgens and hair growth. Dermatol Ther. 2008;21:314–328. doi: 10.1111/j.1529-8019.2008.00214.x. [DOI] [PubMed] [Google Scholar]
- Roche AF, Wellens R, Attie KM, Siervogel RM. The timing of sexual maturation in a group of US white youths. J Pediatr Endocrinol Metab. 1995;8:11–18. doi: 10.1515/jpem.1995.8.1.11. [DOI] [PubMed] [Google Scholar]
- Selevan SG, Rice DC, Hogan KA, Euling SY, Pfahles-Hutchens A, Bethel J. Blood lead concentration and delayed puberty in girls. N Engl J Med. 2003;348:1527–1536. doi: 10.1056/NEJMoa020880. [DOI] [PubMed] [Google Scholar]
- Shisterman EF, Whitcomb BW, Buck Louis GM, Louis TA. Lipid adjustment in the analysis of environmental contaminants and human health risks. Environ Health Perspect. 2005;113:853–857. doi: 10.1289/ehp.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon GS, Tardiff RG, Borzelleca JF. Failure of hexachlorobenzene to induce dominant lethal mutations in the RaP. Toxicol Appl Pharmacol. 1979;47:415–419. doi: 10.1016/0041-008x(79)90337-5. [DOI] [PubMed] [Google Scholar]
- Sjodin A, Jones RS, Lapeza CR, Focant JF, McGahee EE, 3rd, Patterson DJ., Jr Semiautomated high-throughput extraction and cleanup method for the measurement of polybrominated diphenyl ethers, polybrominated biphenyls, and polychlorinated biphenyls in human serum. Anal Chem. 2004;76:1921–1927. doi: 10.1021/ac030381+. [DOI] [PubMed] [Google Scholar]
- Sorensen K, Aksglaede L, Petersen JH, Juul A. Recent changes in pubertal timing in healthy Danish boys: associations with body mass index. J Clin Endocrinol Metab. 2010;95:263–270. doi: 10.1210/jc.2009-1478. [DOI] [PubMed] [Google Scholar]
- Sorensen K, Mouritsen A, Aksglaede L, Hagen CP, Mogensen SS, Juul A. Recent secular trends in pubertal timing: implications for evaluation and diagnosis of precocious puberty. Horm Res Paediatr. 2012;77:137–145. doi: 10.1159/000336325. [DOI] [PubMed] [Google Scholar]
- Sun SS, Schubert CM, Chumlea WC, Roche AF, Kulin HE, Lee PA, et al. National estimates of the timing of sexual maturation and racial differences among US children. Pediatrics. 2002;110:911–919. doi: 10.1542/peds.110.5.911. [DOI] [PubMed] [Google Scholar]
- Susman EJ, Houts RM, Steinberg L, Belsky J, Cauffman E, Dehart G, et al. Longitudinal development of secondary sexual characteristics in girls and boys between ages 9 ½ and 15 ½ years. Arch Pediatr Adolesc Med. 2010;164:166–173. doi: 10.1001/archpediatrics.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child. 1976;51:170–179. doi: 10.1136/adc.51.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner W, DiPietro E, Lapeza C, Green V, Gill J, Patterson DGJ. A fast universal automated cleanup system for the isotope-dilution high-resolution mass spectrometric analysis of PCDDs, PCDFs, coplanar PCBs, PCB congeners, and persistent pesticides from the same serum sample. Organohalogen Compd. 1997;31:26–31. [Google Scholar]
- Van Velsen FL, Danse LH, Van Leeuwen FX, Dormans JA, Van Logten MJ. The subchronic oral toxicity of the beta-isomer of hexachlorocyclohexane in rats. Fundam Appl Toxicol. 1986;6:697–712. [PubMed] [Google Scholar]
- Wang Y, Dinse GE, Rogan WJ. Birth weight, early weight gain and pubertal maturation: a longitudinal study. Pediatr Obes. 2012;7:101–109. doi: 10.1111/j.2047-6310.2011.00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren MP. The effects of exercise on pubertal progression and reproductive function in girls. J Clin Endocrinol Metab. 1980;51:1150–1157. doi: 10.1210/jcem-51-5-1150. [DOI] [PubMed] [Google Scholar]
- Warren MP, Perlroth NE. The effects of intense exercise on the female reproductive system. Horm Sport. 2001;170:3–11. doi: 10.1677/joe.0.1700003. [DOI] [PubMed] [Google Scholar]
- Willers B, Engelhardt L, Pelz L. Sexual maturation in East German boys. Acta Paediatr. 1996;85:758–788. doi: 10.1111/j.1651-2227.1996.tb14152.x. [DOI] [PubMed] [Google Scholar]
- Williams PL, Sergeyev O, Lee MM, Korrick SA, Burns JS, Humblet O, et al. Blood lead levels and delayed onset of puberty in a longitudinal study of Russian boys. Pediatrics. 2010;125:e1088–e1096. doi: 10.1542/peds.2009-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Buck GM, Mendola P. Blood lead levels and sexual maturation in U.S. girls: the Third National Health and Nutrition Examination Survey, 1988–1994. Environ Health Perspect. 2003;111:737–741. doi: 10.1289/ehp.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachmann M, Prader A, Kind P, Hafliger H, Budliger H. Testicular volume during adolescence: cross sectional and longitudinal studies. Helv Paediatr Acta. 1974;29:61–72. [PubMed] [Google Scholar]
- Zawatski W, Lee MM. Male pubertal development: are endocrine disrupting compounds shifting the norms? J Endocrinol. 2013;218:R1–R12. doi: 10.1530/JOE-12-0449. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.