Abstract
The contents of memory can be updated when information from the current episode is bound with content retrieved from previous episodes. Little is known regarding factors that determine the memory content that is subject to this across-episode binding. We tested whether across-episode binding preferentially occurs for memory content that is currently “active” and identified relevant neural correlates. After studying objects at specific locations on scene backgrounds, subjects performed one of two retrieval tasks for the objects on different scene backgrounds. In an active condition, subjects recalled object locations, whereas subjects merely dragged objects to predetermined locations in a passive condition. Immediately following each object-location retrieval event, a novel face appeared on a blank screen. We hypothesized that the original episode content would be active in memory during face encoding in the active condition, but not in the passive condition (despite seeing the same content in both conditions). A ramification of the active condition would thus be preferential binding of original episode content to novel faces, with no such across-episode binding in the passive condition. Indeed, memory for faces was better when tested on the original background scenes in the active relative to passive condition, indicating that original episode content was bound with the active condition faces, whereas this occurred to a lesser extent for the passive condition faces. Likewise, early-onset negative ERP effects reflected binding of the face to the original episode content in the active but not the passive condition. In contrast, binding in the passive condition occurred only when faces were physically displayed on the original scenes during recognition testing, and a very similar early-onset negative ERP effect signaled binding in this condition. ERP correlates of binding were thus similar for across-episode and within-episode binding (and were distinct from other encoding and retrieval ERP signals in both cases), indicating that active retrieval modulated when binding occurred, not the nature of the binding process per se. These results suggest that active retrieval promotes binding of new information with contents of memory, whereas without active retrieval, these unrelated pieces of information might be bound only when they are physically paired.
Keywords: retrieval, active learning, ERP, recognition, binding, reactivation
1. Introduction
Binding arbitrarily paired items experienced together during episodes is a central process in many accounts of memory (Underwood, 1969), particularly those of relational memory processing (e.g. (Bunsey & Eichenbaum, 1996; Eichenbaum & Cohen, 2001; Olsen, Moses, Riggs, & Ryan, 2012; Preston, Shrager, Dudukovic, & Gabrieli, 2004; Prince, Daselaar, & Cabeza, 2005; Ranganath, 2010; Ryan, Althoff, Whitlow, & Cohen, 2000; Watson, Voss, Warren, Tranel, & Cohen, 2013)). Most accounts of binding consider relations among items that co-occur within a spatiotemporal episode. However, it is possible that similar binding mechanisms support updating of memory for previous episodes by linking new information with existing memory content (Bunsey & Eichenbaum, 1996; Zeithamova & Preston, 2010). Although little is known regarding specific factors that influence binding across old and new episodes, some selection factor likely influences binding, such that not all memory content is bound with information in the current episode. One important selection factor could be the extent to which memory content is currently active due to a recent retrieval event (Nader, 2003). Active retrieval, relative to passive re-exposure to memory content could be a particularly salient selection factor that determines the memory content subject to binding with new episodic information. That is, a targeted memory may be reactivated during active retrieval, but not necessarily during passive re-exposure to the same memory content. For example, we recently demonstrated that active retrieval systematically modulated the contents of memory that were currently active and bound with associatively novel information (Bridge & Voss, 2014).
At least two factors could influence which contents of memory become active: recent encoding or recent reactivation (Lewis, 1979). Binding over short time intervals may occur because recently encountered information stays active in memory for a brief period of time. Thus, as new information is encountered, it is bound to the temporally proximal information. Indeed, items presented in a temporally contiguous sequence are often better remembered later together (Schwartz, Howard, Jing, & Kahana, 2005; Sederberg, Miller, Howard, & Kahana, 2010). Moreover, neural activity reflects the reinstatement of the original temporal context (Turk-Browne, Simon, & Sederberg, 2012), suggesting that items presented close together in time are bound during learning (Briggs, Fitz, & Riccio, 2007). Retrieval may also influence what information is currently active (Briggs & Riccio, 2008). In this case, an old memory becomes active in memory, providing a means by which new information can be integrated into existing representations (Iordanova, Good, & Honey, 2011). Taken together, temporal contiguity may promote encoding of newly encountered information, whereas retrieval may promote updating of existing memories with new information. In both cases, information that is currently active in memory is bound with new information.
We hypothesized that the active engagement of retrieval modulates the extent to which binding between existing representations and new information occurs during learning (across-episode binding). This is because active retrieval could promote reactivation of old representations during encoding of new information, thereby enabling binding. Importantly, we hypothesized that binding would occur between new information that was physically present and old information that was only available in memory. On the other hand, we hypothesized that in the absence of active retrieval during learning, new information would be bound to old information only when these two pieces of information were physically paired during a subsequent test (within-episode binding). Importantly, we use binding to refer to the encoding of the association between two arbitrarily paired episodic elements, in this case a face with a specific object-location-scene context.
We manipulated the engagement of active retrieval processing just prior to encoding novel information. During the Study phase, subjects studied objects in locations on a scene background (original context scene). Then subjects completed a Refresh phase. During Active Refresh, subjects were presented with the objects and asked to actively recall the associated locations. In contrast, during Passive Refresh, subjects moved the objects to predetermined locations. Memory content was thus encountered in both the Active and Passive conditions; however, the extent to which active retrieval processes were engaged varied across conditions. Importantly, in both conditions, the old objects were presented on new scene backgrounds relative to the original study episode, and so original contextual information was not physically present. Furthermore, the same context scene background remained constant throughout each phase; however, the specific object-location information differed on each trial. Immediately following each Active and Passive trial, an unfamiliar face was shown, thus providing new information that could be bound with memory content (i.e. with the original object-location scene association). Face memory was tested in the final phase of the experiment, using either the original or new scene backgrounds. We hypothesized that faces would be preferentially bound to original memory content in the Active condition, thereby yielding better face memory relative to the Passive condition when tested with the original background.
We hypothesized that event-related potentials (ERPs) during face encoding in the Active condition would index across-episode binding between the new faces and the old, reactivated memory content. On the other hand, we hypothesized that ERPs during recognition in the Passive condition would index within-episode binding between the faces and the original memory content when they were physically paired during testing. Importantly, we were able to compare ERPs related to binding to ERPs related to other encoding and retrieval processes by comparing across the Active and Passive conditions. To the extent that similar binding processes were operative for the Active condition during encoding (across-episode binding) and for the Passive condition during recognition (within-episode binding), we expected similarities between these conditions in binding-related ERP correlates.
2. Methods
2.1 Subjects
Data were collected from 24 people (16 women; ages 18-33 years, M=23). Two subjects were excluded from all analysis due to failure to follow task instructions, leaving data from 22 subjects for analysis (21 were right handed). All subjects reported no history of neurological or psychiatric conditions and no current use of any psychoactive drugs. Written informed consent was obtained from all subjects prior to participation in accordance with the Northwestern University Institutional Review Board. Subjects were paid for their participation.
2.2 Stimuli
A set of 168 images of real-life objects was used (Brodeur, Dionne-Dostie, Montreuil, & Lepage, 2010). Each object was encapsulated by a white box with dimensions of 4.06×4.06 cm. Eight photographs depicting real-life scenes were used as the background context images (from(Hannula, Federmeier, & Cohen, 2006)). The screen resolution was 1920×1080 pixels, which occupied 52×29.25 cm on an LCD monitor. The refresh rate was 60 Hz. Each object was presented with a red dot marking its center, which could be anywhere such that the whole object was visible on the background. Thus, objects could appear anywhere within the central 46.59×22.75 cm area of the screen. A set of 392 nonfamous faces was used (half male, half female; (Althoff & Cohen, 1999)). Face dimensions during Refresh (face encoding) were 16.25×16.25 cm and face dimensions during Recognition were 4.06×4.06 cm.
