Abstract
The activity of Na+/K+-ATPase establishes transmembrane ion gradients and is essential to cell function and survival. Either dysregulation or deficiency of neuronal Na+/K+-ATPase has been implicated in the pathogenesis of many neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease and rapid-onset dystonia Parkinsonism. However, genetic evidence that directly links neuronal Na+/K+-ATPase deficiency to in vivo neurodegeneration has been lacking. In this study, we use Drosophila photoreceptors to investigate the cell-autonomous effects of neuronal Na+/K+ ATPase. Loss of ATPα, an α subunit of Na+/K+-ATPase, in photoreceptors through UAS/Gal4-mediated RNAi eliminated the light-triggered depolarization of the photoreceptors, rendering the fly virtually blind in behavioral assays. Intracellular recordings indicated that ATPα knockdown photoreceptors were already depolarized in the dark, which was due to a loss of intracellular K+. Importantly, ATPα knockdown resulted in the degeneration of photoreceptors in older flies. This degeneration was independent of light and showed characteristics of apoptotic/hybrid cell death as observed via electron microscopy analysis. Loss of Nrv3, a Na+/K+-ATPase β subunit, partially reproduced the signaling and degenerative defects observed in ATPα knockdown flies. Thus, loss of Na+/K+-ATPase not only eradicates visual function but also causes age-dependent degeneration in photoreceptors, confirming the link between neuronal Na+/K+ ATPase deficiency and in vivo neurodegeneration. This work also establishes Drosophila photoreceptors as a genetic model for studying the cell-autonomous mechanisms underlying neuronal Na+/K+ ATPase deficiency-mediated neurodegeneration.
Keywords: ATPα, nrv3, neurodegeneration
Introduction
The Na+/K+-ATPase transports Na+ and K+ against their concentration gradients across the cell membrane to maintain a low Na+ and high K+ concentration within the cells (Blanco and Mercer, 1998; Mobasheri et al., 2000). These ion gradients determine the resting membrane potential and form the basis of the excitability of neurons. The Na+ gradient also provides the driving force for various secondary active transporters that import glucose, amino acids, and other nutrients into the cell. Additionally, the ion concentrations maintained by Na+/K+-ATPase are important for regulating cellular volume and preventing cells such as neurons from swelling and lysing (Geering, 1997; Pavlov and Sokolov, 2000).
Considering the importance of Na+/K+-ATPase in basic cellular functions, it is not surprising that either dysregulation or deficiency of neuronal Na+/K+-ATPase were observed in many neurodegenerative disorders, such as Alzheimer’s disease (AD), Parkinson’s disease (PD) and rapid-onset dystonia Parkinsonism (RDP) (Cannon, 2004; Chauhan et al., 1997; de Carvalho Aguiar et al., 2004; DeAndrade et al., 2011; Kumar and Kurup, 2002). Thus, disrupting normal Na+/K+-ATPase activity in neurons has been proposed to contribute to the pathogenesis of neurodegeneration. Nevertheless, the link between the disrupting neuronal Na+/K+-ATPase activity and neuronal dysfunction/degeneration has yet to be clarified.
Na+/K+-ATPase is composed of at least two subunits: a large catalytic α subunit and a regulatory, single-transmembrane-domain β subunit (Gloor et al., 1990; Horisberger, 2004; Kaplan, 2002; Paul et al., 2007; Shoshani et al., 2005; Vagin et al., 2005). Mammals have three α-subunit and two β-subunit genes and may express six structurally distinct Na+/K+-ATPase isoforms (Watts et al., 1991). In the brain, although the α3 and β2 subunits are expressed predominantly in neurons, the α2 and β1 subunits are found primarily in glia, and the α1 subunit is ubiquitously expressed (McGrail et al., 1991; Watts et al., 1991). The Na+/K+-ATPase in glia is required to maintain a low K+ level in the neuronal environment (Wang et al., 2012) and thus has a large impact on neuronal function and survival. Na+/K+-ATPase inhibitors like ouabain act on all Na+/K+-ATPase isoforms and cannot differentiate between the cell-autonomous effects of the Na+/K+-ATPase in neurons from those derived from the neighboring glia. Thus, genetic approaches are needed to modulate the Na+/K+-ATPase level in neurons to investigate the function of neuronal Na+/K+-ATPase. Genetic studies on the impact of neuronal Na+/K+-ATPase deficiency in the past decade, which were mostly based on characterization of heterozygous mutant mice of the α3 subunit, have identified defects in the function of central brain neurons (Clapcote et al., 2009; Moseley et al., 2007; Shiina et al., 2010) but have not provided direct evidence of neurodegeneration.
The Drosophila visual system expresses only one type of α subunit, ATPα, and three β subunits, Nrv1-3 (Ashmore et al., 2009; Baumann et al., 2010; Okamura et al., 2003; Palladino et al., 2003; Takeyasu et al., 2001). In this study, we used Drosophila photoreceptors as a genetic model to study the cell-autonomous functions of neuronal Na+/K+-ATPase. Although ATPα mutants in Drosophila exhibit extensive neurodegeneration (Palladino et al., 2003), these mutants were not used because the degeneration is due to the loss of Na+/K+-ATPase not only in neurons but also in neighboring non-neuronal cells. Instead, using a UAS/Gal4-mediated RNAi approach (Brand and Perrimon, 1993; Dietzl et al., 2007; Roy et al., 2013), we knocked down ATPα and Nrv1-3 specifically in photoreceptors and assessed the impact of this knockdown on visual signaling and photoreceptor integrity in the fly.
