Abstract
Rationale
Schizotypal personality disorder (SPD) is associated with working memory (WM) impairments that are similar to those observed in schizophrenia. Imaging studies have suggested that schizophrenia is associated with alterations in dopamine D1-receptor availability in the prefrontal cortex (PFC) that may be related to the WM impairments that characterize this disorder.
Objectives
To characterize prefrontal D1-receptor availability and its relation to WM performance in SPD.
Methods
We used positron emission tomography (PET) and the radiotracer [11C]NNC112 with 18 unmedicated SPD and 21 healthy-control participants; as an index of D1-receptor availability, binding-potential (BP) measures (BPF, BPND, and BPP) were calculated for prefrontal and striatal subregions. To assess WM, SPD participants completed the 2-back and Paced Auditory Serial Addition Test (PASAT).
Results
There were no significant group differences in PFC BP. BPF and BPP in the medial PFC were significantly negatively related to PASAT performance (rs=-0.551, p=.022 and rs=-0.488, p=.047, respectively), but BP was not related to 2-back performance.
Conclusions
In contrast to what has been found in schizophrenia, SPD was not associated with significant prefrontal D1-receptor alterations. Similar to previous schizophrenia findings, however, higher prefrontal D1-receptor availability was associated with poorer WM performance (as measured by the PASAT) in SPD. These findings suggest that schizophrenia and SPD may share a common pathophysiological feature related to prefrontal dopamine functioning that contributes to WM dysfunction, but that in SPD, alterations in D1 may occur only in a subset of individuals and/or to an extent that is minor relative to what occurs in schizophrenia.
Keywords: schizotypal personality disorder, schizophrenia, PET, dopamine, prefrontal cortex, working memory
Cognitive dysfunction is a key feature of schizophrenia that has been shown to predict poorer functional outcome and is relatively intractable to current treatments (Green 2006). Schizotypal personality disorder (SPD) is considered the prototypic schizophrenia-spectrum disorder, and shares common symptomatic, neuropsychological, neuroanatomical, and genetic-risk features with schizophrenia (Siever and Davis 2004). For example, SPD is associated with an array of clinically-relevant working memory (WM) and executive functioning deficits that are qualitatively similar to those observed in schizophrenia (McClure et al. 2008; Mitropoulou et al. 2002). Thus studying cognitive impairments and their neurobiological correlates in SPD can be a powerful strategy for advancing knowledge regarding the pathophysiology of and potential therapeutic targets for schizophrenia, as doing so largely obviates the potentially obscuring factors of antipsychotic medication exposure and secondary effects of chronic psychosis (Siever and Davis 2004).
Preclinical research has indicated that intact WM performance is critically dependent on cortical dopamine (DA) functioning, and in particular stimulation of D1 receptors (D1R) by DA in the prefrontal cortex (PFC). In a pivotal study, it was shown that local infusion of D1R antagonists into the PFC of non-human primates resulted in a degradation of WM performance (Sawaguchi and Goldman-Rakic 1991). Subsequent studies demonstrated that D1R agonists improved WM performance both in aged primates and in DA-depleted young-adult primates (Arnsten et al. 1994). Such findings suggested that the cognitive dysfunction associated with schizophrenia may in part be due to deficits in prefrontal D1 stimulation. In humans, it was recently shown that administration of a D1R antagonist to young adults attenuated WM performance to a level similar to that of an older-adult group (Fischer et al. 2010). Furthermore, we recently found that in SPD participants, performance in several cognitive domains, including WM and executive functioning, improved after 4 weeks of treatment with the mixed D1–D2R agonist pergolide (McClure et al. 2010).
To more directly assess D1R and related WM performance in schizophrenia, we have used positron emission tomography (PET) and the D1R radioligand [11C]NNC112. We previously found that patients with schizophrenia were characterized by significantly higher D1R availability in the dorsolateral PFC (DLPFC) compared to controls (by 28%); we also observed a significant negative association between DLPFC D1R availability and WM performance in the patients with schizophrenia (Abi-Dargham et al. 2002). A possible explanation for these findings is that in schizophrenia, there is a compensatory (but inefficient) upregulation of D1R in response to chronic understimulation of D1 in this brain region (Abi-Dargham et al. 2002). This interpretation is consistent with converging evidence from preclinical (Guo et al. 2003; Tsukada et al. 2005) and human (Narendran et al. 2005; Slifstein et al. 2008) research that reduced DA levels are associated with an upregulation of D1 as measured with [11C]NNC112.
More recently, we found that compared to controls, D1R availability was increased in regions of the PFC in drug-naïve patients with schizophrenia, whereas patients with a history of antipsychotic treatment did not differ significantly from controls; furthermore, among patients with prior antipsychotic treatment, the duration of their medication-free interval was positively associated with DLPFC D1R availability (Abi-Dargham et al. 2012). These findings suggest that antipsychotic exposure may normalize illness-related upregulation of D1. Results from recent studies comparing D1R availability in schizophrenia as measured with the radiotracers [11C]NNC112 and [11C]SCH23390 (Kosaka et al. 2010; Poels et al. 2013) are consistent in suggesting that D1R levels in schizophrenia are influenced by factors such as antipsychotic exposure and possibly stage of illness, and that such patient characteristics have contributed to the mixed findings yielded by studies of D1R availability in schizophrenia (see Abi-Dargham et al. 2012; Kosaka et al. 2010; Poels et al. 2013).
Assessing D1R and its relation to WM performance in SPD can complement the above studies of schizophrenia by examining such phenomena in a related condition while controlling for factors such as antipsychotic exposure. This approach should also contribute to knowledge regarding the potential cortical DA pathology of SPD, a spectrum condition that shares many features of schizophrenia, including cognitive and social impairment, yet is not characterized by frank psychosis. To our knowledge, there are no published studies of D1R availability in SPD. Thus we sought to characterize this index of DA functioning and its relation to WM performance in a group of healthy, unmedicated patients with SPD using PET and [11C]NNC112. Based on previous findings in schizophrenia (Abi-Dargham et al. 2002, 2012), our primary hypotheses were: 1) SPD participants would have higher D1R availability in the PFC compared to matched controls; and 2) prefrontal D1R availability would be negatively related to WM performance in SPD. Given previous findings suggesting that SPD may be characterized by alterations in striatal DA functioning (Abi-Dargham et al. 2004), we also conducted exploratory analyses of D1R availability in striatal subregions.
