Abstract
We have previously demonstrated that long-term tolerance (LTT) of an MHC class-I mismatched renal allograft can be achieved with a short course of cyclosporine. In order to examine regulatory mechanisms underlying tolerance in this model, we assessed the contributions of factors within the graft and in the peripheral blood for their relative roles in the maintenance of stable tolerance. Twelve LTT recipients of MHC class-I mismatched primary kidneys were subjected to a treatment consisting of donor-specific transfusion (DST) followed by leukapheresis, in order to remove peripheral leukocytes, including putative regulatory T cells (Tregs). Following treatment, two controls were followed clinically and 10 animals received a second, donor-MHC-matched kidney. Neither control animal showed evidence of rejection, while 8 of 10 re-transplanted animals developed either rejection crisis or full rejection of the second transplant. In vitro assays confirmed that the removed leukocytes were suppressive and that CD4+Foxp3+ Treg reconstitution in blood and kidney grafts correlated with return to normal renal function in animals experiencing transient rejection crises. These data indicate that components of accepted kidney grafts as well as peripheral regulatory components both contribute to the tolerogenic environment required for tolerance of MHC class-I mismatched allotransplants.
Introduction
We have explored previously the mechanisms underlying immune tolerance of MHC class-I mismatched allografts in MGH miniature swine (1–3). In this model, transplantation of kidney allografts followed by 12 days of high-dose Cyclosporine A (CyA) uniformly induces long-term tolerance (LTT) across an MHC class-I barrier (4). Using this well-established renal tolerance model, we have determined that a) presence of an intact thymus is essential in the induction (5), but not for the maintenance of tolerance (6); b) IL-10 is overexpressed in the cell populations that infiltrate tolerated grafts (1); and c) peripheral blood lymphocytes (PBL) from tolerant animals can suppress in vitro anti-donor CTL reactivity by naive recipient-matched PBL in a donor-specific manner. Furthermore, this cellular suppression is dose-dependent and radiation-sensitive, requires cell-to-cell contact, and is not reversed by exogenous IL-2 administration (7–9). While these studies support the hypothesis that regulatory mechanisms play an essential role in the induction and maintenance of tolerance, they provide only indirect evidence of the role of regulatory cells in this process.
Direct evidence of a Treg mechanism in this model would be two-fold: firstly, if tolerance is mediated by Tregs, it should be possible to successfully adoptively transfer tolerance using Tregs (in the graft and in the blood). Secondly, LTT abrogation should be achievable through Treg removal. Recently we demonstrated the successful adoptive transfer of tolerance in this kidney model. Data showed that adoptive transfer of both peripheral lymphocytes from long-term tolerant animals in combination with the long-term tolerated graft led to stable tolerance in recipients without further pharmacologic immunosuppression (10). Because the tolerated graft along with peripheral lymphocytes led to successful adoptive transfer of tolerance, we hypothesized that removal of these elements from a tolerant animal should abrogate tolerance. The present study was designed to evaluate the impact of modulations in peripheral regulatory cells, through removal of tolerogeneic peripheral leukocytes and LTT kidney grafts, on the maintenance of tolerance. Our results indicate that long-term survival of allografts results from an active and durable process which functions both within the graft and the peripheral blood.
Material and Methods
Animals
The intra-MHC recombinant haplotypes have been described previously (5,7,11). Recipients (SLAdd) and donors (SLAgg) were 3–8 months of ages and size-matched. Experimental groups are described in Figures 1A and 1B in the results section.
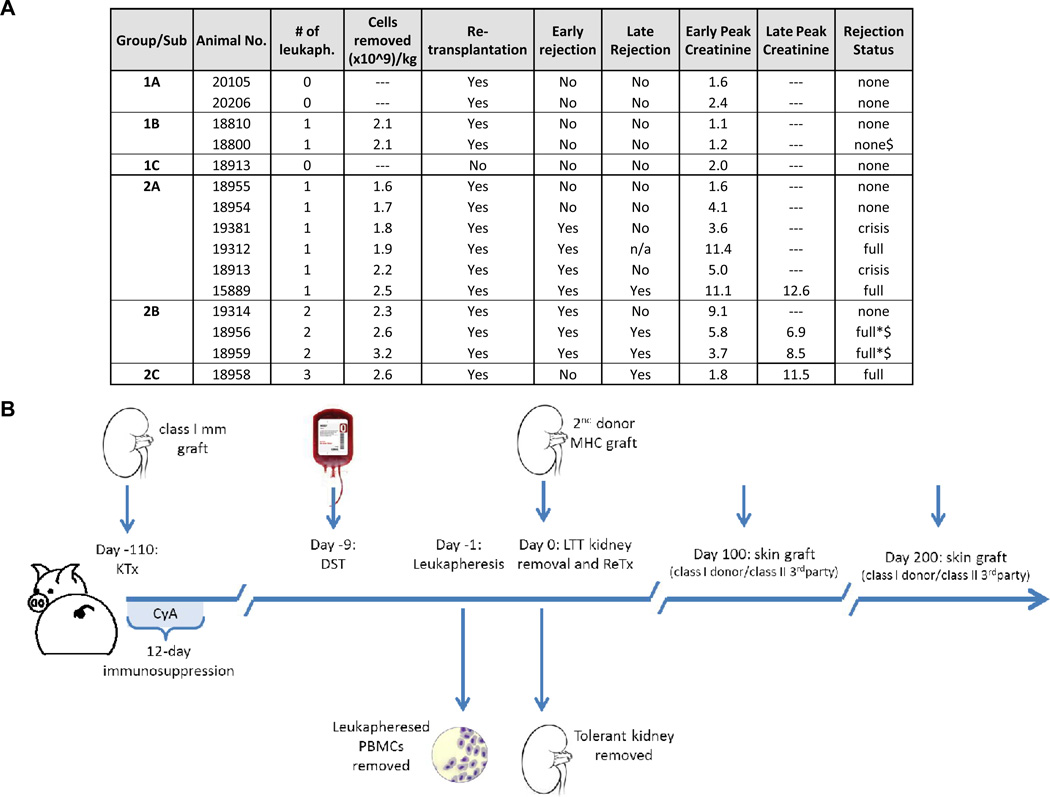
Figure 1. Experiments of tolerance abrogation and experimental animals.
