Summary
Metazoans adapt to a low oxygen environment (hypoxia) through activation of stress response pathways. Here we report that transient hypoxia exposure extends lifespan in C. elegans through mitochondrial ROS-dependent regulation of the nutrient sensing kinase TOR and its upstream activator RHEB-1. The increase in lifespan during hypoxia requires the intestinal GATA-type transcription factor, ELT-2, downstream of TOR signaling. Using RNA-Sequencing, we describe an ELT-2-dependent hypoxia response that includes an intestinal glutathione S-transferase, GSTO-1, and uncover that GSTO-1 is required for lifespan under hypoxia. These results indicate mitochondrial ROS-dependent TOR signaling integrates metabolic adaptations to confer survival under hypoxia.
Introduction
Oxygen serves as the final electron acceptor during mitochondrial oxidative phosphorylation and, thus, plays a crucial role in cellular metabolism and energy production. During periods of low oxygen (i.e. hypoxia), the cell activates adaptive responses that concomitantly increase oxygen delivery and dampen oxygen demand by tissues (Semenza, 2011). The best characterized response to hypoxia is the activation of a family of transcription factors known as hypoxia inducible factors (HIFs) (Majmundar et al., 2010). HIFs are heterodimers consisting of two basic helix-loop-helix/PAS proteins, HIF-1α and HIF-1β. While the HIF-1β subunit is constitutively expressed in the cell, the HIF-1α subunit is only present during hypoxia. Under normoxia, HIF-1α is hydroxylated at proline residues by the prolyl hydroxylase, PHD2, and subsequently targeted for degradation by an E3 ubiquitin ligase that contains a specificity factor, the von Hippel Lindau tumor suppressor (pVHL) (Kaelin and Ratcliffe, 2008). The activity of PHD2 is inhibited under hypoxic conditions, allowing the accumulation of HIF-1α protein and subsequent binding to HIF-1β for translocation into the nucleus. The resulting HIF transcriptional response includes genes which promote angiogenesis, erythropoiesis, and repress mitochondrial metabolism. Interestingly, HIF-1 has been shown to increase the replicative capacity of mammalian cells, suggesting that activation of HIF targets may be a strategy to prevent age-related declines in cellular function (Bell et al., 2007; Welford et al., 2006).
A common genetic model to study organismal aging is the nematode, C. elegans, which have a conserved HIF-1-dependent hypoxia response regulated by VHL and the PHD2 homolog, EGL-9. HIF-1 is necessary and sufficient to extend the lifespan of C. elegans (Mehta et al., 2009; Zhang et al., 2009). But, aside from HIF activation, there is very little known about hypoxia-initiated pathways that promote metabolic adaptation to increase lifespan. To address this question, we developed a transient hypoxia assay in which C. elegans were exposed to a single 36 hour treatment of hypoxia. We uncovered that transient hypoxia exposure was sufficient to extend lifespan in C. elegans through mitochondrial ROS-dependent regulation of the nutrient sensing kinase TOR (target of rapamycin) and its upstream activator RHEB-1. Interestingly, inactivation of RHEB-1 in intestinal cells was sufficient to block longevity under transient hypoxia suggesting that the hypoxia response was not cell-autonomous. This led to the identification of the intestinal GATA-type transcription factor, ELT-2 as a key mediator of the transient hypoxia response, independent from HIF-1. Under transient hypoxia, ELT-2 controls the upregulation of an intestinal glutathione S-transferase, GSTO-1, to extend lifespan. Thus, hypoxia induces intestinal TOR and ELT-2 signaling to extend lifespan in C. elegans.
Results and Discussion
Transient hypoxia extends lifespan through TOR signaling
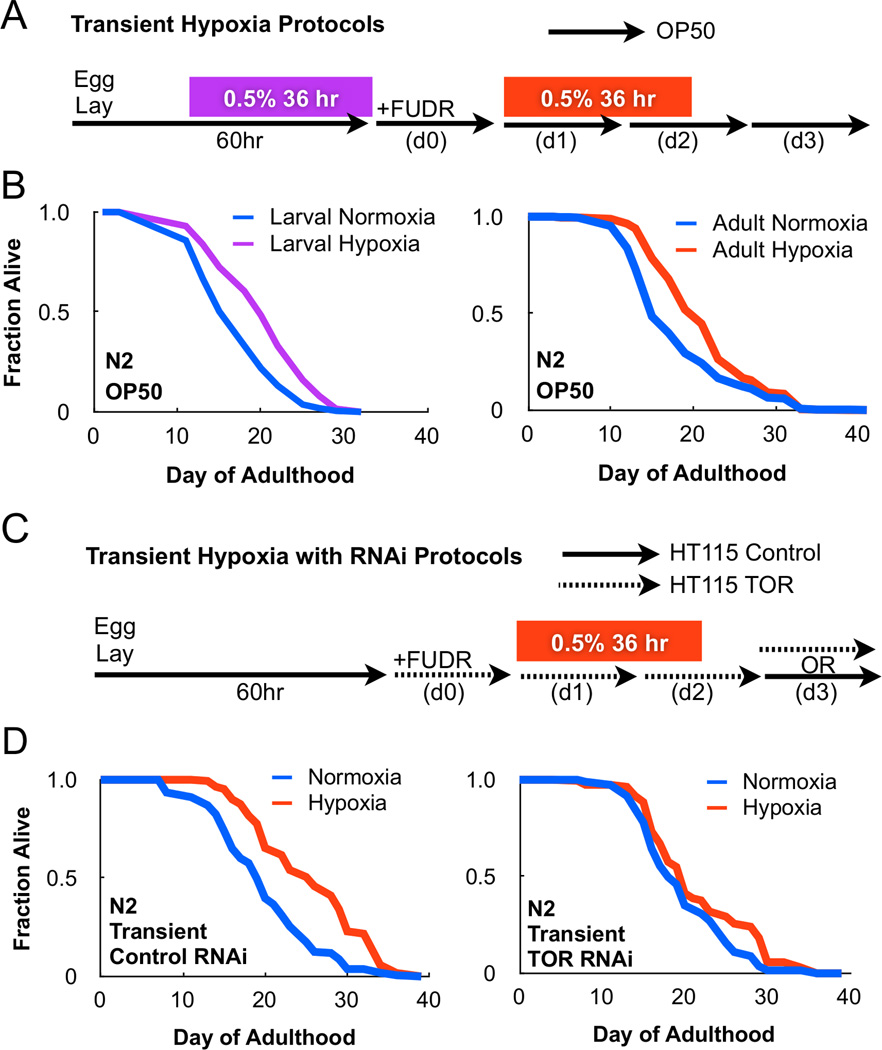
To investigate how the lifespan of C. elegans is affected under transient hypoxia, animals were subjected to a single treatment of 0.5% O2 for 36 hours either during larval development or in adulthood (Figure 1A). Transient hypoxia was sufficient to extend lifespan in both developing and adult worms by 18.76% and 16.19%, respectively (Figure 1B). Interestingly, the lifespan benefit from transient hypoxia treatment was comparable to C. elegans maintained continuously under hypoxia (Leiser et al., 2013), suggesting hypoxia might induce a metabolic switch to increase lifespan. To investigate this possibility, we screened three nutrient sensing pathways: FOXO, AMPK, and TOR, which have been previously implicated in regulating lifespan in C. elegans (Kenyon, 2010).