Materials and Methods
Drosophila stocks and crosses
All flies were raised on corn-meal medium without propionic acid and were maintained at 25°C and 60% humidity under a 12:12 hr light-dark cycle unless otherwise stated. The following fly stocks were used: repo-Gal4, elav-Gal4, lGMR-Gal4 (longGMR, pan-photoreceptor-Gal4, BL8605), GMR-Gal4 (ninaE.GMR-Gal4, BL1104), UAS-ATPα-RNAi (short-hairpin, BL33646), UAS-nrv3-RNAi (BL29431) and tublin-Gal80ts, all of which were obtained from the Drosophila Stock Center in Bloomington. The fly stocks UAS-ATPα-RNAi (v100619), UAS-nrv1-RNAi (v103702) and UAS-nrv2-RNAi (v2660) were also used and supplied by the Vienna Drosophila RNAi Center. UAS-RNAi flies were crossed over specific GAL4 and Gal80ts to either induce or inhibit the expression of RNAi, respectively.
The RNAi constructs of the Na+/K+-ATPase subunit genes were expressed in photoreceptors using the Gal4/UAS system (Brand and Perrimon, 1993; Dietzl et al., 2007; Roy et al., 2013). The drivers lGMR-Gal4 (Chen et al., 2014; Timofeev et al., 2012; Wernet et al., 2003) and GMR-Gal4 (Velentzas et al., 2013) have a photoreceptor-specific expression pattern, and repo- (Awasaki and Ito, 2004) and elav-Gal4 (Zhan et al., 2004) have glial and neuronal expression patterns, respectively. The Gal80ts/TARGET system was used for temporal control of UAS-RNAi expression (McGuire et al., 2004).
Electrophysiological recordings
Electroretinograms (ERG) were recorded as previously described (Li and Montell, 2000). Flies were immobilized with thin strips of tape. Glass recording microelectrodes filled with Ringer’s solution were placed on the eye surface of the fly. A second extracellular recording electrode was maintained on the thorax (as a reference). Five-second orange light pulses (4000 Lux) were used to stimulate the eye after adapting the fly to the dark for 1 min. The signal was amplified and recorded using a Warner IE210 intracellular electrometer.
In vivo photoreceptor intracellular recordings were performed as previously described (Johnson and Pak, 1986). Briefly, a small portion of the cornea was removed with a sharp needle, and the opening was covered with Vaseline petroleum jelly. The intracellular recording electrodes were inserted into the retina through this opening. The recording electrodes had a resistance of 100–150 MΩ when filled with 4% neurobiotin (Vector Labs) in 2 M potassium acetate (KAc). The reference electrode was filled with Ringer’s solution, and its tip was placed in the photoreceptor layer. The fly was dark-adapted for 10 min before measurement. Voltage responses were amplified using a Warner IE210 intracellular electrometer in current clamp mode. When the electrode was inserted into a cell, we measured the resting membrane potential in the dark based on a sudden increase of capacitance and tested the cell’s response to 5 s orange light pulses (4000 Lux). After the recording, the cell was injected with neurobiotin by passing 1nA depolarizing rectangular pulses at 1 Hz for 5 min (Kita and Armstrong, 1991). The retina was subsequently dissected, fixed in 4% paraformaldehyde and stained with streptavidin-Alexa Fluor 488 conjugate (Invitrogen) and rhodamine phalloidin to confirm the photoreceptor identity of the recorded cell (Schnell et al., 2010).
Immunofluorescence staining
For cryosectioning, 1-day-old fly heads were removed, incubated in 0.1 M phosphate buffer with increasing concentrations of sucrose, infiltrated and embedded in TFM tissue freezing medium (Ted Pella Inc.). Approximately 20 μm sections were cut at −20°C and subjected to immunofluorescence staining. After a 30 min incubation with blocking buffer (5% fetal bovine serum in PBS containing 0.3% Triton X-100), the brain sections were incubated overnight at 4°C with primary antibody diluted in blocking buffer. After three washes in PBS containing 0.3% Triton X-100, the brain sections were incubated with FITC-conjugated secondary antibodies for 3 hours at room temperature, washed and mounted in Vectashield medium (Vector Laboratories). The images were captured using confocal microscopy with an LSM 510 instrument (Zeiss). The monoclonal antibody α5-IgG (1:100), which is specific for the α-subunit of the Na+/K+-ATPase, was obtained from the Developmental Studies Hybridoma Bank (Baumann et al., 2010; Lebovitz et al., 1989; Takeyasu et al., 1988). We used the auto-fluorescent properties of visual pigments to mark pigment cells in the retina of red eye flies (Pichaud and Desplan, 2001).
Electron microscopy (EM)
Fly heads were removed, bisected and fixed in a solution of 2.5% glutaraldehyde in 0.05 M sodium cacodylate buffer (pH7.4), and processed for EM as previously described (Meinertzhagen and O’Neil, 1991). After three washes, fly heads were post-fixed with 2% osmium tetroxide for 5 hours, dehydrated in ethanol, infiltrated with propylene oxide and embedded in polybed812 resin (08792-1; Polysciences). After sectioning and staining with uranyl acetate and lead citrate, the ultrastructures in the retina were examined at 80 KV using a Philips Tecnai 12 electron microscope.