A). List of Experimental Groups. DST = Donor Specific Transfusion; Retransplantation = Graftectomy of primary kidney followed by retransplantation with a Donor MHC Matched (Class I MHC mismatched, Class II MHC matched) kidney; Early Rejection = Rejection Crisis within 10 days following kidney retransplantation; Late Rejection = Rejection Crisis >90 days following kidney retransplantation; Early Peak Creatinine = Peak Creatinine Level Observed in Early Rejection Period; Late Peak Creatinine = Peak Creatinine Level Observed in Late Rejection Period. (*). n/a = not applicable. Because animal #19312 died of rejection on POD 8, late peak creatinine was not obtainable. Animals #18959 and #18956 exhibited prolonged, full rejection only after skin grafting ($). B). Timeline of experiments. Time points are relative to the day of the second transplantation (day 0). Skin grafts were performed on animals 18800, 19324, 18956, and 18959.
Donor-Specific Transfusion (DST) and Leukapheresis
DST consisted of whole blood obtained from a donor-MHC-matched (SLAgg) animal and was administered at 10mL/kg. Leukapheresis was performed via internal jugular vein catheter over 6–8 hours according to described procedure (12). Leukapheresis was not cell-type specific, but rather removed all types of circulating leukocytes.
Renal transplantation and retransplantation
Primary orthotopic kidney transplantation and retransplantation in MGH swine have been previously described (5,10). At time of retransplantation, the prior tolerated graft was surgically removed and replaced with a naïve SLA-matched renal allograft.
Skin Grafting
Split-thickness skin grafts (4×8 cm) were harvested from donors and placed on the dorsum or flank of recipients and were monitored daily for color, texture and temperature, and considered rejected when less than 10% of the grafted tissue was viable (13).
Immunosuppression and Rejection Monitoring
Cyclosporine A (Novartis Pharmaceutical Corp., Hanover, NJ) was administered I.V. daily at a dose of 10 to 13 mg/kg and adjusted to maintain a blood level of 400–800 ng/ml for 12 days, starting on the day of the primary renal transplantation. Whole blood trough CyA levels were determined as previously reported (14).
Histopathology and Immunohistochemistry
Routine renal wedge biopsies were performed through a flank incision on days 30 and 60 post-transplantation, during periods of rejection, and/or at time of death. A senior transplant pathologist scored rejection. Immunohistochemical analysis was performed as previously described (10). Graft infiltrating cells were analyzed by staining renal biopsies for CD3, CD4, CD25 and FoxP3.
Peripheral Blood Lymphocytes (PBL) for cell mediated cytolysis (CML)
Freshly heparinized whole blood was diluted 1:2 with HBSS (GIBCO BRL, Gaithersburg, MD) and the mononuclear cells were obtained by gradient centrifugation using lymphocyte separation medium (Organon Teknika, Durham, NC). One-way cell-mediated lympholysis (CML) was carried out using fresh responder PBL and irradiated target PBLs as described (5).
Antibodies and Flow Cytometry
Foxp3 was mouse-anti-Rat (eBioscience). All other antibodies were conjugated in our laboratory (15–18). Cell binding was carried out at 4 °C using Hanks’ Balanced Salt Solution (HBSS; Invitrogen, Gaithersburg, MD) containing 5% Bovine Serum Albumin (BSA) and 0.5% sodium azide. Results were acquired on a Becton Dickinson FACScan microfluorometer (Sunnyvale, CA).
Suppression assay by CML cocultures
CML coculture assays were performed as previously described (7,8). Following primary culture (6 days), primed responder cells were harvested and incubated overnight at 4°C, after which they were coincubated with naive SLA-matched PBLs and irradiated donor-type or third party PBLs for 6 additional days. The cultures were harvested and results were analyzed as described above.
ELISA
Serum TGF-B1 was evaluated using the CytoScreen ELISA kit (BioSource International) as previously reported (19).
Results
Establishment of tolerance
Fifteen animals underwent bilateral native nephrectomy and MHC class-I mismatched kidney transplantation (Figure 1A) followed by 12 days of CyA to permit tolerance induction and all became LTT (4).
DST effect on the stability of tolerance
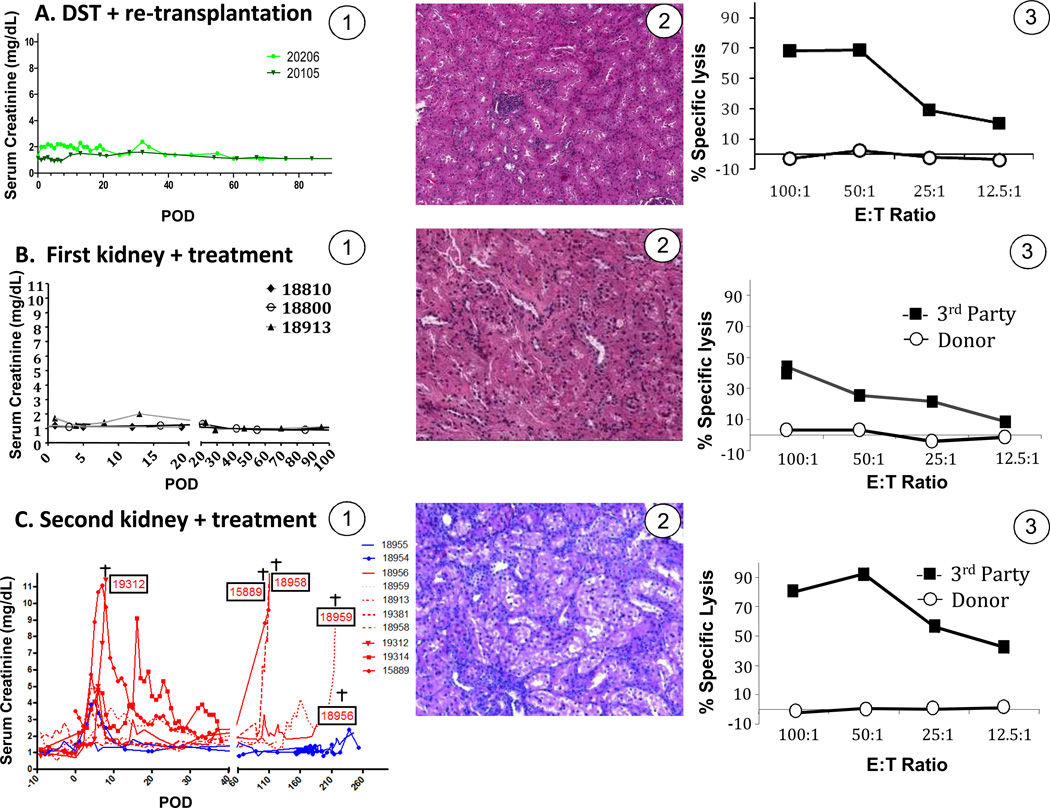
Prior to beginning our tolerance abrogation protocol (described below), all LTT animals were subjected to donor-specific transfusion (DST) comprised of 10mL/kg of non-irradiated, donor-matched whole blood. DST was performed as part of a study of adoptive transfer experiments carried out in parallel to the present study (10). We have previously shown the DST neither abrogates tolerance nor leads to sensitization in tolerant animals, although DST may lead to an increase in circulating regulatory cells (10,20). To confirm these historical observations, and to ensure that DST was not inducing an alteration in the tolerant state by itself, two LTT animals underwent DST more than 100 days after primary kidney transplantation, and were retransplanted 9 days thereafter (Figure 1A, Group 1A). Both animals kept low and stable creatinine levels, remained unresponsive in vitro and histology confirmed acceptance of the second graft (Figure 2, panel 1). These data therefore proved that DST and retransplantation could not induce abrogation of tolerance.
