The Wisepill device is an emerging mobile health technology that may enhance antiretroviral therapy (ART) adherence among people living with HIV. The Wisepill is an electronic pillbox that holds up to 30 large pills or 60 small pills. Opening the Wisepill sends a cellular signal to a Web-based server that can be accessed in real-time by clinicians for monitoring purposes and to potentially trigger intervention. Previous studies have focused on the feasibility and acceptability of using the Wisepill device for patient monitoring in various settings. In Uganda, patients described the device as easy to use and convenient, and the researchers found that the Wisepill produced similar results as medication event monitoring systems (MEMS) pill bottle caps (Haberer et al., 2010). In China, however, another group of researchers found that although using the Wisepill for real-time medication monitoring was technically feasible, there were concerns regarding the acceptability of the device to patients (Bachman DeSilva et al., 2013). Only half of all participants reported positive experiences. No studies to date have examined the Wisepill in the United States where HIV infections are concentrated in impoverished areas with limited clinical resources (Pellowski, Kalichman, Matthews, & Adler, 2013).
In addition to medication monitoring, the Wisepill affords the opportunity for “just-in-time” counseling (Haberer et al., 2010). When an individual does not open the Wisepill at the prescribed medication dose time, adherence service providers have the opportunity to contact the patient to prompt him/her to take the dose and resolve in-the-moment barriers. However, to our knowledge, no studies have tested the feasibility of using the Wisepill device to initiate just-in-time counseling as an intervention to improve adherence. The purpose of our study was to determine the feasibility, acceptability, and potential efficacy of using the Wisepill to deliver a just-in-time adherence intervention to individuals receiving HIV treatment.
Methods
Participants and Setting
Study participants consisted of men and women living with HIV and currently taking ART. This study was conducted in Atlanta, Georgia, an area with a growing HIV epidemic (Georgia Department of Public Health, 2013). Eligibility criteria included (a) being 18 or older, (b) being infected with HIV, (c) currently taking ART, (d) 90% or less adherent, and (e) using a drug regimen of two dose times a day. The drug regimen was restricted to twice-a-day dosing to maximize the possibility of a missed dose contact and to keep dosing consistent across conditions; 90% adherence indicates missing at least six doses of medication during a 1-month time frame.
Overview of Study Design
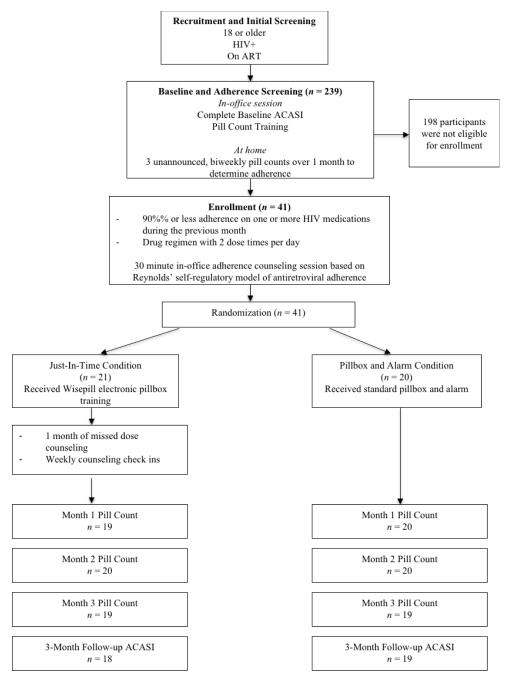
Flow of participants through the study is shown in Figure 1. Prior to enrollment, participants completed a run-in period to determine baseline adherence by unannounced phone-based pill count. If eligible, participants were consented and enrolled into the intervention study. All participants received a 30-minute, one-on-one session with a medication adherence counselor at the initial office session based on the Self-Regulatory Model of Medication Adherence (Reynolds, 2003), which addressed knowledge, behavior skills, and affective support. Following the office session, participants were randomized to receive the just-in-time self-regulation counseling or an informational control condition. The University of Connecticut Institutional Review Board approved the study protocol and participants were financially compensated for all completed study activities. A minimum of 40 participants was targeted for recruitment from community services to assure a sufficient number for feasibility and acceptability testing. A power analysis was not conducted to determine the sample size because of the test-of-concept nature of the study.
