Abstract
Age-related increases in right frontal cortex activation are a common finding in the neuroimaging literature. However, neurocognitive factors contributing to right frontal over-recruitment remain poorly understood. Here we investigated the influence of age-related reaction time (RT) slowing and white matter (WM) microstructure reductions as potential explanatory factors for age-related increases in right frontal activation during task switching. Groups of younger (N = 32) and older (N = 33) participants completed a task switching paradigm while functional magnetic resonance imaging (fMRI) was performed, and rested while diffusion tensor imaging (DTI) was performed. Two right frontal regions of interest (ROIs), the dorsolateral prefrontal cortex (DLPFC) and insula, were selected for further analyses from a common network of regions recruited by both age groups during task switching. Results demonstrated age-related activation increases in both ROIs. In addition, the older adult group showed longer RT and decreased fractional anisotropy in regions of the corpus callosum with direct connections to the fMRI ROIs. Subsequent mediation analyses indicated that age-related increases in right insula activation were mediated by RT slowing and age-related increases in right DLPFC activation were mediated by WM microstructure. Our results suggest that age-related RT slowing and WM microstructure declines contribute to age-related increases in right frontal activation during cognitive task performance.
Keywords: aging, DTI, task switching, neural efficiency, over-recruitment, mediation
INTRODUCTION
Human aging is associated with altered brain activation patterns on a number of cognitive tasks. Alterations in brain activation associated with aging tend to be especially pronounced on tasks that emphasize cognitive control processes (Drag and Bieliauskas, 2010). Cognitive control refers to a set of processes that enable humans to flexibly shape thoughts and behavior in order to accomplish internal goals (Miller and Cohen, 2001). One way to explore this cognitive ability is by using the task switching paradigm, in which participants are required to perform two separate tasks in isolation (non-switch condition) or switch between the two tasks (switch condition). The requirement to switch between tasks tends to prolong reaction time (RT), with this effect being especially pronounced in older adults (Kray and Lindenberger, 2000).
Young adults typically recruit a distributed set of brain regions, prominently involving frontoparietal regions, during task switching (Kim et al., 2012) and other forms of cognitive control tasks (Badre and Wagner, 2006; Cole and Schneider, 2007; Neumann et al., 2008). While a variety of age-related alterations in brain activation during cognitive control tasks have been reported, among the most common appears to be increased activation of the frontal cortex (DiGirolamo et al., 2001; Drag and Bieliauskas, 2010; Spreng et al., 2010; Gazes et al., 2012; Di et al., 2014). In particular, older adults are often found to show greater activation than younger adults in right frontal regions (reviewed in Dennis and Cabeza, 2008; Reuter-Lorenz and Park, 2010).
Despite the relative ubiquity of age-related right frontal over-recruitment, little is known about the factors that contribute to this phenomenon. Among the many neurocognitive changes associated with aging, two that hold high potential as contributors to age-related brain activation increases are RT slowing and white matter (WM) microstructure reductions. Both RT and WM microstructure have potentially high explanatory value as proxy variables of age-related BOLD activation increases because they change significantly with age, and have been found to correlate with BOLD activation magnitudes. For example, RT slowing is the most frequently reported age-related performance change (Salthouse, 1993, 1996), and many studies have revealed RT and BOLD magnitude relationships in older adults (Rypma et al., 2007; Davis et al., 2008; Gazes et al., 2012).
The microstructure of cerebral WM represents a potential contributor to age-related increases in brain activation because it consists of well-myelinated axons that transmit signals between different functional regions of the brain (Burzynska et al., 2011). Diffusion tensor imaging (DTI) provides an in vivo method for estimating WM microstructure by measuring the diffusion of water molecules in neural tissue (Basser and Pierpaoli, 1996; Le Bihan, 2003). For example, fractional anisotropy (FA) is a scalar value that describes the fraction of diffusion and its directionality. Age-related FA declines are well-established (Sullivan and Pfefferbaum, 2006; Madden et al., 2012), and may influence BOLD activation (Bennett and Madden, 2013). Specifically, the orientation of axons and their myelin sheaths running in parallel bundles facilitates the diffusion of the water molecules preferentially along their main direction. Thus, lower FA values may suggest reduced signaling/information flow across WM tracts (Pierpaoli and Basser, 1996). This could result in an increase in synaptic activity and BOLD response at the local level as a compensatory response to disconnection from a larger neuronal circuitry.
While a growing number of studies have begun to explore relationships between RT and WM microstructure or between one of these neurocognitive variables and frontal activation (reviewed in Bennett and Madden, 2013), few have explored the potential contributions of both RT and WM microstructure to age-related frontal over-recruitment. Such investigations have the potential to broaden our understanding of age-related increases in frontal recruitment, which may have implications for testing the efficacy of cognitive intervention programs in aging. In the present study, we first identified brain regions showing sensitivity to task switching across younger and older adult groups. Subsequent analyses focused on two right hemisphere regions that showed sensitivity to task switching and are also often over-recruited by older adults in cognitive control tasks (the right dorsolateral prefrontal cortex (DLPFC) and right insula). Correlation analyses explored potential associations between BOLD magnitudes in these regions and both RT and WM microstructure. Where correlations were observed, we conducted mediation analyses to determine if age-related over-recruitment in these right frontal regions could be explained by RT and/or WM micro-structure. We hypothesized that increased brain activation in older adults compared to younger adults may be better explained by cognitive slowing and WM microstructure than chronological age. We reported the significant models due to space limitation.
EXPERIMENTAL PROCEDURES
Participants
A total of 65 healthy adults (32 younger adults, 33 older adults) participated in the present study. Written informed consent was obtained from each participant, and the study was approved by University of Kentucky Institutional Review Board. Participants were recruited from the Lexington community and from the University of Kentucky via flyers and newspaper advertisements. Participants were community-dwelling individuals who were native English speakers with normal or corrected-to-normal visual acuity. Exclusionary criteria for the study included the following: color blindness, a major head injury, stroke, a neurological or psychiatric disorder, high blood pressure, hypercholesterolemia, diabetes, heart disease, the use of any psychotropic drugs, or the presence of metal fragments and/or metallic implants contraindicated for magnetic resonance imaging (MRI).
Task switching performance is known to be correlated with intelligence and digit span (Kray and Lindenberger, 2000). The task switching paradigm employed in the present study involved non-verbal, perceptual switching. Thus, the Cattell Culture Fair (CCF) Intelligence Test (Cattell and Cattell, 1960) was used as a measure of intelligence because it assesses non-verbal intelligence associated with perceiving inductive relationships in shapes and figures. Digit span forward (DF) and backward (DB) were assessed with The Digit Span Subtests of the Wechsler Memory Scale (WMS III) (Wechsler, 1997). Totals for the DF and DB sets were based on the number of trials that were accurately reported in the correct order.
These cognitive tests were administered for potential use as covariates in our analyses in the event of significant group differences. However, there was no significant difference between younger and older groups in male/female ratio (X2 = 1.85, p = 0.17), years of education [t (63) = 0.20, p = 0.84], IQ [t (61) = 1.20, p = 0.24], DF [t (61) = 0.32, p = 0.75], DB [t (61) = 0.35, p = 0.73] (Table 1). Thus, we did not include these demographic or cognitive test scores as covariates in subsequent analyses.
Table 1.