Figure 2. Clinical course and immune status of recipients of first and second renal transplants.
A) Control animals # 20105 and # 20206 underwent DST and retransplantation, without removal of peripheral cells by leukapheresis. A-1: Serum creatinine levels for animals in group 1; A-2: Representative graft histology from animals of group 1 (animal 20206, POD 100 after retransplantation) and; A-3: Corresponding CTL reactivity representative of animals in group 1 (animal 20206, POD 100). B). Control LTT animals included pigs# 18800 and 18810 that received DST + leukapheresis without retransplantation and # 18913 which was retransplanted without treatment (Figure 1A, group 1). B-1: Serum creatinine levels for animals in group 1; B-2: Representative graft histology from animals of group 1 (animal 18800) and; B-3: Corresponding CTL reactivity representative of animals in group 1 (animal 18800). This CML assay was performed 2 weeks after DST and leukapheresis. C). Animals receiving second renal transplants after DST + leukapheresis. C-1: Serum creatinine for animals in groups 2A, B, and C. levels. C-2: Graft histology representative of group rejectors from group 2 (animal # 18959); this sample was obtained 8 days after retransplantation (ACR2-3). C-3: Corresponding cytotoxic activity for animal 18959 which was representative for animals of group 2. E/T: effector to target ratio in CML assays. ACR = Acute cellular rejection
Studies to determine the role of peripheral lymphocytes in stable tolerance
In order to investigate the role of peripheral lymphocytes, specifically Tregs, we removed lymphocytes from LTT animals by extensive leukapheresis (Figure 1B). Using leukapheresis, CD4+/FoxP3+ cells in addition to other circulating lymphocytes, decreased in similar proportions (not shown). The contribution of Treg cells in the recipient was first assessed using two LTT animals (Figure 1A: Group 1B). Animals #18800 and #18810 underwent leukapheresis, without graftectomy and without retransplantation. Both animals maintained normal renal function without histological signs of rejection (Figure 2B, panel 1 and 2). #18810 was sacrificed 20 days following transplant for biopsies of all lymphoid and renal tissue. #18800 was followed for 100 days at which point this portion of the experiment was terminated. Donor-specific tolerance was confirmed in CML assays showing absence of T cell cytotoxicity to donor antigens while the anti-third party reactivity was preserved (Figure 2B, panel 3).
To assess whether retransplantation could, on its own, prevent tolerance induction of a second donor-matched kidney, LTT animal #18913 was retransplanted with a naïve donor-matched graft without undergoing leukapheresis or adding immunosuppression (Figure 1A: Group 1C). As expected from prior studies (4), retransplantation alone (without DST and leukapheresis) did not lead to rejection of the re-transplanted graft (Figure 2A, panels 1 and 2). These data indicated that neither the leukapheresis alone nor retransplantation had a direct impact on graft survival.
The intra- and extra-graft milieu both contributed to tolerance maintenance
Ten LTT animals underwent DST, leukapheresis and retransplantation. Animals from group 2A (n=6) underwent DST followed by a single leukapheresis one day prior to retransplantation (Figure 1A). Four of 6 animals in group 2A experienced rejection crises which led to complete graft rejection by POD 8 and 112 for animals #19312 and #15889, respectively (Figure 2C, panel 1). Graft rejection was confirmed for these 4 animals (as for all animals in this study) on biopsies collected during rejection crises, which revealed lymphocytic infiltration (Figure 2C, panel 2). Surprisingly, graft rejection did not coincide with a return of peripheral anti-donor cytotoxic responses (Figure 2C, panel 3). To maximize the number of cells removed with leukapheresis, 4 animals (18956, 18959, 18958, and 19314) also underwent leukapheresis on day −2 (group 2B) and animal 18958 (group 2C) had a third leukapheresis on the morning of the day 0, immediately prior to retransplantation. Seven of 10 animals in group 2 (A, B, and C) experienced early rejection. Rejection crises began approximately 4 days following retransplantation and serum creatinine peaks ranged from 3.6 mg/dL to 11.5 mg/dL, for these 7 animals with rejection (Figure 2C, panel 1).