Figure 1.
Participant flow through the study design, enrollment, and completion.
Note: ACASI = Audio-Computer Assisted Self-Interview
Just-in-Time Adherence Counseling
Participants in the just-in-time self-regulation counseling condition received a Wisepill device ($185 each + $3 web hosting fee per month per device) and counseling that lasted for 1 month. When a participant did not open the Wisepill device within 2 hours of their self-selected ART dose times, the phone counselor attempted to reach the participant on a project-provided or personal cell phone to remind her/him to take the medication and to briefly counsel him/her on any in-the-moment barriers. Using motivational interviewing skills, the phone counselor worked with participants to elicit strategies to overcome immediate barriers and to plan for the possibility of reoccurring challenges. Participants also received 4 weekly phone contacts from the counselor to assess technical issues regarding the Wisepill and to encourage those who had not missed a dose in the previous week to maintain adherence.
Informational Control Condition
Participants randomized to the informational control condition received the same enrollment procedures and the initial in-office session described above, as well as a standard pillbox and an alarm watch to use for 1 month. These materials were intended to control for the functional features of the Wisepill without monitoring or just-in-time counseling. After 1 month, participants were scheduled to come into the office to return these items.
Measures
Participants completed computerized interviews to collect demographic and health information, unannounced pill counts for adherence outcome assessments, and measures of Wisepill acceptability at the end of the intervention period. In addition, we collected Wisepill utilization data to assess feasibility.
Unannounced pill count adherence
Medication adherence was assessed the month prior to randomization (baseline) and for 3 months after baseline using unannounced phone-based pill counts (Kalichman et al., 2010). Following an in-office training session, an adherence assessor called participants to count their pills. The assessor asked the participant to report the prescription information for each ART bottle and to count each medication. Adherence was calculated as the ratio of the number of pills taken between phone calls relative to the number of pills dispensed for the time period.
Intervention feasibility and acceptability
Feasibility data were collected regarding how often participants were reached for missed dose counseling calls and if not, why not. Each late dose was logged and uncompleted late dose counseling calls were categorized for the reasons that the calls were not completed.
Participants completed acceptability measures regarding the Wisepill device and the just-in-time intervention when the device was returned. Items were responded to on a 6-point scale from 1 (strongly disagree) to 6 (strongly agree). Five questions assessed the acceptability of the Wisepill device and three questions assessed the acceptability of the just-in-time counseling (see Table 1 for items). Due to the skew of the data, responses are reported categorically.
Table 1.
Percent Endorsement of Acceptability of the Wisepill Device and the Just-in-Time Counseling
| Acceptability of the Device | % Disagree | % Agree | % Strongly Agree |
M(SD) |
|---|---|---|---|---|
| I found the device convenient to use. | 19.1 | 23.8 | 57.1 | 5.1 (1.3) |
| I did not mind using the device for a month. | 23.8 | 19.1 | 57.1 | 4.9 (1.7) |
| I was concerned I would lose/damage the device. | 81 | 4.8 | 14.3 | 2.0 (1.9) |
| I believe that having the device helped me remember to take my medications. |
4.8 | 38.1 | 57.1 | 5.4 (0.9) |
| I liked using the device to hold my medications. | 23.8 | 23.8 | 52.4 | 4.8 (1.7) |
|
| ||||
| Acceptability of the Intervention | ||||
|
| ||||
| I liked knowing that someone was looking out for me to make sure that I took my medications. |
4.8 | 23.8 | 71.4 | 5.5 (1.1) |
| I felt uncomfortable knowing that someone was monitoring if I took my medications. |
52.4 | 9.5 | 38.1 | 3.5 (2.3) |
| I would be happy to participate in another study involving this device. |
9.5 | 19.1 | 71.4 | 5.3 (1.4) |
Note. Responses were on a 6-point scale and due to the skew of the results subsequently categorized as 1-3 = Disagree, 4-5 = Agree, 6 = Strongly Agree
Statistical Analyses
Chi-square tests and t-tests were performed to analyze baseline differences between participants randomized to the just-in-time and informational control conditions. Mixed design ANCOVAs were conducted to determine differences in adherence over time by condition, controlling for baseline adherence and relevant demographics. Cohen’s d effect sizes for all ANCOVAs were calculated. Finally, means and standard deviations were calculated to describe the acceptability of the intervention.
