Abstract
The purpose of this study was to examine auditory-nerve temporal response properties and their relation to psychophysical threshold for electrical pulse trains of varying rates (“rate integration”). The primary hypothesis was that better rate integration (steeper slope) would be correlated with smaller decrements in ECAP amplitude as a function of stimulation rate (shallower slope of the amplitude-rate function), reflecting a larger percentage of the neural population contributing more synchronously to each pulse in the train. Data were obtained for 26 ears in 23 cochlear-implant recipients. Electrically evoked compound action potential (ECAP) amplitudes were measured in response to each of 21 pulses in a pulse train for the following rates: 900, 1200, 1800, 2400, and 3500 pps. Psychophysical thresholds were obtained using a 3-interval, forced-choice adaptive procedure for 300-ms pulse trains of the same rates as used for the ECAP measures, which formed the rate-integration function. For each electrode, the slope of the psychophysical rate-integration function was compared to the following ECAP measures: (1) slope of the function comparing average normalized ECAP amplitude across pulses versus stimulation rate (“adaptation”), (2) the rate that produced the maximum alternation depth across the pulse train, and (3) rate at which the alternating pattern ceased (stochastic rate). Results showed no significant relations between the slope of the rate-integration function and any of the ECAP measures when data were collapsed across subjects. However, group data showed that both threshold and average ECAP amplitude decreased with increased stimulus rate, and within-subject analyses showed significant positive correlations between psychophysical thresholds and mean ECAP response amplitudes across the pulse train. These data suggest that ECAP temporal response patterns are complex and further study is required to better understand the relative contributions of adaptation, desynchronization, and firing probabilities of individual neurons that contribute to the aggregate ECAP response.
Keywords: electrically evoked compound action potential, cochlear implant, temporal integration, stimulation rate, adaptation
1. Introduction
Today’s cochlear implants (CIs) offer stimulation rates that range from 250 pulses per second per channel (pps/ch; Cochlear’s SPEAK strategy) to over 5000 pps/ch (Advanced Bionics’ HiResolution). Speech understanding with a CI has been shown to vary as a function of per-channel stimulation rate both within and across recipients (e.g., Brill et al., 1997; Friesen et al., 2005; Holden et al., 2002; Kiefer et al., 2000; Loizou et al., 2000; Vandali et al., 2000). At a more basic perceptual level, speech-processor program levels also differ with stimulation rate (e.g. Botros and Psarros, 2010; Kreft et al., 2004; McKay et al., 2005; McKay and McDermott, 1998; Shannon, 1985; Zhou et al., 2012). As the stimulation rate increases, behavioral thresholds (T levels) and upper-comfort (C or M) levels typically decrease. The extent to which these levels decrease with increased stimulation rate differs across individuals as well as across electrodes within an individual, although no systematic trends have been reported (Donaldson et al., 1997; Kreft et al., 2004; Pfingst et al., 2011; Zhou et al., 2012). Finally, at the most peripheral physiological level, auditory neural responses also vary with stimulation rate, and these response patterns likewise differ across electrodes and across individuals (Hughes et al., 2012). The primary goal of this study was to examine the extent to which physiological response patterns at the level of the auditory nerve relate to changes in behavioral threshold as a function of stimulation rate for pulse trains. Understanding these relations may provide insights to performance differences across stimulation rates.
1.1. Physiological effects of stimulation rate
The electrically evoked compound action potential (ECAP) is an aggregate response from a collection of auditory-nerve fibers. ECAP amplitudes change as a function of stimulation rate because of variations in neural excitability across fibers and across time. For relatively slow stimulation rates (≤200 pps), ECAPs measured in response to individual pulses within a train exhibit relatively large, equal-amplitude responses because the stimulation rate is sufficiently slow to allow for full recovery from depolarization to each pulse (Haenggeli et al., 1998; Matsuoka et al., 2000; Wilson et al., 1997). For rates ranging from approximately 400–2400 pps, ECAPs show an alternating pattern of amplitudes as a function of pulse number, reflecting differences in refractory periods across the underlying neural population (Finley et al., 1997; Hughes et al., 2012; Matsuoka et al., 2000; Rubinstein et al., 1999; Wilson et al., 1997). The depth of this alternating pattern increases with rate until a maximum depth is reached. The rate that produces the maximum alternation is expected to occur within the relative refractory period of the stimulated neural population (Hughes et al., 2012; Matsuoka et al., 2000). Matsuoka et al. (2000) described the maximum alternation as a “resonance” between the refractory period and the period of the stimulus pulse train, where the two periods are “synchronized”. As the stimulation rate increases beyond the point of maximum alternation, the alternating pattern diminishes and the overall amplitudes decrease (Finley et al., 1997; Hughes et al., 2012; Miller et al., 2008; Wilson et al., 1997). This reduced-amplitude, relatively flat pattern reflects a stochastic state (or desynchronization) across the individual fibers that contribute to the ECAP, and typically occurs for rates ≥2400 pps (Hughes et al., 2012). Each pulse therefore elicits a response from a sub-population of neurons because of differences in refractory-recovery times across fibers. In addition, there is likely some amount of neural adaptation (i.e., extended period of no response) that contributes to the overall amplitude reduction (Hay-McCutcheon et al., 2005; Miller et al., 2008; Zhang et al., 2007); however, the extent to which adaptation and desynchronization each contribute to the overall amplitude reduction remains unclear.
The rate at which the alternating pattern ceases is henceforth termed the “stochastic rate.” Hughes et al. (2012) examined ECAP responses to pulse-train stimuli for 29 ears in 26 CI recipients and found that for the majority of subjects, the stochastic rate differed across the three electrode regions tested (basal, middle, and apical). That study also examined the rate that yielded the maximum alternation in the ECAP amplitude pattern, and found that the maximum alternation occurred most often for 1800 pps for basal and apical electrodes, and 1200 pps for middle electrodes. These rates correspond to stimulus periods of 556–833 μsec, which are slightly longer than the absolute refractory period for the human auditory nerve (approximately 300–500 μsec), and therefore within the earlier portion of the relative refractory period (~0.5–8 ms; Brown et al., 1996, 1998; Finley et al., 1997). There was a trend toward shorter refractory-recovery time constants for electrodes that reached the maximum alternation at faster rates, suggesting that the alternating pattern is influenced by neural refractory effects, as noted by Matsuoka et al. (2000). However, there was no significant relation between refractory-recovery time constants and stochastic rate, suggesting that multiple mechanisms (e.g., variability of individual spike rates, firing probability, adaptation) likely contribute to stochastic independence in a complex way. It has been proposed that variations in neural refractory times underlie differences in how loudness (or behavioral threshold) changes with stimulation rate across individuals (Botros and Psarros, 2010; McKay et al., 2005; discussed further in the next section). Individual variability in auditory-nerve temporal-response properties (e.g., refractory-recovery time constants, adaptation, desynchronization) may therefore contribute to behavioral threshold differences and potentially performance differences across rates.
1.2. Perceptual effects of stimulation rate
The effect of stimulation rate on threshold and upper-comfort levels for CI users has been well documented (e.g. Botros and Psarros, 2010; Kreft et al., 2004; McKay et al., 2005; McKay and McDermott, 1998; Shannon, 1985; Zhou et al., 2012). As stimulation rate increases, thresholds tend to decrease more than upper-comfort levels because the mechanisms that contribute to each measure differ slightly (McKay et al., 2005; McKay and McDermott, 1998; Zhou et al., 2012). For threshold changes, Pfingst et al. (2011) and Zhou et al. (2012) described two mechanisms (central and peripheral) that presumably contribute to threshold decreases with increased stimulation rate. The first is a form of temporal integration, which they termed “multi-pulse integration.” Temporal integration is presumed to be a central mechanism and is defined as a reduction in behavioral threshold for increased stimulus duration (using a fixed rate). Multi-pulse integration describes a reduction in behavioral threshold for increased stimulus rate (using a fixed duration). As the stimulation rate increases, the total number of pulses presented during the fixed period of time increases, resulting in increased overall power of the stimulus. Temporal integration and multi-pulse integration are similar in that the total number of pulses in each listening interval increases, thus resulting in better detectability. The second mechanism contributing to perceptual threshold changes with stimulation rate occurs at the peripheral level, and involves integrative properties of the nerve-fiber membrane. At low stimulation levels, sub-threshold charge from multiple pulses accumulates within the integrative time constant of the neural membrane, resulting in increased probability of neural discharge for sub-threshold stimulation (e.g., Cartee et al., 2000; Middlebrooks, 2004). This phenomenon will only occur if the period between pulses is short enough for multiple pulses to occur within the integrative time window of the neural membrane (i.e., faster rates). Membrane charge integration will not occur for very slow rates due to the longer period between pulses exceeding the integrative time window. Data from animals and humans show integrative (summative) effects for inter-pulse intervals of approximately 400 μsec or less (Cartee et al., 2000; Morsnowski et al., 2006), which corresponds to stimulus rates of approximately 2500 pps or greater. In the present paper, we will use the term “rate integration” to refer to the collective effects of central multi-pulse integration (the rate-based version of temporal integration) and peripheral integration of charge along the auditory neural membrane for sub-threshold current levels, both of which contribute to the perceptual detection of the stimulus.