2.3 Procedure
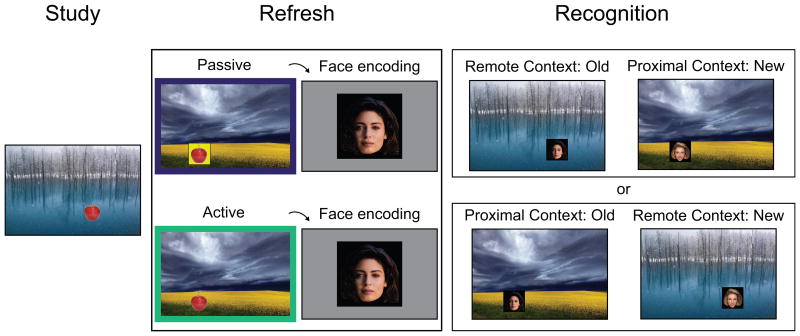
Each block comprised three phases, Study, Refresh, and Recognition (Figure 1), each separated by a two-minute distractor task. There were four blocks, two with an Active Refresh phase and two with a Passive Refresh phase. Two scene background images were used in each block (one for Remote Context and one for Proximal Context), with a total of 8 different scenes in the experiment. The distractor task separating each phase involved configuring different block-shapes as they fell from the top of the screen, with the aim of forming complete rows of the block-shapes without any empty spaces (Pfister, 2008).
Figure 1. Experiment design.
During Study, subjects viewed objects in random locations on a scene background (Remote Context). During Refresh, subjects studied centrally presented faces on a gray background. Before each face onset, subjects completed a Passive or Active reactivation task with the studied objects on a different scene background (Proximal Context). In the Passive condition (depicted in top panel), subjects moved the object to a predetermined location. In the Active condition (shown in bottom panel), subjects recalled the object's location and then moved it accordingly (recall was always inaccurate to some extent, see Methods). During Recognition, memory of the faces was tested in either the Remote Context or Proximal Context. If an old face was tested in the Remote Context, then a new face was tested in the corresponding Proximal Context (example shown in top panel). Alternatively, if an old face was tested in the Proximal Context, then a new face was tested in the corresponding Remote Context (example shown in bottom panel). Face-scene pairings were individually presented during the Recognition phase. Both possible Refresh and Recognition options are shown for each face (for illustration purposes), although only one option was used for each face in the experiment (see Methods). ERPs were time-locked to face onset during Refresh and Recognition.
2.3.1 Study
During Study, subjects viewed objects presented at random locations on a scene background image. The scene background remained on the screen throughout the Study phase as objects were individually presented at randomized locations. A spatial location and scene background combination was thus uniquely tied to each object. We collectively refer to the object-location and Study background scene as the Remote Context (because it was remote relative to face encoding).
There were 42 study trials per block. Subjects were instructed to try to remember each objectlocation, as they would be given a test on the object locations later during Refresh. A 1000 ms fixation cross preceded each study trial. Then, an object appeared in a random location on the Remote Context scene for 3000 ms.
2.3.2 Refresh
In the second phase of the experiment, subjects completed object Refresh and face encoding. For each trial, subjects were prompted to move an object to a new location. A different scene provided the background during object-location Refresh. We collectively refer to the new object location and the Refresh background scene as the Proximal Context (because it was proximal to face encoding). Immediately following each object Refresh trial, a new face appeared on a neutral gray background. Each face was presented immediately following an object-location reactivation event; however, the extent to which active retrieval processing was engaged prior to face encoding differed as a function of Refresh condition (described below).
A jittered fixation cross (500-1000 ms) preceded each object Refresh trial. Then, a centrally presented object (from the preceding Study phase) appeared on the screen and subjects were prompted to move the object to a new location using a mouse. After an object was placed, it remained in that location for 800 ms, ending the trial. Immediately following the end of the object Refresh trial, a gray background appeared with a central fixation cross for 800 ms. Then, a centrally-presented face appeared for 3000 ms on the neutral background. For each face, subjects made a button press to indicate whether they would cast the person pictured in a “comedy” or “drama” production. This face categorization task was used to boost encoding of the faces. Different objects, scenes, and faces were randomly assigned to the Active or Passive Refresh condition.
2.3.2.1 Active Refresh
During Active Refresh, subjects were instructed to recall each object's associated location on the Proximal Context background scene. Although they were not explicitly told to recall the objects' associated background scene, it is likely that the Remote Context scene was reactivated during this time in order to help guide subjects' object-location recall. Subjects dragged the object from the center of the screen to its associated location then pressed a button. The precise placement of the object (the recalled location) always diverged from the location at Study to some extent (M=10.05 cm, SE=0.64) due to forgetting. Divergence distances (i.e., placement error) for the objects were used to generate the location of objects during the Passive Refresh condition (see also (Bridge & Voss, 2014)), such that divergence distances were matched within-subjects for the Active and Passive conditions.
2.3.2.2 Passive Refresh
During Passive Refresh, subjects were prompted to move each object from the center of the screen to a predetermined location on the Proximal Context background scene. Because subjects were not required to recall the objects' associated locations, it is likely that reactivation of the original object-location-scene episode (Remote Context) was reduced during Passive relative to Active Refresh. The centrally presented object was accompanied by the presentation of a yellow target box in a predetermined location and subjects moved the object to the box and pressed a button. Placement within 0.5 cm of the center point of the yellow box was required for the response to be accepted. After the response was accepted, the object moved to the center of the yellow box until the end of the trial. Subjects were told that the yellow box would be located in the original study location for some of the trials and located in a novel location on other trials, analogous to ranging levels of recall accuracy in the Active condition. The mean divergence distance for the Passive Object-Refresh condition was (M=10.06 cm, SE=0.64), which did not differ from the Active divergence distances [t(21)=.26, ns]. Because we used a matching scheme to generate the Passive divergence distances, the first block was always with Active Refresh and the last block was always with Passive Refresh. The order (Active or Passive) of the two intermediate blocks was counterbalanced across subjects.
2.3.3 Recognition
During each face Recognition test block, subjects saw 42 old and 42 new faces and indicated whether or not they remembered seeing each face. Half of the old and new faces were tested in the Remote Context and the other half were tested in the Proximal Context. That is, faces were tested in either the original object-location and scene background (Remote Context) or they were tested in the newer Refresh object-location and corresponding scene background (Proximal Context). Thus, the test face was directly tied to either the original object-location-scene or the newer Refresh object-location-scene association.
A fixation cross superimposed on the upcoming Recognition trial scene background preceded each face Recognition trial. The fixation cross was presented in the center of the screen for 1000 ms. Then, in order to orient attention to the location of the face for the upcoming Recognition trial, the fixation cross flashed once (150 ms on, 150 ms off) in either the Remote Context or Proximal Context location while the background scene remained constantly visible. The fixation cross remained in that location for an additional 800 ms. A face then appeared in the Remote Context or Proximal Context location for 1000 ms. Then, text appeared at the bottom of the screen, prompting the subjects to indicate if the face was “old” or “new” by clicking a button on the mouse. After a response was made, the text changed, prompting the subjects to press a button indicating their level of confidence in their recognition decision, either “low”, “medium”, or “high”. After recognition memory confidence was indicated, the trial ended.
2.4 Behavioral analysis
Hit rates were calculated as the percentage of correctly endorsed old faces for each Refresh condition (Active vs. Passive), test context (Proximal vs. Remote), and confidence level (low, medium, high). False alarm rates were determined for each condition for new faces incorrectly endorsed as “old.” Normalized hit and false alarm rates were used to calculate d′ scores for each condition, response confidence level, and subject. In order to correct for any extreme hit and false alarm values (i.e. 1 or 0), we computed all d′ values according to the procedure suggested by (Snodgrass & Corwin, 1988).
2.5 EEG acquisition and ERP analysis
Continuous EEG was recorded during the Refresh and Recognition phases using Ag/AgCl active electrodes (actiCAP, Brain Vision LLC). Recordings were made from 30 scalp locations (bandwidth DC to 20,000 HZ, sampling rate 1000 Hz). EEG signals were amplified and digitized online. Recordings were made referenced to right mastoid, and referenced offline to averaged mastoids. Electrooculography (EOG) recordings were also made using two bipolar electrodes at left and right outer canthi and two bipolar electrodes situated above and below the left eye to monitor eye movements and blinks. A high-pass filter (0.1 Hz cutoff, 12dB per octave roll-off) was applied prior to analysis.
Event-related potentials (ERPs) were time-locked to the face onset in the Refresh and Recognition phases. Epochs lasted 1200 ms, beginning 200 ms before stimulus onset, with baseline correction using the prestimulus interval. Trials with ocular artifacts were rejected as indicated by large voltage offsets within 200 ms moving windows. An absolute voltage threshold was also applied to the scalp electrodes to identify and reject noisy trials due to massive head movements and muscle tension. Exceptionally noisy channels were spatially interpolated (ranging from 0-1 channels per subject).