Methods
Participants
This study was approved by the Institutional Review Boards of New York State Psychiatric Institute of Columbia University Medical Center, Mount Sinai Hospital, and the Bronx Veterans Affairs Medical Center, and performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. All participants provided written informed consent after the procedures were fully explained to them, and SPD participants were independently assessed for capacity to provide consent. Participants were recruited from the community through advertisements in local newspapers and Internet, and for some SPD participants, through clinician referral. A subset of the SPD participants of this study were included in previously-published reports of WM in SPD (Barch et al. 2004; McClure et al. 2008; Mitropoulou et al. 2002, 2005), and a subset of the controls were included in prior [11C]NNC112 PET publications (Abi-Dargham et al. 2002, 2012; Poels et al. 2013; Slifstein et al. 2008). Screening included a physical examination, basic blood tests, and electrocardiogram. All participants were between the ages of 18–60, medically healthy, not pregnant or nursing, and had no recent recreational drug use (confirmed by urine toxicology tests). All SPD participants met DSM-IV (APA 1994) criteria for SPD, were not currently taking psychotropic medications (see below), and were excluded if they met criteria for a lifetime diagnosis of schizophrenia, bipolar disorder (I or II), current major depressive disorder, or current/recent (past 6 months) substance abuse or dependence. Controls were free of any current or past psychiatric conditions, including substance abuse (nicotine dependence was permitted in both groups). Diagnoses for SPD participants were assessed with the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-IV; First et al. 1994) and Structured Interview for DSM-IV Personality Disorders (SIDP-IV; Pfohl et al. 1997); for controls, absence of psychiatric conditions was confirmed with an abbreviated version of the SCID-IV or the Diagnostic Interview for Genetic Studies (DIGS; Nurnberger et al. 1994).
Cognitive Assessments
SPD participants were administered the N-back (Cohen et al. 1997) and Paced Auditory Serial Addition Test (PASAT; Gronwall 1977), both measures of WM. The N-back requires participants to monitor a series of letters presented sequentially on a computer screen and respond whenever a predetermined target letter is presented (0-back), or when the letter presented is identical to either the letter that immediately preceded it (1-back) or to the letter 2 trials back (2-back); 3 blocks of 25 trials each were administered for each condition. The outcome used was hit rate. Here we report results from the 2-back. The PASAT requires participants to listen to a recorded voice present a series of single-digit numbers (50 at a rate of 1 per 2 sec) and add together each adjacent pair and state the sum aloud; the outcome used was number of correct responses. Of note, SPD participants were administered the WM measures through a separate study that partially overlapped in time with the PET study. As a consequence, the time period between the WM assessments and PET was variable across participants; in some cases, more than a year separated these assessments and PET (see below).
PET Acquisition and Input Function Measurement
Scans were acquired in 3D mode with an ECAT EXACT HR+ scanner (Siemens/CTI, Knoxville, TN). After a 10-min transmission scan for attenuation correction, [11C]NNC112 was injected intravenously as a bolus over 45 sec. Emission data were collected for 90 min. See the Online Resource text for additional information regarding radiochemistry and PET acquisition. Arterial plasma input function measurements were collected for kinetic analysis and estimation of the plasma free fraction (fp, fraction of [11C]NNC112 in arterial plasma not bound to protein) as previously described (Abi-Dargham et al. 2000, 2012).
Image Analysis
All participants received a high-resolution T1-weighted MRI scan. PET reconstruction and coregistration of PET to MRI images and delineation of regions of interest (ROIs) were performed as previously described (Abi-Dargham et al. 2012) to generate time activity curves in each ROI. The primary ROIs examined were regions of the PFC: DLPFC, medial PFC (MPFC), and orbitofrontal cortex (OFC), as defined in Abi-Dargham et al. (2000). We also examined striatal subregions, which included five ROIs (Table 2) and were drawn as defined in Mawlawi et al. (2001). A gray matter mask was applied to cortical ROIs so that only gray matter activity was measured in those regions (Abi-Dargham et al. 2002). The cerebellum (CER) was included as a reference region.
Table 2.
Volumes (mm3) of the Regions of Interest by Diagnostic Group
| ROI | Controls (n=21) | SPD Participants (n=18) | pa |
|---|---|---|---|
| PFC: | |||
| DLPFC | 25037 ± 5708 | 20116 ± 5531 | 0.010 |
| MPFC | 5546 ± 2290 | 4204 ± 2059 | 0.064 |
| OFC | 11821 ± 5356 | 10709 ± 4104 | 0.477 |
|
| |||
| Striatal: | |||
| VST | 1638 ± 428 | 1600 ± 427 | 0.781 |
| preDCA | 4598 ± 749 | 4245 ± 704 | 0.140 |
| preDPU | 3831 ± 591 | 3781 ± 535 | 0.785 |
| postCA | 997 ± 301 | 846 ± 266 | 0.109 |
| postPU | 4102 ± 689 | 3911 ± 512 | 0.337 |
Note. Mean ± Standard Deviation.
DLPFC=dorsolateral prefrontal cortex; MPFC=medial prefrontal cortex; OFC=orbitofrontal cortex; PFC=prefrontal cortex; postCA=postcommissural caudate; postPU=postcommissural putamen; preDCA=precommissural dorsal caudate; preDPU=precommissural dorsal putamen; ROI=region of interest; SPD=schizotypal personality disorder; VST=ventral striatum.
Independent-samples t-test.
