Abstract
Background and objective
There appears to be two distinct clinical phenotypes of obese patients with asthma – those with early-onset asthma and high serum IgE (TH2-high) and those with late-onset asthma and low serum IgE (TH2-low). The aim of the present study was to determine in the two phenotypes of obese asthma the effect of weight-loss on small airway function.
Methods
TH2-low (n=8) and TH2-high (n=5) obese asthmatics underwent methacholine challenge before and 12 months following bariatric surgery. Dose response slopes as measures of sensitivity to airway closure and narrowing were measured as maximum %fall FVC and FEV1/FVC, respectively, divided by dose. Resting airway mechanics were measured by forced oscillation technique.
Results
Weight-loss reduced sensitivity to airway closure in TH2-low but not TH2-high obese asthmatics (pre-post mean change ± 95%CI: 1.8 ± 0.8 doubling doses vs −0.3 ± 1.7 doubling doses, p=0.04). However, there was no effect of weight loss on the sensitivity to airway narrowing in either group (p=0.8, TH2-low: 0.8 ± 1.0 doubling doses, TH2-high: −1.1 ± 2.5 doubling doses). In contrast, respiratory resistance (20Hz) improved in TH2-high but not in TH2-low obese asthmatics (pre-post change median [IQR]: 1.5 [1.3 – 2.8] cmH2O/L/s vs 0.6 [−1.8 – 0.8] cmH2O/L/s, p=0.03).
Conclusions
TH2-low obese asthmatics appear to be characterised by increased small airway responsiveness and abnormalities in resting airway function that may persist following weight loss. However, this was not the case for TH2-high obese asthmatics, highlighting the complex interplay between IgE status and asthma pathophysiology in obesity.
Keywords: Airway closure, Airway hyperresponsiveness, Asthma, Obesity, Weight loss
INTRODUCTION
The obesity epidemic has had detrimental consequences for the management and treatment of patients with asthma. Compared to non-obese asthmatics, obese asthmatics have worse asthma control (1, 2) and symptoms that are less responsive to inhaled corticosteroid (3, 4). Recent research indicates that obese asthmatic patients segregate into two distinct clinical phenotypes– those with early-onset asthma and high serum IgE (TH2-high) and those with late-onset asthma and low serum IgE (TH2-low) (5, 6). This has led to the speculation that TH2-high obese asthmatics have pre-existing allergic asthma that is complicated by obesity, whereas TH2-low obese asthmatics develop asthma symptoms as a consequence of obesity. However, the effect of obesity on asthma pathophysiology in these two phenotypes is not well understood.
We recently reported that weight loss following bariatric surgery improves airway hyperresponsiveness (AHR) in TH2-low obese asthmatics but not in TH2-high obese asthmatics (5). The mechanisms underlying this divergent effect of obesity on AHR were unclear. Healthy obese non-asthmatics have elevated responses to bronchial challenge compared to their non-obese counterparts, as measured by respiratory system resistance (7, 8), airway closure (9), frequency dependence of respiratory system resistance (7) and expiratory flow limitation (10). Since all of these measurements reflect decrements in small airway function, it suggests that obesity increases small airway responsiveness. However, it is unknown whether obesity alters small airway function differentially in TH2-high and TH2-low obese asthmatics, and whether this explains the divergent effects on AHR.
The aim of the present study was to determine the effect of obesity on small airway function in the two phenotypes of obese asthma. Since AHR is reduced by weight loss only in TH2-low obese asthmatics we hypothesised that weight loss would reduce small airway responsiveness, measured as airway closure, in TH2-low obese asthmatics but not in TH2-high obese asthmatics. To test this hypothesis, baseline airway mechanics, assessed with frequency dependent endpoints, and the components of AHR, related to airway narrowing and airway closure, were measured prior to and 12 months following bariatric surgery.
METHODS
Subjects
The asthmatic subjects in the present study comprise a subset of a population reported in a previous publication that investigated the effects of bariatric surgery on airway inflammation, asthma control and AHR, as measured by the traditional lung function parameter FEV1 (5). Volunteers were recruited from the Bariatric Clinic of Fletcher Allen Health Care, Vermont. The Institutional Review Board of the University of Vermont provided ethics approval and all subjects provided written informed consent.
Asthmatics had a doctor diagnosis of asthma, were using prescribed asthma medications and exhibited objective evidence of asthma in the form of either AHR or bronchodilator responsiveness (>12% or 200mL increase in FEV1 and/or forced vital capacity (FVC)). Non-asthmatics had no diagnosis of asthma, no symptoms suggestive of asthma and were not on any asthma medications. All subjects were free from any other respiratory disease (excluding sleep apnea) and upper respiratory tract infection in the preceding month, had less than a 20-pack year smoking history and had not smoked within the preceding six months. Subjects were excluded if their baseline FEV1 was less than 60% of predicted or if the maximum fall in FEV1 during the methacholine challenge was within repeatability limits of the measurement (ie ±150mL) (11). Subjects who did not undergo surgery for personal reasons (n=4) were included in analyses prior to surgery. Data from subjects in whom FEV1 fell less than the limits of repeatability during methacholine challenge following bariatric surgery were excluded from analyses investigating the effects of weight loss (n=5).
Study Design
Obese asthmatics and non-asthmatics had baseline lung function measured by spirometry and by the forced oscillation technique before undergoing methacholine challenge. Obese asthmatics underwent a second complete study visit 12 months after bariatric surgery to determine the effects of weight loss. Asthmatics withheld their use of short-acting β2-agonists for 6h and long-acting β2-agonists for 24h prior to testing.
Forced Oscillation Technique (FOT)
Resting respiratory system mechanics were measured at oscillation frequencies of 5–35Hz during tidal breathing (Impulse Oscillometry System, Jaeger, Wurzburg, Germany). Measurements were made over 20 seconds and values are reported as the average of three acceptable trials. Respiratory system resistance (Rrs) and reactance (Xrs) were calculated at 5Hz, designated as Rrs5Hz and Xrs5Hz, respectively. We also calculated Rrs at 20 Hz (Rrs20Hz) and the difference between Rrs at 5 Hz and at 20 Hz (Rrs5–20Hz), the latter providing a measure of the frequency dependence of resistance.
Methacholine Challenge
Methacholine challenges were performed according to ATS guidelines using the five-breath dosimeter method (12). Challenges consisted of inhaling five vital capacity breaths of doubling concentrations of methacholine from 0.031mg/mL to 16.0mg/mL. FEV1 and FVC were measured after each concentration step, with FVC maneuvers continued for a minimum of 6s and until a clean plateau in the expiratory volume trace. Baseline spirometry was expressed as percent predicted (13).
