Abstract
Substantial research has implicated the hypothalamic-pituitary-adrenal (HPA) and hypothalamic-pituitary-gonadal (HPG) axes independently in adolescent mental health problems, though this literature remains largely inconclusive. Given the cross-talk between the HPA and HPG axes and their increased activation in adolescence, a dual-axis approach that examines both axes simultaneously is proposed to predict the emergence and persistence of adolescent mental health problems. After briefly orienting readers to HPA and HPG axis functioning, we review the literature examining associations between hormone levels and changes with behavior during adolescence. Then, we provide a review of the literature supporting examination of both axes simultaneously and present the limited research that has taken a dual-axis approach. We propose future directions including consideration of between-person and within-person approaches to address questions of correlated changes in HPA and HPG hormones. Potential moderators are considered to increase understanding of the nuanced hormone–behavior associations during key developmental transitions.
Keywords: HPA axis, HPG axis, hormone–behavior associations, adolescence, development, family context, HPA-HPG associations, cortisol, testosterone, dehydroepiandrosterone
INTRODUCTION
A rich body of literature describes dramatic changes in emotional and behavioral problems occurring in boys and girls during adolescence (Moffitt, 1993; Zahn-Waxler, Shirtcliff, & Marceau, 2008). Substantial research has implicated the hypothalamic-pituitary-adrenal (HPA) and hypothalamic-pituitary-gonadal (HPG) axes independently in these mental health problems; however, the findings are inconsistent. Given that the HPA and HPG axes are highly interactive systems, a dual-axis approach examining both axes in combination may more accurately predict the emergence and persistence of adolescent mood and behavior problems than considering each axis independently. Taking such an approach may be particularly important in adolescence compared to other developmental periods, as both axes show increased activation at puberty (Romeo, 2005). Increased activation of the HPA and HPG axes places adolescents in a unique hormonal milieu, which may be linked to the emergence of behavioral and emotional problems. Adopting a novel, developmentally sensitive, dual-axis approach has potential for advancing the field by clarifying how hormones of the HPA and HPG axis are associated with behavior, and how these associations may change over time. This review presents evidence that clarifying the endogenous hormonal milieu (including multiple hormones of the HPA and HPG axis within individuals) embedded within specific developmental and ecological contexts may help to identify how and in which contexts mental health problems are likely to present (i.e., as internalizing and/or externalizing problems). In this way, understanding the correlates of hormonal milieu across development and contexts may prove informative for intervention studies seeking to reduce adolescent mental health problems. Given that the majority of the literature presents hormone behavior associations in terms of hormones influencing behavior, or in terms of hormone levels and changes as measureable markers of risk for behavior problems, we also frame associations in this way. However, it is important to consider that behavior likely influences hormone levels and changes as well; the majority of the research conducted thus far has focused on concurrent associations or directional longitudinal associations from hormones to behavior, and thus it is difficult to conclude the direction of effects and mechanisms of the associations.
The purpose of this review is to identify and highlight potential physiological and developmental reasons for inconsistent hormone–behavior findings during the important growth and development occurring in adolescence. We begin by providing a brief overview of the HPA and HPG axes (for detailed reviews of the HPA and HPG axes see Chrousos & Gold, 1992; Grumbach & Styne, 2003; Gunnar & Quevedo, 2007; Romeo, 2005) and discuss developmental adrenal and gonadal hormone changes relevant for understanding hormone–behavior associations during adolescence. Following this we provide a selective review of the general findings for single adrenal and gonadal hormone–behavior associations during adolescence within a single axis (i.e., cortisol, dehydroepiandrosterone, testosterone) as has been done in the vast majority of the literature thus far. Next, we highlight relevant findings that accentuate the importance of considering cross-axis hormone interactions, particularly in adolescence. Specifically, we discuss how the HPA and HPG axes bi-directionally influence each other, we include potential developmental differences between HPA–HPG cross-talk in adolescence versus adulthood, and finally, we review the few studies that simultaneously examine the adrenal and gonadal hormones in the prediction of adolescent mental health problems. While studies using a dual-axis approach provide a more coherent and comprehensive picture of hormone–behavior associations than studies that examine single hormones independently, these literatures are sparse and inconsistent findings persist. Therefore, we conclude by highlighting several avenues for future research that may serve to further clarify divergent hormone–behavior associations, including replicating findings from studies using dual-axis approaches, carefully considering the appropriate type of dual-axis analyses to best address the research question at hand, and considering environmental contexts in studies taking dual-axis approaches to understand how hormones and behavior are associated.
THE HPA AND HPG AXES
Although the HPA and HPG axes are each networks of complex interactions between various brain regions and other organs, the focus of this review is largely the output hormones of these two systems. A detailed review of the brain systems that control the HPA and HPG axes is out of the scope of this review (for a more thorough overview see Dismukes et al., this issue), but it is crucial to at the very least mention the importance of the brain in reviewing the HPA and HPG axes. Several brain structures, particularly the hypothalamus and limbic, and paralimbic structures (i.e., hippocampus, amygdala, and prefrontal cortex) and intermediary agents (e.g., CRH and levels of several binding globulins in the blood, which dramatically affect the bioavailability and effects of steroid hormones on the brain, Perogamvros, Ray, & Trainer, 2012) have direct and indirect effects on the functioning of the HPA and HPG axes (Herman, 2013; Herman, Ostrander, Mueller, & Figueiredo, 2005; Peper, van den Heuvel, Mandl, Hulshoff, & van Honk, 2011). In addition to instantiating thought and behavior, these brain structures filter the environment to influence the initiation of the HPA and HPG axes prior to triggering a hormonal cascade. Furthermore, this influence is bidirectional, in that many areas of the brain contain hormone receptors and are regulated by these peripheral biomarkers, with highest concentrations within these same structures implicated in initiating the hormone’s release (Nelson, Leibenluft, McClure, & Pine, 2005). Unfortunately the correlational nature of the majority of human studies are unable to determine the direction of such effects, emphasizing the importance of preclinical research to better understand the associations between the brain, HPA/HPG axes, and behavior.
The HPA axis is one of the two primary systems to be activated during a stress response and operates as a self-regulating system via a negative feedback loop. Once a stressor is experienced, neurons in the hypothalamus secrete corticotrophin releasing hormone (CRH), which travels to the anterior pituitary and stimulates the release of adrenocorticotropin hormone (ACTH). ACTH is then transported through the blood stream to the adrenal gland where the endogenous steroid hormones cortisol and dehydroepiandosterone (DHEA) are released. After producing a sufficient amount of cortisol to deal with the stressor, elevated levels of cortisol signal to suppress the release of CRH and ACTH via negative feedback to the glucocorticoid receptors of the hypothalamus, pituitary, and hippocampus (Gunnar & Quevedo, 2007; Sapolsky, 1996, 2003; Schmidt-Reinwald et al., 1999). Although increases in cortisol are necessary to adequately deal with stressors, sustained elevated levels of cortisol may have deleterious effects (McEwen, 1998; Sapolsky, 1997). That is, elevated cortisol could contribute to allostatic load, which can impede on the body’s ability to mount neurobiological responses that protect from disease (McEwen & Seeman, 1999; McEwen, 2005). Further, chronically high levels of cortisol can lead to neuronal atrophy as well as the downregulation of glucocorticoid receptors in the hippocampus and prefrontal cortex along with simultaneous upregulation of receptors in the amygdala, which may lead to poor memory and mental health outcomes (Lupien et al., 1998; Lupien, McEwen, Gunnar, & Heim, 2009). Research suggests that simultaneous release of DHEA may be able to buffer against the deleterious effects of cortisol (Herbert, 1997; Morgan et al., 2004; Wemm, Koone, Blough, Mewaldt, & Bardi, 2010) perhaps in part because DHEA has been shown to decrease neuronal atrophy and promote neurogenesis in rats (Maninger, Wolkowitz, Reus, Epel, & Mellon, 2009). This evidence suggests that the relative level of each hormone should be examined in relation to the other, and indeed cortisol is often considered in studies of the effects of DHEA in the form of ratios (see Combined Influence of HPA and HPG Axis Hormones and Adolescent Mental Health Section). In the absence of a stressful situation, cortisol and DHEA are present at resting basal levels. Basal levels of these hormones are largely influenced by genes and the sleep/wake cycle (Bartels, Van den Berg, Sluyter, Boomsma, & de Geus, 2003; Li & Ji, 2007; Prom-Wormley et al., 2011) and, as such, follow a diurnal rhythm (described below).
The HPG axis is a major component of the reproductive system, and a primary force for pubertal development during the adolescent transition. Though the precise cause of the onset of puberty remains unknown, several factors have been identified, including genetic influences (Cousminer et al., 2013; Elks et al., 2010), and a host of permissive factors (Sisk & Foster, 2004) including body fat and leptin levels (Elias, 2012). Particularly important is the decline in GABA activity leading to the reactivation of the gonadotropin releasing hormone (GnRH) pulse generator (Ojeda & Terasawa, 2002; Ojeda et al., 2010), which stimulates changes in HPG axis functioning, culminating in the release of gonadal steroids such as testosterone or estradiol (Grumbach, 2002; Grumbach & Styne, 2003; Hiort, 2002). The GnRH pulse generator increases the frequency and amplitude of the release of GnRH in the hypothalamus, which signals the pituitary to release gonadotropins, follicle-stimulating, and luteinizing hormones that stimulate gametogenesis and production and secretion of sex steroids (testosterone, estrogens) in the gonads (Romeo, 2005). Testosterone and estradiol are two of the most important pubertal hormones released by the gonads, and drive changes in genital and breast development in boys and girls at puberty, respectively (Hiort, 2002; MacGillivray, Morishima, Conte, Grumbach, & Smity, 1998). The GnRH pulse generator is more active in the early morning hours, resulting in a general decrease in overall levels of these hormones across the course of the day (described below).
Interestingly, increases in DHEA are also responsible for promoting HPG activity and subsequent pubertal development (Grumbach & Styne, 2003). Although DHEA is primarily released by the adrenal glands, some DHEA is also released by the gonads in healthy males and females. DHEA is the first hormone to show large increases at approximately age 6 years to precipitate adrenarche, the first major sign of puberty (Palmert et al., 2001). This rise in basal levels of DHEA during adrenarche is one necessary factor for the onset of puberty. Thus, while DHEA is primarily a hormone of the HPA axis, its role in pubertal development highlights the overlap in HPA and HPG axis functioning.
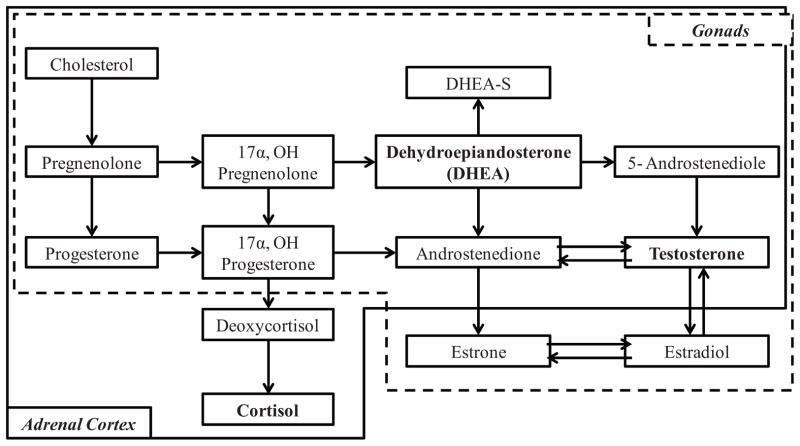
Steroid hormones involved in HPA and HPG axis functioning share several key features. First, the releases of adrenal and gonadal hormones are controlled by the hypothalamus and the pituitary gland and undergo feedback loops to signal to the hypothalamus and pituitary to regulate the production of the relevant hormone. Second, steroid hormones of adrenal and gonadal origin have similar developmental patterns of change characterized by high activity prenatally, followed by a relative period of juvenile quiescence (though the axes remain active at a lower level), and increased activation during adolescence with puberty (Grumbach & Styne, 2003; Terasawa & Fernandez, 2001). Third, HPA and HPG steroid hormones all originate from cholesterol and share several pro-hormones, including pregnenolone and progesterone (Brown et al., 2008; Viau, 2002; see Fig. 1). While adrenal and gonadal hormones each have separate primary functions, the derivation and metabolism of these hormones are highly integrated. For example, testosterone is metabolized in the adrenal glands as well as the gonads. DHEA is derived from both the adrenal cortex and testes, and about 50% of androgens in males and 75% of estrogens in females are derived from DHEA and DHEA-S (Kroboth, Salek, Pittenger, Fabian, & Frye, 1999; Poortman et al., 1980). Figure 1 is a general conceptual model depicting pathways of hormone production intended to demonstrate the high degree of overlap in the metabolism of the HPA and HPG axis hormones. As shown in Figure 1, both HPA and HPG hormones are derived from the same originating source and are metabolized through many of the same pathways, such that the synthesis and functioning of HPG hormones is influenced by earlier HPA functioning via downstream effects, and vice-versa.
FIGURE 1.
Simplified diagram for the synthesis of cortisol, DHEA, and testosterone. All three steroid hormones are synthesized from cholesterol. Cholesterol is converted to pregnenolone, which is in turn converted to either progesterone or to 17α-hydroxypregnenolone, and so forth. Metabolism pathways within the hashed box occur in the gonads and metabolism of hormones within the solid box occur in the adrenal cortex. Model adapted primarily from Payne and Hales (2004) and Heikkilä (2002).
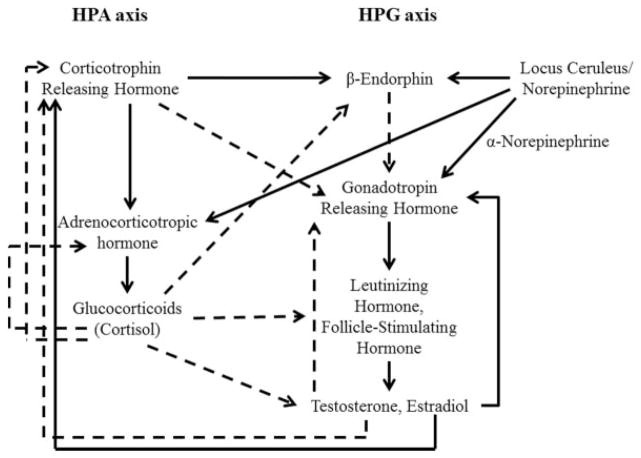
Though the HPA and HPG axes are separate systems, they are highly interactive (Grumbach, 2002). In addition to sharing several of the same components, a substantial amount of literature on adult animals and humans finds that the HPA axis hormones demonstrate a suppressive effect on HPG hormones and vice-versa (Romeo, 2005; Stratakis & Chrousos, 1995), as depicted in Figure 2. For example, glucocorticoids can exert an inhibitory effect on the HPG axis at several points such that cortisol suppresses the activity of sex hormones in adults (Romeo, 2005). Further, testosterone can inhibit production of CRH and therefore suppress the HPA stress response (Stratakis & Chrousos, 1995; Terburg, Morgan, & van Honk, 2009). Most of the literature examining how adrenal and gonadal hormones work together (or against each other) examine adult animals or humans and/or involves administering one hormone and observing the reaction of the other, and highlights the inverse relation between hormones of the HPA and HPG axis (Tsigos & Chrousos, 2002; see Viau, 2002). While hormone administration studies are informative, the conclusions should be interpreted with caution because the manipulation of a single hormone may not be ecologically valid, particularly across highly interactive regulatory systems which may counter-regulate one another (Andrews & Pruessner, 2013). Nonetheless, theories of dual-axis approaches that highlight the combined functioning of HPA and HPG axis hormones explain how antagonistic, inverse, associations between HPA and HPG hormones may affect behavior (see Montoya, Terberg, Bos, & van Honk, 2012; Romeo, 2005; Terburg, Morgan, & van Honk, 2009).
FIGURE 2.