Receptor Parameter Estimation
PET data were analyzed with kinetic modeling and arterial plasma input using 2-tissue compartment modeling (2TC) in ROIs and 1TC in the CER to derive the total distribution volume (VT) for each region (Online Resource text). VT is equivalent to the equilibrium ratio of the total concentration of radioligand in that region to the arterial plasma concentration. Because there is a negligible concentration of D1R in CER (Hall et al. 1994), CER VT was used to estimate VND, which reflects the concentration of free and non-specifically-bound tracer, and is assumed to be equal across brain regions. As in our previous studies with patients with schizophrenia (Abi-Dargham et al. 2002, 2012), we aimed to report the binding potential measure BPP as our primary PET outcome measure, estimated as: BPP = VT (ROI) – VT (CER). Due to significant group differences observed in fp (see below), however, we also present results for BPF and BPND. BPF is defined as: BPF = BPP/fp; thus its calculation accounts for fp. Given the group difference in fp, we consider BPF the primary PET outcome measure for group comparisons. BPND is defined as: BPND = BPP/VT (CER); this measure was included with the aim of corroborating the results obtained with BPF. Due to significant group differences in some of the PFC ROI volumes, PET data for cortical ROIs were adjusted to correct for partial volume effects (PVE). The striatal ROI data were not PVE-corrected, as there were no significant group differences in striatal subregion volumes. See Online Resource text for additional information regarding the PET outcome measures and PVE correction.
Statistical Analysis
Group comparisons of the PET outcome measures BPF, BPND, and BPP were performed using linear mixed modeling for which diagnosis (SPD v. control) and ROI were treated as fixed effects, and ROI was treated as a repeated measure; the diagnosis x ROI term was included in all models. For primary analyses, a model using “PFC” (comprising DLPFC, MPFC, and OFC) as the ROI was analyzed for each BP outcome measure. Between-group planned comparisons were also conducted for each ROI, and Cohen’s d (using the observed group means and pooled SD) was used as an index of effect size. For the SPD participants, correlations of BP outcome measures (BPF, BPND, and BPP) with the 2 WM measures were examined for each of the 3 regions of PFC using Spearman rank correlations, due to non-normal distributions observed for several variables (18 bivariate correlations in total). Analyses for primary hypotheses (i.e. group comparisons of BP in the PFC and the correlations between PFC BP and WM) were not corrected for multiple comparisons. For exploratory analyses, we examined group differences in the “striatal” ROI (comprising the 5 striatal subregions) with mixed modeling as described above and independent-samples t-tests for the composite “whole striatum”; the false discovery rate procedure (Benjamini and Hochberg 1995) was used to correct for multiple comparisons for exploratory analyses. Because age was significantly related to BPF in the DLPFC in SPD participants (see below), we conducted secondary analyses including age as a covariate when examining BP group differences and BP-WM relations (using partial Spearman correlations, prs, for the latter). Mixed models were also run when including age x diagnosis; because this term was non-significant in all cases, it was dropped from analyses. All statistical tests were two-tailed.
Results
Demographic and Clinical Characteristics
Eighteen unmedicated SPD and 21 control participants were included in this study. There were no significant group differences in age, sex, ethnicity, smoking status, or participant or parental years of education (Table 1). Twelve of the 18 SPD participants were naive to psychotropic medication (and 16 to antipsychotics), and the remaining had not taken psychotropic medication for at least 3 weeks prior to PET (with most reporting no medication use for the last several years). The 2 SPD participants with previous antipsychotic use reported last using antipsychotics over 20 years prior to study participation. Comorbid Axis-I and -II diagnoses of the SPD participants are provided in the Online Resource text; note that some of the SPD participants met criteria for past substance-use disorders (see Online Resource text).
Table 1.
Demographic Characteristics and Scan Parameters by Diagnostic Group
| Parameter | Controls (n=21) | SPD Participants (n=18) | pa |
|---|---|---|---|
| Demographic (Mean ± SD unless otherwise noted) | |||
| Age | 40.76 ± 5.80 | 42.30 ± 7.42 | 0.473 |
| Sex (% male) | 67% | 67% | 1.00 |
| Ethnicity (%) | 38% C, 48% AA, 14% H | 39% C, 22% AA, 39% H | 0.959b |
| Smoking status (% yes) | 29% | 29%c | 0.955 |
| Parental education (years) | 14.65 ± 3.22d | 13.40 ± 4.07d | 0.317 |
| Participant education (years) | 15.20 ± 2.09e | 14.33 ± 2.91 | 0.295 |
| Scan parameters (Mean ± SD) | |||
| Injected dose (mCi) | 13.24 ± 3.70 | 11.01 ± 2.93 | 0.046 |
| Injected mass (ug) | 4.91 ± 1.08 | 4.32 ± 1.48 | 0.166 |
| Specific activity (Ci/mmol) | 921.86 ± 285.72 | 985.00 ± 553.65 | 0.650 |
| Plasma free fraction (fp, %) | 0.89 ± 0.26f | 0.73 ± 0.15 | 0.024 |
| VND (mL/cm3) | 2.25 ± 0.48 | 2.17 ± 0.46 | 0.614 |
AA=African American; C=Caucasian; H=Hispanic; SD=standard deviation; SPD=schizotypal personality disorder; VND=nondisplaceable distribution volume (measured in the cerebellum to provide a measure of non-specific radioligand binding).
For continuous variables, independent-samples t-test; for dichotomous variables, chi-square tests used.
Groups were compared on a dichotomized ethnicity variable (Caucasian v. ethnic minority).
Smoking status was missing for 1 SPD participant.
Parental education was missing for 3 SPD participants and 1 control.
Participant education was missing for 1 control.
Plasma free fraction was missing for 1 control.
Scan Parameters and Regional Volumes
Scan parameter data are provided in Table 1. There were no significant group differences in injected mass or specific activity of [11C]NNC112, or in VND. The SPD participants received significantly less injected radioactivity of [11C]NNC112; however, this was not problematic for analyses because injected mass was well within tracer-dose limits for all scans. The mean plasma free fraction of [11C]NNC112 (fp) was significantly smaller in the SPD than control group. The source of this fp group difference is not clear. For example, fp was not significantly related to participant body mass index (in the total sample or either group separately), as was observed in one of our previous samples (Kegeles et al. 2010). As detailed above, this fp group difference posed a problem for relying on BPP as our primary BP outcome measure; therefore, we also report BPF and BPND. Group-comparison results of ROI volumes are presented in Table 2.
Timing of Cognitive Assessments Relative to PET Scans
As noted above, the timing of WM assessments relative to PET was variable across SPD participants. The median number of weeks separating PET and administration of the PASAT and N-back, respectively, was 45.86 (range=2.29–144) and 52 (range=4.43–204.57).
