Abstract
Lupus nephritis (LN) affects many patients with juvenile systemic lupus erythematosus (SLE) and is a significant cause of disease morbidity. Membraneous plus proliferative LN (M+PLN) may represent a more difficult to treat subtype of juvenile LN, compared to isolated proliferative LN (PLN.) In this retrospective observational study, we utilized data from the Childhood Arthritis and Rheumatism Research Alliance (CARRA) registry to compare response rates for pediatric M+PLN versus PLN. Response was assessed at the most recent CARRA registry visit gathered ≥ 6 months after diagnostic kidney biopsy. Estimated glomerular filtration rate (GFR) less than 90 ml/min/1.73m2, indicating renal insufficiency, was found in 16.1% of patients with M+PLN and 6.1% of patients with PLN (P=0.071). We found no significant difference in achievement of response in either hematuria or proteinuria between PLN and M+PLN groups or between subgroups determined by presence of class III vs. class IV proliferative disease. Exposure rates to mycophenolate, cyclophosphamide, and rituximab were similar between groups. Future studies will be necessary to correlate pediatric LN renal histology data with treatment response as well as other disease outcome measures.
Keywords: lupus, nephritis, response, membranous, proliferative, pediatric, CARRA registry
Introduction
Children and adolescents with systemic lupus erythemetosus (SLE) frequently develop lupus nephritis (LN), with approximately 50% showing evidence of kidney involvement1. Severity of juvenile LN is in large part determined by histology found on kidney biopsy, as graded by the International Society of Nephrology/Renal Pathology Society (ISN/RPS) classification.2 Presence of proliferative LN, defined as ISN/RPS class III or IV, and failure to attain remission of juvenile LN are risk factors for the development of chronic kidney disease and poor outcomes.3–8
Treatment of proliferative LN with conventional first line immunosuppressive agents such as cyclophosphamide (CYC) or mycophenolate (MMF) is not effective for a significant percentage of patients with juvenile SLE, with reported failure rates for induction treatment ranging from 10% to 43%.9, 10 This study is the first comparison of response rates for M+PLN versus PLN in juvenile LN. Data from studies of LN in adult patients suggests that combined membranous plus proliferative LN (M+PLN) may be associated with a poorer treatment response. Najafi et. al. examined remission rates of severe LN under an oral CYC treatment regimen and found that only 27% of patients with class IV+V LN by ISN/RPS criteria entered remission after 120±65 months of follow-up, compared with 51% of patients with class IV LN.11 Sloan et. al. reported remission rates for class IV+V LN of only 21% after treatment with oral CYC and after 2.7 ± 5.4 years of follow-up.12
However, it is important to note that prospective studies directly comparing patients with M+PLN to those with isolated proliferative lupus nephritis (PLN) have not been performed, and that the potential association between M+PLN and treatment resistance has not been definitively demonstrated. More recently, Bao et. al. reported a complete or partial remission rate of 45% after 6 months of IV CYC treatment in patients with class IV+V LN,13 and this compares favorably to the complete or partial remission rate of 30% reported by Ginzler et. al. for a group of patients treated with IV CYC, 70% of whom had PLN with no membraneous involvement.14
Whether M+PLN represents a more treatment resistant histologic class in juvenile LN is unknown. To attempt to answer this question, we utilized data from the Childhood Arthritis and Rheumatology Research Alliance (CARRA) observational registry of pediatric rheumatology patients to document prevalence and compare remission rates for PLN and M+PLN in this cohort.
Methods
Data Source
The CARRA Registry (CR) is an observational longitudinal data capture study that encompasses all major pediatric rheumatic diseases. Fifty-nine active CARRA clinical sites participate in the CR and represent the majority of pediatric rheumatology centers from all major geographic regions of the United States. Patients with juvenile SLE were eligible for recruitment into the CR if they met revised 1997 ACR classification criteria for diagnosis of SLE,15 they developed juvenile SLE at ≤ 18 years of age, and their enrollment into the CR occurred at ≤ 21 years of age. After obtaining Institutional Review Board approval, we analyzed clinical and demographic data from CR registry patients with biopsy-confirmed PLN or M+PLN as per ISN/RPS classification criteria. We used de-identified data from all active clinical sites from the start of the CR in May 2010 through March 2013.
