Abstract
Background
The resolution of deep vein thrombosis (DVT) requires an inflammatory response and mobilization of proteases, such as urokinase-type plasminogen activator (uPA) and matrix metalloproteinases (MMPs), to degrade the thrombus and remodel the injured vein wall. PAI-2 is a serine protease inhibitor (serpin) with unique immunosuppressive and cell survival properties that was originally identified as an inhibitor of uPA.
Objective
To investigate the role of PAI-2 in venous thrombus formation and resolution.
Methods
Venous thrombus resolution was compared in wild type C57BL/6, PAI-2 -/- and PAI-1 -/- mice using the stasis model of DVT. Formed thrombi were harvested, thrombus weights were recorded, and tissue was analyzed for uPA, and MMP activities, PAI-1 expression, and the nature of inflammatory cell infiltration.
Results
We found that absence of PAI-2 enhanced venous thrombus resolution, while thrombus formation was unaffected. Enhanced venous thrombus resolution in PAI-2 -/- mice was associated with increased uPA activity and reduced levels of PAI-1, with no significant effect on MMP-2 and -9 activities. PAI-1 deficiency resulted in an increase in thrombus resolution similar to PAI-2 deficiency, but additionally reduced venous thrombus formation and altered MMP activity. PAI-2 deficient thrombi had increased levels of the neutrophil chemoattractant, CXCL2, which was associated with early enhanced neutrophil recruitment.
Conclusions
These data identify PAI-2 as a novel regulator of venous thrombus resolution, which modulates several pathways involving both inflammatory and uPA activity mechanisms, distinct from PAI-1. Further examination of these pathways may lead to potential therapeutic prospects in accelerating thrombus resolution.
Keywords: Plasminogen Activator Inhibitor 2, Plasminogen Activator Inhibitor 1, Serine Proteases, Urokinase-type Plasminogen Activator, Venous thrombosis, serine protease inhibitors
Introduction
Deep vein thrombosis (DVT) is a common inflammatory condition that can lead to substantial morbidity and mortality. Along with the potentially fatal complication of a pulmonary embolism, many patients with DVT develop a chronic condition of venous wall injury and venous hypertension that can cause debilitating edema and skin ulcers, known as post-thrombotic syndrome [1;2]. Current standard therapy relies on anticoagulation, which prevents subsequent propagation and extension of the thrombus as well as pulmonary embolism, but does little to resolve the existing clot. Anticoagulation and thrombolytic therapies for DVT have the added morbidity of hemorrhagic complications. Clinical studies suggest that a slower rate of endogenous thrombus resolution is correlated with the development of post-thrombotic syndrome [3]. Therefore, methods to accelerate endogenous thrombus resolution would have clinical benefit to patients with DVT.
Studies using mouse models of DVT reveal that effective thrombus resolution requires a sterile immune response that depends on the recruitment of inflammatory cells, specifically neutrophils and monocytes to the early thrombus, and proteases to degrade the thrombus and remodel the injured tissue [4;5]. Chemokines and proinflammatory cytokines produced by the infiltrating inflammatory cells are critical for thrombus resolution and vein wall healing [2;6]. Neovascularization, the development of functional flow channels, clot retraction, and fibrinolysis are all eventually required for the recanalization and restoration of blood flow in a thrombosed vein [4].
Fibrinolysis is a fundamental element of venous thrombus resolution [7]. Fibrinolysis is mediated by the action of two serine proteases, urokinase-type plasminogen activator (uPA) and tissue-type plasminogen activator (tPA) [8]. Studies have identified an essential role for uPA in this process. uPA activity is increased after thrombus formation in a rat model of venous thrombosis [9]. Impaired venous thrombus resolution is observed in mice with uPA deficiency, but not tPA deficiency [10] and overexpression of uPA via adenoviral gene delivery by macrophages into formed venous thrombi has been shown to enhance thrombus resolution [11]. uPA derived from bone-marrow derived cells, specifically macrophages, appears critical for this process [10;12].
Major inhibitory regulators of the plasminogen activators are the serine protease inhibitors, known as serpins [13]. Plasminogen activator inhibitor type-1 (PAI-1 or serpinE1), is a key physiological inhibitor of both uPA and tPA in vivo and plays a primary role as an inhibitor of fibrinolysis [14]. PAI-1 circulates in plasma and increased levels of PAI-1 activity are correlated with impaired fibrinolytic responses in patients with DVT [15]. One study showed that patients with unresolved thrombi were more likely to have elevated PAI-1 levels and this was associated with a polymorphism in the promoter region of PAI-1 [16]. Transgenic mice overexpressing PAI-1 develop spontaneous venous occlusions in their tails and hind feet [17]. Mice deficient in PAI-1 have a mild baseline increase in fibrinolytic activity and a decrease in incidence of venous thrombi after footpad injection with endotoxin [18]. In surgical mouse models of thrombosis, PAI-1 deficiency, by pharmaceutical inhibition or genetic deletion, results in a delay in the time to total venous occlusion [19;20] and a decrease in early thrombus size [20-22].
PAI-2, or serpinB2, was first discovered as an effective inhibitor of uPA activity in in vitro assays [23;24]. PAI-2 is an atypical serpin that is structurally and functionally distinct from PAI-1 and is characterized by the lack of a classical secretory signal. Consequently PAI-2 accumulates in cells predominantly as an intracellular protein [25], except during pregnancy when it is found in a glycosylated form in circulating plasma [26]. PAI-2 is not typically in direct contact with extracellular uPA. Up-regulation of PAI-2 is a major stress response in multiple cell types [27], and PAI-2 has been shown to possess unique immunomodulatory and cell survival activities that are independent of uPA [28-30]. PAI-2 deficient mice exhibit impaired responses to infections [28;29;31], but PAI-2 has not generally been associated with the regulation of thrombosis in vivo. Unlike PAI-1 deficiency, PAI-2 gene-deficient mice do not display any overt baseline changes in fibrinolysis or spontaneous thrombosis [32]. PAI-2 is one of the most abundantly induced proteins in monocytes and macrophages in response to inflammatory stimuli, with induction reported over 105-fold [33]. Recent studies link PAI-2 to inflammatory diseases such as asthma [34], lupus [35] and antiphospholipid syndrome [36] and in the regulation of adaptive immunity [29]. The roles of PAI-2 in the inflammatory and fibrinolytic processes that occur during venous thrombus resolution are unknown.
Here we have investigated the contribution of PAI-2 to venous thrombus resolution. We hypothesized that the immunomodulating and uPA inhibitory activities of PAI-2 could play a role in mediating this process. Using an established mouse model of stasis DVT that accurately mimics many of the clinical and pathophysiological features observed in human DVT [37], we find that PAI-2 gene deficiency accelerates venous thrombus resolution. PAI-2 deficiency is associated with increased uPA activity in the inflammatory milieu of the resolving thrombus and with the downregulation of PAI-1, however, the role of PAI-2 in DVT appears to be distinct from PAI-1. These data establish PAI-2 as a potential therapeutic target for patients with DVT.