Associations of Age with Binding-Potential and WM Measures
When examining associations of age with BP in the PFC ROIs, and with WM performance in SPD participants, age was significantly related only to DLPFC BPF in the SPD participants (rs=0.494, p=0.037). In contrast, age was significantly negatively related to BPND in several striatal subregions in the total sample and in both groups; see Online Resource text.
Group Differences in [11C]NNC112 VT, BPF, BPND, and BPP
PFC
There were no significant group differences in VT for the PFC ROIs (Online Resource Table 1). In mixed-model analyses with the PFC ROI, there was no significant effect of diagnosis in predicting BPF [F(1, 36.01)=1.03, p=0.316, n=20 for controls] or BPND [F(1, 37.05)=0.59, p=0.446]. Scatterplots of the BPF values by group are provided in Online Resource Figure 1. Between-group planned comparisons of the predicted marginal means derived from the mixed models confirmed the lack of significant group differences in BPF (Table 3) and BPND (Table 4). Mixed modeling with these terms and BPP were consistent in indicating no significant effect of diagnosis in the PFC ([F(1, 37.02)=0.46, p=0.504]. Results were essentially unchanged when including age as a covariate in these PFC models.
Table 3.
[11C]NNC112 BPF by Diagnostic Group for PFCa and Striatal ROIs
| ROI | Controls (n=20b) | SPD (n=18) | pc |
|---|---|---|---|
| PFC: | |||
| DLPFC | 357.61 ± 109.66 | 408.58 ± 187.08 | 0.306 |
| MPFC | 373.28 ± 129.22 | 414.40 ± 179.27d | 0.466 |
| OFC | 377.23 ± 160.76 | 447.50 ± 207.56 | 0.248 |
|
| |||
| Striatal: | |||
| VST | 663.41 ± 219.96 | 877.40 ± 335.61 | 0.025 |
| preDCA | 762.28 ± 237.42 | 928.88 ± 359.64 | 0.097 |
| preDPU | 813.18 ± 266.70 | 990.80 ± 380.12 | 0.101 |
| postCA | 584.59 ± 196.56 | 698.07 ± 309.58 | 0.181 |
| postPU | 781.91 ± 265.39 | 939.80 ± 363.83 | 0.132 |
| whole STR | 755.49 ± 241.89 | 927.82 ± 358.57 | 0.088 |
Note. Mean ± Standard Deviation (observed).
STR=striatum; see Table 2 for other abbreviations.
BPF values for all PFC (but not striatal) ROIs were adjusted to correct for partial volume effects (PVE); see text.
n=20: BPF values were missing for one control due to unavailability of fp term.
Group comparisons of the estimated marginal means derived from linear mixed modeling (see text) with the exception of the composite whole STR; for this region, an independent-samples t-test was used.
n=17: One SPD participant’s values were excluded due to excessive noise in the VT measurement.
Table 4.
[11C]NNC112 BPND by Diagnostic Group for PFCa and Striatal ROIs
| ROI | Controls (n=21) | SPD (n=18) | pb |
|---|---|---|---|
| PFC: | |||
| DLPFC | 1.35 ± 0.22 | 1.28 ± 0.27 | 0.369 |
| MPFC | 1.40 ± 0.24 | 1.28 ± 0.25c | 0.172 |
| OFC | 1.40 ± 0.34 | 1.39 ± 0.30 | 0.960 |
|
| |||
| Striatal: | |||
| VST | 2.50 ± 0.49 | 2.78 ± 0.44 | 0.064 |
| preDCA | 2.90 ± 0.59 | 2.94 ± 0.45 | 0.792 |
| preDPU | 3.08 ± 0.65 | 3.14 ± 0.53 | 0.772 |
| postCA | 2.19 ± 0.41 | 2.20 ± 0.48 | 0.969 |
| postPU | 2.96 ± 0.60 | 2.98 ± 0.50 | 0.885 |
| whole STR | 2.86 ± 0.54 | 2.94 ± 0.46 | 0.618 |
Note. Mean ± Standard Deviation (observed).
STR=striatum; see Table 2 for other abbreviations.
BPND values for all PFC (but not striatal) ROIs were adjusted to correct for partial volume effects (PVE); see text.
Group comparisons of the estimated marginal means derived from linear mixed modeling (see text) with the exception of the composite whole STR; for this region, an independent-samples t-test was used.
n=17: One SPD participant’s values were excluded due to excessive noise in the VT measurement.
Striatal subregions
There were no significant group differences in striatal VT (Online Resource Table 1). Mixed modeling with the striatal ROI indicated a trend effect for diagnosis in predicting BPF [F(1, 36)=3.08, p=0.088, n=20 for controls]. Group comparisons indicated that SPD participants had higher BPF in the VST compared to controls (p=0.025; Table 3, Online Resource Figure 1d); the effect size for this difference was in the medium-to-large range (0.763), but the difference was not considered significant after multiple-comparisons correction. There were no significant differences in BPF in the other striatal subregions or whole striatum. For BPND, there was no significant effect of diagnosis [F(1, 37)=0.29, p=0.595]. Group comparisons indicated that SPD participants had higher BPND in the VST compared to controls at trend level (p=0.064; Table 4); the effect size for this difference was in the medium range (0.612). There were no significant differences in other striatal subregions or whole striatum. For BPP, the diagnosis term was not significant [F(1, 37)=0.03, p=0.861], nor were the group differences in the striatal subregions or whole striatum. See Online Resource text for effect sizes related to striatal group comparisons. Results were nearly identical when including age in these striatal models (Online Resource text).