We used ERPLAB for all preprocessing steps and ERP analysis (Lopez-Calderon & Luck, 2014). We examined mean amplitude during a priori time windows to determine differences due to memory across conditions. Early negative potentials beginning ∼150 ms are often elicited during visual processing of faces (e.g. (Rossion & Jacques, 2011)); therefore we analyzed ERPs beginning at 150 ms after face onset, in order to determine if Refresh condition influenced early face processing (between 150-400 ms). Based on other ERP face recognition studies (Yovel & Paller, 2004), we also chose to examine differences during mid (400-600 ms) and late (600-800 ms) time intervals during encoding as a function of subsequent memory, and between 500-700 ms during Recognition as a function of face memory.
For these targeted analyses, we formed five regionally defined clusters of electrodes distributed across the scalp. We assessed midline effects by collapsing data from frontal (FP1, FP2, F3, F4, FZ), central (FC1, FC2, C3, C4, CZ), and parietal sites (CP1, CP2, P3, P4, PZ). We assessed lateral effects by collapsing data from left (F7, FT9, FC5, T7, CP5, P7) and right sites (F8, FT10, FC6, T8, CP6, P8). We report significant differences only with respect to memory performance. Error probability was adjusted using the Greenhouse-Geisser correction to account for violations of sphericity (denoted GG when applied to analyses in text).
During face encoding, we compared ERPs corresponding to faces that were subsequently remembered on the recognition test to ERPs corresponding to faces that were subsequently forgotten,termed subsequent memory effects or “Dm,” for “Differences due to later memory” (Paller, Kutas, & Mayes, 1987; Paller, McCarthy, & Wood, 1988; Paller & Wagner, 2002). Subsequent memory effects were examined irrespective of later test context. During Recognition, we compared ERPs corresponding to correctly identified old faces (hits) to ERPs corresponding to correctly identified new faces (correct rejections). We refer to significant differences between correctly identified old faces and correctly identified new faces as “ERP old/new effects”. We examined ERP old/new effects separately for each test context.
Subjects with fewer than 10 trials in a critical condition were excluded from the corresponding ERP analysis. Data from 17 subjects were included in the Dm analysis, and data from 20 subjects were included in the context-specific Recognition memory analysis. Targeted follow-up analyses were also performed to compare among effects individually identified during Refresh versus Recognition, and these analyses included only subjects that contributed to both effects (i.e., so that comparisons among phases utilized within-subjects tests). See Table 1 for the mean number of ERP trials per condition.
Table 1.
ERP trial counts.
| Active | Passive | |||
|---|---|---|---|---|
| Hits | Misses | Hits | Misses | |
| Dm (all contexts) | 44.59 (27-63) | 20.18 (10-34) | 46.82 (25-60) | 20.29 (11-30) |
| Hits | CRs | Hits | CRs | |
| Recognition: Remote Context |
24 (16-33) | 25.4 (13-34) | 23 (14-34) | 22.3 (12-32) |
| Recognition: Proximal Context |
25.3 (14-37) | 25.7 (14-33) | 24.15 (13-34 | 22.9 (14-31) |
Note. Mean trial numbers with range of trials in parentheses.
3. Results
3.1 Behavioral Results
3.1.1 Effects of Active versus Passive Refresh on Recognition sensitivity
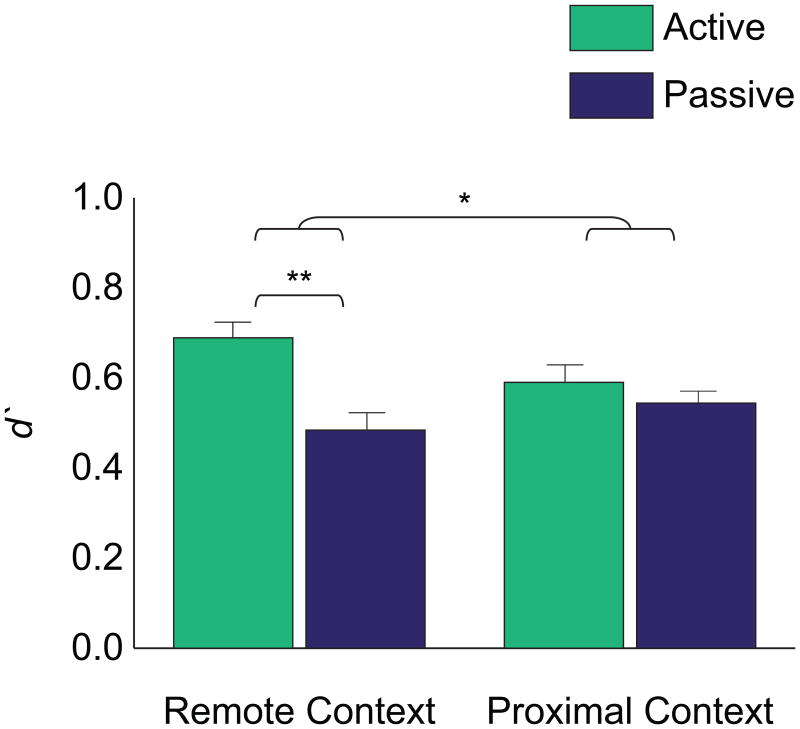
We hypothesized that active retrieval would facilitate binding of newly encountered information with memory content that was currently active; therefore, we expected that Active Refresh would selectively facilitate face recognition memory for the Remote Context. To test this hypothesis, we examined recognition memory performance by computing ď as a function of Refresh condition (Active, Passive), test context (Remote Context, Proximal Context), and confidence level (low, medium, high) (Table 2). There was a significant main effect of Refresh condition, indicating that d′ was significantly higher for the Active (M=.64, SE=.06) relative to the Passive (M=.51, SE=.07) condition [F(1,21)=5.88, p<.03]. This main effect was qualified by an interaction of Refresh condition and test context [F(1,21)=5.17, p<.04] (Figure 2). For recognition in the Remote context, d′ was significantly higher for the Active (M=.69, SE=.06) relative to the Passive condition (M=.48, SE=.08) [t(21)=3.12, p<.006]. On the other hand, for recognition in the Proximal Context, d′ did not differ for the Active (M=.59, SE=.07) and Passive (M=.54, SE=.06) conditions [t(21)=.78, ns]. Within each Refresh condition, d′ did not differ significantly for test contexts [Active: t(21)=1.71, ns; Passive: t(21)=1.31, ns].
Table 2.
Hits and false alarms as a function of Refresh condition, test context, and confidence level.
| Active | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| High confidence | Medium confidence | Low confidence | |||||||
| Hits | FA | d′ | Hits | FA | d′ | Hits | FA | d′ | |
| Remote Context | .37(.03) | .04(.01) | 1.45(.11) | .22(.02) | .11(.02) | .51(.10) | .13(.01) | .11(.01) | .10(.07) |
| Proximal Context | .37(.03) | .06(.01) | 1.29(.12) | .22(.02) | .13(.02) | .36(.10) | .13(.02) | .09(.01) | .12(.10) |
| Passive | |||||||||
| High confidence | Medium confidence | Low confidence | |||||||
| Hits | FA | d′ | Hits | FA | d′ | Hits | FA | d′ | |
| Remote Context |
.37(.04) | .09(.02) | 1.08(.11) | .21(.02) | .12(.02) | .39(.11) | .13(.02) | .13(.02) | -.01(.10) |
| Proximal Context |
.37(.03) | .08(.02) | 1.17(.10) | .21(.02) | .11(.02) | .44(.09) | .12(.01) | .13(.02) | .02(.08) |
Note. Means with standard error in parentheses.
Figure 2. Face recognition memory performance for the Active and Passive conditions subdivided by testing context.
d′ collapsed across confidence level; plotted as a function of Refresh condition and test context. **p<.01; *p<.05.