Analysis of Methacholine Challenge Data
FEV1 is a global non-specific measure of lung function (14), with reductions in FEV1 during bronchial challenge reflecting changes in both airway narrowing and airway closure. FEV1 is reduced by airway narrowing because a narrowed airway loses some of its capacity to transmit flow. However, FEV1 is also determined by the number of parallel airways contributing to flow and is thus reduced by atelectasis or sufficiently severe narrowing of subtending airways, both of which constitute functional airway closure. By contrast, FVC is determined by the volume of expirable air in communication with the airway opening which is reduced by functional airway closure but not by airway narrowing. Air narrowing, per se, is thus reflected in the ratio FEV1/FVC (9, 15). We therefore measured the overall airway response to methacholine as the % fall in FEV1, and its components related to airway narrowing (% fall in FEV1/FVC) and airway closure (% fall in FVC). In addition, we used the ratio % fall in FVC/% fall in FEV1 as an index of the proportion of the change in FEV1 attributable to airway closure, termed the closing index (9).
Airway hyperresponsiveness was assessed by the dose response slope for FEV1 (DRSFEV1), calculated as the percent change in FEV1 at the end of challenge divided by the dose in µmoles (16, 17). A subject was defined as having AHR if DRSFEV1 > 4.4 %ΔFEV1/µmole, equivalent to a provocative concentration causing a 20% fall in FEV1 of less than 16.0mg/mL. Sensitivity to airway narrowing and airway closure were similarly calculated for FEV1/FVC and FVC, designated as DRS(FEV1/FVC) and DRSFVC, respectively.
Serum IgE
Serum IgE levels were measured using a near-infrared particle immunoassay and a Beckman Image 800 Immunochemistry Analyzer (Beckman Coulter, Fullerton, California). Serum IgE was only measured in asthmatics and the upper limit of normal was defined as 100 IU/mL based on previous population data (18). IgE was measured at baseline in all obese asthmatics and repeated following surgery only in obese asthmatics with elevated baseline levels.
Data analysis
Obese asthmatics were grouped into those with normal serum IgE levels (TH2-low) and those with elevated serum IgE levels (TH2-high). Comparisons between TH2-low, TH2-high and obese non-asthmatics at baseline were done using one-way ANOVA with Tukey post-hoc comparisons or Kruskal-Wallis tests with Dunn post-hoc comparison. Comparisons of obese asthmatic data before and 12 months following bariatric surgery were performed using mixed model repeat measures analysis of variance with terms for TH2 group, effect of surgery, and a test of interaction using an interaction term of TH2 group × surgery. Summary data are presented as mean ± 95% confidence intervals (95% CI) unless otherwise stated. The data were analysed using JMP® Pro 10 (SAS Institute Inc., Cary, NC, USA). DRS data were log transformed and presented as geometric mean ± 95% CI with changes in DRS presented as doubling doses. P values < 0.05 were regarded as statistically significant.
RESULTS
Lung function and the response to methacholine challenge prior to bariatric surgery
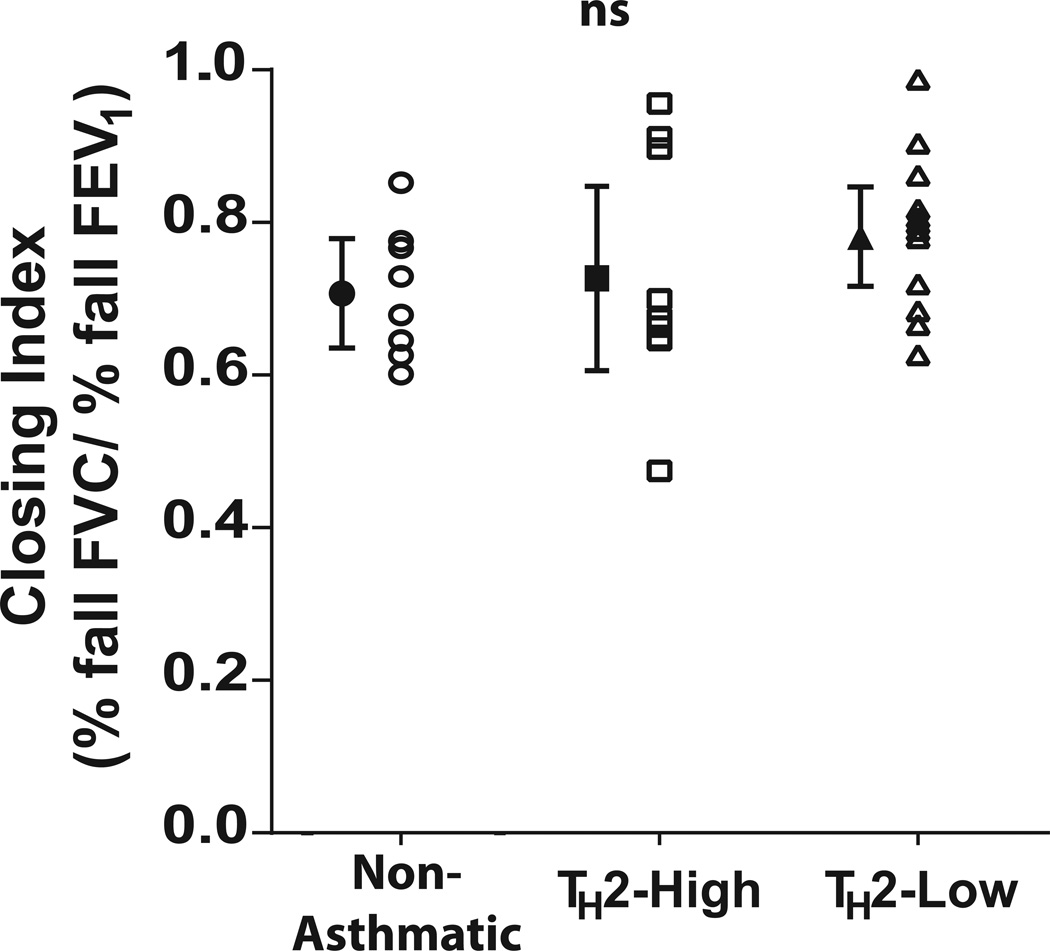
Data from eight obese non-asthmatics, ten obese asthmatics with elevated IgE levels (TH2-high) and 12 obese asthmatics with normal IgE levels (TH2-low) were analysed. There was no difference in age or BMI between the groups (Table 1). Similarly, there was no difference in resting lung function, measured by either spirometry or FOT. Compared to obese non-asthmatics, airway responsiveness as measured by DRSFEV1 was increased in both TH2-high obese asthmatics and TH2-low asthmatics (p<0.05 for both) although there was no difference between the two asthmatic groups (p = 0.74). Despite a reduced overall response to methacholine in the obese non-asthmatics (p< 0.05 for both) the closing index did not differ between the three groups (ANOVA, p= 0.26) (Figure 1).