Figure 1. TOR is required for longevity following transient hypoxia.
(A) Methods for transient hypoxia treatment in C. elegans fed OP50 bacteria. (B) Transient hypoxia during either development or adulthood is sufficient to extend lifespan. (C) Methods for transient hypoxia treatment in C. elegans fed HT115 dsRNA producing bacteria. On day 3 of adulthood, animals may be removed to vector control plates (transient RNAi) or maintained on dsRNA producing bacteria (continuous RNAi). (D) Transient RNAi of the C. elegans TOR homolog, LET-363, prevents lifespan extension following transient hypoxia treatment. Cox proportional hazard analysis of transient control RNAi and transient TOR RNAi under hypoxia = 1.618 (95% CI 1.311 – 1.997).
The C. elegans FOXO-homolog, DAF-16, is negatively regulated by the insulin-like growth factor (IGF) receptor, DAF-2, and mutations impairing IGF signaling extend lifespan (Kenyon et al., 1993). However, animals bearing a null mutation in DAF-16, daf-16(mu86), were long-lived following transient hypoxia treatment (Figure S1A). AMP-activated protein kinase (AMPK) activity is promoted by increased AMP levels and thus acts as a sensor for bioenergetic stress (Hardie et al., 2012). We found animals bearing a null mutation in the catalytic subunit of AMPK, aak-2(gt33), were also long-lived when treated with transient hypoxia (Figure S1B). Lastly, TOR is a serine-threonine kinase that responds to nutrient availability to concomitantly stimulate anabolic and suppress catabolic metabolic pathways. To test whether TOR signaling was required for longevity following transient hypoxia, we adapted our hypoxia protocol for RNAi feeding and included methods for both transient and continuous TOR RNAi (Figure 1C). Transient RNAi of the C. elegans TOR homolog, LET-363, significantly reduced lifespan following hypoxia treatment (Figure 1D). Furthermore, while continuous TOR RNAi in adulthood blocked longevity following transient hypoxia exposure, it also extended lifespan of wildtype C. elegans, as previously reported (Figure S1C–D). Collectively, these results indicate that hypoxia extends lifespan in a TOR-dependent but AMPK- and FOXO-independent manner.
TORC1 is required for lifespan following transient hypoxia
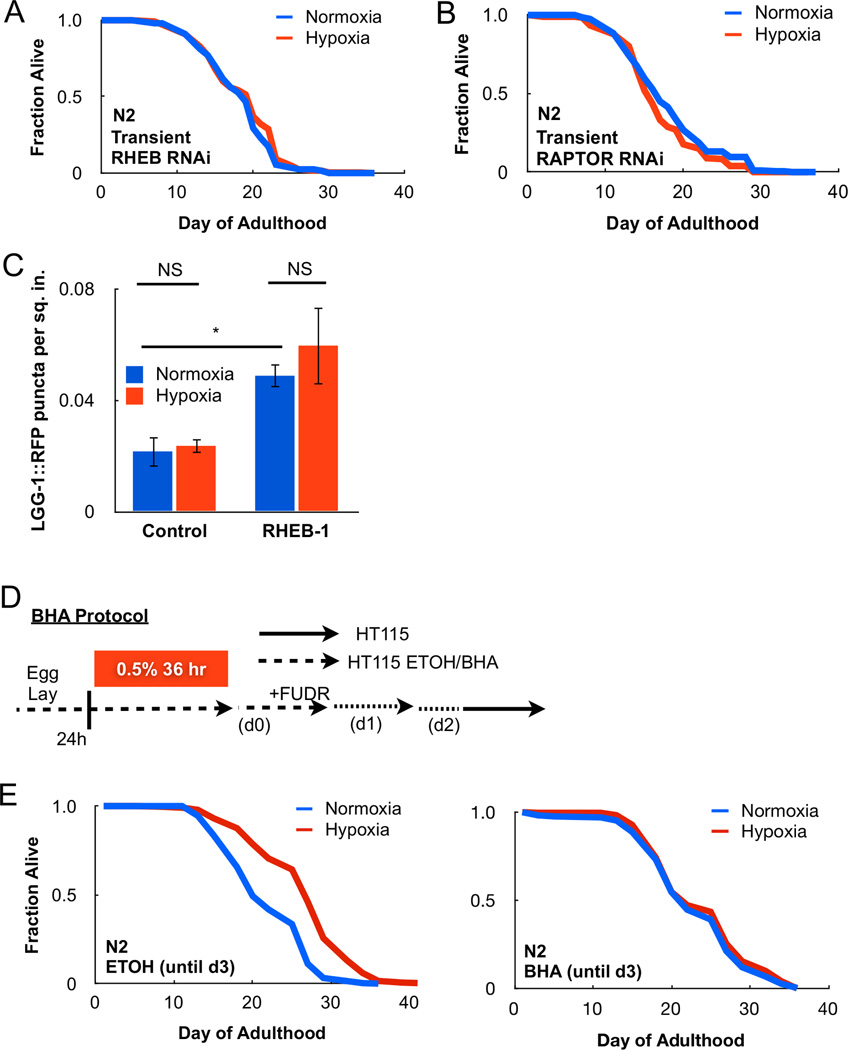
TOR is a component of two large protein complexes known as TOR Complex I (TORC1) and TOR Complex 2 (TORC2) (Laplante and Sabatini, 2012). TORC1 and TORC2 share common subunits but also have distinct proteins within their complexes. The Ras-related small G-protein RHEB-1 is an upstream activator of TORC1 signaling, and thus served as an attractive candidate to test whether TORC1 activity is required under transient hypoxia. Indeed, transient or continuous RHEB-1 RNAi reversed lifespan extension following transient adult hypoxia (Figure 2A, S2A). The TOR binding protein, RAPTOR, is a 150 kDa scaffold protein of the TORC1 complex and is encoded by the DAF-15 homolog in C. elegans. Transient RNAi of RAPTOR\DAF-15 also blocked longevity under transient hypoxia (Figure 2B). Additionally, RHEB-1 RNAi had no impact on the activation of HIF-dependent transcription (F22B5.4 mRNA) under hypoxia even though HIF-1 was required for lifespan extension following transient hypoxia (Figure S2B–C). We conclude TORC1 mediates a unique HIF-1-independent response to transient hypoxia to extend lifespan in C. elegans.