Assessment of Response to Therapy
Response was assessed by evaluation of the independent clinical parameters of proteinuria and hematuria. Criteria for response were defined by the CR clinical data, which is collected at each CR patient visit in the form of binary survey questions (e.g. “Has the patient had a protein/creatinine ratio > 0.5 in the last 10 days? Yes or No” and “Has the patient had > 5 RBC/hpf on urinalysis in the past 10 days? Yes or No.”) Response of proteinuria was defined as protein/creatinine ratio of < 0.5. Response of hematuria was defined as < 6 RBC/hpf on urinalysis. Response of hematuria and proteinuria was assessed at the most recent CARRA registry visit gathered ≥ 6 months after diagnostic kidney biopsy. Clinical data from the time period prior to patient enrollment in the CR was not included in the CR, and therefore this data was not available for analysis.
Exclusion Criteria for Response Analyses
Patients were excluded from response analyses if 1) the most recent CR visit was <6 months after diagnostic kidney biopsy or 2) clinical data for the response parameter was not available for a CR visit ≥ 6 months after diagnostic kidney biopsy.
Calculations and Statistical Analysis
Estimated glomerular filtration rate (GFR) was calculated using the Schwartz formula.16, 17 Chi-square test or Fisher exact test was used to evaluate associations between type of nephritis and categorical variables, as appropriate. Two-sample t-test, Wilcoxon Rank Sum test, or Mann-Whitney U test was used to evaluate associations between type of nephritis and ordinal and continuous variables, as appropriate.
Results
Patient Characteristics
We identified 320 CR patients with juvenile SLE and juvenile LN diagnosed by renal biopsy. Of these, 185 had PLN and 38 had M+PLN (Table 1). [insert Table 1.] Gender distribution, age at onset of juvenile SLE, duration of juvenile SLE, SLEDAI scores at the most recent CR visit, and frequency of antinuclear antibody (ANA) positivity were similar between both groups. Estimated glomerular filtration rate (GFR) less than 90 ml/min/1.73m2, indicating renal insufficiency, was found in 16.1% of patients with M+PLN and 6.1% of patients with PLN (P=0.071).
Table 1.
Characteristics of Patients with Juvenile Lupus Nephritis
| Characteristic | PLN (N = 185) | M+PLN (N=38) | P |
|---|---|---|---|
| Male:Female | 36:149 | 8:30 | 0.822 |
| Age onset SLE (yrs; mean ± SD) | 11.9 ± 3.3 | 12.6 ± 2.6 | 0.226 |
| Duration of SLE at 1st Renal Biopsy (yrs; ± SD) | 1.1 ± 1.8 | 1.8 ± 2.1 | 0.076 |
| Duration of SLE (yrs; mean ± SD) | 4.6 ± 3.3 | 4.7 ± 3.2 | 0.853 |
| Duration of LN (yrs; mean ± SD) | 3.6 ± 3.1 | 2.8 ± 2.3 | 0.073 |
| SLEDAI at LV (mean ± SD) | 4.3 ± 5.3 | 3.6 ± 5.1 | 0.460 |
| ANA positive (%, N) | 94% (174) | 92% (35) | 1.000 |
| Est. GFR at LV < 90 ml/min/m2 (%, n/N)a | 6.2 %, (10/162) | 16.1 %, (5/31) | 0.071 |
LV, last study visit; LN, lupus nephritis; ANA, antinuclear antibody; est. GFR, estimated glomerular filtration rate; yrs = years; duration of LN = (age at LV - age at 1st biopsy); duration of SLE = (age at LV - age at onset of SLE)
excluded patients without data from ≥ 6m post-biopsy
Medication Exposure
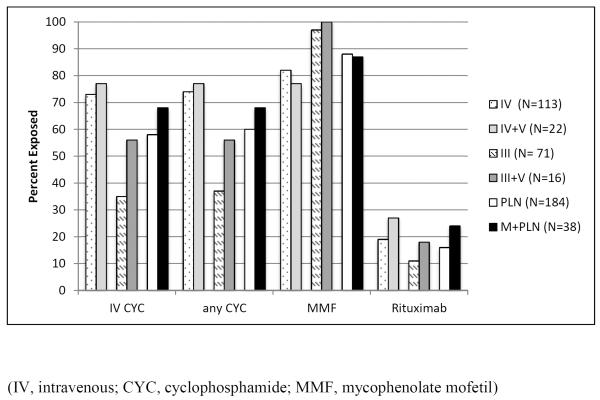
Data regarding prior and current medication exposures was available for 184 of the patients with PLN and 38 of the patients with M+PLN (Figure 1). [insert Figure 1.] Percentage of patients with exposures to CYC, MMF, and rituximab were similar between PLN and M+PLN groups, as well as between subgroups determined by presence of class III vs. class IV proliferative disease. Rates of CYC use were higher for patients with class IV LN compared to patients with class III LN (77% vs. 35%, p<0.0001).