Table 1.
Baseline lung function data comparing obese non-asthmatics, obese asthmatics with elevated serum IgE (TH2-high) and obese asthmatics with normal serum IgE (TH2-low)
| Obese Non- Asthmatic |
Obese Asthma | p- value |
||
|---|---|---|---|---|
| TH2-high | TH2-low | |||
| IgE (IU/mL)* | - | 282 [175 – 546] | 20 [1 – 49] | - |
| N (female) | 8 (8) | 10 (9) | 12 (11) | - |
| Age (years) | 41.1 ± 7.4 | 42.1 ± 8.7 | 44.6 ± 10.7 | 0.72 |
| BMI (kg/m2)* | 48.6 [42.7 – 57.6] | 47.8 [43.3 – 58.7] | 46.7 [42.3 – 50.5] | 0.85 |
| FEV1 (% pred) | 87.8 ± 9.7 | 83.2 ± 8.6 | 80.6 ± 6.9 | 0.40 |
| FVC (% pred) | 91.1 ± 10.4 | 87.7 ± 8.3 | 83.1 ± 7.7 | 0.34 |
| FEV1/FVC | 77.5 ± 5.1 | 77.5 ± 5.6 | 78.8 ± 2.2 | 0.83 |
| PEF (% pred)* | 86.1 [76.5 – 90.4] | 89.4 [78.3 – 108.2] | 91.7 [80.7 – 97.1] | 0.45 |
| Rrs 5Hz (cmH2O/L/s) $ | 7.0 ± 3.2 | 7.3 ± 2.2 | 7.3 ± 1.1 | 0.97 |
| Rrs 20Hz (cmH2O/L/s)$ | 5.1 ± 1.1 | 5.3 ± 1.6 | 5.1 ± 0.7 | 0.95 |
| Rrs 5Hz–20Hz (cmH2O/L/s)$ | 2.0 ± 1.9 | 2.0 ± 1.2 | 2.1 ± 0.7 | 0.97 |
| Xrs 5Hz (cmH2O/L/s)*$ | −2.2 [−3.3 – −1.7] | −2.2 [−4.9 – −1.8] | −2.7 [−4.0 –−2.0] | 0.83 |
| Max fall in FEV1 | 15.9 ± 2.9 | 22.2 ± 3.6A | 25.1 ± 3.8A | 0.002 |
| DRS (% fall FEV1/µmol MCh)# | 3.8 [3.0 – 4.7] | 18.0 [7.4 – 43.4]A | 24.5 [13.9 – 43.3]A | 0.005 |
| AHR (n)^ | 2 | 8 | 12 | |
| Closing Index | 0.71 ± 0.07 | 0.71 ± 0.12 | 0.79 ± 0.07 | 0.26 |
All data are presented as mean ± 95% CI unless otherwise stated.
Median [IQR],
geometric mean ± 95 % CI
Data are from 5 non-asthmatic subjects
number of subjects with AHR as defined by PC20FEV1 < 16mg/mL
p-value < 0.05 vs obese non-asthmatic
FEV1 = forced expiratory volume in one second, FVC = forced expiratory volume, PEF = peak expiratory flow, Rrs = respiratory system resistance, Xrs = respiratory system reactance, DRS = dose response slope, MCh = methacholine, AHR = airway hyperresponsiveness, defined as >4.4% fall FEV1/µmol MCh, N= number.
Figure 1. Comparison of the proportion of the fall in FEV1 during methacholine challenge that is due to airway closure prior to bariatric surgery.
The Closing Index, calculated as the % fall in FVC/ % fall in FEV1 at the highest dose of the methacholine challenge, was compared between obese non-asthmatic, obese asthmatics with elevated serum IgE (TH2-high) and obese asthmatics with normal serum IgE (TH2-low). A larger closing index represents a greater proportion of overall bronchoconstriction attributed to airway closure. The mean± SEM closing index in normal weight non-asthmatics is 0.54 ± 0.03 (9). ns = non-significant ANOVA.