Interaction of the HPA and HPG axes in adulthood. Solid lines represent stimulating pathways and hashed lines representing inhibitory pathways. Note that estradiol can provide both positive and negative feedback at different phases of the menstrual cycle. Model adapted primarily from Stratakis and Chrousos (1995) and Terberg et al. (2009).
The present review focuses on three hormones: cortisol, testosterone, and DHEA, although other hormones are involved in HPA and HPG axis functioning. The rationale for limiting our observations to these three output hormones was based on the following criteria. First, cortisol, DHEA, and testosterone are more easily measured in saliva than estradiol and other sex hormones (e.g., estrogen, estrone, follicle-stimulating hormone, luteinizing hormone, Dorn, Dahl, Woodward, & Biro, 2006) and hormonal precursors to cortisol (e.g., CRH, ACTH, GnRH). Relatedly, girls have measureable quantities of testosterone, whereas boys and pre-pubertal girls have low concentrations of circulating estrogen, which contributes to the difficulty of establishing reliability of concentrations using conventional assay techniques (Dorn et al., 2006; Shirtcliff et al., 2000). Thus sex differences in testosterone-behavior associations are more frequently tested than estrogen-behavior associations in humans, resulting in a relatively small literature on estrogen-behavior associations during adolescence. Second, cortisol, DHEA, and testosterone demonstrate a diurnal rhythm characterized by high levels in the morning, followed by a decline over the course of the day in both boys and girls (Anders, 1983; Granger et al., 2003; Matchock, Dorn, & Susman, 2007; Smyth et al., 1997). While a diurnal pattern has been shown for estrogen in pubertal girls (Mitamura et al., 2000), the presence of a diurnal pattern for estrogen has also been disputed (see Mitamura et al., 2000, for discussion) and is further complicated by the dramatic monthly cyclical pattern of estrogen release. Finally, cortisol, DHEA, and testosterone undergo shorter-term fluctuations in response to challenge during adolescence (Eatough, Shirtcliff, Hanson, & Pollak, 2009; Marceau, Dorn, & Susman, 2012; 2014), whereas this pattern has not been established for estrogens (e.g., Heinz et al., 2003; Schoofs & Wolf, 2011). Therefore, in the remainder of this review, we focus on cortisol, DHEA, and testosterone.
Developmental Changes
Developmental changes in the HPA and HPG axes are frequently conceptualized in terms of the organization/activational hypothesis (Phoenix, Goy, Gerall, & Young, 1959). The organizational/activational hypothesis posits that early development of the endocrine system has lasting effects on future endocrine function, brain and behavior (organizational effects) as well as more immediate effects when particular hormones are activated (activational effects) across the lifespan (Phoenix et al., 1959; Phoenix, Goy, & Young, 1967; Shirtcliff & Ruttle, 2010). The HPA axis demonstrates significant development during the prenatal period. Activation of the adrenal glands spikes prenatally, resulting in the release of increased levels of cortisol and DHEA (Griffin & Ojeda, 1996). DHEA is released by the placenta, and concentrations decline over the first 5 months after birth (Kroboth et al., 1999). The HPA axis appears to demonstrate a period of quiescence during childhood (Jonetz-Mentzel & Wiedemann, 1993; Kiess et al., 1995; Kenny, Preeyasombat, & Migeon, 1966; Netherton, Goodyer, Tamplin, & Herbert, 2004; Walker, Walder, & Reynolds, 2001). In late childhood or early adolescence, the HPA axis becomes reactivated, initiating an increase in hormones released by the adrenal glands (i.e., cortisol, DHEA), noted as early as 6 years of age (Apter, Pakarinen, Hammond, & Vihko, 1979; Kroboth et al., 1999; Palmert et al., 2001; Romeo, 2013). The rise in DHEA, in part, prompts the first signs of puberty (i.e., body odor, acne, hair growth) but may also aid in the stimulation or increased activation of other systems, including the GnRH pulse generator and HPG axis via permissive effects.
The HPG axis also demonstrates substantial prenatal development. The gonads develop very early, are recognizable in the 4th week in utero, and differentiate into male or female organs in the 7th week in utero. By the end of the first trimester, testosterone synthesis is underway (Griffin & Ojeda, 1996). This is particularly important for prenatal development as testosterone exerts organizational effects, resulting in further development of male genitals and behavior in childhood (Berenbaum & Beltz, 2011; Berenbaum & Snyder, 1995; Berenbaum, 1999; Griffin & Ojeda, 1996; Phoenix et al., 1959). The HPG axis is active prenatally, but then goes through a period of relative quiescence in juvenility (see Shirtcliff & Ruttle, 2010; Terasawa & Fernandez, 2001 for discussion). Active inhibition of pubertal hormones (Terasawa & Fernandez, 2001) provides children with the opportunity to grow and develop in other ways before the reproductive years (Bjorklund, 1997). At the commencement of puberty, the hypothalamus signals a cascade of hormones, resulting in the reactivation of the HPG axis, which continues to be active throughout the lifespan. The organization of the HPG axis prenatally and in early years has been shown to influence the reactivation of the HPG axis at puberty, though the neurobiological mechanisms of this effect are not well understood (Sisk & Foster, 2004; Witham, Meadows, Shojaei, Kauffman, & Mellon, 2012). There is some evidence from animal studies that suggests that prenatal hormones influence the development of the central nervous system, which is related to the release of gonadotropins from the anterior pituitary gland later in life (Resko & Roselli, 1997). Furthermore, increased activity of pubertal hormones can exert both organizational and activational effects on physical, behavioral, and emotional development (Phoenix et al., 1959; Romeo, 2003), suggesting that adolescence may also be a sensitive period for HPG axis development and later effects on behavioral and emotional development. The increased activation of both axes during adolescence results in the production of elevated basal levels of numerous hormones that had been relatively quiescent for several years, placing adolescents in a unique hormonal milieu as they navigate the social, psychological and emotional stressors of adolescence.
Diurnal Rhythms across Development
A diurnal rhythm refers to the portion of the 24-hr pattern of change in which the individual is awake, controlled in part by the suprachiasmatic nucleus and influenced by light-dark cycles. Due to the influence of light-dark cycles and the increased need for sleep following birth, the diurnal rhythms of all hormones develop substantially in the first few months of post-natal life (Price, Close, & Fielding, 1983). The diurnal rhythm of cortisol in humans is characterized by increased morning levels, which peak approximately 20 min post-awakening (Clow, Hucklebridge, Stalder, Evans, & Thorn, 2010; Fries, Dettenborn, & Kirschbaum, 2009), followed by a decline across the course of the day, quicker in the morning hours and then more gradually in the afternoon and evening (Ice, Katz-Stein, Himes, & Kane, 2004; Kirschbaum & Hellhammer, 1994). A surge in morning cortisol levels followed by a steady decline helps initiate waking activities and primes the body for the demands of a pending day (Klimes-Dougan, Hastings, Granger, Usher, & Zahn-Waxler, 2001; Smyth et al., 1997). There is much debate surrounding when a robust diurnal pattern is established in infants (de Weerth, Zijl, & Buitelaar, 2003). Researchers generally agree that there is significant individual variability in when diurnal patterns emerge and studies vary considerably on when a reliable diurnal rhythm can be detected, generally suggesting between 2 and 9 months of age (de Weerth et al., 2003; Kiess et al., 1995). Adolescents and adults demonstrate a similar diurnal pattern; however, there is a slight flattening of the diurnal slope of cortisol with increasing age during adolescence (Essex et al., 2011; Shirtcliff et al., 2012; Shirtcliff & Essex, 2008).
DHEA demonstrates a similar diurnal rhythm to cortisol, though potentially lacking the awakening response, characterized by higher morning levels and lower evening levels (Brown et al., 2008; Goodyer, Park, Netherton, & Herbert, 2001). There is some evidence that the diurnal pattern of DHEA may be more pronounced in females relative to males (Granger, Schwartz, Booth, Curran, & Zakaria, 1999). The diurnal pattern of DHEA has been observed as early as 8 years old, and it becomes more pronounced over time, driven by increasing morning levels but stable evening levels of DHEA from late childhood until approximately 30 years of age (Granger et al., 1999).
In adolescents and adults, testosterone generally shows diurnal pattern characterized by a steady decrease across waking hours (Brown et al., 2008; Granger et al., 2003; Goodyer et al., 2001; Resko & Eik-Nes, 1966). Basal levels and the diurnal pattern for testosterone differ for boys and girls (Granger et al., 2003). While one study did not identify a consistent diurnal pattern of testosterone in infancy (Thompson, Whitten, & Lampl, 2011), there is some evidence that the diurnal rhythm of testosterone may be observable as early as 5–6 years of age in girls and 4–5 years of age in boys (Mitamura et al., 1999, 2000). The diurnal pattern of testosterone may become more normal and adult-like in late pre-puberty/early puberty, at least in boys (Veldhuis et al., 2001).
Responsivity to Environmental Stimuli Across Development
The developmental periods of relative heightened activation and quiescence identified above are reflected in the pattern of stress responsivity of the HPA axis. Fetuses are capable of mounting HPA stress responses in several species (Matthews, 2002; Weinstock, 1997) including humans (cortisol response: Gitau, Fisk, & Glover, 2004; ACTH response: Kosinska-Kaczynska, Bartkowiak, Kaczynski, Szymusik, & Wielgos, 2012), especially in the third trimester when the HPA axis undergoes significant development. Relevant animal and human studies show how activation of the maternal stress response system, through exogenous stressors, immune challenges (i.e., non-abortive maternal infections), as well as direct injection of stress hormones, can profoundly impact the development of sensitivity of the HPA axis of the offspring (Fameli, Kitraki, & Stylianopoulou, 1994; Matthews, 2002; Reul et al., 1994; Wadhwa, Culhane, Rauh, & Barve, 2001; see Mulder et al., 2002 for meta-analysis), in part through epigenetic mechanisms (Oberlander et al., 2008; Wadhwa, Dunkel-Schetter, Chicz-DeMet, Porto, & Sandman, 1996; Weaver et al., 2004). Possibly reflecting developmental changes, HPA axis activity and cortisol responses to mild stressors tend to decrease across the first year of life. Newborns show substantial behavioral distress to many mild stressors but, by 1 year of age many infants show little or no cortisol reaction to these stressors (Gunnar, Wewerka, Frenn, Long, & Griggs, 2009). This is evidence of buffering of the stress response system beginning in infancy and lasting into adolescence (Gunnar & Donzella, 2002).
Corresponding with developmentally driven increases in basal levels of the HPA and HPG axes in adolescence, responsivity of the HPA system to stressors also becomes more pronounced during adolescence (Romeo, 2013). Cortisol is responsive to multiple laboratory stressors, including social stress, parent–child conflict, and venipuncture in adolescence and adulthood, especially when stressors are seen as uncontrollable (Granger, Weisz, & Kauneckis, 1994; Granger, Weisz, McCrakcen, Ikeda, & Douglas, 1996; Klimes-Dougan et al., 2001; Susman, Dorn, Inoff-Germain, Nottelmann, & Chrousos, 1997; Susman et al., 2010; see Dickerson and Kemeny, 2004). In adults, DHEA has also been shown to respond to social stress (Izawa et al., 2008; Lennartsson, Kushnir, Bergquist, & Jonsdottir, 2012), and DHEA was responsive to common laboratory based stress-inducing paradigms including parent–child conflict interactions during adolescence (Shirtcliff, Zahn-Waxler, Klimes-Dougan, & Slattery, 2007). Parent–child conflict interactions may elicit frustration (Granger et al., 1994), and although somewhat speculative, it may be that DHEA is responsive to frustration within social challenges in particular. For example, DHEA levels rose pre-post task in response to an impossible anagram task that was designed specifically to elicit frustration (Wemm et al., 2013). More work is needed to examine whether DHEA is responsive to frustration in particular and implications of DHEA reactivity for behavioral development.
Although the HPA axis is generally thought of as the stress–response endocrine system, substantial evidence suggests testosterone is also responsive to various environmental stimuli, at least in adolescence and adulthood. It is currently unknown whether HPG axis responsivity follows a similar developmental pattern to basal HPA and HPG hormone levels and HPA responsivity patterns. Studies of adult nonhuman primates and humans suggest that rises in testosterone are evident in response to threat (Sapolsky, 1982; van Honk et al., 2000) as well as in in adult (human) males faced with competitive challenges such as strenuous (i.e., maximal effort swimming or rowing, heavy weight lifting –physical stressors), but not moderate exercise (Sutton, Coleman, Casey, & Lazarus, 1973; Volek, Kraemer, Bush, Incledon, & Boetes, 1997; see Zitzmann & Nieschlag, 2001 for review). Similarly, in young women, testosterone increased in anticipation of, and in response to, a rugby competition (Bateup, Booth, Shirtcliff, & Granger, 2002) and intense exercise (Kraemer et al., 2001). As with cortisol and DHEA, the type of stress seems to play an important role. Similar to physical competition, testosterone has been shown to rise in response to mental competition (i.e., chess matches, Mazur, Booth, & Dabbs, 1992) and vicarious competition (i.e., fans of sporting events; Bernhardt, Dabbs, Fielden, & Lutter, 1998; van der Meij et al., 2012; in response to election results; Stanton, Beehner, Saini, Kuhn, & LaBar, 2009). However, there is some evidence that non-competitive mental stresses with a lack of controllability may be associated with decreased testosterone levels (Grossi, Theorell, Jürisoo, & Setterlind, 1999; Zitzmann & Nieschlag, 2001). Thus, because individuals must interpret the context of the stressors, different aspects of stressors may be more or less related to changes in testosterone versus cortisol versus DHEA.
Recognizing the potential importance that reactivity across cortisol, DHEA and testosterone during adolescence may have on our understanding of the endocrine system, some studies have begun studying these three hormones simultaneously in response to stressful situations (i.e., undergoing magnetic resonance imaging [MRI], intense exercise, parent–child conflict, and venipuncture). Consistently these studies have found reactivity of all three hormones in some—but not all—adolescents, suggesting substantial individual variability in hormone responses (Eatough et al., 2009; Kraemer et al., 2001; Marceau et al., 2012, 2014). Further, these studies have shown that responses of cortisol, DHEA, and testosterone differ for the same individuals in several contexts. For example, most youth had DHEA responses to medical procedures (i.e., MRI, venipuncture), but fewer responded with cortisol or testosterone increases (Eatough et al., 2009; Marceau et al., 2012). However, in response to parent–child conflict more adolescents responded with testosterone and DHEA than with cortisol (Marceau et al., 2014). Studies examining responses of cortisol and testosterone have suggested that competition (i.e., rugby, video games) elicits higher testosterone than cortisol responses (Bateup et al., 2002; Mazur, Susman, & Edelbrock, 1997). Extreme stress, for example skydiving, has been shown to elicit responses of all three hormones from almost all youth (Allison et al., 2012). Therefore, it is likely that the type or interpretation of the stressor is important to consider in studies examining HPA–HPG activation and associations between HPA–HPG activation and emotions and behavior.
Summary
The HPA and HPG axes are highly integrated across development. The hypothalamus and pituitary gland control the release of hormones of the HPA and HPG axes, in part through negative feedback loops. Steroid hormones of adrenal and gonadal origin are all derived from cholesterol and follow overlapping metabolic pathways. We focus primarily on three hormones: cortisol, DHEA, and testosterone. These three hormones show similar developmental patterns: high activity prenatally, a reduction in activity during the first post-natal year, a period of juvenile quiescence, and increased activation in mid- to late childhood and through adolescence and co-occurring with puberty. These hormones also display similar diurnal patterns, with peak concentrations occurring in the morning and declines across the day. Finally, these hormones all have been shown to respond to various types of environmental stimuli during adolescence and adulthood, including exercise, competition, conflict, venipuncture, MRI, and social stressors, and hormone responses vary with the type of stress experienced.