For behavioral upper-comfort levels, McKay and McDermott (1998) and McKay et al. (2005) suggested two somewhat opposing mechanisms (again, central and peripheral) that contribute to loudness as a function of stimulation rate. The first is multi-pulse integration, which is the same central mechanism that acts at threshold, summing peripheral input across a given time window. The second mechanism, neural refractory effects, occurs at the peripheral level and counterbalances the first to some extent by reducing the overall amount of neural input to the central integrator. Zhou et al. (2012) further proposed that fibers located near the edge of the excitation region likely receive sub-threshold stimulation, which temporally sums according to the neural-membrane integration mechanism described previously. Because there are fewer fibers that receive sub-threshold stimulation at high levels (near upper-comfort) than at low levels (near threshold), neural-membrane charge integration contributes less to the reduction in upper-comfort levels with increased rate. In sum, perceptual thresholds decrease with increased rate to a greater extent than upper-comfort levels. The larger decrements in threshold therefore result in an expanded behavioral dynamic range for faster stimulation rates.
1.3. Physiology versus perceptual measures
Relatively few studies have directly compared temporal response properties at the level of the auditory nerve to behavioral measures in the temporal domain with electrical pulse trains. Hay-McCutcheon et al. (2005) examined the relation between temporal integration and adaptation of ECAP responses across individual pulses in a pulse train for 11 adult recipients of Cochlear devices. In contrast to the studies discussed thus far (and the present study), temporal integration was measured using the traditional method of increasing the stimulus duration for a fixed-rate (1000 pps) stimulus, rather than increasing the stimulation rate for a fixed-duration stimulus. Regarding the ECAP measures, adaptation is typically considered a marked reduction in neural discharge rate over a relatively long period of time (~30–100 ms), potentially even leading to complete cessation of a response (e.g., Litvak et al., s2001; 2003). Hay-McCutcheon et al. (2005) characterized adaptation in their study as the overall decrease in ECAP amplitude across the pulse train, relative to the amplitude obtained in response to the first pulse in the train. The authors hypothesized that increased neural adaptation would limit the input to the central integrator, thereby reducing the amount of temporal integration. Their results showed no significant correlation between the two measures, suggesting that adaptation at the level of the auditory nerve did not affect temporal integration. Although not offered as a potential explanation in that study, it may be that the mechanisms contributing to the overall decrease in ECAP amplitude reflect variations across subjects in the role of desynchronization and increased variance in refractory-recovery times across pulses, rather than solely adaptation. In other words, adaptation is likely only partly responsible for the observed ECAP amplitude decrements. Reduced amplitudes may be due in part to desynchronization of individual neurons, where individual fibers might fire in response to every second, third, or fourth pulse, for example. The individual auditory-nerve fibers that contribute to the gross ECAP response likely consist of a range of high-, medium-, and low-spontaneous-rate fibers that vary in diameter, conduction velocity, spike probability, discharge threshold, and recovery time. Miller et al. (2000) presented single-fiber feline data showing variations in response amplitude and latency for repeated stimulation within the relative refractory period. In a subsequent study assessing rates of 250, 1000, and 5000 pps, Miller et al. (2008) showed variance in the number and timing of spikes for each rate, with greater variance for faster stimulation rates (5000 pps) versus the slower rates (250 pps and 1000 pps). In a related study, Zhang et al. (2007) showed feline single-fiber responses to each pulse in a 1000-pps pulse train that demonstrated charge integration and varying response probabilities across pulses. They also showed that spike-rate adaptation for some fibers can be overcome for slow rates (i.e., 250 pps) via increased stimulus levels, but not for the higher rates (1000 and 5000 pps). In sum, for 1000-pps pulse trains, as used in the study by Hay-McCutcheon et al. (2005), it appears that the overall decrease in ECAP amplitude was likely due to a combination of adaptation and variance in spike probability across pulses. It was therefore of interest in the present study to relate multiple aspects of the ECAP pattern (overall amplitude, alternation, stochasticity) to perceptual thresholds for different rates.
In a more recent study, Pfingst et al. (2011) examined psychophysical threshold as a function of stimulus rate (rate integration) in implanted guinea pigs, and compared those results to the amount of residual hearing and histological analyses of the cochleae. Results showed that the slope of the rate-integration function was generally steeper for pulse trains faster than 1000 pps, and shallower for rates slower than 1000 pps. The authors proposed that central multi-pulse integration was the primary mechanism contributing to threshold decreases for the slower rates (<1000 pps), whereas a combination of peripheral membrane integration and central multi-pulse integration contributed to threshold decrements for faster rates (>1000 pps). For pulse-train rates below 1000 pps, results showed that the slope of the rate-integration function was steeper for ears with more preserved hearing and greater hair-cell/nerve survival than those without. Taken together, their results suggest that better cochlear health results in increased total neural spike count, which contributes to lower detection thresholds and a steeper slope of the rate-integration function. Similar perceptual outcomes were reported for rate-integration functions obtained in 11 human CI recipients (Zhou et al., 2012), although that study did not include corresponding physiological measures to examine temporal responses at the level of the auditory nerve.
Finally, Botros and Psarros (2010) examined T-levels and C-levels as a function of stimulation rate for a group of 12 adult Cochlear CI recipients. They compared the slope of the T-level-versus-rate (rate integration) and C-level-versus-rate functions to ECAP refractory-recovery time constants across electrodes and subjects to evaluate the hypothesis that slower recovery is associated with a larger neural population, which in turn results in better rate integration. Results showed a significant negative correlation between the slope of the rate-integration function at T-level and the refractory-recovery time constant, where steeper slopes were obtained for electrodes with slower recovery times. These results suggest that longer ECAP recovery times are indicative of better temporal responsiveness. This somewhat counterintuitive result can be explained by the modeling portion of Botros and Psarros (2010), which was based on the assumption that loudness reflects a combination of (i) the number of fibers firing and (ii) the rate at which each fiber fires. Thus, for equal loudness, a smaller population of fibers will need to fire at a faster rate to produce the same total neural activity as a larger population firing at a slower rate. A faster firing rate for the smaller neural population would be achieved via increased current levels. Higher current levels yield faster recovery from refractoriness (Finley et al., 1997). Because current requirements would be lower for the larger population, stimulus levels will be closer to fiber threshold, resulting in lower spike probabilities, greater susceptibility to masking, and slower recovery (Finley et al., 1997; Botros & Psarros, 2010). It should be noted, however, that the refractory-recovery time constants in the Botros and Psarros (2010) study were obtained for a single masker-probe pulse pair and not for pulse trains, which are used for everyday listening. As the authors noted, it is important to understand that a single ECAP measurement represents the aggregate response across a collection of neurons that have different thresholds and temporal response properties. It is therefore important to consider how ECAP responses to pulse trains of different rates compare to perceptual thresholds at different rates. One of the benefits of measuring ECAPs in response to each pulse in a train is that we can obtain more detailed information about the temporal processing of sub-populations of neurons, and how the variations in neural responses across pulses within a pulse train relate to its overall percept. This was the goal of the present study.
In sum, the key findings of relevant research studies are:
ECAP adaptation is not correlated with temporal integration (Hay-McCutcheon et al., 2005).
Rate integration describes decreased behavioral thresholds for increased stimulation rates. For slower rates, central integration mechanisms will dominate. For faster rates, both central and peripheral integration mechanisms contribute to stimulus detection (Pfingst et al., 2011; Zhou et al., 2012).