Optical neutralization analysis and retinal degeneration assay
This analysis was performed as previously described (Franceschini and Kirschfeld, 1971). Briefly, fly heads were separated from the body and immersed in a layer of lens oil to optically neutralize the cornea. On the microscope stage, a spotlight was shone into the head from the neck side to antidromically illuminate the compound eye. After the images were acquired with a CoolSNAP ES2 CCD Camera, the number of rhabdomeres that appeared as bright dots resulting from a high transmission of light were counted for each upright ommatidium (Sengupta et al., 2013). Degeneration of photoreceptors was assessed directly with this method (Lessing and Bonini, 2009). Briefly, a score ranging from 0 to 3 was given to each rhabdomere depending on the state of its degeneration: 3 was indicative of no degeneration; 0 corresponded to total loss of the rhabdomeres and a score of 1 or 2 suggested partial degeneration. The total score of an intact ommatidium is 21. The rhabdomere integrity index for a fly was the average score from 6 axially aligned ommatidia. For each group, the value of the integrity index was the average of 6 flies.
Measurement of extracellular K+ concentration ([K+]o) in the retina
The extracellular K+ activity was measured in the fly retina using K+-selective double-barreled microelectrodes, which were fabricated as previously described (Sandler and Kirschfeld, 1991; Semb et al., 1997). Briefly, a K+-selective microelectrode was pulled from the borosilicate double-barreled capillaries with a filament. The K+-selective barrel was silanized with N, N-Dimethyltrimethylsilylamine (41716, Fluka) at 200°C. The barrels were filled with 100 mM NaCl and 5 mM KCl. Next, the tip of the silanized barrel was filled by capillary action with a small amount (to approximately 100 μm from the tip) of the K+ ionophore I-Cocktail B (60398, Fluka). The electrodes were connected to the differential amplifier using a chlorinated silver wire. The K+-selective electrode was calibrated before and after each experiment in a series of solutions where concentrations of KCl and NaCl totaling 150 mM were varied reciprocally. To measure light-induced changes of the extracellular K+ concentration in the retina, the eye was illuminated for 40 seconds. The K+ concentrations were calculated from the Nernst equation. All the calibrations and experiments were carried out at room temperature (22–25°C) in a light tight Faraday cage.
Visual behavior assays
Phototaxis assays were carried out in a dark room with the countercurrent procedure as previously described (Benzer, 1967). After a 1-min equilibration period, groups of 10 flies in a long glass tube were given 1 min to move toward a white light source in an otherwise dark room. At the end of the assay, the number of responsive flies was counted. The results were the mean of the responsive flies from five groups per genotype.
The walking optomotor assay was conducted using a previously described method (Rendahl et al., 1992; Zhu et al., 2009) with minor modifications. An LED panel was put in the front of a clear tube of 10 flies. Dark stripes against a bright background continuously drifted from left to right and then from right to left. Control flies move against the direction of the stripe motion, and will change direction of movement when the direction of the stripe motion is reversed. Fly performance was evaluated by counting the number of flies changing direction in response to the flipping of the stripe motion. The results were the mean of the responsive flies from five groups per genotype.
Giant fiber system (GFS) recording
A fly will jump to a variety of stimuli, including a rapid light-off stimulus. Giant fibers (GF) are command neurons that are activated by a light-off stimulus through the excitation of photoreceptors and evoke a stereotypical pattern of activity in the thoracic muscles, producing an escape jump. We recorded GF-driven muscle potentials and the response to a rapid light-off stimulation following a previously described method (Thomas and Wyman, 1984). Briefly, flies were immobilized with thin strips of tape. The potentials in the dorsal longitudinal muscles (DLMs) were recorded to measure the output of the giant fiber pathway. The signal was amplified and recorded using a Warner IE210 intracellular electrometer. Ten flies were examined per genotype.
Results
ATPα is required for visual function of photoreceptor in adult flies
In Drosophila, there are three genes (ATPα, JYalpha and CG45062) that encode the α subunits of Na+/K+ ATPase (McQuilton et al., 2012). Whereas both JYalpha and CG45062 have relatively specific expression in the testes, ATPα is distributed ubiquitously and functions in the brain, eye and Johnston’s organ of hearing (Baumann et al., 2010; Chintapalli et al., 2007; Roy et al., 2013; Yasuhara et al., 2000). All ATPα loss-of-function mutants are embryonic lethal (Palladino et al., 2003). When ATPα expression was suppressed via RNAi in either all neurons or glia cells using the elav- and repo-Gal4 drivers, respectively, we did not obtain any knockdown flies (S.Table.1). Thus, both neuronal and glial functions of ATPα are essential to fly viability.
