Abstract
Five candidate plasma biomarkers (ST2, REG3α, elafin, TNFR1, sIL2Rα) were measured at specific time-points following cyclophosphamide/fludarabine-based nonmyeloablative allotransplantation (NMAT) in patients who did or did not develop acute graft-versus-host-disease (aGVHD). Plasma samples from 34 patients were analyzed at days +7, +14, +21 and +30. At a median follow-up of 358 days, 17 patients had experienced aGVHD with a median time to onset of day +36. Risk of aGVHD was associated with elevated plasma ST2 concentrations at day +7 (c-stat=0.72, p=0.03), day +14 (c-stat=0.74, p=0.04), and day +21 (c-stat=0.75, p=0.02); elevated plasma REG3α concentrations at day +14 (c-stat=0.73, p=0.03), day +21 (c-stat=0.76, p=0.01) and day +30 (c-stat=0.73, p=0.03); and elevated elafin at day +14 (c-stat=0.71, p=0.04). Plasma concentrations of TNFR1 and sIL2Rα were not associated with aGVHD risk at any of the time-points studied. This study identified ST2, REG3α and elafin as prognostic biomarkers to evaluate risk of aGVHD following Cy/Flu-based NMAT. These results need to be confirmed in an independent validation cohort.
INTRODUCTION
Acute graft versus host disease (aGVHD) continues to be a major contributor to early transplant related mortality (TRM) following allogeneic hematopoietic cell transplantation (HCT). There is not a reliable way to determine prior to the onset of symptoms who will suffer the complication. The choice of candidate biomarkers for aGVHD has to date been guided by studies performed in groups of patients who received myeloablative full or reduced intensity conditioning (RIC). We previously demonstrated that a biomarker panel consisting of interleukin-2-receptor-alpha (IL2Rα), tumor-necrosis-factor-receptor-1 (TNFR1), interleukin-8 (IL8) and hepatocyte growth factor correlated with clinical diagnosis of aGVHD as well as survival, independent of clinical grade severity. A panel of 6 biomarkers predicted treatment response and survival after aGVHD.[1, 2] Recently, the suppression of tumorogenesis 2 (ST2) was identified as a novel marker useful in predicting glucocorticoid-resistant aGVHD and non-relapse mortality (NRM).[3]
Nonmyeloablative transplant (NMAT) conditioning extends allotransplant options to older individuals who may be at higher risk for aGVHD on the basis of age; NMAT, a minimally-intense RIC is associated with low incidences of early transplant-related complications and mortality. Cyclophosphamide (Cy) and fludarabine (Flu)- based NMAT enables engraftment in recipients of related and unrelated HLA-matched grafts without mucositis and/or sinusoidal obstructive syndrome (SOS).[4, 5] The validation of biomarkers across a variety of settings is critical prior to attempting integration of their use in clinical practice. We conducted a study to test the ability of plasma levels of 5 individual biomarkers at specific time-points to serve as prognostic markers for aGVHD among patients undergoing Cy/Flu-based NMAT.
MATERIALS AND METHODS
Patient Population
A total of 34 patients with hematological malignancies who underwent Cy/Flu-based NMAT at Indiana University between 2008 and 2012 were included in the study, which was approved by the Indiana University Institutional review board. Disease status at transplant was categorized according to the American Society of Blood and Marrow Transplantation (ASBMT) criteria.[6]
Patients received mobilized peripheral blood hematopoietic cells (PBHC) from matched related (MRD) or matched unrelated donors (MUD). GVHD prophylaxis for MUD recipients consisted of cyclosporine A (CsA) +/− mycophenolate mofetil (MMF) or basiliximab (Bas, per NCT00975975), or combination of tacrolimus (Tac) and sirolimus (Sir). MRD recipients received a combination of CsA +/− MMF or Bas. Patients were followed prospectively until death or a median of 358 days (Range: 182-1381 days) and divided into aGVHD+ and no aGVHD groups. Modified Glucksberg criteria were used to diagnose and grade aGVHD at onset and at maximum severity.[7] Histopathological confirmation of aGVHD was obtained whenever clinically feasible.