Number of cells removed correlated with rejection
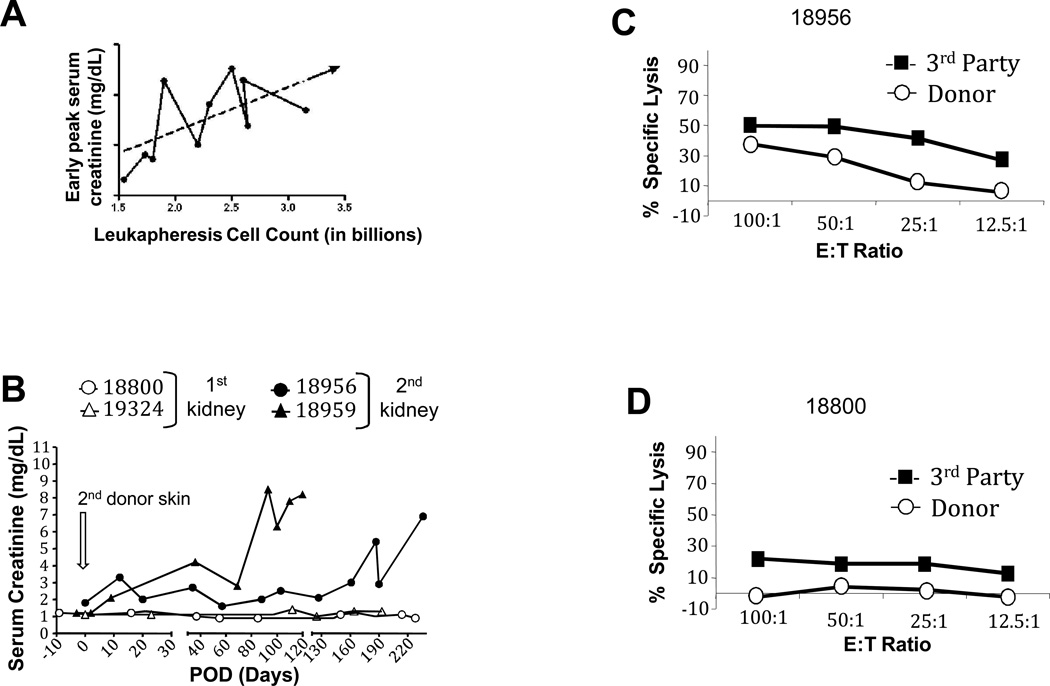
The animals with the lowest number of cells removed (18954 and 18955) had no histological signs of rejection. There was a trend toward more severe rejection episodes (higher creatinine peaks and longer rejection crises) with larger leukapheresis products (Figure 3A), suggesting that the number of cells removed correlated with rejection.
Figure 3. Effect of leukapheresis.
A). More extensive leukaphereses correlated with higher initial peak creatinine, which was used as read out of rejection. Assessment of tolerance of renal allografts with skin grafts (B-D): Two animals (18956, 18959) which experienced rejection crises (Figure 2, panel C1) that subsequently resolved were challenged with 2 consecutive donor skin grafts according to the timeline of Figure 1B (day 0 = 2nd skin grafting). Control animals (18800 and 19324) are recipients of first kidney grafts that received (18800) or not (19324) the DST + leukapheresis treatment prior to skin grafting. B). Serum creatinine in recipients of skin transplants. C and D). CML assays, with representative data, performed 2 weeks after skin grafting. Studies for animals # 18800 and experimental animal #18956 are shown.
Abrogation of peripheral tolerance of allogeneic kidneys
Results from figure 2 showed that the majority of animals who underwent the complete treatment (DST + leukapheresis and retransplantation) displayed early episodes of rejection with partial resolution. This suggested that the state of tolerance of first grafts became less robust following leukapheresis and placement of a naïve second donor-matched transplant. As seen in figure 2C, the appearance of leukocyte infiltration in second transplants coincided with rejection crises, suggesting that intra-graft donor-specific alloreactivity was only temporarily inhibited in accepted first transplants.
We hypothesized that the persistence of alloreactive T cells in LTT swine led to early rejection episodes. Providing additional CD4 help through the placement of donor-MHC class I matched/class II third party skin allografts tested this hypothesis.
Skin graft experiments were performed 100 and 200 days after retransplantation, on 2 groups of animals (Figure 1B). Group 1 included two animals LTT of a first kidney with stable tolerance parameters; group 2 included two animals subjected to DST + leukapheresis followed by retransplantation which led to rejection crisis in both animals. Skin graft experiments in animals of group 2 were performed after rejection had resolved (Figure 1A; Figure 3B). All animals rejected their allogeneic skin transplants between POD 8 and 12 while accepting autologous skin grafts (not shown). Sensitization to donor skin antigens had no effects on the survival of long-term accepted kidney grafts in control animals (Figure 3B). In contrast, experimental animals (#18956 and #18959) developed prolonged rejection crises and eventual full graft rejection (Figure 3B). Graft rejection coincided with the emergence of peripheral cytotoxicity to donor antigens (Figure 3C) whereas animals from group 1 maintained donor-specific unresponsiveness (Figure 3D).
In vitro evidence for Tregs and their contribution to tolerance maintenance
1) The leukapheresis product was suppressive
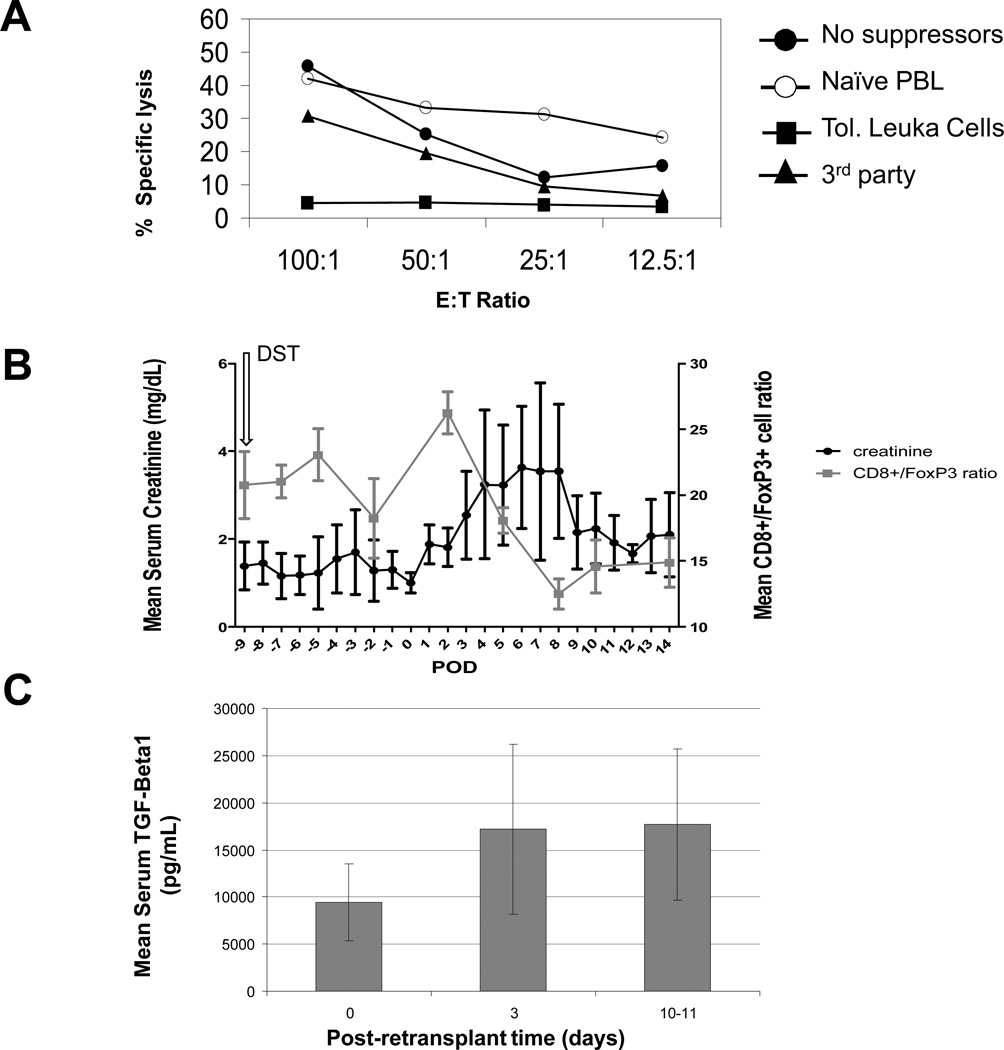