Steeper slope of the rate-integration function is observed for faster rates (>1000 pps) and is related to better cochlear health (Pfingst et al., 2011).
Longer ECAP recovery times are consistent with better nerve survival and better temporal responsiveness (Botros & Psarros, 2010).
The primary goal of this study was to examine auditory-nerve temporal response properties and their relation to perceptual rate integration for pulse-train stimuli in human CI users. The objectives were to compare the slope of the rate-integration function with: (1) the average decrease in ECAP amplitude across pulses as a function of stimulation rate (similar to the adaptation measure used for increasing stimulus durations in Hay-McCutcheon et al., 2005), (2) the rate that produced the maximum alternation depth within the ECAP-amplitude pattern, and (3) ECAP stochastic rate. For the first objective, it was hypothesized that better rate integration (steeper slope) would be correlated with less reduction in ECAP amplitude as a function of rate (shallower slope of the amplitude-rate function), reflecting a larger percentage of the neural population contributing more synchronously to each pulse. For the second objective, it was hypothesized that better rate integration would be observed for electrodes that exhibit maximum alternation at slower rates, consistent with evidence that slower refractory recovery (a) tends to occur with maximum alternation at slower rates (Hughes et al., 2012) and (b) is related to better rate integration (e.g., Botros & Psarros, 2010). For the third objective, an a priori hypothesis regarding the relation between rate integration and stochastic rate was less straightforward. Animal studies indicate contributions of refractory-recovery time, fiber onset spike rate, spike probability, and spike desynchronization (e.g., Miller et al., 2008; Zhang et al., 2007) to the stochastic state; however, the effect of each factor is difficult to parse in human ECAP (whole-nerve) recordings. It was therefore important to examine the relation between rate integration and stochastic rate because the stochastic state reflects the complexity of the underlying neural mechanisms.
2. Materials and methods
2.1. Subjects
Psychophysical and ECAP data were obtained from 26 ears of 23 CI recipients for whom the ECAP data were reported in Hughes et al. (2012). Three participants were adolescents (F10, N4, and C17; aged approximately 12–14 years at the time of participation); the remaining 20 were adults. The mean age at implant was 44 years, 6 months (range: 7 years, 11 months to 82 years) and mean duration of CI use at participation was 3 years, 11 months (range: 3 months to 9 years). Three adults were implanted bilaterally; both ears were tested for this study (denoted with matching symbols for right and left ears in Table 1). Sixteen ears had Cochlear devices (N=6 Nucleus 24R(CS), N=8 Nucleus 24RE, N=2 CI512; Cochlear Ltd., Macquarie, NSW, Australia) and 10 had Advanced Bionics devices (N=8 HiRes 90K, N=2 Clarion CII; Advanced Bionics, Valencia, CA, USA). Additional demographic information is detailed in Table 1.
Table 1.
Demographic information for study participants.
| Subject | Internal Device, Electrode Array | Ear | Dur. Deafness (yrs) | Etiology | Age at CI (yrs, mos) | Duration CI Use (yrs, mos) | Test Electrodes (B, M, A) |
|---|---|---|---|---|---|---|---|
| R2 | Nucleus 24R(CS) | R* | 3 | Noise induced, hereditary | 52, 0 | 7, 3 | 3, 11, 20 |
| R3 | Nucleus 24R(CS) | R† | 50 | Unknown | 56, 1 | 7, 10 | 3, 11, 17 |
| R4 | Nucleus 24R(CS) | L^ | 1 | Unknown | 41, 11 | 7, 5 | 5, 11, 20 |
| R6 | Nucleus 24R(CS) | R | 6 | Autoimmune disease | 44, 4 | 5, 6 | 7, 11, 20 |
| R7 | Nucleus 24R(CS) | R | 5 | Unknown, progressive | 62, 2 | 4, 9 | 5, 11, 20 |
| R10 | Nucleus 24R(CS) | R | 2 | Unknown, progressive | 61, 10 | 6, 6 | 3, 11, 19 |
| F1 | Nucleus 24RE(CA) | L† | 54 | Unknown | 60, 7 | 3, 3 | 3, 11, 20 |
| F2 | Nucleus 24RE(CA) | R | 10 | Unknown | 60, 2 | 2, 0 | 5, 11, 20 |
| F4 | Nucleus 24RE(CA) | L | 17 | Ototoxicity | 17, 6 | 1, 11 | 3, 11, 20 |
| F5 | Nucleus 24RE(CA) | R^ | 7 | Unknown | 48, 3 | 1, 1 | 3, 11, 20 |
| F7 | Nucleus 24RE(CA) | R | 28 | Unknown | 39, 1 | 3, 7 | 3, 11, 20 |
| F8 | Nucleus 24RE(CA) | L* | 9 | Noise induced, hereditary | 58, 3 | 0, 7 | 3, 11, 20 |
| F10 | Nucleus 24RE(CA) | R | 8 | Waardenburg syndrome | 8, 3 | 4, 6 | 3, 11, 20 |
| F12 | Nucleus 24RE(CA) | R | 4 | Unknown, progressive | 82, 0 | 1, 4 | 3, 11, 20 |
| N4 | Nucleus CI512 | R | 0.5 | Unknown | 13, 4 | 0, 10 | 5, 11, 20 |
| N5 | Nucleus CI512 | R | 1 | Sudden SNHL | 50, 9 | 0, 3 | 5, 11, 20 |
| C17 | HiRes 90K HF 1J | R | 5 | Unknown | 7, 11 | 4, 0 | 14, 9, 1 |
| C18 | HiRes 90K HF 1J | R | 34 | Sudden SNHL | 36, 8 | 2, 8 | 14, 8, 5 |
| C19 | HiRes 90K HF 1J | R | 0.3 | Sudden from established SNHL | 15, 5 | 5, 9 | 15, 8, 1 |
| C24 | Clarion CII HF 1 + p | R | 15 | Unknown, progressive | 67, 4 | 9, 0 | 14, 8, (1-NR) |
| C26 | HiRes 90K HF 1J | R | 54 | Unknown | 67, 6 | 2, 1 | 14, 8, 1 |
| C29 | HiRes 90K HF 1J | R | 21 | Meningitis | 31, 0 | 2, 6 | 14, 8, 1 |
| C30 | Clarion CII HF 1 + p | L | 4 | Unknown, progressive | 52, 0 | 8, 11 | 14, 8, 1 |
| C31 | HiRes 90K HF 1J | L | 7 | Unknown, progressive | 24, 1 | 4, 6 | 14, 8, 5 |
| C33 | HiRes 90K HF 1J | R | 34 | Genetic | 36, 0 | 1, 7 | 14, 10, 1 |
| C39 | HiRes 90K HF 1J | L | 0.5 | Unknown | 63, 0 | 2, 5 | 14, 8, 1 |
Paired symbols (*,†, and ^) indicate ear-specific information for the three participants with bilateral CIs. SNHL = sensorineural hearing loss; NR = no measurable physiological response; +p = electrode array with positioner.
2.2. ECAP stimuli and procedures
Stimuli and procedures for the ECAP data are described in greater detail in the earlier study (Hughes et al., 2012). Briefly, ECAP amplitudes were recorded in response to each of 21 pulses in a pulse train for five rates (900, 1200, 1800, 2400, and 3500 pps)1 and three electrodes across the basal, middle, and apical regions of the electrode array (see Table 1 for specific electrodes). ECAP responses were based on an average of 50–100 sweeps. The pulse width was 25 μsec/phase for Cochlear devices (default interphase gap of 25 μsec for 24R(CS) and 7 μsec for 24RE and CI512) and 21.55 μsec for AB devices (no interphase gap). The Advanced Neural Response Telemetry (NRT) module within the clinical Custom Sound EP software (Cochlear Ltd., Macquarie, NSW, Australia) was used for subjects with Cochlear devices, and the research-based Bionic Ear Data Collection System (BEDCS v.1.18; Sylmar, CA, USA) was used for subjects with AB devices. The recording electrode was typically two positions apical to the stimulating electrode; stimulation and recording were both monopolar. Recording delay was optimized individually for Cochlear subjects; delay is not adjustable for AB devices. The gain was 50 dB and 60 dB (software defaults) for Cochlear 24RE/CI512 and 24R(CS) subjects, respectively, and 1000 (linear multiplier) for AB. A modified forward-masking technique was used to manage stimulus artifact (Hughes et al., 2012; Hay-McCutcheon et al., 2005). This technique uses the standard forward-masking paradigm to obtain a template of the probe artifact (masker-probe interval [MPI] of 400 μsec), which is subsequently subtracted from the pulse-train conditions. For the pulse-train conditions, the MPI equaled the period of the masker pulse train so that the probe was the last pulse in the train (requiring equal masker and probe levels). To avoid potentially confounding effects of level, stimuli were presented at the same current level for all five rates, which equaled a loudness rating of 8 (“loud but comfortable”) on a scale of 1—10 for the 3500-pps train. The 3500-pps train of 21 pulses was used to obtain loudness judgments because it was assumed that the fastest rate and greatest number of pulses should sound the loudest across all stimuli tested.