Group means and standard deviations (in brackets) for demographic and neuropsychological scores
| Age group | Age interval | Mean age | N (female) | Years of education | IQ | DF | DB |
|---|---|---|---|---|---|---|---|
| Younger | 25–40 | 32.1 (3.6) | 32 (15) | 16.4 (2.8) | 124.5 (20.7) | 10.6 (2.3) | 9.8 (2.8) |
| Older | 63–83 | 68.4 (5.4) | 33 (21) | 16.5 (2.8) | 130.2 (17.0)31 | 10.4(2.0)31 | 10.0 (2.5)31 |
Notes: IQ, Cattell Culture Fair Intelligence Test; DF, forward digit span; DB, backward digit span. Values for IQ and DB are age-scaled scores. If score values were missing, the number of participants used in the computation is shown as a subscript.
Task and procedure
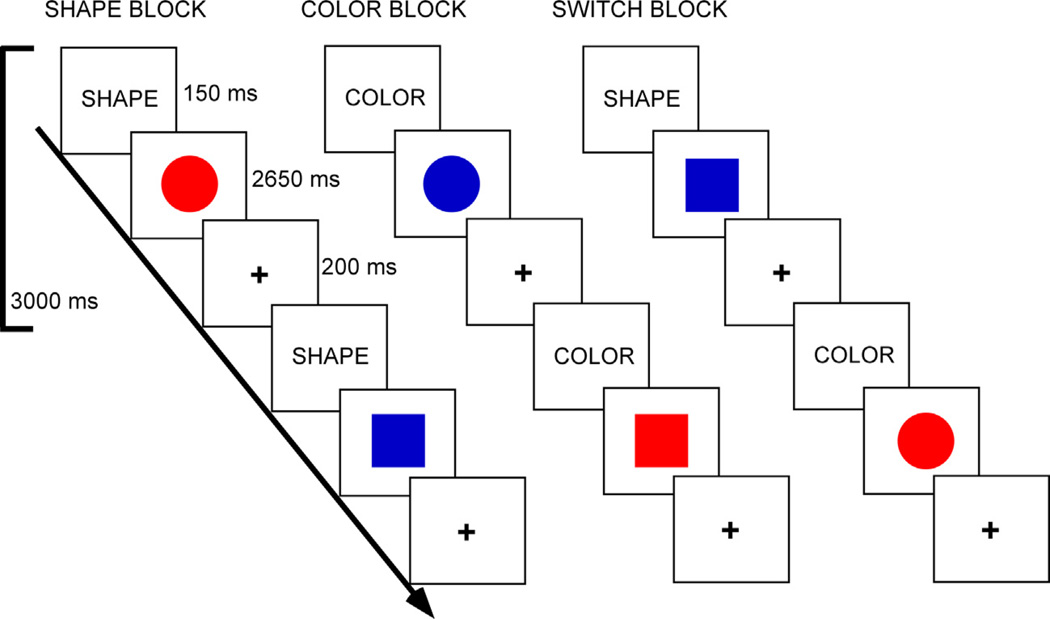
Participants completed a color-shape task switching paradigm (Fig. 1). The stimuli consisted of two possible shapes (circle or square), in one of two possible colors (red or blue), presented in the center of the screen. The cue was presented for 150 ms and was followed immediately by the stimulus for 2650 ms. A fixation cross then appeared for 200 ms prior to the next cue word. A block design was employed which included four types of blocks: shape, color, switch (shape/color) and baseline fixation [in which participants focused their vision on a central cross hair ( + )]. In the shape block, participants decided if a stimulus was a circle or square. In the color block, participants decided if a stimulus was red or blue. In the switch block, participants alternated between shape and color decisions. Participants were asked to press a response (left or right) button to indicate whether the stimulus was red or blue, or if it was a circle or square, depending upon the cue word. Participants were asked to respond as quickly and accurately as possible.
Fig. 1.
Task switching paradigm. Tasks were indicated via cue words. In the shape task, participants decided if a stimulus was a circle or square. In the color task, participants decided if a stimulus was red or blue. In the switch task, participants alternated between shape and color decisions. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Task blocks were 60 s in duration, and fixation blocks were 30 s in duration. There were three runs. Each run contained four task blocks and five fixation blocks. One run consisted of two blocks of the color task and two blocks of the shape task. The other two runs contained one block each of the color task and shape task, and two switching blocks (in which the color and shape tasks switched pseudorandomly). The order of runs, task blocks within runs, and stimulus-response mappings were counterbalanced across participants. The experiment was programed via E-Prime v1.2 (Psychology Software Tool, Pittsburgh, PA, USA). RT and accuracy for subject responses on each trial were recorded by the stimulus presentation program.
Behavioral analysis
We calculated mean error rates, RT of corrected trials, and switch cost (switch–non-switch) for each participant. Mean error rates and RTs were submitted to 2 (age group: younger/older) × 2 (condition: non-switch/ switching task) mixed effects analyses of variance (ANOVAs) using SPSS for Windows (version 21). Age-related switch cost differences are used to report results in tables and are statistically equivalent to age by condition interactions reported in the text.
Imaging data acquisition
Imaging data were collected on a 3 Tesla Siemens TIM scanner at the Magnetic Resonance Imaging and Spectroscopy Center of University of Kentucky. Four types of images were collected for each participant: (1) a high-resolution, T-1 weighted sequence for the subsequent localization of functional magnetic resonance imaging (fMRI) activity in standard stereotactic space; (2) T2*-weighted images sensitive to the BOLD signal for estimation of fMRI activity; (3) diffusion tensor images for estimation of FA; (4) a B0 field map sequence for subsequent geometric unwarping of fMRI and DTI images.
High-resolution, 3D anatomic images were acquired using an MP-RAGE sequence [repetition time (TR) = 1690 ms, echo time (TE) = 2.56 ms, flip angle = 12°, 1-mm isotropic voxels]. T2*-weighted functional images were collected using a gradient-echo (EPI) sequence [33 interleaved slices, TR = 3000 ms, TE = 30 ms, flip angle = 83°, field of view (FOV) = 224 mm, matrix = 64 × 64, 3.5-mm isotropic voxels]. DTI used a double spin echo EPI sequence (TR = 6900 ms, TE = 105 ms, flip angle = 90°, FOV = 224 mm, in-plane resolution = 1.75 × 1.75-mm voxels, 40 contiguous 3-mm-thick axial slices). The DTI images were acquired with 36 non-collinear encoding directions (b = 1000 s/mm2) and five images without diffusion weighting (b = 0 s/mm2, b0). The field map images were collected using a double-echo EPI sequence (TE1 = 5.19 ms, TE2 = 7.65 ms).
fMRI preprocessing and voxelwise analyses
Statistical Parametric Mapping (SPM 8; Wellcome Department of Cognitive Neurology, UCL, London, UK) was used in the preprocessing and statistical analyses of the fMRI data. After discarding the first three functional volumes of each run, slice timing correction was performed using sinc interpolation. The timing-corrected images were then realigned to the first volume in order to correct for head motion. The resulting images were unwarped via BO field maps to reduce magnetic field distortions. The T1-weighted (MP-RAGE) image was then co-registered to the first functional volume using a mutual information algorithm. This co-registered high-resolution image was then used to determine the non-linear basis function parameters for transformation into Montreal Neurological Institute (MNI) 2 × 2 × 2-mm standard space. This transformation was then applied to the functional data, which was re-sliced to 2-mm isotropic voxels and spatially smoothed with an 8-mm full-width at half-maximum (FWHM) Gaussian kernel. Finally, high-pass filtering (a 128-s cutoff) was applied to the images to remove low-frequency drifts.
Statistical analyses at the subject-level were conducted such that predictor variables in the design matrix were composed of epochs representing each task block. Each epoch was convolved with a canonical hemodynamic response function (HRF) producing contrasts for non-switch condition and switch condition.