There was also a marginally significant interaction of confidence level and Refresh condition [F(1.99, 41.87)=3.13, p=.054GG] (Table 2). For high confidence responses, d′ was significantly higher for the Active relative to the Passive condition [t(21)=3.55, p<.002]. Recognition d′ did not differ for Active and Passive conditions for either medium [t(21)=.30, ns] or low confidence responses [t(21)=1.39, ns]. Unsurprisingly, there was a main effect of confidence on d′ F(1.93,40.48)=128.51, p<.001GG]. Recognition d′ was significantly greater for high confidence (M=1.25, SE=.09) relative to medium confidence (M=.43, SE=.08) [t(21)=10.06, p<.001] and low confidence (M=.06, SE=.06) responses [t(21)=15.53, p<.001]. Recognition d′ was also greater for medium confidence responses compared to low confidence response [t(21)=5.31, p<.001].
3.1.2 Effects of Active versus Passive Refresh on Hits and False Alarms
We next examined raw recognition performance as a function of Refresh condition, test context, and confidence level for hits and false alarms separately (Table 2). We subjected hit rates to a repeated-measures ANOVA with Refresh condition, test context, and confidence level as factors. No effects involving Refresh condition were significant on hit rate. Unsurprisingly, there was a main effect of confidence on hit rates [F(1.49,31.25)=30.81, p<.0001GG], given that high confidence hit rate was greater relative to medium [t(21)=4.10, p<.001] and low confidence hit rates [t(21)=7.79, p<.0001]. Additionally, hit rate was greater for medium relative to low confidence responses [t(21)=3.80, p<.01]. No other effects on hit rate were significant.
We also examined false alarms with a repeated-measures ANOVA with Refresh condition, test context, and confidence level as factors. The main effect of Refresh condition was significant [F(1,21)=5.70, p<.03], indicating that false alarm rate was significantly higher in the Passive condition (M=.11, SE=.01) relative to the Active condition (M=.09, SE=.01). The main effect of confidence was also significant, in that false alarm rate was lower for high relative to medium [t(21)=2.95, p<.01] and low confidence responses [t(21)=2.23, p<.04]. False alarm rate did not differ between medium and low confidence responses [t(21)=.12, ns]. No other effects on false alarm rate reached significance (Fs<2.68).
In summary, these behavioral effects show that overall face memory was enhanced when encoding followed Active rather than Passive object-location Refresh. Interestingly, this memory enhancement was pronounced when faces were tested in the original object-location context, despite the fact that these faces were never studied at these locations when first encountered (i.e., presentation was central when faces were encoded). This suggests that Active retrieval selectively facilitated binding between existing memory traces and new, unrelated face information. These effects were driven by the higher false alarm rate in the Passive relative to the Active condition, indicating overall weaker face memory in the Passive condition. It is possible that the familiarity of the context scenes made face memory decisions more challenging in the Passive condition, due to weaker face-context binding relative to the Active condition. That is, the highly familiar scenes could have caused subjects to endorse new faces as old more frequently because binding between the individual faces and scenes was overall weaker in the Passive condition and did not override this influence of context familiarity on face endorsement, as it might have in the Active condition. We next sought to determine when binding between the faces and the Remote Context occurred in each condition by examining ERPs time-locked to face onset during encoding and Recognition.
3.2 ERP Results
3.2.1 Overview of ERP comparisons and predictions
To identify ERP correlates of face-context binding, we examined Dm effects for the Active and Passive Refresh conditions. Critically, we hypothesized that Refresh condition would modulate the extent to which the original object-location context (Remote Context) was bound to the faces during encoding. We predicted that across-episode binding would be enhanced in the Active condition, because the Remote Context was active in memory during face encoding (Figure 3). We expected that this binding-related effect would be distinct from typical encoding effects, which we determined by comparing Dm effects for the Active versus the Passive Refresh conditions. On the other hand, we expected that reactivation of the Remote Context was minimal during Passive Refresh; thus, we hypothesized that binding between the face and the Remote Context would not occur until they were physically paired later in the experiment during Recognition (within-episode binding). Accordingly, we predicted that old/new ERP effects from Recognition in the Remote Context would reflect binding between the face and the original context in the Passive condition.
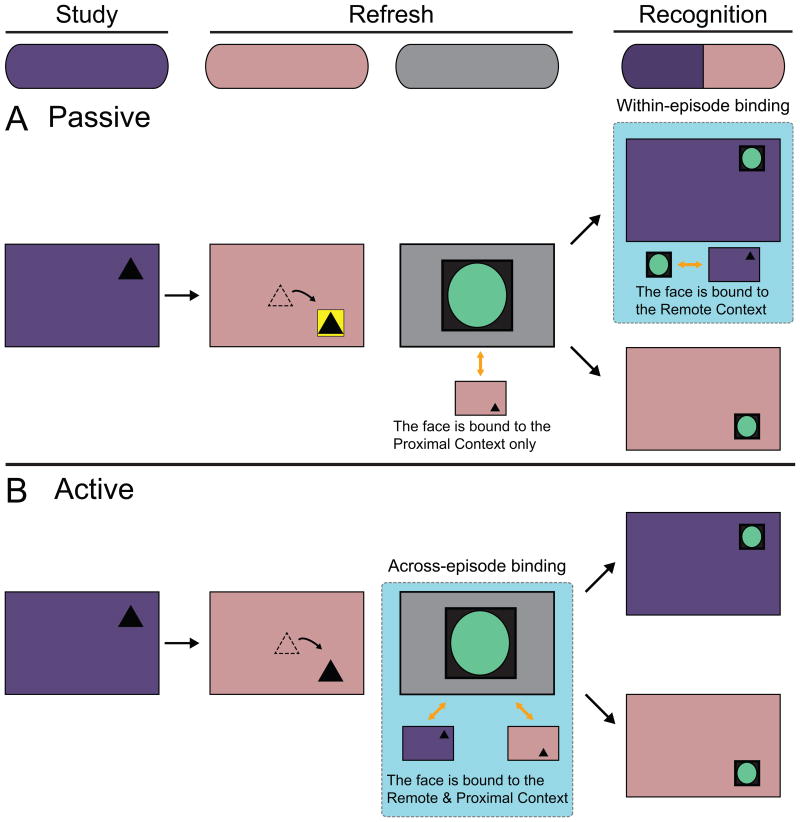
Figure 3. Hypothesized ERP effects related to across- and within-episode binding.
We predicted that faces would be bound to the Remote Context during face encoding in the Active condition (across-episode binding), and during face recognition in the Passive condition (within-episode binding). We expected that faces would always be bound to the Proximal Context during face encoding, because it was active in memory due to temporal proximity. Orange bidirectional arrows between the faces and contexts depict hypothesized binding. The teal backgrounds denote binding between the faces and the Remote Context. Purple boxes represent the Remote Context scene, red boxes represent the Proximal Context scene, and the black triangles represent the objects and their locations. (A) In the Passive condition, we did not expect binding to occur during face encoding, because the Remote Context was not active in memory during Refresh. Instead, we predicted that faces would be bound to the Remote Context during Recognition, when the faces and contexts were physically presented together (within-episode binding). We expected that Remote Context-specific ERPs during Recognition would reflect binding when faces were successfully recognized. (B) In the Active condition, we predicted that binding between the faces and the Remote Context would occur during face encoding because the Remote Context was active in memory during Refresh via reactivation (across-episode binding). We hypothesized that subsequent-memory ERPs would reflect binding between the faces and the Remote Context during encoding.
Finally, we predicted that binding between the faces and the Proximal Context would always occur during Refresh (irrespective of condition). Because information within the surrounding temporal context is likely bound during encoding (Sederberg, et al., 2010), we expected that these binding-related effects would elicit ERPs consistent with other encoding processes (i.e., not related to the binding that is the focus of this study) that are frequently identified in ERP studies of memory encoding. We therefore hypothesized that these temporal proximity-based binding effects would be distinct from the across- and within-episode binding effects described above. Specifically, whereas we expected that temporal proximity-based binding effects would resemble typical Dm effects, we hypothesized that across- and within-episode binding effects would elicit unique ERPs, because this is a distinct form of binding.