Figure 1.
Medication Exposures of Patients with Juvenile Lupus Nephritis
Response of Hematuria and Proteinuria
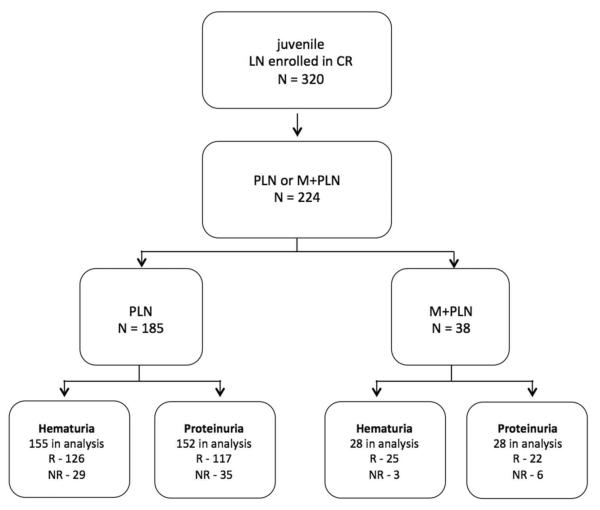
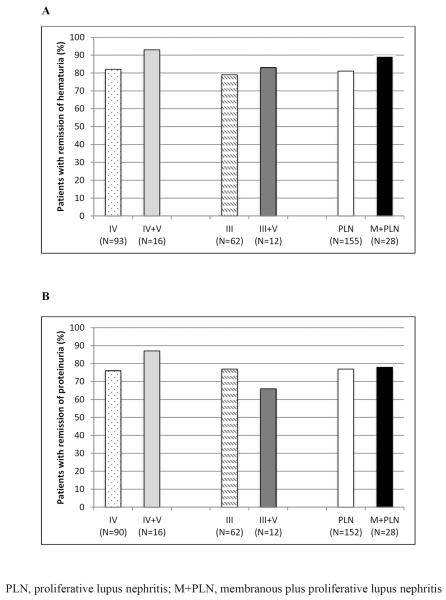
Inclusion of patients in our response analyses and the number of patients in each group who achieved response at their most recent CR visit is depicted (Figure 2). [insert Figure 2.] We found no significant difference in achievement of response in either hematuria or proteinuria between PLN and M+PLN groups or between subgroups determined by presence of class III vs. class IV proliferative disease (Table 3). [insert Figure 3.]
Figure 2.
Inclusion of CR patients with juvenile LN in response analysis and number of patients achieving response for both hematuria and proteinuria.
(R, response; NR, no response)
Figure 3.
Comparison of response rates for hematuria and proteinuria in patients with PLN and M+PLN. (A) response of hematuria. (B) response of proteinuria
Patients with class IV and class IV+V juvenile LN were re-categorized into groups based on presence of global vs. segmental glomerular inflammation on biopsy, irrespective of the presence of membranous disease. Of these patients, those included in the response analysis for hematuria were comprised of 34 subjects with global proliferative disease (class IV(G),) 27 with segmental proliferative disease (class IV(S),) and 48 for whom type of glomerular proliferative disease was not reported. Those included in the proteinuria analysis were comprised of 33 with global, 27 with segmental, and 46 for whom the type was not reported. Based on the available data, we found no significant difference in response of either hematuria (75% of patients with response vs. 85%, P=0.52) or proteinuria (76% vs. 92%, P=0.16), for patients with global vs. segmental class IV juvenile LN, respectively.