Tregs have been implicated in numerous models of transplantation tolerance using both small and large animal models (14,21–23). We therefore examined whether the cells of the leukapheresed product could likewise be implicated in tolerance of retransplanted grafts. To determine this, we tested whether these cells were suppressive in vitro. First, we tested the ability of the leukapheresis product to suppress donor-specific allogeneic cytotoxicity. Results from a representative suppression assay (Figure 4A) indicated that the leukocyte fraction removed from a DST-treated LTT animal was capable of suppressing cytotoxic responses to donor cells. Thus, these data suggest that a population of suppressive cells with specificity for donor antigens was removed by leukapheresis. Depletion of CD25+ cells from the leukapheresis product did not fully restore donor responsiveness in vitro (not shown). These data suggest that, in addition to CD25+ cells, other cell subpopulations might also have regulatory effects in the periphery.
Figure 4. Potential involvement of regulatory T cells in tolerance to kidney grafts.
A). The CML Suppression assay. Control anti-donor CML involved recipient matched PBLs stimulated with donor-type PBLs (no-suppressors, solid circles). Percent lysis observed when suppressor populations added were either naïve recipient MHC-matched (open-circles), or leukapheresis product from LTT animals (Tol. Leuka Cells, solid squares). Specificity controls for suppression involved LTT leukapheresis products added to recipient CML against 3rd party (3rd party-MHC-I/recipient matched Class-II). Additional negative controls included 3rd party cells cultured independent of cells in leukapheresis product and self anti-self controls (not shown). Suppression assays were performed in duplicate. B). Monitoring of CD8+: CD4+ Foxp3+ ratios (left axis) and serum creatinine levels (right axis) in experimental animals. Error bars reflect standard deviation. C). Serum TGF(−1 levels (ng/mL) for four animals which experienced rejection of second renal transplants, but demonstrated resolution of renal function (18913, 18956, 18959, and 19381) are presented as mean TGF(−1 levels with associated standard deviations during times when animals experienced acute rejection. Increases in TGF-B1 correlated temporally with the expansion of peripheral Tregs (See 4B).
2) Flow cytometry of peripheral blood
Next, we studied whether peripheral leukocyte depletion achieved by leukapheresis had an effect on the Treg pool. Treg levels were also compared with clinical rejection. Because leukapheresis removed all leukocytes equally, the percentage of Tregs in the leukapheresed product was the same as the Treg level in peripheral blood. The mean number of CD4+/FoxP3+ cells in the blood increased to 200 cells/uL following DST and decreased (to approximately 50 cells/uL) following leukapheresis (not shown). Combined results from the 8 retransplanted LTT recipients which experienced rejection revealed that DST induced a cell expansion both in the CD8+ alloreactive component and in the CD4+/FoxP3+ population, and the CD8+/ CD4+FoxP3+ ratio remained unaltered after the DST. However, an increase in CD8+/ CD4+FoxP3+ ratio was observed after retransplantation. CD4+/FoxP3+ cell populations increased between days 3 to 8 following retransplantation (Figure 4B) and the CD8+/ CD4+FoxP3+ were at levels below the pre-treatment baseline. Subsequently, serum creatinine decreased between days 5 and 10, suggesting a relationship between increased Tregs and resolution of rejection (i.e. serum creatinine).
3) Levels of TGF beta1 in peripheral blood
Several cytokines, including TGF-beta1, have been associated with Treg function (24). We monitored TGF-beta1 serum levels in animals that developed rejection and spontaneous resolution. Concentrations of TGF-beta1 decreased with leukapheresis (not shown), but subsequently increased following retransplantation and normalized in approximately 10 days (Figure 4C). The increase in TGF-beta1 temporally correlated with a) the expansion of Tregs (described above) and b) the resolution of rejection (Figure 4C). These findings support the potential role of TGF-beta1 producing cells, including suppressive Tregs, in the control of rejection crises in this model.
