Abstract
This study's purpose was to identify psychosocial predictors of weight loss maintenance in a multi-site clinical trial, following a group-based weight loss program. Participants (N = 1025) were predominately women (63 %) and 38 % were Black (mean age = 55.6 years; SD = 8.7). At 12 months, higher SF-36 mental health composite scores were associated with less weight regain (p < .01). For Black participants, an interaction existed between race and friends' encouragement for exercise, where higher exercise encouragement was related to more weight regain (p < .05). At 30 months, friends' encouragement for healthy eating was associated with more weight regain (p < .05), whereas higher SF-36 mental health composite scores were related to less weight regain (p < .0001). Perceived stress and select health-related quality of life indices were associated with weight regain; this relationship varied across gender, race, and treatment conditions. Temporal changes in these variables should be investigated for their impact on weight maintenance.
Keywords: Weight loss maintenance, Psychosocial predictors, Obesity, Quality of life, Social support
Introduction
Many weight loss programs are successful in helping people achieve weight loss (e.g., West et al., 2007, 2008); however, maintenance of weight loss is challenging (Barte et al., 2010; Turk et al., 2009). A limited number of published weight loss management programs have included a long-term weight loss maintenance component; however, this has usually been in the form of extended treatment rather than a new program focusing on specific weight loss maintenance strategies (Perri et al., 1989). Perri and colleagues (1989) found that the longer participants are in treatment, the longer they will comply with weight loss behaviors. However, as soon as the extended treatment is stopped, the participants begin to regain their weight (Perri et al., 1989), indicating that weight loss maintenance strategies may be necessary to aid individuals in shifting from guided weight loss to more self-directed maintenance.
To date, few studies have examined outcomes related to different types of weight loss maintenance strategies. Research suggests that strategies for weight loss maintenance differ from strategies for initial weight loss (see Perri et al., 1984; Sciamanna et al., 2011; Wadden et al., 2011). Other research has applied the Transtheoretical Model (Prochaska & Velicer, 1997) to weight loss maintenance; however, the focus was on individuals' readiness to maintain weight (e.g., Johnson et al., 2008) and did not account for the potential importance of other psychosocial variables, such as perceived stress and social support (Perri et al., 1984; Sciamanna et al., 2011; Wadden et al., 2011).
Psychosocial variables would likely be important in predicating success of weight loss maintenance (Byrne, 2002; Wing & Hill, 2001), an enterprise dependent on human motivation (Williams et al., 1996). However, previously published studies on predictors of weight loss maintenance have mostly focused on behavioral, physiological, and process variables (i.e., food/fitness records, self-weighing, attendance), rather than psychosocial variables (e.g., perceived stress, social support) associated with weight regain (e.g., Perri et al., 1984; Sciamanna et al., 2011; Wadden et al., 2011). Further, Delahanty and colleagues (2013) recently noted that few studies have been published examining the role of psychosocial and behavioral variables as they related to weight loss and subsequent weight maintenance. To the authors' knowledge, only four studies have examined psychosocial factors associated with weight regain following behavioral weight loss and weight maintenance interventions (Delahanty et al., 2013; Liebbrand & Fitcher, 2002; Vieira et al., 2012; Wing et al., 2008). In the first study, participants completed a 10-week, group-based, inpatient cognitive behavioral weight loss intervention followed by randomization to 18 months of either “supported maintenance” or follow-up without support (Liebbrand & Fitcher, 2002). Of all the variables of interest [i.e., depression, general psychopathology, quality of life, body image, dietary restraint, disinhibition, hunger, eating disorder symptoms, body mass index (BMI), all assessed at baseline], only baseline BMI significantly predicted weight loss at 18 months (Liebbrand & Fitcher, 2002).
Wing and colleagues (2008) reported results from STOP Regain, an 18-month randomized controlled trial comparing weight regain in a face-to-face or an Internet intervention to a control condition. Participants (N = 261) were mostly non-Latino White and women. In this trial, there were no significant baseline predictors of weight regain; however, changes in several psychological variables were related to weight regain during maintenance including increases in depressive symptoms, dietary hunger, and disinhibition.
Vieira and colleagues (2012) compared health-related quality of life, psychological well-being, and self-regulation of eating among predominately non-Latino White women who had previously lost weight were categorized as weight loss maintainers (individuals who had intentionally lost ≥5 kg in the last 15 years as adults and had kept this weight off for ≥1 year), weight loss treatment (those who had just completed a 12-month weight loss program focused on increased physical activity, as well as improved dietary behaviors, body image, and self-regulation of weight control), or not attempting weight loss. Women in the weight loss maintainers group averaged a 14.7 kg weight loss which had been kept off for an average of 2.5 years. Women in the weight loss treatment group had lost an average of 6.1 kg of body weight at the termination of the weight loss program. Results revealed that maintainers had lower physical health-related quality of life and higher eating disinhibition and perceived hunger compared to women actively engaging in weight loss group. However, compared to women not attempting to lose weight, maintainers had higher physical health-related quality of life; lower eating disinhibition and perceived hunger; and higher exercise enjoyment and perceived competence (Vieira et al., 2012).
Most recently, Delahanty et al. (2013) examined the behavioral and psychosocial variables (N = 269) associated with an achievement of 7 % weight loss following a 16-week lifestyle intervention among those high at risk for the development of diabetes. Defined as 2.8 years (range 1.8–4.6 years) post-intervention (Diabetes Prevention Program Research Group, 2002), long-term weight maintenance of 7 % weight loss was positively associated with older age, fewer previous weight loss attempts, increased exercise self-efficacy, decreased selection of high-fat foods, increased dietary restraint, and lower baseline activity levels.
Prior research indicates that Black women tend to lose less weight during the intensive weight loss phase of a behavioral weight loss program and maintain a lower percent of their weight loss compared to non-Latino White women (Fabricatore et al., 2009; Kumanyika et al., 2002, 1991; Rickel et al., 2011; Stevens et al., 2001; West et al., 2007, 2008). However, participants in two of the four studies examining psychosocial variables related to weight maintenance were almost exclusively non-Latino White. Given the differences in weight loss and weight maintenance (e.g., Perri et al., 1989), it is of particular interest to examine potential differences in psychosocial predictors of weight maintenance and the potential impact of race on these psychosocial predictors.
The Weight Loss Maintenance intervention was guided by the following: Social Cognitive Theory (Bandura, 1986), behavioral self-management techniques (Watson et al., 1989), the Transtheoretical Model/Stages of Change (Prochaska & DiClemente, 1983), and motivational enhancement approaches (Bock et al., 2001; Miller & Rollnick, 1991; Rollnick, Mason, & Butler, 1999). These approaches emphasize the importance of an individual's ability to regulate his or her own behavior through goal setting, developing specific behavior change plans, monitoring progress towards the goals, and developing and implementing the skills necessary to reach the goals. Self-efficacy (an individual's confidence in performing a particular behavior) and outcome expectancies (an individual's outcome expectations of performing a particular behavior) are critical mediators of behavior change (Bandura, 1986; Bandura & Adams, 1977). The Transtheoretical Model recognizes that behavior change is a dynamic process of moving through different motivational stages of readiness for change (Prochaska & DiClemente, 1983). During the program different behavioral strategies were emphasized, depending on the individual's stage of change. These secondary analyses are limited by the existing data and cannot evaluate any single theoretic frame work in its entirety. However, the current study examined constructs believed to be related to the guiding approaches. Specifically, social support may be related to an individual's ability to progress through the Transtheoretical Model; functional and perceived quality of life may be related to outcome expectancies; and depressive symptoms and stress may be related to the ability to engage in goal setting and skill building.