Correlations between WM Performance and [11C]NNC112 BP in the PFC
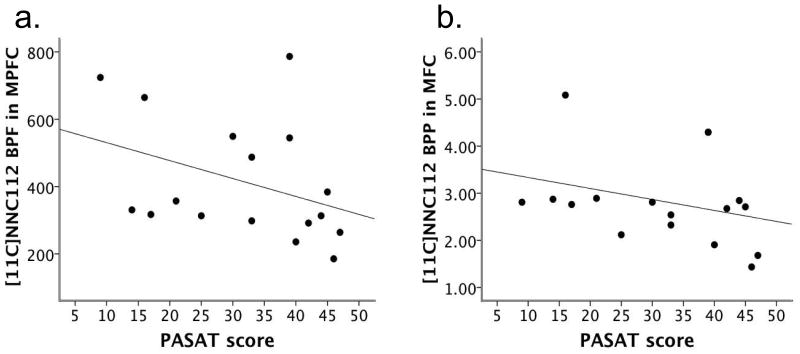
The Online Resource text provides the descriptive data of the PASAT and 2-back performance of the SPD participants. PASAT performance was significantly negatively related to [11C]NNC112 BPF and BPP in the MPFC (rs=-0.551, p=.022 and rs=-0.488, p=.047, respectively; n=17; Figure 1), and was negatively related at trend level to BPF and BPP in the DLPFC (rs=-0.460, p=.055 and rs=-0.421, p=.082, respectively; Online Resource Figure 2). These results indicate that SPD participants with poorer PASAT performance tended to have higher BPF and BPP in these prefrontal regions. There were no other significant associations between the PASAT and BP in the DLPFC, MPFC, or OFC. When controlling for age, PASAT performance was related at trend level to BPF in the MPFC (prs=-0.474, p=.064), and remained significantly associated with BPP in the MPFC (prs=-0.498, p=.050); the trend-level associations with BP in the DLPFC became nonsignificant, but their strength remained moderate (prs =-0.354, p=.164 and prs =-0.357, p=.160 for BPF and BPP, respectively). In contrast to the PASAT, performance on the 2-back (n=16) was not significantly related to BP in any region of the PFC; these results remained unchanged when controlling for age. See Online Resource Tables 2 and 3 for a complete list of the BP-WM correlational results.
Fig. 1.
a) The relation between [11C]NNC112 BPF in the medial prefrontal cortex (MPFC) and performance on the Paced Auditory Serial Addition Test (PASAT) in participants with schizotypal personality disorder (SPD), n=17; rs=-0.551, p=.022 b) The relation between BPP in the MPFC and PASAT performance in SPD participants, n=17; rs=-0.488, p=.047
Discussion
To our knowledge, this is the first report of D1R availability and related WM performance in SPD. In contrast to our hypothesis and previous findings in schizophrenia (Abi-Dargham et al. 2002, 2012), we found no evidence for significant group differences between SPD and control participants in D1R availability in the PFC as measured with [11C]NNC112 PET. As predicted, however, prefrontal D1R availability, particularly in the MPFC, was significantly negatively related to WM performance in SPD as measured by the PASAT, indicating that the SPD participants who performed more poorly on the PASAT tended to have higher levels of prefrontal D1. Associations between prefrontal D1 and 2-back performance were not significant, however; thus our 2nd hypothesis was only partially supported. Results from exploratory analyses suggest that SPD may be characterized by greater D1R availability in the VST compared to that of controls. These group differences did not survive multiple-comparisons correction but were of medium-to-large effect.
The significant negative associations we observed between prefrontal D1R availability and PASAT performance are similar to one of our previous findings in schizophrenia (Abi-Dargham et al. 2002); such results may reflect that individuals with SPD who have impairment in the processes tapped by the PASAT (i.e. WM, attention) are characterized by prefrontal DA dysfunction that, similar to what has been proposed to occur in schizophrenia (Abi-Dargham et al. 2002, 2012), results in understimulation of D1R and a compensatory but inefficient upregulation of these receptors. However, this interpretation is tentative given that we did not find evidence of significantly higher prefrontal D1R availability in SPD. It may be that compensatory alterations in D1 occur in a subset of SPD participants with particular WM deficits but to a relatively minor degree that does not result in detectable elevations of D1 at the group level, and/or that there are other processes occurring in SPD that contribute to variance in D1 levels. Of note, preliminary findings from our group (Rosell et al. 2013) indicate that in SPD (N=16), 3 days of treatment with the potent D1R agonist DAR-0100A significantly improved PASAT performance compared to placebo; these results are consistent with the current findings in suggesting a link between impaired WM performance and alterations in D1R stimulation by dopamine in SPD.
The inconsistent results we observed between the PASAT and 2-back with regard to associations with BP are worth noting. The lack of a significant association between prefrontal D1 and 2-back performance contrasts with the findings from one of our previous studies of schizophrenia (Abi-Dargham et al., 2002) in which higher DLPFC D1R availability was associated with worse performance on both the 2- and 3-back. Research on cognitive functioning in SPD has indicated that this spectrum condition is associated with cognitive impairment across various domains that is similar to, but typically less severe than, what is found in schizophrenia (McClure et al. 2008; Siever and Davis 2004). Thus, although speculative, it is possible that in SPD, associations between cognitive performance and potential neurobiological correlates such as D1R are only clearly evident when using tasks with heavier cognitive demands than that of the 2-back. Of note, we did not obtain data with the more difficult 3-back for this study. Relatedly, we previously found that compared to several other cognitive measures, including a visuospatial WM task, the PASAT was the most effective at discriminating SPD and control participants (Mitropoulou et al. 2005). These findings, along with our current results, suggest that the PASAT may be particularly useful in tapping the cognitive processes that are impaired in the schizophrenia spectrum and that are especially dependent on the appropriate level of prefrontal D1R stimulation in SPD.
Exploratory analyses indicated that SPD may be characterized by greater D1R availability in the VST compared to controls. Of interest is a similar trend-level finding from Abi-Dargham et al. (2012), suggesting that antipsychotic-naive patients with schizophrenia may have elevated VST D1R availability. Thus it may be worth investigating such potential alterations in D1R with pre-planned comparisons in future studies, particularly given this brain region’s putative role in motivational processes and negative symptoms.