The aim of the study was to evaluate the effect of age, performance, and WM microstructure on BOLD magnitudes in frontal regions contributing to executive control. Thus group level of analysis was performed via a voxelwise comparison of switch versus non-switch conditions across all 65 subjects. The voxelwise statistical map was thresholded at P = 0.05 with FWE (familywise error-rate) correction, and a cluster threshold of 10 continuously activated voxels.
fMRI regions of interest (ROI) analysis
A main goal of the study was to test potential explanatory factors that may contribute to age-related increases in the right frontal cortex during cognitive control operations. Thus, we selected right frontal ROIs that showed sensitivity to task switching across participants (from the voxelwise switch versus non-switch contrast described above). There were two regions within the right frontal cortex that showed sensitivity to task switching across our younger and older participants: the right dorsolateral PFC (DLPFC) and right insula. These two regions have also been activated in previous task switching studies of younger adults and/or older adults (DiGirolamo et al., 2001; Gold et al., 2010; Madden et al., 2010; Kim et al., 2012). ROI mean BOLD magnitude (percent signal-change) was extracted from each subject’s fMRI data using Marsbar (http://marsbar.sourceforge.net). The ROIs were defined as 8-mm spheres surrounding peak activation coordinates (44236 for the right DLPFC and 34264 for the right insula) from each contrast. Mean BOLD response in each ROI was then subjected to a two-way (condition × group) ANOVAs to test for potential effects of age, and age × condition interactions.
DTI preprocessing
Participants’ DTI data sets were normalized to MNI152 (1×1×1 mm) space using FSL v4.1.5 (Functional MRI of the Brain software library, FMRIB) (Smith et al., 2006) to enable selection of spatially corresponding WM ROIs across subjects (described below). Registration of FA images into MNI space followed a series of procedures known as Tract-Based Spatial Statistics [TBSS v1.2; (http://www.fmrib.ox.ac.uk/fsl/tbss/), as described in detail in our previous work (Johnson et al., 2012). Briefly, prior to normalization, raw images were corrected for motion and residual eddy current distortion, and corrected for magnetic field distortions using B0 field maps. The FMRIB Diffusion Toolbox (FDT v2.0) was then used to fit the diffusion tensor and calculate FA eigenvalues.
Participants’ FA images were then aligned to a common target (the one to which the least amount of warping was required) using a nonlinear registration approach based on free-form deformations and B-Splines. FA datasets were then affine registered and resampled to 1-mm isotropic MNI152 space. All MNI-transformed FA images were then averaged to generate a mean FA image that was used to create a common WM tract skeleton. This skeleton was then thresholded at an FA value of 0.2 in order to minimize partial volume effects after warping across subjects. Each participant’s aligned FA image was subsequently projected onto the FA skeleton in order to account for residual misalignments between participants after the initial nonlinear registration.
Correlation analysis
Potential relationships between age, task performance, BOLD magnitude and FA values were explored via correlation (and subsequent mediation) analyses. These analyses focused on the switch condition because age- related increases in frontal ROIs were pronounced in this condition. As an index of behavioral performance, correlation (and mediation) analyses focused on RT.
For WM microstructure ROIs, to limit the number of correlations performed, two tracts were selected. The WM ROIs were the genu of the corpus callosum (CC-Genu) and the body of the corpus callosum (CC-Body), and were selected on the basis of being well-defined structures with direct connections to our two fMRI ROIs (Park et al., 2008). The CC-Genu includes connections between the DLPFC, as well as rostral portions of the insula, and contralateral frontal structures. The CC-Body includes connections between mid-to-caudal portions of the insula (as well as caudal portions of the DLPFC) and contralateral frontal structures. The WM ROIs were defined using the Johns Hopkins University WM tractog-raphy atlas and the International Consortium of Brain Mapping-DTI WM labels atlas. Mean FA values in each of the two WM ROI masks were extracted from each participant by using fslmeants.
FA, BOLD, and RT values greater or less than three standard deviations from the group mean were excluded from analyses. This criterion resulted in the removal of between 0.6% and 1.8% of total data points from correlation (and subsequent mediation) analyses. Correlation and subsequent mediation analyses employed an uncorrected statistical threshold of p < 0.05 as they were motivated by strong a priori hypotheses and limited to cortical fMRI and WM ROIs with established connections.
Mediation analyses
These analyses sought to determine whether the observed relationships between age and BOLD magnitude during task switching could be better accounted for (i.e. mediated) by the neurocognitive proxies of WM microstructure (FA) or performance (RT). We followed Baron & Kenny’s criterion for mediation analysis (Baron and Kenny, 1986), which requires that all three variables entered into a model be reliably correlated.
To examine whether RT mediated the relationship between age and BOLD magnitude (“Performance meditation model”), we used hierarchical regression analyses in which age was entered as a predictor of BOLD magnitude both alone and after entering RT into the model (Salthouse, 2011). To examine whether WM microstructure mediated the relationship between age and BOLD magnitude (“WM meditation model”), we used hierarchical regression analyses in which age was entered as a predictor of BOLD signal change both alone and after entering WM microstructure into the model. Finally, we calculated the degree to which each mediator attenuated the amount of variance in BOLD signal change that can be explained by age following a widely used procedure suggested by Salthouse (1993). To avoid redundancy between text and tables, we reported significant models in the tables.
RESULTS
Behavioral results
Mean error rates, RT, and behavioral switch costs are presented in Table 2. Both groups performed the non-switch and switch conditions with high accuracy (both groups ≥ 95% correct in each condition). The main effect of condition was significant [F (1, 63) = 31.96, p < 0.001], with higher error rates in the switch condition (M = 4.75%, SE = 0.6) than the non-switch condition (M = 2.45%, SE = 0.3). Both groups showed higher error rates in the switch condition compared to the non-switch condition (p-values ≤ 0.001). However, there was no effect of age group [F < 1], or condition × age group interaction [F < 1].
Table 2.
Behavioral performance in the young and older groups
| Younger | Older | |
|---|---|---|
| Non-switch error rates (%) | 2.5 (0.5) | 2.4 (0.4) |
| Non-switch RTs (ms) | 613 (22) | 753 (19) |
| Switch error rates (%) | 4.6 (0.7) | 4.9 (0.8) |
| Switch RTs (ms) | 811 (31) | 1011 (36) |
| Switch cost error rates (%) | 2.1 (0.4) | 2.4 (0.7) |
| Switch cost RTs (ms) | 199 (17) | 258 (26) |
Note: Standard errors of the mean are presented in brackets.
For RTs, the main effect of condition was significant [F (1, 63) = 211.37, p < 0.001], with longer RTs for the switch condition (M = 911 ms, SE = 24) than the non-switch condition (M = 683 ms, SE = 14). Both groups had longer RTs in the switch condition compared to the non-switch condition (p-values < 0.001). The main effect of age group was also significant [F (1, 63) = 22.18, p < 0.001], with the older adults having longer RTs (M = 882 ms, SE = 25) than the younger adults (M = 712 ms, SE = 26). Finally, a marginal group × condition interaction was observed [F (1, 63) = 3.60, p = 0.06] indicating a trend toward age-related increases in RT switch costs.