Hypotheses in the present study were based on: (1) previous research; (2) constructs associated with the Social Cognitive Theory, behavioral self-management, the Transtheoretical Model, and motivational enhancement approaches; and (3) findings from behavioral weight loss interventions (Eflhag & Rossner, 2005; Teixeira et al., 2002). It was hypothesized that psychosocial variables, assessed at Phase II randomization, such as social support, higher functional and perceived quality of life, lower depressive symptoms, and lower perceived stress would be associated with improved weight loss maintenance or less weight regain following a 30-month weight loss maintenance intervention. Differences based on gender, race, and treatment condition were also investigated.
Method
Weight Loss Maintenance was a randomized controlled trial consisting of two phases (see Brantley et al., 2008; Hollis et al., 2008; Svetkey et al., 2008). The first phase (Phase I), was a 6-month group-based weight loss program; the second phase (Phase II), was a 30-month weight loss maintenance intervention, which tested three strategies [i.e., Personal Contact, Interactive Technology, Self-Directed comparison condition] for maintaining the weight that was lost during the weight loss program (Phase I). The trial was conducted at four clinical centers (Baltimore, MD; Baton Rouge, LA; Durham, NC; Portland, OR) between August 2003 and June 2007. The Institutional Review Board at each clinical site, as well as a National Heart Lung and Blood Institute appointed Protocol Review Committee approved the Weight Loss Maintenance study protocol. Each participant provided written informed consent prior to participation. A detailed description of the Weight Loss Maintenance design has been published elsewhere (Brantley et al., 2008), as well as the primary outcomes (Svetkey et al., 2008) and another predictor study focusing on race and gender of participants (Svetkey et al., 2012). Briefly, the overall findings revealed that there were no differences in weight regain between the Interactive Technology and Self-Directed groups at 30 months; however, the Personal Contact group had regained less weight at 30 months compared to both those in the Self-Directed group and those in the Interactive Technology group. At both 18 and 24 months after randomization, those in the Interactive Technology group regained less weight than those in the Self-Directed group.
Eligibility
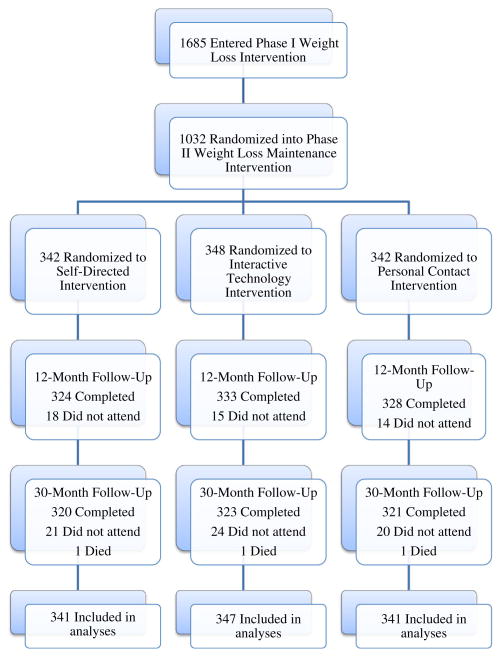
Eligible participants were either overweight or obese (BMI between 25 to 45 kg/m2) adults (age ≥ 25 years) who were currently taking medication for hypertension and/or dyslipidemia, but not for the management of diabetes. Those without telephone or Internet access were excluded. Eligible participants were enrolled into Phase I, and those who were able to lose ≥4 kg during Phase I were randomized to Phase II to ensure no initial group differences, which is described in detail below (see Fig. 1 for a flowchart). A more complete description of the eligibility criteria can be found in the Brantley et al. (2008) article and on the Weight Loss Maintenance study website (www.kpchr.org/wlmpublic). This website also provides the study's manual of procedures, and details the protocol for each of the intervention components.
Fig. 1. Flowchart of randomization and follow-up.
Intervention
Weight loss program (phase I)
Phase I participants (N = 1685) who were enrolled in a 6-month group-based weight loss program attended 20 weekly sessions. Sessions lasted approximately 1.5 h in duration with 18–25 participants per group. Participants were encouraged to follow the high-fiber and low-fat Dietary Approaches to Stop Hypertension diet guidelines which have been shown to lower blood pressure (Appel et al., 1997). Participants were also encouraged to engage in ≥180 min of physical activity per week. The weight loss program provided social support and taught participants problem solving, decision-making, and goal setting skills (Hollis et al., 2008).
Weight loss maintenance intervention (phase II)
Participants who lost ≥4 kg during the initial weight loss program and were then randomly assigned to 1 of 3 maintenance conditions: Self-Directed comparison with minimal intervention; Interactive Technology, in which participants were given unlimited access to an interactive weight loss maintenance website; or Personal Contact, in which participants received monthly, personalized telephone contact and quarterly, face-to-face contact from a study interventionist. Participants were provided with modest incentives for attending their clinical assessment visits at 12 and 30 months to bolster retention to the study's primary outcomes. All incentives were approved by each of the institutions' Institutional Review Boards. During the maintenance phase (Phase II), participants' diet and physical activity goals were identical to those in Phase I; however, they were asked to increase their weekly physical activity by 45 min, for a total of ≥225 min each week. Personal Contact and Interactive Technology conditions provided the same content and differed only in mode of presentation (i.e., in-person and internet-based, respectively). Strategies utilized in the interventions (e.g., self-monitoring, social support, frequent patient-therapist contact, relapse prevention) were based on previous research related to long-term weight loss maintenance (Wadden et al., 2004). Conversely, those in the Self-Directed condition received printed lifestyle guidelines and met briefly with a study interventionist after the 12- and 30-month follow-up visits only to discuss their participation in the study. Participants in the Interactive Technology group logged on to the website an average of once a week, and had contact with the website for 77 % of the 30 months during the maintenance phase (Svetkey et al., 2008). Indicative of interaction with the website, a study examining one of the 27 available interactive website tools (i.e., the Tailored Self-Assessment tool assisted participants with developing a personalized action plan based on their current progress and make choices about future weight management actions) found that Interactive Technology participants were highly engaged in the website (Funk et al., 2010). Participants completed this interactive tool a total of 800 times; 53 % utilized this tool at least once, and of those that utilized the tool at least once, 72 % completed the tool more than once. Similarly, previously published results indicated that the average number of contacts for individuals randomized to the Personal Contact group completed 91 % of the intervention contacts specified in the protocol (Svetkey et al., 2008).
Measures
Self-report measures were completed upon entry into the Weight Loss Maintenance study (prior to Phase I) and again at randomization into Phase II. The measures reported in this paper are those collected at randomization into Phase II.
Social support and exercise survey (Sallis et al., 1987)
This 13-item self-report measure assesses perceived receipt of social support for exercise. Participants report how often their friends or family members (separately rated) encouraged their exercise behaviors during the previous 3 months. Responses are on a 5-point Likert-type scale (ranging from 1 = “none” to 5 = “very often,” or “does not apply”). Responses to two negatively worded items are reversed and responses are summed, yielding two separate scores for friends-encouragement and family-encouragement. Scores range from 13 to 65 (for family and friends), with higher scores reflective of higher encouragement for exercise. This measure has demonstrated adequate internal consistency (α = .76–.84; Sallis et al., 1987). In this study, the internal consistency for the Social Support and Exercise Survey was excellent (α = .97 for both Family and Friend support, respectively).