The limitations of this study must be considered when interpreting its findings. One issue relates to the lack of selectivity in vivo of [11C]NNC112 for cortical D1R versus serotonin-2A receptors (5-HT2AR), such that ~25–30% of the binding in the cortex of healthy controls is to 5-HT2AR (Slifstein et al. 2007). This lack of selectivity for cortical D1 also characterizes the other currently-used D1 tracer, [11C]SCH23390 (Ekelund et al. 2007). In the striatum, however, where 5-HT2A density is negligible compared to D1, most of the binding of [11C]NNC112 is attributable to D1 (Slifstein et al. 2007; see Abi-Dargham et al. 2012). Thus if SPD is associated with alterations in cortical 5-HT2AR availability, our characterization of SPD-control group differences in prefrontal D1 levels may have been compromised. We are not aware of any studies of 5-HT2AR in SPD per se. PET studies of cortical 5-HT2A in schizophrenia have yielded mixed results; however, recent findings with antipsychotic-naive first-episode patients provide evidence that this disorder may be associated with lower cortical 5-HT2AR levels compared to controls (Rasmussen et al. 2010). In addition, recent work of Hurlemann et al. (2008) suggests that individuals at increased clinical risk for psychosis may have lower cortical 5-HT2AR availability relative to controls. Given that it is unclear how this limitation of [11C]NNC112 influenced the current results, future investigations using a more selective tracer, when available, would provide a more definitive characterization of prefrontal D1R and its relation to WM performance in SPD.
Another limitation is that we did not include group comparisons on catechol-O-methyltransferase (COMT) genotype in this report. Although efforts to collect genetic samples from participants were initiated prior to the completion of this study, due to the lack of samples among those who completed PET prior to the initiation of these efforts, as well as issues of feasibility in collecting samples among some of the later subjects, ultimately we did not have genotyping data on an adequate number of participants to allow for this. Given findings that healthy controls with the COMT genotype Val/Val have significantly higher D1R availability in cortical (but not striatal) regions as measured by [11C]NNC112 compared to Met carriers (Slifstein et al. 2008), characterizing the current sample on COMT genotype would have provided valuable information regarding potential factors influencing prefrontal D1 in both groups. Although the question of potential group differences in COMT genotype cannot be addressed with the current sample, Minzenberg et al. (2006) found that distribution in COMT Val/Met genotype did not differ significantly between SPD and control participants.
In addition, we did not systematically collect information regarding quantity of tobacco used by the participants who reported current regular tobacco use; thus although groups were matched on smoking status, we could not examine whether the quantity of tobacco used differed by group. We also did not collect an estimate of quantity of past recreational drug use of participants; this is particularly relevant for the SPD participants, as several met criteria for a past substance-use disorder (see Online Resource text). As a result, we could not examine potential associations between quantity of tobacco or past drug use and indices of D1R availability or WM performance. Relatedly, given that the SPD and control groups were not matched on past drug use, the potential influence of this factor on the current group-comparison results is unclear. Thus additional research that accounts for past drug use will advance attempts to characterize DA functioning and its correlates in SPD.
A limitation with regard to our correlational analyses between D1 and WM performance is that the time period between the WM assessments and PET was variable across SPD participants, and in some cases spanned more than a year (see Results). Given that presumably both state and trait factors influence our PET and behavioral measurements, it is possible that these variable time intervals influenced our correlational results, in particular weakening our ability to detect true cross-sectional associations. We acknowledge this limitation but believe it is unlikely that this issue led to significant associations that would not have been detected if standardized and shorter time intervals separated the WM assessments and PET. Of note, previous research has indicated high test-retest reliability for the PASAT, including long-term reliability (e.g., rs=0.96, Sjogren et al. 2000; for review, Tombaugh 2006). The test-retest reliability of the 2-back is generally considered fair to good (Nuechterlein et al. 2008), although we are not aware of any reports of the long-term reliability of the 2-back per se. It is possible that this limitation contributed to the inconsistent results obtained between the PASAT and 2-back with regard to associations with D1R.
We conducted multiple correlational analyses to test our hypothesis that prefrontal D1R availability was related to WM performance in SPD (see Methods) without correcting the related p values for this multiple testing. Our rationale for not using such a correction was that these tests were hypothesis-driven and that the relatively small size of our SPD sample would require larger statistical test values to achieve significance. It should be noted that the magnitude of the significant correlations observed were in the moderate-to-large range. On the other hand, due to the small sample size and lack of correction for multiple tests, these results should be considered preliminary and require replication.
In addition, we assessed WM performance among the SPD participants of this study, but not among the controls. Thus, although in prior studies we have reported impaired WM performance in individuals with SPD compared to healthy controls on both the PASAT (Mitropoulou et al. 2002, 2005) and 2-back (McClure et al. 2008), we cannot draw conclusions regarding WM dysfunction in this SPD sample relative to their matched controls. Relatedly, we were not able to examine the same associations between prefrontal D1R and WM performance among controls that were examined in SPD; thus we cannot draw conclusions regarding the potential specificity of the observed associations to SPD.
Another issue relates to the PET outcome measures we report here. In our prior [11C]NNC112 studies, we have included BPP and BPND, with BPP as our primary outcome. However, given that in the present study there was a significant group difference in the mean plasma free fraction of radiotracer (fp), we also included BPF, as its calculation accounts for fp (by dividing BPP by fp) - and we consider BPF as the primary PET outcome for group comparisons. Given this, there is increased confidence in the group difference we detected in BP in the VST because it was found with BPF and corroborated at trend level by BPND. In contrast, correlational analyses with BPP are not compromised by the fp group difference, given that these only included the SPD participants. This lends credence to the correlational results we obtained with BPP, which were consistent with those found with BPF.
In summary, we found only limited support for our hypotheses positing that the same prefrontal D1 alterations and associations with WM performance previously found in schizophrenia (Abi-Dargham et al. 2002, 2012) would characterize individuals with SPD. In contrast to what has been observed in schizophrenia, we found no evidence for prefrontal D1R upregulation in SPD. Similar to previous schizophrenia findings, however, the individuals with SPD who displayed poorer WM performance as measured by the PASAT tended to have higher D1R availability in the PFC. These findings combine to suggest that schizophrenia and SPD may share a common pathophysiological feature related to prefrontal DA functioning that contributes to the WM dysfunction that is present in both conditions, but that in SPD, compensatory alterations in D1 may occur only in the subset of individuals who are the most severely impaired with regard to WM and/or occur to an extent that is minor relative to the alterations that characterize schizophrenia. Such differences may reflect that SPD is characterized by a less severe form of cortical DA pathology compared to schizophrenia, or that there are other system-level adaptations in SPD that compensate for cortical DA dysfunction that do not occur in the full syndrome of schizophrenia. Future well-powered investigations including both schizophrenia and SPD samples, as well as those selected based on particular WM impairments, would be valuable in further elucidating the cortical DA alterations and their relations to functioning across the schizophrenia spectrum. In addition, studies more directly assessing presynaptic cortical DA functioning, such as with the amphetamine-challenge paradigm and the high-affinity D2R radioligand [11C]FLB 457, should provide valuable insight with regard to cortical DA functioning in this condition.