Sample Preparation and Processing
Ten to 20ml of whole blood was obtained from patients on days +7, +14, +21 and +30 in heparin containing tubes to prevent clotting. Plasma was obtained from blood samples by centrifugation. Samples were aliquoted without additives into cryovials and stored at −80°C.
Five plasma biomarkers were studied including the following: ST2, regenerating-Islet-Derived-3-alpha (REG3α), elafin, TNFR1 and soluble-IL-2-receptor-alpha (sIL2Rα). Plasma ST2, elafin, and TNFR1 levels were measured by enzyme-linked immunosorbent assay (ELISA) using commercially available kits DST200, DY1747 and DY225 (R&D Quantikine®, Minneapolis, MN, USA), respectively. Plasma REG3α was measured using ELISA kit 5323 (MBL International Corp, Woburn, MA, USA) and sIL2Rα was measured using a commercially available multiplex platform, MPXHCYTO-60K (Millipore Corp, Billerica, MA, USA). All assays were performed in compliance with protocols provided by kit manufacturers.
Statistical Analysis
Differences in patient characteristics between aGVHD+ and no aGVHD groups were determined using Wilcoxon rank sum test for age at transplant and Fisher's exact test for all categorical variables. Medians, 25th and 75th percentiles of individual biomarker levels in aGVHD+ and no aGVHD groups were calculated and distributions compared at each time point using exact Wilcoxon rank sum tests (due to non-normality of biomarkers), and corresponding c-statistics, which represent the area under the ROC curves, were calculated by fitting logistic regression models. Biomarkers with statistically significant prognostic value were further analyzed to determine their ability to predict grade III-IV or GI-specific aGVHD (versus no aGVHD using exact Wilcoxon test). Association of elevated (median or higher) biomarker levels with overall survival (OS) and non-relapse mortality (NRM) was determined using log-rank test and Gray's test. A p-value of 0.05 or less was considered as the criteria of statistical significance. All statistical analyses were performed in SAS Version 9.3 (Cary, NC).
RESULTS
Of 34 patients included in the study, 17 patients experienced aGVHD and 17 patients did not. Table 1a describes patients’ characteristics. Age, diagnosis, match, GVHD prophylaxis, and ASBMT risk, did not differ between aGVHD+ and no aGVHD groups (p-value >0.16 for each). Patients who received grafts from an unrelated donor developed aGVHD more frequently (76% vs. 24%; p=0.015). Median onset of aGVHD was day +36 (range: +17-151). Table 1b describes the overall and site-specific severity of aGVHD at onset as well as at maximum clinical grade according to modified Gluckburg criteria. Of 17 patients who experienced aGVHD, 9 had skin, 12 had gastrointestinal (GI) and 6 had liver involvement.
Table 1a.
Patient characteristics
| Total (n=34) | aGVHD+ (n=17) | No aGVHD (n=17) | |
|---|---|---|---|
| Median Age (Range) | 60 (29-72) | 61 (29-72) | 59 (33-66) |
| Diagnosis | |||
| Acute leukemia | 14 | 8 | 6 |
| Chronic leukemia | 5 | 4 | 1 |
| MDS/MF | 9 | 3 | 6 |
| NHL | 4 | 1 | 3 |
| HD | 2 | 1 | 1 |
| Donor | |||
| Related | 16 | 4 | 12 |
| Unrelated | 18 | 13 | 5 |
| Match | |||
| Fully matched | 32 | 15 | 17 |
| Mismatched | 2 | 2 | 0 |
| GVHD prophylaxis | |||
| CsA/Bas | 16 | 10 | 6 |
| Tacro/Siro | 15 | 6 | 9 |
| CsA/MMF | 3 | 1 | 2 |
| ASBMT Status* | |||
| Low-risk | 11 | 5 | 6 |
| Intermediate-risk | 14 | 8 | 6 |
| High-risk | 8 | 3 | 5 |
Table 1b.