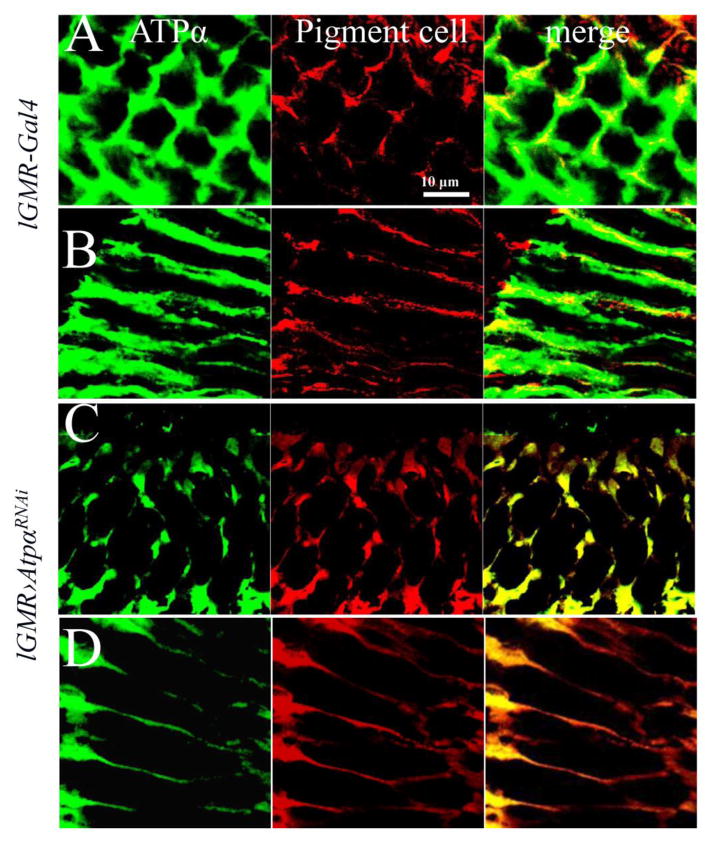
In the eye, ATPα is the only α subunit of Na+/K+-ATPase and is expressed in both photoreceptor neurons and the surrounding pigment glial cells (Baumann et al., 2010; Yasuhara et al., 2000). To study the function of Na+/K+-ATPase specifically in photoreceptors, we knocked down ATPα expression using the photoreceptor-specific driver line lGMR-Gal4 (Wernet et al., 2003). Two independent RNAi lines (v100619 and BL33646) that express different dsRNA against different regions of ATPα mRNA showed identical phenotypes in combination with the Gal4 drivers, ruling out off-target effects of the RNAi lines (S.table.1). We chose to use the ATPα RNAi line that expresses a short dsRNA (TCCCAACGGCTTTAAGTTCAA, 21 bp) in this study. In 1-day-old control flies that only contain lGMR-Gal4, we observed that ATPα was distributed over the peripheral surface of photoreceptors and the cell membrane of pigment cells in the retina (Fig. 1A & B). In 1-day-old ATPα knockdown flies, which have both lGMR-Gal4 and the ATPα RNAi transgene, the staining signal of ATPα was drastically reduced in photoreceptors, but unchanged in pigment cells that were marked with autofluorescence of their red pigments (Fig. 1C & D). These data confirm the effectiveness and specificity of ATPα knockdown in photoreceptors.
Figure 1.
Knockdown of ATPα in photoreceptors results in reduced ATPα expression levels in 1-day-old flies. A and C, Cross-sections of compound eyes were labeled with α5 antibody (green). B and D, Longitudinal sections of compound eyes were labeled with α5 antibody. The red signal is enhanced autofluorescence of red pigments in the pigment cells. In the 1-day-old control lGMR-Gal4 flies (A & B), ATPα was expressed in pigment cells and the basolateral membrane domain of photoreceptors. In 1-day-old knockdown flies (C & D), ATPα was greatly reduced in photoreceptors and observed primarily in pigment cells.
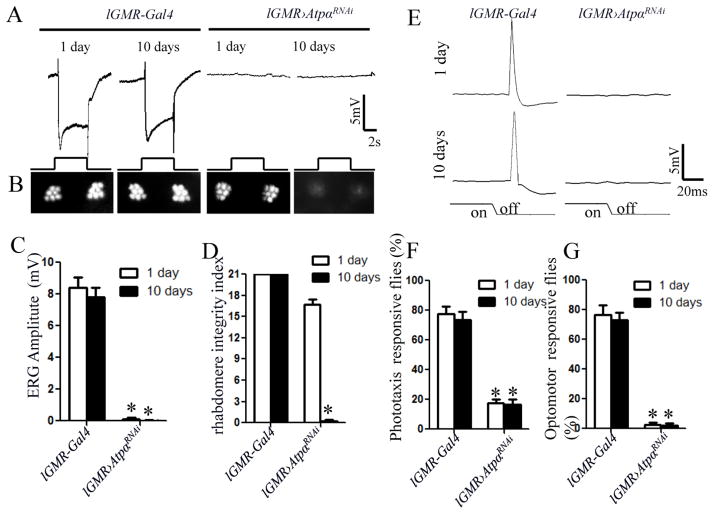
To investigate the functional impact of ATPα knockdown, we examined light response in 1-day-old and 10-day-old flies by recording the ERG on the eye surface. In control lGMR-Gal4 flies, a light pulse stimulated a negative corneal potential change due to depolarization of the photoreceptors. In contrast, ATPα knockdown flies completely failed to respond to light pulses at both ages (Fig. 2A & C). To test whether this defect is due to loss of the rhabdomere, the light sensory organelle of the fly photoreceptor, we examined rhabdomere integrity through optical neutralization analysis (ONA, see Methods). In each ommatidium (the unit of compound eyes) of the control fly, we observed seven bright spots that represent the positions of six peripheral (R1–R6) rhabdomeres and the R7/R8 central rhabdomere. In ATPα knockdown flies, all seven spots were visible in the 1-day-old flies but disappeared in the 10-day-old flies (Fig. 2B & D), indicating age-dependent degeneration of the photoreceptors. Thus, ATPα knockdown flies lose their responsiveness to light before degeneration of the rhabdomere occurs.
Figure 2.
ATPα is essential for the function and survival of Drosophila photoreceptors. A, ERG recordings failed to reveal any light response in 1-day-old and 10-day-old knockdown flies. The event markers underneath represent 5-sec orange light pulses. B, In optical neutralization assays, severe loss of peripheral rhabdomeres was detected in 10-day-old knockdown flies but not in 1-day-old knockdown flies. C, Quantification and comparison of ERG response amplitudes. D, Based on optical neutralization assays, the rhabdomere integrity index (Materials and Methods) was dramatically decreased in 10-day-old but not in 1-day-old knockdown flies. E, The light-off stimulation elicited a large peak of giant fiber response within 40 ms in the control lGMR-Gal4 flies. No visually elicited giant fiber response was observed in ATPα knockdown flies. F and G, Both phototaxis and the motion-dependent walking optomotor response in ATPα knockdown flies were severely impaired. Asterisk (*): p< 0.01; Two-tailed t-test. Error bars denote S.E.M.