Material and Methods
Stasis model of DVT
Mice deficient in SerpinB2 (PAI-2 -/-) backcrossed 12 times onto the C57BL/6 background have been reported previously [31]. C57BL/6 wild-type (WT) and mice deficient in SerpinE1 (PAI-1 -/-) were purchased from Jackson Laboratories (Bar Harbor, ME). The surgical stasis model of DVT has been described [38;39]. Briefly, a laparotomy was performed on anesthetized 8-12 week old mice to expose the caudal vena cava. Major side branches and lumbar veins between the renal veins and iliac bifurcation were cauterized. The vena cava was ligated with a 7-0 silk suture immediately distal to the renal veins. This leads to reproducible thrombi that form between the infrarenal vena cava and the iliac bifurcation in all strains. At euthanasia, 2, 4, 8 or 12 days post-ligation, the thrombosed vena cava from the level of the renal veins to the iliac bifurcation was excised, weighed and tissue was evaluated by histological and molecular analyses. Gross observation and histologic staining showed that the thrombi formed were comparably fibrin, leukocyte and red blood cell rich in each of the 3 strains. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Maryland School of Medicine.
Enzyme linked immunoassays (ELISAs)
Thrombus protein lysates were prepared by homogenization in T-PER Tissue Protein Extraction Reagent (Pierce) followed by centrifugation to remove cell debris according to the manufacturer’s instructions. The level in thrombus protein lysates of murine chemokine (C-X-C motif) ligand (CXCL)1 (KC), CXCL2 (MIP2-α) (R&D Systems), mouse PAI-2 total antigen (US Biologicals), and mouse PAI-1 total antigen (MPAIKT-TOT), total uPA (MUPAKT) and active uPA (MUPAKT-TOT)(Molecular Innovations) were analyzed by ELISA using commercial ELISA kits according to manufacturer’s instructions. Signals are expressed relative to total protein levels in the thrombus lysate, as determined by nanodrop or BioRad Protein assay, to normalize for any differences in clot size.
SDS-PAGE gelatin zymography
Thrombus protein lysates (20μg per sample) were analyzed by zymography on precast 10% SDS-polyacrylamide/1% gelatin gels (Invitrogen). Gels were scanned by densitometry and data analyzed using ImageJ software [40] and Adobe Photoshop histogram analysis. Matrix metalloproteinase (MMP)-2 and MMP-9 optical densities were normalized to actin levels in each sample as determined by densitometry of western blots.
Immunohistochemistry
Immunohistochemical staining was performed on tissues fixed in 4% paraformaldehyde, paraffin embedded, and cut into 5-μm sections. Sections were immunostained for macrophages using rat anti-mouse Mac-2 (1:600; Cedarlane), and for neutrophils, using rat anti-mouse Ly-6B.2 (1:100; AbD Serotec), and signals detected using anti-rat secondary antibody and the ABC kit (Vector Laboratories) according to the manufacturer’s instructions. Specificity controls included isotype IgG or secondary antibody only. Staining was developed using 3,3′ diaminobenzidine substrate and slides were counterstained with hematoxylin (Vector Laboratories). Macrophages and neutrophils were distinguished by positive stained nuclei in high power fields.
Flow Cytometry
To assess leukocyte cell populations in the thrombus milieu, thrombi were evaluated at day 4 after ligation, at which time complete thrombus extraction from the vein wall is possible. Extracted clots were enzymatically digested with collagenase II (Worthington) and tPA (EMD Milipore). Red cells were lysed with ammonium chloride (Sigma), and then the remaining cells were filtered through a 70 micron filter to generate a single cell suspension. The cells were counted and then stained at a constant manufacturer recommended concentration for CD11b (AbD Serotec), a marker of macrophages and activated neutrophils, and Ly-6B.2 (AbD Serotec), a neutrophil-specific marker. Draq5 (Cell Signaling Technology) was used to identify nucleated cells and to subtract debris from digestion. Single stained controls were used for compensation, and fluorescence-minus-one controls were used to determine the gates. Flow cytometric analysis was performed using a Becton-Dickinson FACS Calibur flow cytometer and post-collection data processing was performed with FlowJo analysis software (Tree Star, Inc).
Macrophage isolation and gene array analyses
Peritoneal macrophages were isolated from WT and PAI-2 -/- mice 4 days following i.p. injection of 1 ml of 5% thioglycollate. Recruited cells were recovered by peritoneal lavage and macrophages twice purified by limited adherence to bacterial plastic. Total RNA was prepared using TRIzol (Invitrogen) following manufacturer’s instructions. cDNA was synthesized from the purified RNA using Superscript II cDNA synthesis kit (Invitrogen). Biotin labeled cRNA was prepared from the cDNA using standard procedures and was subsequently hybridized on a Affymetrix GeneChip array (GeneChip Mouse Genome 430 2.0 array). Slides were scanned and data were analyzed using GeneChip Operating Software v 1.1.1 (Affymetrix).
Statistical analysis
All data are presented as mean ± standard error. Unpaired student’s t-test was used to compare experimental groups that were normally distributed. p≤0.05 was defined as statistical significance. All n values stated represent samples from separate animals.
Results
PAI-2 deficiency causes enhanced venous thrombus resolution
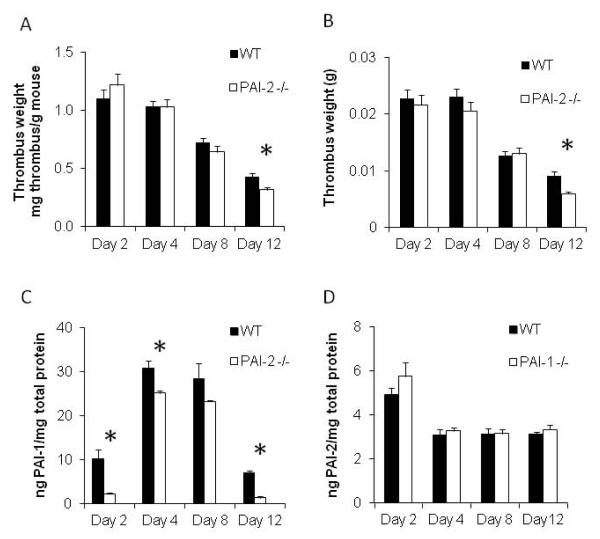
The stasis DVT mouse model produces a consistent thrombus within the vena cava that is fibrin, leukocyte, platelet and red cell rich, and forms on the surface of the endothelium [4]. Typically maximum thrombus burden, as determined by thrombus weight normalized to body weight, is observed between day 2 and day 4 post infrarenal vena cava ligation. Around this time there is a tipping of the balance of prothrombotic and fibrinolytic activities. After this time, fibrinolytic tendencies predominate, and thrombus resolution occurs and thrombus weight decreases. Thrombus resolution is typically measured by comparing thrombus weights between control and test animals at day 12 after ligation, while a change at day 4 is an indicator of thrombus formation. When comparing surgically ligated WT and PAI-2 -/- mice, thrombus weight at day 12 in PAI-2 -/- mice (0.32 ± 0.02 mg/g, n=16) was significantly decreased (by 26%) compared with WT mice (0.43 ± 0.03 mg/g, n=13) (p<0.005). No difference was observed in thrombus weights at day 2, 4 or 8 between groups (n= 7-17) (Figure 1A). The difference at day 12 remained significant when thrombus weight alone was analyzed (Figure 1B). These data reveal that PAI-2 deficiency enhances venous thrombus resolution, while having no effect on thrombus formation.