Age-related over-recruitment
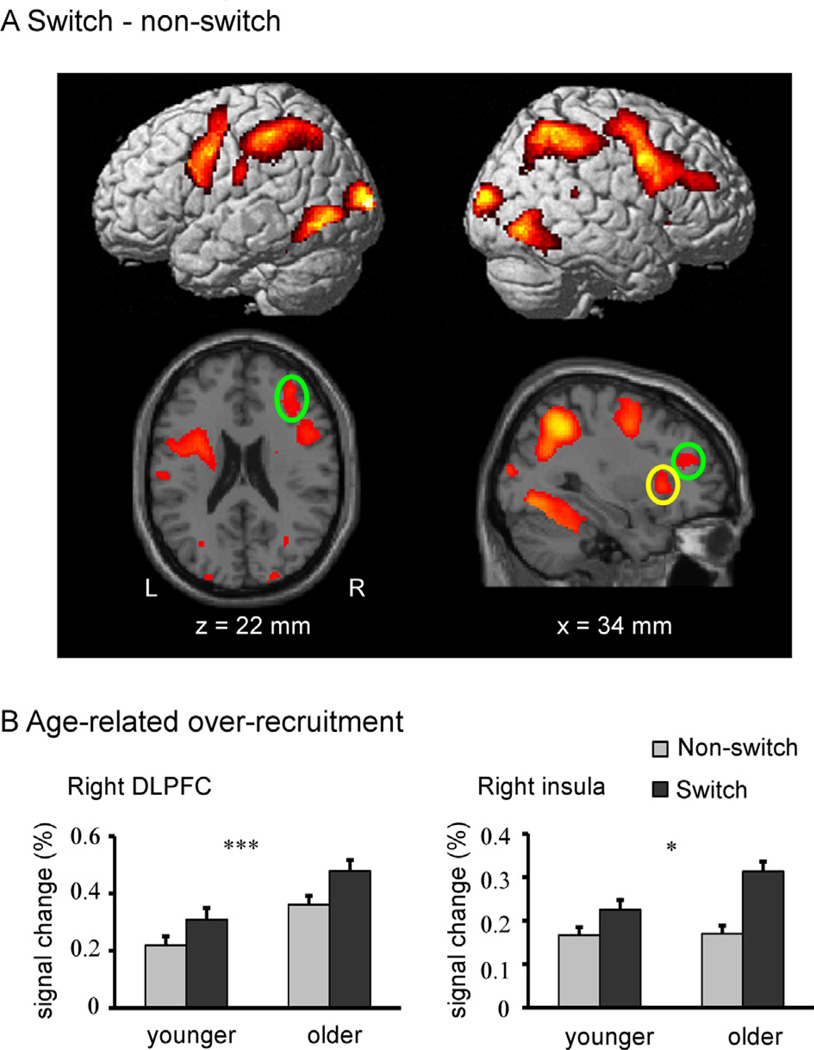
The switch vs. non-switch contrast revealed significant increases in activation in regions of all four lobes, and most prominently within frontoparietal regions (see Fig. 2A and Table 3). In contrast, there were no regions showing significantly greater activation in the non-switch than in the switch condition.
Fig. 2.
fMRI results. (A) Voxelwise activation map of the switch vs. non-switch contrast across all subjects presented on the rendered SPM surface (top panel) and slices of a T1 image (bottom panel). The two right frontal ROIs selected from the voxelwise results are indicated by circles (yellow circle for the right insula and green circle for the right DLPFC). (B) Age-related task switching over-recruitment in the two frontal ROIs. Note: Asterisks represent main effects of age. *p < 0.05, ***p < 0.001. The error bars represent the standard error of the mean. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 3.
Significant areas of activation in the switch vs. non-switch contrast across all subjects
| Region | Hem | BA | Voxel | T | MNI coordinate (mm) |
||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Precentral gyrus | Left | 6 | 2516 | 9.36 | −38 | −2 | 40 |
| Anterior cingulate cortex | Left | 6 | 1184 | 8.43 | −6 | 4 | 52 |
| Superior parietal lobule | Left | 7 | 2626 | 10.31 | −30 | −54 | 48 |
| Cuneus | Left | 17 | 5896 | 14.87 | −14 | −100 | 6 |
| Thalamus | Left | − | 98 | 6.06 | −16 | −12 | 4 |
| Dorsolateral prefrontal cortex | Right | 9 | 2687 | 8.75 | 44 | 2 | 36 |
| Insula | Right | 13 | 235 | 7.93 | 34 | 26 | 4 |
| Insula | Right | 13 | 20 | 5.27 | 62 | −38 | 16 |
| Superior parietal lobule | Right | 7 | 2516 | 10.72 | 32 | −52 | 46 |
| Putamen | Right | − | 105 | 5.58 | 24 | −2 | 16 |
| Thalamus | Right | − | 79 | 5.54 | 16 | −16 | 0 |
Note: The voxelwise statistical threshold was P = 0.05 with FWE (familywise error-rate) correction, and a cluster threshold of 10 continuously activated voxels. Hem for hemisphere. X, Y, Z represent the stereotaxic coordinates according to Montreal Neurological Institute (MNI) template. Brodmann’s areas (BAs) of peak activations were identified through conversion to Talairach and Tournoux space (Talairach and Tournoux, 1988) via the icbm2tal function (Lancaster et al., 2007).
Mean percent-signal change in two right hemisphere frontal ROIs was then submitted to a 2 (age group: younger/older) × 2 (condition: non-switch/switching task) mixed effects ANOVA. Each of the ROIs showed main-effects of condition (ps < .001) (a finding which was statistically guaranteed by the selection of these regions from the voxelwise switch > non-switch contrast across all subjects). In the right DLPFC, there was a main effect of age such that BOLD magnitude was higher in the older adult group than the younger adult group [F (1, 63) = 12.31, p < .001] while no group × condition interaction was found (F < 1). In the right insula, BOLD magnitude was higher in the older adult group than the younger adult group [F (1, 57) = 4.01, p < .05] and significant group × condition interaction was found [F (1, 57) = 4.88, p < .05] such that a larger increase in activation between non-switch and switch task conditions was found in the older adults than in the younger adults (Fig. 2B).
Group differences in WM microstructure
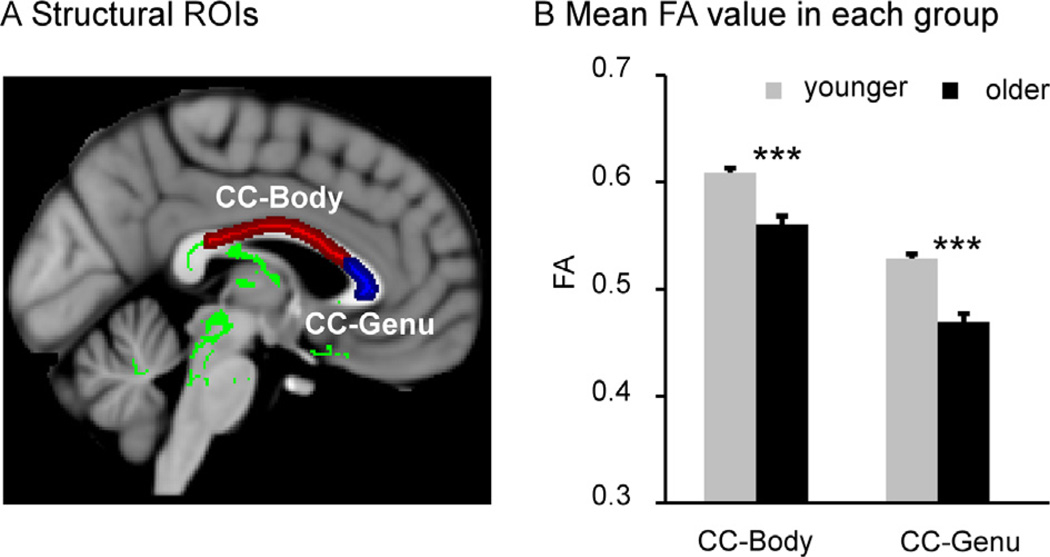
As shown in Fig. 3, the older adult group had significantly lower FA than the younger adult group in both the CC-Genu [t (62) = 6.76, p < .001] and the CC-Body [t(63) = 3.33, p < .001].