To confirm that the brains of ATPα knockdown flies do not receive any visual information, we recorded electrical signals in the GFS. The GFS mediates the light-off escape response by relaying excitation from the eyes to the muscles of the thorax in Drosophila (Thomas and Wyman, 1984). The light-off stimulus evoked GFS response can be detected as a large transient depolarization of the thoracic muscles. The light-off stimulus will not be able to activate GF neurons if the function of photoreceptors is impaired. In ATPα knockdown flies, we could not detect any electrical response of the thoracic muscles at the end of light stimulation (Fig. 2E). We then examined fly visual behavior with a stationary light-elicited phototaxis assay and a visual motion-dependent walking optomotor assay. The results showed that both phototaxis (Fig. 2F) and optomotor responses (Fig. 2G) were severely impaired in ATPα knockdown flies, and that there was no significant difference between the 1-day-old and 10-day-old flies. Any defects in the components of the neuronal networks transducing visual information into motor output can cause abnormal visual behaviors; however, all the components except photoreceptors are intact in the photoreceptor-specific knockdown flies. Thus, the knockdown flies are virtually blind, even at 1-day old.
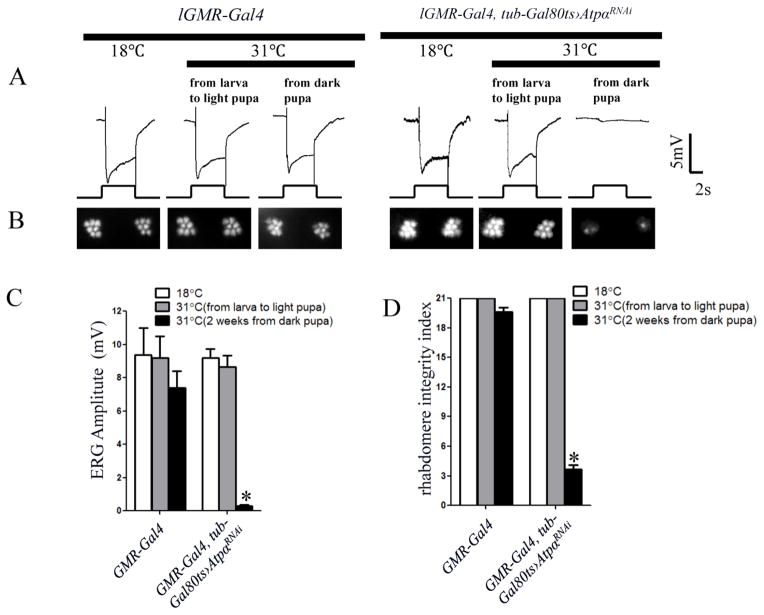
Na+/K+ ATPase is involved in a variety of developmental processes in both vertebrates and invertebrates, such as epithelial junction formation (Paul et al., 2007) and morphogenesis, as well as oncogenesis. To investigate whether any developmental defect accounts for the absence of light response in ATPα knockdown flies, we expressed Gal80ts (McGuire et al., 2004), a temperature sensitive inhibitor of Gal4, in knockdown flies using a tubulin promoter. When raised at a permissive temperature (18°C) of Gal80ts, the lGMR-Gal4/tub-Gal80ts;UAS-ATPαRNAi/+ flies had no RNAi phenotype due to Gal80 suppression of the Gal4 driver (Fig. 3). Next, we exposed flies of the same genotype to a restrictive temperature (31°C) to induce RNAi during different developmental stages. Metamorphosis in Drosophila can be divided into 16 stages (P1–P16). We define the light pupal stage at approximately P9–P11 (characterized by pink eye, dark ocellar bristles and dorsal chaetae), when the retina is still developing, and the dark pupal stage after P12 (characterized by dark wings, abdominal bristles), when the retina is essentially complete (Cagan and Ready, 1989). When flies were exposed to 31°C from the larval to light pupal stage, they exhibited normal ERG light responses (Fig. 3A & C) and intact rhabdomeres in the ONA (Fig. 3B & D), even at 2 weeks of age. In contrast, when flies were exposed to 31°C starting at the late pupal stage, they displayed negligible light response (Fig. 3A & C) and had almost no intact rhabdomeres at 2 weeks (Fig. 3B & D). Thus, ATPα is not essential for early development of the photoreceptor, but is required for the function and survival of adult photoreceptors.
Figure 3.
ATPα in photoreceptors is required for visual function in adult flies. lGMR-GAL4, tub-gal80ts AtpαRNAi and control lGMR-Gal4 flies were exposed to 31°C (to allow for ATPα RNAi) at either the pupal or adult stage before being examined 2 weeks after eclosion. A, ERG recordings revealed that flies with ATPα knockdown starting at the dark pupal stage had virtually no light response, whereas those that underwent RNAi from the larval to light pupal stages showed normal response. No significant ERG phenotype was observed in any of the control flies and lGMR-GAL4, tub-gal80ts AtpαRNAi flies that were kept at a constant 18°C. B, Optical neutralization assays showed that photoreceptors undergoing ATPα RNAi from the dark pupal stage had degenerated, whereas those with ATPα RNAi from the larval to light pupal stage had intact rhabdomeres. C, Comparison of the ERG amplitude among control flies and those subjected to ATPα RNAi at different stages. D, Comparison of the rhabdomere integrity index. Asterisk (*): p< 0.01; Two-tailed t-test. Error bars denote S.E.M.