Figure 1. PAI-2 deficiency enhances thrombus resolution and decreases PAI-1 levels.
(A) Thrombus weight normalized to mouse body weight over time in PAI-2 -/- mice compared to WT (Day 12 p<0.005; n=13-16, Day 2 n=9-12, Day 4 n=17, Day 8 n=7-10). (B) Thrombus weight over time in PAI-2 -/- mice compared to WT (Day 12 p<0.005; n=13-16, Day 2 n=9-12, Day 4 n=17, Day 8 n=7-10). (C) Assay of total PAI-1 in WT and PAI-2 -/- thrombus protein lysates by ELISA (Day 2 p=0.019, Day 4 p=0.041, Day 12 p<0.005; n=3). (D) Assay of total PAI-2 in WT and PAI-1 -/- thrombus protein lysates by ELISA (n=3).
Decreased levels of PAI-1 protein are found in PAI-2 deficient venous thrombi
Since uPA plays an essential role in thrombus resolution, and PAI-1 is a potent inhibitor of uPA, we investigated the effect of PAI-2 gene deficiency on PAI-1 levels in thrombus protein lysates by ELISA. Although one could hypothesize that there would be a compensatory increase in PAI-1 levels in the absence of PAI-2, we found a reduction in PAI-1 protein expression in PAI-2 -/- mice compared to WT mice by ~80% at days 2 and 12, and ~20% at days 4 and 8 (Day 2 p=0.019, Day 4 p=0.041, Day 12 p<0.005; n=3) (Figure 1C). As PAI-1 is anti-fibrinolytic, this reduction in expression could contribute to the thrombus resolution phenotype seen in PAI-2 -/- mice. No significant differences in PAI-2 protein expression were detected in PAI-1 -/- mice compared to WT mice (Figure 1D).
PAI-1 deficiency causes decreased venous thrombus formation
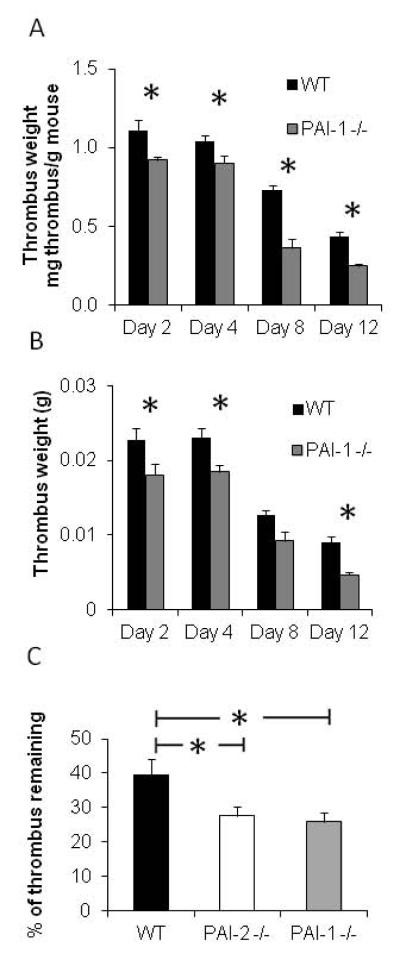
To explore if the reduced PAI-1 levels in thrombi of PAI-2 -/- mice could be responsible for the enhanced thrombus resolution, we investigated the effect of PAI-1 deficiency on DVT using the stasis DVT model. Unlike the results seen with PAI-2 -/- mice, thrombus weights were significantly reduced in PAI-1 -/- mice at all time points tested compared to WT mice (Day 2 p=0.045, Day 4 p=0.049, Day 8 p<0.005, Day 12 p<0.005) (Figure 2A). These differences remained significant at day 2, day 4 and day 12 when thrombus weight alone was analyzed (Figure 2B). The significant reduction in thrombus weights at days 2 and 4 indicates that unlike PAI-2, PAI-1 deficiency results in reduced thrombus formation, consistent with increased fibrinolysis.
Figure 2. PAI-1 deficiency impairs thrombus formation and enhances thrombus resolution.
(A) Thrombus weight normalized to mouse body weight over time in PAI-1 -/- mice compared to WT (Day 2 p=0.045 n=8-12, Day 4 p=0.049 n=11-17, Day 8 p<.005 n=3-7, Day 12 p<0.005; n=10-13). (B) Thrombus weight over time in PAI-1 -/- mice compared to WT (Day 2 p=0.046 n=8-12, Day 4 p=0.018 n=11-17, Day 8 p=0.065 n=3-7, Day 12 p<0.005; n=10-13). (C) Percent of thrombus remaining on day 12 in PAI-2 -/- (p=0.012), and PAI-1 -/- (p=0.007) compared to WT mice, as assessed by thrombus weight at day 12 divided by thrombus weight on day 2.
To further investigate the effect on thrombus resolution alone and to account for the differences in thrombus weight observed at day 2, the thrombus weight measured at day 12 was compared to the thrombus weight at day 2. We used the percentage of thrombus remaining at day 12 as a measure of thrombus resolution. Compared to 38% of the thrombus remaining at day 12 in the wild type mice, PAI-1 deficiency resulted in 27.6% (p=0.007) and PAI-2 deficiency resulted in 25.8% (p=0.012) of the thrombus remaining at day 12 (Figure 2C), indicating a similarly enhanced thrombus resolution. Together, these data show that both thrombus formation and thrombus resolution are influenced by PAI-1 deficiency, whereas the effect of PAI-2 deficiency is limited only to thrombus resolution.