Lung function and the response to methacholine challenge 12 months following bariatric surgery
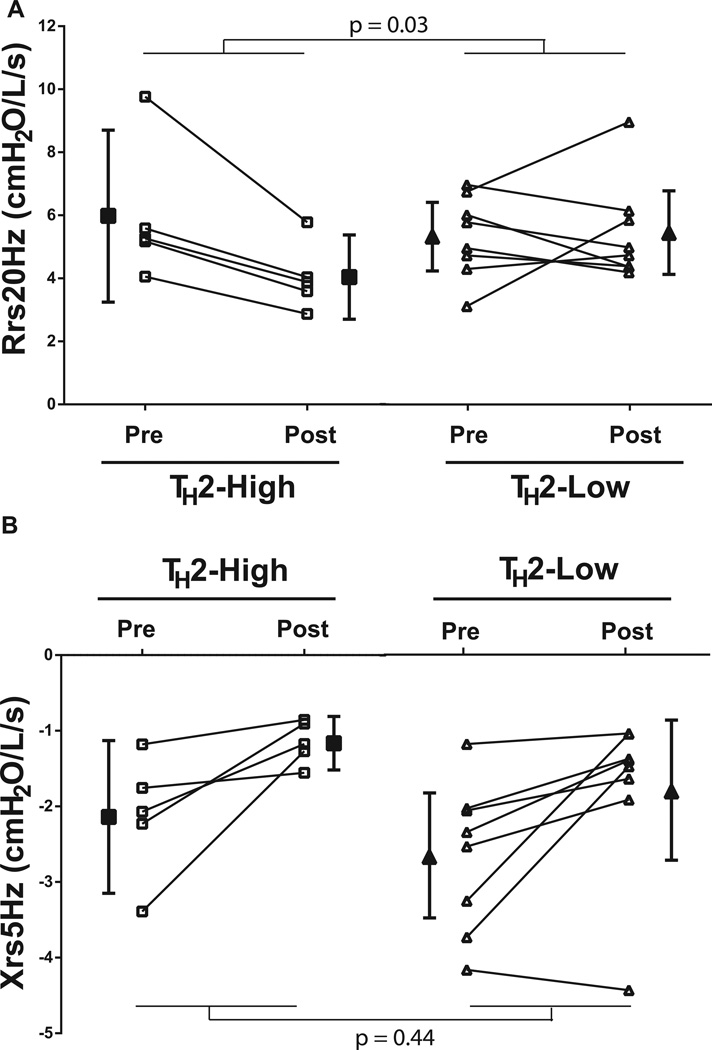
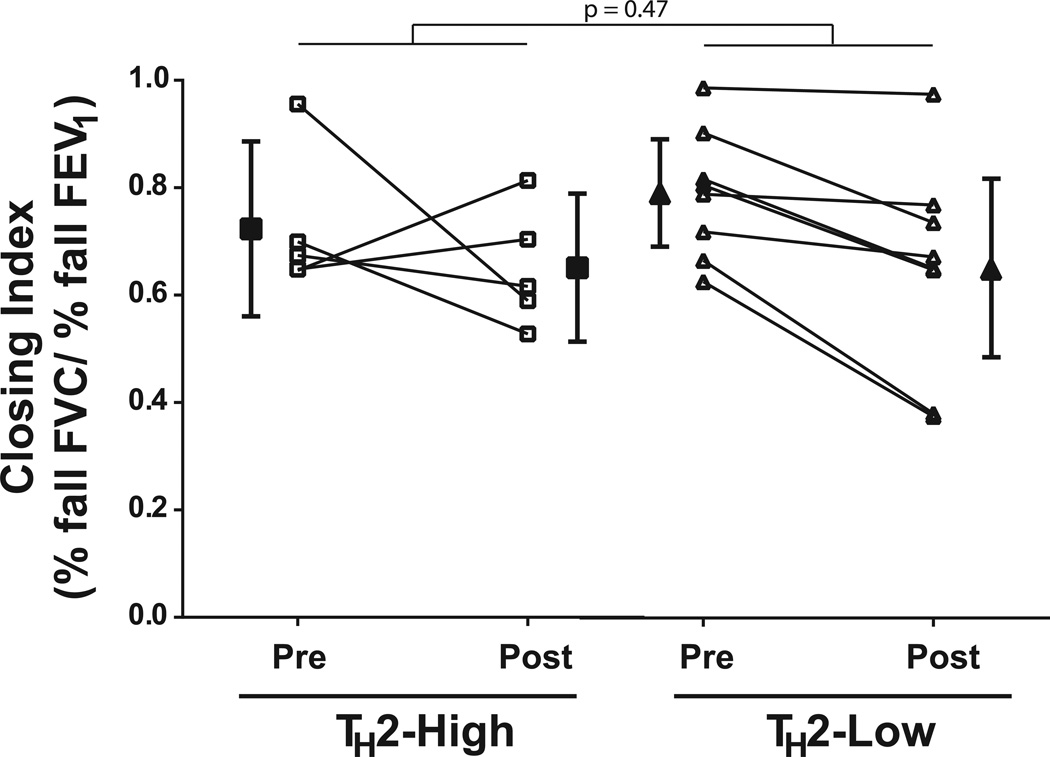
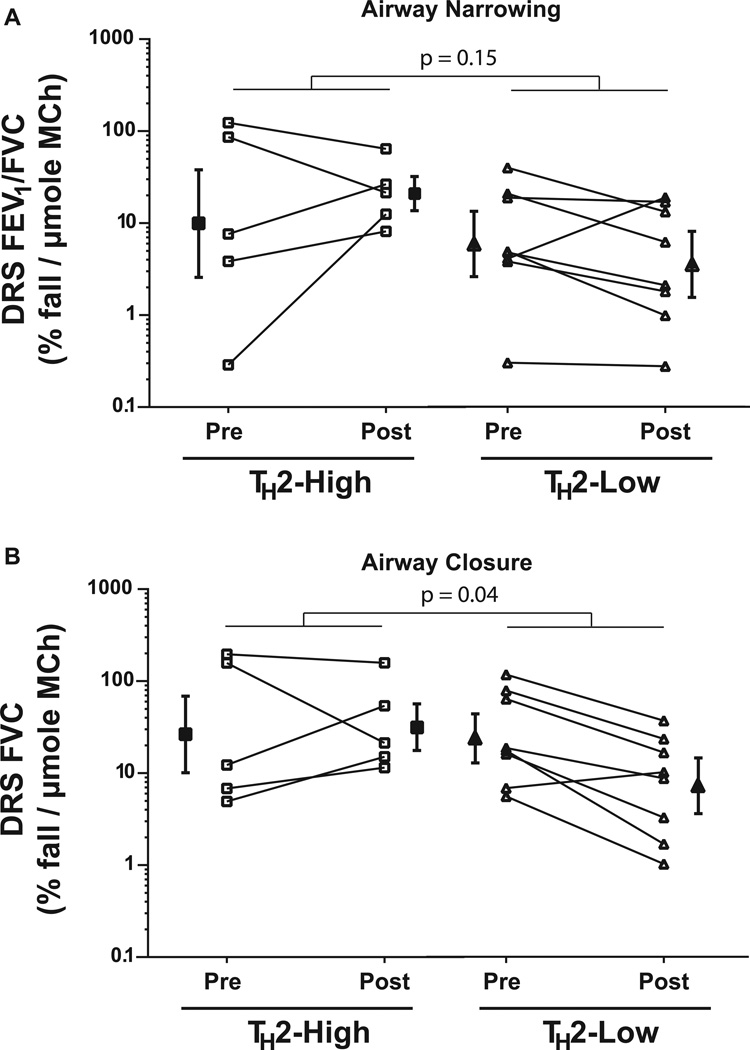
Although bariatric surgery resulted in substantial weight loss, two of five TH2-high obese asthmatics and seven of eight TH2-low obese asthmatics still had a BMI in the overweight or obese range (> 30kg/m2). There was a trend towards greater reductions in BMI in the TH2-high obese asthmatics (interaction p=0.07). IgE levels remained elevated in all TH2-high asthmatics following weight loss, although there was a trend towards a small reduction in absolute levels (median [range] 283 IU/mL [170–593] vs 251 IU/mL [152 – 490], p=0.06). Weight loss improved baseline lung function measured by FEV1 and FVC (p<0.001 and =0.001, respectively) which was similar in the TH2-high and TH2–low groups (interaction p=0.86 and 0.70, respectively). In contrast, there was no improvement in FEV1/FVC or PEF (p=0.35 and 0.28, respectively). On the other hand, weight loss following bariatric surgery affected respiratory system mechanics differently between the groups. Although the improvement in Xrs5Hz was similar in both groups (interaction p=0.44), Rrs20Hz was unaltered by bariatric surgery in the TH2-low group, whereas Rrs20Hz improved in the TH2-high group (interaction p=0.03, Figure 2). Similarly, there was a trend towards a greater improvement in Rrs5Hz following weight loss in the TH2-high asthmatics (interaction p=0.11). In contrast, weight loss did not alter Rrs5–20 in either group (interaction p=0.73). There was an improvement in the closing index following weight loss (p=0.03) that was not different between the groups (interaction p=0.47, Figure 3). However, the sensitivity to airway closure (logDRSFVC) improved in the TH2-low group but not in the TH2-high group (interaction p=0.04), despite no change in the sensitivity to airway narrowing (logDRSFEV1/FVC) in either group (p=0.79, Figure 4). Therefore the improvement in AHR, as measured by FEV1, following bariatric surgery is due to a reduction in airway closure during methacholine challenge.