3.2.2 Face encoding ERPs
3.2.2.1 Active Refresh
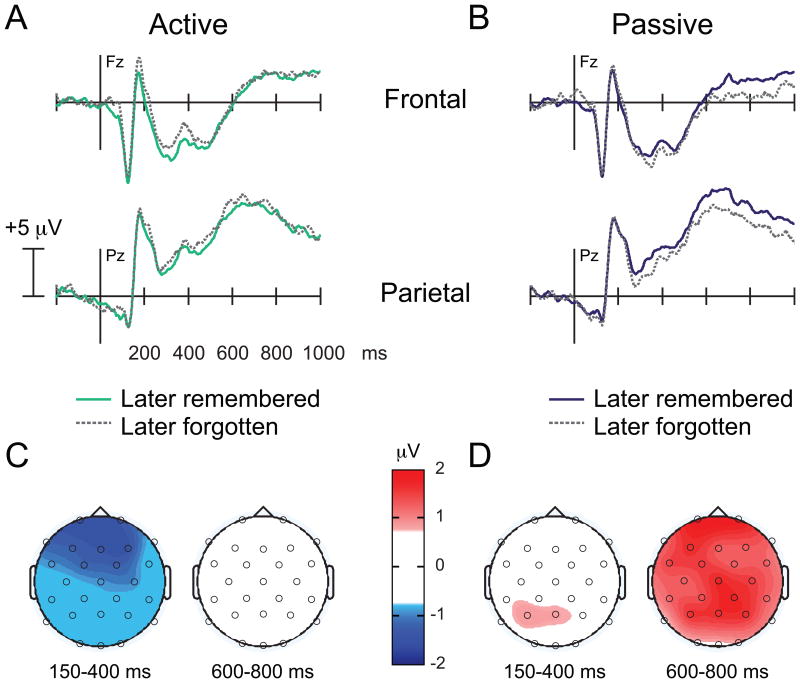
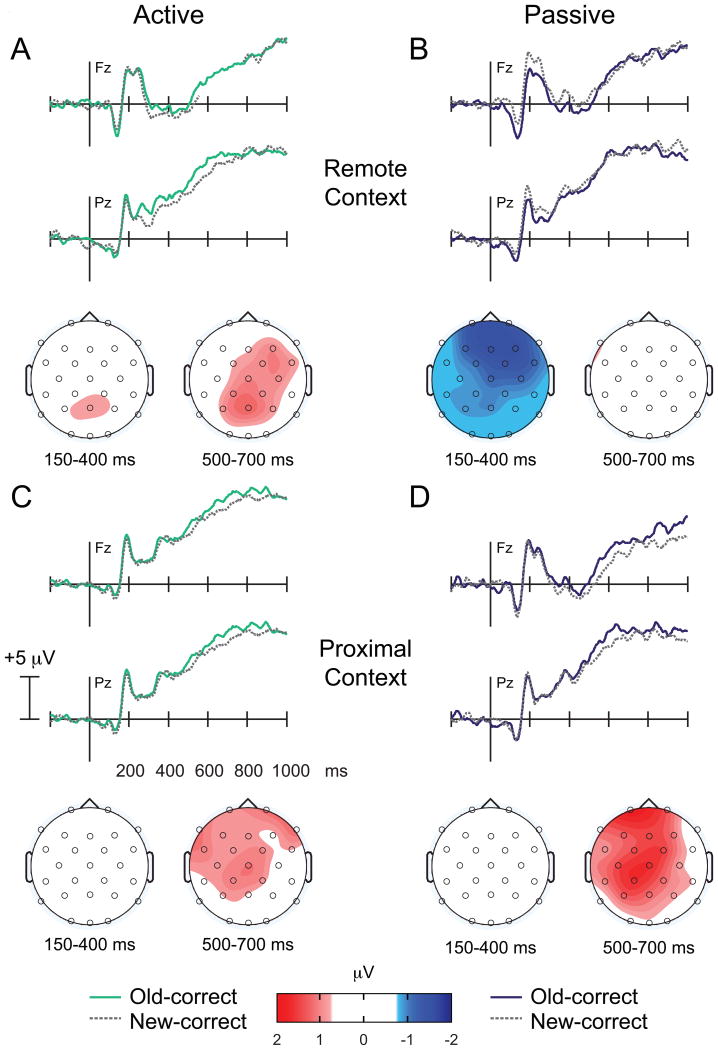
Visual inspection of the Active Refresh waveforms (Figure 4A) indicated an early Dm effect beginning as early as 150 ms at frontal sites and appearing to last until ∼400-500 ms. An interaction of region and accuracy [F(2.79,44.57)=3.18, p<.05GG] between 150-400 ms, indicated that mean amplitudes corresponding to subsequent hits were significantly more negative relative to mean amplitudes corresponding to subsequent misses at frontal [t(16)=3.32, p<.005] and central electrode sites [t(16)=2.15, p<.05]. Mean amplitudes did not differ during this early time interval at any other region (ts<1.49). Although there was a trend, the main effect of face accuracy was not significant during this early time interval [F(1,16)=4.44, p<.06]. No significant Dm effects were apparent during the 400-600 ms interval (Fs<2.72) or the 600-800 ms interval (Fs<.90). These results indicate that early negative differences at frontocentral electrode sites predicted subsequent memory in the Active condition. Late positive differences typically observed during encoding in other settings (e.g., (Rugg & Curran, 2007)) were absent in this contrast. These results suggest that a novel binding mechanism may be operative during face encoding following Active Refresh to promote subsequent memory.
Figure 4. Dm effects.
ERPs time-locked to faces during encoding as a function of subsequent memory. (A-B) Dark solid lines depict later remembered faces; dashed grey lines depict later forgotten faces for the (A) Active and (B) Passive condition. (C-D) Topographic maps depict the difference between later remembered and later forgotten faces for the (C) Active and (D) Passive condition. Whereas negative differences between 150-400 ms predicted subsequent memory of the faces in the Active condition, positive differences between 600-800 ms predicted subsequent memory of the faces in the Passive condition.
3.2.2.2 Passive Refresh
Visual inspection of the Passive Refresh waveforms indicated late positive differencesas a function of subsequent face memory (Figure 4B). Indeed, no differences were observed as a function of subsequent memory between 150-400 ms (Fs<2.00), or 400-600 ms (Fs<2.90). However,during the late time interval (600-800 ms), mean amplitudes corresponding to subsequent hits were significantly more positive relative to mean amplitudes corresponding to subsequent misses [F(1,16)=8.53, p<.01]. The interaction with electrode site was not significant [F(1.93,30.87)=.80, nsGG]. These Dm effects are consistent with other ERP studies generally (Rugg & Curran, 2007), and specifically with those examining subsequent memory effects for faces (Yovel & Paller, 2004), suggesting that ERPs predictive of successful face memory were apparently unaffected by the preceding Passive Refresh task.
3.2.2.3 Active versus Passive Dm effects
We compared the Active and Passive Dm effects to test our prediction that different processes were operative during face encoding across Refresh conditions. To do this, we first calculated difference waves (later-remembered minus later-forgotten faces) during the time intervals with reliable differences for each condition, and we used the regional clusters used for the targeted analysis above. We thus compared the Active Dm effect (150-400 ms) (Figure 4C left panel) to the Passive Dm effect (600-800 ms) (Figure 4D right panel). A main effect of Refresh condition indicated that mean differences predictive of later memory were significantly more positive in the Passive relative to the Active condition [F(1,16)=11.10, p<.005]. There was also a nonsignificant interaction of Refresh condition with region [F(2.34,37.39)=2.89, p<.07GG]. Dm effects differed significantly between the Refresh conditions at all regional sites (ts>2.60, ps<.02). These results suggest that distinct processes were operative during encoding that predicted later memory of the faces as a function of Refresh condition. We hypothesize that differences across conditions during face encoding arose because faces were selectively bound to the Remote Context in the Active condition (Figure 3B).
3.2.3 Face recognition old/new ERPs
We reasoned that context-specific ERPs during Recognition would indicate whether or not face-context binding occurred earlier in the experiment during face encoding. Specifically, we hypothesized that ERPs corresponding to successful recognition of the faces in the Remote Context would reflect face-context binding for the Passive condition only (Figure 3A). For all other contrasts, (i.e. Passive recognition in the Proximal Context, and Active recognition in the Remote Context and Proximal Context) we expected that ERP old/new effects would simply reflect successful retrieval (as binding presumably occurred previously for these conditions). To test this hypothesis, we examined mean amplitudes for each test context and for each condition.
3.2.3.1 Active: Remote Context old/new ERP effects
We did not observe any significant old/new effects for the Active condition between 150-400 ms (Fs<1.35). Between 500-700 ms, there was a nonsignificant interaction with region in the Remote Context [F(2.55,48.40)=2.42, p<.09GG]. Mean amplitudes corresponding to old-correct faces tended to be more positive at parietal sites relative to mean amplitudes corresponding to new-correct faces [t(19)=2.05, p<.055] (Figure 5A).