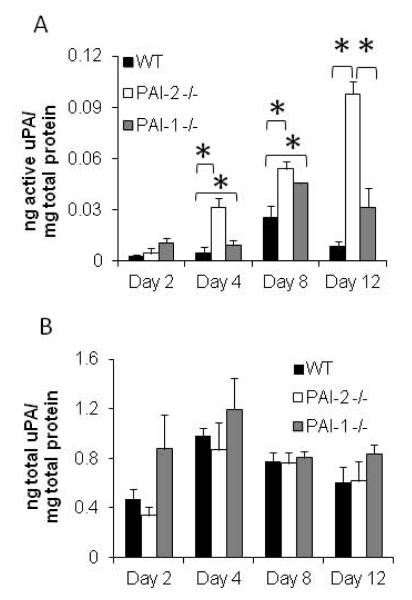
uPA activity is significantly increased in venous thrombi from PAI-2 -/- mice
Total uPA in thrombus lysates was measured using an ELISA assay that detects uPA antigen (all forms) and active uPA levels were measured using a murine-specific, functional uPA bioimmunoassay which detects only active uPA and will not detect inactive uPA or uPA complexed to inhibitors. While there was no difference in the levels of active uPA between WT, PAI-1 -/- and PAI-2 -/- thrombi on day 2, by day 4 increased levels of active uPA were detected in both PAI-2 -/- and PAI-1 -/- thrombi compared with WT. At day 12, there was a 4-fold increase in active uPA levels in PAI-1 -/- thrombi (n=6, p=0.07) and an even greater 12-fold increase in PAI-2 -/- thrombi (n=6, p<0.005) compared to WT mice (Figure 3A). No significant differences were seen in total uPA antigen levels amongst WT, PAI-2 -/- or PAI-1 -/- mice by ELISA (n=3)(Figure 3B). PAI-2 -/- thrombi have ~10-fold greater amount of active uPA at day 12 than seen with WT thrombi (Figure 3A). Since PAI-1 is known to play a primary role as an inhibitor of uPA fibrinolytic activity, and PAI-1 levels are reduced only about ~4-fold in PAI-2-/- thrombi at day 12 (Figure 1C), our data indicate an effect of PAI-2 on uPA activity independent of PAI-1.
Figure 3. uPA activity is increased in PAI-2 deficient thrombi.
Protein lysates were prepared from thrombi produced in each strain and (A) active uPA and (B) total uPA levels measured by ELISA as described in the methods. Results are represented as the mean of 3-6 independent samples. (Active uPA: Day 4 WT vs PAI-2 -/- p=0.017, WT vs PAI-1 -/- p=0.027; Day 8 WT vs PAI-2 -/- p=0.026, WT vs PAI-1 -/- p=0.047; Day 12 WT vs PAI-2 -/- p<0.005, PAI-1 -/- vs PAI-2 -/- p<0 .005).
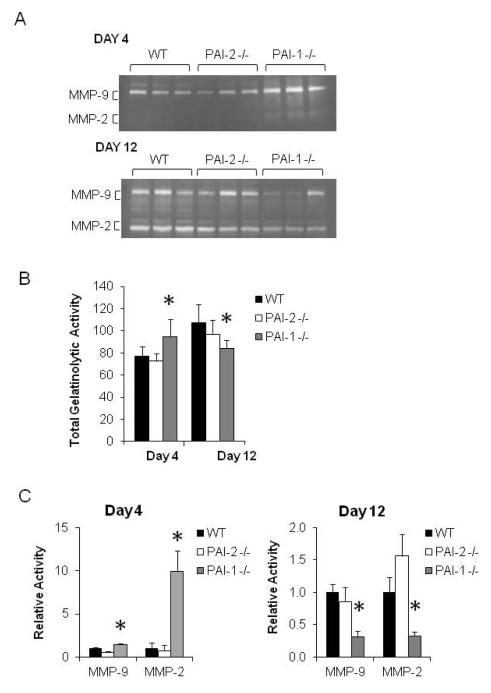
PAI-2 and PAI-1 deficiencies affect venous thrombi MMP activities in distinct manners
MMPs, specifically MMP-2 and MMP-9, are believed to contribute to enhanced venous thrombus resolution possibly through their activities in the remodeling of the extracellular matrix [41;42]. The effects of PAI-1 and PAI-2 deficiencies on gelatinolytic activities during venous thrombus resolution were investigated by gelatin zymography. At day 4 following ligation, PAI-1 -/- venous thrombi showed increased gelatinolytic activity by gelatin zymography compared to WT and PAI-2 -/- venous thrombi (n=3, p=0.02 and 0.01 respectively), and decreased gelatinolytic activity compared to the WT controls by day 12 (n=3, p=0.001) (Figure 4A & B).
Figure 4. PAI-2 and PAI-1 deficiency have distinct effects on MMP activity.
(A) Gelatin zymography of thrombus protein lysates (n=3 for each strain). (B) Densitometric analysis of total gelatinolytic activity in A (Day 4 PAI-1 -/- vs WT p=0.02) (Day 4 PAI-1 -/- vs PAI-2 -/- p=0.01) (Day 12 PAI-1 -/- vs WT p=0.001) (Day 12 PAI-2 -/- vs WT or PAI-1 -/-p=0.01). (C) Densitometry of MMP2 and MMP9 activities shown in A, normalized to actin levels (MMP-9 Day 4 PAI-1 -/- vs PA1-2 -/- p=0.01) (MMP-2 Day 4 PAI-1 -/- vs WT or PAI-2 -/- p=0.02) (MMP-9 Day 12 PAI-1 -/- vs WT p=0.01) (MMP-2 Day 12 PAI-1 -/- vs WT p=0.05).
Examination of MMP-2 and -9 activities showed that at day 4 following ligation, PAI-1 -/- venous thrombi had increased MMP-2 and -9 activities compared to PAI-2 -/- venous thrombi (n=3, p=0.01 and 0.02 respectively), and increased MMP-2 activity compared to the WT controls (n=3, p=0.02). By day 12 however, MMP-2 and -9 activities were decreased in PAI-1 -/- venous thrombi compared to WT venous thrombi (n=3. p=0.05 and 0.01 respectively) (Figure 4). PAI-2 deficiency did not have a significant effect on MMP activities in venous thrombi. These data show that deficiency of PAI-2 and PAI-1 have contrasting effects on MMP activities, and further suggest that PAI-2 and PAI-1 affect distinct pathways during venous thrombosis and its resolution.
PAI-2 deficiency stimulates early enhanced neutrophil recruitment
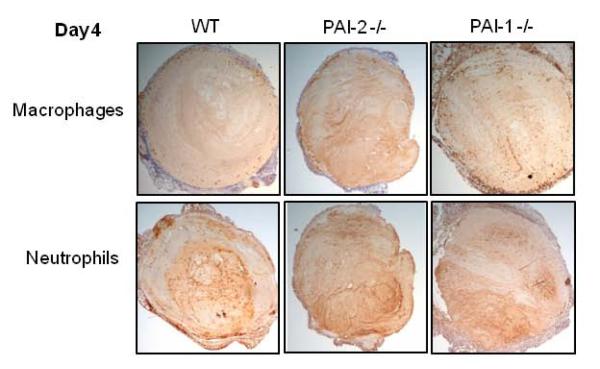
Inflammatory cells are recruited to resolving venous thrombi and are important effectors of thrombus resolution. The inflammatory process in venous thrombus resolution is similar to sterile wound healing, with the temporal accumulation of polymorphonucleic neutrophils (PMN) early in the process (days 2-4), which is followed by a later monocyte and lymphocyte influx (days 8-12) [12;43]. Immunostaining of day 4 thrombus sections from PAI-2 -/-, PAI-1 -/- and WT mice for leukocyte infiltrates showed the presence of neutrophils (Lys-6G/C positive) and a low number of macrophages (Mac-2 positive) within venous thrombi of all 3 genotypes (Figure 5).
Figure 5. Leukocyte infiltrates in day 4 thrombi.