4) Rejection resolution correlated with the presence of intra-graft Tregs
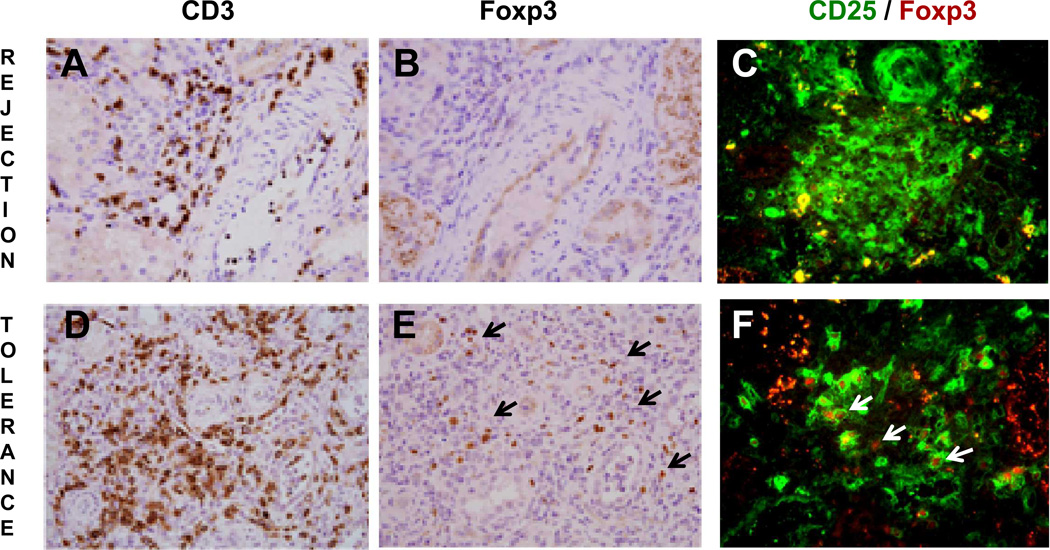
Renal biopsies collected during and after rejection crises of second transplants were stained to identify CD3+ effector lymphocytes as well as Foxp3+ Treg cells (Figure 5, samples called rejection or tolerance, respectively). Comparisons between these two sample sets revealed that both grafts had similar densities of CD3+ cell infiltrates (Figure 5A and 5D, respectively). In contrast, their content in Foxp3+ cells was markedly lower in the rejection sample (Figure 5B and 5E). Further immunohistochemistry analysis of the same samples showed higher ratios of CD25+Foxp3+ Treg / CD25+Foxp3− T effector cells in rejection-controlled than rejected transplants (Figure 5C and 5F).
Figure 5.
Accepted second transplants are selectively infiltrated by Foxp3+ Tregs. Renal graft biopsies from animal # 18958, which experienced complete rejection (Rejection) and from animal # 18959 (pre-skin graft) with resolved rejection (Tolerance) were analyzed by immunohistochemistry for their respective content in CD3+ (brown, A and D). Foxp3+ (brown, B and E) and Foxp3+/CD25+ lymphocytes (C and F). Black arrowheads in 5E highlight brown FoxP3+ cells. Double labeling for CD25-Foxp3 identifies Treg cells with surface CD25 (green) and intracellular Foxp3 (red) labeling (white arrow heads). Magnification = 200X (A, B, D, E) and 400X (C and F). These data are from animals experiencing full rejection or acceptance of second transplants.
Discussion
Data in this study demonstrated that removal of peripheral leukocytes and tolerated kidneys from LTT kidney recipients were both required to abrogate tolerance across an MHC class-I mismatch in our MHC-inbred large animal model. LTT animals first underwent leukaphereses to remove leukocytes, which included circulating T regulatory cells. The impact of graft infiltrating cells of host origin on maintenance of tolerance was evaluated by replacing long-term accepted kidneys (containing host infiltrating leukocytes) with naïve donor-matched grafts devoid of host cell infiltrates. Loss of tolerance was assessed by rejection of donor-matched second transplants that, otherwise, were accepted.
Results from figure 2B demonstrated the robustness of peripheral tolerance. Despite the removal of >1.5 billon leukocytes, which were shown to be suppressive in a donor-specific fashion, tolerance remained intact. In fact, neither the leukapheresis protocol nor the retransplantation were able, on their own, to induce rejection of the graft. In contrast, the combination of both procedures prompted rejection. DST was added to the preparatory regimen as many of these animals were used as kidney and cells donors in studies on adoptive transfer of tolerance (the mirror study of the present work. (10)). Previous in vitro observations from our group have shown that DST in this model might corroborate tolerance rather than helping to abrogate it. These observations were confirmed by animals in group 1A, in which DST followed by retransplantation without removal of peripheral cells via leukapheresis did not induce an alteration of the tolerant state. It is therefore highly unlikely that DST is responsible for the effects observed in the experimental Group #2. A more focused investigation of DST in this model is currently underway.
We have also observed a correlation between the number of leukocytes removed via leukapheresis and the severity of rejection, as measured by peak serum creatinine. This correlation was not statistically significant; however a trend toward rejection with greater numbers of peripheral cells removed was observed. These data suggest that circulating regulatory cells contribute to maintenance of tolerance. One possible explanation for rejection following leukapheresis is recent data showing that that lymphopenia-induced homeostatic proliferation of lymphocytes (possibly caused by the leukapheresis) may lead to restoration of effector cell function (25). However, if re-expansion of the T effector pool alone was responsible for rejection, we should have seen rejection following leukapheresis in animals that were not retransplanted; but we did not. Because rejection only occurred following both the removal of a tolerated graft and subsequent retransplantation, the cells infiltrating the graft may play a dominant role in controlling rejection. Episodes of rejection following retransplantation did not correlate with anti-donor cytotoxicity measured in CML assays on blood cells. This suggested that peripheral cytotoxic alloresponses were mainly concentrated within the transplants as documented by increased leukocyte infiltrations at time of rejection (Figure 2, panel 2C). For a second graft, the inflammatory milieu created by the DST + leukapheresis and retransplantation may have been sufficient to reactivate donor-specific alloresponses.
Rejection of second renal grafts occurred following skin transplantation (donor-MHC class I matched/class II third party). We have previously shown that transplantation of donor-MHC class I matched/class II third party skin allografts onto tolerant animals leads to the development of donor specific cytotoxicity in-vitro, without rejection of the transplanted graft (13,26). These findings suggest that an active immune protection mechanism is present in accepted kidneys, possibly reflected by the presence of intra-graft Tregs. The outcomes after skin grafting encountered in the present study were quite different from our prior experience. In the present study, skin grafting onto animals that experienced rejection crises after leukapheresis and retransplantation prompted prolonged and full kidney rejection. Comparison of skin grafting outcomes with animals that received leukapheresis, but no second transplant strongly suggests that tolerated kidneys from the transplanted group (group 2A, B, and C) were not efficiently protected. Together these results demonstrate the strength and durability of peripheral tolerance, as it was only after extensive intervention (i.e. leukapheresis, retransplantation, and skin grafting) that we were able to overcome, fully, mechanisms of peripheral tolerance. However, after DST, leukapheresis, and retransplantation it is reasonable to infer that these mechanisms of peripheral tolerance were weakened, such that skin-grafting with T-cell help led to complete rejection (14).