Figure 5. Context-dependent old/new ERP effects.
Mean amplitudes during Recognition plotted as a function of test context. (A-B) Remote Context old/new effects for the (A) Active and (B) Passive conditions. (C-D) Proximal Context old/new effects for the (C) Active and (D) Passive conditions. Early negative old/new differences for evident only for the Passive condition in the Remote Context, suggesting that binding between the face and context occurred at this time. On the other hand, late positivity effects reflected successful recognition memory for the Passive condition in Proximal Context and for the Active condition in both test contexts, suggesting that successful retrieval of the existing face-context representations occurred for these conditions.
3.2.3.2 Active: Proximal Context old/new ERP effects
Similar to the Active Recognition results for the Remote Context, we did not observe any significant old/new effects between 150-400 ms (Fs<1.04). Between 500-700 ms, there was a main effect of face condition, such that mean amplitudes corresponding to old-correct faces were significantly more positive relative to mean amplitudes corresponding to new-correct faces [F(1,19)=6.61, p<.02] (Figure 5C).
Therefore, no early negative old/new effects were observed during Recognition in the Active condition, irrespective of test context. On the other hand, late positive old/new effects were apparent in the Active condition marginally in the Remote Context and significantly in the Proximal Context (Figure 5A and 5C), suggesting that this late positivity reflects successful retrieval of the bound face-context associations.
3.2.3.3 Passive: Remote Context old/new ERP effects
Consistent with our hypothesis that face-context binding occurred selectively for the Passive condition in the Remote Context, a main effect of face condition between 150-400 ms indicated that mean amplitudes corresponding to old-correct faces were significantly more negative relative to mean amplitudes corresponding to new-correct faces [F(1,19)=6.33, p<.03] (Figure 5B). The interaction of face condition and region was not significant [F(2.75,52.27)=1.95,ns]. During the later time interval, no old/new effects were apparent (Fs<1.53).
3.2.3.4 Passive: Proximal Context old/new ERP effects
Between 150-400 ms, there was a significant interaction of face condition with region [F(2.70,50.34)=3.17, p<.05GG]. However, old/new effects were not significant at any region during this time interval (ts<1.39). Between 500-700 ms, there was a significant effect of face condition, indicating that mean amplitudes corresponding to old-correct faces were significantly more positive relative to mean amplitudes corresponding to new correct faces [F(1,19)=4.86, p<.05] (Figure 5D). The interaction of face condition with region was also significant [F(2.45,50.29)=3.16, p<.05GG], such that old/new effects were significant at frontal [t(19)=2.11, p<.05], central [t(19)=2.40, p<.03], and parietal sites [t(19)=2.53, p<.03], but not at lateral sites (ts<1.70). Thus, unlike recognition in the Remote Context, successful recognition in the Proximal Context corresponded to late positive effects, similar to the Active old/new effects in either test context.
These results indicate that early negative effects in the Passive condition were context-specific, given that they were pronounced during recognition in the Remote Context, but not the Proximal Context. Conversely, late positive differences were apparent during recognition in the Proximal Context, whereas they were absent in the Remote Context. We hypothesize that these early negative old/new effects unique to Passive Recognition in the Remote Context reflected successful face-context binding, given that similar ERP effects were also identified for the Active Dm analysis when face-context binding was thought to occur.
3.2.4 Active versus Passive binding effects
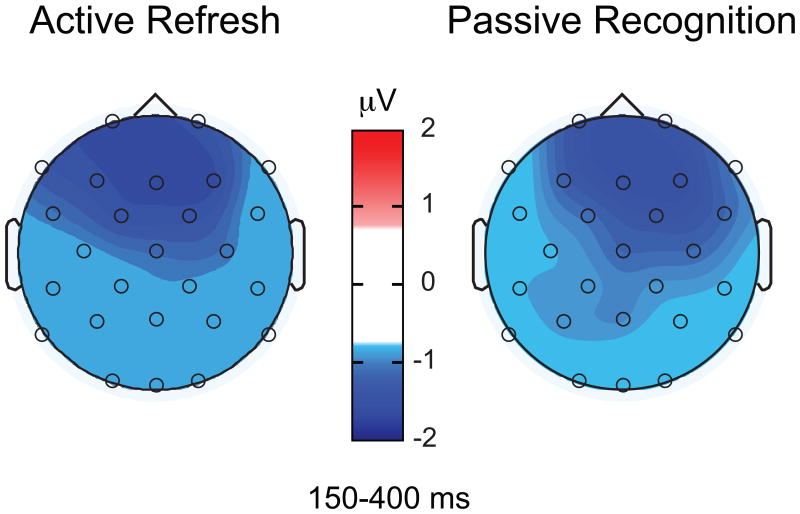
Whereas binding between the faces and the Remote Context occurred during encoding intheActive condition, binding between the faces and the Remote Context occurred during Recognition inthe Passive condition. Interestingly, ERPs corresponding to these hypothesized instances of across-and within-episode binding were remarkably similar in timing, directionality, and topography (Figure 6). Both binding-related effects occurred at similar latencies; between 150-400 ms during encoding in the Active condition and between 150-400 ms during Recognition in the Passive condition. Furthermore, the directionality of both binding effects was negative, which is unusual given that both subsequent memory and Recognition memory effects typically involve enhanced positivity (Rugg & Curran, 2007; Yovel & Paller, 2004). Finally, both were frontocentrally distributed. Given these similarities, we aimed to determine if the amplitudes and spatial topographies of these two binding-related ERP effects statistically differed. (Data from 15 subjects were included in this comparison, such that all included subjects contributed data for both conditions; see Methods.)
Figure 6. Binding-related spatial topography.
(A) Mean amplitude differences between 150-400 ms corresponding to the Active Dm effect (during encoding). (B) Mean amplitude differences between 150-400 ms corresponding to the Passive old/new effect in the Remote Context (during Recognition). Spatial topographies looked remarkably similar across the two contrasts, supporting the notion that early negative differences reflect face-context binding.
First, we determined if mean amplitudes corresponding to binding in the Active andPassive conditions differed by comparing the difference waves corresponding to the Active Dm effectand the Passive old/new effect in the Remote Context. A repeated measures ANOVA with binding condition (Active Refresh, Passive Recognition) and region (frontal, central, parietal, left lateral, right lateral) as factors did not reveal any significant effects (Fs<.74). Thus, the negative amplitudes corresponding to binding during Active Refresh and Passive Recognition did not statistically differ. Next, we compared spatial topographies of these two binding-related effects to determine if the spatial distribution of the negative ERP effects differed across conditions. For this comparison, we normalized the difference waves corresponding to the Dm effect in the Active condition and the old/new effect in the Remote Context for the Passive condition according to the procedure recommended by (McCarthy & Wood, 1985). A repeated measures ANOVA with binding condition (Active Refresh, Passive Recognition) and location (30 scalp electrodes) as factors did not reveal a significant interaction [F(3.98,55.78)=.70, ns], indicating that the spatial topographies did not differ across Refresh conditions.
To further interrogate potential topographical differences for these two binding effects, we performed an additional analysis using data collapsed for the five electrode regionstested in the amplitude analysis. We averaged the difference amplitudes across electrodes for eachof the 5 regional sites and then normalized the amplitudes across conditions (McCarthy & Wood, 1985). Normalized values were submitted to a repeated-measures ANOVA with region and binding condition (Active Refresh, Passive Recognition) as factors. The interaction of region and binding condition was not significant [F(2.26,31.61)=.10, ns]. Therefore, even after limiting the topographical analysis to the electrode sites used for the analyses of amplitude effects, we did not observe significant differences in the spatial distributions of the binding-related ERP effects.
These results are surprising, given that these ERP effects corresponded to different portions of the experiment, with distinct visual stimuli, and while subjects were engaged in different tasks. Although it is difficult to interpret lack of statistically significant differences, these results are consistent with the hypothesis that binding between the faces and the Remote Context occurred during Active Refresh, whereas binding between the faces and the Remote Context occurred during Passive Recognition. It is important to note, however, that the Active Dm effect appeared to be more frontocentrally located (indicated by an interaction of Region with face accuracy in the amplitude analysis), whereas the Passive Recognition effect was more broadly dispersed across the scalp (indicated by a main effect of face condition in the amplitude analysis). Despite these apparent spatial differences, the topographies did not statistically differ. In any case, the ERP correlates of binding across these two conditions were highly similar and atypical for ERP Dm and old/new effects during recognition memory with faces. Taken together, early negative differences corresponding to face-context memory may be a novel ERP index of binding.