HORMONAL INFLUENCES ON ADOLESCENT MENTAL HEALTH
Models of the development of mental health problems including anxiety, depression, and externalizing problems, may be strengthened by including measures of both adrenal and gonadal hormone function (Susman, 2006). According to biosocial theories, puberty is viewed as a particularly sensitive and transitional period of development because of major changes in the endocrine system (i.e., reactivation of the gonadal axis, gradual increases in GnRH) as well as accelerated growth and physical maturation that contribute to the metamorphosis of a child into an adolescent (Dorn & Biro, 2011). These myriad biological changes can impact adolescents’ perceptions of the social changes that occur during puberty, potentially resulting in problematic psychological and emotional functioning in some adolescents (Paikoff & Brooks-Gunn, 1991). While numerous studies have examined associations between single hormones and behavior in adolescence, the literature as a whole is largely inconclusive.
Cortisol and Adolescent Mental Health
Cortisol has been a major focus of biological influences on behavior. Research has linked both high and low cortisol baseline levels and release in response to a stressor to increased mental health problems. Recently, a comprehensive evolutionary biosocial model, the adaptive calibration model (ACM, see Del Giudice, Ellis, & Shirtcliff, 2011), has been proposed to explain how different profiles of stress responsivity are associated with internalizing and externalizing problems. According to this theory, the combination of different developmental contexts and HPA responsivity patterns lead to particular behavioral outcomes, which may differ for males and females. For example, high responsivity of the HPA axis may be associated with more internalizing-type problems, whereas low responsivity of the HPA axis may be associated with more externalizing-type problems, especially in the context of chronically stressful or harsh environments across development. Further, girls with particularly sensitive stress response systems and who show high basal levels or responsivity to the environment are more likely to exhibit hyper-vigilance which may lead to or be a symptom of anxiety and potentially other internalizing-type problems, whereas for boys, sensitive stress response systems may lead to or be a symptom of agonistic or reactive aggression problems (Del Giudice et al., 2011). Thus, different types of responsivity patterns may predict internalizing versus externalizing problems via different mechanisms.
Research on how cortisol is associated with externalizing and drug use behaviors has been guided by several additional theories, including fearlessness theory (Raine, 1996), the challenge hypothesis (Archer, 2006), and stimulation seeking (Zuckerman, 1979). Generally, biosocial theories of externalizing problems posit that youth who were exposed to prolonged stressors may experience a “down-regulation” of cortisol at the level of the adrenal and in the brain (Shirtcliff et al., 2009; Susman, 2006), evidenced as low basal levels of cortisol and blunted reactivity in response to challenge or stress (Fries, Hesse, Hellhammer, & Hellhammer, 2005). It has been posited that children and adolescents who engage in externalizing behaviors may do so, in part, in an attempt to increase or return arousal to more typical levels (Koob & Le Moal, 2001). These theories are supported by research suggesting that children and adolescents with externalizing problems generally display blunted patterns of HPA axis reactivity (Fairchild et al., 2008; Susman et al., 1997; Yoon & Joormann, 2012) as well as low basal and diurnal levels of cortisol (Andersen & Teicher, 2009; Brown et al., 2008; Hastings et al., 2011; Moss, Vanyukov, & Martin, 1995; Ruttle et al., 2011), though findings remain mixed (see Alink et al., 2008; Susman et al., 1997). Interestingly, a developmental effect has been identified within the externalizing research suggesting that increased externalizing problems are initially associated with HPA hyperactivity and later associated with hypoactivity (Alink et al., 2008).
Research on cortisol and internalizing problems has been shaped by theories which suggest that certain individuals are born with a lower threshold for limbic-hypothalamic arousal in response to changes in one’s context (Kagan, Reznick, & Snidman, 1988), perhaps due to elevated levels of neuroendocrine receptors in fear-related brain regions (e.g., amygdala). These individuals’ heightened sensitivity to the environment results in both hyper-physiological (e.g., cortisol) and behavioral (e.g., fear, anxiety, social withdrawal) reactions to benign perturbations in their environment. Another potential mechanism of cortisol-internalizing associations is through glucocorticoid receptor sensitivity. For example, elevated cortisol is associated with reduced negative feedback loops due to reduced sensitivity to glucocorticoids (which is likely epigenetically mediated; Menke et al., 2012) in key brain regions for HPA negative feedback (i.e., the prefrontal cortex). Further, there is evidence of reduced glucocorticoid sensitivity in depressed adults compared with healthy controls (e.g., Webster, Knable, O’Grady, Orthmann, & Weickert, 2002; Young, Haskett, Murphy-Weinberg, Watson, & Akil, 1991). While the cross-sectional literature on cortisol-internalizing associations in adolescence is largely divergent (Cicchetti & Rogosh, 2001; De Bellis et al., 1996; Granger et al., 1998; Kaufman, 1991; Perez-Edgar, Schmidt, Henderson, Schulkin, & Fox, 2008), longitudinal research supports the general notion of elevated physiological arousal leading to internalizing problems; high levels of basal cortisol have been found to precede the development of internalizing problems in children and adolescents (Adam et al., 2010; Ellenbogen, Hodgins, Linnen, & Ostiguy, 2011; Goodyer et al., 1996, 2001; Goodyer, Tamplin, Herbert, & Altham, 2000; Gunnar, 2001; Halligan, Herbert, Goodyer, & Murray, 2007; Lopez-Duran, Kovacs, & George, 2009; Ruttle et al., 2011; Smider et al., 2002; see Burke, Davis, Otte, & Mohr, 2005 for review). Although not all studies have found the anticipated positive association between cortisol and internalizing problems, divergence from this association has been attributed to down-regulation of the HPA axis (Feder et al., 2004; Ruttle et al., 2011). Like the developmental effects noted for externalizing problems, there may also be a developmental cortisol-behavior association for certain types of internalizing problems. For example, prepubescent children with dysphoria display blunted patterns of HPA axis reactivity whereas post-pubertal adolescents and adults display increased levels of cortisol reactivity to psychosocial stressors (Hankin, Badanes, Abela, & Watamura, 2010; Luby et al., 2003; Rao, Hammen, Ortiz, Chen, & Poland, 2008).
DHEA and Adolescent Mental Health
Compared to cortisol, less research has been devoted to understanding DHEA-behavior associations during adolescence. Studies examining associations between basal DHEA and externalizing behavior generally suggest that higher basal DHEA and its sulphate, DHEA-S, have also been associated with increased aggression and externalizing problems (Brown et al., 2008; Maras et al., 2003; Susman et al., 1987; van Goozen et al., 1998; van Goozen, Matthys, Cohen-Kettenis, Buitelaar, & Van Engeland, 2000). Lower basal DHEA during adolescence has been associated with more negative affect (Susman et al., 1987; Susman, Dorn, & Chrousos, 1991) and internalizing symptoms during adolescence (Goodyer, Herbert, & Altham, 1998; Goodyer, Herbert, & Tamplin, 2003; Goodyer, 2007); however, higher basal DHEA during adolescence has also been associated with more internalizing problems (Goodyer et al., 2000; Shirtcliff, Granger, Booth, & Johnson, 2005; Susman, Granger, Murowchick, Ponirakis, & Worrall, 1996). It may be that DHEA is associated with internalizing problems when it is concomitant with stressful life events, potentially operating as a stress hormone (see Angold, 2003 for further discussion). Thus, it may be beneficial to examine DHEA in relation to cortisol output.
Fewer studies have examined associations between stress responsivity of DHEA and emotionality or behavior. Stress responsiveness of DHEA has been associated with increased internalizing symptoms in adolescents in response to social tasks, including parent–child conflict (Shirtcliff et al., 2007), and the DHEA stress response to venipuncture was concurrently positively associated with negative emotionality in adolescent boys (Marceau et al., 2012), though it is unknown whether DHEA responsivity is associated with externalizing problems.
Testosterone and Adolescent Mental Health
The association between testosterone and aggression in social relationships in adulthood has received substantial attention (Archer, 2006). Like studies in adults (Booth, Granger, Mazur, & Kivlighan, 2006), associations between basal testosterone levels and behavior are mixed for adolescents. This is somewhat surprising given the general acceptance of a testosterone-aggression link, and suggests that the studies thus far may be missing an important moderator or contextual force. Basal testosterone and changes in basal levels across adolescence have been associated with increased aggression during adolescence (Brooks-Gunn & Warren, 1989; Dabbs, Jurkovic, & Frady, 1991; Dorn et al., 2009; Finkelstein et al., 1997; Inoff-Germain et al., 1988; Maras et al., 2003; Olweus, Mattsson, Schalling, & Low, 1988; Susman et al., 1991, 1996). Further, boys with higher basal testosterone did not respond as well to treatment for oppositional defiant disorder and conduct disorder as boys with lower basal testosterone (Shenk et al., 2012). In one of the few longitudinal studies of aggression and testosterone, boys who had a history of high physical aggression from ages 6 to 12 years were found to have lower basal testosterone levels at age 13 compared with boys who did not have a history of high physical aggression (Schaal, Tremblay, Soussignan, & Susman, 1996). However, some studies have found that basal testosterone was not associated with aggression in young girls (Booth, Johnson, Granger, Crouter, & McHale, 2003; Susman et al., 1987), and findings are generally mixed regarding testosterone and externalizing problems (see Book, Starzyk, & Quinsey, 2001).
For internalizing-type problems, lower initial levels of testosterone and slower declines in testosterone across the day were associated with higher levels of anxiety and depression in pubertal youth (Granger et al., 2003) and lower testosterone has been associated with increased negative affect (Inoff-Germain et al., 1988). However, in another study, higher basal levels of testosterone were associated with higher levels of negative emotional tone concurrently in girls (Susman et al., 1991). In other studies testosterone has not been associated with negative emotionality during puberty in girls (Brooks-Gunn and Warren, 1989; Booth et al., 2003; Susman et al., 1987). Thus, as with cortisol and DHEA, findings are mixed regarding the nature of the association between basal testosterone and adolescent emotionality and behavior problems.
The responsivity of testosterone to social challenges, especially during adolescence, has been associated with greater psychopathic tendencies in males (Yildirim & Derksen, 2012), as well as aggression and social status-seeking behaviors in animals and humans (Eisenegger, Haushofer, & Fehr, 2011). Associations between testosterone responses to laboratory stressors and adolescent emotionality and behavior have not been demonstrated during adolescence, though a recent study suggests that testosterone responses to venipuncture were not directly related to negative emotionality (Marceau et al., 2012). More research is needed to understand whether stress responsivity of testosterone to laboratory stressors is associated adolescent mental health.
Summary
There is a substantial body of research examining associations between basal levels of cortisol, DHEA, and testosterone, and emotional/behavioral problems during adolescence. Although some specific hormone–behavior patterns tend to prevail (e.g., low cortisol is generally associated with increased externalizing problems), the literature is plagued with inconsistent findings. One explanation for the inconsistent findings is the lack of studies examining of testosterone and DHEA in young adolescents, with existing studies tending to use smaller samples with little power to detect stable findings. It was not until recently, and after multiple studies, that the low cortisol and externalizing behavior associations became a moderately consistent finding. There are several other possible reasons for the mixed findings. For example, the mixed findings in the literature could reflect the different methodologies and techniques used (e.g., time of day of sampling, method of saliva collection, storage, and assay), the presence of non-linear effects when only linear effects are tested, the influence of negative feedback loops (related to the timing of sampling), and the specific outcome variables assessed. Additionally, the mixed findings in the literature likely indicate the presence of moderators of single hormone–behavior associations, for example, the environmental context of the assessment and hormonal milieu of the adolescent. Given the interactive nature of the HPA and HPG axes and the protective effects of DHEA on the negative effects of cortisol (Goodyer et al., 2001; 1997; 2003; Herbert, 1997; Maninger et al., 2009; Morgan et al., 2004), there is a clear need for studies examining how the HPA and HPG axes interact and bi-directionally influence each other and behavior during adolescence.
INTEGRATION OF THE HPA AND HPG AXES
Examining multiple endocrine systems may be more fruitful for understanding hormone–behavior associations than examining a single system in isolation (Montoya et al., 2012; Terburg et al., 2009; van Honk, Harmon-Jones, Morgan, & Schutter, 2010). As mentioned above, the HPA and HPG axes are highly interactive and inverse associations of HPA and HPG axes have been well documented (Viau, 2002). Interestingly, although the HPA and HPG axis remain highly interactive in adolescence, these axes may not demonstrate the typically observed adult suppressive-like patterns of interaction (Matchock et al., 2007). Seemingly paradoxical positive associations between the HPA and HPG axis have been noted during adolescence (Marceau et al., 2012; Matchock et al., 2007; Popma et al., 2007; Susman et al., 1987, see also the empirical papers of this special issue). Although speculative, in terms of basal levels this may possibly be due to the recent increased activation of the endocrine system in puberty resulting in normative elevations in both stress and pubertal hormones (Grumbach & Styne, 2003; Gunnar et al., 2009). In terms of stress responsivity, in addition to the reawakened responsivity of these hormones with puberty, cortisol and DHEA and testosterone may be released together during challenges/stressors (Marceau et al., 2014), so in studies examining the effects of hormones in response to environmental stimuli the hormones may exhibit positive relationships. It is important to note that these positive associations were found in adolescent samples exposed to stressors in ecologically valid contexts, in contrast to many of the studies in animals and adults which found inverse associations using pharmacological challenges and direct manipulation of one hormone. Thus, it may be that pharmacological studies tap into a different endocrine process than laboratory stressors, wherein hormones may simply be released together by the adrenal glands, resulting in differing findings.
There is some evidence that the associations between HPA and HPG hormones differ across adolescence (Matchock et al., 2007; Marceau et al., 2012; Marceau, Ruttle, Shirtcliff, Essex, & Susman, this issue; Marceau et al., 2014; Ruttle, Shirtcliff, Arm-strong, Klein, & Essex, this issue), suggesting that HPA-HPG cross-talk is likely developmentally influenced, resulting in unique HPA-HPG interactions in adolescence. Specifically, while adolescents may demonstrate increased basal levels of both stress and sex hormones at the beginning of puberty due to increased activation of both systems, patterns of neuroendocrine activity become more adult-like over time (e.g., Ruttle et al., this issue). The unique patterns of association observed in adolescence suggests the importance of considering hormones of the HPA and HPG axis together in order to understand how hormones influence behavioral development (Bombadilla et al., this issue), especially during adolescence (Johnson, Dismukes, Fleury, & Shirtcliff, 2014). The changing adolescent hormonal milieu, or deviations from the developmentally appropriate patterns of activation, may contribute to the increase in mental health problems observed during adolescence; however, with the exception of the empirical articles included in this special issue, very few studies have examined normative, developmental HPA–HPG cross-talk in adolescents or the combined effect of both stress and sex hormones on behavior.
Combined Influence of HPA and HPG Axis Hormones and Adolescent Mental Health
Much of the literature examining associations of multiple hormones on behavior in humans has used cortisol-DHEA (Goodyer et al., 1998, 2003; Izawa et al., 2008; Izawa, Saito, Shirotsuki, Sugaya, & Nomura, 2012; Young, Gallagher, & Porter, 2002) and testosterone–cortisol ratios (Montoya et al., 2012; Terburg et al., 2009; van Honk et al., 2010). A ratio provides a measure of the level of one hormone (e.g., cortisol) compared to the level of another hormone (e.g., DHEA) within an individual. The cortisol–DHEA ratio hypothesis posits that DHEA has a balancing effect on cortisol, and therefore high cortisol-to-DHEA ratios are thought to indicate an imbalance that predisposes individuals to depression, and has been generally supported during adolescence (Goodyer et al., 1998, 2003, see also Angold, 2003) and adulthood (Izawa et al., 2008, 2012; Young et al., 2002).