Immunohistochemical staining for macrophages (anti-Mac-2) and neutrophils (anti-Ly-6B.2) in venous thrombus samples at Day 4. Staining was performed as described in the methods. No specific staining was observed for isotype IgG or secondary antibody alone. Figure shows representative results from 3 independent thrombi from each mouse strain. Images were taken on a Nikon Eclipse E400 at 40X.
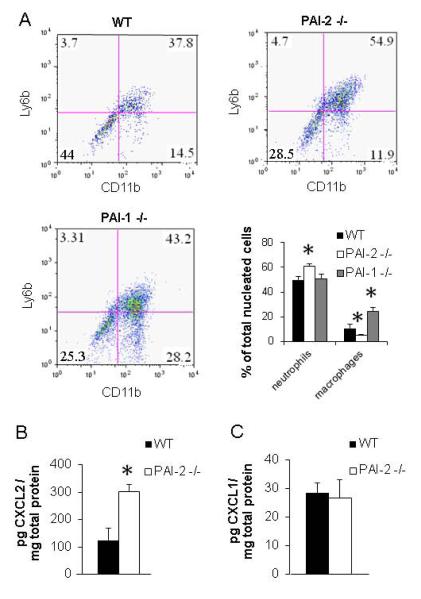
Flow cytometry was employed for quantitative assessment of the relative cell populations within the milieu of the venous thrombi. Cells were released from whole day 4 clots with vein wall removed by enzymatic digestion. Clots from PAI-2 -/- mice showed a decrease in the percentage of macrophages (4.8 ± 0.7%, n =6, p=0.04), while the clots from PAI-1 -/- mice had an increase in percentage of macrophages (24.0 ± 3.7%, n=3, p<0.005) compared to WT mice (10.7 ± 3%, n=6). We also found that PAI-2 -/- mice had an increased percentage of neutrophils compared to either WT or PAI-1 -/- mice (WT = 50.0 ± 2.8%, PAI-1 -/- = 50.4 ±3.8%, PAI-2 -/- = 61.1 ± 1.8%, p=0.006, n=3-6) (Figure 6A). These data indicate that PAI-2 and PAI-1 deficiencies have differential effects on sculpting the inflammatory environment during venous thrombus resolution, and demonstrate that the effect of PAI-2 on thrombus resolution is likely not solely due to reduced levels of PAI-1.
Figure 6. PAI-2 deficiency enhances neutrophil recruitment through elevated CXCL2 levels.
(A) Flow cytometry scatter plots and quantitation of flow cytometric results of cells released from Day 4 clots. Cells were double-stained with anti-CD11b and anti-Ly6B.2 as described in the methods. Cells were counted as neutrophils if they were Ly6B.2 positive or Ly6B.2 and CD11b double positive (upper 2 quadrants on scatter plots). Cells were counted as macrophages if they were only CD11b positive (lower right quadrant on scatter plots). The scatter plots show a typical results from one thrombi from each strain, and the bar graph represents the mean and SEM from 3-6 independent flow cytometry experiments. (Neutrophils: PAI-2 -/- vs WT p=0.006) (Macrophages: PAI-2 -/- vs WT p=0.04; PAI-1 -/- vs WT p<0.005). (B) CXCL2 and (C) CXCL1 ELISA of thrombus protein lysates on day 4 (n=3) (p=0.027).
PAI-2 deficiency alters macrophage expression of inflammatory mediators
Since PAI-2 is a major macrophage protein [44], we speculated that PAI-2 deficiency would alter macrophage function which in turn could contribute to the enhanced venous thrombus resolution associated with PAI-2 -/- mice. Published studies implicate PAI-2 as a promoter of macrophage differentiation and a modulator of cytokine genes important for sculpting the nature of immune responses [28;29;45]. To better understand how PAI-2 deficiency may affect macrophage gene expression, we used microarray technology to examine gene expression profiles in thioglycollate-elicited peritoneal macrophages isolated from WT and PAI-2 -/- mice. These analyses revealed a several-fold increase in mRNA levels of the neutrophil chemoattractants, CXCL1 and CXCL2, in macrophages recovered from PAI-2 -/- mice compared to WT (data not shown). Increased expression of these chemokines by PAI-2 -/- macrophages could contribute to enhanced neutrophil recruitment, resulting in the increased neutrophils in venous thrombi of PAI-2 -/- mice determined by flow cytometry (Figure 6A). Analysis of the CXCL1 and CXCL2 protein levels in day 4 thrombus protein lysates by ELISA revealed a 2-fold increase in the levels of CXCL2 protein in PAI-2 -/- thrombi compared to WT thrombi (n= 3) (p= 0.027) (Figure 6B). The levels of CXCL1 were similar between PAI-2 -/- and WT thrombi (Figure 6C). Although the number of macrophages present with in PAI-2 -/- clots was not increased, those macrophages present may have an altered pro-inflammatory phenotype that contributes to enhancement of neutrophil recruitment (Figure 6A) and ultimately to enhancement of thrombus resolution (Figure 1).
Discussion
These data identify the serpin PAI-2 as a novel regulator of venous thrombus resolution in vivo and reveal that PAI-2 modulates pathways involving inflammation and components of the plasminogen activation system. To our knowledge this is the first context in vivo that supports a role for PAI-2 in the modulation of thrombosis.
The proteolytic activity of the serine protease, plasmin, is pivotal for clot dissolution. The generation of plasmin by the plasminogen activator system is classically associated with tPA-mediated fibrinolysis in the circulation and uPA-mediated plasmin generation in extravascular compartments [33]. Activities of plasmin in the latter situation are commonly associated with processes involved in wound repair, cell migration and tumor metastasis. PAI-1 is an effective inhibitor of both uPA and tPA activities [27]. While PAI-2 is an effective inhibitor of uPA in cell based studies [23], the evidence for inhibition of uPA by PAI-2 in physiological contexts has not been forthcoming [33]. PAI-2 deficiency does not result in differences in uPA activity in certain cell populations [29;32], and causes no obvious changes in bleeding times [32]. Nonetheless our data convincingly demonstrate that in a stasis model of DVT, PAI-2 modulates venous thrombus resolution and uPA activity.
PAI-2 -/- venous thrombi had reduced levels of PAI-1, indicating that PAI-1 levels do not increase to compensate for the lack of PAI-2. This result suggested that the enhanced resolution in PAI-2 -/- mice could be due to the reduction in PAI-1 expression. However the complete genetic loss of PAI-1 (Figure 2A,B) does not reproduce the substantive active uPA levels seen in PAI-2 deficiency (Figure 3), suggesting additional pathways for the effect of PAI-2 on uPA activity. Further, PAI-1 and PAI-2 deficiencies result in different downstream effects. These differences suggest that PAI-2 deficiency may modulate venous thrombus resolution through both PAI-1-dependent and PAI-1-independent pathways. Interestingly, PAI-1 deficiency has an additional effect on thrombus formation, which was not seen with PAI-2 deficiency, indicating a complex interplay of PAI-1 and PAI-2 in the process of venous thrombus formation and resolution.