Discussion
Histopathologic classification of juvenile LN contributes significantly to determination of disease severity. Presence of prolifierative glomerulonephritis has been well established as a poor prognostic factor,3, 6 and therefore patients with class III or class IV LN are generally treated with more aggressive immunosuppressive therapy. Failure to attain remission and failure to avoid flares of juvenile LN are well-established risk factors for ESRD, highlighting the importance of effective treatment for juvenile LN.4, 5 Identification of a subset of juvenile LN patients who are at increased risk for treatment failure would be valuable, as it would allow for the focused development of new treatment strategies.
Data from studies in adult patients suggests that M+PLN may be more refractory to treatment than PLN.11, 12 In fact, some authors have gone so far as to conduct prospective, randomized trials of novel induction regimens for M+PLN, based on the premise that this histologic subtype is less responsive to conventional treatment.13 However, it is important to note the lack of studies directly comparing treatment outcomes of M+PLN and PLN, as defined under the ISN/RPS classification system. An issue of particular importance arises for studies which utilized the 1982 World Health Organization (WHO) classification,18 because under this schema, what would be considered class IV LN by the ISN criteria is split into two separate categories, category III>50% and category IV, which are defined by the presence of segmental vs. global glomerular inflammation, respectively. If an analogous separation of patients with M+PLN into two similar categories is not performed (as it often was not,) direct comparison of PLN and M+PLN patients is not possible.
For example, Najafi et. al. demonstrated a substantial and significant difference in remission rates for WHO category IV LN compared to a M+PLN category defined by combining WHO category Vc(>50%) and WHO category Vd (60% vs. 27%, respectively.) Although this data was cited by Bao et. al. as justification for a prospective trial of a novel induction regimen for treatment of patients with M+PLN, it is important to note that WHO category III>50% patients were excluded from this comparison.13 Additionally, these category III>50% patients had significantly lower remission rates (38%) than the category IV patients in the same study, suggesting that exclusion of these patients from the comparison limits it's value, at least in so far as addressing the issue of whether remission rates for M+PLN differ from those of PLN.
Although the clinicopathologic association of class IV LN with worse outcomes has been well described in both the adult19–21 and pediatric literature3–8, attempts to correlate more specific and detailed renal histopathology findings with disease outcomes or with achievement of disease response have been limited and sometimes contradictory. Notably, Marks et. al. studied the clinical outcomes of 39 patients with juvenile LN and found that diffuse glomerular inflammation, as defined by ISN class IV(G), was associated with worse outcomes than segmental LN, as defined by class IV(S). This stands in direct contrast to the data from adult studies mentioned above.22 In this study, we found no difference in response rates between class IV(G) and class IV(S) patients.
Our study is a novel attempt to answer the question of whether M+PLN represents a more treatment refractory subtype of juvenile LN, and it is the largest such study to date. The CR represents an extremely large and valuable source of data pertaining to pediatric rheumatology patients and their diseases. Nevertheless, CR data does have some unavoidable limitations. Notably, there are missing data. For example, for reporting of serum creatinine, “not done” is an option on the CR data collection surveys. Thus, a certain number of patients were excluded from specific cross-sectional analyses depending on the availability of pertinent data.