Fig. 3.
Group differences in fractional anisotropy. (A) Representation of white matter ROIs: red for the body of the corpus callosum (CC-Body) and blue for the genu of the corpus callosum (CC-Genu). (B) Mean FA values in the white matter ROIs for each group. Note:***p < 0.001. The error bars represent the standard error of the mean. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Correlations between age, performance, BOLD and FA
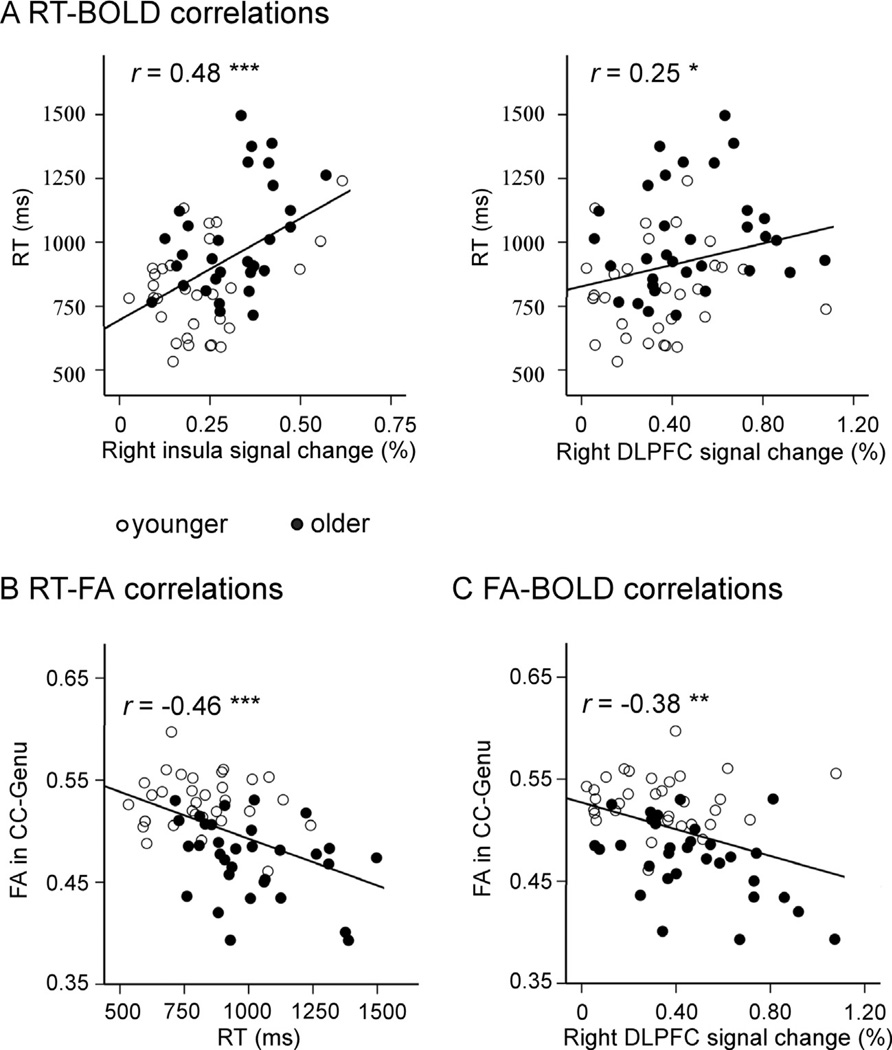
Fig. 4 and Table 4 present the correlations between age, RT, BOLD and FA. A significant positive relationship between age and BOLD magnitude was observed in both the right DLPFC and the right insula. A significant negative relationship between age and FA was also observed in the CC-Body and the CC-Genu. Switch RT showed a significant positive relationship with age and BOLD magnitude in the right DLPFC and the right j insula, but showed a significant negative correlation with FA in CC-Body and CC-Genu. FA in the CC-Genu showed a significant negative relationship with BOLD magnitude in the right DLPFC and right insula. In contrast, there was no correlation between FA in the CC-body and BOLD magnitude in the right insula.
Fig. 4.
Scatter plots of significant correlations across groups. (A) Positive correlations between RT and BOLD magnitude in right frontal ROIs. (B) Negative correlation between RT and FA in CC-Genu. (C) Negative correlation between FA in CC-Genu and BOLD magnitude in the right dorsolateral prefrontal cortex (DLPFC). Note: * for p < 0.05, ** for p < 0.01, *** for p < 0.001.
Table 4.
Correlation results between age, performance in the switch condition, BOLD magnitude in two frontal ROIs and FA in two WM ROIs
| Switch RT | CC-Body | CC-Genu | Right DLPFC | Right insula | |
|---|---|---|---|---|---|
| Age | 0.517*** | −0.459*** | −0.721*** | 0.322** | 0.388** |
| Switch RT | −0.253* | −0.463*** | 0.246* | 0.479*** | |
| CC-Body | 0.621*** | −0.347** | −0.121 | ||
| CC-Genu | −0.381** | −0.353** |
p < 0.05.
p < 0.01.
p < 0.001.
Mediation models
Following the criteria for mediation analyses that all variables should be correlated with each other, the following mediating factors were tested: (1) RT, FA in the CC-Genu and FA in the CC-Body as potential mediators of the relationship between age and right DLPFC BOLD magnitude; and (2) RT and FA in the CC-Genu as potential mediators of the relationship between age and right insula BOLD magnitude. FA in the CC-Body could not be used as a predictor in the second set of models because it was not correlated with BOLD in the right insula.
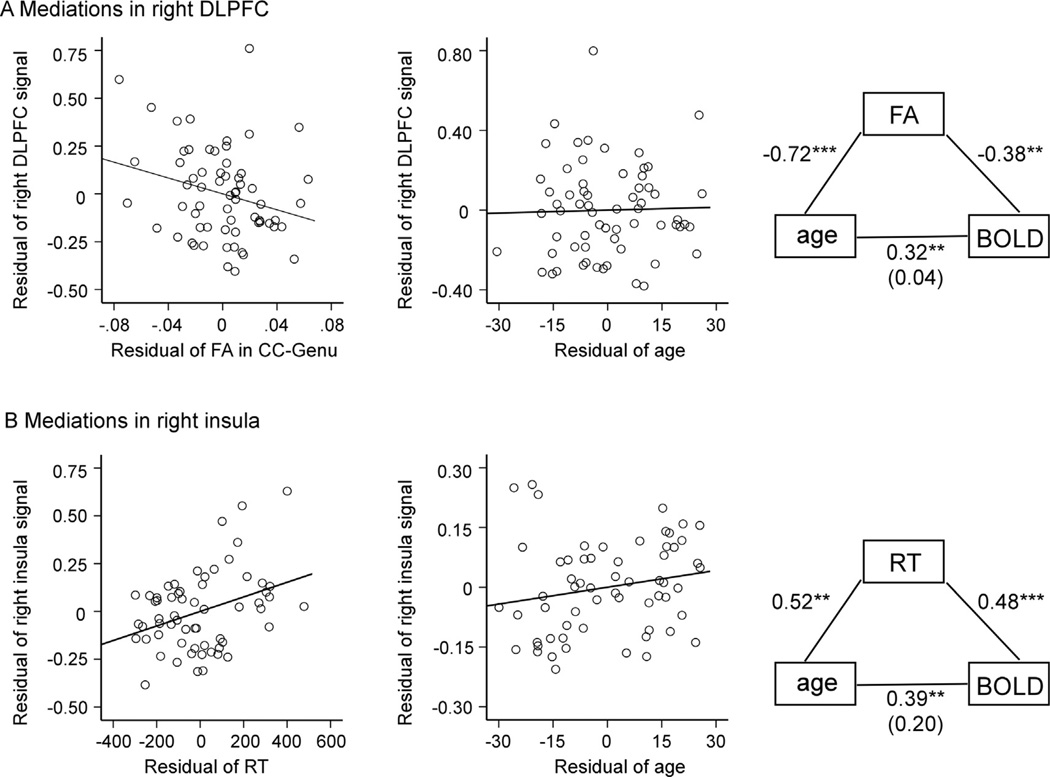
The mediation analyses revealed different patterns in right frontal ROIs (Fig. 5). In the right DLPFC ROI, the relationship between age and BOLD magnitude was fully mediated by WM microstructure in the CC-Genu. Specifically, after controlling for FA in the CC-Genu, age-related variance in right DLPFC BOLD magnitude was attenuated by 98.82%. After entering FA into the full model, a significant relationship between FA and BOLD magnitude was observed (p = 0.042), while the relationship between age and BOLD magnitude was no longer significant (p = 0.833) (Table 5 and Fig. 5A).