Social support and eating habits survey (Sallis et al., 1987)
This 10-item scale was supplemented with additional items specific to individuals actively losing weight, yielding a total of 18 items. Following Sallis et al. (1987), separate ratings for friends and family were obtained. Participants are asked to report how often their friends and family provided encouragement or discouragement for healthy eating during the last 3 months. Responses were recorded using a 5-point Likert-type scale (ranging from 1 = “none” to 5 = “very often,” or “does not apply”). A total of 9 items are positively worded (encouragement) and the remaining 9 items are negatively worded (discouragement). For the adapted 18-item scale, scores range from 9 to 45 for encouragement for healthy eating habits (family and friends' encouragement scored separately; higher scores reflective of more encouragement) and 9–45 for discouragement for healthy eating habits (family and friends' discouragement scored separately; higher scores reflective of more discouragement). Adequate internal consistency has been demonstrated (α = .84–α = .85) for the 10-item Sallis et al. (1987) measure. In the current sample, the internal consistency for the Social Support and Eating Habits Survey was excellent, α = .93, .92, .91, and .89 for family encouragement, family discouragement, friends encouragement, and friends discouragement, respectively.
SF-36™ (Ware & Sherbourne, 1992)
This 36-item self-report measure assessed health-related quality of life. The SF-36 is a generic (rather than disease-specific) measure appropriate for the general population. Norms-based scoring yields two composite scales (i.e., physical health component summary, mental health component summary) and eight subscales [i.e., physical functioning (10 items); limitations due to physical problems (4 items); pain (2 items); general health (5 items); vitality (4 items); social functioning (2 items); limitations due to emotional problems (3 items); and, mental health (5 items)] related to functional health and well-being. One item indicative of change in health is also included on the scale. Participants respond to questions formatted on a Likert-type scale, with higher scores reflecting better health. Excellent internal consistency has been demonstrated on the two composite scales (α = .90; Ware et al., 1994), and the eight subscales have displayed adequate internal consistency (α = .80; McHorney et al., 1994; Ware et al., 1993). The internal consistency for the total SF-36 scale in this study was good (α = .83), and the component summaries were acceptable (physical component summary, α = .73; mental component summary, α = .77).
Patient Health Questionnaire Depression Scale (Kroenke & Spitzer, 2002)
The Patient Heath Questionnaire Depression Scale, a self-administered patient questionnaire, includes 8 of the 9 criteria for Major Depressive Disorder (suicidality is omitted; American Psychiatric Association, 2000). Responses provide an estimate of impairment severity and are helpful in guiding further assessment and treatment. Participants respond to questions using a Likert-type scale, ranging from “not at all” (0) to “nearly every day” (3), and these are summed to obtain a total depression score. Scores can range from 0 to 24, with higher scores reflecting more depression symptoms. Rohyans and Pressler (2009) reported adequate internal consistency (α = .83). Likewise, the internal consistency for the current study was good (α = .83).
Perceived Stress Scale (Cohen et al., 1983)
To decrease potential participant burden, a 4 item subset of this 14-item self-report measure assesses perceived global stress within the previous month. The response format is a 5-point Likert-type scale (0 = “never” to 5 = “very often”). Scores range from 0 to 20, where higher scores indicate a higher level of perceived stress. Good internal consistency and test–retest reliability (α = .85) have been established for the overall Perceived Stress Scale (Cohen et al., 1983). For the current study, the internal consistency for the 4-item subset was questionable (α = .62).
Statistical analyses
All statistical analyses were conducted using SAS®, version 9.1 (SAS Institute Inc., Cary, NC) and results were considered significant at p < .05. Five copies of complete data were created using multiple imputation (SAS® PROC MI data augmentation with MCMC sampling) before any analyses were carried out on outcomes (Schafer, 1997). The PROC MIANALYZE procedure was performed due to missing data. Specifically, at 12 months after randomization, 18, 15, and 14 individuals did not attend the Self-Directed, Interactive Technology, and Personal Contact follow-up, respectively. At 30 months after randomization, 21, 24, and 20 individuals did not attend the Self-Directed, Interactive Technology, and Personal Contact follow-up, respectively. A total of 3 individuals died (1 from each intervention arm; Fig. 1). The outcome measure was weight change (follow-up minus baseline, so weight regain was positive). Linear interpolation was used to correct for missing item responses for each of the self-reported measures if less than half of the responses were missing.
Descriptive analyses and bivariate associations
Means, standard deviations, and frequencies were performed for all included predictor variables. Pearson or Spearman correlation coefficients (where appropriate) were obtained to examine pair-wise associations of the potential predictor variables (SF-36 composite scales and subscales, social support and exercise, social support and eating habits, perceived stress, depression, race, sex, randomization weight, and treatment group). Regression models were run separately for weight change outcomes at 12 and 30 months, first examining which psychosocial predictors had direct associations with weight change, and second examining whether introducing interactions with race, sex, or treatment would reveal more complex associations. Treatment was included as a potential interaction because differences in weight regain between groups occurred (Svetkey et al., 2008). Following selection of the final model, the identical analysis was carried out on all 5 copies of the imputed dataset and the estimates of regression parameters, with F-tests of each effect combined according to Rubin's rules (Rubin, 1987).
Main analyses
After the significant predictors were identified, an exploration of whether interactions existed across race and/or sex and/or treatment subgroups in the association between covariate and weight change was conducted using backward stepwise elimination. Potential interactions of race and/or sex and/or treatment subgroups were explored due to the known differences in psychosocial factors between races and men and women (Wang & Chen, 2011). Terms were retained in the model if the change in R2 was α < .02. In addition, steps were constrained to account for the hierarchy of terms. In order to examine the pattern of associations (predictor with weight) underlying the interaction, predicted values [least squares means (LS-means)] for the outcome were obtained at high and low values (10th and 90th decile) of each covariate in each subgroup involved in an interaction (e.g., both sexes, when there is an interaction with sex). The LS-means were tested at the high or low level of the covariate to determine whether they differed between all meaningful pairs of groups involved in the interaction.
Results
Participant characteristics
Of the 1,685 participants who completed Phase I, 1032 (61 %) lost ≥4 kg, and were randomized to Phase II. At randomization, 1,027 of the 1,032 participants provided complete psychosocial data. Of the five participants lost to follow-up, three of the participants died and two failed to provide complete and analyzable responses on the social support scales. Two additional participants were identified as outliers (according to Tukey's criterion) at both 12 and 30 months (Tukey, 1977), and were dropped from analyses, yielding a final sample of 1,025.
The 1025 randomized participants were predominantly women (63 %) and had a mean age of 55.6 years (SD = 8.7). Approximately 38 % of the participants were Black, 61 % were non-Latino White, and 1 % were Hispanic. The mean initial (Phase I) weight loss was 8.5 kg (range from 4.0 to 30.3 kg). During Phase II, all treatment conditions showed a mean weight regain of weight lost during Phase I (Self-Directed = 5.5 kg, Interactive Technology = 5.2 kg, Personal Contact = 4.2 kg; see Svetkey et al., 2008). However, mean weight at 30 months remained significantly lower than their mean weight at entry into Phase I (see Svetkey et al., 2008). Table 1 provides more detailed information regarding participant characteristics.