PAI-2 regulation of uPA activity could be due a number of possible mechanisms, or combination of mechanisms. While the downregulation of PAI-1 protein levels cannot totally account for the even larger increase in uPA activity seen in PAI-2 -/- thrombi, it still could account for some part of the effect on uPA activity. The effect of PAI-2 on uPA could also be more specific to the context of a fibrin clot. Previous work has suggested that, in vitro, in the presence of a fibrin clot along with transglutaminase and FXIIIa, PAI-2 is found extracellularly and can cross-link to the alpha chain of fibrin. PAI-2 retains its inhibitory effect on uPA in this context [46]. It is possible that this could also occur in the presence of a thrombus in vivo, and PAI-2 could directly inhibit uPA. In addition, PAI-2 has intracellular immune modulatory properties that could indirectly regulate uPA activity and also contribute to the down-regulation of PAI-1 levels in PAI-2 -/- thrombi. As more is learned about immune signaling pathways that PAI-2 regulates, these indirect links may be elucidated.
A number of studies suggest that inflammatory responses occurring early in the resolving thrombus and that are controlled by both neutrophils and macrophages are critical for venous thrombus resolution [2;12;43;47]. Macrophages play a large role in sculpting the early inflammatory response, by regulating such processes as thrombolysis and MMP activation, although it has been found that increased macrophage numbers do not necessarily result in less vascular injury [48]. We found that PAI-1 -/- clots had an increased percentage of macrophages (Figure 6A). This is not surprising, as PAI-1 has previously been shown to play a role in macrophage migration [49;50]. On the other hand, we found that PAI-2 -/- clots had an increased percentage of neutrophils (Figure 6A). The elevation of CXCL2 could be responsible for the increase in neutrophils seen in the PAI-2 -/- clots (Figure 6B). Neutrophils have been shown to play a role in thrombus resolution [51]. In a previous study, neutropenia in a rat model of venous thrombosis impaired thrombus resolution and caused an increase in collagen content [43]. The increase in neutrophils seen in PAI-2 -/- mice could therefore contribute to the enhanced resolution observed.
Together these data identify PAI-2 as a potential therapeutic target for the treatment of DVT and the prevention of post thrombotic syndrome. As PAI-2 -/- mice show no obvious bleeding phenotype and form clots normally, the specific role of PAI-2 appears to be associated with thrombus resolution. Antagonists of PAI-2 could lead to clinical benefits as there may be less adverse hemorrhagic complications and may be beneficial to patients at later time points in DVT when other therapies have proven to be less effective. Further studies will be needed to explore specific pathways that are affected by PAI-2 and to develop in vivo inhibitors of PAI-2 to fully elucidate the therapeutic potential of PAI-2.
Acknowledgements
The authors thank Dr. Sarah Netzel-Arnett for performing the ELISAs, Elizabeth Smith immunohistochemistry assistance, Dr. Laura Tonnetti for microarray assistance, and Dr. Linda Siefert for assistance with statistical analyses.
Sources of funding This work was supported by National Institutes of Health R01HL083917 (R.S.), R01CA098369 and R56CA098369 (T.M.A), R01HL114379 (D.K.S) and Award Number 1I01BX001921 from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development. S.A.S was supported by NIH T32 HL007698 (D.K.S).
Footnotes
Authorship contributions S. A. Siefert contributed to experimental design, performed experiments, analyzed data, and wrote the manuscript; C. Chabasse contributed to experimental design, performed experiments, analyzed data and reviewed the manuscript. S. Mukhopadhyay contributed to experimental design, performed experiments, analyzed data and reviewed the manuscript; M. H. Hoofnagle performed experiments and analyzed data; D. K. Strickland provided critical reagents and contributed to data analysis and reviewed the manuscript; R. Sarkar designed and supervised the study, analyzed data, and contributed to writing the manuscript; T. M. Antalis designed and supervised the study, analyzed data, and contributed to writing the manuscript.
Disclosures The authors state that they have no conflict of interest.
References
- (1).Moll S, Mackman N. Venous thromboembolism: a need for more public awareness and research into mechanisms. Arterioscler Thromb Vasc Biol. 2008;28:367–369. doi: 10.1161/ATVBAHA.108.163097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Henke PK, Wakefield T. Thrombus resolution and vein wall injury: dependence on chemokines and leukocytes. Thromb Res. 2009;123(Suppl 4):S72–S78. doi: 10.1016/S0049-3848(09)70148-3. [DOI] [PubMed] [Google Scholar]
- (3).Meissner MH, Caps MT, Zierler BK, Polissar N, Bergelin RO, Manzo RA, Strandness DE., Jr. Determinants of chronic venous disease after acute deep venous thrombosis. J Vasc Surg. 1998;28:826–833. doi: 10.1016/s0741-5214(98)70057-6. [DOI] [PubMed] [Google Scholar]
- (4).Saha P, Humphries J, Modarai B, Mattock K, Waltham M, Evans CE, Ahmad A, Patel AS, Premaratne S, Lyons OT, Smith A. Leukocytes and the natural history of deep vein thrombosis: current concepts and future directions. Arterioscler Thromb Vasc Biol. 2011;31:506–512. doi: 10.1161/ATVBAHA.110.213405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Wakefield TW, Myers DD, Henke PK. Mechanisms of venous thrombosis and resolution. Arterioscler Thromb Vasc Biol. 2008;28:387–391. doi: 10.1161/ATVBAHA.108.162289. [DOI] [PubMed] [Google Scholar]
- (6).Henke PK, Pearce CG, Moaveni DM, Moore AJ, Lynch EM, Longo C, Varma M, Dewyer NA, Deatrick KB, Upchurch GR, Jr., Wakefield TW, Hogaboam C, Kunkel SL. Targeted deletion of CCR2 impairs deep vein thombosis resolution in a mouse model. J Immunol. 2006;177:3388–3397. doi: 10.4049/jimmunol.177.5.3388. [DOI] [PubMed] [Google Scholar]
- (7).Collen D, Lijnen HR. Basic and clinical aspects of fibrinolysis and thrombolysis. Blood. 1991;78:3114–3124. [PubMed] [Google Scholar]