Figure 2. TORC1 and ROS are necessary for transient hypoxia extension of lifespan.
(A) Transient RNAi of the TORC1 activator, RHEB-1, prevents longevity following transient hypoxia treatment. (B) Transient RNAi of the TORC1 scaffold protein RAPTOR prevents adaptation to hypoxia. (C) Transient hypoxia does not increase autophagy in the C. elegans nhx-2::LGG-1::RFP reporter strain, suggesting TORC1 is not inhibited under hypoxia (* = p < 0.05, NS = not significant). (D) Methods for BHA treatment in C. elegans. (E) The antioxidant BHA suppresses longevity following transient hypoxia compared to ethanol (ETOH) control.
Our results showing TORC1 promotes lifespan are surprising given that genetic or pharmacological inhibition of TORC1 signaling extends lifespan in multiple organisms, including C. elegans (Hansen et al., 2007; Kaeberlein et al., 2005; Kapahi et al., 2004; Wu et al., 2013). While TOR RNAi reproduces this phenotype (Figure S1C–D), our transient hypoxia data suggests the requirement of TOR signaling in promoting or suppressing lifespan is likely dependent on various nutritional conditions. Supporting this, TORC1 activity is required to promote longevity in response to intermittent fasting (Honjoh et al., 2009).
Canonical TORC1 signaling promotes cellular growth by increasing protein synthesis through phosphorylation of P70/S6-kinase and inhibiting catabolic processes such as autophagy (Laplante and Sabatini, 2012). A C. elegans strain featuring a mutation in the S6-kinase homolog, RSKS-1, displayed increased lifespan under transient hypoxia, despite being long-lived under normoxic conditions, suggesting transient hypoxia does not extend lifespan by reducing protein synthesis (Figure S2D). Additionally, we utilized a C. elegans fluorescent reporter strain that allows quantification of autophagic vesicles within the intestine of the animal (LGG-1::RFP), a process activated following TORC1 inhibition (Robida-Stubbs et al., 2012). Indeed, RHEB-1 RNAi increased puncta formation (Figure 2C). However, transient hypoxia treatment did not increase the number of quantifiable puncta indicating TORC1 signaling is still active under hypoxia. These data support a model in which TORC1 signaling promotes longevity under transient hypoxia.
In mammalian cells, hypoxia is known to negatively regulate TORC1 signaling (Arsham et al., 2003; Wouters and Koritzinsky, 2008). Nonetheless, it is important to note that multiple cell types proliferate in vitro under hypoxic conditions including human hematopoietic stem cells and human fibroblasts (Bell et al., 2007; Danet et al., 2003). Hypoxia also increases pulmonary vascular smooth muscle cell proliferation through TOR signaling (Krymskaya et al., 2011). Moreover, hypoxia is prominent in many pro-growth states, such as the developing embryo or rapidly proliferating tumors. Thus, hypoxic regulation of TORC1 signaling is likely context dependent.
Mitochondrial ROS released under transient hypoxia extend lifespan through TORC1
A key question to decipher is how cells sense decreases in oxygen levels and couple it to TOR signaling to impart metabolic adaptations and increased lifespan. We have previously proposed that mitochondria are critical for oxygen sensing by releasing ROS during hypoxia to activate multiple signaling pathways, including HIFs (Hamanaka and Chandel, 2009). Along these lines, long-lived mitochondrial mutants in C. elegans feature elevated ROS levels that activate HIF-1-dependent longevity pathways (Lee et al., 2010). Furthermore, numerous studies in yeast, C. elegans, and mice indicate that increasing ROS promote longevity (Liu et al., 2005; Pan et al., 2011; Schulz et al., 2007; Van Raamsdonk and Hekimi, 2009; Zarse et al., 2012). Oxidants in mammalian cells have also been shown to activate TOR signaling (Sarbassov and Sabatini, 2005). Therefore, we surmised that ROS might couple oxygen sensing to TOR signaling. To test whether ROS were required for lifespan under transient hypoxia, we treated C. elegans with the antioxidant butylated hydroxyanisole (BHA) for the 3 days adjacent to hypoxia exposure (Figure 2D). As expected, hypoxia increased mean lifespan from 19.7 days compared to 24.3 days in C. elegans treated with ethanol control (Figure 2E). By contrast, hypoxia only slightly increased mean lifespan from 20.8 days to 21.6 days in animals treated with BHA (Figure 2E). Thus, increased ROS under hypoxia serve as biological signaling molecules to activate adaptive responses to increase lifespan in C. elegans.