Fig. 5.
Scatter plots of relationships from mediation analyses. (A) Plots show a significant residual association between BOLD magnitude in the right DLPFC and FA in CC-Genu after controlling for age, and the lack of residual association between BOLD magnitude in the right DLPFC and age after controlling for FA in the CC-Genu. A graphical representation of the FA mediation effect is shown in the right column. The age-BOLD correlation after partialling out variance associated with FA is shown in parentheses. (B) Plots show a significant residual association between BOLD magnitude in the right insula and RT after controlling for age, and the lack of residual association between BOLD magnitude in the right insula and age after controlling for RT. A graphical representation of the RT mediation effect is shown in the right column. The age-BOLD correlation after partialling out variance associated with RT is shown in parentheses. Note: ** for p < 0.01, *** for p < 0.001.
Table 5.
Mediation models testing effects of age, RT and FA in CC-Genu on the age-BOLD relationship in the switch condition
| R2 | R2 change | F | Percentage attenuation | Beta significance | |
|---|---|---|---|---|---|
| WM mediation model in right DLPFC | |||||
| Model 1 | |||||
| Age | 0.085 | 5.78* | 0.019 | ||
| Model 2 | |||||
| CC-Genu | 0.145 | 10.54** | 0.042 | ||
| Age | 0.146 | 0.001 | 5.21** | 98.82 | 0.833 |
| Performance mediation model in right insula | |||||
| Model 1 | |||||
| Age | 0.15 | 10.45** | 0.002 | ||
| Model 2 | |||||
| RT | 0.229 | 17.57*** | 0.005 | ||
| Age | 0.258 | 0.029 | 10.11*** | 80.67 | 0.138 |
Note: For Model 2, beta significance indicated beta value in full model in which both independent variable and mediator were entered in the regression model.
p < 0.05.
p < 0.01.
p < 0.001.
In the same right DLPFC ROI, the relationship between age and BOLD magnitude was also marginally mediated by FA in CC-Body. Specifically, age-related variance in right DLPFC BOLD magnitude was attenuated by 67.31% after controlling FA in CC-Body. Upon entering FA in CC-Body into the full model, a marginally significant relationship between FA and BOLD magnitude was observed (p = 0.060), while the relationship between age and BOLD magnitude was no longer significant (p = 0.121). RT did not mediate age-BOLD relationship, as RT–BOLD correlation was not significant (p = 0.444) after inclusion of RT into the full model.
In contrast, in the right insula ROI, the relationship between age and BOLD magnitude was not significantly mediated by WM microstructure in the CC-Genu. Although controlling for FA in CC-Genu did attenuate age-related variance in right insula BOLD magnitude by 76%, after inclusion of FA into the full model, there was no longer a correlation between FA and BOLD magnitude (p = 0.41) or between age and BOLD magnitude (p = 0.119). There was, however, a significant mediation effect of performance on the age–BOLD relationship in the right insula. After controlling for RT, the age–BOLD relationship was attenuated by 80. 67%, the relationship between RT and BOLD magnitude was significant (p = 0.005), and the age–BOLD relationship was no longer reliable (p = 0.138) (Table 5 and Fig. 5B).
DISCUSSION
We explored the potential contributions of age-related RT slowing and WM microstructure reductions to age-related increases in right frontal activation during task switching. Our results build upon previous findings reporting separate relationships between RT and WM microstructure or between one of these neurocognitive variables and frontal activation in our previous work (Gold et al., 2010) and in other studies (reviewed in Salthouse, 2011). We found that higher BOLD magnitude in regions that were over-recruited in older adults was associated with longer RT and lower WM microstructure. Moreover, our results showed that longer RT mediated the relationship between age and BOLD magnitude in the right insula and reduction of WM in CC-Genu mediated the relationship between age and BOLD magnitude in the right DLPFC. Our results provide support for efficiency models of frontal recruitment and suggest that age-related slowing and disconnection contribute to age-related increases in right frontal activation during cognitive control operations.
Age-related over-recruitment of right frontal regions is a common finding in the literature and represents a key element of both compensation and neural efficiency theories (reviewed in Dennis and Cabeza, 2008; Reuter-Lorenz and Park, 2010). We focused our analyses on the right DLPFC and right insula because these regions showed sensitivity to task switching across younger and older adult groups. Age-related activation increases were observed in each of these regions. In addition, in each region, higher BOLD magnitude was associated with slower switching performance across groups. Given that the switching task was performed with high accuracy, our findings are consistent with neural efficiency accounts of individual differences in BOLD response, which suggest that faster performers may require less frontal recruitment for accurate task performance (Rypma et al., 2006; Stern et al., 2012).
We also observed a relationship between BOLD magnitude in right frontal regions and WM microstructure across groups. Specifically, higher BOLD magnitudes in the right DLPFC and right insula tended to be correlated with lower FA in the CC-genu and CC-body. This negative relationship across groups is consistent with several recent reports of relationships between BOLD magnitude and WM microstructure that persist after controlling for age (Schulze et al., 2011; Burzynska et al., 2013). Our results suggest that age-related BOLD increases in right frontal regions could reflect a compensatory response to reduced structural connectivity (i.e., an increase in signal amplitude in response to a noisier environment). However, our results show that right frontal activation increases in older adults can in some cases reflect a failed attempt at compensation, as age-related increases were linked with poorer performance.
The finding of cross-group relationships between BOLD magnitude in the right frontal regions with both RT and WM microstructure served as motivation for our mediation analyses. Here we asked whether these two proxy variables of aging—longer RT and lower FA—contribute to age-related over-recruitment. Results indicated that age-related increases in BOLD magnitude in the right insula were mediated by task performance (RT in the switching condition). The region of the right inferior frontal cortex at or near the insula has an established role in inhibitory control functions (Gehring and Knight, 2000; Botvinick et al., 2004). For example, there is evidence suggesting that right inferior frontal region at or near the insula is involved in response inhibition (Aran et al., 2003; Robbins, 2007). Our task stimuli were bivalent (i.e., contained both color and shape information) and thus likely taxed inhibitory control processes associated with managing competing stimulus-response mappings (Koch and Alport, 2006; Meiran et al., 2008; Kiesel et al., 2010).