aGVHD grade at onset and at maximum
| aGVHD Grade – Onset | I | II | III | IV | |||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | 3 | 9 | 5 | 0 | |||||
| Skin | 1 | 4 | 3 | 0 | |||||
| GI | 6 | 1 | 4 | 0 | |||||
| Liver | 2 | 2 | 1 | 0 | |||||
| aGVHD Grade – Maximum | I | II | III | IV | |||||
| Overall | 2 | 5 | 8 | 2 | |||||
| Skin | 0 | 3 | 6 | 0 | |||||
| GI | 3 | 1 | 6 | 2 | |||||
| Liver | 2 | 3 | 1 | 0 |
not applicable for one subject with myelofibrosis
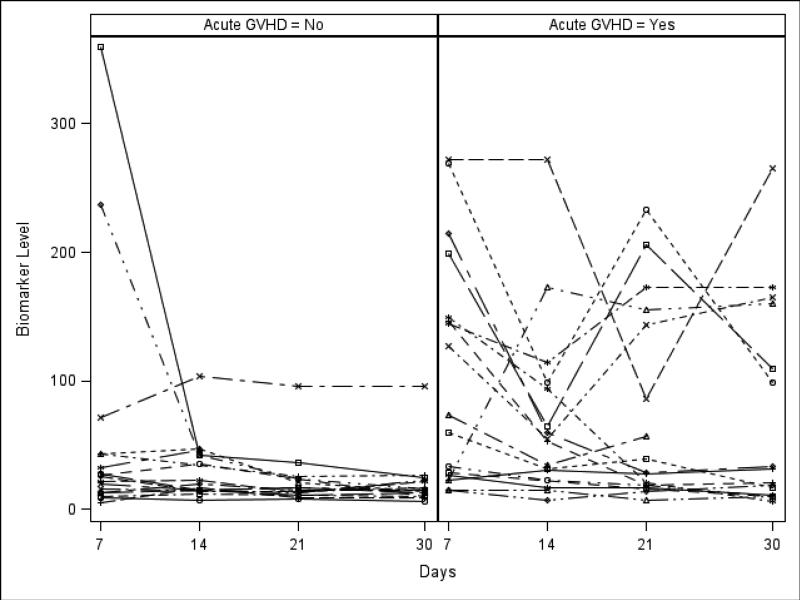
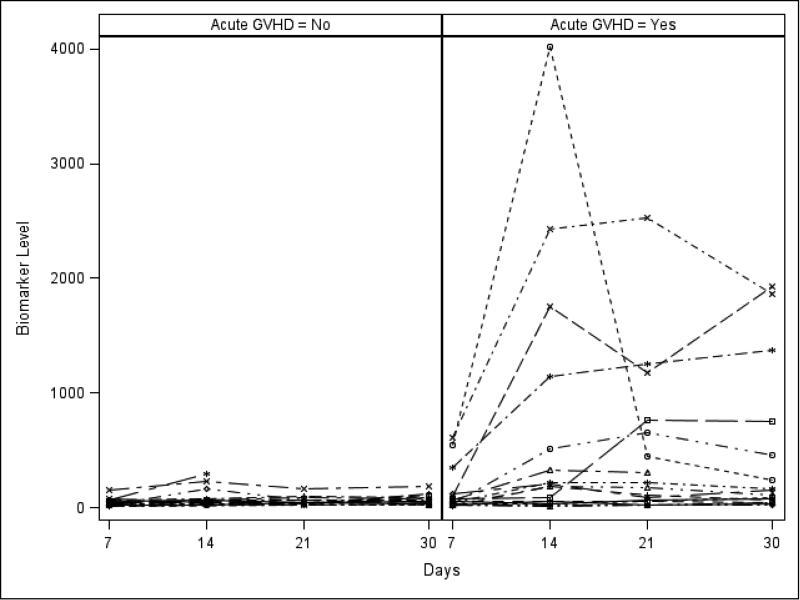
Table 2 shows the median, 25th and 75th percentiles of plasma biomarker concentrations in aGVHD+ and no aGVHD groups at specific time-points after HCT and corresponding c-statistics and p-values. Elevated plasma ST2 levels at days +7, +14, and +21 were significant risk factors for aGVHD occurrence. Similarly, plasma REG3α levels at days +14, +21 and +30 were also significantly elevated among aGVHD+ patients. Elevated plasma elafin levels at day +14 were also associated with aGVHD. See Figures 1a and 1b for ST2 and REG3α levels plotted by day in each group. When ST2 and REG3α were considered together in a logistic regression model, the corresponding c-statistics were 0.68 at day +7, 0.77 at day +14, 0.75 at day +21 and 0.74 at day +30.