Figure 2. Comparison of resting respiratory system resistance (Rrs20Hz, A) and reactance (Xrs5Hz, B) in obese asthmatic subjects before (pre) and 12 months following bariatric surgery (post).
Obese asthmatic subjects were grouped into those with elevated IgE (TH2-high) and those with normal IgE (TH2-low).
The p-values shown are for the TH2-status × surgery interaction factor
p = 0.05 (A) and 0.01 (B) for effect of surgery
Figure 3. Comparison of the proportion of the fall in FEV1 during methacholine challenge that is due to airway closure prior to and 12 months following bariatric surgery in obese asthmatic subjects.
Obese asthmatic subjects were grouped into those with elevated IgE (TH2-high) and those with normal IgE (TH2-low). The Closing Index, calculated as the % fall in FVC/% fall in FEV1 at the highest dose of the methacholine challenge, was compared prior to and 12 month following bariatric surgery in obese asthmatic subjects. A larger closing index represents a greater proportion of overall bronchoconstriction attributed to airway closure.
The p-value shown is for the TH2-status × surgery interaction factor
p = 0.03 for effect of surgery
Figure 4. Comparison of the sensitivity to airway narrowing (a) and airway closure (b) in obese asthmatic subjects before (pre) and 12 months following bariatric surgery (post).
Obese asthmatic subjects were grouped into those with elevated IgE (TH2-high) and those with normal IgE (TH2-high). Sensitivity to methacholine was measured as the dose response slope (DRS), calculated as the two point slope from the fall in lung function at the end of challenge divided by the dose of methacholine (MCh) in µmoles. The sensitivity to airway narrowing and airway closure was determined by calculating a DRS using % fall in FEV1/FVC (DRS(FEV1/FVC)) and % fall in FVC (DRSFVC). DRS is log-normally distributed and is thus plotted on a log-scale
The p-values shown are for the TH2-status × surgery interaction factor
.p = 0.79 (A) and 0.11 (B) for effect of surgery
DISCUSSION
Recent evidence of two distinct clinical phenotypes of obese asthma highlights the complexity of the relationship between asthma and obesity (5, 6). In the present study, weight loss in obese asthmatics with early-onset disease and elevated IgE levels (TH2-high) led to an improvement in resting airway mechanics but no effect on the sensitivity to airway closure or AHR. In direct contrast, weight loss in obese asthmatics with late-onset disease and normal serum IgE levels (TH2-low) led to an improvement in airway closure during methacholine challenge and AHR, while resting respiratory resistance was unaltered. These findings suggest a clear differential effect of obesity on the two phenotypes of obese asthma; weight loss does not alter small airway responsiveness in TH2-high obese asthmatics whereas weight loss is associated with a reduction in small airway responsiveness in TH2-low obese asthmatics and may lead to abnormalities in resting airway mechanics that persist following weight loss.
The response to bronchial challenge in obese non-asthmatics is known to be characterised by exaggerated reductions in small airway function as compared to non-obese subjects (7–10). In the present study we found that the contribution of airway closure to the overall response, as measured by the closing index, was similar between obese subjects with and without asthma. Furthermore, weight loss led to a similar improvement in the closing index in TH2-high and TH2-low obese asthmatics. Taken together, these findings suggest that obesity itself increases the contribution of airway closure to the overall response, independent of asthma or atopic status. This is consistent with a lack of difference in closing index between healthy weight non-asthmatics and asthmatics (9). However, the closing index does not correlate with airway responsiveness (9, 15) so to determine the contribution of airway closure and airway narrowing to AHR we calculated dose-response slopes for FVC and FEV1/FVC, respectively. Weight loss reduced the sensitivity to airway closure in TH2-low obese asthmatics but not TH2-high obese asthmatics. On the other hand, the sensitivity to airway narrowing was unaltered by weight loss in either group. Therefore, these findings suggest that obesity increases small airway responsiveness in TH2-low obese asthmatics, but not in TH2-high obese asthmatics.
The present finding that weight loss improves AHR through a reduction in the sensitivity of airway closure is consistent with the role of increased airway closure in AHR (9). Obesity reduces end-expiratory lung volume, thereby reducing the tethering forces of the parenchyma on the small airways potentially predisposing them to closure. This effect of reduced lung volume on AHR has been demonstrated in normal healthy subjects breathing at reduced lung volumes (19, 20). However, although airway responsiveness is associated with FRC in men, this association was not found in women (21), suggesting that non-mechanical factors may underlie the present findings in our predominantly female cohort. Obesity is associated with resistance to leptin (22), a hormone that plays an important role in surfactant synthesis (23). If leptin resistance causes reduced surfactant levels in obese subjects then one would also expect an increased predisposition to airway closure. In keeping with this hypothesis, we recently reported an association between visceral fat leptin expression and AHR (24) in the same cohort of obese asthmatic patients as used in the present study. Similarly, enhanced pro-inflammatory activity of alveolar macrophages in obese asthmatics (25) may predispose to airway closure through effects on surfactant function (26), although this is not a consistent finding (24).