The testosterone–cortisol ratio hypothesis posits that imbalances marked by high testosterone and low-cortisol predispose individuals toward aggression and externalizing problems. This association has been demonstrated in human adults (Montoya et al., 2012; van Honk et al., 2010) and is thought to be due to up-regulated gene expression in several key brain regions including the amygdala (van Honk et al., 2010). One study combined cortisol–DHEA ratios with testosterone and found that girls with conduct disorder had hormone profiles marked by lower cortisol–DHEA ratios in combination with higher basal testosterone (Pajer et al., 2006). Other studies have not identified associations utilizing basal levels of hormones in ratios but rather found that basal testosterone-to-cortisol responsivity ratios, reflecting higher basal levels of testosterone and lower cortisol responsivity, were associated with higher psychopathy scores among adults (Glenn, Raine, Schug, Gao, & Granger, 2011). Together this evidence suggests that hormone profiles including cortisol, DHEA, and testosterone may best predict behavior during adolescence.
There are some limitations of ratios that should be noted. First, development is generally not considered when examining ratios, despite knowledge that levels of DHEA and cortisol change at different times and rates across puberty (Saczawa, Graber, Brooks-Gunn, & Warren, 2013). Indeed ratios have been shown to be influenced by both age (Hartaigh et al., 2012) and pubertal maturation during adolescence, although age and puberty are not commonly controlled for in studies using ratios in adolescence (Saczawa et al., 2013). Second, ratios are limited to only examining two hormones, and thus are unable to incorporate other hormones, which may also be important. Finally, ratios examine the balance of two hormones, which often occur in difference concentrations in the vehicle used to assay the hormones (e.g., blood, saliva), which may skew findings and interpretations.
In addition to ratios, there is also evidence that basal levels of stress- and puberty-related hormones interact to predict mental health problems during adolescence. Interaction terms compare the average level of one hormone (e.g., cortisol) among individuals to the average level of another hormone (e.g., DHEA) so that comparisons of hormone levels are being made between (rather than within) individuals. First, boys with disruptive behavior disorders showed numerous differences in an array of HPA and HPG axis hormones compared to a group of healthy controls (Dorn et al., 2009). Multiple studies report that higher basal levels of testosterone predicted overt aggression in boys and men who had lower basal cortisol relative to the rest of the sample, but not in boys who had higher basal cortisol (Dabbs et al., 1991; Mehta & Josephs, 2010; Popma et al., 2007; see Montoya et al., 2012). However, one study did not find a significant cortisol–testosterone interaction predicting internalizing or externalizing problems (Scerbo & Kolko, 1994) and another found that high testosterone predicted reactive aggression in response to insult only in the context of higher cortisol in women (Denson, Mehta, & Ho Tan, 2013). Thus, interactions among hormones suggest that the endogenous context (i.e., levels of other hormones) is important for the behavioral correlates of specific hormones, though this has generally been shown with cortisol moderating the correlates of testosterone.
Significant interactions between cortisol and testosterone have been interpreted as either responsivity of the HPG axis to stress-related changes in the HPA axis (i.e., suppression of the gonadal axis when the HPA axis is activated by a stressor; Susman et al., 1991), or that the combination of high adrenal and low gonadal axis activation may represent a biological sensitivity to stressful events (Susman et al., 1991), although studies have not tested which axis drives the changes in the other axis. Notably, these studies examine basal levels of each hormone, and not associations among diurnal changes or responses to specific stressors among multiple hormones. More work is warranted to understand the potential mechanisms driving associations among hormones and interactions among hormones predicting behavior during adolescence.
Summary
Theory and evidence support the hypothesis that the HPA and HPG axes may work simultaneously in associations with adolescent emotionality and behavior, and that hormone systems may correlate with behavior differently in boys and girls. Studies using ratios and interactions of multiple hormones generally suggest that high cortisol in the context of low DHEA is associated with internalizing problems, whereas low cortisol in the context of high testosterone is associated with externalizing problems. Thus, basal levels or responsivity of multiple hormones contribute to a hormonal milieu, which likely has important implications for behavioral correlates of each hormone. However, this literature is quite sparse and further replication is necessary in order to be sure of these conclusions. The biosocial perspective emphasizes that the environmental context in which a hormone is expressed is important for the behavioral correlates of the hormone (Booth et al., 2006; Del Giudice et al., 2011). The evidence presented here suggests that the hormonal milieu (i.e., endogenous context) is equally as important to consider as environmental contexts (i.e., exogenous contexts) for the development of emotional and behavioral problems during adolescence. Here, we extend these ideas to highlight the importance of examining associations among multiple hormones within individuals in order to empirically demonstrate whether taking a dual-axis approach systematically helps to clarify associations between hormones and mental health.
CONSIDERATIONS AND FUTURE DIRECTIONS
We hypothesize that a developmental dual-axis approach, although less frequently used as illustrated by the review above, may be useful for clarifying the mixed literature on single hormone–behavior associations. We suspect that one reason the extant literature has not utilized a dual-axis approach is in part because of methodological challenges, given how frequently this approach is discussed. Indeed, examining even a single axis can be challenging as it involves basal levels, change, rhythms, reactivity, and momentary fluctuations. As such, there are methodological considerations for a developmental dual-axis approach, which will be crucial for elucidating divergent findings and for forging a coherent literature in the future. Similarly, we propose careful consideration of the time-course, or amount of time it takes for a hormone to be signaled, released, and affect other changes in the HPA and HPG axes, as failure to appropriately measure hormone changes will result in ambiguous findings in the future. Finally, continuing to incorporate biosocial approaches will likely further elucidate divergent findings, and so we propose consideration of both immediate environmental contexts (i.e., characteristics of specific stressors eliciting hormone responses) and broader ecological contexts (i.e., characteristics of the general social environment). Together, we believe that these recommendations will help to forge a more complete and nuanced body of literature to aid in understanding how hormonal milieus are associated with the development of emotional and behavioral problems during adolescence.
Methodological Considerations for Analyzing HPA and HPG Axis Hormones and Hormone Changes
Though there are certainly many methodological considerations, the foremost is to consider the research question and determine the corresponding appropriate analyses before examining how associations or interactions among hormones predict behavior. There are two basic ways of examining multiple hormones (described in detail in Marceau et al., this issue): (1) comparing levels or changes in hormones across people (between-individual), and (2) examining levels or changes in hormones within each person (within-individual). Together, these two approaches, identifying groups of people with similar hormone profiles (between-person differences) and identifying hormonal profile changes within each individual (within-person changes or coupling), and behavioral correlates, will lead to a much more nuanced understanding of the implications of endogenous hormonal milieu and shed light on more precise, developmentally sensitive biological mechanisms contributing to the development of behavior problems. Each approach answers different types of questions, and as researchers move toward more complex developmental questions it is crucial that the statistical method applied be appropriate to each specific question. Here we do not favor one approach, but instead highlight the importance of using a method appropriate for the specific question.
Thus far, the literature examining associations between HPA and HPG hormones during adolescence have focused primarily on basal levels (Matchock et al., 2007; Popma et al., 2007; Susman et al., 1987), or used the between-person approach to examining associations between changes in HPA and HPG axis hormones (Marceau et al., 2012) or associations between changes among HPG hormones (Mitamura et al., 1999, 2000). The within-person (coupling) approach outlined above and in Marceau et al. (this issue) is particularly useful for examining the correspondence between changes in hormones, as demonstrated by the empirical articles in this special issue, and, simultaneously, in behavior by including behavior as a moderator of within-person coupling using multilevel models, or by extracting coupling parameters and examining associations with behavior (e.g., Bombadilla et al., this issue; Johnson et al., 2014; Marceau et al., this issue). Therefore, examining within-person coupling of HPA and HPG axis hormones on multiple time-courses (i.e., developmental, diurnal, and responsivity), and the effect of within-person HPA–HPG coupling on adolescent mental health provides a very exciting avenue for future research. Further, a recent extension of the multi-level framework, the time-varying effects model (e.g., Shiyko, Lanza, Tan, Li, & Shiffman, 2012) may be particularly useful in the future for examining how the magnitude and direction of within-person coupling of HPA and HPG hormones changes across development, or over shorter time-frames. Understanding within-person changes in HPA–HPG hormone associations may further elucidate how individuals’ hormonal milieus change across adolescence, and whether the changing hormonal milieu is associated with specific behaviors and behavior problems during adolescence. Outside of longer time courses (e.g., development across years or puberty), within-person hormonal milieus likely also change across shorter time frames, as well as across exogenous environmental contexts (e.g., Marceau et al., 2014).
Time-Course of HPA and HPG Hormone Changes
The time-course of hormone responses to different environmental cues is an area in need of much research given the different metabolic pathways for production and the different functions of each hormone. The peak DHEA response to stress has been found to occur about 10 min before the peak in cortisol (Izawa et al., 2008), as would be expected given derivation pathways (Fig. 1). However, in another study DHEA and cortisol both increased from 0 to 20 min, and DHEA continued to increase through 40 min after social stressors, whereas cortisol began to decline (Shirtcliff et al., 2007). Theoretically, the DHEA response to stress would occur before a response in testosterone because testosterone can also be metabolized downstream from DHEA. The particular environmental stimuli (i.e., physical vs. mental stressors, controllable vs. uncontrollable events, exercise, competition, conflict, medical procedures) may also impact the time course of hormone responses to different stressors or tasks, as well as which hormones show greater responses. Generally, studies are limited in the timing of hormone assessments, and so responsivity of multiple hormones are measured simultaneously (Eatough et al., 2009; Marceau et al., 2012). This is understandable given the need for study protocols to be feasible and reduce participant burden. To untangle these relationships in correlational/endogenous designs, researchers would have to take frequent measurements and relate time-courses of one hormone to the other (e.g., Schlotz et al., 2008). Thus, creative solutions for studies with more intensive hormone measures are required to accurately uncover different time-courses of disparate hormone responses to particular environmental stimuli.
Immediate Environmental Context
Lessons from research using biosocial approaches should also be incorporated into the study of HPA–HPG hormones. For example, the ACM theory has gained traction by pulling apart the notion of “stress” into three key components (resource availability, extrinsic morbidity-mortality, and unpredictability; Del Giudice, Ellis, & Shirtcliff, 2013; Ellis et al., 2013) in order to make more specific predictions about the outcomes associated with stress responses (Del Giudice et al., 2011). This theory suggests these experiences are salient because they provide information about the probability of survival and reproductive success, helping the individual balance the inherent trade-offs of resource investment such as time and energy and other intrinsically limited resource. The term stress is thus a catch-all phrase for cues that the environment cannot support slow or flexible maturation trajectories; that is these time-consuming investments would be unlikely to pay off in an unstable or unpredictable environment. Both the HPA and HPG axes are thought to be sensitive to life-history relevant cues of resource availability, extrinsic morbidity-mortality risk and unpredictability. Other important components of stress shown to be particularly implicated in cortisol stress responses have also been identified, including social evaluative threat (Dickerson & Kemeny, 2004). Thus, the ways in which individuals experience stressors have implications for the hormonal responses observed in those individuals, and decomposing “stress” into its mechanism, which may include one or more of several components depending on the situation, will likely clarify hormone–behavior associations.
In line with this perspective, empirical research has shown that hormones respond differently to diverse types of stressors. Incorporating findings concerning which hormones respond to different types or aspects of stressors allows for some predictions for which types of context would more likely moderate HPA–HPG coupling and how these different hormone combinations may result in different behaviors. For example, the above review suggests that youth respond to social evaluative threat and unpredictability with cortisol, and to social status and competition with testosterone. Further, low cortisol, but high testosterone often predicts externalizing problems. Together, we can hypothesize that in contexts in which there is unpredictable social evaluative competition (therefore potentially eliciting higher cortisol and testosterone), such as in a deviant peer group or negative sibling relationship, youth with both higher cortisol and testosterone may not express externalizing problems, but youth with inversely correlated cortisol and testosterone may express the highest externalizing problems. That is, youth with a hormonal milieu marked by higher cortisol and testosterone may be appropriately responding to those particular immediate environmental cues, whereas youth with a hormonal milieu marked by lower cortisol and higher testosterone would be displaying an atypical hormonal milieu in that type of situation, indicative of externalizing psychopathology. In this way, identifying deviations from context-expected hormone milieus may provide insight into how adolescents perceive various contexts and ultimately prove to be a better biomarker of psychopathology than levels of single hormones.
Hormonal milieus may also help to disentangle which type of behavior problem (i.e., internalizing or externalizing) youth are likely to exhibit in particular situations. For example, as hypothesized above, youth may respond to frustration with changes in DHEA and social conflict with changes in testosterone. Further, higher DHEA responsivity is associated with internalizing problems (Marceau et al., 2012; Shirtcliff et al., 2007), but higher testosterone responsivity is associated with externalizing problems (Yildirim & Derksen, 2012). Together, we may expect that youth who exhibit hormonal milieus in which increases in DHEA and testosterone are positively correlated in the context of a frustrating and conflict-ridden environment (e.g., high parent-adolescent conflict), would express comorbid internalizing and externalizing problems, whereas youth who experience only a rise in DHEA may exhibit only internalizing problems and youth who experience only a rise in testosterone may exhibit only externalizing problems. Implicit in these predictions is that the individual must appraise or interpret the context in these manners in order to display the downstream hormonal results of these contextual forces. Thus, immediate contexts in which youth are required to respond to mixed social mechanisms may moderate associations between the hormonal milieu and behavior or psychopathology, potentially elucidating how underlying mental health problems are likely to present- as internalizing, externalizing, or comorbid problems.
Broader Environmental Context
In addition to the importance of the immediate context, biosocial approaches emphasize the role of broader contextual factors for hormone functioning and associated behaviors. The broader environmental context is influential in part because it changes how the HPA and HPG axes are organized. Because the HPA and HPG axes can influence how we interpret (e.g., appraise and encode) our immediate environmental cues, earlier broad environmental contextual forces can moderate the interpretation of environmental cues and subsequent release of HPA and HPG hormones. For example, the biological sensitivity to context theory (Ellis & Boyce, 2008) and ACM (Del Giudice et al., 2011) suggest that HPA axis functioning depends on the environmental context (i.e., parenting or family environment) being predictable versus unpredictable. That is, individuals with high reactivity would respond the most to an unpredictable environment, doing best in a positive environment and worst in a negative environment. Individuals with low reactivity would appear to respond similarly to individuals with high reactivity only in predictable environments (Del Giudice et al., 2011).
There is evidence that beginning in utero, the HPA axis is responsive to its environment and prenatal influences can have lasting, organizational effects on the development of the HPA axis activity and coping (Phillips & Jones, 2006; Weinstock, 1997). For example, glucocorticoids from the mother pass easily through the placenta to the developing fetus during the third trimester and the fetus’ HPA axis can activate in response to maternal glucocorticoids before the HPA axis is fully developed. Early life stress has also been shown to exert negative effects on HPA functioning in childhood (Essex, Klein, Cho, & Kalin, 2002) and well into adolescence (Essex et al., 2011; Roisman et al., 2009). Early family environmental factors including father absence, and particularly negative rearing environments before age 5 years are also thought to impact the timing of onset and duration of pubertal development (i.e., HPG axis functioning) and particularly the timing of the increase of DHEA in middle childhood/adolescence associated with puberty (Belsky, Steinberg, & Draper,1991; Belsky, Bakermans-Kranenburg, & Van Ijzendoorn, 2007; Ellis, Shirtcliff, Boyce, Deardorff, & Essex, 2011; Ellis & Essex, 2007; see Ellis, 2004 for review). There is also evidence from cross-sectional studies that several broader environmental contexts can affect associations between single hormones and behavior, including deviant peers (Dorn et al., 2009), family contexts (Booth et al., 2003; Dorn et al., 2009; Fang et al., 2009; Laurent et al., 2012), childcare (Dettling, Gunnar, & Donzella, 1999; Roisman et al., 2009), and likely school or neighborhood. Recent research has taken a more holistic approach, examining how the environment may moderate hormone–hormone and hormone–behavior associations. More specifically, Ruttle et al. (this issue) found that exposure to early life stress moderated the association between cortisol and DHEA as well as cortisol and testosterone in girls, suggesting that the cross-talk between cortisol and gonadal hormones may be at least partially responsible for the link between early rearing conditions and early pubertal timing and development.