Table 2.
Biomarker medians (25th, 75th percentile) by group over time*
| Day +7 | Day +14 | Day +21 | Day +30 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aGVHD+ | No aGVHD |
C- stat |
p- value |
aGVHD+ | No aGVHD |
C- stat |
p- value |
aGVHD+ | No aGVHD |
C- stat |
p- value |
aGVHD+ | No aGVHD |
C- stat |
p- value |
|
| ST2 ng/ml | 73.2 (25.9, 149.2) | 25.7 (12.9, 42.7) | 0.72 | 0.03 | 53.8 (26.8, 95.9) | 19.8 (14.4, 42.3) | 0.74 | 0.02 | 28.3 (18.8, 143.9) | 16.2 (12.5, 23.7) | 0.75 | 0.02 | 26.3 (10.3, 134.4) | 15.0 (11.2, 22.0) | 0.68 | 0.09 |
| Reg3α pg/ml | 51.4 (24.2, 93.5) | 40.5 (20.0, 53.2) | 0.63 | 0.20 | 194.8 (46.7, 825.0) | 50.3 (23.6, 72.9) | 0.73 | 0.03 | 178.1 (54.0, 649.5) | 41.7 (29.6, 64.6) | 0.76 | 0.01 | 130.6 (52.5, 605.2) | 51.4 (30.1, 87.8) | 0.73 | 0.03 |
| Elafin pg/ml | 7628.3 (4303.0, 18557.2) | 5088.0 (3530.3, 8057.8) | 0.62 | 0.26 | 10156.8 (5531.0, 14428.2) | 4610.0 (3416.2, 8140.6) | 0.71 | 0.04 | 7793.0 (4610.0, 10586.5) | 5931.4 (3916.6, 10941.0) | 0.54 | 0.74 | 6987.6 (5355.0, 12224.5) | 8794.1 (5786.9, 15783.8) | 0.57 | 0.45 |
| TNFR1 pg/ml | 4348.9 (2408.7, 6562.1) | 3747.1 (2025.1, 4341.2) | 0.62 | 0.23 | 5695.1 (2946.4, 9701.1) | 3809.8 (2391.9, 4935.8) | 0.64 | 0.20 | 5443.2 (3974.0, 6784.1) | 4528.0 (2692.6, 5492.5) | 0.67 | 0.11 | 4421.9 (3261.4, 7989.6) | 4094.6 (3150.5, 5572.2) | 0.49 | 0.33 |
| sIL2Rα (pg/ml | 883.2 (254.6, 1673.0) | 137.5 (50.1, 481.0) | 0.70 | 0.14 | 269.1 (26.1, 3051.3) | 95.7 (83.8, 265.3) | 0.61 | 0.41 | 930.5 (194.3, 2098.1) | 216.6 (167.4, 398.1) | 0.68 | 0.13 | ||||
For ST2, Reg3α, Elafin, and TNFR1, sample sizes were 17, 16, 17, and 16 for GVHD+ and 17, 15, 15, and 16 for GVHD- for Day +7, +14, +21, and +30 respectively. For sIL2Rα, sample sizes were 12, 10, and 10 for GVHD+ and 10, 13, and 14 for GVHD- for Day +7, +14, and +21 respectively.
Figure 1a.
ST2 (ng/ml) levels over time for individual patients in aGVHD+ and no aGVHD groups
Figure 1b.