4.1 Discussion
Active retrieval immediately prior to learning promoted across-episode binding duringface encoding. In contrast, in the Passive condition (when active retrieval did not precede new learning), within-episode binding occurred later during Recognition, when information was physically paired. These across- and within-episode binding effects were marked by similar early negative ERPs that were strongly distinct from ERP correlates of encoding and retrieval (as identified here and in previous ERP memory studies). In the Active condition, early negative ERPs corresponded to across-episode binding during successful face encoding (predictive of subsequent face memory), whereas similar early negative ERPs corresponded to within-episode binding in the Passive condition during successful face recognition in the Remote Context. These behavioral and ERP findings are consistent with the hypothesis that active retrieval facilitated reactivation of the original memory content, thereby causing across-episode binding with the subsequently presented face. In contrast, because original memory content was not reliably activated in the Passive condition, binding did not occur until the faces were physically paired with the contextual information from the original episode during the Recognition test (within-episode binding). These findings provide a novel neural marker of episodic memory binding, and show that active retrieval modulates the contents of memory that are currently active and available for binding. These results build on our previous work (Bridge & Voss, 2014), further supporting the notion that memory traces that are currently active in memory are preferentially bound with relatively novel information.
We hypothesize that the early negative ERP effects observed during Active encoding andPassive Recognition in the Remote Context specifically reflected binding currently activememorytraces and associatively novel information. These binding effects may be distinct from effectsofbinding new pieces of information encountered together in a similar spatiotemporal context. For instance, studies have indicated that information encountered within the same temporal context is bound during encoding (Sederberg, et al., 2010). It is thus plausible that typical late positive Dm ERP effects (Paller & Wagner, 2002; Voss & Paller, 2008) correspond not only to subsequent memory of the current item but also to memory of the items encoded in the surrounding temporal context. Indeed, we observed late positive Dm effects in the Passive condition, which likely corresponded to binding between the faces and the proximal context information (the Proximal Context). Future studies should investigate if ERPs during item encoding correspond not only to subsequent memory of the current item, but also to items encoded in the surrounding temporal context to determine if late positive ERPs during encoding reflect memory of rich temporal context information in addition to item-specific information. In any case, the unique early negative Dm effect that we identified was very distinct from standard Dm effects, suggesting that binding of existing memory content with novel information is likely a distinct process from binding that occurs in standard recognition memory paradigms that involve only novel information.
Based on the Refresh phase alone, one could conclude that the negative-going ERPs in the Active condition reflected the amount of information being bound rather than across-episode binding per se, as two contexts were bound to the faces during Active Refresh but only one was bound to faces during Passive Refresh. However, during the Recognition phase, we observed these negative-going ERPs for Passive Recognition in the Remote Context, when the faces were bound to only one context scene. If these negative-going ERPs reflect the amount of information being bound, then the Passive Dm ERPs should be identical to the Passive Recognition old/new ERPs in the Remote Context, because in both cases the faces are being bound to only one scene (i.e. the amount of information being bound is the same). Instead, the negative-going ERPs are only observed for the Active Refresh Dm effect and the Passive Recognition old/new effect for the Remote Context; therefore, we infer that these early negative ERPs reflect binding between currently active memory information and associatively novel information (across- and within-episode binding), and that they are unrelated to the amount of information being bound (as this differed for the two effects).
Several studies have suggested that memory reactivation promotes integrationof new information into related memory traces during learning (Shohamy & Wagner, 2008; Zeithamova, Dominick, &Preston, 2012; Zeithamova & Preston, 2010). In these studies, subjects learned pairs of items (e.g. A-B) and then learned overlapping pairs, in which old items were paired with new items (e.g. B-C). In a final generalization test, subjects judged the relatedness of the overlapping elements (i.e. A-C). These transitive associations could be learned either through a reactivation mechanism during learning or through an inference mechanism during testing. Using fMRI, Shohamy and Wagner (2008) found that hippocampal activity during B-C learning predicted subsequent generalization performance. In contrast, hippocampal activity during testing did not correspond to successful generalization performance, suggesting that reactivation of related information during learning promoted integration of new information into existing memory representations. Interestingly, only subjects with “good” generalization performance showed this enhanced hippocampal learning effect. It is possible that “good” learners engaged in more effortful retrieval processing during B-C learning to promote generalization performance on the test. This idea is consistent with the results from the present study, in that the active engagement of retrieval promoted across-episode binding during learning, whereas a passive reactivation task only supported within-episode binding when the stimuli were physically paired during the Recognition test.
It is possible that the current processing mode or state influences encoding and retrieval processes. Because the hippocampal system handles both encoding and retrieval functions,different types of input (e.g. old or new stimuli) could prime the system to perform either pattern separation (encoding) or pattern completion (retrieval). This idea was supported by a recent study, which demonstrated that identification of new stimuli promoted item discrimination (pattern separation), whereas identification of old stimuli promoted integration of familiar information into existing memory representations (pattern completion) (Duncan, Sadanand, & Davachi, 2012). In the current study, it could be argued that across-episode binding was promoted during Active retrieval merely because the hippocampal system was “primed” for pattern completion. However, this interpretation is difficult to reconcile with the ERP findings, because similar ERP effects both predicted subsequent face memory during encoding in the Active condition (across-episode binding) and corresponded to successful face memory during Recognition in the Remote Context in the Passive condition (within-episode binding). If ERPs during face encoding in the Active condition simply reflected a boost in retrieval-related processing, then we would expect to see early negative old/new effects for all conditions during Recognition. Instead, we observed early negative ERP effects selectively during Recognition in the Remote Context for the Passive condition. These conditions associated with similar ERP correlates of binding varied in terms of the type of stimuli and the encoding/retrieval demands, which is difficult to reconcile with the mode/state interpretation of the present effects. A more parsimonious explanation of these findings is that early negative ERPs reflected binding, and the engagement of active retrieval prior to learning systematically increased this binding.
The current findings lend further support to the idea that engaging in active learning or retrieval processes can have powerful effects on subsequent memory (Bridge & Paller, 2012). For instance, exerting volitional controlduring object-location learning facilitates later memory of the individual objects and the related spatial locations relative to passively viewing the objects in a predetermined sequence (Voss, Galvan, & Gonsalves, 2011; Voss, Gonsalves, Federmeier, Tranel, & Cohen, 2011; Voss, Warren, et al., 2011). Taken together, these findings suggest that methods to promote active retrieval could be used to enhance encoding of novel information. By promoting retrieval of information from memory, new information may be encoded more efficiently and effectively. It is unclear from the current results what brain regions are involved in this binding-related facilitation by active retrieval, although our previous experiment specifically implicated anterior hippocampus (Bridge & Voss, 2014). By further refining knowledge of critical brain regions in future studies, it will be possible to determine whether facilitation by active retrieval would be appropriate for use by individuals suffering from memory impairments due to damage to regions likely involved, such as hippocampus and prefrontal cortex (which are prominently involved in the influence of active retrieval and learning on memory; (Bridge & Voss, 2014 ; Voss, Gonsalves, et al., 2011; Voss, Warren, et al., 2011)). In summary, active retrieval has a unique promotional effect on integrating new information into existing memories, over and above passively encountering familiar information. The current behavioral and ERP findings indicate this is because active retrieval allows binding to occur for specific memory content, and that the binding process could be quite similar for this across-episode binding relative to within-episode binding of co-occurring information.
Highlights.
Active retrieval prior to encoding new information promotes across-episode binding.