ECAP amplitudes were measured for each pulse in the train, and then normalized to the amplitude of the response to the first pulse so that response patterns could be compared across electrodes, rates, and subjects (Hughes et al., 2012). An example of the amplitude response pattern across rates is shown in Fig. 1 for subject F8. Each column represents a different electrode; rates from 900–3500 pps are shown from top to bottom, respectively. For each rate and electrode, two metrics were obtained: (1) average normalized amplitude (similar to the “adaptation” measure used by Hay-McCutcheon et al., 2005) and (2) average alternation depth. For the average amplitude measure, normalized amplitudes were averaged across pulses 2–21 (excludes the normalization point). The alternation depth for each rate was quantified as the absolute value of the difference between the average normalized amplitude for even- and odd-numbered pulses, again excluding the first pulse. The average amplitudes (Avg Amp) and alternation depths (Alt Dep) are detailed in each panel of Fig. 1. The middle and bottom panels of Fig. 2 show the average ECAP amplitude and alternation depth, respectively, plotted as a function of doubling (log2) stimulation rate for this subject. For the middle panel of Fig. 2, the linear-regression slope of each amplitude-rate function is detailed in the figure legend. Slopes were not calculated for the alternation depth functions (Fig. 2, bottom) because the functions were non-monotonic.
Figure 1.
Normalized ECAP amplitudes are plotted as a function of pulse number for electrodes 3, 11, and 20 (left to right columns, respectively) for subject F8. Data for each rate are shown from top (900 pps) to bottom (3500 pps). Asterisks represent a statistically significant difference (p < 0.05) in mean amplitudes between even- and odd-numbered pulses (pulses 2–21; excluding the normalization point), indicating alternation. The stochastic rate (SR) is the rate at which alternation ceased, and is indicated for each electrode. The mean average normalized amplitude (Avg Amp) and alternation depth (Alt Dep), excluding the normalization point, are noted in each panel.
Figure 2.
Top: Psychophysical thresholds (dB) as a function of stimulation rate (in pps) for subject F8. The abscissa is scaled to show dB per doubling of rate (log2). Middle: Normalized ECAP amplitudes averaged across pulses 2–21 as a function of stimulation rate. Bottom: Average ECAP alternation depth as a function of rate. In each panel, electrode is the parameter, with corresponding linear-regression slope (dB/log2 pps) values noted in the legend. Slopes are not shown for the bottom panel due to non-monotonicity of the functions.
For each electrode (columns in Fig. 1), the stochastic rate was determined as the fastest rate at which the alternating pattern ceased. For each rate, the normalized amplitudes for even- and odd-numbered pulses (excluding the first pulse) were compared using a paired t-test. A significant difference (p < 0.05) meant the alternating pattern was present (indicated by an asterisk next to each rate in Fig. 1). Lack of a significant difference indicated no alternation. The stochastic rate for each electrode in Fig. 1 is identified with “(SR)”. In this example, stochastic rate was 2400 pps for the basal and middle electrodes, and 3500 pps for the apical electrode.
2.3. Psychophysical stimuli and procedures
All stimuli were delivered via direct connect. For Cochlear subjects, a custom program written in Visual Basic that implemented Nucleus Implant Communicator subroutines (NIC v. 2; Cochlear Ltd., Macquarie, New South Wales, Australia) was used to present stimuli to subjects via a laboratory Freedom speech processor interfaced with a programming pod. For AB subjects, the BEDCS software was used to present stimuli to subjects via a laboratory Platinum Series Processor interfaced with a Clinical Programming Interface (CPI-II).
Psychophysical thresholds were measured for the same electrodes (see Table 1) and rates as the ECAP measures. Loudness estimates were first obtained for each test electrode at “first hearing” and “loud” (corresponding to 1 and 8, respectively, on a 10-point scale) for the slowest (900 pps) and fastest (3500 pps) rates to determine approximate starting level and to set upper-comfort limits. Starting level and upper-comfort limits were linearly interpolated for the remaining rates. The test stimulus was a 300-ms pulse train presented in monopolar mode for each rate, using the same pulse durations and interphase gaps as for the ECAPs. Starting level was typically 2.6 dB above the loudness rating of “1” (equivalent to 15 CL for Cochlear subjects). The task consisted of a 3-interval, forced-choice, adaptive procedure (3-down, 1-up) in which the stimulus randomly occurred in one of the three test intervals indicated by sequentially lighted boxes on a computer monitor. For Cochlear subjects, the step size was 10 CL (1.76 dB) for the first reversal, 5 CL (0.88 dB) for the second reversal, and 2 CL (0.35 dB) for the last 6 reversals. Step sizes were slightly larger for AB subjects: 3 dB for the first reversal, 1.5 dB for the second reversal, and 0.75 dB for the last 6 reversals. Subjects were instructed to choose the interval that contained the stimulus. Correct-answer feedback was provided. The mean of the last six reversals was used as threshold for each block and the final threshold was the average of 2–4 blocks. Test order was randomized across electrodes and rates.
For each subject, mean psychophysical thresholds were converted to dB and plotted as a function of log2 stimulation rate (dB per doubling of rate). The slope of each rate-integration function was calculated for each electrode and subject for comparison to the respective ECAP data. An example is shown in the top panel of Fig. 2 for subject F8, whose corresponding ECAP data are shown in Fig. 1 and the middle and bottom panels of Fig. 2. For this subject, thresholds were lower overall for the most apical electrode (E20), and the slope for the middle electrode (−3.60 dB/log2 pps) was steeper than for the basal and apical electrodes (−2.92 and −2.93 dB/log2 pps, respectively).
3. Results
The primary goal of this study was to compare several aspects of auditory-nerve temporal response properties (overall amplitude decrease, alternation depth, and stochastic rate) to the slope of the rate-integration function for pulse trains of different rates. Figure 3 shows the group mean data (±1 SD) for psychophysical thresholds (rate integration; top row), normalized ECAP amplitudes averaged across pulses 2–21 (“adaptation”; middle row), and average ECAP alternation depth (bottom row), each plotted as a function of doubling stimulation rate (log2 scaling). Cochlear and AB data are plotted separately in the left and right columns. For the psychophysical rate-integration functions (top row), thresholds were higher overall for AB subjects most likely because of the shorter stimulus pulse width and lack of interphase gap. Across both devices, as expected, psychophysical thresholds and average ECAP amplitudes decreased as pulse rate increased (top and middle rows, respectively). For each function in the top and middle graphs, linear regression was used to fit the mean data for each electrode; the slope of each regression line is shown in the corresponding figure legends. A one-way repeated-measures analysis of variance (RM ANOVA) was used separately for each device to test for slope differences across electrode regions. For thresholds (top) and average ECAP amplitudes (middle), results showed no significant differences in mean slope across the three regions tested (p > 0.2). In contrast to the observed trends for thresholds and average ECAP amplitude (top and middle rows), ECAP alternation depth (bottom row) exhibited a non-monotonic pattern, where the depth generally increased from 900 pps to 1800 pps, then decreased from 1800 pps to 3500 pps. This non-monotonic trend was more pronounced for Cochlear subjects than for AB. For AB subjects, only the basal electrode exhibited a non-monotonic trend, whereas the middle and apical electrodes generally showed progressively less alternation as rate increased. There was a larger percentage of electrodes in AB devices (9/29 or 31%) that exhibited maximum alternation at 900 pps than in Cochlear devices (6/48 or 12.5%). This result could be a by-product of stimulus differences (pulse width and interphase gap), and likely warrants further investigation. Linear regression analyses were not applied to the alternation depth data due to the non-monotonic pattern of the functions.