However, there is currently no research that examines the influence of broader contextual factors on the association between HPA–HPG hormone links and mental health during adolescence although researchers have begun taking steps in this direction. Essex and colleagues (2011) found evidence that early life stress moderates the covariation, or coupling, of cortisol levels with the severity of mental health symptoms at different points in time across adolescence (Essex et al., 2011), suggesting early life stress exposure may result in persistent, long-term physiological perturbations which correspond with, or possibly result in, behavioral changes. As the field moves toward a more developmental dual-axis approach, researchers will examine whether between-family differences and/or within-family changes in contextual factors over time (e.g., rising peer influence as the child becomes an adolescent or rising parent–child conflict as the child becomes independent) moderate associations between hormone levels, changes, and interactions on adolescent emotionality and behavior. As summarized in Figure 3, the environmental context can exert organizational effects on the developing hormonal milieu, potentially magnifying individual differences in basal hormone levels, diurnal changes, and stress responsivity. The environmental context can also change over time, and may be a moderator of associations between individuals’ hormonal milieu and behavior concurrently and longitudinally. It is also important to note that context may also affect other brain systems, which could also contribute to mental health problems, thus the environmental context may also contribute to mental health problems and associations with hormone levels and changes in other ways, beyond those described here.
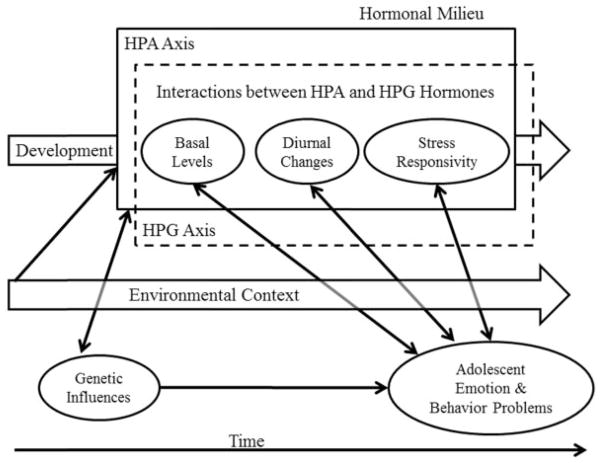
FIGURE 3.
Conceptual model of hormone–behavior associations and adolescent mental health over time. The hormonal milieu is depicted across the top of the Figure as a combination of HPA axis (solid box), HPG axis (hashed box), and interactions of HPA and HPG axis hormones, which can be conceptualized in terms of basal levels, diurnal changes, or stress responsivity (depicted in circles within the boxes), each of which undergoes developmental shifts (depicted as the block arrow labeled Development), and are influenced by genes and can influence gene expression (depicted by the bidirectional arrow from genetic influences to the hormonal milieu). Note that genetic influences are depicted as a single circle because the structure of DNA does not change over time, although genetic expression and influences certainly do change over time. Basal levels, diurnal changes, and stress responsivity of HPA, HPG, and across HPA and HPG hormones are all associated with adolescent emotion and behavior problems (depicted as bidirectional arrows). These associations are also thought to be context dependent (depicted by the bidirectional arrows passing through a semi-transparent arrow depicting changing environmental context over time).
Other Considerations
It is also important to acknowledge that the HPA and HPG axis do not work in isolation but rather are simultaneously influenced by a variety of neurobiological and genetic influences. Furthermore, the activity of one system does not work in isolation from other systems (e.g., immune system) or from the larger ecological context. Theories including allostatic load (McEwen & Stellar, 1993; Sterling & Eyer, 1988), posit that biomarker panels are important to consider and the field has progressed in examining multiple endogenous systems in interaction including HPA and autonomic nervous system (Bauer, Quas, & Boyce, 2002; Hastings et al., 2011; Lupien et al., 2006), the dopaminergic system (Gatzke-Kopp, 2011) and immune function (Glaser & Kiecolt-Glaser, 2005; Segerstrom & Miller, 2004). Therefore, in the future, genetics and other systems may be examined in conjunction with a developmental approach to HPA-HPG hormonal milieus to gain a better understanding of the physiological underpinnings of the development of psychopathology in adolescence.
Summary
Of the many avenues for future research, here we highlight the importance of examining changes in integrated functioning of multiple hormones of the HPA and HPG axis in relation to adolescent emotionality and behavior problems as well as considering the immediate and broader environmental context, which has been highlighted as of primary importance in many biosocial theories (Booth et al., 2003; Del Giudice et al., 2011; Dodge & Pettit, 2003; Ellis & Boyce, 2008). Careful matching between research questions and appropriate analyses will be of utmost importance as the field grows to incorporate a developmental dual-axis approach to understanding associations between hormones and behavior. As shown in Figure 3, hormones are measured at single occasions to ascertain basal levels, sampled repeatedly to assess diurnal changes or measured in response to a stressor or other environmental influence, and so the time-course of assessment should be carefully considered. All of these changes can be developmentally influenced and vary by immediate and broader environmental contexts, opening questions of how environmental contexts shape the HPA & HPG axes and behavioral outcomes, and how the latter two relate to each other depending on the context. The challenges of incorporating developmental and contextual factors into dual-axis approaches to hormone–behavior associations are not insurmountable, and the resulting research has great potential to move the field forward.
CONCLUSIONS
The groundwork laid here provides a platform for exciting new directions for developmental hormone–behavior research. In the present review, we sought to clarify seemingly divergent hormone–behavior findings by considering a dual system approach within a developmental, ecological framework. We have argued that examining multiple hormones may lead to a better understanding of hormone–behavior associations in adolescence and careful consideration of developmental processes is important for the next phase in research that aims to further clarify these associations. While recent studies have begun to consider the role of multiple hormones and the role of context, prolonged consideration of study design and a careful developmental approach is necessary. We propose that in the future, carefully considering the nature and time-course of changes in hormones, the developmental and environmental context, and taking multiple methodological approaches (i.e. between-person and within-person approaches) will greatly increase our understanding of the nuanced associations between hormones and behavior during key developmental transitions like that of adolescence.
This review concludes that there may be developmental changes in HPA-HPG hormone associations within-individuals from adolescence to adulthood, and that understanding HPA-HPG associations and interactions in a developmental framework may advance our understanding of the development of mental health problems through improved biomarkers. It is clear that both the HPA and HPG axes undergo substantial development across adolescence, and the findings reviewed here hint at a developmental switch in how the two axes are functionally related somewhere in late adolescence or early adulthood. This developmental profile may be meaningful for understanding neuroendocrine development as cross-axis communication may be influenced by both proximal (see Dismukes et al., this issue; Simmons et al., this issue) and longitudinal (Ruttle et al., this issue) stressful life events. A small, but growing literature largely collected within this special issue brings together research that suggests that combinations of HPA and HPG hormones, including high cortisol and low DHEA, or low cortisol and high testosterone, may predict behavior more consistently than single hormones. Together, the research reviewed here suggests that using a developmentally sensitive dual-axis approach, considering the environmental context, may help to clarify specific mechanisms by which hormones and behavior are associated during adolescence. Moving toward a biosocial, developmentally sensitive dual-axis approach will advance the field by clarifying how both the hormonal milieu within an individual and the ecological context around the individual, together, have implications for behavior problems. This more nuanced understanding of how individuals’ hormonal milieu changes over time, and how various contexts influence associations among multiple hormones and behavior may help us to identify at-risk individuals as well as those who will respond to different therapies with the end goal of reducing adolescent onset psychopathology symptoms.
Acknowledgments
Contract grant sponsor: National Institute of Drug Abuse
Contract grant numbers: F31DA033737, T32 DA016184
Contract grant sponsor: Canadian Institutes of Health Research Post-Doctoral Fellowship
Contract grant sponsor: National Institute of Mental Health
Contract grant number: MH093675
Contract grant sponsor: National Institute of Mental Health
Contract grant number: P50-MH084051
Authors’ work was supported by the National Institute of Drug Abuse F31DA033737 and T32 DA016184 (Marceau), Canadian Institutes of Health Research Post-Doctoral Fellowship (Ruttle), the National Institute of Mental Health MH093675 (Shirtcliff), and the National Institute of Mental Health P50-MH084051 (Essex).
References
- Adam EK, Doane LD, Zinbarg RE, Mineka S, Craske MG, Griffith JW. Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology. 2010;35(6):921–931. doi: 10.1016/j.psyneuen.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alink LRA, van Ijzendoorn MH, Bakermans-Kranenburg MJ, Mesman J, Juffer F, Koot HM. Cortisol and externalizing behavior in children and adolescents: Mixed meta-analytic evidence for the inverse relation of basal cortisol and cortisol reactivity with externalizing behavior. Developmental Psychobiology. 2008;50(5):427–450. doi: 10.1002/dev.20300. [DOI] [PubMed] [Google Scholar]
- Allison AL, Scott G, Boettger C, Leonbacher U, Peres JC, Shirtcliff EA. Sensation seeking and a real world stressor: Endocrine and autonomic effects; New Orleans, LA. Poster Presented at Society for Psychophysiological Research.2012. [Google Scholar]
- Anders T. Biological rhythms in development. Psychosomatic Medicine. 1983;44:61–72. doi: 10.1097/00006842-198203000-00008. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Desperately driven and no brakes: Developmental stress exposure and subsequent risk for substance abuse. Neuroscience & Biobehavioral Reviews. 2009;33:516–524. doi: 10.1016/j.neubiorev.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews J, Pruessner JC. The combined propranolol/TSST paradigm—A new method for psychoneuroendocrinology. PLoS ONE. 2013;8(2):e57567. doi: 10.1371/journal.pone.0057567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angold A. Adolescent depression, cortisol and DHEA. Psychological Medicine. 2003;33:573–581. doi: 10.1017/s003329170300775x. [DOI] [PubMed] [Google Scholar]
- Apter D, Pakarinen A, Hammond GL, Vihko R. Adrenocortical function in puberty: Serum ACTH, cortisol and dehydroepiandrosterone in girls and boys. Acta Paediatrica. 1979;68:599–604. doi: 10.1111/j.1651-2227.1979.tb05062.x. [DOI] [PubMed] [Google Scholar]
- Archer J. Testosterone and human aggression: An evaluation of the challenge hypothesis. Neuroscience & Biobehavioral Reviews. 2006;30:319–345. doi: 10.1016/j.neubiorev.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Bartels M, Van den Berg M, Sluyter F, Boomsma DI, de Geus EJC. Heritability of cortisol levels: Review and simultaneous analysis of twin studies. Psychoneuroendocrinology. 2003;28(2):121–137. doi: 10.1016/s0306-4530(02)00003-3. [DOI] [PubMed] [Google Scholar]
- Bateup HS, Booth A, Shirtcliff EA, Granger DA. Testosterone, cortisol, and women’s competition. Evolution and Human Behavior. 2002;23:181–192. [Google Scholar]
- Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children’s behavior: Advantages of a multisystem approach. Journal of Developmental & Behavioral Pediatrics. 2002;23:102–113. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, Van Ijzendoorn MH. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16:300–304. [Google Scholar]
- Belsky J, Steinberg L, Draper P. Childhood experience, interpersonal development, and reproductive strategy: An evolutionary theory of socialization. Child Development. 1991;62:647–670. doi: 10.1111/j.1467-8624.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Beltz AM. Sexual differentiation of human behavior: Effects of prenatal and pubertal organizational hormones. Front Neuroendocrinol. 2011;32(2):183–200. doi: 10.1016/j.yfrne.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Snyder E. Early hormonal influences on childhood sex-typed activity and playmate preferences: Implications for the development of sexual orientation. Developmental Psychology. 1995;31:31–42. [Google Scholar]
- Berenbaum SA. Effects of early androgens on sex-typed activities and interests in adolescents with congenital adrenal hyperplasia. Hormones and Behavior. 1999;35:102–110. doi: 10.1006/hbeh.1998.1503. [DOI] [PubMed] [Google Scholar]
- Bernhardt PC, Dabbs JM, Jr, Fielden JA, Lutter CD. Testosterone changes during vicarious experiences of winning and losing among fans at sporting events. Physiology & Behavior. 1998;65(1):59–62. doi: 10.1016/s0031-9384(98)00147-4. [DOI] [PubMed] [Google Scholar]
- Bjorklund DF. The role immaturity in human development. Psychological Bulletin. 1997;122:153–169. doi: 10.1037/0033-2909.122.2.153. [DOI] [PubMed] [Google Scholar]
- Bobadilla L, Johnson M, Asberg K, Shirtcliff EA. Experiences in the Military May Impact Dual-Axis Neuroendocrine Processes in Veterans. doi: 10.1002/dev.21259. under review. [DOI] [PubMed] [Google Scholar]
- Book AS, Starzyk KB, Quinsey VL. The relationship between testosterone and aggression: A meta-analysis. Aggression and Violent Behavior. 2001;6:579–599. [Google Scholar]
- Booth A, Granger DA, Mazur A, Kivlighan KT. Testosterone and social behavior. Social Forces. 2006;85:167–191. [Google Scholar]
- Booth A, Johnson DR, Granger DA, Crouter AC, McHale S. Testosterone and child and adolescent adjustment: The moderating role of parent–child relationships. Developmental Psychology. 2003;39:85–98. doi: 10.1037//0012-1649.39.1.85. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J, Warren MP. Biological and social contributions to negative affect in young adolescent girls. Child Development. 1989;60:40–55. doi: 10.1111/j.1467-8624.1989.tb02693.x. [DOI] [PubMed] [Google Scholar]
- Brown GL, McGarvey EL, Shirtcliff EA, Keller A, Granger DA, Flavin K. Salivary cortisol, dehydroepiandrosterone, and testosterone interrelationships in healthy young males: A pilot study with implications for studies of aggressive behavior. Psychiatry Research. 2008;159:67–76. doi: 10.1016/j.psychres.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders: Overview of physical and behavioral homeostasis. Journal of the American Medical Association. 1992;267:1244–1252. [PubMed] [Google Scholar]
- Cicchetti D, Rogosh FA. Diverse patterns of neuroendocrine activity in maltreated children. Development and Psychopathology. 2001;13:677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. The cortisol awakening response: More than a measure of HPA axis function. Neuroscience & Biobehavioral Reviews. 2010;35(1):97–103. doi: 10.1016/j.neubiorev.2009.12.011. http://dx.doi.org/10.1016/j.neubiorev.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Cousminer DL, Berry DJ, Timpson NJ, Ang W, Thiering E, Byrne EM, Widen E. Genome-wide association and longitudinal analyses reveal genetic loci linking pubertal height growth, pubertal timing and childhood adiposity. Human Molecular Genetics. 2013;22(13):2735–2747. doi: 10.1093/hmg/ddt104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbs JM, Jurkovic GJ, Frady RL. Salivary testosterone and cortisol among late adolescent male offenders. Journal of Abnormal Child Psychology. 1991;19:469–478. doi: 10.1007/BF00919089. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Dahl RE, Perel JM, Birmaher B, Al-Shabbout M, Williamson DE, Ryan ND. Nocturnal ACTH, cortisol, growth hormone, and prolactin secretion in prepubertal depression. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:1130–1138. doi: 10.1097/00004583-199609000-00010. [DOI] [PubMed] [Google Scholar]