Nrv3 is a primary β subunit of Na+/K+ ATPase in the photoreceptor
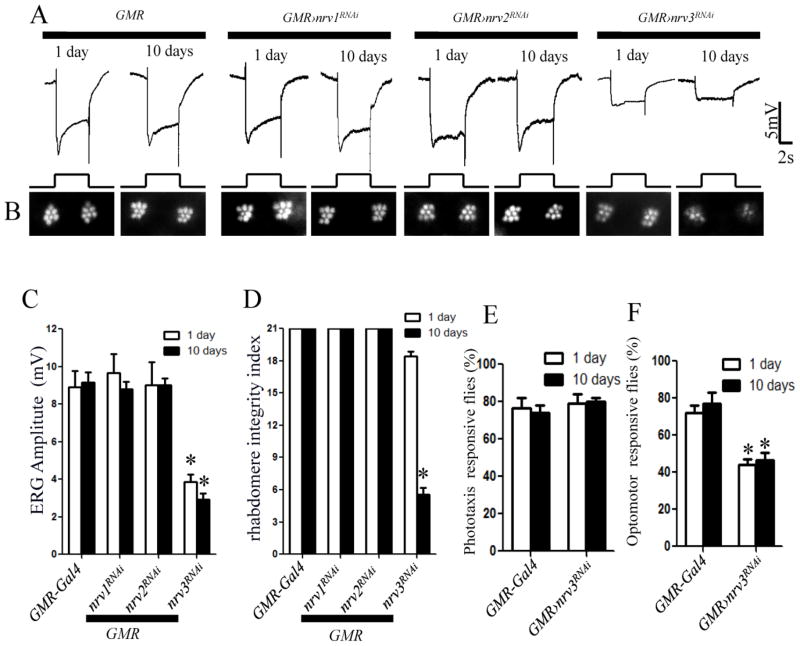
The Drosophila genome encodes three β subunits (Nrv1-3) of Na+/K+ ATPase. When these β subunits were knocked down using lGMR-Gal4, we did not observe either ERG abnormality or rhabdomere loss in any knockdown flies (data not shown), which could be attributed to functional redundancy. However, when we used a stronger Gal4 GMR-Gal4 into these knockdown flies, the Nrv3 knockdown flies responded to light with significantly reduced amplitude in the ERG recordings, even at 1 day old (Fig. 4A and C). Additionally, they showed severe loss of rhabdomeres in 10-day-old flies according to the ONA (Fig. 4B and D). In contrast, Nrv1 and Nrv2 knockdown flies with GMR-Gal4 did not show any phenotype in either the ERG or ONA (Fig. 4A–D). Although we cannot exclude the involvement of Nrv1 and Nrv2 because of variable RNAi efficiency, the results suggest that Nrv3 is the primary β subunit of Na+/K+ ATPase in the photoreceptor.
Figure 4.
The Na+/K+-ATPase β subunit Nrv3 is essential for the function and survival of Drosophila photoreceptors. Na+/K+-ATPase β subunits Nrv1, Nrv2 and Nrv3 were separately knocked down in photoreceptors. A, ERG recordings revealed that the light response in both 1-day and 10-day-old Nrv3 knockdown flies was impaired. No ERG defect was observed in either the control GMR-Gal4 flies or the Nrv1 and Nrv2 knockdown flies. B, Optical neutralization assays showed a severe loss of peripheral rhabdomeres in 10-day-old Nrv2 knockdown flies but not in 1-day-old flies. C, The amplitude of the ERG response was reduced to a similar extent in 1-day-old and 10-day-old Nrv3 knockdown flies. D, The rhabdomere integrity index was dramatically decreased in 10-day-old Nrv3 knockdown flies. E, Nrv3 knockdown flies had normal phototaxis but exhibited a weaker optomotor response. There were no significant differences within genotypes between 1-day-old and 10-day-old flies. Asterisk (*): p< 0.01; Two-tailed t-test. Error bars denote S.E.M.
We further examined the visual behavior of GMR-Gal4;UAS-nrv3RNAi flies using the phototaxis and walking optomotor assays. In the phototaxis assay, Nrv3 knockdown flies showed a normal tendency to light (Fig. 4E). However, they exhibited a relatively weak optomotor response compared to control flies (Fig. 4F). Thus, although Nrv3 knockdown flies are able to sense light, they are defective in responding to complex visual cues.