Intestinal TOR activity is required for lifespan following transient hypoxia
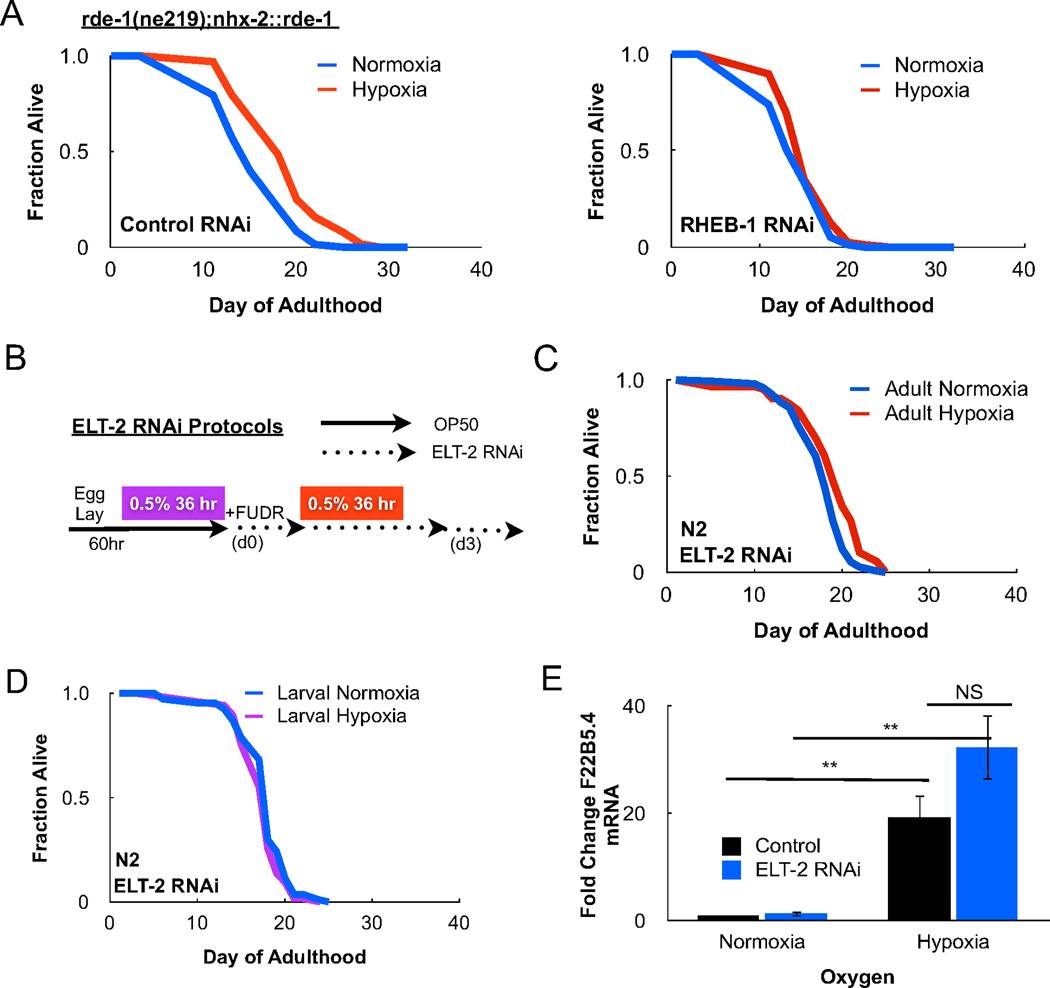
Recent studies indicate stress responses in C. elegans can be regulated in a cell-non-autonomous manner whereby the C. elegans intestine integrates an organismal response to environmental change (Durieux et al., 2011). To investigate whether this was case for the TORC1-dependent hypoxia response, we inactivated TORC1 using tissue specific RNAi strains. The C. elegans rde-1(ne219) strain cannot process dsRNA due to a mutation in RDE-1, an Argonaute family protein, and is RNAi deficient (Tabara et al., 1999). Transgenic C. elegans strains reconstituted with RDE-1 driven under various promoters permit RNAi knockdown in a tissue-specific manner (Qadota et al., 2007). Intestinal RNAi of RHEB-1, using the nhx-2 promoter to drive RDE-1 expression, prevented increased lifespan following transient hypoxia (Figure 3A). Thus, RHEB-1 signaling in the C. elegans intestine is required to extend lifespan under transient hypoxia.
Figure 3. Intestinal TORC1 and ELT-2 signaling is required for lifespan under hypoxia.
(A) The rde-1(ne219);nhx-2::rde-1 mutant features intact RNAi machinery only in the C. elegans intestine. Impairment of intestinal TORC1 signaling prevents longevity following transient hypoxia. (B) Methods for ELT-2 RNAi feeding during adulthood in C. elegans. (C) ELT-2 is a GATA-type transcription factor required for development and maintenance of the nematode intestine. ELT-2 RNAi in the adult animal prevents longevity following adult transient hypoxia. Cox proportional hazard analysis of control RNAi and ELT-2 RNAi under hypoxia = 3.907 (95% CI 2.802 – 5.447). (D) ELT-2 RNAi following larval transient hypoxia also prevents hypoxia-induced lifespan. (E) The HIF-1-dependent gene, F22B5.4, is induced following transient hypoxia. ELT-2 RNAi does not affect F22B5.4 upregulation under hypoxia, suggesting that ELT-2 controls a HIF-1-independent hypoxia response (** p < 0.01, NS = not significant).
The nematode intestine, the primary organ responsible for digestion of ingested bacteria and absorption of nutrients, also plays a central role in the storage of lipids and fat metabolism as well as in the synthesis of lipid-derived hormones and signaling molecules. The mitochondrial unfolded protein response (UPRmt) specifically activated in the intestine triggers a cell-non-autonomous signaling response to extend C. elegans lifespan (Durieux et al., 2011). Thus, we sought to determine whether longevity following transient hypoxia required intestinal UPRmt activation. To do this, we induced the UPRmt through RNAi of ISP-1, a critical protein required for proper assembly of electron transport chain complex III. ISP-1 RNAi was sufficient to extend lifespan compared to wildtype controls, however, co-treatment of ISP-1 RNAi with transient hypoxia led to a further 28.12% lifespan increase (Figure S3B). HAF-1 and ATFS-1 are two key mediators of the UPRmt implicated in modulating lifespan (Figure S3A) (Houtkooper et al., 2013; Pellegrino et al., 2013). Transient hypoxia extended the lifespan of C. elegans containing a deletion in the HAF-1 (Figure S3C) and in C. elegans fed RNAi to reduce ATFS-1 expression (Figure S3D). Alternatively, ATFS-1 was required for lifespan extension by ISP-1 RNAi (Figure S3D). These data indicate that activation of UPRmt does not contribute to lifespan extension under transient hypoxia.