Given that older adults often show impairments in inhibitory control (Hasher and Zacks, 1988), the observed age-related increases in right frontal activation may in part reflect an attempt to suppress interference from the non-relevant stimulus information. Such increased inhibitory control functioning incurs a RT cost, which likely contributes to the age-related increase in RT switch costs observed in the present study and other switching studies (Gold et al., 2010; Gazes et al., 2012). Interestingly, we observed a parallel age-related increase in neural switch cost (switch–non-switch) in the right insula (Fig. 2; panel B). Thus, ‘age-related’ increases in the right insula during executive tasks may be in part attributable to generally slower, less efficient, inhibitory control processing in older adults compared to younger adults.
The tendency for slower, less efficient inhibitory control (and most other) processes in aging is consistent with a view that RT may be a proxy variable of multiple age-related neurobiological changes (Salthouse, 2011). The established relationship between degree of myelination and speed of nerve conduction velocity (Jack et al., 1983) suggests that WM microstructure in other tracts not assessed here may contribute to age-related BOLD over-recruitment in the right insula. Multiple other forms of age-related neurodegenerative change are likely to contribute RT slowing in aging including but not limited to depletion of neurotransmitter systems (Loerch et al., 2008), alterations in metabolite ratios reflecting various molecular and cellular processes (Kantarci et al., 2011) and/or vascular changes (Hedden et al., 2012). Significant further research will be required to delineate the broad range of neural mechanisms contributing to age-related RT slowing. In the meantime, our RT mediation results call attention to the need to consider performance differences in studies exploring age-related BOLD increases. In particular, future studies should attempt to dissociate regions in which age-related BOLD over-recruitment persists when average RT is equated across age groups from regions in which age-related activation increases are attributable to RT differences.
In contrast, we found that age-related increases in BOLD magnitude in the right DLPFC were mediated by WM microstructure in the corpus callosum. Specifically, age-related activation increases in the right DLPFC were fully mediated by FA in CC-Genu and marginally mediated by FA in CC-Body. Age-related declines in FA were observed in the corpus callosum in the present study, consistent with many other studies (Sullivan and Pfefferbaum, 2006; Madden et al., 2012). Thus, age-related increases in right DLPFC activation during task switching could be a compensatory response to lower structural connectivity within the cognitive control network or the result of reduced inhibitory input from contralateral structures. This latter possibility would appear to be consistent with a view of the right DLPFC as contributing to domain-general processes of monitoring and checking (Shallice, 2002; Petrides, 2005) rather than switching-related processes per se. In support of this view, we observed age-related increases in right DLPFC activation in both switch and non-switch conditions but no age by condition interaction in this region.
The different mediation patterns observed in right DLPFC and right insula appear to be stable effects in our sample, as supplementary analyses indicated that sub-groups did not significantly differ in the relationships between residuals of insula-RT or residuals of DLPFC-FA. Although age-related over-recruitment of right frontal regions is sometimes viewed as a unitary construct, our results suggest that factors contributing to BOLD increases in older adults may vary across different anatomical regions. This may reflect the structural and functional heterogeneity of frontal regions (Petrides and Pandya, 1994; Duncan and Owen, 2000; Rajah and D’Esposito, 2005). However, it should be noted that this explanation, while plausible, is post-hoc and will need to be tested directly in future studies.
We note several caveats in the present study. First, we note that age-related brain activation increases in right frontal regions are likely the result of many neurocognitive factors beyond those explored here, similar to the previously mentioned biological mechanisms contributing to RT slowing (Loerch et al., 2008; Kantarci et al., 2011; Hedden et al., 2012). Future work will be required to comprehensively delineate the full complement of biological mechanisms underlying age-related changes in BOLD recruitment. Similarly, our study was designed to focus on switching as a global cognitive measure. However, age-related declines in task switching likely reflect the joint effects of multiple cognitive alterations including those affecting attention, encoding, episodic retrieval, rule maintenance and inhibition, among other processes (Altmann and Gray, 2008; Koch et al., 2010; Vandierendonck et al., 2010). Future studies should explore more specific relationships between cognitive sub-processes of switching and age-related BOLD over-recruitment. In addition, a more complete understanding of age-related BOLD activation increases would benefit greatly from future longitudinal designs.
CONCLUSION
Our results suggest that better structural connectivity may allow for higher neural efficiency (i.e., accurate performance with low energy expenditure) in older adults. In particular, our results suggest that age-related BOLD increases in the right frontal cortex may be in part attributable to age-related slowing and WM microstructure reductions. Finally, our results also underscore the need for further research to delineate the broad range of mechanisms likely to contribute to age-related increases in frontal cortex activation.
Acknowledgments
This study was supported by the National Institute on Aging of the National Institutes of Health under award number R01AG033036 and the National Science Foundation under award number BCS 0814302. The content is solely the responsibility of the authors and does not necessarily represent the official views of these granting agencies. We thank our study volunteers for their participation in this research.
Abbreviations
- ANOVAs
analyses of variance
- CC-Body
body of the corpus callosum
- CC-Genu
genu of the corpus callosum
- DB
digit span backward
- DF
digit span forward
- DLPFC
dorsolateral prefrontal cortex
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- fMRI
functional magnetic resonance imaging
- FMRIB
Functional MRI of the Brain software library
- FWE
familywise error-rate
- MNI
Montreal Neurological Institute
- ROIs
regions of interest
- RT
reaction time
- TE
echo time
- TR
repetition time
- WM
white matter
REFERENCES
- Altmann EM, Gray WD. An integrated model of cognitive control in task switching. Psychol Rev. 2008;115:602–639. doi: 10.1037/0033-295X.115.3.602. [DOI] [PubMed] [Google Scholar]
- Aran AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Computational and neurobiological mechanisms underlying cognitive flexibility. Proc Natl Acad Sci USA. 2006;103:7186–7191. doi: 10.1073/pnas.0509550103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator mediator variable distinction in social psychological research - conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson Ser B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ. Disconnected aging: cerebral white matter integrity and age-related differences in cognition. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Burzynska AZ, Nagel IE, Preuschhof C, Li SC, Lindenberger U, Backman L, Heekeren HR. Microstructure of frontoparietal connections predicts cortical responsivity and working memory performance. Cereb Cortex. 2011;21:2261–2271. doi: 10.1093/cercor/bhq293. [DOI] [PubMed] [Google Scholar]
- Burzynska AZ, Garrett DD, Preuschhof C, Nagel IE, Li SC, Backman L, Heekeren HR, Lindenberger U. A scaffold for efficiency in the human brain. J Neurosci. 2013;33:17150–17159. doi: 10.1523/JNEUROSCI.1426-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattell RB, Cattell AKS. Handbook for the individual or group culture fair intelligence test. USA: IPAT; 1960. [Google Scholar]