Table 1. Phase II participant characteristics (N = 1025).
| SD 339 n (%) |
IT 346 n (%) |
PC 340 n (%) |
Total 1025 n (%) |
|
|---|---|---|---|---|
| Mean age (SD) | 55.87 (8.47) | 55.69 (8.54) | 55.42 (8.96) | 55.66 (8.65) |
| Black | ||||
| Women | 89 (29) | 90 (26) | 86 (25) | 265 (26) |
| Men | 35 (10) | 41 (12) | 45 (13) | 121 (12) |
| Non-Black | ||||
| Women | 130 (38) | 129 (37) | 126 (37) | 385 (38) |
| Men | 85 (25) | 86 (25) | 83 (24) | 254 (25) |
| Mean randomization weight (SD) | 87.45 (15.26) | 88.40 (15.21) | 88.67 (16.90) | 88.17 (15.80) |
| Mean phase II weight change, in kg (SD) | 5.52 (5.80) | 5.50 (5.55) | 4.18 (5.36) | 5.07 (5.60) |
| Education | ||||
| Some college | 134 (40) | 131 (38) | 130 (38) | 395 (39) |
| College degree | 205 (61) | 215 (62) | 210 (62) | 630 (62) |
| Household Income/Year | ||||
| <$60,000 | 149 (44) | 135 (39) | 154 (45) | 438 (43) |
| >$60,000 | 190 (56) | 211 (61) | 186 (55) | 587 (57) |
SD Self-Directed Group, IT Interactive Technology Group, PC Personal Contact Group
Descriptive analyses and bivariate associations
Follow-up data collection was conducted at both 12 and 30 months; results from the calculated correlation coefficients (not presented) revealed that the SF-36 subscales of bodily pain, mental health, physical functioning, limitations due to emotional problems, and limitations due to physical problems were redundant with either the mental health composite or physical health composite score of the SF-36, and were dropped from further analyses. The backward stepwise regression performed on weight change (not presented) indicated that participants with a higher SF-36 Mental Health Composite score at randomization were also more successful with weight loss maintenance at both 12 months (p < .01) and at 30 months (p < .0001). Higher perceptions of friends' encouragement for healthy eating was associated with more weight regain (p < .05) at 30 months after randomization; this was not present at 12 months. No other psychosocial variables were significantly related to weight loss at 12 or 30 months.
Main analyses
Interactions: weight change from randomization to 12 months
The interaction model at 12 months (R2 = 0.052) retained three measures of social support (family discouragement for healthy eating; friends' discouragement for healthy eating; friends' encouragement for exercise) in addition to the SF-36 (Table 2).
Table 2. Interaction model—weight change at 12 months (N = 1025).
| Estimate (SE) | df | Adj df | F | p | |
|---|---|---|---|---|---|
| Race (non-Black as referent) | −1.33 (0.68) | 1 | 946.70 | 3.80 | 0.0515 |
| Treatment (SD condition as referent) | |||||
| IT | −0.92 (0.34) | 2 | 11,558.00 | 9.75 | <.0001 |
| PC | −1.51 (0.34) | ||||
| Weight at randomization | 0.03 (0.01) | 1 | 834.00 | 14.51 | 0.0002 |
| SF-36 vitality | −0.05 (0.02) | 1 | 663.40 | 6.24 | 0.0127 |
| Family discouragement, eating habits | 0.25 (0.11) | 1 | 928.70 | 5.46 | 0.0197 |
| Friends discouragement, eating habits | −0.24 (0.11) | 1 | 428.10 | 4.61 | 0.0324 |
| Friend encouragement, exercise | −0.02 (0.13) | 1 | 701.8 | 0.03 | 0.8530 |
| Race*friend encouragement, exercise | 0.41 (0.20) | 1 | 935.10 | 4.23 | 0.0400 |
R2 = 0.0517, controlling for gender; SF-36: (a) Physical Composite, (b) Bodily Pain, (c) General Health, (d) Social Functioning, (e) Vitality; Family Encouragement of Eating Habits; Friend Encouragement of Eating Habits; Family Encouragement of Exercise; Perceived Stress; and, Depression
IT Interactive Technology, PC Personal Contact; only those variables with a significant difference in LS-means are included
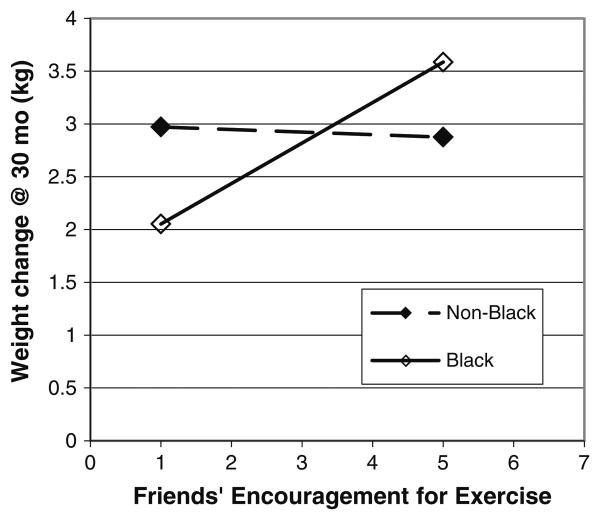
One significant interaction between race and friends' encouragement for exercise (p < .05) was detected. For Black participants, as friends' encouragement for exercise increased, weight regain at 12 months also significantly increased (Fig. 2). Friends' support for exercise was not related to non-Blacks' ability to maintain weight loss. The difference between groups (Blacks vs. non-Blacks) was not significant at either the 10th or the 90th deciles on exercise support (p > .05).
Fig. 2. 2-way interaction of weight by friends' encouragement for exercise at 12 months.
Interactions: weight change from randomization to 30 months
The interaction model (R2 = 0.091) for weight change at 30 months included three measures from the SF-36 (i.e., mental health composite, physical health composite, vitality), all interacting with race (Table 3).
Table 3. Interaction model—weight change at 30 months (N = 1025).