ELT-2 increases intestinal GSTO-1 expression to extend lifespan under hypoxia
The requirement for RHEB-1 in the intestine during hypoxia led us to examine whether the intestinal-specific GATA-type erythroid-like-2 transcription factor (ELT-2) is required for increased lifespan following transient hypoxia (McGhee, 2007). ELT-2 plays a critical role in the differentiation of the intestine in the developing embryo and regulates the expression of intestinal genes in the adult (McGhee et al., 2007). To avoid the developmental effects of larval ELT-2 RNAi, we fed C. elegans ELT-2 RNAi bacteria beginning at the L4/young adult stage, 60 hours after synchronized egg lay (Figure 3B). RNAi of ELT-2 blocked longevity following transient hypoxia regardless of whether the animals were exposed to hypoxia in adulthood or development (Figure 3C–D). To eliminate the possibility that ELT-2 RNAi interferes with the canonical HIF-1 hypoxia response in C. elegans, we measured induction of the HIF-1 target F22B5.4 in animals fed ELT-2 RNAi under hypoxia. Importantly, no difference in F22B5.4 induction was observed upon ELT-2 RNAi, suggesting ELT-2 mediates unique adaptions to hypoxia within the intestine (Figure 3E).
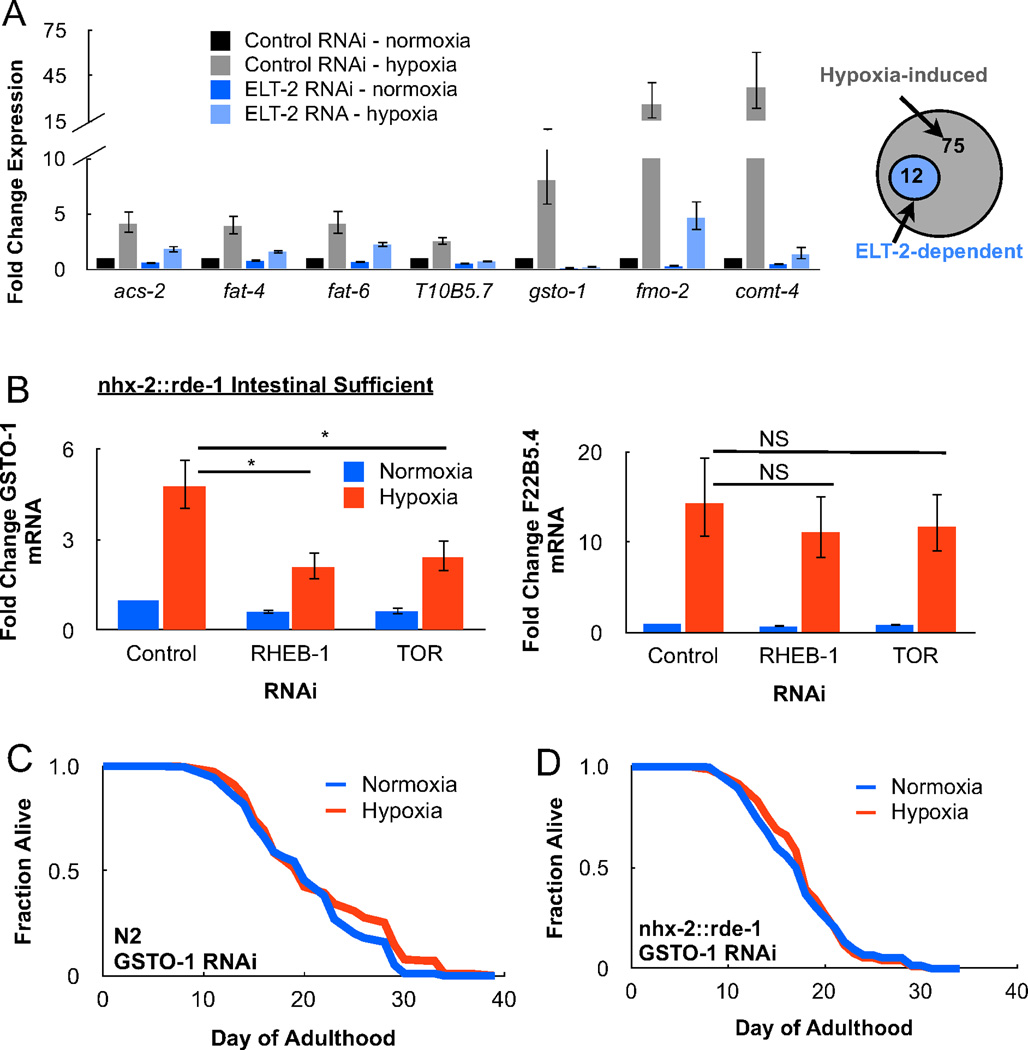
To understand the genes and cellular pathways controlled by ELT-2, we performed an RNA-Sequencing analysis on adult animals treated with transient hypoxia and ELT-2 RNAi. 12 transcripts were upregulated under hypoxia in an ELT-2-dependent manner and we noted multiple candidates with known functions in fatty acid metabolism and ROS homeostasis (Table S2). The expression changes of these candidates were confirmed and quantified by RT-PCR (Figure 4A). Of particular interest was the identification of GSTO-1, an intestinal omega-class glutathione S-transferase (GST), whose induction under hypoxia was abrogated by ELT-2 RNAi. GSTs are a broad class of intracellular proteins with ROS scavenging activity in addition to their role in the metabolism of xenobiotics (Burmeister et al., 2008). Along these lines, loss of GSTO-1 increases the sensitivity of C. elegans to mitochondrial ROS generating agents, such as paraquat (PQ). To determine if hypoxic induction of GSTO-1 was dependent on intestinal TORC1, we measured GSTO-1 mRNA levels under hypoxia following feeding of TOR and RHEB-1 RNAi. Indeed, TORC1 RNAi specifically in the C. elegans intestine impaired upregulation of GSTO-1 while having no effect on HIF-1-dependent targets (Figure 4B). GSTO-1 RNAi also completely suppressed longevity following transient hypoxia when performed in wildtype animals (Figure 4C) and intestinal RNAi sufficient strains (Figure 4D).