Figure 3.
Top: Mean (±1 SD) psychophysical thresholds as a function of the log2 (dB per doubling) stimulation rate for each device type (Cochlear, left column; Advanced Bionics right column). Middle: Mean (± 1 SD) normalized ECAP amplitudes (“adaptation”) as a function of stimulation rate. For each subject, the average amplitude across pulses 2–21 was calculated; these individual averages were used to calculate the mean data points in the figure. Bottom: Mean (± 1 SD) ECAP alternation depth as a function of stimulation rate. In each panel, electrode is the parameter, with corresponding linear-regression slope (dB/log2 pps) values noted in the legend. Slopes are not shown for the bottom panel due to non-monotonicity of the functions.
3.1. Rate integration versus average ECAP amplitude
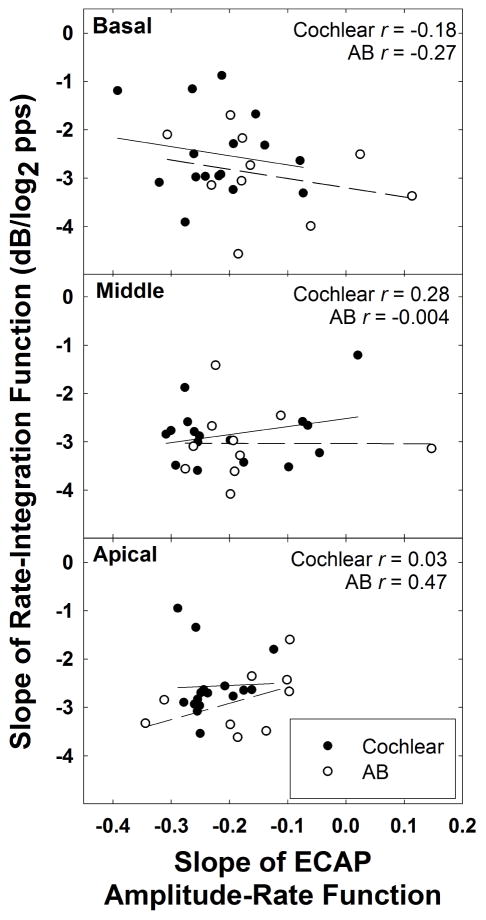
The first objective of this study was to compare the slope of the rate-integration function with the slope of the respective ECAP amplitude-rate function (“adaptation”) across subjects. It was expected that better rate integration (steeper slope) would be correlated with smaller decrements in ECAP amplitude as a function of rate (shallower slope); that is, we would expect a negative correlation between the two variables. Group data for this comparison are shown in Figure 4. Each panel represents a different electrode region with one data point per subject/ear; filled and open circles represent Cochlear and AB devices, respectively. Pearson correlation coefficients (r values) are shown in each panel for each device, detailed in the top right corner. Results showed no significant correlations for any of the comparisons (p values ranged from 0.21 to 0.99). Only the basal electrodes exhibited a trend in the predicted direction.
Figure 4.
Scatter plots showing the relation between the slope of the rate-integration function and the slope of the ECAP amplitude-rate function. From top to bottom, panels show data for basal to apical electrodes, respectively. Cochlear and AB data are represented with filled and open circles, respectively. Solid and dashed lines show linear regression fits for Cochlear and AB devices, respectively. Pearson r values are shown in each panel for each device type separately.
Given the similar trends between psychophysical thresholds and average ECAP amplitudes across rates in Fig. 3 (top and middle rows), it was of interest to examine correlations within individuals, independent of the slope measures. Although these comparisons do not directly test the first hypothesis, the results provide insight into relations between ECAP amplitude and behavioral threshold on an individual basis. Figure 5 shows examples of individual data that illustrate the relation between psychophysical threshold and average ECAP amplitudes for each electrode (symbols) and rate (individual data points of the same symbol). For clarity, a single regression line is shown for each subject across all three electrodes and five rates, and the corresponding Pearson correlation coefficient (r value) and significance (p-value) are shown in each graph. The top and bottom rows show data from three Cochlear and three AB subjects, respectively. Table 2 shows r and p values for all 26 subjects/ears. Results showed significant correlations (in boldface with asterisks) between psychophysical threshold and average ECAP amplitude for 18 of the 26 ears. For the 18 ears that exhibited significant correlations across electrodes, 17 also showed significant correlations for some or all individual electrodes within the data set (e.g., F4, F8, C17, C26, and C39 in Fig. 5; electrode-specific statistical results not displayed). Only one subject (F2; see Fig. 5) showed a significant correlation across the entire data set but none of the individual electrodes demonstrated significant correlations (p > 0.13).
Figure 5.
Individual examples illustrating statistically significant within-subject correlations between psychophysical threshold (dB) and average normalized ECAP amplitude across the pulse train for each of the five rates tested. Data for each electrode are shown with different symbols; regression lines are fit to all data within a subject. Correlation coefficients (r) and p values are noted in each panel along with subject number. Asterisks indicate statistical significance for p < 0.05.
Table 2.
Correlation coefficients and p-values for individual subjects’ relation between behavioral threshold (dB) and average normalized ECAP amplitude across pulses 2–21 (“adaptation”). Calculations are for all data (three electrodes and five rates) within a subject. Bolded values with asterisks were statistically significant (p < 0.05).
| Subject | r value | p value |
|---|---|---|
| R2 | 0.64 | 0.011* |
| R3 | 0.47 | 0.078 |
| R4 | 0.85 | <0.001* |
| R6 | 0.72 | 0.002* |
| R7 | 0.72 | 0.002* |
| R10 | 0.43 | 0.112 |
| F1 | 0.86 | <0.001* |
| F2 | 0.67 | 0.006* |
| F4 | 0.87 | <0.001* |
| F5 | 0.79 | <0.001* |
| F7 | 0.67 | 0.006* |
| F8 | 0.80 | <0.001* |
| F10 | 0.69 | 0.005* |
| F12 | 0.17 | 0.546 |
| N4 | 0.38 | 0.160 |
| N5 | 0.81 | <0.001* |
| C17 | 0.67 | 0.006* |
| C18 | −0.13 | 0.635 |
| C19 | 0.80 | <0.001* |
| C24 | 0.38 | 0.283 |
| C26 | 0.53 | 0.044* |
| C29 | 0.44 | 0.104 |
| C30 | 0.37 | 0.174 |
| C31 | 0.79 | <0.001* |
| C33 | 0.62 | 0.015* |
| C39 | 0.86 | <0.001* |
For the eight subjects who did not exhibit significant correlations across electrodes (see Table 2), significant correlations were found for 11 of the 23 electrodes tested across these subjects. Data for these eight subjects are shown in Fig. 6 and the electrode-specific r and p values are detailed in Table 3. As an example, the middle and apical electrodes for subject F12 (squares and triangles, respectively) showed a significant correlation between psychophysical threshold and averaged ECAP amplitude (p = 0.023 and 0.006, respectively), but the basal electrode did not (circles; p = 0.136). The spread in the data across the three electrodes (i.e., different slopes within each symbol type) yielded a weak correlation for the pooled data set (r = 0.17). As can be seen from Fig. 6 and Table 3, seven of the eight subjects exhibited trends across electrodes similar to those for F12. C30 was the only subject who exhibited no significant correlations within or across electrodes (bottom right panel of Fig. 6). In sum, it appears that rate integration is generally correlated with ECAP amplitude reduction on an individual basis. As rate increases, behavioral thresholds and normalized ECAP amplitudes decrease (see Fig. 3). However, there are differences across electrodes and subjects regarding the degree to which these two measures are correlated.
Figure 6.
Individual examples for subjects who showed non-significant correlations between psychophysical threshold (dB) and average normalized ECAP amplitude when data were pooled across electrodes (see Table 3). Data are plotted as in Fig. 5.
Table 3.