Gender distribution, age at onset of juvenile SLE, duration of juvenile SLE, SLEDAI scores at the most recent CR visit, and frequency of antinuclear antibody (ANA) positivity were similar between both groups. Rates of ANA positivity, approached, but fell short of 100%, which is perhaps a surprising finding in a group of patients for whom the expectation would be 100% positivity for ANA. We believe that this finding can be explained in part by missing data. Interestingly, we found no significant difference in rates of response for M+PLN vs. PLN, suggesting that current therapies are equally effective for both subtypes of juvenile LN. Rates of CYC use were significantly higher for patients with class IV LN compared to patients with class III LN, which may reflect a notable but unexplained practice trend in the pediatric community. Although rates of response were similar, we did note increased prevalence of renal insufficiency, defined by ESR <90 mL/min/1.73 m2, in the group with M+PLN. This finding is difficult to interpret due to possible confounding factors such as missing patients and the difference in LN disease duration between the two groups. It must be noted that data pertaining to height and/or serum creatinine was not entered in the CR for every patient visit, and this missing data made it impossible to calculate estimated GFR for a 23 patients from the PLN group and 7 patients from the M+PLN group. Although we did not find a significant correlation between presence of segmental vs. global glomerular injury and achievement of response, this analysis was limited by the large percentage of patients for whom this histologic data was unavailable.
This study was inherently limited due to its cross-sectional, retrospective design. The ideal method for addressing our research question would be a prospective, randomized, double blind, controlled clinical trial. We must acknowledge as well that the use of differing definitions of response to therapy can substantially influence the ability to detect a therapeutic difference between patient groups23, 24. Our definitions of response for proteinuria and hematuria were confined by our data set. Additionally, the absence of baseline pre-treatment renal parameters did not allow for assessment of a serial measure of response after initiation of therapy. Recent LN trials have used measures of response determined at unified time points after initiation of treatment. Because diagnosis of LN preceded enrollment in the CR for many of the patients included in our analysis, clinical data collected at first presentation (i.e. at time of renal biopsy) were not available. Specifically, data regarding complement levels, presence or level of anti-dsDNA antibody, measures of hematuria, proteinuria, and serum creatinine were not available for time points preceding CR enrollment. Given the variable length of time for each patient between diagnosis of LN and enrollment in the CR, analysis at a unified time point for all patients was not possible. Nor was it possible to do a survival analysis for time to outcome. Thus, our cross-sectional analyses included patients who had undergone variable lengths of treatment, and therefore may have been more or less likely to meet response criteria.
Additionally, although available data on use of immunosuppressant medications suggests that treatment was similar between groups, it is important to note that detailed information regarding the steroid dosage of individual patients was not available, and therefore differences in corticosteroid dosage between groups may have been unaccounted for. Data on use of angiotensin-converting-enzyme-inhibitors and angiotensin-receptor-blockers was also not available, and may have influenced measures of proteinuria. Renal biopsies, although interpreted according to standardized diagnostic criteria, were read by numerous pathologists at different institutions and therefore may have been subject to varying methods of interpretation.
Despite its limitations, this study remains informative. We observed high rates of response in our study population in both patient groups, as well as a trend toward more renal insufficiency in the M+PLN group. Future longitudinal, prospective, controlled studies will be needed to examine the relationship between renal histopathology and treatment response in juvenile LN. As the pediatric rheumatology community makes progress in the design and implementation of prospective, multi-center studies utilizing consensus treatment plans for juvenile LN, it will be important to capitalize on this opportunity to collect detailed, precise, and complete renal histopathology data and to correlate this data with treatment response as well as other disease outcome measures.