- de Weerth C, Zijl RH, Buitelaar JK. Development of cortisol circadian rhythm in infancy. Early Human Development. 2003;73:39–52. doi: 10.1016/s0378-3782(03)00074-4. [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, Shirtcliff EA. The adaptive calibration model of stress responsivity. Neuroscience & Biobehavioral Reviews. 2011;35:1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, Shirtcliff EA. Making sense of stress: An evolutionary—developmental framework. In: Laviola G, Macri S, editors. Adaptive and maladaptive aspects of developmental stress. New York: Springer; 2013. pp. 23–44. [Google Scholar]
- Denson TF, Mehta PH, Ho Tan D. Endogenous testosterone and cortisol jointly influence reactive aggression in women. Psychoneuroendocrinology. 2013;38(3):416–424. doi: 10.1016/j.psyneuen.2012.07.003. http://dx.doi.org/10.1016/j.psyneuen.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Gunnar MR, Donzella B. Cortisol levels of young children in full-day childcare centers: Relations with age and temperament. Psychoneuroendocrinology. 1999;24:505–518. doi: 10.1016/s0306-4530(99)00009-8. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dismukes AR, Johnson MM, Vitacco MJ, Shirtcliff EA. Severe stress exposure moderates the link of cortisol with testosterone and DHEA in incarcerated adolescent males accepted. [Google Scholar]
- Dodge KA, Pettit GS. A biopsychosocial model of the development of chronic conduct problems in adolescence. Developmental Psychology. 2003;39:349–371. doi: 10.1037//0012-1649.39.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn LD, Biro FM. Puberty and its measurement: A decade in review. Journal of Research on Adolescence. 2011;21:180–195. [Google Scholar]
- Dorn LD, Dahl RE, Woodward HR, Biro F. Defining the boundaries of early adolescence: A user’s guide to assessing pubertal status and pubertal timing in research with adolescents. Applied Developmental Science. 2006;10:30–56. [Google Scholar]
- Dorn LD, Kolko DJ, Susman EJ, Huang B, Stein H, Music E, Bukstein OG. Salivary gonadal and adrenal hormone differences in boys and girls with and without disruptive behavior disorders: Contextual variants. Biological Psychology. 2009;81:31–39. doi: 10.1016/j.biopsycho.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eatough EM, Shirtcliff EA, Hanson JL, Pollak SD. Hormonal reactivity to MRI scanning in adolescents. Psychoneuroendocrinology. 2009;34:1242–1246. doi: 10.1016/j.psyneuen.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenegger C, Haushofer J, Fehr E. The role of testosterone in social interaction. Trends in Cognitive Science. 2011;15:263–271. doi: 10.1016/j.tics.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Elias CF. Leptin action in pubertal development: Recent advances and unanswered questions. Trends Endocrinology and Metabolism. 2012;23(1):9–15. doi: 10.1016/j.tem.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elks CE, Perry JR, Sulem P, Chasman DI, Franceschini N, He C, Murray A. Thirty new loci for age at menarche identified by a meta-analysis of genome-wide association studies. Nature Genetics. 2010;42(12):1077–1085. doi: 10.1038/ng.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbogen MA, Hodgins S, Linnen AM, Ostiguy CS. Elevated daytime cortisol levels: a biomarker of subsequent major affective disorder? J Affect Disord. 2011;132(1–2):265–269. doi: 10.1016/j.jad.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Ellis BJ. Timing of pubertal maturation in girls: An integrated life history approach. Psychological Bulletin. 2004;130:920–958. doi: 10.1037/0033-2909.130.6.920. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT. Biological sensitivity to context. Current Directions in Psychological Science. 2008;17:183–187. [Google Scholar]
- Ellis BJ, Del Giudice M, Shirtcliff EA. Beyond allostatic load: The stress response system as a mechanism of conditional adaptation. In: Beauchaine TP, Hinshaw SP, editors. Child and adolescent psychopathology. 2. New York: Wiley & Sons; 2013. pp. 251–284. [Google Scholar]
- Ellis BJ, Essex MJ. Family environments, adrenarche, and sexual maturation: A longitudinal test of a life history model. Child Development. 2007;78:1799–1817. doi: 10.1111/j.1467-8624.2007.01092.x. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Shirtcliff EA, Boyce WT, Deardorff J, Essex MJ. Quality of early family relationships and the timing and tempo of puberty: Effects depend on biological sensitivity to context. Development and Psychopathology. 2011;23:85–99. doi: 10.1017/S0954579410000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: Effects on cortisol and behavior. Biological Psychiatry. 2002;52(8):776–784. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Shirtcliff EA, Burk LR, Ruttle PL, Klein MH, Slattery MJ, Armstrong JM. Influence of early life stress on later hypothalamic-pituitary-adrenal axis functioning and its covariation with mental health symptoms: A study of the allostatic process from childhood into adolescence. Development and Psychopathology. 2011;23(4):1039–1058. doi: 10.1017/s0954579411000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild G, van Goozen SHM, Stollery SJ, Brown J, Gardiner J, Herbert J, Goodyer IM. Cortisol diurnal rhythm and stress reactivity in male adolescents with early-onset or adolescence-onset conduct disorder. Biological Psychiatry. 2008;64:599–606. doi: 10.1016/j.biopsych.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fameli M, Kitraki E, Stylianopoulou F. Effects of hyperactivity of the maternal hypothalamic-pituitary-adrenal (HPA) axis during pregnancy on the development of the HPA axis and brain monoamines of the offspring. International Journal of Developmental Neuroscience. 1994;12:651–659. doi: 10.1016/0736-5748(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Fang CY, Egleston BL, Brown KM, Lavigne JV, Stevens VJ, Barton BA, Dorgan JF. Family cohesion moderates the relation between free testosterone and delinquent behaviors in adolescent boys and girls. Journal of Adolescent Health. 2009;44:590–597. doi: 10.1016/j.jadohealth.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder A, Coplan JD, Goetz RR, Mathew SJ, Pine DS, Dahl RE, Weissman MM. Twenty-four-hour cortisol secretion patterns in prepubertal children with anxiety or depressive disorders. Biological Psychiatry. 2004;56(3):198–204. doi: 10.1016/j.biopsych.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Finkelstein JW, Susman EJ, Chinchilli VM, Kunselman SJ, D’Arcangelo MR, Kulin HE. Estrogen or testosterone increases self-reported aggressive behaviors in hypogonadal adolescents. Journal of Clinical Endocrinology and Metabolism. 1997;82:2423–2438. doi: 10.1210/jcem.82.8.4165. [DOI] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): Facts and future directions. International Journal of Psychophysiology. 2009;72(1):67–73. doi: 10.1016/j.ijpsycho.2008.03.014. http://dx.doi.org/10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Gatzke-Kopp LM. The canary in the coalmine: The sensitivity of mesolimbic dopamine to environmental adversity during development. Neuroscience & Biobehavioral Reviews. 2011;35:794–803. doi: 10.1016/j.neubiorev.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Gitau R, Fisk NM, Glover V. Human fetal and maternal corticotrophin releasing hormone responses to acute stress. Archives of Disease in Childhood Fetal and Neonatal Edition. 2004;89(1):F29–F32. doi: 10.1136/fn.89.1.F29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: Implications for health. Nature Reviews Immunology. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Glenn AL, Raine A, Schug RA, Gao Y, Granger DA. Increased testosterone-to-cortisol ratio in psychopathy. Journal of Abnormal Psychology. 2011;120:389–399. doi: 10.1037/a0021407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyer IM. The hypothalamic–pituitary–adrenal axis: Cortisol, DHEA and mental and behavioral function. In: Steptoe A, editor. Depression and physical illness. London, UK: Cambridge University Press; 2007. pp. 280–298. [Google Scholar]
- Goodyer IM, Herbert J, Tamplin A, Secher SM, Pearson J. Short-term outcome of major depression: II. Life events, family dysfunction, and friendship difficulties as predictors of persistent disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(4):474–480. doi: 10.1097/00004583-199704000-00009. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Herbert J, Altham PME. Adrenal steroid secretion and major depression in 8- to 16-year-olds, III. Influence of cortisol/DHEA ratio at presentation on subsequent rates of disappointing life events and persistent major depression. Psychological Medicine. 1998;28:265–273. doi: 10.1017/s0033291797006314. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Herbert J, Tamplin A. Psychoendocrine antecedents of persistent first-episode major depression in adolescents: A community-based longitudinal enquiry. Psychological Medicine. 2003;33:601–610. doi: 10.1017/s0033291702007286. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Herbert J, Altham PM, Pearson J, Secher SM, Shiers HM. Adrenal secretion during major depression in 8- to 16-year-olds, I. Altered diurnal rhythms in salivary cortisol and dehydroepiandrosterone (DHEA) at presentation. Psychological Medicine. 1996;26:245–256. doi: 10.1017/s0033291700034644. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Park RJ, Netherton CM, Herbert J. Possible role of cortisol and dehydroepiandrosterone in human development and psychopathology. The British Journal of Psychiatry. 2001;179(3):243–249. doi: 10.1192/bjp.179.3.243. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Tamplin A, Herbert J, Altham PME. Recent life events, cortisol, dehydroepiandrosterone and the onset of major depression in high-risk adolescents. British Journal of Psychiatry. 2000;177:499–504. doi: 10.1192/bjp.177.6.499. [DOI] [PubMed] [Google Scholar]
- Granger DA, Schwartz EB, Booth A, Curran M, Zakaria D. Assessing dehydroepiandrosterone in saliva: A simple radioimmunoassay for use in studies of children, adolescents and adults. Psychoneuroendocrinology. 1999;24:567–579. doi: 10.1016/s0306-4530(99)00013-x. [DOI] [PubMed] [Google Scholar]
- Granger DA, Serbin LA, Schwartzman A, Lehoux P, Cooperman J, Ikeda S. Children’s salivary cortisol, internalising behaviour problems, and family environment: Results from the concordia longitudinal risk project. International Journal of Behavioral Development. 1998;22:707–728. [Google Scholar]
- Granger DA, Shirtcliff EA, Zahn-Waxler C, Usher B, Klimes-Dougan B, Hastings P. Salivary testosterone diurnal variation and psychopathology in adolescent males and females: Individual differences and developmental effects. Development and Psychopathology. 2003;15:431–449. [PubMed] [Google Scholar]
- Granger DA, Weisz JR, Kauneckis D. Neuroendocrine reactivity, internalizing behavior problems, and control-related cognitions in clinic-referred children and adolescents. Journal of Abnormal Psychology. 1994;103:267–276. doi: 10.1037//0021-843x.103.2.267. [DOI] [PubMed] [Google Scholar]
- Granger DA, Weisz JR, McCracken JT, Ikeda SC, Douglas P. Reciprocal influences among adrenocortical activation, psychosocial processes, and the behavioral adjustment of clinic-referred children. Child Development. 1996;67:3250–3262. [PubMed] [Google Scholar]
- Griffin JE, Ojeda SR, editors. Textbook of endocrine physiology. New York, NY: Oxford University Press; 1996. [Google Scholar]
- Grossi G, Theorell T, Jürisoo M, Setterlind S. Psychophysiological correlates of organizational change and threat of unemployment among police inspectors. Integrative Physiological and Behavioral Science. 1999;34(1):30–42. doi: 10.1007/BF02688708. [DOI] [PubMed] [Google Scholar]
- Grumbach MM. The neuroendocrinology of human puberty revisited. Hormone Research in Paediatrcs. 2002;57:2–14. doi: 10.1159/000058094. [DOI] [PubMed] [Google Scholar]
- Grumbach MM, Styne DM. Puberty: Ontogeny, neuroendocrinology, physiology, and disorders. In: Larsen PR, Kronenberg HM, Melmed S, Polonsky KS, editors. Williams textbook of endocrinology. New York: Oxford University Press; 2003. pp. 1115–1286. [Google Scholar]
- Gunnar M. The role of glucocorticoids in anxiety disorders: A critical analysis. In: Vasey MW, Dadds V, editors. The developmental psychopathology of anxiety. New York, NY: Oxford University Press; 2001. pp. 143–159. [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: Normative changes and associations with puberty. Development and Psychopathology. 2009;21:69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halligan SL, Herbert J, Goodyer I, Murray L. Disturbances in morning cortisol secretion in association with maternal postnatal depression predict subsequent depressive symptomatology in adolescents. Biological Psychiatry. 2007;62:40–46. doi: 10.1016/j.biopsych.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Badanes LS, Abela JRZ, Watamura SE. Hypothalamic–pituitary–adrenal axis dysregulation in dysphoric children and adolescents: Cortisol reactivity to psychosocial stress from preschool through middle adolescence. Biological Psychiatry. 2010;68:484–490. doi: 10.1016/j.biopsych.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartaigh BÓ, Loerbroks A, Thomas GN, Engeland CG, Hollands MA, Fischer JE, Bosch JA. Age-dependent and -independent associations between depression, anxiety, DHEAS, and cortisol: From the MIPH Industrial Cohort Studies (MICS) Psychoneuroendocrinology. 2012;37(7):929–936. doi: 10.1016/j.psyneuen.2011.10.009. http://dx.doi.org/10.1016/j.psyneuen.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Hastings PD, Ruttle PL, Serbin LA, Mills RSL, Stack DM, Schwartzman AE. Adrenocortical responses to strangers in preschoolers: Relations with parenting, temperament, and psychopathology. Developmental Psychobiology. 2011;53:694–710. doi: 10.1002/dev.20545. [DOI] [PubMed] [Google Scholar]
- Heikkilä M. Dissertation. Biocenter Oulu, Department of Biochemistry, University of Oulu; Oulu, Finland: 2002. Development of the adreno-genital system: Female sex determination, ovarian and adrenal gland ontogeny regulated by Wnt-4 in mice. Print. [Google Scholar]
- Heinz A, Hermann D, Smolka M, Rieks M, Gräf KJ, Pöhlau D, Bauer M. Effects of acute psychological stress on adhesion molecules, interleukins and sex hormones: Implications for coronary heart disease. Psychopharmacology. 2003;165(2):111–117. doi: 10.1007/s00213-002-1244-6. [DOI] [PubMed] [Google Scholar]
- Herbert J. Fortnightly review: Stress, the brain, and mental illness. British Medical Journal. 1997;315:530–535. doi: 10.1136/bmj.315.7107.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP. Neural control of chronic stress adaptation. Frontiers of Behavioral Neuroscience. 2013;7:61. doi: 10.3389/fnbeh.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: Hypothalamo-pituitary-adrenocortical axis. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2005;29(8):1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hiort O. Androgens and puberty. Best Practice & Research Clinical Endocrinology & Metabolism. 2002;16:31–41. doi: 10.1053/beem.2002.0178. [DOI] [PubMed] [Google Scholar]
- Ice GH, Katz-Stein A, Himes J, Kane RL. Diurnal cycles of salivary cortisol in older adults. Psychoneuroendocrinology. 2004;29:355–370. doi: 10.1016/s0306-4530(03)00034-9. [DOI] [PubMed] [Google Scholar]
- Inoff-Germain G, Arnold GS, Nottelmann ED, Susman EJ, Cutler GB, Jr, Chrousos GP. Relations between hormone levels and observational measures of aggressive behavior of young adolescents in family interactions. Developmental Psychology. 1988;24:129–139. [Google Scholar]