Because regulatory T cells have been implicated in tolerance mechanisms of linked suppression (19), this cell subset appears to be an obvious candidate for immune regulation in this model (7,27). The data from this study provides evidence to support this hypothesis. We showed that the leukapheresis product possessed suppressive capability (Figure 4A). Rejection following retransplantation may be explained by a change in the balance of alloreactive cells and T regulatory cells following leukapheresis and retransplantation. This hypothesis is substantiated by the correlation between improved clinical outcome and return of peripheral Tregs which was detectable before POD 8 suggesting that a re-expansion of the Treg pool was potentially sufficient to control rejection (Figure 4B). Control of rejection of second donor-matched grafts and expansion of the Treg pool was also associated with increases in serum TGF-beta1, a cytokine involved in Treg cell maturation (Figure 4C)(24). Although it was not possible to measure more relevant graft TGF-beta1 levels on a larger number of animals, the trend observed suggests that Tregs are involved in controlling rejection in this model. Finally, the presence of Tregs in LTT grafts and in grafts where rejection spontaneously resolved reinforces the significance of their involvement (Figure 5). Given the potential importance of these intra-graft Tregs, ongoing studies in our laboratory are aimed at quantifying their contribution to the maintenance of tolerance.
We have previously demonstrated that the presence of renal allograft is required for the indefinite maintenance of tolerance in this model. We showed in a prior model that when the tolerated kidney was removed for 3 months, abrogation of systemic tolerance was observed. Further, immunization of recipients with donor class-I peptides, after nephrectomy of the primary tolerated kidney, led to loss of tolerance at both the T-cell and B-cell levels (14). The ultimate proof of a Treg mechanism in this model would be to a) show successful adoptive transfer of tolerance using Tregs (in the graft and in the blood), and to b) demonstrate that LTT abrogation can be achieved through their removal. We have recently carried out adoptive transfer experiments in which tolerance was transferred using leukapheresed peripheral leukocytes and an explanted tolerated graft from LTT animals (10). Our data in this study, as a mirror experiment to adoptive transfer, confirms the adoptive transfer data by showing that removal of both the leukapheresed peripheral leukocytes and tolerated graft from LTT animals resulted in subsequent rejection. While a more specific method of Treg removal would be selective T-reg depletion, this technology was not technically feasible due to the amount of antibody required for removal of the selective populations from a large tolerant animal (>60kg) in this study. However, leukapheresis, as shown, significantly decreased the number of circulating T regulatory cells. The data presented here strengthen our previous data and confirm that tolerance in this model is mediated by mechanisms of peripheral T cell regulation. In addition, these data show that both peripheral T cells and presence of LTT kidneys play an essential role. Our data to assess cell populations in leukapheresis products demonstrated that in vitro suppressive effects were maintained even after removal of CD25+ cell subpopulations. These results suggest that other suppressive cell populations may be involved in the maintenance of tolerance (8). Based on these data, we are now attempting to identify specific tolerogeneic cell populations in both peripheral blood and in the kidneys of LTT animals by selectively depleting Tregs and through the isolation of renal parenchyma/endothelial cells.
Acknowledgments
This work was supported in part by an American Society of Transplantation Grant awarded to Dr. Joseph R. Scalea, Massachusetts General Hospital Swine Facility Grant 1C06RR20135-01, the MGH Swine Facility grant #C06 RR020135-01 and NIH/NIAID 4R37AI031046-21. The authors gratefully acknowledge Novartis for Cyclopsorine, Drs. David Leonard and Joren Madsen for critical review of the manuscript and Rebecca A. Brophy for expert editorial assistance.
Abbreviations
- LTT
Long-term tolerance
- MHC
Major histocompatibility complex
- CyA
Cyclosporine A
- Tregs
T regulatory cells
- MGH
Massachusetts General Hospital
- CML
Cell Mediated Lympholysis
- POD
Post-operative day
- DST
Donor specific transfusion
- TGF
Transforming growth factor beta
- AJT
American journal of transplantation
- SLA
Swine leukocyte antigen
Footnotes
Animals were cared for according to the guidelines of the Massachusetts General Hospital (MGH) Institutional Animal Care and Use Committee.
Author contributions:
Joseph R. Scalea: Participated in research design, performance of the research, data analysis and writing of the paper
Masayoshi Okumi: Participated in research design, performance of the research and data analysis
Vincenzo Villani: Participated in performance of the research and data analysis
Akira Shimizu Participated in performance of the research (Pathology)
Hiroaki Nishimura: Participated in the performance of the research
Bradford C. Gillon: Participated in the performance of the research, participated in data analysis
Radbeh Torabi: Participated in the research
Taylor Cormack: Participated in the performance of the research (in vitro studies)
Shannon Moran: Assist the research (in vivo and in vitro)
Christian LeGuern: Participated in data analysis and paper writing
David H. Sachs: Participated in research design, data analysis and writing of the paper
Kazuhiko Yamada: Participated in research design, the performance of the research, data analysis, and writing of the paper
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Blancho G, Gianello P, Germana S, Baetscher M, Sachs DH, LeGuern C. Molecular identification of porcine interleukin-10: regulation of expression in a kidney allograft model. Proc Natl Acad Sci USA. 1995;92:2800–2804. doi: 10.1073/pnas.92.7.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fishbein JM, Rosengard BR, Gianello P, et al. Development of tolerance to class II mismatched renal transplants following a short course of cyclosporine therapy in miniature swine. Transplantation. 1994;57:1303–1308. doi: 10.1097/00007890-199405150-00002. [DOI] [PubMed] [Google Scholar]