Acknowledgements
This work could not have been accomplished without the aid of the following organizations: NIAMS, Friends of CARRA, and the Arthritis Foundation. We would also like to thank all participants and hospital sites that recruited patients for the CARRA Registry. The authors thank the following CARRA Registry site principal investigators and research coordinators: L. Abramson, E. Anderson, M. Andrew, N. Battle, M. Becker, H. Benham, T. Beukelman, J. Birmingham, P. Blier, A. Brown, H. Brunner, A. Cabrera, D. Canter, D. Carlton, B. Caruso, L. Ceracchio, E. Chalom, J. Chang, P. Charpentier, K. Clark, J. Dean, F. Dedeoglu, B. Feldman, P. Ferguson, M. Fox, K. Francis, M. Gervasini, D. Goldsmith, G. Gorton, B. Gottlieb, T. Graham, T. Griffin, H. Grosbein, S. Guppy, H. Haftel, D. Helfrich, G. Higgins, A. Hillard, J.R. Hollister, J. Hsu, A. Hudgins, C. Hung, A. Huttenlocher, A. Imlay, L. Imundo, C.J. Inman, J. Jaqith, R. Jerath, L. Jung, P. Kahn, A. Kapedani, D. Kingsbury, K. Klein, M. Klein-Gitelman, A. Kunkel, S. Lapidus, S. Layburn, T. Lehman, C. Lindsley, M. Macgregor-Hannah, M. Malloy, C. Mawhorter, D. McCurdy, K. Mims, N. Moorthy, D. Morus, E. Muscal, M. Natter, J. Olson, K. O'Neil, K. Onel, M. Orlando, J. Palmquist, M. Phillips, L. Ponder, S. Prahalad, M. Punaro, D. Puplava, S. Quinn, A. Quintero, C. Rabinovich, A. Reed, C. Reed, S. Ringold, M. Riordan, S. Roberson, A. Robinson, J. Rossette, D. Rothman, D. Russo, N. Ruth, K. Schikler, A. Sestak, B. Shaham, Y. Sherman, M. Simmons, N. Singer, S. Spalding, H. Stapp, R. Syed, E. Thomas, K. Torok, D. Trejo, J. Tress, W. Upton, R. Vehe, E. von Scheven, L. Walters, J. Weiss, P. Weiss, N. Welnick, A. White, J. Woo, J. Wootton, A. Yalcindag, C. Zapp, L. Zemel, and A. Zhu.
Grants: The CARRA Registry is supported by grants from NIAMS, Friends of CARRA, and the Arthritis Foundation. The CARRA Registry is supported by NIH grant RC2AR058934.
References
- 1.Hiraki LT, Benseler SM, Tyrrell PN, Hebert D, Harvey E, Silverman ED. Clinical and laboratory characteristics and long-term outcome of pediatric systemic lupus erythematosus: a longitudinal study. The Journal of pediatrics. 2008;152:550–6. doi: 10.1016/j.jpeds.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 2.Weening JJ, D'Agati VD, Schwartz MM, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Journal of the American Society of Nephrology : JASN. 2004;15:241–50. doi: 10.1097/01.asn.0000108969.21691.5d. [DOI] [PubMed] [Google Scholar]
- 3.Emre S, Bilge I, Sirin A, et al. Lupus nephritis in children: prognostic significance of clinicopathological findings. Nephron. 2001;87:118–26. doi: 10.1159/000045899. [DOI] [PubMed] [Google Scholar]
- 4.Lee BS, Cho HY, Kim EJ, et al. Clinical outcomes of childhood lupus nephritis: a single center's experience. Pediatric nephrology. 2007;22:222–31. doi: 10.1007/s00467-006-0286-0. [DOI] [PubMed] [Google Scholar]
- 5.Moroni G, Quaglini S, Gallelli B, Banfi G, Messa P, Ponticelli C. The long-term outcome of 93 patients with proliferative lupus nephritis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2007;22:2531–9. doi: 10.1093/ndt/gfm245. [DOI] [PubMed] [Google Scholar]
- 6.Lupus nephritis: prognostic factors and probability of maintaining life-supporting renal function 10 years after the diagnosis. Gruppo Italiano per lo Studio della Nefrite Lupica (GISNEL) American journal of kidney diseases : the official journal of the National Kidney Foundation. 1992;19:473–9. doi: 10.1016/s0272-6386(12)80957-7. [DOI] [PubMed] [Google Scholar]
- 7.McCurdy DK, Lehman TJ, Bernstein B, et al. Lupus nephritis: prognostic factors in children. Pediatrics. 1992;89:240–6. [PubMed] [Google Scholar]
- 8.Rush PJ, Baumal R, Shore A, Balfe JW, Schreiber M. Correlation of renal histology with outcome in children with lupus nephritis. Kidney international. 1986;29:1066–71. doi: 10.1038/ki.1986.108. [DOI] [PubMed] [Google Scholar]