- (8).Castellino FJ, Ploplis VA. Structure and function of the plasminogen/plasmin system. Thromb Haemost. 2005;93:647–654. doi: 10.1160/TH04-12-0842. [DOI] [PubMed] [Google Scholar]
- (9).Northeast AD, Soo KS, Bobrow LG, Gaffney PJ, Burnand KG. The tissue plasminogen activator and urokinase response in vivo during natural resolution of venous thrombus. J Vasc Surg. 1995;22:573–579. doi: 10.1016/s0741-5214(95)70041-2. [DOI] [PubMed] [Google Scholar]
- (10).Singh I, Burnand KG, Collins M, Luttun A, Collen D, Boelhouwer B, Smith A. Failure of thrombus to resolve in urokinase-type plasminogen activator gene-knockout mice: rescue by normal bone marrow-derived cells. Circulation. 2003;107:869–875. doi: 10.1161/01.cir.0000050149.22928.39. [DOI] [PubMed] [Google Scholar]
- (11).Gossage JA, Humphries J, Modarai B, Burnand KG, Smith A. Adenoviral urokinase-type plasminogen activator (uPA) gene transfer enhances venous thrombus resolution. J Vasc Surg. 2006;44:1085–1090. doi: 10.1016/j.jvs.2006.07.020. [DOI] [PubMed] [Google Scholar]
- (12).Humphries J, Gossage JA, Modarai B, Burnand KG, Sisson TH, Murdoch C, Smith A. Monocyte urokinase-type plasminogen activator up-regulation reduces thrombus size in a model of venous thrombosis. J Vasc Surg. 2009;50:1127–1134. doi: 10.1016/j.jvs.2009.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Gettins PG. Serpin structure, mechanism, and function. Chem Rev. 2002;102:4751–4804. doi: 10.1021/cr010170+. [DOI] [PubMed] [Google Scholar]
- (14).Vaughan DE. PAI-1 and atherothrombosis. J Thromb Haemost. 2005;3:1879–1883. doi: 10.1111/j.1538-7836.2005.01420.x. [DOI] [PubMed] [Google Scholar]
- (15).Nilsson IM, Ljungner H, Tengborn L. Two different mechanisms in patients with venous thrombosis and defective fibrinolysis: low concentration of plasminogen activator or increased concentration of plasminogen activator inhibitor. Br Med J (Clin Res Ed) 1985;290:1453–1456. doi: 10.1136/bmj.290.6480.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Bern MM, McCarthy N. Failure to lyse venous thrombi because of elevated plasminogen activator Inhibitor 1 (PAI-1) and 4G polymorphism of its promotor genome (The PAI-1/4G Syndrome) Clin Appl Thromb Hemost. 2010;16:574–578. doi: 10.1177/1076029610361334. [DOI] [PubMed] [Google Scholar]
- (17).Erickson LA, Fici GJ, Lund JE, Boyle TP, Polites HG, Marotti KR. Development of venous occlusions in mice transgenic for the plasminogen activator inhibitor-1 gene. Nature. 1990;346:74–76. doi: 10.1038/346074a0. [DOI] [PubMed] [Google Scholar]
- (18).Carmeliet P, Stassen JM, Schoonjans L, Ream B, van den Oord JJ, De Mol M, Mulligan RC, Collen D. Plasminogen activator inhibitor-1 gene-deficient mice. II. Effects on hemostasis, thrombosis, and thrombolysis. J Clin Invest. 1993;92:2756–2760. doi: 10.1172/JCI116893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Eitzman DT, Westrick RJ, Nabel EG, Ginsburg D. Plasminogen activator inhibitor-1 and vitronectin promote vascular thrombosis in mice. Blood. 2000;95:577–580. [PubMed] [Google Scholar]
- (20).Hennan JK, Morgan GA, Swillo RE, Antrilli TM, Mugford C, Vlasuk GP, Gardell SJ, Crandall DL. Effect of tiplaxtinin (PAI-039), an orally bioavailable PAI-1 antagonist, in a rat model of thrombosis. J Thromb Haemost. 2008;6:1558–1564. doi: 10.1111/j.1538-7836.2008.03063.x. [DOI] [PubMed] [Google Scholar]
- (21).Diaz JA, Ballard-Lipka NE, Farris DM, Hawley AE, Wrobleski SK, Myers DD, Henke PK, Lawrence DA, Wakefield TW. Impaired fibrinolytic system in ApoE gene-deleted mice with hyperlipidemia augments deep vein thrombosis. J Vasc Surg. 2012;55:815–822. doi: 10.1016/j.jvs.2011.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Diaz JA, Hawley AE, Alvarado CM, Berguer AM, Baker NK, Wrobleski SK, Wakefield TW, Lucchesi BR, Myers DD., Jr. Thrombogenesis with continuous blood flow in the inferior vena cava. A novel mouse model. Thromb Haemost. 2010;104:366–375. doi: 10.1160/TH09-09-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Harbour JW, Dean DC. Rb function in cell-cycle regulation and apoptosis. Nat Cell Biol. 2000;2:E65–E67. doi: 10.1038/35008695. [DOI] [PubMed] [Google Scholar]
- (24).Mikus P, Urano T, Liljestrom P, Ny T. Plasminogen-activator inhibitor type 2 (PAI-2) is a spontaneously polymerising SERPIN. Biochemical characterisation of the recombinant intracellular and extracellular forms. Eur J Biochem. 1993;218:1071–1082. doi: 10.1111/j.1432-1033.1993.tb18467.x. [DOI] [PubMed] [Google Scholar]
- (25).Ye RD, Wun TC, Sadler JE. Mammalian protein secretion without signal peptide removal. Biosynthesis of plasminogen activator inhibitor-2 in U-937 cells. J Biol Chem. 1988;263:4869–4875. [PubMed] [Google Scholar]
- (26).Kruithof EK, Tran-Thang C, Gudinchet A, Hauert J, Nicoloso G, Genton C, Welti H, Bachmann F. Fibrinolysis in pregnancy: a study of plasminogen activator inhibitors. Blood. 1987;69:460–466. [PubMed] [Google Scholar]
- (27).Kruithof EK, Baker MS, Bunn CL. Biological and clinical aspects of plasminogen activator inhibitor type 2. Blood. 1995;86:4007–4024. [PubMed] [Google Scholar]
- (28).Schroder WA, Gardner J, Le TT, Duke M, Burke ML, Jones MK, McManus DP, Suhrbier A. SerpinB2 deficiency modulates Th1Th2 responses after schistosome infection. Parasite Immunol. 2010;32:764–768. doi: 10.1111/j.1365-3024.2010.01241.x. [DOI] [PubMed] [Google Scholar]
- (29).Schroder WA, Le TT, Major L, Street S, Gardner J, Lambley E, Markey K, MacDonald KP, Fish RJ, Thomas R, Suhrbier A. A physiological function of inflammation-associated SerpinB2 is regulation of adaptive immunity. J Immunol. 2010;184:2663–2670. doi: 10.4049/jimmunol.0902187. [DOI] [PubMed] [Google Scholar]
- (30).Darnell GA, Schroder WA, Gardner J, Harrich D, Yu H, Medcalf RL, Warrilow D, Antalis TM, Sonza S, Suhrbier A. SerpinB2 is an inducible host factor involved in enhancing HIV-1 transcription and replication. J Biol Chem. 2006;281:31348–31358. doi: 10.1074/jbc.M604220200. [DOI] [PubMed] [Google Scholar]