- Izawa S, Saito K, Shirotsuki K, Sugaya N, Nomura S. Effects of prolonged stress on salivary cortisol and dehydroepiandrosterone: A study of a two-week teaching practice. Psychoneuroendocrinology. 2012;37:852–858. doi: 10.1016/j.psyneuen.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Izawa S, Sugaya N, Shirotsuki K, Yamada KC, Ogawa N, Ouchi Y, Nomura S. Salivary dehydroepiandrosterone secretion in response to acute psychosocial stress and its correlations with biological and psychological changes. Biological Psychology. 2008;79(3):294–298. doi: 10.1016/j.biopsycho.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Johnson MM, Dismukes AR, Fleury D, Shirtcliff EA. Psychopathy’s influence on the coupling between hypothalamic-pituitary-adrenal and -gonadal axes among incarcerated adolescents. Developmental Psychobiology. 2014;56:448–458. doi: 10.1002/dev.21111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonetz-Mentzel L, Wiedemann G. Establishment of reference ranges for cortisol in neonates, infants, children and adolescents. European Journal of Clinical Chemistry & Biochemistry. 1993;31:525–529. [PubMed] [Google Scholar]
- Kagan J, Reznick S, Snidman N. Biological bases of childhood shyness. Science. 1988;240:167–167. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- Kaufman J. Depressive disorders in maltreated children. Journal of the American Academy of Child and Adolescent Psychiatry. 1991;30:257–265. doi: 10.1097/00004583-199103000-00014. [DOI] [PubMed] [Google Scholar]
- Kenny FM, Preeyasombat C, Migeon CJ. Cortisol production rate. Pediatrics. 1966;37:34–42. [PubMed] [Google Scholar]
- Kiess W, Meidert A, Dressendorfer RA, Schriever K, Kessler U, Kounig A, Strasburger CJ. Salivary cortisol levels throughout childhood and adolescence: Relation with age, pubertal stage, and weight. Pediatric Research. 1995;37:502–506. doi: 10.1203/00006450-199504000-00020. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology. 1994;19:313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Klimes Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: Individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Development and Psychopathology. 2001;13:695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Kosinska-Kaczynska K, Bartkowiak R, Kaczynski B, Szymusik I, Wielgos M. Autonomous adrenocorticotropin reaction to stress stimuli in human fetus. Early Human Development. 2012;88(4):197–201. doi: 10.1016/j.earlhumdev.2011.08.006. http://dx.doi.org/10.1016/j.earlhumdev.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Kraemer RK, Acevedo EA, Synovitz LS, Hebert EH, Gimpel TG, Castracane VC. Leptin and steroid hormone responses to exercise in adolescent female runners over a 7-week season. European Journal of Applied Physiology. 2001;86:85–91. doi: 10.1007/s004210100500. [DOI] [PubMed] [Google Scholar]
- Kroboth P, Salek F, Pittenger A, Fabian T, Frye R. DHEA and DHEA-S: A review. The Journal of Clinical Pharmacology. 1999;39:327–348. doi: 10.1177/00912709922007903. [DOI] [PubMed] [Google Scholar]
- Laurent HK, Leve LD, Neiderhiser JM, Natsuaki MN, Shaw DS, Harold GT, Reiss D. Effects of prenatal and postnatal parent depressive symptoms on adopted child HPA regulation: Independent and moderated influences. Developmental Psychology. 2012;49(5):876–886. doi: 10.1037/a0028800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennartsson AK, Kushnir MM, Bergquist J, Jonsdottir IH. DHEA and DHEA-S response to acute psychosocial stress in healthy men and women. Biological Psychology. 2012;90:143–149. doi: 10.1016/j.biopsycho.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Li H, Ji C. Change of dehydroepiandrosterone in serum of 6–18 year-old twin girls in Qingdao City. Wei Sheng Yan Jiu (Journal of Hygiene Research) 2007;36(1):41–42. [PubMed] [Google Scholar]
- Lopez-Duran NL, Kovacs M, George CJ. Hypothalamic–pituitary–adrenal axis dysregulation in depressed children and adolescents: A meta-analysis. Psychoneuroendocrinology. 2009;34:1272–1283. doi: 10.1016/j.psyneuen.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Heffelfinger A, Mrakotsky C, Brown K, Hessler M, Spitznagel E. Alterations in stress cortisol reactivity in depressed preschoolers relative to psychiatric and no-disorder comparison groups. Archives of General Psychiatry. 2003;60:1248–1255. doi: 10.1001/archpsyc.60.12.1248. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nature Neuroscience. 1998;1(1):69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Ouellet-Morin I, Hupbach A, Tu MT, Buss C, Walker D, McEwen BS. Beyond the stress concept: Allostatic load—A developmental biological and cognitive perspective. In: Cohen DCDJ, editor. Developmental psychopathology. Vol 2: Developmental neuroscience. 2. Hoboken, NJ: John Wiley & Sons Inc; 2006. pp. 578–628. [Google Scholar]
- MacGillivray MH, Morishima A, Conte F, Grumbach M, Smith EP. Pediatric endocrinology update: An overview. Hormone Research Paediatrics. 1998;49:2–8. doi: 10.1159/000053061. [DOI] [PubMed] [Google Scholar]
- Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) Frontiers in Neuroendocrinology. 2009;30(1):65–91. doi: 10.1016/j.yfrne.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maras A, Laucht M, Gerdes D, Wilhelm C, Lewicka S, Haack D, Schmidt MH. Association of testosterone and dihydrotestosterone with externalizing behavior in adolescent boys and girls. Psychoneuroendocrinology. 2003;28:932–940. doi: 10.1016/s0306-4530(02)00119-1. [DOI] [PubMed] [Google Scholar]
- Marceau K, Dorn LD, Susman EJ. Stress and puberty-related hormone reactivity, negative emotionality, and parent–adolescent relationships. Psychoneuroendocrinology. 2012;37:1286–1298. doi: 10.1016/j.psyneuen.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Marceau K, Ruttle PL, Shirtcliff EA, Essex MJ, Susman EJ. Developmental and contextual considerations for adrenal and gonadal hormone functioning during adolescence: Implications for adolescent mental health. Developmental Psychobiology. doi: 10.1002/dev.21214. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau K, Shirtcliff EA, Hastings PD, Klimes-Dougan B, Zahn-Waxler C, Dorn LD, Susman EJ. Within-adolescent coupled changes in cortisol with DHEA and testosterone in response to three stressors during adolescence. Psychoneuroendocrinology. 2014;41(0):33–45. doi: 10.1016/j.psyneuen.2013.12.002. http://dx.doi.org/10.1016/j.psyneuen.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchock RL, Dorn LD, Susman EJ. Diurnal and seasonal cortisol, testosterone, and DHEA rhythms in boys and girls during puberty. Chronobiology International. 2007;24:969–990. doi: 10.1080/07420520701649471. [DOI] [PubMed] [Google Scholar]
- Matthews SG. Early programming of the hypothalamo–pituitary–adrenal axis. Trends in Endocrinology & Metabolism. 2002;13:373–380. doi: 10.1016/s1043-2760(02)00690-2. [DOI] [PubMed] [Google Scholar]
- Mazur A, Booth A, Dabbs JM., Jr Testosterone and chess competition. Social Psychology Quarterly. 1992;55:70–77. [Google Scholar]
- Mazur A, Susman EJ, Edelbrock S. Sex differences in testosterone response to a video game contest. Evolution and Human Behavior. 1997;18:317–326. [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual: Mechanisms leading to disease. Archives of Internal Medicine. 1993;153:2093–2101. [PubMed] [Google Scholar]
- McEwen BS. Stressed or stressed out: What is the difference? Journal of Psychiatry & Neuroscience. 2005;30(5):315–318. [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Seeman T. Protective and damaging effects of mediators of stress: Elaborating and testing the concepts of allostasis and allostatic load. Annals of the New York Academy of Sciences. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Mehta PH, Josephs RA. Testosterone and cortisol jointly regulate dominance: Evidence for a dual-hormone hypothesis. Hormones and Behavior. 2010;58:898–906. doi: 10.1016/j.yhbeh.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Menke A, Arloth J, Pütz B, Weber P, Klengel T, Mehta D, Binder EB. Dexamethasone stimulated gene expression in peripheral blood is a sensitive marker for glucocorticoid receptor resistance in depressed patients. Neuropsychopharmacology. 2012;37(6):1455–1464. doi: 10.1038/npp.2011.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitamura R, Yano K, Suzuki N, Ito Y, Makita Y, Okuno A. Diurnal rhythms of luteinizing hormone, follicle-stimulating hormone, and testosterone secretion before the onset of male puberty. Journal of Clinical Endocrinology and Metabolism. 1999;84:29–37. doi: 10.1210/jcem.84.1.5404. [DOI] [PubMed] [Google Scholar]
- Mitamura R, Yano K, Suzuki N, Ito Y, Makita Y, Okuno A. Diurnal rhythms of luteinizing hormone, follicle-stimulating hormone, testosterone, and estradiol secretion before the onset of female puberty in short children. Journal of Clinical Endocrinology and Metabolism. 2000;85:1074–1080. doi: 10.1210/jcem.85.3.6445. [DOI] [PubMed] [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: A developmental taxonomy. Psychology Review. 1993;100:674–701. [PubMed] [Google Scholar]
- Montoya E, Terburg D, Bos P, van Honk J. Testosterone, cortisol, and serotonin as key regulators of social aggression: A review and theoretical perspective. Motiv Emot. 2012;36(1):65–73. doi: 10.1007/s11031-011-9264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CA, III, Southwick S, Hazlett G, Rasmusson A, Hoyt G, Zimolo Z, Charney D. Relationships among plasma dehydroepiandrosterone sulfate and cortisol levels, symptoms of dissociation, and objective performance in humans exposed to acute stress. Archieves of General Psychiatry. 2004;61(8):819–825. doi: 10.1001/archpsyc.61.8.819. [DOI] [PubMed] [Google Scholar]
- Moss HB, Vanyukov MM, Martin CS. Salivary cortisol responses and the risk for substance abuse in prepubertal boys. Biological Psychiatry. 1995;38:547–555. doi: 10.1016/0006-3223(94)00382-D. [DOI] [PubMed] [Google Scholar]
- Mulder EJH, Robles de Medina PG, Huizink AC, Van den Bergh BRH, Buitelaar JK, Visser GHA. Prenatal maternal stress: Effects on pregnancy and the (unborn) child. Early Human Development. 2002;70:3–14. doi: 10.1016/s0378-3782(02)00075-0. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35(2):163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Netherton C, Goodyer I, Tamplin A, Herbert J. Salivary cortisol and dehydroepiandrosterone in relation to puberty and gender. Psychoneuroendocrinology. 2004;29:125–140. doi: 10.1016/s0306-4530(02)00150-6. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Lomniczi A, Loche A, Matagne V, Kaidar G, Sandau US, Dissen GA. The transcriptional control of female puberty. Brain Research. 2010;1364:164–174. doi: 10.1016/j.brainres.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda SR, Terasawa E. Neuroendocrine regulation of puberty. In: Pfaff D, Arnold A, Etgen A, Fahrbach S, Moss R, Rubin R, editors. Hormones, brain and behavior. Vol. 4. New York, NY: Elsevier; 2002. pp. 589–659. [Google Scholar]
- Olweus D, Mattsson A, Schalling D, Low H. Circulating testosterone levels and aggression in adolescent males: A causal analysis. Psychosomatic Medicine. 1988;50:261–272. doi: 10.1097/00006842-198805000-00004. [DOI] [PubMed] [Google Scholar]
- Paikoff RL, Brooks-Gunn J. Do parent-child relationships change during puberty? Psychological Bulletin. 1991;110:47–66. doi: 10.1037/0033-2909.110.1.47. [DOI] [PubMed] [Google Scholar]
- Pajer K, Tabbah R, Gardner W, Rubin RT, Kenneth Czambel R, Wang Y. Adrenal androgen and gonadal hormone levels in adolescent girls with conduct disorder. Psychoneuroendocrinology. 2006;31:1245–1256. doi: 10.1016/j.psyneuen.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Palmert MR, Hayden DL, Mansfield MJ, Crigler JF, Crowley WF, Chandler DW, Boepple PA. The longitudinal study of adrenal maturation during gonadal suppression: Evidence that adrenarche is a gradual process. Journal of Clinical Endocrinology and Metabolism. 2001;86:4536–4542. doi: 10.1210/jcem.86.9.7863. [DOI] [PubMed] [Google Scholar]
- Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocrinology Review. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- Peper JS, van den Heuvel MP, Mandl RC, Hulshoff Pol HE, van Honk J. Sex steroids and connectivity in the human brain: A review of neuroimaging studies. Psychoneuroendocrinology. 2011;36(8):1101–1113. doi: 10.1016/j.psyneuen.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K, Schmidt LA, Henderson HA, Schulkin J, Fox NA. Salivary cortisol levels and infant temperament shape developmental trajectories in boys at risk for behavioral maladjustment. Psychoneuroendocrinology. 2008;33:916–925. doi: 10.1016/j.psyneuen.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perogamvros I, Ray DW, Trainer PJ. Regulation of cortisol bioavailability—Effects on hormone measurement and action. Nature Reviews Endocrinology. 2012;8(12):717–727. doi: 10.1038/nrendo.2012.134. [DOI] [PubMed] [Google Scholar]
- Phillips DIW, Jones A. Fetal programming of autonomic and HPA function: Do people who were small babies have enhanced stress responses? Journal of Physiology. 2006;572:45–50. doi: 10.1113/jphysiol.2005.104695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Young W. Sexual beahvior: General aspects. In: Martini L, Ganong W, editors. Neuroendocrinology. Vol. 2. New York, NY: Academic Press; 1967. [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Poortman J, Andriesse R, Agema A, Donker GH, Schwarz F, Thijssen JHH. Adrenal androgen secretion and metabolism in post-menopausal women. In: Genazzani AR, Thijssen JHH, Siiteri PK, editors. Adrenal androgens. New York, NY: Raven; 1980. pp. 219–240. [Google Scholar]
- Popma A, Vermeiren R, Geluk CAML, Rinne T, van den Brink W, Knol DL, Doreleijers TA. Cortisol moderates the relationship between testosterone and aggression in delinquent male adolescents. Biological Psychiatry. 2007;61:405–411. doi: 10.1016/j.biopsych.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Price DA, Close GC, Fielding BA. Age of appearance of circadian rhythm in salivary cortisol values in infancy. Archives of Disease in Childhood. 1983;58:454–456. doi: 10.1136/adc.58.6.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prom-Wormley EC, York TP, Jacobson KC, Eaves LJ, Mendoza SP, Hellhammer D, Kremen WS. Genetic and environmental effects on diurnal dehydroepiandrosterone sulfate concentrations in middle-aged men. Psychoneuroendocrinology. 2011;36(10):1441–1452. doi: 10.1016/j.psyneuen.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A. Autonomic nervous system factors underlying disinhibited, antisocial, and violent behavior biosocial perspectives and treatment implications. Annals of the New York Academy of Sciences. 1996;794:46–59. doi: 10.1111/j.1749-6632.1996.tb32508.x. [DOI] [PubMed] [Google Scholar]
- Rao U, Hammen C, Ortiz LR, Chen LA, Poland RE. Effects of early and recent adverse experiences on adrenal response to psychosocial stress in depressed adolescents. Biological Psychiatry. 2008;64:521–526. doi: 10.1016/j.biopsych.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resko JA, Eik-Nes KB. Diurnal testosterone levels in peripheral plasma of human male subjects. Journal of Clinical Endocrinology and Metabolism. 1966;26:573–576. doi: 10.1210/jcem-26-5-573. [DOI] [PubMed] [Google Scholar]
- Resko J, Roselli C. Prenatal hormones organize sex differences of the neuroendocrine reproductive system: Observations on guinea pigs and nonhuman primates. Cellular and Molecular Neurobiology. 1997;17(6):627–648. doi: 10.1023/A:1022534019718. [DOI] [PubMed] [Google Scholar]
- Reul JM, Stec I, Wiegers GJ, Labeur MS, Linthorst AC, Arzt E, Holsboer F. Prenatal immune challenge alters the hypothalamic-pituitary-adrenocortical axis in adult rats. The Journal of Clinical Investigation. 1994;93(6):2600–2607. doi: 10.1172/JCI117272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roisman G, Susman EJ, Booth-LaForce C, Belsky J, Houts R, Barnett-Walker K, Steinberg L. Early family and child-care antecedents of awakening cortisol levels in adolescence. Child Development. 2009;80:907–920. doi: 10.1111/j.1467-8624.2009.01305.x. [DOI] [PubMed] [Google Scholar]
- Romeo RD. Neuroendocrine and behavioral development during puberty: A tale of two axes. Vitamins & Hormones. 2005;71:1–25. doi: 10.1016/S0083-6729(05)71001-3. [DOI] [PubMed] [Google Scholar]