Correlation coefficients and p-values for behavioral thresholds (dB) versus average normalized ECAP amplitude across pulses 2–21 (“adaptation”), calculated across rates for individual electrodes. Data are for the subset of subjects who showed no significant correlations when data were pooled across rates and electrodes (see Table 2 and Fig. 6). Bolded values with asterisks were statistically significant (p < 0.05). CNT, could not test (no measurable ECAPs).
| Subject | Electrode | r value | p value |
|---|---|---|---|
|
| |||
| R3 | Basal | 0.85 | 0.066 |
| Middle | 0.95 | 0.014* | |
| Apical | 0.85 | 0.066 | |
|
| |||
| R10 | Basal | 0.87 | 0.057 |
| Middle | 0.89 | 0.041* | |
| Apical | 0.96 | 0.011* | |
|
| |||
| F12 | Basal | 0.76 | 0.136 |
| Middle | 0.93 | 0.023* | |
| Apical | 0.97 | 0.006* | |
|
| |||
| N4 | Basal | 0.90 | 0.037* |
| Middle | 0.32 | 0.605 | |
| Apical | 0.91 | 0.033* | |
|
| |||
| C18 | Basal | −0.87 | 0.054 |
| Middle | −0.90 | 0.036* | |
| Apical | 0.84 | 0.075 | |
|
| |||
| C24 | Basal | 0.88 | 0.051 |
| Middle | 0.99 | 0.002* | |
| Apical | CNT | CNT | |
|
| |||
| C29 | Basal | 0.76 | 0.132 |
| Middle | 0.91 | 0.033* | |
| Apical | 0.94 | 0.016* | |
|
| |||
| C30 | Basal | 0.39 | 0.512 |
| Middle | 0.87 | 0.056 | |
| Apical | 0.54 | 0.344 | |
3.2. Rate integration versus ECAP alternation depth
The second objective of this study was to examine how the slope of the rate-integration function relates to the average alternation depth of the ECAP response pattern. Because the slopes of the ECAP alternation depth-versus-rate functions were generally non-monotonic (see Fig. 3, bottom row), correlation analyses across slope comparisons were not appropriate. Instead, the mean slopes of the rate-integration functions were examined as a function of the rate that yielded the maximum alternation in the ECAP pattern. Better rate integration (steeper slope) was expected for electrodes that exhibited maximum alternation at slower rates. Figure 7A shows the slope of the rate-integration function for each electrode as a function of the rate of maximum alternation. Cochlear data are shown with open circles; AB data are shown with filled circles and are offset slightly for better visualization. Mean data pooled across devices are shown in the top section of Table 4, which lists the mean slope of the rate-integration function for electrodes that exhibited maximum alternation in the ECAP pattern at rates ranging from 900 pps to 2400 pps (no electrodes showed maximum alternation at 3500 pps). The number of electrodes (N) contributing to the mean slope, standard deviations (SD), and range of slopes are also included in Table 4. A one-way ANOVA showed no significant differences among the mean slopes across electrodes that exhibited maximum alternation at different rates (p = 0.16). Although the test was underpowered (0.21), there appears to be no systematic trend across electrodes with different maximum-alternation rates.
Figure 7.
Slope of the rate-integration function for each electrode plotted as a function of the rate of maximum ECAP alternation (A) or ECAP stochastic rate (B). Open circles represent Cochlear data; filled circles represent AB data. AB data are offset slightly to the right for better visualization.
Table 4.
Mean slope of the temporal-integration function (in dB/log2 pps) with standard deviation (SD), range of slopes, and number of observations (N) for electrodes with the rate of maximum alternation (top section) and stochastic rates (bottom section) ranging from 900 pps to 3500 pps.
| Rate of Maximum Alternation: | 900 pps | 1200 pps | 1800 pps | 2400 pps | 3500 pps |
|---|---|---|---|---|---|
| N | 15 | 23 | 34 | 5 | 0 |
| Mean Slope (SD) | −2.69 (0.84) | −2.99 (0.62) | −2.57 (0.78) | −3.03 (0.30) | — |
| Range | −1.21 – −3.99 | −1.88 – −4.57 | −0.88 – −3.91 | −2.83 – −3.56 | — |
| Stochastic Rate: | 900 pps | 1200 pps | 1800 pps | 2400 pps | 3500 pps |
|---|---|---|---|---|---|
| N | 1 | 3 | 14 | 30 | 29 |
| Mean Slope (SD) | −2.66 (0) | −3.33 (0.68) | −2.68 (0.57) | −2.78 (0.83) | −2.70 (0.74) |
| Range | — | −2.64 – −3.99 | −1.60 – −3.52 | −0.88 – −4.57 | −0.95 – −3.91 |
3.3. Rate integration versus ECAP stochastic rate
The third objective was to examine the relation between rate integration and ECAP stochastic rate. Recall that there was no a priori hypothesis for this objective; rather, the goal was to examine whether a relation existed between rate integration and stochastic rate. Figure 7B shows the slope of the rate-integration function for each electrode as a function of stochastic rate. Data are plotted as in Fig. 7A. The bottom section of Table 4 shows the mean slope of the rate-integration function for electrodes with ECAP stochastic rates ranging from 900 pps to 3500 pps. A one-way ANOVA on ranks (Kruskal-Wallis, due to non-normal distribution of data) showed no significant differences among the slopes of the rate-integration functions across electrodes with different stochastic rates (p = 0.67). Again, there was no systematic trend across electrodes with different stochastic rates. The steepest average rate-integration function (mean slope −3.33 dB/log2 pps) was observed for electrodes that exhibited the stochastic rate at 1200 pps.
4. Discussion
4.1. Rate integration versus average ECAP amplitude
The primary goal of this study was to examine the extent to which physiological response patterns at the level of the auditory nerve relate to behavioral threshold as a function of stimulation rate. For the first objective, it was hypothesized that better rate integration (steeper slope) would be correlated with smaller decrements in ECAP amplitude as a function of rate (shallower slope of the amplitude-rate function), reflecting a larger percentage of the neural population (re: the first pulse) contributing to the response to each subsequent pulse. In general, psychophysical thresholds and average ECAP amplitudes both decreased as stimulation rate increased. When the slopes of the functions were compared across subjects, however, the data showed no significant correlations between the two measures (Fig. 4). The null findings for the group slope data are consistent with those obtained for increasing stimulus durations by Hay-McCutcheon et al. (2005), and two recent studies that compared ECAP amplitude decrements to either rate integration (McKay et al., 2013) or to gap detection (Zhang et al., 2013).
In the McKay et al. (2013) study, behavioral thresholds were obtained for a group of eight subjects for rates ranging from 40–2400 pps, and ECAPs were obtained across pulse trains ranging from 500–2400 pps. The group ECAP data (average normalized amplitude across pulses) were input to a loudness model to predict the rate-integration function. Their model was found to be appropriate for average data across the group, but was not sufficient for predicting rate integration on an individual basis. Although the present data set showed no significant slope differences across electrode regions for the group means in Fig. 3, individual correlations between average ECAP amplitude and psychophysical threshold showed different trends across electrodes and subjects (Figs. 5 and 6), consistent with the poor model prediction for individuals shown by McKay et al. (2013). Although both the psychophysical thresholds and ECAP-amplitude functions generally decreased as a function of increased stimulation rate, the rate of decrease (slope) differed across individuals, electrodes, and measures. These results suggest that different aspects of the underlying neural response (e.g., number of contributing fibers, synchrony across fibers) might differentially dominate across individuals and electrodes, perhaps as a function of the amount and type of neural survival, which in turn differentially affects the slopes of each of these functions. This might partially explain why stimulation rates for best performance differ across individuals (e.g., Brill et al., 1997; Friesen et al., 2005; Holden et al., 2002; Kiefer et al., 2000; Loizou et al., 2000; Vandali et al., 2000). More detailed sampling across electrodes within subjects may yield more useful information for relating physiological and perceptual measures on an individual basis, and remains an opportunity for further study.