Interestingly, weight loss in TH2-high obese asthmatics did not alter the sensitivity to airway closure and therefore did not improve AHR. This was despite improvements in resistance consistent with the increase in resting lung volume expected to occur with weight loss. Additionally, Xrs5Hz, which reflects the stiffness of the respiratory system, improved similarly in both the TH2-high and TH2-low groups. This was likely due to reductions in the stiffness of the chest wall following weight loss and recruitment of airways that had previously been closed due to compression by excess adipose tissue. Nonetheless, the improvements in both reactance and resistance in TH2-high obese asthmatics are consistent with an increase in end-expiratory lung volume following weight loss. Surprisingly, this did not alter small airway responsiveness. Absolute end expiratory lung volume in obese asthmatics is determined by the combined effects of reduced lung volume due to obesity and hyperinflation due to asthma pathophysiology (27). Therefore, one could speculate that lung volume in TH2-high obese asthmatics may be relatively reduced, but remain at an absolute lung volume that does not predispose to airway closure. On the other hand, airway inflammation appears reduced in the obese (5, 28) while weight loss in obese asthmatics appears to increase production of pro-inflammatory cytokines from stimulated T-lymphocytes (5). It is therefore possible that weight loss in TH2-high obese asthmatics leads to restoration of active airway inflammation which counteracts the beneficial effects of increased lung volume on airway closure.
It is also intriguing that weight loss did not cause an improvement in Rrs20Hz in TH2-low asthmatics since our findings are consistent with an increase in resting lung volumes that would be expected to increase airway calibre. One possible explanation is that the increased predisposition to airway closure in the TH2-low group may have resulted in permanent small airways disease. This may have occurred due to cyclic opening and closing of small airways, which has been shown to cause airway remodeling that is sustained even upon restoration of normal lung volume (29, 30). Indeed, increased airway remodelling has been reported in obese mice following chronic allergen challenge (31). An effect of obesity on airway calibre independent of lung volume is consistent with the recent finding that resistance remains elevated in two thirds of obese patients during lung inflation to predicted FRC (32). On the other hand, the extent of weight loss in the TH2-low obese asthmatics was somewhat less than the TH2-high group, so it is also possible that the TH2-low group did not lose enough weight to cause a significant decrease in resistance. In fact, FRC does not differ between subjects with a BMI of 35–40 and those with BMI > 40kg/m2 (33).
The present study does have limitations. Firstly, the small sample size may have reduced our ability to detect differences in the effect of weight loss on Rrs5Hz between the asthmatic groups (interaction term p=0.11); however, significance would only further support the notion of persistent airway abnormalities in TH2-low obese asthmatics. On the other hand, it is unlikely that a larger sample size would reveal a reduction in the sensitivity to airway closure following weight loss in TH2-high obese asthmatics since the data trended towards an increase in DRSFVC. Secondly, our body plethysmograph was unable to accommodate the morbidly obese patients so we inferred improvements in lung volume following weight loss from changes in FOT parameters. However, direct measurement of FRC with helium dilution would have been helpful in quantifying these improvements. Additionally, our population is almost exclusively female, reflecting the demographics presenting at our bariatric clinic. Since the mechanisms underlying the effect of obesity on airway responsiveness appear distinct between males and females (21), our findings may not translate to males. Lastly, our measure of obesity by BMI does not differentiate between general and abdominal adiposity. However, BMI and waist circumference appear to indicate similar risks for AHR in women (33), suggesting that differences in fat distribution between TH2-high and TH2-low obese asthmatics may not explain the distinct effects of weight loss on airway responsiveness.
In summary, the present findings support the conclusion that there are two distinct clinical phenotypes of obese asthma with distinct pathophysiology, contributed to by disparate effects of obesity and atopic status on small airway function. Obesity appears sufficient to increase small airway responsiveness in obese asthmatics with normal IgE, which may promote permanent abnormalities in resting airway function. In contrast, obesity does not alter small airway responsiveness in obese asthmatics with high serum IgE. These findings of distinct effects of obesity on small airway function highlight the complex interplay between IgE status and asthma pathophysiology in the obese.
Table 2.
Comparison of changes in baseline lung function for obese asthmatic subjects prior to bariatric surgery and 12 months following bariatric surgery stratified by IgE levels prior to surgery
| TH2-high (n = 5) | TH2-low (n = 8) | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre-surgery | Post-surgery | Pre-post change |
Pre-surgery | Post-surgery | Pre-post change |
Weight loss p-value |
interaction p-value |
|
| BMI (kg/m2) | 50.9 ± 13.7 | 32.4 ± 13.1 | 18.4 ± 6.3 | 50.7 ± 9.3 | 38.7 ± 6.3 | 11.9 ± 5.0 | < 0.0001 | 0.07 |
| FEV1 (% pred) | 86.2 ± 10.1 | 97.0 ± 8.1 | −10.8 ± 7.6 | 80.1 ± 9.0 | 91.2 ± 6.7 | −11.8 ± 4.6 | < 0.0001 | 0.86 |
| FVC (% pred) | 89.2 ± 4.4 | 99.8 ± 9.2 | −10.6 ± 12.7 | 82.1 ± 9.9 | 91.0 ± 10.2 | −8.9 ± 4.9 | 0.001 | 0.70 |
| FEV1/FVC | 79.2 ± 9.8 | 79.6 ± 9.6 | −0.4 ± 5.5 | 79.0 ± 2.8 | 80.6 ± 3.1 | −1.5 ± 2.2 | 0.35 | 0.58 |
| PEF (% pred) | 91.2 [79.3 – 105.0] | 85.4 [79.5 – 104.3] | −3.4 [−15.0 – 20.1] | 94.7 [86.7 – 99.2] | 103.1 [90.3 – 114.6] | −10.2 [−22.2 – 0.1] | 0.28 | 0.17 |
| Rrs 5Hz (cmH2O/L/s) | 7.0 ± 3.5 | 4.4 ± 1.7 | 2.6 ± 1.8 | 7.2 ± 1.4 | 7.0 ± 1.9 | 0.3 ± 2.3 | 0.05 | 0.11 |
| Rrs 20Hz (cmH2O/L/s)* | 5.3 [4.6 – 7.7] | 3.9 [3.2 – 4.9] | 1.5 [1.3 – 2.8] | 5.4 [4.4 – 6.6] | 4.9 [4.4 – 6.1] | 0.6 [−1.8 – 0.8] | 0.05 | 0.03 |
| Rrs 5Hz–20Hz (cmH20/L/s)* | 0.84 [0.3 – 1.9] | 0.21 [0.06 – 0.9] | 0.85 [0.16 – 1.05] | 1.9 [1.1 – 3.0] | 1.1 [0.7 – 2.5] | 0.05 [−0.8 – 2.0] | 0.14 | 0.73 |
| Xrs 5Hz (cmH2O/L/s)* | −2.1 [−4.2 – −1.5] | −1.2 [−1.6 – −0.9] | −0.9 [−3.0 –−0.3] | −2.4 [−3.6 – −2.0] | −1.4 [−1.8 – −1.1] | −0.6 [−1.9 –−0.2] | 0.01 | 0.44 |
| Max % fall in FEV1 | 22.7 [21.3 – 27.4] | 22.7 [21.3 – 41.6] | −0.3 [−19.4 – 5.3] | 23.0 [21.7 – 29.2] | 21.5 [14.1 – 27.4] | 4.4 [−1.3 – 10.5] | 0.79 | 0.16 |
| Closing Index | 0.72 ± 0.16 | 0.65 ± 0.14 | 0.07 ± 0.26 | 0.79 ± 0.1 | 0.65 ± 0.17 | 0.14 ± 0.09 | 0.03 | 0.47 |
| DRSFEV1 (% fall /µmol MCh)$ | 36.8 [7.3 – 186.4] | 49.2 [21.7 – 111.4] | −0.4 ± 1.8^ | 31.1 [14.9 – 65.0] | 11.7 [5.2 – 26.2] | 1.4 ± 0.9 | 0.29 | 0.07 |
| DRS(FEV1/FVC) (% fall /µmol MCh)$ | 9.9 [1.1 – 86.6] | 20.8 [10.5 – 41.4] | −1.1 ± 2.5^ | 6.0 [2.1 – 17.0] | 1.7 [1.2 – 10.2] | 0.8 ± 1.0^ | 0.79 | 0.15 |
| logDRS FVC (% fall/µmol MCh)$ | 26.4 [5.6 – 123.7] | 31.6 [12.4 – 31.6] | −0.3 ± 1.7^ | 24.3 [11.1 – 53.2] | 7.4 [3.0 – 17.6] | 1.8 ± 0.8^ | 0.11 | 0.04 |
Data were analysed only from those subjects in whom methacholine challenge caused a > 5% fall in FEV1 at both study visits.