Supplementary Material
Acknowledgments
We thank the research participants of this study, and express gratitude for the expert assistance of Rawad Ayoub, Jennifer Bae, Felipe Castillo, John Castrillon, Elizabeth Hackett, Elisabeth Iskander, Olga Kambalov, and Ethan Rothstein.
Footnotes
- This work was supported by a Merit Review Grant (7609-028) from the U.S. Department of Veterans Affairs to Larry J. Siever; Grant MH56140 from the National Institute of Mental Health to Larry J. Siever; and by the Veterans Affairs VISN 3 Mental Illness Research, Education & Clinical Center (MIRECC).
- The authors declare no conflicts of interest; however, the following co-authors have appointments at the James J. Peters Veterans Affairs Medical Center, Bronx, NY (in addition to the Mount Sinai School of Medicine, New York, NY): Daniel R. Rosell, Yosefa Ehrlich, Erin A. Hazlett, and Larry J. Siever.
- In addition, some co-authors have financial relationships with pharmaceutical companies; specifically: Mark Slifstein serves as a consultant for Amgen, and receives research support from Pierre Fabre; Ragy R. Girgis receives research support from Otsuka; Lawrence S. Kegeles receives research support from Pfizer and Amgen; and Anissa Abi-Dargham is a consultant for Amgen, InVivo, UCB, and Roche, and receives research support from Otsuka, Takeda, Forest, and Pierre Fabre.
References
- Abi-Dargham A, Kegeles LS, Zea-Ponce Y, Mawlawi O, Martinez D, Mitropoulou V, O’Flynn K, Koenigsberg HW, Van Heertum R, Cooper T, Laruelle M, Siever LJ. Striatal amphetamine-induced dopamine release in patients with schizotypal personality disorder studied with single photon emission computed tomography and [123I]iodobenzamide. Biol Psychiatry. 2004;55:1001–6. doi: 10.1016/j.biopsych.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, Martinez D, Mawlawi O, Simpson N, Hwang DR, Slifstein M, Anjilvel S, Pidcock J, Guo NN, Lombardo I, Mann JJ, Van Heertum R, Foged C, Halldin C, Laruelle M. Measurement of striatal and extrastriatal dopamine D1 receptor binding potential with [11C]NNC 112 in humans: validation and reproducibility. J Cereb Blood Flow Metab. 2000;20:225–243. doi: 10.1097/00004647-200002000-00003. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang DR, Keilp J, Kochan L, Van Heertum R, Gorman JM, Laruelle M. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abi-Dargham A, Xu X, Thompson JL, Gil R, Kegeles LS, Urban N, Narendran R, Hwang DR, Laruelle M, Slifstein M. Increased prefrontal cortical D(1) receptors in drug naive patients with schizophrenia: a PET study with [(11)C]NNC112. J Psychopharmacol. 2012;26:794–805. doi: 10.1177/0269881111409265. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, D.C: 1994. [Google Scholar]
- Arnsten AF, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology (Berl) 1994;116:143–151. doi: 10.1007/BF02245056. [DOI] [PubMed] [Google Scholar]
- Barch DM, Mitropoulou V, Harvey PD, New AS, Silverman JM, Siever LJ. Context-processing deficits in schizotypal personality disorder. J Abnorm Psychol. 2004;113:556–68. doi: 10.1037/0021-843X.113.4.556. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Ekelund J, Slifstein M, Narendran R, Guillin O, Belani H, Guo NN, Hwang Y, Hwang DR, Abi-Dargham A, Laruelle M. In vivo DA D(1) receptor selectivity of NNC 112 and SCH 23390. Mol Imaging Biol. 2007;9:117–125. doi: 10.1007/s11307-007-0077-4. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis-I Disorders, Version 2.0. Biometrics Research New York State Psychiatric Institute; New York: 1994. [Google Scholar]
- Fischer H, Nyberg L, Karlsson S, Karlsson P, Brehmer Y, Rieckmann A, MacDonald SW, Farde L, Backman L. Simulating neurocognitive aging: effects of a dopaminergic antagonist on brain activity during working memory. Biol Psychiatry. 2010;67:575–580. doi: 10.1016/j.biopsych.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006;67(Suppl 9):3–8. [PubMed] [Google Scholar]
- Gronwall DM. Paced auditory serial-addition task: a measure of recovery from concussion. Percept Mot Skills. 1977;44:367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Guo N, Hwang DR, Lo ES, Huang YY, Laruelle M, Abi-Dargham A. Dopamine depletion and in vivo binding of PET D1 receptor radioligands: implications for imaging studies in schizophrenia. Neuropsychopharmacology. 2003;28:1703–1711. doi: 10.1038/sj.npp.1300224. [DOI] [PubMed] [Google Scholar]
- Hall H, Sedvall G, Magnusson O, Kopp J, Halldin C, Farde L. Distribution of D1- and D2-dopamine receptors, and dopamine and its metabolites in the human brain. Neuropsychopharmacology. 1994;11:245–256. doi: 10.1038/sj.npp.1380111. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Matusch A, Kuhn KU, Berning J, Elmenhorst D, Winz O, Kolsch H, Zilles K, Wagner M, Maier W, Bauer A. 5-HT2A receptor density is decreased in the at-risk mental state. Psychopharmacology (Berl) 2008;195:579–590. doi: 10.1007/s00213-007-0921-x. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Slifstein M, Xu X, Urban N, Thompson JL, Moadel T, Harkavy-Friedman JM, Gil R, Laruelle M, Abi-Dargham A. Striatal and extrastriatal dopamine D2/D3 receptors in schizophrenia evaluated with [18F]fallypride positron emission tomography. Biol Psychiatry. 2010;68:634–641. doi: 10.1016/j.biopsych.