In the present study, it was hypothesized that smaller decrements in ECAP amplitude as a function of rate would be indicative of a larger percentage of neurons responding to each pulse and thus better rate integration. The ECAP data from the present study, as well as those in previous studies (Hay-McCutcheon et al., 2005; McKay et al., 2013; Zhang et al., 2013), were normalized to the amplitude of the ECAP response to the first pulse in the train. Normalized amplitudes do not yield information about the size of the underlying neural population, but rather a percentage of neural activity relative to that of the first pulse in the train. “Neural activity” would include the percentage of fibers responding, the relative timing of each fiber’s response (inter-spike interval), and spike amplitudes for individual fibers (e.g., Zhang et al., 2007). Although normalization allows for more direct comparisons across electrodes and subjects (as was the initial goal for the present study), it obscures information about the overall magnitude of the ECAP. The raw ECAP amplitude presumably reflects the number of fibers responding and the degree of synchrony across fibers, which may be more relevant for predicting psychophysical measures, particularly across a larger electrode set within subjects. However, the raw ECAP amplitude is also affected by the distance between the recruited neural population and the stimulating (and recording) electrodes, which complicates comparisons for cross-subject analyses. Despite the multitude of factors that contribute to ECAP measures, raw ECAP amplitudes might provide additional information regarding the status of the auditory nerve and may be useful to include in future models that include measures across a larger sample of electrodes within each recipient.
In another recent study, Zhang et al. (2013) compared ECAP adaptation (characterized as the average decrement in ECAP amplitude over portions of a 1000-pps pulse train) and cortical measures of adaptation to behavioral measures of gap detection in 14 adult Cochlear recipients. One of their goals was to determine whether peripheral and/or central physiological measures are predictive of perceptual temporal processing. The results showed no significant correlation between either of the physiological measures (ECAP, cortical) and gap detection for the group, consistent with the present study and with Hay-McCutcheon et al. (2005) and McKay et al. (2013). As with Hay-McCutcheon et al. (2005) and McKay et al. (2013), individual correlations were not reported. It should be noted that while Zhang et al. (2013) obtained both ECAP and cortical physiological potentials within the same subjects, the degree of adaptation was not compared between the two physiological measures. Such a comparison may help elucidate the relative contributions of peripheral versus central adaptation to perceptual measures of temporal processing, and may help explain individual differences that contribute to poor correlations between ECAP “adaptation” and temporal processing across subjects.
Finally, it is possible that stimulus level differences between the two measures in the present study might have contributed to the poor correlation between the slopes of the ECAP-amplitude and rate-integration functions. ECAP measures required higher stimulus levels than those used for behavioral thresholds because ECAPs were suprathreshold measures obtained with shorter-duration pulse trains, whereas behavioral measures were thresholds obtained with longer-duration pulse trains. The excitation pattern for higher current levels is broader than that for lower levels (e.g., Hughes & Stille, 2010). As a result, each measure likely represents input from slightly different populations of peripheral neurons operating at different discharge rates. These population differences will likely affect the rate of change (slope) for each measure in a slightly different way, lending to a poor correlation between the ECAP and behavioral measures across subjects.
4.2. Rate integration versus ECAP alternation depth
For the second objective of the present study, it was hypothesized that better rate integration would be observed for electrodes that exhibit maximum alternation at slower rates. This hypothesis was based on evidence from earlier studies that suggested slower refractory recovery is related to better rate integration across subjects and electrodes (e.g., Botros & Psarros, 2010) and tends to occur for electrodes that exhibit maximum alternation at slower rates (Hughes et al., 2012). Results showed no relation between the slope of the rate-integration function and maximum alternation in the ECAP amplitude patterns, which was not consistent with the hypothesis. Animal data from Haenggeli et al. (1998) showed that the alternating ECAP amplitude patterns occurred at slower rates (300–400 pps) for non-deafened, implanted rats than for rats with substantial spiral-ganglion-cell loss from neomycin deafening (≥800 pps). Thus, alternation occurs for slower rates in healthier cochleae, consistent with the human data and modeling results of Botros & Psarros (2010), and the presumption that stimulation of the peripheral portion of auditory neurons results in longer refractory-recovery times (Matsuoka et al., 2000). The alternating pattern results when the period of the pulse train coincides with the refractory period of the contributing fibers (Matsuoka et al., 2000), and appears to be influenced by the presence of hair cells and intact peripheral neural processes. In contrast, more synchronous responses to each pulse (less alternation) and shorter recovery times may be the result of axonal stimulation due to poorer nerve survival and potentially higher stimulus levels. Again, the heterogeneity in nerve survival and individual fiber characteristics (e.g., threshold, refractory-recovery time, adaptation) across CI recipients may have contributed to the lack of any clear trend between ECAP alternation and temporal integration for the group data. Future studies should investigate relations between ECAP alternation and temporal or rate integration across a larger sample of electrodes within subjects.
4.3. Rate integration versus ECAP stochastic rate
For the third objective, the slope of the rate-integration function was compared across electrodes with different ECAP stochastic rates. Results from the present study showed no clear relation between rate integration and stochasticity across the group data. Based on results from Haenggeli et al. (1998), better rate integration might be expected for electrodes with slower ECAP stochastic rates. That is, if better rate integration is related to better nerve survival (Botros & Psarros, 2010), and better nerve survival results in greater stochasticity (less synchrony) as evidenced by greater alternation in the ECAP pattern (Haenggeli et al., 1998), then the ECAP should reach a desynchronized or stochastic state at slower rates. However, this hypothesis assumes direct relations between temporal or rate integration, auditory nerve survival, and neural synchrony, which is likely overly simplistic. The ECAP is a whole-nerve response that reflects activity from a collection of fibers that vary in diameter, and therefore vary in threshold and susceptibility to adaptation (e.g., Litvak et al., 2003; Miller et al., 2008; Zhang et al., 2007). The auditory nerve-fiber population characteristics will likely vary substantially across individual CI users, which likely explains why the present results do not show clear relations between ECAP measures and rate integration.
5. Conclusions
Behavioral thresholds and average normalized ECAP amplitudes generally decreased with increasing stimulation rate. However, there was no significant correlation between the slopes of the functions when each measure was plotted against stimulation rate. Given the stimulus level differences between measures, these results suggest that neural population size and response characteristics may differentially affect the slope of each function within and across individuals. Each of the ECAP measures in the present study (amplitude decrement, alternation, and stochastic rate) reflects the activity from a collection of individual fibers, the properties of which likely vary widely across CI recipients. ECAP temporal response patterns are complex, and further study is required to better understand the relative contributions of adaptation, desynchronization, and firing probabilities of individual neurons that contribute to the aggregate ECAP response. Future studies should examine physiological and perceptual temporal responses across a larger sample of electrodes within individuals to better understand the relations between perceptual and physiological measures in individual CI users. Such results may help better explain variations in performance across CI users for different stimulation rates.
Highlights.
ECAP amplitude across pulses and behavioral threshold decrease with increased rate
Group data showed no significant correlation between slopes of the two measures
Significant correlations were, however, found within subjects
Stimulus level differences might account for poor correlations in the group data
Acknowledgments
This research was supported by NIH/NIDCD R01 DC009595, T35 DC008757, and P30 DC04662. The content of this project is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Deafness and Other Communication Disorders or the National Institutes of Health. The authors thank Tom Creutz for data collection and analysis programs; Leonid Litvak (Advanced Bionics) for BEDCS support; Lisa Stille, Erin Castioni, and Donna Neff for assistance with data collection; and Rachel Scheperle for feedback on earlier versions of this manuscript.