- 3.Fishbein JM, Gianello P, Nickeleit V, et al. Interleukin-2 reverses the ability of cyclosporine to induce tolerance to class I disparate kidney allografts in miniature swine. Transplant Proc. 1993;25:322–323. [PubMed] [Google Scholar]
- 4.Rosengard BR, Ojikutu CA, Guzzetta PC, et al. Induction of specific tolerance to class I disparate renal allografts in miniature swine with cyclosporine. Transplantation. 1992;54:490–497. doi: 10.1097/00007890-199209000-00020. [DOI] [PubMed] [Google Scholar]
- 5.Yamada K, Gianello PR, Ierino FL, et al. Role of the thymus in transplantation tolerance in miniature swine. I. Requirement of the thymus for rapid and stable induction of tolerance to class I-mismatched renal allografts. J Exp Med. 1997;186:497–506. doi: 10.1084/jem.186.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vagefi PA, Ierino FL, Gianello PR, et al. Role of the thymus in transplantation tolerance in miniature Swine: IV. The thymus is required during the induction phase, but not the maintenance phase, of renal allograft tolerance. Transplantation. 2004;77:979–985. doi: 10.1097/01.tp.0000116416.10799.c6. [DOI] [PubMed] [Google Scholar]
- 7.Ierino FL, Yamada K, Hatch T, Rembert J, Sachs DH. Peripheral tolerance to class I mismatched renal allografts in miniature swine: donor antigen-activated peripheral blood lymphocytes from tolerant swine inhibit antidonor CTL reactivity. J Immunol. 1999;162:550–559. [PubMed] [Google Scholar]
- 8.Ierino FL, Yamada K, Lorf T, Arn JS, Sachs DH. Mechanism of tolerance to class I-mismatched allografts in miniature swine: Regulation of interleukin-2 receptor a-chain expression on CD8 peripheral blood lymphocytes of tolerant animals. Transplantation. 1998;66:454–460. doi: 10.1097/00007890-199808270-00007. [DOI] [PubMed] [Google Scholar]
- 9.Gianello PR, Blancho G, Fishbein JF, et al. Mechanism of cyclosporin-induced tolerance to primarily vascularized allografts in miniature swine. Effect of administration of exogenous IL-2. J Immunol. 1994;153:4788–4797. [PubMed] [Google Scholar]
- 10.Okumi M, Scalea JR, Gillon BC, et al. The induction of tolerance of renal allografts by adoptive transfer in miniature swine. Am J Transplant. 2013;13:1193–1202. doi: 10.1111/ajt.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sachs DH, Leight G, Cone J, Schwartz S, Stuart L, Rosenberg S. Transplantation in miniature swine. I. Fixation of the major histocompatibility complex. Transplantation. 1976;22:559–567. doi: 10.1097/00007890-197612000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Nash K, Chang Q, Watts A, et al. Peripheral blood progenitor cell mobilization and leukapheresis in pigs. Lab Anim Sci. 1999;49:645–649. [PubMed] [Google Scholar]
- 13.Gianello PR, Fishbein JF, Rosengard BR, et al. Tolerance to class I disparate renal allografts in miniature swine: maintenance of tolerance despite induction of specific antidonor CTL responses. Transplantation. 1995;59:772–777. doi: 10.1097/00007890-199503150-00023. [DOI] [PubMed] [Google Scholar]
- 14.Okumi M, Fishbein JM, Griesemer AD, et al. Role of Persistence of Antigen and Indirect Recognition in the Maintenance of Tolerance to Renal Allografts. Transplantation. 2008;85:270–280. doi: 10.1097/TP.0b013e31815e8eed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blancho G, Gianello PR, Lorf T, et al. Molecular and cellular events implicated in local tolerance to kidney allografts in miniature swine. Transplantation. 1997;63:26–33. doi: 10.1097/00007890-199701150-00006. [DOI] [PubMed] [Google Scholar]
- 16.Pescovitz MD, Thistlethwaite JR, Jr., Auchincloss H, Jr., et al. Effect of class II antigen matching on renal allograft survival in miniature swine. J Exp Med. 1984;160:1495–1508. doi: 10.1084/jem.160.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pescovitz MD, Sachs DH, Lunney JK, Hsu SM. Localization of class II MHC antigens on porcine renal vascular endothelium. Transplantation. 1984;37:627–630. [PubMed] [Google Scholar]
- 18.Pescovitz MD, Lunney JK, Sachs DH. Preparation and characterization of monoclonal antibodies reactive with porcine PBL. J Immunol. 1984;133:368–375. [PubMed] [Google Scholar]
- 19.Griesemer AD, LaMattina JC, Okumi M, et al. Linked suppression across an MHC-mismatched barrier in a miniature swine kidney transplantation model. J Immunol. 2008;181:4027–4036. doi: 10.4049/jimmunol.181.6.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee RS, Rusche JR, Cheatham LA, et al. CTLA4Ig and donor-specific transfusion prolongs cardiac allograft survival in the miniature swine. J Heart Lung Transplant. 2001;20:150. doi: 10.1016/s1053-2498(00)00256-4. [DOI] [PubMed] [Google Scholar]
- 21.Rosengard BR, Ojikutu CA, Guzzetta PC, et al. Renal transplantation in miniature swine: Preliminary evidence that graft infiltrating leukocytes suppress donor-specific cell-mediated lymphocytotoxicity in co-culture. Transplant Proc. 1991;23:189–191. [PubMed] [Google Scholar]
- 22.Rosengard BR, Kortz EO, Guzzetta PC, et al. Transplantation in miniature swine: Analysis of graft-infiltrating lymphocytes provides evidence for local suppression. Hum Immunol. 1990;28:153–158. doi: 10.1016/0198-8859(90)90012-e. [DOI] [PubMed] [Google Scholar]
- 23.Nobori S, Shimizu A, Okumi M, et al. Thymic rejuvenation and the induction of tolerance by adult thymic grafts. Proc Natl Acad Sci U S A. 2006;103:19081–19086. doi: 10.1073/pnas.0605159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu S, Zhang N, Yopp AC, et al. TGF-beta induces Foxp3 + T-regulatory cells from CD4 + C. Am J Transplant. 2004;4:1614–1627. doi: 10.1111/j.1600-6143.2004.00566.x. [DOI] [PubMed] [Google Scholar]
- 25.Schietinger A, Delrow JJ, Basom RS, Blattman JN, Greenberg PD. Rescued tolerant CD8 T cells are preprogrammed to reestablish the tolerant state. Science. 2012;335:723–727. doi: 10.1126/science.1214277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosengard BR, Kortz EO, Ojikutu CA, et al. The failure of skin grafting to break tolerance to class I disparate renal allografts in miniature swine despite inducing marked anti-donor cellular immunity. Transplantation. 1991;52:1044–1052. doi: 10.1097/00007890-199112000-00020. [DOI] [PubMed] [Google Scholar]
- 27.Ierino FL, Yamada K, Hatch T, Sachs DH. Preliminary in vitro evidence for regulatory cells in a miniature swine renal allograft model. Transplant Proc. 1997;29:1165. doi: 10.1016/s0041-1345(96)00515-5. [DOI] [PubMed] [Google Scholar]