| Estimate (SE) | df | Adj df | F | p | |
|---|---|---|---|---|---|
| Race (non-Black as referent) | −6.17 (5.81) | 1 | 307.70 | 1.13 | 0.2892 |
| Gender (men as referent) | −0.36 (1.48) | 1 | 138.10 | 0.06 | 0.8102 |
| Race*Gender | −4.50 (2.31) | 1 | 451.80 | 3.79 | 0.0521 |
| Treatment (SD condition as referent) | |||||
| IT | 0.07 (1.49) | 2 | 841.90 | 4.35 | 0.0132 |
| PC | −0.33 (1.46) | ||||
| Race*treatment (non-Black as referent) | |||||
| Race*IT | −2.87 (2.51) | 1 | 892.90 | 1.30 | 0.2541 |
| Race*PC | −3.35 (2.63) | ||||
| Sex*Treatment (men as referent) | |||||
| Sex*IT | 0.98 (2.05) | 1 | 162.80 | 0.23 | 0.6328 |
| Sex*PC | 0.09 (1.95) | ||||
| Race*sex*treatment (non-Black and men as referents) | |||||
| Race*Sex*IT | 3.60 (3.13) | 1 | 688.90 | 1.32 | 0.2505 |
| Race*Sex*PC | 5.75 (3.17) | ||||
| Weight at randomization | 0.03 (0.01) | 1 | 275.40 | 6.08 | 0.0143 |
| SF-36 mental composite | −0.15 (0.05) | 1 | 347.10 | 8.54 | 0.0037 |
| Race*SF-36 mental composite | 0.22 (0.08) | 1 | 307.70 | 7.75 | 0.0057 |
| SF-36 physical composite | −0.03 (0.08) | 1 | 273.30 | 0.65 | 0.4218 |
| Race*SF-36 physical composite | 0.14 (0.07) | 1 | 269.20 | 4.23 | 0.0408 |
| SF-36 Vitality Subscale | 0.07 (0.04) | 1 | 535.00 | 2.33 | 0.1273 |
| Race*SF-36 Vitality Subscale | −0.2 (0.07) | 1 | 522.80 | 6.89 | 0.0089 |
| Friend encouragement, eating habits | 0.65 (0.16) | 1 | 200.50 | 17.41 | <.0001 |
| Perceived stress | 0.24 (0.27) | 1 | 558.70 | 0.84 | 0.3595 |
| Race*perceived stress | −1.63 (0.43) | 1 | 800.80 | 13.98 | 0.0002 |
| Sex*perceived stress | −0.17 (0.32) | 1 | 192.20 | 0.27 | 0.6041 |
| Race*sex*perceived stress | 2.08 (0.52) | 1 | 757.30 | 16.16 | <.0001 |
| Perceived stress (SD condition as referent)*treatment | |||||
| Stress*IT | −0.22 (0.38) | 1 | 642.20 | 0.33 | 0.5664 |
| Stress*PC | −0.43 (0.37) | ||||
| Perceived stress*race*treatment (SD condition and non-Black as referents) | |||||
| Stress*Race*IT | 1.62 (0.61) | 1 | 910.90 | 6.99 | 0.0084 |
| Stress*Race*PC | 1.32 (0.65) | ||||
| Perceived stress*sex*treatment (SD condition and men as referents) | |||||
| Stress*race*IT | −0.01 (0.48) | 1 | 235.30 | 0.00 | 0.9955 |
| Stress*race*PC | 0.10 (0.46) | ||||
| Perceived stress*race*sex*treatment (SD condition, non-Black, and men as referents) | |||||
| Stress*race*sex*IT | −1.98 (0.75) | 1 | 735.00 | 6.90 | 0.0088 |
| Stress*race*sex*PC | −1.87 (0.76) | ||||
R2 = 0.0643, controlling for SF-36: (a) Bodily Pain, (b) General Health, (c) Social Functioning; Family Discouragement of Eating Habits; Family Encouragement of Eating Habits; Family Encouragement of Exercise; Friend Encouragement of Exercise; Perceived Stress; and, Depression
SD Self-Directed, IT Interactive Technology, PC Personal Contact; only those variables with a significant difference in LS-means are included
Results indicated a significant interaction between race and self-reported mental health (SF-36 Mental Health Composite) on weight regain at 30 months (p < .01). Specifically, non-Black participants with the lowest scores on the mental health composite were more likely to regain weight than Black participants with similar scores (Fig. 3a). This is illustrated by the significant difference between groups on weight change at the 10th decile on SF-36 Mental Health Composite scores (p < .0001), as higher mental health composite scores were not related to weight change in either group. Thus, the groups were similar at higher levels on the mental health composite.
Fig. 3. 2-way interactions between race and 3 SF-36 subscales a mental health, b, physical health, c vitality, at 30 months.
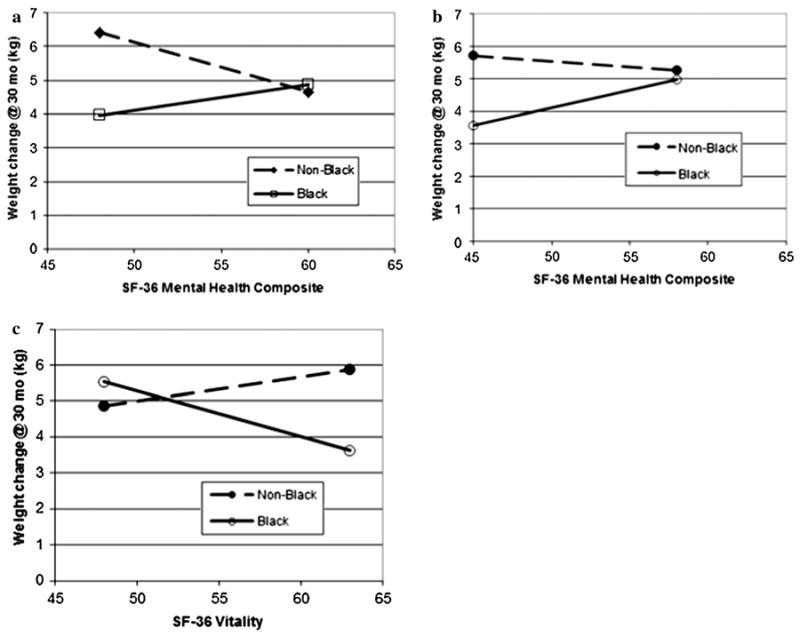
A significant interaction was detected between race and SF-36 Physical Health Composite scores on weight regain at 30 months (p < .05). Lower perceived physical functioning was related to significantly more weight regain at 30 months for non-Black than for Black participants (p < .001), but this difference was not apparent at higher scores; non-Blacks and Blacks did not differ on weight regain at higher physical functioning levels (Fig. 3b). Race and the SF-36 Vitality subscale also significantly interacted (p < .01). Higher vitality subscale scores were associated with significantly less weight regain at 30 months among Blacks than the non-Blacks (p < .001); this was not apparent at lower vitality scores (Fig. 3c).
There was a significant 4-way interaction between race, sex, treatment, and self-reported stress on weight regain at 30 months (p < .01; Table 3). Post-hoc analyses revealed several significant contrasts (Fig. 4).
Fig. 4. 4-way interaction of race by gender by treatment by stress and post-hoc comparisons of treatment, at 30 months.
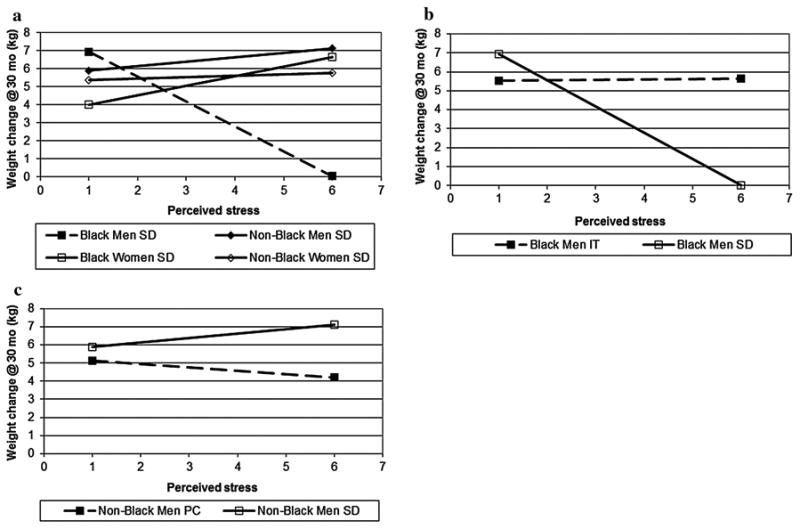
Being a non-Black woman with the highest levels of stress at baseline randomized to the Personal Contact condition was associated with a decreased likelihood of weight regain at 30 months compared to those randomized to both the Interactive Technology (p < .05) and Self-Directed (p < .01) conditions. However, there was no difference between conditions among non-Black women at lower levels of stress. Among participants randomized to the Self-Directed condition, Black men with higher perceived stress had a predicted mean weight change that was close to zero, significantly different from that predicted for both non-Black men (p <. 0001) and Black women (p < .0001) in the Self-Directed condition (Fig. 4a). In contrast, there was not a significant difference between these 4 groups (i.e., Black men, Black women, non-Black men, non-Black women) at lower levels of stress.