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka J, Takahashi H, Ito H, Takano A, Fujimura Y, Matsumoto R, Nozaki S, Yasuno F, Okubo Y, Kishimoto T, Suhara T. Decreased binding of [11C]NNC112 and [11C]SCH23390 in patients with chronic schizophrenia. Life Sci. 2010;86:814–818. doi: 10.1016/j.lfs.2010.03.018. [DOI] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, Huang Y, Simpson N, Ngo K, Van Heertum R, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D2 receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21:1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- McClure MM, Barch DM, Flory JD, Harvey PD, Siever LJ. Context processing in schizotypal personality disorder: evidence of specificity of impairment to the schizophrenia spectrum. J Abnorm Psychol. 2008;117:342–354. doi: 10.1037/0021-843X.117.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure MM, Harvey PD, Goodman M, Triebwasser J, New A, Koenigsberg HW, Sprung LJ, Flory JD, Siever LJ. Pergolide treatment of cognitive deficits associated with schizotypal personality disorder: continued evidence of the importance of the dopamine system in the schizophrenia spectrum. Neuropsychopharmacology. 2010;35:1356–1362. doi: 10.1038/npp.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Xu K, Mitropoulou V, Harvey PD, Finch T, Flory JD, New AS, Goldman D, Siever LJ. Catechol-O-methyltransferase Val158Met genotype variation is associated with prefrontal-dependent task performance in schizotypal personality disorder patients and comparison groups. Psychiatr Genet. 2006;16:117–24. doi: 10.1097/01.ypg.0000199448.00163.e6. [DOI] [PubMed] [Google Scholar]
- Mitropoulou V, Harvey PD, Maldari LA, Moriarty PJ, New AS, Silverman JM, Siever LJ. Neuropsychological performance in schizotypal personality disorder: evidence regarding diagnostic specificity. Biol Psychiatry. 2002;52:1175–1182. doi: 10.1016/s0006-3223(02)01426-9. [DOI] [PubMed] [Google Scholar]
- Mitropoulou V, Harvey PD, Zegarelli G, New AS, Silverman JM, Siever LJ. Neuropsychological performance in schizotypal personality disorder: importance of working memory. Am J Psychiatry. 2005;162:1896–1903. doi: 10.1176/appi.ajp.162.10.1896. [DOI] [PubMed] [Google Scholar]
- Narendran R, Frankle WG, Keefe R, Gil R, Martinez D, Slifstein M, Kegeles LS, Talbot PS, Huang Y, Hwang DR, Khenissi L, Cooper TB, Laruelle M, Abi-Dargham A. Altered prefrontal dopaminergic function in chronic recreational ketamine users. Am J Psychiatry. 2005;162:2352–2359. doi: 10.1176/appi.ajp.162.12.2352. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ, 3rd, Gold JM, Goldberg T, Heaton RK, Keefe RS, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–13. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic Interview for Genetic Studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Pfohl B, Blum N, Zimmerman M. Structured Interview for DSM-IV Personality: SIDP-IV. American Psychiatric Publishing; Washington, D.C: 1997. [Google Scholar]
- Poels EM, Girgis RR, Thompson JL, Slifstein M, Abi-Dargham A. In vivo binding of the dopamine-1 receptor PET tracers [(11)C]NNC112 and [(11)C]SCH23390: a comparison study in individuals with schizophrenia. Psychopharmacology (Berl) 2013;228:167–174. doi: 10.1007/s00213-013-3026-8. [DOI] [PubMed] [Google Scholar]
- Rasmussen H, Erritzoe D, Andersen R, Ebdrup BH, Aggernaes B, Oranje B, Kalbitzer J, Madsen J, Pinborg LH, Baare W, Svarer C, Lublin H, Knudsen GM, Glenthoj B. Decreased frontal serotonin2A receptor binding in antipsychotic-naive patients with first-episode schizophrenia. Arch Gen Psychiatry. 2010;67:9–16. doi: 10.1001/archgenpsychiatry.2009.176. [DOI] [PubMed] [Google Scholar]
- Rosell DR, Zaluda LC, McClure MM, Strike KS, Barch DM, Harvey PD, Girgis RR, Lieberman JA, Siever LJ. Clinical testing of a D1 agonist for cognitive enhancement in the schizophrenia spectrum. Society of Biological Psychiatry 68th Annual Scientific Meeting; San Francisco, CA, USA. 2013. [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Siever LJ, Davis KL. The pathophysiology of schizophrenia disorders: perspectives from the spectrum. Am J Psychiatry. 2004;161:398–413. doi: 10.1176/appi.ajp.161.3.398. [DOI] [PubMed] [Google Scholar]
- Sjogren P, Thomsen AB, Olsen AK. Impaired neuropsychological performance in chronic nonmalignant pain patients receiving long-term oral opioid therapy. J Pain Symptom Manage. 2000;19:100–8. doi: 10.1016/s0885-3924(99)00143-8. [DOI] [PubMed] [Google Scholar]
- Slifstein M, Kegeles LS, Gonzales R, Frankle WG, Xu X, Laruelle M, Abi-Dargham A. [11C]NNC 112 selectivity for dopamine D1 and serotonin 5-HT(2A) receptors: a PET study in healthy human subjects. J Cereb Blood Flow Metab. 2007;27:1733–1741. doi: 10.1038/sj.jcbfm.9600468. [DOI] [PubMed] [Google Scholar]
- Slifstein M, Kolachana B, Simpson EH, Tabares P, Cheng B, Duvall M, Gordon Frankle W, Weinberger DR, Laruelle M, Abi-Dargham A. COMT genotype predicts cortical-limbic D1 receptor availability measured with [(11)C]NNC112 and PET. Mol Psychiatry. 2008;13:821–827. doi: 10.1038/mp.2008.19. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN. A comprehensive review of the Paced Auditory Serial Addition Test (PASAT) Arch Clin Neuropsychol. 2006;21:53–76. doi: 10.1016/j.acn.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Tsukada H, Nishiyama S, Fukumoto D, Sato K, Kakiuchi T, Domino EF. Chronic NMDA antagonism impairs working memory, decreases extracellular dopamine, and increases D1 receptor binding in prefrontal cortex of conscious monkeys. Neuropsychopharmacology. 2005;30:1861–1869. doi: 10.1038/sj.npp.1300732. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.