Results
A total of 41 participants were enrolled in the study; 21 were randomized into the just-in-time adherence counseling condition and 20 into the informational control condition. All participants completed the intervention and 90% completed all follow-up assessments (see Figure 1). The majority of the participants were male (68%), although this varied significantly by condition. More females and transgender individuals were randomized into the informational control condition (X2 = 6 .54, p < 0.05). Gender was therefore controlled for in all subsequent analyses. No other participant characteristics or outcome measures varied significantly by condition at baseline.
Primary Adherence Outcomes
The ANCOVA controlling for baseline adherence and gender indicated no significant differences between conditions on the adherence assessments (F[1, 40] = 0.48, p = 0.62, d = −0.21). Univariate analyses at the 1-month follow-up indicated a non-significant statistical trend (F[1, 38] = 2.86, p = 0.09, d = 0.53), and non-significant results for the 2-month follow-up (F[1, 39] = 0.28, p = 0.60, d = 0.16) and the 3-month-follow-up (F[1, 37] = 1.07, p = 0.31, d = 0.33).
Feasibility and Acceptability of the Intervention
Out of a possible 1,162 medication doses during the intervention period, Wisepill registered a total of 222 missed doses. Of these, only 26 resulted in completed just-in-time counseling calls (12%). For 18 doses (8%), the participant answered the phone but was too busy to talk, and for 15 doses (7%), the participant had not opened the Wisepill device but reported taking a pocket dose of medication. Participants could not be reached for counseling for 105 doses (47%) because they did not answer the phone or the phone was turned off. In these cases, several attempts were made and voicemails were left. These calls were examined to determine how many resulted in doses taken late but before a participant’s next dose. Participants, on average, took 73% of their “missed” doses even when the counselor did not make direct contact with them, possibly due to seeing the missed calls.
In general, the intervention was deemed acceptable to participants (see Table 1). Participants reported liking the device and found the Wisepill convenient to use. Additionally, participants did not mind using the device and believed that the device helped them to remember to take their medications. About half of participants endorsed the statement, I felt uncomfortable knowing that someone was monitoring if I took my medications, although there was considerable variation. Participants, in general, liked knowing that someone was looking out for them and stated they would be happy to participate in another study involving the device.
Discussion
Several important lessons were learned in conducting this test-of-concept study. The first was in regard to participants’ reactions to being monitored. Although, overall, participants found the Wisepill device and the just-in-time adherence counseling to be acceptable, almost half of participants expressed concern and stated that they felt uncomfortable being electronically monitored or “policed.” Although we were concerned about this during the development of the intervention, it was unclear the extent to which this would impact participants. Clearly, this is something that should be addressed in future monitoring interventions with the Wisepill device. These concerns could possibly be addressed by having in-depth conversations with patients to acknowledge and address their reservations and assure them that they are not being judged.
Another key lesson learned from this intervention was in regard to the counseling calls. Although we anticipated some amount of uncompleted counseling calls, the high levels of uncompleted counseling calls were not foreseen. Missed medication doses, in general, are likely to occur at times that may prove least amenable to intervention such as being busy, away from home, oversleeping, or intoxication. Given the relative forgiveness of many of the newer antiretroviral medication regimens, counseling may be more useful for multiple missed doses or patterns of missed doses than periodic missed single doses. However, Parienti et al. (2008) found that each additional day that a patient missed medications could lead to a loss of viral suppression. A just-in-time approach is valuable in cases where a participant has missed two or more doses to prevent continuing patterns of missed doses.