For Black men with higher perceived stress, being in the Self-Directed condition was also associated with a decreased likelihood of weight regain at 30 months compared to Black men in the Interactive Technology condition (p < .01; Fig. 4b); however, this pattern was not observed in these subgroups at the 10th decile. Among non-Black men with higher levels of perceived stress at baseline, being in the Personal Contact condition was associated with an increased likelihood of weight regain at 30 months compared to non-Black men randomized to the Self-Directed condition (p < .05; Fig. 4c); this difference was not present at the 10th decile. Taken together, it appears that differences between race, sex, and treatment occur among those with higher perceived stress, but not at the lower levels of perceived stress.
Discussion
The Weight Loss Maintenance study was unique because of its size and scope. It was comprised of a large and racially and gender diverse sample, and participants at high risk for cardiovascular disease (e.g., hypertension, dyslipidemia) were targeted rather than excluded. As little previous research has described psychosocial factors related to weight loss maintenance, not much is known about specific factors that might contribute to weight regain. Previous subgroup analyses suggested that the relationships between psychosocial factors and weight loss maintenance in different race/sex subgroups are complex (Svetkey et al., 2012). Thus, the current analysis attempted to unravel that complexity by taking a more nuanced approach to these relationships. As such, the present study is now one of five to examine and describe psychosocial factors related to success in studies designed specifically to compare weight outcomes in a weight loss maintenance trial. Results identified psychosocial variables (i.e., perceptions of social support, functional and perceived physical health, functional and perceived mental health, and stress) that modestly but significantly predicted participants' ability to maintain weight loss. As hypothesized, participants with higher ratings of functional and perceived mental health assessed just after completing a successful lifestyle weight loss program were more successful at maintaining their weight loss than participants with lower functional and perceived mental health ratings. This association was present in the early months of maintenance (at 12 months), where most regain occurs (Svetkey et al., 2008), as well as at 30 months of sustained effort to avoid weight regain.
Perceived encouragement for eating a healthy diet at baseline was associated with weight regain measured at 30 months of maintenance, but not in the early months of maintenance. This finding appears to run counter to the extant literature which consistently predicts improved health behaviors with higher levels of social support (House et al., 1988). Perhaps this association resulted from the well-intentioned reactions of friends who perceived participants regaining weight or engaging in eating patterns likely to lead to weight regain. Participants who were clearly on the path to weight regain may have elicited more comments and encouragement for healthy eating from the friends. It is also possible that individuals who perceived higher levels of encouragement from friends at baseline solicited support from friends as they began to regain weight during latter phases of the maintenance phase. Alternatively, the perceived encouragement for eating a healthy diet may have been interpreted as stigmatization by participants. Research has shown that feelings of stigmatization are often accompanied by psychological distress in a variety of health areas (e.g., Halding et al., 2008), including obesity (e.g., Schvey et al., 2011; Wott & Carels, 2010), and Friedman et al. (2005) speculate that obesity-related stigma may lead to body shame among some. Research also suggests that weight stigma may impede individuals' weight loss success (Wott & Carels, 2010) and is predictive of weight gain (Sutin & Terracciano, 2013), perhaps through increased caloric consumption (Schvey et al., 2011).
Psychosocial indices interacted with sex, race, and treatment condition, suggesting that psychosocial factors differentially affected participants' weight loss maintenance based on individual characteristics. Overall, non-Blacks showed a stronger association between higher Health-Related Quality of Life indices and better weight maintenance. Further, an interaction with treatment was present, indicating that psychosocial predictors differently affected weight loss maintenance for participants randomized into the three groups. These psychosocial interaction findings may be important in designing future weight loss maintenance programs. In particular, psychosocial indices should be assessed at the outset of engaging in a weight loss maintenance program, and tailoring based on individuals' sex, race, and psychosocial characteristics ought to take place. Further, the interaction of psychosocial indices with the treatment condition may suggest that weight loss maintenance programs should also be tailored to the means by which individuals lost their weight initially. However, further investigation is warranted.
In the current study, Black participants who reported higher perceived friends' encouragement for exercise were more likely to regain weight at 12 months. Perhaps some of the explanations presented above for the unexpected direction of the relationship between perceived encouragement for healthy eating and weight regain also apply for perceived encouragement for exercise. This association of higher perceived encouragement for exercise with greater weight regain was not evident for non-Blacks. Further, when examining interaction effects, race did not interact with perceived exercise encouragement for participants at extremely high or low levels of exercise.
Limitations
This study had several limitations. First, only the participants who were able to lose ≥ 4 kg during Phase I were randomized to complete the weight loss maintenance phase (Phase II). Although this was the primary intention of the study, these results are limited in that they have only been observed in those who successfully lost weight (see Svetkey et al., 2008). Second, the measures assessing psycho-social factors were self-report measures, which can be unreliable or biased. In addition, it is likely that certain psychosocial characteristics (i.e., perceived stress, receipt of social support) varied over time. Third, although Weight Loss Maintenance participants were the most racially and gender diverse of any previous weight loss maintenance study, the majority of participants were Black and non-Latino White; very few Hispanic and no Asian participants took part in this study, thus limiting the generalizability of the results to other ethnic groups. Despite this limitation, the current study is unique in that specific focus was given to Black participants, as this population continues to be underrepresented in research (Rogers & Lange, 2013). Because this study included such a large sample of Blacks, these results may be an initial characterization of this population.
Lastly, although the interaction models were able to explain some of the variance in weight regain (5.2 % at 12 months and 9.1 % at 30 months), they were unable to account for a large portion. However, this is not surprising due to the complex nature of weight regain. Further exploration into the potential psychosocial variables that may predict weight regain after a weight reduction intervention program should be conducted.
Conclusions
This study demonstrated that psychosocial variables, measured at randomization into Phase II, may predict the likelihood of weight loss maintenance at both 12 and 30 months. Although more research is needed to fully explore this relationship, these initial findings illustrate the importance of a more tailored weight maintenance program at the conclusion of successful weight loss programs, particularly evidenced by the complex interactions. Identification of an individual's most important and modifiable psychosocial predictors of weight loss maintenance will also allow practitioners to better tailor weight loss maintenance programs to an individual's specific needs, whereby increasing the likelihood of long-term weight loss maintenance success.
Acknowledgments
Funding was provided by National Heart, Lung, and Blood Institute Grants 5-U01 HL68734, 5-U01 HL68676, 5-U01 HL68790, 5-U01 HL68920, and 5-HL68955; This work was also supported in part by a faculty fellowship from the University of Texas MD Anderson Cancer Center's Duncan Family Institute for Cancer Prevention and Risk Assessment (awarded to DWS), as well as a National Institutions of Health postdoctoral training Grant (T32 DK064584; awarded to MRME).
Footnotes
Conflict of interest: Phillip J. Brantley, Diana W. Stewart, Valerie H. Myers, Molly R. Matthews-Ewald, Jamy D. Ard, Janelle Coughlin, Carmen Samuel-Hodge, Lillian D. Lien, Christina M. Gullion, Jack F. Hollis, Laura P. Svetkey, and Victor J. Stevens declare that they have no conflict of interest. Gerald J. Jerome declares consultation for Healthways, Inc. as a potential conflict of interest.