All data are presented as mean ±95% CI unless otherwise stated.
P-values shown are from mixed model repeat measures analysis of variance. Interaction designates the TH2-group × surgery interaction factor.
Median [IQR],
geometric mean ± 95 % CI,
doubling doses
n = 5 for high IgE and 7 for low IgE
FEV1 = forced expiratory volume in one second, FVC = forced expiratory volume, PEF = peak expiratory flow, Rrs = respiratory system resistance, Xrs = respiratory system reactance, DRS = dose response slope, MCh = methacholine. The Closing Index, calculated as the % fall in FVC/ % fall in FEV1 at the highest dose of the methacholine challenge, with larger numbers represen
Summary at a Glance.
In obese asthmatic patients with low IgE, weight loss improves airway hyperresponsiveness related to airway closure, but does not improve resting airway resistance. In obese asthmatics with high IgE, weight-loss improves resting lung mechanics but does not improve airway hyperresponsiveness.
Acknowledgements
This research was supported by National Institutes of Health grants from the National Center for Research Resources (P20 RR15557 and RR019965) and the National Institute of General Medical Sciences (P30 GM103532).
The authors would like to gratefully acknowledge the time, effort and commitment of all those patients that participated in this study. In addition, the authors gratefully acknowledge the invaluable support of members of the Vermont Lung Center, in particular Laurianne Griffes, Stephanie Burns Lorraine Bourassa and Joan Lippman; Department of Anesthesia at University of Vermont/Fletcher Allen Health Care; the Fletcher Allen Operating Room staff; and the faculty and staff of the Fletcher Allen Bariatric Clinic, in particular Laurie Spaulding, MD and Deborah Wachtel. The authors would also like to offer their appreciation to Michael DeSarno, MS for his statistical advice.
Abbreviations
- AHR
airway hyperresponsiveness
- DRS
dose response slope
- FEV1
forced expiratory volume in one second
- FOT
forced oscillation technique
- FRC
functional residual capacity
- FVC
forced vital capacity
- PEF
peak expiratory flow
- Rrs
respiratory system resistance
- Xrs
respiratory system reactance
REFERENCES
- 1.Cazzoletti L, Marcon A, Janson C, Corsico A, Jarvis D, Pin I, Accordini S, Almar E, Bugiani M, Carolei A, Cerveri I, Duran-Tauleria E, Gislason D, Gulsvik A, Jogi R, Marinoni A, Martinez-Moratalla J, Vermeire P, de Marco R. Asthma control in Europe: a real-world evaluation based on an international population-based study. J Allergy Clin Immunol. 2007;120(6):1360–1367. doi: 10.1016/j.jaci.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 2.Lessard A, Turcotte H, Cormier Y, Boulet LP. Obesity and asthma: a specific phenotype? Chest. 2008;134(2):317–323. doi: 10.1378/chest.07-2959. [DOI] [PubMed] [Google Scholar]
- 3.Boulet LP, Franssen E. Influence of obesity on response to fluticasone with or without salmeterol in moderate asthma. Respir Med. 2007;101(11):2240–2247. doi: 10.1016/j.rmed.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 4.Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM. Influence of body mass index on the response to asthma controller agents. Eur Respir J. 2006;27(3):495–503. doi: 10.1183/09031936.06.00077205. [DOI] [PubMed] [Google Scholar]
- 5.Dixon AE, Pratley RE, Forgione PM, Kaminsky DA, Whittaker-Leclair LA, Griffes LA, Garudathri J, Raymond D, Poynter ME, Bunn JY, Irvin CG. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol. 2011;128(3):508–515. e1–e2. doi: 10.1016/j.jaci.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holguin F, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Erzurum SC, Fitzpatrick AM, Gaston B, Israel E, Jarjour NN, Moore WC, Peters SP, Yonas M, Teague WG, Wenzel SE. Obesity and asthma: an association modified by age of asthma onset. J Allergy Clin Immunol. 2011;127(6):1486–93. e2. doi: 10.1016/j.jaci.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skloot G, Schechter C, Desai A, Togias A. Impaired response to deep inspiration in obesity. J Appl Physiol. 2011;111(3):726–734. doi: 10.1152/japplphysiol.01155.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salome CM, Munoz PA, Berend N, Thorpe CW, Schachter LM, King GG. Effect of obesity on breathlessness and airway responsiveness to methacholine in non-asthmatic subjects. Int J Obes (Lond) 2008;32(3):502–509. doi: 10.1038/sj.ijo.0803752. [DOI] [PubMed] [Google Scholar]
- 9.Chapman DG, Berend N, King GG, Salome CM. Increased airway closure is a determinant of airway hyperresponsiveness. Eur Respir J. 2008;32(6):1563–1569. doi: 10.1183/09031936.00114007. [DOI] [PubMed] [Google Scholar]
- 10.Mahadev S, Farah CS, King GG, Salome CM. Obesity, expiratory flow limitation and asthma symptoms. Pulm Pharmacol Ther. 2012 doi: 10.1016/j.pupt.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 12.Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JS, Anderson SD, Cockcroft DW, Fish JE, Sterk PJ. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161(1):309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 13.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 14.Mead J. Problems in interpreting common tests of pulmonary mechanical function. In: Macklem P, Permutt S, editors. The lung in transition between death and disease. New York: Marcel Dekker Inc; 1979. pp. 43–51. [Google Scholar]