ATPα knockdown photoreceptors undergo light-independent degeneration characterized by apoptotic/hybrid cell death
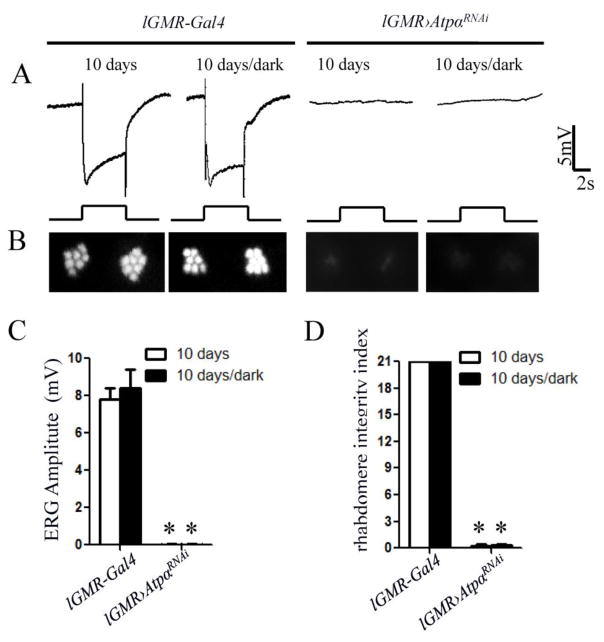
The loss of rhabdomeres in older ATPα and Nrv3 knockdown flies suggests that Na+/K+ ATPase is required to protect photoreceptors from age-dependent degeneration. Drosophila photoreceptors degenerate through various mechanisms such as rhodopsin endocytosis-mediated apoptosis and Ca2+-dependent necrosis (Knust, 2007; Shieh, 2011; Wang and Montell, 2007), which are triggered by light. To investigate whether the rhabdomere degeneration caused by loss of Na+/K+ ATPase depends on light exposure, we reared flies in complete darkness from the embryonic stages. At 10 days old, dark-reared ATPα knockdown flies not only lacked a light response (Fig. 5A and C) but also lost most rhabdomeres (Fig. 5 B and D). Thus, loss of Na+/K+ ATPase leads to light-independent rhabdomere degeneration.
Figure 5.
Photoreceptor degeneration and the loss of light response in 10-day-old ATPα knockdown flies are independent of light exposure. A, No ERG difference was observed between light-exposed and dark-reared flies in both the control and ATPα knockdown groups. B, Optical neutralization assays showed that both light-exposed and dark-reared knockdown flies exhibited a severe loss of rhabdomeres with no obvious difference. C and D, Comparison of the ERG response amplitude and the rhabdomere integrity index, did not reveal significant difference between light-exposed and dark-reared knockdown flies. Asterisks (*) indicate significant differences compared to the control flies (p< 0.01; Two-tailed t test). Error bars denote S.E.M.
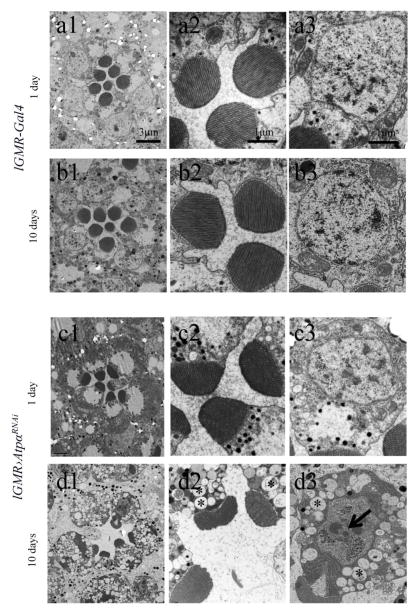
To gain additional insight into the degeneration mechanism, we conducted electron microscopy (EM) with cross sections of the retina to examine the morphology of photoreceptors in both 1-day-old and 10-day-old flies. At 1 day old, the microvillus structure was normal in all rhabdomeres (Fig. 6), but the rhabdomeres in ATPα knockdown flies appeared smaller than in the control flies, which indicates either a subtle developmental defect or an early sign of degeneration, In 10-day-old ATPα knockdown flies, however, all the rhabdomeres were greatly reduced. In contrast to the damaged photoreceptors, the pigment glia that wraps the photoreceptor cluster of the ommatidium did not show significant morphological abnormalities in the knockdown flies. The degenerating photoreceptors showed apoptotic features such as condensed nuclei and dark chromatin clumps, as well as necrotic changes including cytoplasmic edema manifested by vacuolization (Fig. 6). These morphological abnormalities indicate that ATPα knockdown results in concurrent apoptosis and necrosis in the photoreceptors similar to the “hybrid” cell death of mammalian cells due to inhibition of Na+/K+-ATPase (Yu, 2003a).
Figure 6.
EM analyses revealed that ATPα knockdown photoreceptors in 10-day-old flies underwent neurodegeneration as characterized by apoptotic/hybrid cell death. Those photoreceptors lost rhabdomeres, and the remaining rhabdomeres were reduced in size (d). Apoptotic features, including condensed nuclei and dark chromatin clumps (indicated by arrow, d3), accompanied by necrotic changes such as cytoplasmic edema manifested by vacuolation (indicated by asterisks, d2 and d3) were observed in the cell bodies of degenerating photoreceptors. Although rhabdomeres in 1-day-old knockdown flies appeared to be slightly smaller than those of control lGMR-Gal4 flies, no obvious degeneration was observed (c2 and c3).