REG3α (pg/ml) levels over time for individual patients in aGVHD+ and no aGVHD groups
We compared sIL2Rα levels using exact Wilcoxon tests in patients who received or did not basiliximab. Those who received basiliximab had higher values of sIL2Rα on day +7 (p=0.003), lower values on day +14 (p=0.02) and no difference in values on day +21 (p=0.27
The difference of day +14 biomarker levels between grade III-IV aGVHD+ and no aGVHD was not statistically significant; median ST2 34.0 and 19.8 ng/ml (p=0.10), median REG3α 180.9 and 50.3 pg/ml (p=0.07), median elafin 7818.1 and 4610.0 pg/ml (p=0.07), respectively. Difference of day +14 REG3α levels between GI aGVHD+ and no aGVHD also did not reach statistical significance; median 208.7 vs. 50.3 pg/ml (p=0.08).
Elevated (>50th percentile) biomarker levels at day +14 were not associated with OS; ST2 median 792 vs. 442 days (log-rank p=0.824), REG3α 792 vs. 442 days (p=0.558) and elafin 1081 vs. 545 days (p=0.582). Similarly elevated biomarker levels at day +14 were also not associated with NRM.
DISCUSSION
This study was conducted to determine prognostic plasma biomarkers for aGVHD following Cy/Flu-based NMAT. ST2, REG3α and elafin levels were elevated at certain time points in patients who developed aGVHD versus those who did not. These were, however, not significant prognostic biomarkers for other endpoints including grade III-IV aGVHD, OS and NRM, possibly due to the sample size of the study that was not powered for these endpoints.
The choice of biomarkers to consider was based on previous findings in cohorts undergoing myeloablative allotransplantation.[2, 3, 8, 9] REG3α is secreted by Paneth cells into intestinal crypts and reduces inflammation, protects intestinal stem cells and prevents GI epithelial damage.[10] REG3α does not mediate aGVHD, but appears to protect damaged epithelium. It is a biomarker for GVHD of GI tract as well as a predictor of NRM.[11] Day +14 REG3α levels were not statistically higher in patients with GI aGVHD compared to no aGVHD, which we think is attributable to the relatively small number of patients in this study, Elafin is associated with severity and mortality from aGVHD of the skin.[8] As part of a panel consisting of 6 biomarkers, REG3α and elafin predicted treatment response and survival from aGVHD.[2] ST2 in its soluble form acts as a negative regulator of type-2 helper T-cells (Th2).[12] ST2 was recently identified as a marker that predicted glucocorticoid-resistant aGVHD and non-relapse mortality (NRM).[3]
In the present study, the fact that we did not find TNFR1 or sIL2Rα elevated in those who developed aGVHD and elafin was significantly elevated at only day +14 is noteworthy. The relatively small sample size might not yield sufficient power to detect the prognostic value of all relevant biomarkers. Secondly, use of prognostic markers identified in the setting of higher-intensity conditioning might not be applicable during Cy/Flu-based NMAT. Third, it is plausible that using basiliximab (given at day +7, +8 or +9, as part of GVHD prophylaxis for some patients led to lower levels of day +14 sIL2Rα and confounded results; subset analysis among patients not receiving basiliximab was not done due to the relatively small number of patients. The higher level of sIL2Rα at day +7 was obtained prior to basiliximab administration, when cyclosporine was the only immunosuppressive present in the circulation.
Biomarkers are most valuable if they identify patients at high risk of an adverse outcome before the clinical signs are apparent. This could potentially provide clinicians with sufficient time to institute appropriate interventions before significant tissue damage has occurred and hopefully avert the adverse outcome. For instance, patients at high risk of developing aGVHD may be started on treatment preemptively. Among biomarkers identified in current study, only ST2 was prognostic of aGVHD risk before day +14 following HCT; this is not surprising given the relatively late (median, 36 days) onset of aGVHD following NMAT. Clinically-relevant prognostic tools proposed in prior studies consisted of a panel rather than single biomarker; therefore combinations of biomarkers will need to be explored further.[1, 2, 9]
In conclusion, the current study identified ST2, REG3α and elafin as prognostic biomarkers to stratify for risk of developing aGVHD after Cy/Flu-based NMAT. These results need to be confirmed in a large independent validation cohort, ideally amongst a number of institutions, to establish clinically useful cutoffs for their future use in clinical trials.