Figure 4. GSTO-1 is required for lifespan following transient hypoxia.
(A) C. elegans treated with transient adult hypoxia and fed ELT-2 RNAi bacteria were harvested for total RNA and analyzed by RNA-Sequencing for ELT-2-dependent transcriptional changes. In the adult animal, 75 transcripts were upregulated under hypoxia, 12 of which were determined to be ELT-2-dependent (see Supplemental Table S2). Select candidates involved in lipid metabolism (acs-2, fat-4, fat-6, T10B5.7), ROS homeostasis (gsto-1, fmo-2), and neurotransmission (comt-4) were verified by RT-PCR. (B) Hypoxia induction of the omega-class glutathione S-transferase, GSTO-1, requires intestinal TORC1 activity. However intestinal TORC1 activity is not required for upregulation of the HIF-1-dependent target, F22B5.4 (* = p < 0.05, NS = not significant). (C) GSTO-1 RNAi in wildtype animals (N2) prevents lifespan following transient hypoxia. (D) Intestinal-specific GSTO-1 RNAi in the nhx-2::rde-1 transgenic strain is sufficient to prevent hypoxia-induced longevity.
Previous studies have shown that transient and low dose levels of mitochondrial ROS, achieved through exposure to PQ, are sufficient to extend lifespan in C. elegans (Yee et al., 2014). We therefore hypothesized that the mechanism of longevity following PQ treatment involves induction of antioxidants, such as GSTO-1, through TORC1/ELT-2 signaling. To test this, C. elegans were treated for 72 hours with 0.25 mM PQ and subsequently scored for mean lifespan (Figure S4A). While PQ exposure was sufficient to increase lifespan (Figure S4B), RNAi of the TORC1 components, RHEB-1 and TOR, or the intestinal transcription factor ELT-2, completely prevented the mitohormesis phenotype (Figure S4C–E). Importantly, GSTO-1 is also required for longevity following PQ treatment, an observation consistent with previous studies (Figure S4F).
These data support a model where a HIF-independent but TOR-dependent signaling pathway extends lifespan in C. elegans following transient hypoxia exposure. The TOR-dependent hypoxia response is initiated by mitochondrial ROS within the intestine to induce an ELT-2 transcriptional network. ELT-2 and TOR directly upregulate expression of the glutathione S-transferase GSTO-1, leading to increased lifespan. Our results are consistent with multiple studies that have been reported whereby increasing mitochondrial ROS provides a lifespan benefit through activation of adaptive stress response pathways (i.e. mitohormesis) (Ristow and Schmeisser, 2011). It is worth noting that human randomized control trials for administration of antioxidants continue to measure no mortality or disease prevention benefits (Bjelakovic et al., 2007). Perhaps the reverse strategy of administering transient and low doses of ROS-generating agents within specific mammalian tissues might activate stress pathways that improve age-related pathologies.
Experimental Procedures
C. elegans Strains and Maintenance
All strains used in this study were obtained from the C. elegans Genetic Center at the University of Minnesota. Animals were maintained and passaged at 20 °C on NGM media seeded with an OP50 bacteria lawn.
RNAi Methods
Primers used to generate RNAi constructs are depicted in the supplemental experimental procedures. To generate RNAi clones, amplified fragments from C. elegans N2 wildtype cDNA were ligated into the L4440 vector using XhoI and PstI restriction sites and the manufacturer’s T4 ligase protocol (New England Biolabs). Candidates were verified by sequencing. The L4440 RNAi vector was then transformed into the HT115 feeding strain and verified by restriction digest.
Solid NGM RNAi media was generated by adding 1.429 g/L IPTG (GoldBio), 0.075 g/L ampicillin (Sigma), 12.5 mg/L tetracycline (Sigma), and 1 mg/L Amphotericin B (Sigma) to lukewarm NGM media prior to pouring. RNAi cultures were inoculated in LB containing ampicillin (100 µg/uL), tetracycline (12.5 µg/uL), and Amphotericin B (1 µg/uL) from frozen glycerol stocks for 12–14 hr prior to adding IPTG (1 mM, 4 hr) to induce dsRNA expression. Small plates (35 mm diameter) were seeded with 200 µL RNAi culture and large plates (10 mm diameter) were seeded with 1 mL. Bacterial lawns were dried at room temperature for up to 3 days before use.
Lifespan Analysis and Hypoxia Treatment
For all lifespan experiments, C. elegans were synchronized using a 4–6 hr egg lay (EL). N2 animals were transferred to FUDR (Sigma) at 60 hr post-EL (L4/young adult stage). 84 hr post-EL represents day 1 of adulthood. rsks-1(ok1255) animals are slightly developmentally delayed and were transferred to FUDR upon reaching L4/young adult stage. 50 µM FUDR was used to prevent progeny from hatching on OP50 seeded NGM plates while 5 µM FUDR was sufficient to block hatching on HT115 RNAi plates. Animals were scored alive or dead by manual prodding under a dissection scope. C. elegans which burst or desiccated along the sides of the petri dish were removed from the final analysis. Statistical analyses on lifespan curves were performed using the Mantel-Cox\Logrank test with Prism 6 software and are presented in the lifespan supplement (Table S1). Hazard ratios were calculated with the Cox Proportional-Hazard Model using SSPS software.