- 15.Gibbons WJ, Sharma A, Lougheed D, Macklem PT. Detection of excessive bronchoconstriction in asthma. Am J Respir Crit Care Med. 1996;153(2):582–589. doi: 10.1164/ajrccm.153.2.8564102. [DOI] [PubMed] [Google Scholar]
- 16.O'Connor G, Sparrow D, Taylor D, Segal M, Weiss S. Analysis of dose-response curves to methacholine. An approach suitable for population studies. Am Rev Respir Dis. 1987;136(6):1412–1417. doi: 10.1164/ajrccm/136.6.1412. [DOI] [PubMed] [Google Scholar]
- 17.Peat JK, Salome CM, Berry G, Woolcock AJ. Relation of dose-response slope to respiratory symptoms and lung function in a population study of adults living in Busselton, Western Australia. Am Rev Respir Dis. 1992;146(4):860–865. doi: 10.1164/ajrccm/146.4.860. [DOI] [PubMed] [Google Scholar]
- 18.Marsh DG, Bias WB, Ishizaka K. Genetic control of basal serum immunoglobulin E level and its effect on specific reaginic sensitivity. Proc Natl Acad Sci U S A. 1974;71(9):3588–3592. doi: 10.1073/pnas.71.9.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chapman DG, Berend N, Horlyck KR, King GG, Salome CM. Does increased baseline ventilation heterogeneity following chest wall strapping predispose to airway hyperresponsiveness? J Appl Physiol. 2012;113(1):25–30. doi: 10.1152/japplphysiol.01582.2011. [DOI] [PubMed] [Google Scholar]
- 20.Ding DJ, Martin JG, Macklem PT. Effects of lung volume on maximal methacholine-induced bronchoconstriction in normal humans. J Appl Physiol. 1987;62(3):1324–1330. doi: 10.1152/jappl.1987.62.3.1324. [DOI] [PubMed] [Google Scholar]
- 21.Torchio R, Gobbi A, Gulotta C, Dellaca R, Tinivella M, Hyatt RE, Brusasco V, Pellegrino R. Mechanical effects of obesity on airway responsiveness in otherwise healthy humans. J Appl Physiol (1985) 2009;107(2):408–416. doi: 10.1152/japplphysiol.00083.2009. [DOI] [PubMed] [Google Scholar]
- 22.Rajala MW, Scherer PE. Minireview: The adipocyte--at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144(9):3765–3773. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- 23.Torday JS, Rehan VK. Stretch-stimulated surfactant synthesis is coordinated by the paracrine actions of PTHrP and leptin. Am J Physiol Lung Cell Mol Physiol. 2002;283(1):L130–L135. doi: 10.1152/ajplung.00380.2001. [DOI] [PubMed] [Google Scholar]
- 24.Sideleva O, Suratt BT, Black KE, Tharp WG, Pratley RE, Forgione P, Dienz O, Irvin CG, Dixon AE. Obesity and asthma: an inflammatory disease of adipose tissue not the airway. Am J Respir Crit Care Med. 2012;186(7):598–605. doi: 10.1164/rccm.201203-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lugogo NL, Hollingsworth JW, Howell DL, Que LG, Francisco D, Church TD, Potts-Kant EN, Ingram JL, Wang Y, Jung SH, Kraft M. Alveolar macrophages from overweight/obese subjects with asthma demonstrate a proinflammatory phenotype. Am J Respir Crit Care Med. 2012;186(5):404–411. doi: 10.1164/rccm.201109-1671OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Hohlfeld JM, Schmiedl A, Erpenbeck VJ, Venge P, Krug N. Eosinophil cationic protein alters pulmonary surfactant structure and function in asthma. J Allergy Clin Immunol. 2004;113(3):496–502. doi: 10.1016/j.jaci.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Nicolacakis K, Skowronski ME, Coreno AJ, West E, Nader NZ, Smith RL, McFadden ER., Jr Observations on the physiological interactions between obesity and asthma. J Appl Physiol. 2008;105(5):1533–1541. doi: 10.1152/japplphysiol.01260.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berg CM, Thelle DS, Rosengren A, Lissner L, Toren K, Olin AC. Decreased fraction of exhaled nitric oxide in obese subjects with asthma symptoms: data from the population study INTERGENE/ADONIX. Chest. 2011;139(5):1109–1116. doi: 10.1378/chest.10-1299. [DOI] [PubMed] [Google Scholar]
- 29.D'Angelo E, Pecchiari M, Baraggia P, Saetta M, Balestro E, Milic-Emili J. Low-volume ventilation causes peripheral airway injury and increased airway resistance in normal rabbits. J Appl Physiol. 2002;92(3):949–956. doi: 10.1152/japplphysiol.00776.2001. [DOI] [PubMed] [Google Scholar]
- 30.Yalcin HC, Perry SF, Ghadiali SN. Influence of airway diameter and cell confluence on epithelial cell injury in an in vitro model of airway reopening. J Appl Physiol. 2007;103(5):1796–1807. doi: 10.1152/japplphysiol.00164.2007. [DOI] [PubMed] [Google Scholar]
- 31.Saraiva SA, Silva AL, Xisto DG, Abreu SC, Silva JD, Silva PL, Teixeira TP, Parra ER, Carvalho AL, Annoni R, Mauad T, Capelozzi VL, Silva PM, Martins MA, Rocco PR. Impact of obesity on airway and lung parenchyma remodeling in experimental chronic allergic asthma. Respir Physiol Neurobiol. 2011;177(2):141–148. doi: 10.1016/j.resp.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Oppenheimer BW, Berger KI, Segal LN, Stabile A, Coles KD, Parikh M, Goldring RM. Airway dysfunction in obesity: response to voluntary restoration of end expiratory lung volume. PLoS One. 2014;9(2):e88015. doi: 10.1371/journal.pone.0088015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim KM, Kim SS, Kwon JW, Jung JW, Kim TW, Lee SH, Min KU, Kim YY, Cho SH. Association between subcutaneous abdominal fat and airway hyperresponsiveness. Allergy Asthma Proc. 2011;32(1):68–73. doi: 10.2500/aap.2011.32.3407. [DOI] [PubMed] [Google Scholar]