Abbreviations
- AB
Advanced Bionics
- BEDCS
Bionic Ear Data Collection System
- CI
cochlear implant
- ECAP
electrically evoked compound action potential
- MPI
masker-probe interval
- NRT
Neural Response Telemetry
- pps
pulses per second
- SD
standard deviation
Footnotes
The corresponding total pulse-train durations were 23.3, 17.5, 11.7, 8.8, and 6.0 ms, respectively.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Botros A, Psarros C. Neural Response Telemetry reconsidered: II. The influence of neural population on the ECAP recovery function and refractoriness. Ear Hear. 2010;31:380–391. doi: 10.1097/AUD.0b013e3181cb41aa. [DOI] [PubMed] [Google Scholar]
- Brill SM, Gstötner W, Helms J, von Ilberg C, Baumgartner W, Müer J, Kiefer J. Optimization of channel number and stimulation rate for the fast continuous interleaved sampling stragegy in the COMBI 40+ Am J Otol. 1997;18:S104–S106. [PubMed] [Google Scholar]
- Brown CJ, Abbas PJ, Borland J, Bertschy MR. Electrically evoked whole nerve action potentials in Ineraid cochlear implant users: Responses to different stimulating electode configurations and comparison to psychophysical responses. J Sp Hrg Res. 1996;39:453–467. doi: 10.1044/jshr.3903.453. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Abbas PJ, Gantz BJ. Preliminary experience with Neural Response Telemetry in the Nucleus CI24M cochlear implant. Am J Otol. 1998;19:320–327. [PubMed] [Google Scholar]
- Cartee LA, van den Honert C, Finley CC, Miller RL. Evaluation of a model of the cochlear neural membrane. I Physiological measurement of membrane characteristics in response to intrameatal electrical stimulation. Hear Res. 2000;146:143–152. doi: 10.1016/s0378-5955(00)00109-x. [DOI] [PubMed] [Google Scholar]
- Donaldson GS, Viemeister NF, Nelson DA. Psychometric functions and temporal integration in electric hearing. J Acoust Soc Am. 1997;101:3706–3721. doi: 10.1121/1.418330. [DOI] [PubMed] [Google Scholar]
- Finley C, Wilson B, van den Honert C, Lawson D. Speech processors for auditory prostheses. NIH Project N01-DC-5-2103, Sixth Quarterly Progress Report; November 1 1996 –January 31, 1997.1997. [Google Scholar]
- Friesen LM, Shannon RV, Cruz RJ. Effects of stimulation rate on speech recognition with cochlear implants. Aud Neurotol. 2005;10:169–184. doi: 10.1159/000084027. [DOI] [PubMed] [Google Scholar]
- Haenggeli A, Zhang JS, Vischer MW, Pelizzone M, Rouiller EM. Electrically evoked compound action potential (ECAP) of the cochlear nerve in response to pulsatile electrical stimulation of the cochlea in the rat: Effects of stimulation at high rates. Audiol. 1998;37:353–371. doi: 10.3109/00206099809072989. [DOI] [PubMed] [Google Scholar]
- Hay-McCutcheon M, Brown CJ, Abbas PJ. An analysis of the impact of auditory nerve adaptation on behavioral measures of temporal integration in cochlear implant recipients. J Acoust Soc Am. 2005;118(4):2444–2457. doi: 10.1121/1.2035593. [DOI] [PubMed] [Google Scholar]
- Holden LK, Skinner MW, Holden TA, Demorest ME. Effects of stimulation rate with the Nucleus 24 ACE speech coding strategy. Ear Hear. 2002;23:463–476. doi: 10.1097/00003446-200210000-00008. [DOI] [PubMed] [Google Scholar]
- Hughes ML, Castioni EE, Goehring JL, Baudhuin JL. Temporal response properties of the auditory nerve: data from human cochlear implant recipients. Hear Res. 2012;285:46–57. doi: 10.1016/j.heares.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes ML, Stille LJ. Effect of stimulus and recording parameters on spatial spread of excitation and masking patterns obtained with the electrically evoked compound action potential in cochlear implants. Ear Hear. 2010;31:679–692. doi: 10.1097/AUD.0b013e3181e1d19e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer J, von Ilberg C, Rupprecht V, Huber-Egener J, Baumgartner W, Gstoettner W, Forgasi K, Stephan K. Optimized speech understanding with the CIS-speech coding strategy in cochlear implants: The effect of variations in stimulus rate and number of channels. In: Waltzman SB, Cohen NL, editors. Cochlear Implants. Thieme; New York: 2000. [Google Scholar]
- Kreft HA, Donaldson GS, Nelson DA. Effects of pulse rate and electrode array design on intensity discrimination in cochlear implant users. J Acoust Soc Am. 2004;116(4):2258–2268. doi: 10.1121/1.1786871. [DOI] [PubMed] [Google Scholar]
- Litvak LM, Delgutte B, Eddington DK. Auditory nerve fiber responses to electric stimulation: Modulated and unmodulated pulse trains. J Acoust Soc Am. 2001;110(1):368–379. doi: 10.1121/1.1375140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak LM, Smith ZM, Delgutte B, Eddington DK. Desynchronization of electrically evoked auditory-nerve activity by high-frequency pulse trains of long duration. J Acoust Soc Am. 2003;114(4):2066–2078. doi: 10.1121/1.1612492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loizou PC, Poroy O, Dorman M. The effect of parametric variations of cochlear implant processors on speech understanding. J Acoust Soc Am. 2000;108(2):790–802. doi: 10.1121/1.429612. [DOI] [PubMed] [Google Scholar]
- Matsuoka AJ, Abbas PJ, Rubinstein JT, Miller CA. The neuronal response to electrical constant-amplitude pulse train stimulation: evoked compound action potential recordings. Hear Res. 2000;149:115–128. doi: 10.1016/s0378-5955(00)00172-6. [DOI] [PubMed] [Google Scholar]
- McKay CM, Chandan K, Akhoun I, Siciliano C, Kluk K. Can ECAP measures be used for totally objective programming of cochlear implants? J Assoc Res Otolaryngol. 2013;14:879–890. doi: 10.1007/s10162-013-0417-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay CM, Fewster L, Dawson P. A different approach to using Neural Response Telemetry for automated cochlear implant processor programming. Ear Hear. 2005;26:38S–44S. doi: 10.1097/00003446-200508001-00006. [DOI] [PubMed] [Google Scholar]
- McKay CM, McDermott HJ. Loudness perception with pulsatile electrical stimulation: The effect of interpulse intervals. J Acoust Soc Am. 1998;104(2):1061–1074. doi: 10.1121/1.423316. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC. Effects of cochlear-implant pulse rate and inter-channel timing on channel interactions and thresholds. J Acoust Soc Am. 2004;116(1):452–468. doi: 10.1121/1.1760795. [DOI] [PubMed] [Google Scholar]
- Miller CA, Abbas PJ, Brown CJ. An improved method of reducing stimulus artifact in the electrically evoked whole-nerve potential. Ear Hear. 2000;21:280–290. doi: 10.1097/00003446-200008000-00003. [DOI] [PubMed] [Google Scholar]
- Miller CA, Hu N, Zhang F, Robinson BK, Abbas PJ. Changes across time in the temporal responses of auditory nerve fibers stimulated by electric pulse trains. J Assoc Res Otolaryngol. 2008;9:122–137. doi: 10.1007/s10162-007-0108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsnowski A, Charasse B, Collet L, Killian M, Muller-Deile J. Measuring the refractoriness of the electrically stimulated auditory nerve. Audiol Neurotol. 2006;11:38–402. doi: 10.1159/000095966. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Colesa DJ, Hembrador S, Kang SY, Middlebrooks JC, Raphael Y, Su GL. Detection of pulse trains in the electrically stimulated cochlea: Effects of cochlear health. J Acoust Soc Am. 2011;130(6):3954–3968. doi: 10.1121/1.3651820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein JT, Wilson BS, Finley CC, Abbas PJ. Pseudospontaneous activity: stochastic independence of auditory nerve fibers with electrical stimulation. Hear Res. 1999;127:108–118. doi: 10.1016/s0378-5955(98)00185-3. [DOI] [PubMed] [Google Scholar]
- Shannon RV. Threshold and loudness functions for pulsatile stimulation of cochlear implants. Hear Res. 1985;18:135–143. doi: 10.1016/0378-5955(85)90005-x. [DOI] [PubMed] [Google Scholar]
- Vandali AE, Whitford LA, Plant KL, Clark GM. Speech perception as a function of electrical stimulation rate: Using the Nucleus 24 cochlear implant system. Ear Hear. 2000;21:608–624. doi: 10.1097/00003446-200012000-00008. [DOI] [PubMed] [Google Scholar]
- Wilson BS, Finley CC, Lawson DT, Zerbi M. Temporal representations with cochlear implants. Am J Otol. 1997;18:S30–S34. [PubMed] [Google Scholar]
- Zhang F, Benson C, Murphy D, Boian M, Scott M, Keith R, Xiang J, Abbas PJ. Neural adaptation and behavioral measures of temporal processing and speech perception in cochlear implant recipients. PLOS One. 2013;8(12):e84631. doi: 10.1371/journal.pone.0084631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Miller CA, Robinson BK, Abbas PJ, Hu N. Changes across time in spike rate and spike amplitude of auditory nerve fibers stimulated by electric pulse trains. J Assoc Res Otolarygol. 2007;8:356–372. doi: 10.1007/s10162-007-0086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Xu L, Pfingst BE. Characteristics of detection thresholds and maximum comfortable loudness levels as a function of pulse rate in human cochlear implant users. Hear Res. 2012;284:25–32. doi: 10.1016/j.heares.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]