Graphical depictions of methods for transient hypoxia treatment can be found throughout this manuscript. Briefly, hypoxia treatments were performed for 36 hr during larval development (24 hr post-EL) or during adulthood (84 hr post-EL). Treatment was performed at room temperature by flowing a 0.5% O2/balanced N2 tank (Airgas) through an airtight chamber. Normoxia controls were kept in an adjacent chamber exposed to ambient air. Animals were returned to 20 °C after 36 hr. TORC1 and ELT-2 RNAi feeding was either performed throughout adulthood starting 60 hr post-EL (continuous RNAi) or for 3 days (transient RNAi). GSTO-1 RNAi was performed continuously in all experiments.
BHA Treatment
The timing of C. elegans ethanol and BHA treatment is detailed in Figure 2D. To prepare solid agar plates for this experiment, BHA was dissolved in ethanol at a 1 M concentration and was added 1:1000 to cooled autoclaved RNAi media (1 mM final concentration). An equal volume of 100% ethanol was used as a pharmacological control.
Paraquat Treatment
The timing of transient paraquat (PQ) treatment is detailed in Figure S4A. To prepare solid plates for this experiment, 1 M PQ dissolved in water was added to cooled autoclaved RNAi media to a final concentration of 0.25 mM (1:4000 dilution). The growth of HT115 bacteria is impacted in the presence of PQ and compromises RNAi knockdown. We therefore allowed HT115 bacterial lawns to grow in the absence of PQ and subsequently manually transferred RNAi bacteria onto control and PQ plates using a metal spatula. Following 72 hr of PQ treatment, animals were returned to normally seeded HT115 plates.
Autophagy Assay
The nhx-2::LGG-1::RFP strain was synchronized via 4 hr EL and grown for 24 hr on control or RHEB-1 RNAi plates. Animals were then exposed to 24 hr transient hypoxia prior to quantification of intestinal fluorescent puncta (Zeiss Axiovert). Puncta were most reliably quantified at the L3/L4 stage prior to gonadal development. Image analysis was performed using ImageJ64 with a maxima threshold tolerance of 100. This closely correlated with the puncta quantified by human observation. Total puncta per square inch was calculated by dividing the total puncta number by the intestinal fluorescent area. A student T-test was used to evaluate for statistical significance (Prism 6).
RNA-Sequencing and RT-PCR
ELT-2 RNAi feeding for RNA Sequencing (RNA-Seq) analysis was initiated during adulthood to avoid developmental effects (Figure 4A), whereas intestinal TOR or RHEB-1 RNAi was performed throughout development (Figure 4B). For RNA isolation, synchronized C. elegans grown on large RNAi plates were quickly washed from solid plates and rinsed once in M9 buffer prior to addition of 250 µL TRIzol (Invitrogen). Lysates were preserved at −80 °C until RNA isolation. RNA was isolated with two chloroform extractions (50 µL each), followed by isopropanol precipitation (125 µL) of the aqueous phase, and a single wash of the resulting pellet with 70% ethanol (250 µL). RNA pellets were dried in a tissue culture hood and resuspended in RNAse free water. If necessary, RNA pellets were dissolved by heating briefly to 60 °C.
RNA-Seq analysis was performed by Beijing Genomics on a pool of 5 independent RNA samples (4 µg RNA per experiment, 20 µg total RNA). RNA was evaluated for integrity using an Agilent Bioanalyzer 2100 and 100 bp paired-end cDNA sequencing libraries were prepared separately for each sample using indexed adapters. All four samples were multiplexed onto a single lane of an Illumina HiSeq2000. After sequencing, the reads were demultiplexed by removing adapters and filtered by censoring the reads with poor quality. Reads were aligned to the C. elegans reference genome (ce10 from UCSC) using TopHat (v2.0.8b) in conjunction with the ce10 gene annotation data obtained from UCSC. After alignment, transcripts were quantified and a differential expression analysis was performed using Cufflinks (v2.1.1), which calculates gene expression as RPKM values.
cDNA for RT-PCR experiments was synthesized using 1 µg of total RNA template and the MMLV reverse transcriptase according to the manufacturer’s protocol (Life Technologies). RT-PCR was performed using the CFX384 real-time PCR detection system (Bio-Rad) and a SYBR Green fluorophore (Bio-Rad). Total cDNA template for all reactions was 5 ng except for the actin loading control, for which 0.1 ng of total template was used. All reactions were performed in triplicate wells and fold-change calculations were performed manually (Macintosh Numbers). A student T-test was used to evaluate for statistical significance (Prism 6). A list of RT-PCR primers used is provided in the Supplemental Methods.