Informed consent: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Contributor Information
Phillip J. Brantley, Email: phil.brantley@pbrc.edu, Behavioral Medicine Laboratory, Pennington Biomedical Research Center, Louisiana State University System, 6400 Perkins Road, Baton Rouge, LA 70808, USA.
Diana W. Stewart, Department of Health Disparities Research, University of Texas MD Anderson Cancer Center, Houston, TX, USA
Valerie H. Myers, Klein Buendel, Inc., Golden, CO, USA
Molly R. Matthews-Ewald, Behavioral Medicine Laboratory, Pennington Biomedical Research Center, Louisiana State University System, 6400 Perkins Road, Baton Rouge, LA 70808, USA
Jamy D. Ard, Department of Epidemiology and Prevention, Wake Forest Baptist Medical Center, Winston-Salem, NC, USA
Janelle W. Coughlin, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Gerald J. Jerome, Department of Kinesiology, Towson University, Towson, MD, USA
Carmen Samuel-Hodge, Department of Nutrition, School of Public Health and School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Lillian F. Lien, Division of Endocrinology, Duke University Medical Center, Durham, NC, USA
Christina M. Gullion, Health Sciences Programs, Center for Health Research, Kaiser Permanente Northwest, Portland, OR, USA
Jack F. Hollis, Health Sciences Programs, Center for Health Research, Kaiser Permanente Northwest, Portland, OR, USA
Laura P. Svetkey, Division of Nephrology and the Sarah W. Stedman Nutrition and Metabolism Center, Duke University Medical Center, Durham, NC, USA
Victor J. Stevens, Health Sciences Programs, Center for Health Research, Kaiser Permanente Northwest, Portland, OR, USA
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th, text revision. Washington, DC: Author; 2000. [Google Scholar]
- Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure: DASH Collaborative Research Group. New England Journal of Medicine. 1997;336(16):1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- Bandura A. Social foundations of thought and action: A social cognitive theory. Englewood Cliffs, NJ: Prentice Hall; 1986. [Google Scholar]
- Bandura A, Adams N. Analysis of self-efficacy theory of behavioral change. Cognitive Therapy and Research. 1977;1(4):287–310. [Google Scholar]
- Barte JCM, Ter Bogt NCW, Bogers RP, Teixeira PJ, Blissmer B, Mori TA, et al. Maintenance of weight loss after lifestyle interventions for overweight and obesity, a systematic review. Obesity Reviews. 2010;11(12):899–906. doi: 10.1111/j.1467-789X.2010.00740.x. [DOI] [PubMed] [Google Scholar]
- Bock BC, Marcus BH, Pinto BM, Forsyth LH. Maintenance of physical activity following an individualized motivationally tailored intervention. Annals of Behavioral Medicine. 2001;23(2):79–87. doi: 10.1207/S15324796ABM2302_2. [DOI] [PubMed] [Google Scholar]
- Brantley PJ, Appel LJ, Hollis J, Stevens V, Ard J, Champagne C, et al. Design considerations and rationale of a multi-center trial to sustain weight loss: The weight loss maintenance trial. Clinical Trials. 2008;5:546–556. doi: 10.1177/1740774508096315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne SM. Psychological aspects of weight maintenance and relapse in obesity. Journal of Psychosomatic Research. 2002;53:1029–1036. doi: 10.1016/s0022-3999(02)00487-7. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Delahanty LM, Peyrot M, Shrader PJ, Williamson DA, Meigs JB, Nathan DM. Pretreatment, psychological, and behavioral predictors of weight outcomes among lifestyle intervention participants in the diabetes prevention program (DPP) Diabetes Care. 2013;36(1):34–40. doi: 10.2337/dc12-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or Metformin. New England Journal of Medicine. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfhag K, Rossner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obesity Reviews. 2005;6:67–85. doi: 10.1111/j.1467-789X.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- Fabricatore AN, Wadden TA, Moore RH, Butryn ML, Heymsfield SB, Nguyen AM. Predictors of attrition and weight loss success: Results from a randomized controlled trial. Behaviour Research and Therapy. 2009;47:685–691. doi: 10.1016/j.brat.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman KE, Reichmann SK, Costanzo PR, Zelli A, Ashmore JA, Musante GJ. Weight stigmatization and ideological beliefs: Relation to psychological functioning in obese adults. Obesity Research. 2005;5:907–916. doi: 10.1038/oby.2005.105. [DOI] [PubMed] [Google Scholar]
- Funk KL, Stevens VJ, Appel LJ, Bauck A, Brantley PJ, Champagne CM, et al. Associations of internet website use with weight change in a long-term weight loss maintenance program. Journal of Medical Internet Research. 2010;12(3):e29. doi: 10.2196/jmir.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halding A, Heggdal K, Wahl A. Experiences of self-blame and stigmatization for self-infliction among individuals living with COPD. Scandinavian Journal of Caring Sciences. 2008;25:100–107. doi: 10.1111/j.1471-6712.2010.00796.x. [DOI] [PubMed] [Google Scholar]
- Hollis JF, Gullion CM, Stevens VJ, Brantley PJ, Appel LJ, Ard JD, et al. Weight loss during the intensive intervention phase of the weight-loss maintenance trial. American Journal of Preventive Medicine. 2008;35:118–126. doi: 10.1016/k.amepre.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1177/0022146510383501. [DOI] [PubMed] [Google Scholar]
- Johnson SS, Paiva AL, Cummins CO, Johnson JL, et al. Transtheoretical model-based multiple behavior intervention for weight management: Effectiveness on a population basis. Preventive Medicine. 2008;46(3):238–246. doi: 10.1016/j.ypmed.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatric Annals. 2002;32(9):509–515. [Google Scholar]
- Kumanyika SK, Espeland MA, Bahnson JL, Bottom JB, Whelton PK. Ethnic comparison of weight loss in the trial of nonpharmacologic interventions in the elderly. Obesity Research. 2002;10:96–106. doi: 10.1038/oby.2002.16. [DOI] [PubMed] [Google Scholar]
- Kumanyika SK, Obarzanek E, Stevens VJ, Hebert PR, Whelton PK. Weight-loss experience of black and white participants in NHLBI-sponsored clinical trials. American Journal of Clinical Nutrition. 1991;53:1631S–1638S. doi: 10.1093/ajcn/53.6.1631S. [DOI] [PubMed] [Google Scholar]
- Liebbrand R, Fitcher MM. Maintenance of weight loss after obesity treatment: Is continuous support necessary? Behaviour Research and Therapy. 2002;40:1275–1289. doi: 10.1016/S0005-7967(01)00099-7. [DOI] [PubMed] [Google Scholar]
- McHorney CA, Ware JE, Lu JFR, Sherbourne CD. The MOS 36-item short-form health survey (SF-36®): III. Tests of data quality, scaling assumptions and reliability across diverse patient groups. Medical Care. 1994;32(4):40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Preparing people to change addictive behavior. New York, NY: The Guilford Press; 1991. [Google Scholar]
- Perri MG, Nezu AM, Patti ET, McCann KL. Effect of length of treatment on weight loss. Journal of Consulting and Clinical Psychology. 1989;57:450–452. [PubMed] [Google Scholar]
- Perri MG, Shapiro RM, Ludwig WW, Twentyman CT, McAdoo WG. Maintenance strategies for the treatment of obesity: An evaluation of relapse prevention training and posttreatment contact by mail and telephone. Journal of Consulting and Clinical Psychology. 1984;52(3):404–413. doi: 10.1037/0022-006X.52.3.404. [DOI] [PubMed] [Google Scholar]
- Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: Toward an integrative model of change. Journal of Consulting and Clinical Psychology. 1983;51(3):390–395. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. American Journal of Health Promotion. 1997;12(1):38–48. doi: 10.4278/0890-1171-12.1.38. doi:10.4278.0890-1171-12.1.38. [DOI] [PubMed] [Google Scholar]
- Rickel KA, Milsom VA, Ross KM, Hoover VJ, Peterson ND, Perri MG. Differential response of African American and Caucasian women to extended-care programs for obesity management. Ethnicity and Disease. 2011;21:170–175. [PMC free article] [PubMed] [Google Scholar]
- Rogers W, Lange MM. Rethinking the vulnerability of minority populations in research. American Journal of Public Health. 2013;103(12):2141–2146. doi: 10.2105/AJPH.2012.301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohyans LM, Pressler SJ. Depressive symptoms and heart failure: Examining the sociodemographic variables. Clinical Nurse Specialist. 2009;23:138–144. doi: 10.1016/S0735-1097(01)01334-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollnick S, Mason P, Butler C. Health behavior change: A guide for practitioners. London: Churchill Livingston; 1999. [Google Scholar]
- Rubin DB. Multiple imputation for nonresponse in surveys. New York: Wiley; 1987. [Google Scholar]
- Sallis JF, Grossman RM, Pinski RB, Patterson TL, Nader PR. The development of scales to measure social support for diet and exercise behaviors. Preventive Medicine. 1987;16:825–836. doi: 10.1016/0091-7435(87)90022-3. [DOI] [PubMed] [Google Scholar]
- Schafer JL. Analysis of incomplete multivariate data. New York: Chapman and Hall; 1997. [Google Scholar]
- Schvey NA, Puhl RM, Brownell KD. The impact of weight stigma on caloric consumption. Obesity. 2011;19(10):1957–1962. doi: 10.1038/oby.2011.204. [DOI] [PubMed] [Google Scholar]
- Sciamanna CN, Kiernan M, Rolls BJ, Boan J, Stuckey H, Dellasega C. Practices associated with weight loss versus weight-loss maintenance: Results of a national study. American Journal of Preventive Medicine. 2011;41(2):159–166. doi: 10.1016/j.amepre.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Stevens VJ, Obarzanek E, Cook NR, Lee IM, Appel LJ, Smith WD, et al. Long-term weight loss and changes in blood pressure: Results of the trials of hypertension prevention, phase II. Annals of Internal Medicine. 2001;134(1):1–11. doi: 10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- Sutin AR, Terracciano A. Perceived weight discrimination and obesity. PLoS ONE. 2013;8(7):e70048. doi: 10.1371/journal.pone.0070048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svetkey LP, Ard JD, Stevens VJ, Loria CM, Young DY, Hollis JF, et al. Predictors of long-term weight loss in adults with modest initial weight loss by sex and race. Obesity. 2012;20(9):1820–1828. doi: 10.1038/oby.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svetkey LP, Stevens VJ, Brantley PJ, Appel LJ, Hollis JF, Loria CM, et al. Comparison of strategies for sustaining weight loss: The weight loss maintenance randomized controlled trial. Journal of the American Medical Association. 2008;229:1139–1148. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- Teixeira PJ, Going SB, Houtkooper LB, Cussler EC, Martin CJ, Metcalfe LL, et al. Weight loss readiness in middle-aged women: Psychosocial predictors of success for behavioral weight reduction. Journal of Behavioral Medicine. 2002;25(6):499–523. doi: 10.1023/a:1020687832448. [DOI] [PubMed] [Google Scholar]
- Tukey JW. Exploratory data analysis. Reading, MA: Addison-Wesley; 1977. p. 688. [Google Scholar]
- Turk MW, Yang K, Hravnak M, Sereika SM, Ewing LJ, Burke LE. Randomized clinical trials of weight-loss maintenance: A review. The Journal of Cardiovascular Nursing. 2009;24(1):58–80. doi: 10.1097/01.JCN.0000317471.58048.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira PN, Silva MN, Mata J, Coutinho SR, Santos TC, Sardina LB, et al. Correlates of health-related quality of life, psychological well-being, and eating self-regulation after successful weight loss maintenance. Journal of Behavioral Medicine. 2012;36:601–610. doi: 10.1007/s10865-012-9454-9. [DOI] [PubMed] [Google Scholar]
- Wadden TA, Butryn ML, Byrne KJ. Efficacy of lifestyle modification for long-term weight control. Obesity Research. 2004;12(Suppl. 32):1515–1625. doi: 10.1038/oby.2004.282. [DOI] [PubMed] [Google Scholar]
- Wadden TA, Neiberg RH, Wing RR, Clark JM, Delahanty LM, Hill JO, et al. Four-year weight losses in the Look AHEAD study: Factors associated with long-term success. Obesity. 2011;19(10):1987–1999. doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chen X. How much of racial/ethnic disparities in dietary intakes, exercise, and weight status can be explained by nutrition- and health-related psychosocial factors and socioeconomic status among US adults? Journal of the American Dietetic Association. 2011;111(12):1904–1911. doi: 10.1016/j.jada.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE, Kosinski M, Keller SK. SF-36® Physical and mental health summary scales: A user's manual. Boston: The Health Institute; 1994. [Google Scholar]
- Ware JE, Sherbourne CD. The MOS 36-item short form health survey (SF-36): Conceptual framework and item selection. Medical Care. 1992;30:473–483. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- Ware JE, Snow KK, Kosinski M, Gandek B. SF-36® Health survey manual and interpretation guide. Boston: New England Medical Center, The Health Institute; 1993. [Google Scholar]
- Watson DL, Tharp RG, Loomis LH, Steinberg S. Self-directed behavior: Self-modification for personal adjustment. 5th. Pacific Grove, CA: Brooks/Cole; 1989. [Google Scholar]
- West DS, DiLillo V, Bursan Z, Gore SA, Greene PG. Motivational interviewing improves weight loss in women with type 2 diabetes. Diabetes Care. 2007;30:1081–1087. doi: 10.2337/dc06-1966. [DOI] [PubMed] [Google Scholar]
- West DS, Prewitt TE, Bursac Z, Felix HC. Weight loss of black, white, and Hispanic men and women in the diabetes prevention program. Obesity. 2008;16:1413–1420. doi: 10.1038/oby.2008.224. [DOI] [PubMed] [Google Scholar]
- Williams GC, Grow VM, Freedman ZR, Ryan RM, Deci EL. Motivational predictors of weight loss and weight-loss maintenance. Journal of Personality and Social Psychology. 1996;70(1):115–126. doi: 10.1037//0022-3514.70.1.115. [DOI] [PubMed] [Google Scholar]
- Wing RR, Hill JO. Successful weight loss maintenance. Annual Reviews in Nutrition. 2001;21:323–341. doi: 10.1146/annurev.nutr.21.1.323. [DOI] [PubMed] [Google Scholar]
- Wing RR, Papandonatos G, Fava JL, Gorin AA, Phelan S, McCaffery J, et al. Maintaining large weight losses: The role of behavioral and psychological factors. Journal of Consulting and Clinical Psychology. 2008;76(6):1015–1021. doi: 10.1037/a0014159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wott CB, Carels RA. Overt weight stigma, psychological distress and weight loss treatment outcomes. Journal of Health Psychology. 2010;15(4):608–614. doi: 10.1177/1359105309355339. [DOI] [PubMed] [Google Scholar]