- 9.Lau KK, Ault BH, Jones DP, Butani L. Induction therapy for pediatric focal proliferative lupus nephritis: cyclophosphamide versus mycophenolate mofetil. Journal of pediatric health care : official publication of National Association of Pediatric Nurse Associates & Practitioners. 2008;22:282–8. doi: 10.1016/j.pedhc.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Gibson KL, Gipson DS, Massengill SA, et al. Predictors of relapse and end stage kidney disease in proliferative lupus nephritis: focus on children, adolescents, and young adults. Clinical journal of the American Society of Nephrology : CJASN. 2009;4:1962–7. doi: 10.2215/CJN.00490109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Najafi CC, Korbet SM, Lewis EJ, et al. Significance of histologic patterns of glomerular injury upon long-term prognosis in severe lupus glomerulonephritis. Kidney international. 2001;59:2156–63. doi: 10.1046/j.1523-1755.2001.00730.x. [DOI] [PubMed] [Google Scholar]
- 12.Sloan RP, Schwartz MM, Korbet SM, Borok RZ. Long-term outcome in systemic lupus erythematosus membranous glomerulonephritis. Lupus Nephritis Collaborative Study Group. Journal of the American Society of Nephrology : JASN. 1996;7:299–305. doi: 10.1681/ASN.V72299. [DOI] [PubMed] [Google Scholar]
- 13.Bao H, Liu ZH, Xie HL, Hu WX, Zhang HT, Li LS. Successful treatment of class V+IV lupus nephritis with multitarget therapy. Journal of the American Society of Nephrology : JASN. 2008;19:2001–10. doi: 10.1681/ASN.2007121272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginzler EM, Dooley MA, Aranow C, et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. The New England journal of medicine. 2005;353:2219–28. doi: 10.1056/NEJMoa043731. [DOI] [PubMed] [Google Scholar]
- 15.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis and rheumatism. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. Journal of the American Society of Nephrology : JASN. 2009;20:629–37. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz GJ, Work DF. Measurement and estimation of GFR in children and adolescents. Clinical journal of the American Society of Nephrology : CJASN. 2009;4:1832–43. doi: 10.2215/CJN.01640309. [DOI] [PubMed] [Google Scholar]
- 18.J C, LH S. Lupus Nephritis, in Renal disease, classification and atlas of glomerular diseases. Igaku-Shoin; Tokyo/New York: 1982. [Google Scholar]
- 19.Donadio JV, Jr., Hart GM, Bergstralh EJ, Holley KE. Prognostic determinants in lupus nephritis: a long-term clinicopathologic study. Lupus. 1995;4:109–15. doi: 10.1177/096120339500400206. [DOI] [PubMed] [Google Scholar]
- 20.Jacobsen S, Starklint H, Petersen J, et al. Prognostic value of renal biopsy and clinical variables in patients with lupus nephritis and normal serum creatinine. Scandinavian journal of rheumatology. 1999;28:288–99. doi: 10.1080/03009749950155464. [DOI] [PubMed] [Google Scholar]
- 21.MacGowan JR, Ellis S, Griffiths M, Isenberg DA. Retrospective analysis of outcome in a cohort of patients with lupus nephritis treated between 1977 and 1999. Rheumatology. 2002;41:981–7. doi: 10.1093/rheumatology/41.9.981. [DOI] [PubMed] [Google Scholar]
- 22.Marks SD, Sebire NJ, Pilkington C, Tullus K. Clinicopathological correlations of paediatric lupus nephritis. Pediatric nephrology. 2007;22:77–83. doi: 10.1007/s00467-006-0296-y. [DOI] [PubMed] [Google Scholar]
- 23.Wofsy D, Hillson JL, Diamond B. Abatacept for lupus nephritis: alternative definitions of complete response support conflicting conclusions. Arthritis and rheumatism. 2012;64:3660–5. doi: 10.1002/art.34624. [DOI] [PubMed] [Google Scholar]
- 24.Wofsy D, Hillson JL, Diamond B. Comparison of alternative primary outcome measures for use in lupus nephritis clinical trials. Arthritis and rheumatism. 2013;65:1586–91. doi: 10.1002/art.37940. [DOI] [PMC free article] [PubMed] [Google Scholar]