Supplementary Material
Acknowledgements
Chicago Biomedical Consortium as well as NIH T32HL076139 Grant (MS) and 1R01HL122062-01 (NSC) supported this work. MS executed the experiments. MS and NSC designed and wrote the paper. We thank Matthew J. Schipma at NGS Core Facility at Feinberg School of Medicine, Northwestern University for RNA sequencing analysis. We are grateful to Manu Jain, MD for assistance with the statistical analysis of longevity assays in this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arsham AM, Howell JJ, Simon MC. A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J Biol Chem. 2003;278:29655–29660. doi: 10.1074/jbc.M212770200. [DOI] [PubMed] [Google Scholar]
- Bell E, Klimova T, Eisenbart J, Schumacker P, Chandel N. Mitochondrial reactive oxygen species trigger hypoxia-inducible factor-dependent extension of the replicative life span during hypoxia. Mol Cell Biol. 2007;27:5737–5745. doi: 10.1128/MCB.02265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- Burmeister C, Luersen K, Heinick A, Hussein A, Domagalski M, Walter RD, Liebau E. Oxidative stress in Caenorhabditis elegans: protective effects of the Omega class glutathione transferase (GSTO-1) FASEB J. 2008;22:343–354. doi: 10.1096/fj.06-7426com. [DOI] [PubMed] [Google Scholar]
- Danet GH, Pan Y, Luongo JL, Bonnet DA, Simon MC. Expansion of human SCID-repopulating cells under hypoxic conditions. J Clin Invest. 2003;112:126–135. doi: 10.1172/JCI17669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 2011;144:79–91. doi: 10.1016/j.cell.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanaka R, Chandel N. Mitochondrial reactive oxygen species regulate hypoxic signaling. Curr Opin Cell Biol. 2009;21:894–899. doi: 10.1016/j.ceb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Taubert S, Crawford D, Libina N, Lee SJ, Kenyon C. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell. 2007;6:95–110. doi: 10.1111/j.1474-9726.2006.00267.x. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjoh S, Yamamoto T, Uno M, Nishida E. Signalling through RHEB-1 mediates intermittent fasting-induced longevity in C. elegans. Nature. 2009;457:726–730. doi: 10.1038/nature07583. [DOI] [PubMed] [Google Scholar]
- Houtkooper RH, Mouchiroud L, Ryu D, Moullan N, Katsyuba E, Knott G, Williams RW, Auwerx J. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature. 2013;497:451–457. doi: 10.1038/nature12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Powers RW, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Krymskaya VP, Snow J, Cesarone G, Khavin I, Goncharov DA, Lim PN, Veasey SC, Ihida-Stansbury K, Jones PL, Goncharova EA. mTOR is required for pulmonary arterial vascular smooth muscle cell proliferation under chronic hypoxia. FASEB J. 2011;25:1922–1933. doi: 10.1096/fj.10-175018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Hwang AB, Kenyon C. Inhibition of respiration extends C. elegans life span via reactive oxygen species that increase HIF-1 activity. Curr Biol. 2010;20:2131–2136. doi: 10.1016/j.cub.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiser SF, Fletcher M, Begun A, Kaeberlein M. Life-span extension from hypoxia in Caenorhabditis elegans requires both HIF-1 and DAF-16 and is antagonized by SKN-1. The journals of gerontology Series A, Biological sciences and medical sciences. 2013;68:1135–1144. doi: 10.1093/gerona/glt016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Jiang N, Hughes B, Bigras E, Shoubridge E, Hekimi S. Evolutionary conservation of the clk-1-dependent mechanism of longevity: loss of mclk1 increases cellular fitness and lifespan in mice. Genes Dev. 2005;19:2424–2434. doi: 10.1101/gad.1352905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee JD. The C. elegans intestine. WormBook : the online review of C elegans biology. 2007:1–36. doi: 10.1895/wormbook.1.133.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee JD, Sleumer MC, Bilenky M, Wong K, McKay SJ, Goszczynski B, Tian H, Krich ND, Khattra J, Holt RA, et al. The ELT-2 GATA-factor and the global regulation of transcription in the C. elegans intestine. Dev Biol. 2007;302:627–645. doi: 10.1016/j.ydbio.2006.10.024. [DOI] [PubMed] [Google Scholar]
- Mehta R, Steinkraus KA, Sutphin GL, Ramos FJ, Shamieh LS, Huh A, Davis C, Chandler-Brown D, Kaeberlein M. Proteasomal regulation of the hypoxic response modulates aging in C. elegans. Science. 2009;324:1196–1198. doi: 10.1126/science.1173507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Schroeder EA, Ocampo A, Barrientos A, Shadel GS. Regulation of yeast chronological life span by TORC1 via adaptive mitochondrial ROS signaling. Cell Metab. 2011;13:668–678. doi: 10.1016/j.cmet.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino MW, Nargund AM, Haynes CM. Signaling the mitochondrial unfolded protein response. Biochim Biophys Acta. 2013;1833:410–416. doi: 10.1016/j.bbamcr.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadota H, Inoue M, Hikita T, Koppen M, Hardin JD, Amano M, Moerman DG, Kaibuchi K. Establishment of a tissue-specific RNAi system in C. elegans. Gene. 2007;400:166–173. doi: 10.1016/j.gene.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow M, Schmeisser S. Extending life span by increasing oxidative stress. Free Radic Biol Med. 2011;51:327–336. doi: 10.1016/j.freeradbiomed.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Robida-Stubbs S, Glover-Cutter K, Lamming DW, Mizunuma M, Narasimhan SD, Neumann-Haefelin E, Sabatini DM, Blackwell TK. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. 2012;15:713–724. doi: 10.1016/j.cmet.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Sabatini DM. Redox regulation of the nutrient-sensitive raptor-mTOR pathway and complex. J Biol Chem. 2005;280:39505–39509. doi: 10.1074/jbc.M506096200. [DOI] [PubMed] [Google Scholar]
- Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365:537–547. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- Tabara H, Sarkissian M, Kelly WG, Fleenor J, Grishok A, Timmons L, Fire A, Mello CC. The rde-1 gene, RNA interference, and transposon silencing in C. elegans. Cell. 1999;99:123–132. doi: 10.1016/s0092-8674(00)81644-x. [DOI] [PubMed] [Google Scholar]
- Van Raamsdonk JM, Hekimi S. Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000361. doi: 10.1371/journal.pgen.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welford SM, Bedogni B, Gradin K, Poellinger L, Broome Powell M, Giaccia AJ. HIF1alpha delays premature senescence through the activation of MIF. Genes Dev. 2006;20:3366–3371. doi: 10.1101/gad.1471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters BG, Koritzinsky M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer. 2008;8:851–864. doi: 10.1038/nrc2501. [DOI] [PubMed] [Google Scholar]
- Wu JJ, Liu J, Chen EB, Wang JJ, Cao L, Narayan N, Fergusson MM, Rovira II, Allen M, Springer DA, et al. Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mTOR expression. Cell reports. 2013;4:913–920. doi: 10.1016/j.celrep.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee C, Yang W, Hekimi S. The intrinsic apoptosis pathway mediates the pro-longevity response to mitochondrial ROS in C. elegans. Cell. 2014;157:897–909. doi: 10.1016/j.cell.2014.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarse K, Schmeisser S, Groth M, Priebe S, Beuster G, Kuhlow D, Guthke R, Platzer M, Kahn CR, Ristow M. Impaired Insulin/IGF1 Signaling Extends Life Span by Promoting Mitochondrial L-Proline Catabolism to Induce a Transient ROS Signal. Cell Metab. 2012;15:451–465. doi: 10.1016/j.cmet.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Shao Z, Zhai Z, Shen C, Powell-Coffman JA. The HIF-1 hypoxia-inducible factor modulates lifespan in C. elegans. PLoS One. 2009;4:e6348. doi: 10.1371/journal.pone.0006348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.