- Romeo RD. The teenage brain: The stress response and the adolescent brain. Current Directions in Psychological Science. 2013;22(2):140–145. doi: 10.1177/0963721413475445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD. Puberty: A period of both organizational and activational effects of steroid hormones on neurobehavioural development. Journal of Neuroendocrinology. 2003;15:1185–1192. doi: 10.1111/j.1365-2826.2003.01106.x. [DOI] [PubMed] [Google Scholar]
- Ruttle PL, Shirtcliff EA, Armstrong JM, Klein MH, Essex MJ. Neuroendocrine coupling across adolescence and the longitudinal influence of early life stress. Developmental Psychobiology. doi: 10.1002/dev.21138. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttle PL, Shirtcliff EA, Serbin LA, Ben-Dat Fisher D, Stack DM, Schwartzman AE. Disentangling psychobiological mechanisms underlying internalizing and externalizing behaviors in youth: Longitudinal and concurrent associations with cortisol. Hormones and Behavior. 2011;59:123–132. doi: 10.1016/j.yhbeh.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saczawa ME, Graber JA, Brooks-Gunn J, Warren MP. Methodological considerations in use of the cortisol/DHEA(S) ratio in adolescent populations. Psychoneuroendocrinology. 2013;38(11):2815–2819. doi: 10.1016/j.psyneuen.2013.06.024. http://dx.doi.org/10.1016/j.psyneuen.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Stress, glucocorticoids, and damage to the nervous system: The current state of confusion. Stress. 1996;1(1):1–19. doi: 10.3109/10253899609001092. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The endocrine stress–response and social status in the wild baboon. Hormones and Behavior. 1982;16:279–292. doi: 10.1016/0018-506x(82)90027-7. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The trouble with testosterone: And other essays on the biology of the human predicament. New York, NY: Scribner; 1997. [Google Scholar]
- Sapolsky RM. Stress and plasticity in the limbic system. Neurochemical Research. 2003;28:1735–1742. doi: 10.1023/a:1026021307833. [DOI] [PubMed] [Google Scholar]
- Scerbo AS, Kolko DJ. Salivary testosterone and cortisol in disruptive children: Relationship to aggressive, hyperactive, and internalizing behaviors. Journal of the American Academy of Child and Adolescent Psychiatry. 1994;33:1174–1184. doi: 10.1097/00004583-199410000-00013. [DOI] [PubMed] [Google Scholar]
- Schaal B, Tremblay RE, Soussignan R, Susman EJ. Male testosterone linked to high social dominance but low physical aggression in early adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:1322–1330. doi: 10.1097/00004583-199610000-00019. [DOI] [PubMed] [Google Scholar]
- Schlotz W, Kumsta R, Layes I, Entringer S, Jones A, Wüst S. Covariance between psychological and endocrine responses to pharmacological challenge and psychosocial stress: A question of timing. Psychosomatic Medicine. 2008;70(7):787–796. doi: 10.1097/PSY.0b013e3181810658. [DOI] [PubMed] [Google Scholar]
- Schmidt-Reinwald A, Pruessner JC, Hellhammer DH, Federenko I, Rohleder N, Kirschbaum C. The cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life Sciences. 1999;64:1653–1660. doi: 10.1016/s0024-3205(99)00103-4. [DOI] [PubMed] [Google Scholar]
- Schoofs D, Wolf OT. Are salivary gonadal steroid concentrations influenced by acute psychosocial stress? A study using the Trier Social Stress Test (TSST) International Journal of Psychophysiology. 2011;80(1):36–43. doi: 10.1016/j.ijpsycho.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychological Bulletin. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk CE, Dorn LD, Kolko DJ, Susman EJ, Noll JG, Bukstein OG. Predicting treatment response for oppositional defiant and conduct disorder using pre-treatment adrenal and gonadal hormones. Journal of Child and Family Studies. 2012;21(6):973–981. doi: 10.1007/s10826-011-9557-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Vitacco MJ, Graf AR, Gostisha AJ, Merz JL, Zahn-Waxler C. Neurobiology of empathy and callousness: Implications for the development of antisocial behavior. Behavioral Sciences & the Law. 2009;27(2):137–171. doi: 10.1002/bsl.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff E, Zahn-Waxler C, Klimes-Dougan B, Slattery M. Salivary dehydroepiandrosterone responsiveness to social challenge in adolescents with internalizing problems. Journal of Clinical Psychology and Psychiatry. 2007;48:580–591. doi: 10.1111/j.1469-7610.2006.01723.x. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Essex MJ. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Developmental Psychobiology. 2008;50:690–703. doi: 10.1002/dev.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Ruttle P. Immunological and neuroendocrine dysregulation following early deprivation and stress. In: Brisch KH, editor. Attachment and early disorders of development. Munich: Klett-Cotta; 2010. [Google Scholar]
- Shirtcliff EA, Allison AL, Armstrong JM, Slattery MJ, Kalin NH, Essex MJ. Longitudinal stability and developmental properties of salivary cortisol levels and circadian rhythms from childhood to adolescence. Developmental Psychobiology. 2012;54:493–502. doi: 10.1002/dev.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Booth A, Johnson D. Low salivary cortisol levels and externalizing behavior problems in youth. Development and Psychopathology. 2005;17:167–184. doi: 10.1017/s0954579405050091. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Schwartz EB, Curran MJ, Booth A, Overman WH. Assessing estradiol in biobehavioral studies using saliva and blood spots: Simple radioimmunoassay protocols, reliability, and comparative validity. Hormones and Behavior. 2000;38:137–147. doi: 10.1006/hbeh.2000.1614. [DOI] [PubMed] [Google Scholar]
- Shiyko M, Lanza S, Tan X, Li R, Shiffman S. Using the time-varying effect model (TVEM) to examine dynamic associations between negative affect and self confidence on smoking urges: Differences between successful quitters and relapsers. Prevention Science. 2012;13(3):288–299. doi: 10.1007/s11121-011-0264-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons J, Byrne M, Schwartz O, Whittle S, Sheeber L, Kaess M, Allen N. Hormonal coupling in adolescence and the moderating influence of prior trauma and aversive maternal parenting. Developmental Psychobiology. doi: 10.1002/dev.21275. (this issue) [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nature Neuroscience. 2004;7(10):1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Smider NA, Essex MJ, Kalin NH, Buss KA, Klein MH, Davidson RJ, Goldsmith HH. Salivary cortisol as a predictor of socioemotional adjustment during kindergarten: A prospective study. Child Development. 2002;73:75–92. doi: 10.1111/1467-8624.00393. [DOI] [PubMed] [Google Scholar]
- Smyth JM, Ockenfels MC, Gorin AA, Catley D, Porter LS, Kirschbaum C, Stone AA. Individual differences in the diurnal cycle of cortisol. Psychoneuroendocrinology. 1997;22(2):89–105. doi: 10.1016/s0306-4530(96)00039-x. [DOI] [PubMed] [Google Scholar]
- Stanton SJ, Beehner JC, Saini EK, Kuhn CM, LaBar KS. Dominance, politics, and physiology: Voters’ testosterone changes on the night of the 2008 United States presidential election. PLoS ONE. 2009;4(10):e7543. doi: 10.1371/journal.pone.0007543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling P, Eyer J. Allostasis: A new paradigm to explain arousal pathology. In: Reason SFJ, editor. Handbook of life stress, cognition and health. Oxford, England: John Wiley & Sons; 1988. pp. 629–649. [Google Scholar]
- Stratakis CA, Chrousos GP. Neuroendocrinology and pathophysiology of the stress system. Annals of the New York Academy of Sciences. 1995;771:1–18. doi: 10.1111/j.1749-6632.1995.tb44666.x. [DOI] [PubMed] [Google Scholar]
- Susman EJ, Inoff-Germain G, Nottelmann ED, Loriaux DL, Cutler GB, Chrousos GP. Hormones, emotional dispositions, and aggressive attributes in young adolescents. Child Development. 1987;58(4):1114–1134. [PubMed] [Google Scholar]
- Susman EJ. Psychobiology of persistent antisocial behavior: Stress, early vulnerabilities and the attenuation hypothesis. Neuroscience & Biobehavioral Reviews. 2006;30:376–389. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Susman EJ, Dockray S, Granger DA, Blades KT, Randazzo W, Heaton JA, Dorn LD. Cortisol and alpha amylase reactivity and timing of puberty: Vulnerabilities for antisocial behaviour in young adolescents. Psychoneuroendocrinology. 2010;35:557–569. doi: 10.1016/j.psyneuen.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susman EJ, Dorn LD, Chrousos GP. Negative affect and hormone levels in young adolescents: Concurrent and predictive perspectives. Journal of Youth and Adolescence. 1991;20:167–190. doi: 10.1007/BF01537607. [DOI] [PubMed] [Google Scholar]
- Susman EJ, Dorn LD, Inoff-Germain G, Nottelmann ED, Chrousos GP. Cortisol reactivity, distress behavior, and behavioral and psychological problems in young adolescents: A longitudinal perspective. Journal of Research on Adolescence. 1997;7:81–105. [Google Scholar]
- Susman EJ, Granger DA, Murowchick E, Ponirakis A, Worrall BK. Gonadal and adrenal hormones developmental transitions and aggressive behavior. Annals of the New York Academy of Sciences. 1996;794:18–30. doi: 10.1111/j.1749-6632.1996.tb32506.x. [DOI] [PubMed] [Google Scholar]
- Sutton JR, Coleman MJ, Casey J, Lazarus L. Androgen responses during physical exercise. British Medical Journal. 1973;1:520–522. doi: 10.1136/bmj.1.5852.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocrine Reviews. 2001;22:111–151. doi: 10.1210/edrv.22.1.0418. [DOI] [PubMed] [Google Scholar]
- Terburg D, Morgan B, van Honk J. The testosterone–cortisol ratio: A hormonal marker for proneness to social aggression. International Journal of Law and Psychiatry. 2009;32(4):216–223. doi: 10.1016/j.ijlp.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Thompson AL, Whitten PL, Lampl M. Measurement of testosterone in infant fecal samples. American Journal of Human Biology. 2011;23:820–822. doi: 10.1002/ajhb.21205. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. Journal of Psychosomatic Research. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- van der Meij L, Almela M, Hidalgo V, Villada C, IJzerman H, van Lange PA, Salvador A. Testosterone and cortisol release among Spanish soccer fans watching the 2010 World Cup final. PloS ONE. 2012;7(4):e34814. doi: 10.1371/journal.pone.0034814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Goozen SHM, Matthys W, Cohen-Kettenis PT, Buitelaar JK, Van Engeland H. Hypothalamic-pituitary-adrenal axis and autonomic nervous system activity in disruptive children and matched controls. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:1438–1445. doi: 10.1097/00004583-200011000-00019. [DOI] [PubMed] [Google Scholar]
- van Goozen SHM, Matthys W, Cohen-Kettenis PT, Gispende Wied C, Wiegant VM, van Engeland H. Salivary cortisol and cardiovascular activity during stress in oppositional-defiant disorder boys and normal controls. Biological Psychiatry. 1998;43:531–539. doi: 10.1016/S0006-3223(97)00253-9. [DOI] [PubMed] [Google Scholar]
- van Honk J, Harmon-Jones E, Morgan BE, Schutter DJLG. Socially explosive minds: The triple imbalance hypothesis of reactive aggression. Journal of Personality. 2010;78:67–94. doi: 10.1111/j.1467-6494.2009.00609.x. [DOI] [PubMed] [Google Scholar]
- van Honk J, Tuiten A, van den Hout M, Koppeschaar H, Thijssen J, de Haan E, Verbaten R. Conscious and preconscious selective attention to social threat: Different neuroendocrine response patterns. Psychoneuroendocrinology. 2000;25(6):577–591. doi: 10.1016/s0306-4530(00)00011-1. http://dx.doi.org/10.1016/S0306-4530.(00)00011-1. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Pincus SM, Mitamura R, Yano K, Suzuki N, Ito Y, Okuno A. Developmentally delimited emergence of more orderly luteinizing hormone and testosterone secretion during late prepuberty in boys. Journal of Clinical Endocrinology & Metabolism. 2001;86(1):80–89. doi: 10.1210/jc.86.1.80. [DOI] [PubMed] [Google Scholar]
- Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. Journal of Neuroendocrinology. 2002;14:506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- Volek JS, Kraemer WJ, Bush JA, Incledon T, Boetes M. Testosterone and cortisol in relationship to dietary nutrients and resistance exercise. Journal of Applied Physiology. 1997;82:49–54. doi: 10.1152/jappl.1997.82.1.49. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Culhane JF, Rauh V, Barve SS. Stress and preterm birth: Neuroendocrine, immune/inflammatory, and vascular mechanisms. Maternal and Child Health Journal. 2001;5:119–125. doi: 10.1023/a:1011353216619. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Dunkel-Schetter C, Chicz-DeMet A, Porto M, Sandman CA. Prenatal psychosocial factors and the neuroendocrine axis in human pregnancy. Psychosomatic Medicine. 1996;58:432–446. doi: 10.1097/00006842-199609000-00006. [DOI] [PubMed] [Google Scholar]
- Walker EF, Walder DJ, Reynolds F. Developmental changes in cortisol secretion in normal and at-risk youth. Development and Psychopathology. 2001;13:721–732. doi: 10.1017/s0954579401003169. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Meaney MJ. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Knable MB, O’Grady J, Orthmann J, Weickert CS. Regional specificity of brain glucocorticoid receptor mRNA alterations in subjects with schizophrenia and mood disorders. Molecular Psychiatry. 2002;7(9):985–994. 924. doi: 10.1038/sj.mp.4001139. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Does prenatal stress impair coping and regulation of hypothalamic-pituitary-adrenal axis? Neuroscience & Biobehavioral Reviews. 1997;21:1–10. doi: 10.1016/s0149-7634(96)00014-0. [DOI] [PubMed] [Google Scholar]
- Wemm S, Fanean A, Baker A, Blough ER, Mewaldt S, Bardi M. Problematic drinking and physiological responses among female college students. Alcohol. 2013;47(2):149–157. doi: 10.1016/j.alcohol.2012.12.006. http://dx.doi.org/10.1016/j.alcohol.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Wemm S, Koone T, Blough ER, Mewaldt S, Bardi M. The role of DHEA in relation to problem solving and academic performance. Biological Psychology. 2010;85(1):53–61. doi: 10.1016/j.biopsycho.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Witham EA, Meadows JD, Shojaei S, Kauffman AS, Mellon PL. Prenatal exposure to low levels of androgen accelerates female puberty onset and reproductive senescence in mice. Endocrinology. 2012;153(9):4522–4532. doi: 10.1210/en.2012-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim BO, Derksen JJL. A review on the relationship between testosterone and the interpersonal/affective facet of psychopathy. Psychiatry Research. 2012;197(3):181–198. doi: 10.1016/j.psychres.2011.08.016. http://dx.doi.org/10.1016/j.psychres.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Yoon KL, Joormann J. Stress reactivity in social anxiety disorder with and without comorbid depression. Journal of Abnormal Psychology. 2012;121:250–255. doi: 10.1037/a0025079. [DOI] [PubMed] [Google Scholar]
- Young AH, Gallagher P, Porter RJ. Elevation of the cortisol-dehydroepiandrosterone ratio in drug-free depressed patients. American Journal of Psychiatry. 2002;159:1237–1239. doi: 10.1176/appi.ajp.159.7.1237. [DOI] [PubMed] [Google Scholar]
- Young EA, Haskett RF, Murphy-Weinberg V, Watson SJ, Akil H. Loss of glucocorticoid fast feedback in depression. Archives of General Psychiatry. 1991;48(8):693–699. doi: 10.1001/archpsyc.1991.01810320017003. [DOI] [PubMed] [Google Scholar]
- Zahn-Waxler C, Shirtcliff EA, Marceau K. Disorders of childhood and adolescence: Gender and psychopathology. Annual Review of Clinical Psychology. 2008;4:275–303. doi: 10.1146/annurev.clinpsy.3.022806.091358. [DOI] [PubMed] [Google Scholar]
- Zitzmann M, Nieschlag E. Testosterone levels in healthy men and the relation to behavioural and physical characteristics: Facts and constructs. 2001. pp. 183–197. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Sensation seeking: Beyond the optimum level of arousal. Hillsdale, NJ: Erlbaum; 1979. [Google Scholar]