- Cole MW, Schneider W. The cognitive control network: integrated cortical regions with dissociable functions. Neuroimage. 2007;37:343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cereb Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Cabeza R, editors. Neuroimaging of healthy cognitive aging. Mahwah, NJ: Lawrence Erlbaum; [Google Scholar]
- Di X, Rypma B, Biswal BB. Correspondence of executive function related functional and anatomical alterations in aging brain. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:41–50. doi: 10.1016/j.pnpbp.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGirolamo GJ, Kramer AF, Barad V, Cepeda NJ, Weissman DH, Milham MP, Wszalek TM, Cohen NJ, Banich MT, Webb A, Belopolsky AV, McAuley E. General and task-specific frontal lobe recruitment in older adults during executive processes: a fMRI investigation of task-switching. Neuroreport. 2001;12:2065–2071. doi: 10.1097/00001756-200107030-00054. [DOI] [PubMed] [Google Scholar]
- Drag LL, Bieliauskas LA. Contemporary review 2009: cognitive aging. J Geriatr Psych Neur. 2010;23:75–93. doi: 10.1177/0891988709358590. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Gazes Y, Rakitin BC, Habeck C, Steffener J, Stern Y. Age differences of multivariate network expressions during task-switching and their associations with behavior. Neuropsychologia. 2012;50:3509–3518. doi: 10.1016/j.neuropsychologia.2012.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Knight RT. Prefrontal-cingulate interactions in action monitoring. Nat Neurosci. 2000;3:516–520. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- Gold BT, Powell DK, Xuan L, Jicha GA, Smith CD. Age-related slowing of task switching is associated with decreased integrity of frontoparietal white matter. Neurobiol Aging. 2010;31:512–522. doi: 10.1016/j.neurobiolaging.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension and aging: a review and a new view. In: Bower GH, editor. The psychology of learning and motivation. Vol. 22. New York: Academic Press; 1988. pp. 193–225. [Google Scholar]
- Hedden T, Mormino EC, Amariglio RE, Younger AP, Schultz AP, Becker JA, Buckner RL, Johnson KA, Sperling RA, Rentz DM. Cognitive profile of amyloid burden and white matter hyperintensities in cognitively normal older adults. J Neurosci. 2012;32:16233–16242. doi: 10.1523/JNEUROSCI.2462-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack JJB, Noble D, Tsien RW. Electrical current flow in excitable cells. Oxford: Oxford University Press; 1983. [Google Scholar]
- Johnson NF, Kim C, Clasey JL, Bailey A, Gold BT. Cardiorespiratory fitness is positively correlated with cerebral white matter integrity in healthy seniors. Neuroimage. 2012;59:1514–1523. doi: 10.1016/j.neuroimage.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Lowe V, Przybelski SA, Senjem ML, Weigand SD, Ivnik RJ, Roberts R, Geda YE, Boeve BF, Knopman DS, Petersen RC, Jack CR., Jr. Magnetic resonance spectroscopy, beta-amyloid load, and cognition in a population-based sample of cognitively normal older adults. Neurology. 2011;77:951–958. doi: 10.1212/WNL.0b013e31822dc7e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesel A, Steinhauser M, Wendt M, Falkenstein M, Jost K, Philipp AM, Koch I. Control and interference in task switching - a review. Psychol Bull. 2010;136:849–874. doi: 10.1037/a0019842. [DOI] [PubMed] [Google Scholar]
- Kim C, Cilles SE, Johnson NF, Gold BT. Domain general and domain preferential brain regions associated with different types of task switching: a meta-analysis. Hum Brain Mapp. 2012;33:130–142. doi: 10.1002/hbm.21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch I, Allport A. Cue-based preparation and stimulus-based priming of tasks in task switching. Mem Cognition. 2006;34:433–444. doi: 10.3758/bf03193420. [DOI] [PubMed] [Google Scholar]
- Koch I, Gade M, Schuch S, Philipp AM. The role of inhibition in task switching: a review. Psychon Bull Rev. 2010;17:1–14. doi: 10.3758/PBR.17.1.1. [DOI] [PubMed] [Google Scholar]
- Kray J, Lindenberger U. Adult age differences in task switching. Psychol Aging. 2000;15:126–147. doi: 10.1037//0882-7974.15.1.126. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4:469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Loerch PM, Lu T, Dakin KA, Vann JM, Isaacs A, Geula C, Wang J, Pan Y, Gabuzda DH, Li C, Prolla TA, Yankner BA. Evolution of the Aging Brain Transcriptome and Synaptic Regulation. PloS one. 2008:3. doi: 10.1371/journal.pone.0003329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Costello MC, Dennis NA, Davis SW, Shepler AM, Spaniol J, Bucur B, Cabeza R. Adult age differences in functional connectivity during executive control. Neuroimage. 2010;52:643–657. doi: 10.1016/j.neuroimage.2010.04.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen NK, Song AW. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochim Biophys Acta. 2012;1822:386–400. doi: 10.1016/j.bbadis.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiran N, Kessler Y, Adi-Japha E. Control by action representation and input selection (CARIS): a theoretical framework for task switching. Psychol Res-Psych Fo. 2008;72:473–500. doi: 10.1007/s00426-008-0136-8. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Neumann J, von Cramon DY, Lohmann G. Model-based clustering of meta-analytic functional imaging data. Hum Brain Mapp. 2008;29:177–192. doi: 10.1002/hbm.20380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Kim JJ, Lee SK, Seok JH, Chun J, Kim Dl, Lee JD. Corpus callosal connection mapping using cortical gray matter parcellation and DT-MRI. Hum Brain Mapp. 2008;29:503–516. doi: 10.1002/hbm.20314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya D. Comparative architectonic analysis of the human and the macaque frontal cortex. In: Boiler F, Grafman J, editors. Handbook of neuropsychology. Amsterdam: Elsevier; 1994. pp. 17–57. [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Rajah MN, D’Esposito M. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain. 2005;128:1964–1983. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Park DC. Human neuroscience and the aging mind: a new look at old problems. J Gerontol B Psychol Sci Soc Sci. 2010;65:405–415. doi: 10.1093/geronb/gbq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci. 2007;362:917–932. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, Berger JS, Prabhakaran V, Bly BM, Kimberg DY, Biswal BB, D’Esposito M. Neural correlates of cognitive efficiency. Neuroimage. 2006;33:969–979. doi: 10.1016/j.neuroimage.2006.05.065. [DOI] [PubMed] [Google Scholar]
- Rypma B, Eldreth DA, Rebbechi D. Age-related differences in activation-performance relations in delayed-response tasks: A multiple component analysis. Cortex. 2007;43:65–76. doi: 10.1016/s0010-9452(08)70446-5. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Speed mediation of adult age-differences in cognition. Dev Psychol. 1993;29:722–738. [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Neuroanatomical substrates of age-related cognitive decline. Psychol Bull. 2011;137:753–784. doi: 10.1037/a0023262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze ET, Geary EK, Susmaras TM, Paliga JT, Maki PM, Little DM. Anatomical correlates of age-related working memory declines. J Aging Res. 2011;2011:606871. doi: 10.4061/2011/606871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T. Fractionation of the supervisory system. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. Oxford: Oxford University Press; 2002. pp. 261–277. [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Wojtowicz M, Grady CL. Reliable differences in brain activity between young and old adults: a quantitative metaanalysis across multiple cognitive domains. Neurosci Biobehav Rev. 2010;34:1178–1194. doi: 10.1016/j.neubiorev.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Stern Y, Rakitin BC, Habeck C, Gazes Y, Steffener J, Kumar A, Reuben A. Task difficulty modulates young-old differences in network expression. Brain Res. 2012;1435:130–145. doi: 10.1016/j.brainres.2011.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neurosci Biobehav Rev. 2006;30:749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Vandierendonck A, Liefooghe B, Verbruggen F. Task switching: interplay of reconfiguration and interference control. Psychol Bull. 2010;136:601–626. doi: 10.1037/a0019791. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale®—Third edition (WMS—III) San Antonio, TX: Harcourt Assessment; 1997. [Google Scholar]