There were also lessons to be learned from the limitations of this study. A significant limitation of this test-of-concept trial was the small sample size. Although effect sizes for the main outcomes were small to moderate, none of the findings were significant. A larger sample may have yielded more significant results regarding differences in adherence. Another limitation of the intervention design may have been the length of intervention period. One month of intervention may not have been long enough to establish durable changes in adherence behaviors among participants. Finally, the control group was not contact-matched to the intervention group. This lack of counselor contact may have influenced the results such that the intervention group’s higher adherence may be attributed to having more counselor contact.
Overall, the just-in-time adherence intervention as implemented in this test-of-concept trial demonstrated high acceptability, low feasibility, and minimal evidence of improvements in medication adherence. The potential for this type of device to assist patient adherence should be considered separately from the challenges encountered in delivering the just-in-time counseling. The lessons learned from this study’s findings, strengths, and limitations provide avenues for further investigation.
Acknowledgements
This research was supported by the National Institute of Mental Health Grants T32MH07487 and R01MH082633.
Footnotes
Conflict of Interest Statement: The authors report no real, or perceived, vested interests related to this article that could be construed as a conflict of interest. The manufactures of Wisepill had no role in the analysis or preparation of the manuscript and the authors have no financial interest in Wisepill.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jennifer A. Pellowski, Department of Psychology, University of Connecticut, Storrs, Connecticut, USA..
Seth C. Kalichman, Department of Psychology, University of Connecticut, Storrs, Connecticut, USA..
Denise White, Department of Psychology, University of Connecticut, Storrs, Connecticut, USA..
Christina M. Amaral, Department of Psychology, University of Connecticut, Storrs, Connecticut, USA..
Ginger Hoyt, Department of Psychology, University of Connecticut, Storrs, Connecticut, USA..
Moira O. Kalichman, Department of Psychology, University of Connecticut, Storrs, Connecticut, USA..
References
- Bachman DeSilva M, Gifford AL, Keyi X, Li A, Feng C, Brooks M, Sabin L. Feasibility and acceptability of a real-time adherence device among HIV-Positive IDU patients in China. AIDS Research and Treatment. 2013:957862. doi: 10.1155/2013/957862. doi:10.1155/2013/957862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgia Department of Public Health HIV/AIDS surveillance, Georgia. 2013 2012. Retrieved from http://dph.georgia.gov/sites/dph.georgia.gov/files/HIV%20EPI_2012%20Georgia%20HIV%20AIDS%20Fact%20Sheet.pdf.
- Haberer JE, Kahane J, Kigozi I, Emenyonu N, Hunt P, Martin J, Bangsberg DR. Real-time adherence monitoring for HIV antiretroviral therapy. AIDS and Behavior. 2010;14:1340–1346. doi: 10.1007/s10461-010-9799-4. doi:10.1007/s10461-010-9799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman SC, Amaral C, Swetsze C, Eaton L, Kalichman MO, Cherry C, Schinazi RF. Monthly unannounced pill counts for monitoring HIV treatment adherence: Tests for self-monitoring and reactivity effects. HIV Clinical Trials. 2010;11:325–331. doi: 10.1310/hct1106-325. doi:10.1310/hct1106-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parienti JJ, Das-Douglas M, Massari V, Guzman D, Deeks SG, Verdon R, Bangsberg DR. Not all missed doses are the same: Sustained NNRTI treatment interruptions predict HIV rebound at low-to-moderate adherence levels. PLoS One. 2008;3:e2783. doi: 10.1371/journal.pone.0002783. doi:10.1371/journal.pone.0002783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellowski JA, Kalichman SC, Matthews KA, Adler N. A pandemic of the poor: Social disadvantage and the U.S. HIV epidemic. American Psychologist. 2013;68:197–209. doi: 10.1037/a0032694. doi:10.1037/a0032694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds NR. The problem of antiretroviral adherence: A self-regulatory model for intervention. AIDS Care. 2003;15:117–124. doi: 10.1080/0954012021000039815. [DOI] [PubMed] [Google Scholar]