Elevated ST2 and REG3α at days +14 and +21 were associated with aGVHD after NMAT.
Elevated plasma elafin at day +14 was associated with aGVHD after NMAT.
TNFR1 and sIL2Rα were not associated with aGVHD risk at any of these time-points.
ACKNOWLEDGEMENTS
Authors would like to thank the patients who participated in this study, the clinicians of Indiana University Bone Marrow and Stem Cell Transplantation Program, and the members of the Paczesny and Orschell laboratories. We would also like to acknowledge our funding sources including National Institutes of Health (NIH) R01CA174667 (to S. P.), and the Lilly Physician Scientist Initiative Program (to S. P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURE:
S.P. holds a patent on “Methods of detection of graft-versus-host disease” (U.S. Patent #13/573,766).
REFERENCES
- 1.Paczesny S, Krijanovski OI, Braun TM, Choi SW, Clouthier SG, Kuick R, et al. A biomarker panel for acute graft-versus-host disease. Blood. 2009;113:273–8. doi: 10.1182/blood-2008-07-167098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine JE, Logan BR, Wu J, Alousi AM, Bolaños-Meade J, Ferrara JLM, et al. Acute graft-versus-host disease biomarkers measured during therapy can predict treatment outcomes: a Blood and Marrow Transplant Clinical Trials Network study. Blood. 2012;119:3854–60. doi: 10.1182/blood-2012-01-403063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vander Lugt MT, Braun TM, Hanash S, Ritz J, Ho VT, Antin JH, et al. ST2 as a Marker for Risk of Therapy-Resistant Graft-versus-Host Disease and Death. N Engl J Med. 2013;369:529–39. doi: 10.1056/NEJMoa1213299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson RP, Jr., Yu M, Schwartz JE, Robertson MJ, Hromas R, Fausel CA, et al. Long-term disease-free survival after nonmyeloablative cyclophosphamide/fludarabine conditioning and related/unrelated allotransplantation for acute myeloid leukemia/myelodysplasia. Bone Marrow Transplant. 2010;45:1300–8. doi: 10.1038/bmt.2009.348. [DOI] [PubMed] [Google Scholar]
- 5.Childs R, Clave E, Contentin N, Jayasekera D, Hensel N, Leitman S, et al. Engraftment Kinetics After Nonmyeloablative Allogeneic Peripheral Blood Stem Cell Transplantation: Full Donor T-Cell Chimerism Precedes Alloimmune Responses. Blood. 1999;94:3234–41. [PubMed] [Google Scholar]
- 6.ASBMT . Disease Classifications Corresponding to CIBMTR Classifications. 2014. [Google Scholar]
- 7.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–8. [PubMed] [Google Scholar]
- 8.Paczesny S, Braun TM, Levine JE, Hogan J, Crawford J, Coffing B, et al. Elafin is a biomarker of graft-versus-host disease of the skin. Sci Transl Med. 2010;2:13RA2. doi: 10.1126/scitranslmed.3000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paczesny S, Braun T, Lugt MV, Harris AC, Fiema B, Hernandez J, et al. Three biomarker panel at day 7 and 14 can predict development of grade II-IV acute graft-versus-host disease [abstract]. Blood. 2010;116:675. [Google Scholar]
- 10.Elphick DA, Mahida YR. Paneth cells: their role in innate immunity and inflammatory disease. Gut. 2005;54:1802–9. doi: 10.1136/gut.2005.068601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrara JLM, Harris AC, Greenson JK, Braun TM, Holler E, Teshima T, et al. Regenerating islet-derived 3-alpha is a biomarker of gastrointestinal graft-versus-host disease. Blood. 2011;118:6702–8. doi: 10.1182/blood-2011-08-375006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayakawa H, Hayakawa M, Kume A, Tominaga S-i. Soluble ST2 Blocks Interleukin-33 Signaling in Allergic Airway Inflammation. J Biol Chem. 2007;282:26369–80. doi: 10.1074/jbc.M704916200. [DOI] [PubMed] [Google Scholar]