- (31).Zhao A, Yang Z, Sun R, Grinchuk V, Netzel-Arnett S, Anglin IE, Driesbaugh KH, Notari L, Bohl JA, Madden KB, Urban JF, Jr., Antalis TM, Shea-Donohue T. SerpinB2 is critical to Th2 immunity against enteric nematode infection. J Immunol. 2013;190:5779–5787. doi: 10.4049/jimmunol.1200293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Dougherty KM, Pearson JM, Yang AY, Westrick RJ, Baker MS, Ginsburg D. The plasminogen activator inhibitor-2 gene is not required for normal murine development or survival. Proc Natl Acad Sci U S A. 1999;96:686–691. doi: 10.1073/pnas.96.2.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Medcalf RL. Plasminogen activator inhibitor type 2: still an enigmatic serpin but a model for gene regulation. Methods Enzymol. 2011;499:105–134. doi: 10.1016/B978-0-12-386471-0.00006-7. [DOI] [PubMed] [Google Scholar]
- (34).Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, Ellwanger A, Sidhu SS, Dao-Pick TP, Pantoja C, Erle DJ, Yamamoto KR, Fahy JV. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci U S A. 2007;104:15858–15863. doi: 10.1073/pnas.0707413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Palafox-Sanchez CA, Vazquez-Del Mercado M, Orozco-Barocio G, Garcia-De la Torre I, Torres-Carrillo N, Torres-Carrillo NM, Illades-Aguiar B, Munoz-Valle JF. A functional Ser(413)/Ser(413) PAI-2 polymorphism is associated with susceptibility and damage index score in systemic lupus erythematosus. Clin Appl Thromb Hemost. 2009;15:233–238. doi: 10.1177/1076029607308868. [DOI] [PubMed] [Google Scholar]
- (36).Vazquez-Del Mercado M, Garcia-Cobian TA, Munoz Valle JF, Torres-Carrillo N, Martin-Marquez BT, Arana-Argaez VE, Best-Aguilera CR, Martinez-Garcia EA, Petri MH, Nunez-Atahualpa L, Delgado-Rizo V. Genotype Ser413/Ser of PAI-2 polymorphism Ser413/Cys is associated with anti-phospholipid syndrome and systemic lupus erythematosus in a familial case: comparison with healthy controls. Scand J Rheumatol. 2007;36:206–210. doi: 10.1080/03009740601089648. [DOI] [PubMed] [Google Scholar]
- (37).Day SM, Reeve JL, Myers DD, Fay WP. Murine thrombosis models. Thromb Haemost. 2004;92:486–494. [PubMed] [Google Scholar]
- (38).Dahi S, Lee JG, Lovett DH, Sarkar R. Differential transcriptional activation of matrix metalloproteinase-2 and membrane type-1 matrix metalloproteinase by experimental deep venous thrombosis and thrombin. J Vasc Surg. 2005;42:539–545. doi: 10.1016/j.jvs.2005.04.051. [DOI] [PubMed] [Google Scholar]
- (39).McGilvray KC, Sarkar R, Nguyen K, Puttlitz CM. A biomechanical analysis of venous tissue in its normal and post-phlebitic conditions. J Biomech. 2010;43:2941–2947. doi: 10.1016/j.jbiomech.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Abramoff MD, Magalhaes PJ, Ram SJ. Image processing with Image J. Biophotonics International. 2004;11:36–42. [Google Scholar]
- (41).Deatrick KB, Eliason JL, Lynch EM, Moore AJ, Dewyer NA, Varma MR, Pearce CG, Upchurch GR, Wakefield TW, Henke PK. Vein wall remodeling after deep vein thrombosis involves matrix metalloproteinases and late fibrosis in a mouse model. J Vasc Surg. 2005;42:140–148. doi: 10.1016/j.jvs.2005.04.014. [DOI] [PubMed] [Google Scholar]
- (42).Sood V, Luke C, Miller E, Mitsuya M, Upchurch GR, Jr., Wakefield TW, Myers DD, Henke PK. Vein wall remodeling after deep vein thrombosis: differential effects of low molecular weight heparin and doxycycline. Ann Vasc Surg. 2010;24:233–241. doi: 10.1016/j.avsg.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Varma MR, Varga AJ, Knipp BS, Sukheepod P, Upchurch GR, Kunkel SL, Wakefield TW, Henke PK. Neutropenia impairs venous thrombosis resolution in the rat. J Vasc Surg. 2003;38:1090–1098. doi: 10.1016/s0741-5214(03)00431-2. [DOI] [PubMed] [Google Scholar]
- (44).Ritchie H, Jamieson A, Booth NA. Thrombin modulates synthesis of plasminogen activator inhibitor type 2 by human peripheral blood monocytes. Blood. 1995;86:3428–3435. [PubMed] [Google Scholar]
- (45).Park JM, Greten FR, Wong A, Westrick RJ, Arthur JS, Otsu K, Hoffmann A, Montminy M, Karin M. Signaling pathways and genes that inhibit pathogen-induced macrophage apoptosis--CREB and NF-kappaB as key regulators. Immunity. 2005;23:319–329. doi: 10.1016/j.immuni.2005.08.010. [DOI] [PubMed] [Google Scholar]
- (46).Ritchie H, Robbie LA, Kinghorn S, Exley R, Booth NA. Monocyte plasminogen activator inhibitor 2 (PAI-2) inhibits u-PA-mediated fibrin clot lysis and is cross-linked to fibrin. Thromb Haemost. 1999;81:96–103. [PubMed] [Google Scholar]
- (47).Henke PK, Varma MR, Deatrick KB, Dewyer NA, Lynch EM, Moore AJ, Dubay DA, Sukheepod P, Pearce CG, Upchurch GR, Jr., Kunkel SL, Franz MG, Wakefield TW. Neutrophils modulate post-thrombotic vein wall remodeling but not thrombus neovascularization. Thromb Haemost. 2006;95:272–281. doi: 10.1160/TH05-02-0099. [DOI] [PubMed] [Google Scholar]
- (48).Baldwin JF, Sood V, Elfline MA, Luke CE, Dewyer NA, Diaz JA, Myers DD, Wakefield T, Henke PK. The role of urokinase plasminogen activator and plasmin activator inhibitor-1 on vein wall remodeling in experimental deep vein thrombosis. J Vasc Surg. 2012;56:1089–1097. doi: 10.1016/j.jvs.2012.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Cao C, Lawrence DA, Li Y, Von Arnim CA, Herz J, Su EJ, Makarova A, Hyman BT, Strickland DK, Zhang L. Endocytic receptor LRP together with tPA and PAI-1 coordinates Mac-1-dependent macrophage migration. EMBO J. 2006;25:1860–1870. doi: 10.1038/sj.emboj.7601082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Deng G, Curriden SA, Wang S, Rosenberg S, Loskutoff DJ. Is plasminogen activator inhibitor-1 the molecular switch that governs urokinase receptor-mediated cell adhesion and release? J Cell Biol. 1996;134:1563–1571. doi: 10.1083/jcb.134.6.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Henke PK, Varga A, De S, Deatrick CB, Eliason J, Arenberg DA, Sukheepod P, Thanaporn P, Kunkel SL, Upchurch GR, Jr., Wakefield TW. Deep vein thrombosis resolution is modulated by monocyte CXCR2-mediated activity in a mouse model. Arterioscler Thromb Vasc Biol. 2004;24:1130–1137. doi: 10.1161/01.ATV.0000129537.72553.73. [DOI] [PubMed] [Google Scholar]