Similar early negative ERPs index across- and within-episode binding
When encoding follows active retrieval, ERPs index across-episode binding
Otherwise, ERPs index within-episode binding when information is physically paired
Acknowledgments
Financial support was provided by grants R00-NS069788 from the National Institute of Neurological Disorders and Stroke and T32-AG20506 from the National Institute on Aging. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Kelly Brandstatt for assistance with data collection. We thank James Antony, Jessica Creery, and Peter Winter for providing helpful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Althoff RR, Cohen NJ. Eye-movement-based memory effect: a reprocessing effect in face perception. J Exp Psychol Learn Mem Cogn. 1999;25:997–1010. doi: 10.1037//0278-7393.25.4.997. [DOI] [PubMed] [Google Scholar]
- Bridge DJ, Paller KA. Neural correlates of reactivation and retrieval-induced distortion. J Neurosci. 2012;32:12144–12151. doi: 10.1523/JNEUROSCI.1378-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge DJ, Voss JL. Hippocampal binding of novel information with dominant memory traces can support both memory stability and change. J Neurosci. 2014;34:2203–2213. doi: 10.1523/JNEUROSCI.3819-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs JF, Fitz KI, Riccio DC. Transfer of memory retrieval cues in rats. Psychon Bull Rev. 2007;14:495–499. doi: 10.3758/bf03194096. [DOI] [PubMed] [Google Scholar]
- Briggs JF, Riccio DC. Transfer of Old ‘Reactivated’ Memory Retrieval Cues in Rats. Learn Motiv. 2008;39:13–23. doi: 10.1016/j.lmot.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur MB, Dionne-Dostie E, Montreuil T, Lepage M. The Bank of Standardized Stimuli (BOSS), a new set of 480 normative photos of objects to be used as visual stimuli in cognitive research. Plos One. 2010;5:e10773. doi: 10.1371/journal.pone.0010773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunsey M, Eichenbaum H. Conservation of hippocampal memory function in rats and humans. Nature. 1996;379:255–257. doi: 10.1038/379255a0. [DOI] [PubMed] [Google Scholar]
- Duncan K, Sadanand A, Davachi L. Memory's penumbra: episodic memory decisions induce lingering mnemonic biases. Science. 2012;337:485–487. doi: 10.1126/science.1221936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From conditioning to conscious recollection: memory systems of the brain. New York, NY: Oxford University Press; 2001. [Google Scholar]
- Hannula DE, Federmeier KD, Cohen NJ. Event-related potential signatures of relational memory. J Cogn Neurosci. 2006;18:1863–1876. doi: 10.1162/jocn.2006.18.11.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanova MD, Good M, Honey RC. Retrieval-mediated learning involving episodes requires synaptic plasticity in the hippocampus. J Neurosci. 2011;31:7156–7162. doi: 10.1523/JNEUROSCI.0295-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DJ. Psychobiology of Active and Inactive Memory. Psychological Bulletin. 1979;86:1054–1083. [PubMed] [Google Scholar]
- Lopez-Calderon J, Luck SJ. ERPLAB: an open-source toolbox for the analysis of event-related potentials. Front Hum Neurosci. 2014;8:213. doi: 10.3389/fnhum.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy G, Wood CC. Scalp distributions of event-related potentials: an ambiguity associated with analysis of variance models. Electroencephalogr Clin Neurophysiol. 1985;62:203–208. doi: 10.1016/0168-5597(85)90015-2. [DOI] [PubMed] [Google Scholar]
- Nader K. Memory traces unbound. Trends Neurosci. 2003;26:65–72. doi: 10.1016/S0166-2236(02)00042-5. [DOI] [PubMed] [Google Scholar]
- Olsen RK, Moses SN, Riggs L, Ryan JD. The hippocampus supports multiple cognitive processes through relational binding and comparison. Front Hum Neurosci. 2012;6 doi: 10.3389/fnhum.2012.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller KA, Kutas M, Mayes AR. Neural correlates of encoding in an incidental learning paradigm. Electroencephalogr Clin Neurophysiol. 1987;67:360–371. doi: 10.1016/0013-4694(87)90124-6. [DOI] [PubMed] [Google Scholar]
- Paller KA, McCarthy G, Wood CC. ERPs predictive of subsequent recall and recognition performance. Biol Psychol. 1988;26:269–276. doi: 10.1016/0301-0511(88)90023-3. [DOI] [PubMed] [Google Scholar]
- Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends in Cognitive Sciences. 2002;6:93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Pfister R. Pretris - Tetris for Presentation. Archives of Neurobehavioral Experiments and Stimuli 2008 [Google Scholar]
- Preston AR, Shrager Y, Dudukovic NM, Gabrieli JD. Hippocampal contribution to the novel use of relational information in declarative memory. Hippocampus. 2004;14:148–152. doi: 10.1002/hipo.20009. [DOI] [PubMed] [Google Scholar]
- Prince SE, Daselaar SM, Cabeza R. Neural correlates of relational memory: successful encoding and retrieval of semantic and perceptual associations. J Neurosci. 2005;25:1203–1210. doi: 10.1523/JNEUROSCI.2540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C. Binding Items and Contexts: The Cognitive Neuroscience of Episodic Memory. Current Directions in Psychological Science. 2010;19:131–137. [Google Scholar]
- Rossion B, Jacques C. The N170: understanding the time-course of face perception in the human brain. In: Luck S, Kappenman E, editors. The Oxford Handbook of ERP Components. Oxford University Press; 2011. [Google Scholar]
- Rugg MD, Curran T. Event-related potentials and recognition memory. Trends in Cognitive Sciences. 2007;11:251–257. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Ryan JD, Althoff RR, Whitlow S, Cohen NJ. Amnesia is a deficit in relational memory. Psychol Sci. 2000;11:454–461. doi: 10.1111/1467-9280.00288. [DOI] [PubMed] [Google Scholar]
- Schwartz G, Howard MW, Jing B, Kahana MJ. Shadows of the past: temporal retrieval effects in recognition memory. Psychol Sci. 2005;16:898–904. doi: 10.1111/j.1467-9280.2005.01634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederberg PB, Miller JF, Howard MW, Kahana MJ. The temporal contiguity effect predicts episodic memory performance. Mem Cognit. 2010;38:689–699. doi: 10.3758/MC.38.6.689. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Wagner AD. Integrating memories in the human brain: hippocampalmidbrain encoding of overlapping events. Neuron. 2008;60:378–389. doi: 10.1016/j.neuron.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Turk-Browne NB, Simon MG, Sederberg PB. Scene representations in parahippocampal cortex depend on temporal context. J Neurosci. 2012;32:7202–7207. doi: 10.1523/JNEUROSCI.0942-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood BJ. Attributes of Memory. Psychol Rev. 1969;76:559–573. [Google Scholar]
- Voss JL, Galvan A, Gonsalves BD. Cortical regions recruited for complex activelearning strategies and action planning exhibit rapid reactivation during memory retrieval. Neuropsychologia. 2011;49:3956–3966. doi: 10.1016/j.neuropsychologia.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Gonsalves BD, Federmeier KD, Tranel D, Cohen NJ. Hippocampal brain-network coordination during volitional exploratory behavior enhances learning. Nat Neurosci. 2011;14:115–120. doi: 10.1038/nn.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Paller KA. Neural substrates of remembering: Electroencephalographic studies. In: Eichenbaum H, Byrne J, editors. Memory Systems. Vol. 3. Oxford; Elsevier: 2008. pp. 79–98. [Google Scholar]
- Voss JL, Warren DE, Gonsalves BD, Federmeier KD, Tranel D, Cohen NJ. Spontaneous revisitation during visual exploration as a link among strategic behavior, learning, and the hippocampus. Proc Natl Acad Sci U S A. 2011;108:E402–409. doi: 10.1073/pnas.1100225108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PD, Voss JL, Warren DE, Tranel D, Cohen NJ. Spatial reconstruction by patients with hippocampal damage is dominated by relational memory errors. Hippocampus. 2013;23:570–580. doi: 10.1002/hipo.22115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yovel G, Paller KA. The neural basis of the butcher-on-the-bus phenomenon: when a face seems familiar but is not remembered. Neuroimage. 2004;21:789–800. doi: 10.1016/j.neuroimage.2003.09.034. [DOI] [PubMed] [Google Scholar]
- Zeithamova D, Dominick AL, Preston AR. Hippocampal and ventral medial prefrontal activation during retrieval-mediated learning supports novel inference. Neuron. 2012;75:168–179. doi: 10.1016/j.neuron.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeithamova D, Preston AR. Flexible memories: differential roles for medial temporal lobe and prefrontal cortex in cross-episode binding. J Neurosci. 2010;30:14676–14684. doi: 10.1523/JNEUROSCI.3250-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]