Na+/K+ ATPase is required for photoreceptors to establish a high intracellular level of K+
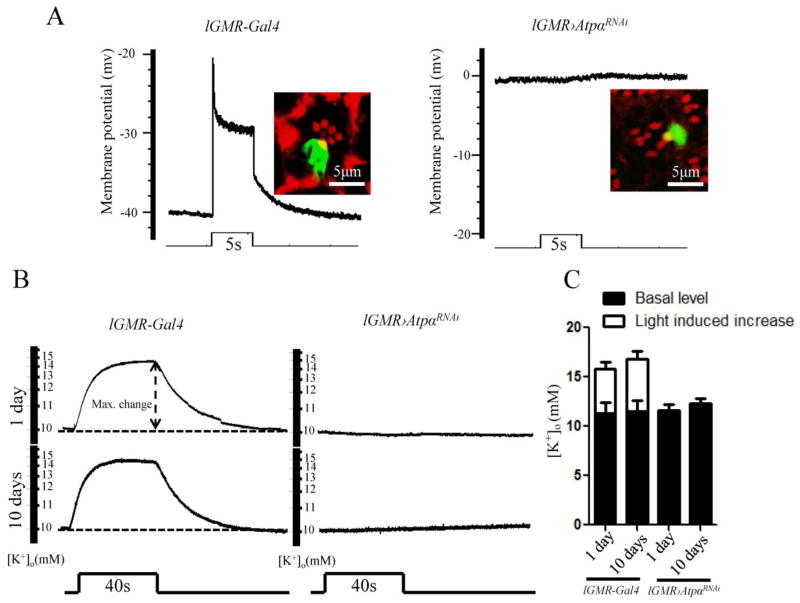
When Na+/K+-ATPase is inhibited, mammalian cells undergo apoptosis due to loss of intracellular K+ (Yu, 2003a). Because the cross-membrane K+ gradient creates a negative membrane potential, photoreceptor cells in ATPα knockdown flies also lose their K+ gradient, which causes a depolarized membrane potential in the dark. To test this, we measured photoreceptor membrane potentials using intracellular recording electrodes in 1-day-old flies. Neurobiotin was injected into the recorded cells after measurement to confirm their photoreceptor identity (Fig. 7A). In control flies, photoreceptors had resting membrane potentials at approximately −40 mV and responded to light pulses with 20 mV depolarization, which was similar to findings from a previous report (Johnson and Pak, 1986). In contrast, ATPα knockdown photoreceptors showed no negative membrane potential in the dark and therefore had no response to light (Fig. 7A). Thus, ATPα knockdown photoreceptors are already depolarized in the dark, indicating a complete loss of the K+ gradient across the cell membrane.
Figure 7.
The photoreceptors in ATPα knockdown flies lost their ability to maintain the K+ gradient. A, Intracellular recordings of photoreceptors were performed in 1-day-old control lGMR-Gal4 and ATPα knockdown flies. The resting membrane potential of the photoreceptor in the dark is approximately −40 mV in controls (n=10) and 0 mV in knockdown flies (n=10). Light stimulation failed to trigger any electrical response in the ATPα knockdown photoreceptors. To confirm the cellular identities, photoreceptors were electrically injected with neurobiotin, and the labeled cells were visualized by streptavidin-Alexa Fluor 488 conjugate and rhodamine phalloidin. B, Retinal [K+]o levels were measured with double-barrel K+-selective microelectrodes. The [K+]o level was increased by a 40-sec light stimulation in 1-day-old and 10-day-old control flies but not in ATPα knockdown flies. Dashed line: signal level in the dark. C, Comparison of basal [K+]o levels and the 40-sec light-stimulated [K+]o levels shows an increase in the retina between the control and ATPα knockdown flies. Asterisk (*): p< 0.01; Two-tailed t-test. Error bars denote S.E.M.
Because ATPα knockdown photoreceptors appeared to have equal K+ levels inside and outside the cell, we estimated their intracellular K+ level by measuring the extracellular level ([K+]o) in the retina using double-barrel K+ selective microprobes. In both 1-day-old and 10-day-old knockdown flies, the basal [K+]o was approximately 10 mM, which is close to that in control flies (Fig. 7B and C). However, the [K+]o in knockdown flies did not show a light-stimulated increase as observed in the controls. These results suggest that the K+ level in ATPα knockdown photoreceptors is as low as 10 mM.
Discussion
Na+/K+-ATPase activity establishes and maintains the characteristic transmembrane gradients of Na+ and K+, which underlie essentially all vertebrate and invertebrate cellular physiology. Although the importance of Na+/K+-ATPase to the function and survival of both neurons and non-excitable cells has been demonstrated by decades of pharmacological studies (Blanco and Mercer, 1998; Mobasheri et al., 2000), the involvement of neuronal Na+/K+ ATPase defects in neurodegeneration has yet to be demonstrated in vivo. To our knowledge, this cell-specific RNAi study reveals for the first time that Na+/K+-ATPase is essential for normal neuronal function of Drosophila photoreceptors. More importantly, this work provides in vivo evidence that links neuronal Na+/K+-ATPase deficiency to age-dependent neurodegeneration.
The importance of neuronal Na+/K+-ATPase to Drosophila visual function
Genetic studies on neuronal Na+/K+-ATPase function in the past decade, which were primarily based on the characterization of mice heterozygous for a mutation of the α3 subunit, have focused on central brain neurons (Clapcote et al., 2009; DeAndrade et al., 2011; Moseley et al., 2007; Shiina et al., 2010); however, the importance of functional Na+/K+-ATPase in sensory neurons remains largely unknown. Here, we show that knockdown of ATPα in Drosophila photoreceptor neurons abolishes their response to light, resulting in complete blindness in the fly. These results confirm the importance of Na+/K+-ATPase in animal sensory functions. The light response of Drosophila photoreceptors is mediated by cation influx (mostly Na+) through light-stimulated TRP channels (Hardie, 2001). Photoreceptors in ATPα knockdown flies were unresponsive to light for two reasons. First, without a sufficient K+ gradient across the cell membrane, the cell has no negative resting membrane potential and, thus, no electrical driving force for cation influx through TRP channels. Second, in the absence of Na+/K+-ATPase, photoreceptors may have already accumulated a high intracellular level of Na+ (Archibald and White, 1974) in the dark, which prevents extracellular Na+ flow into the cell through TRP channels during light stimulation. However, loss of the light response may not be attributed to morphological defects in the photoreceptor. First, temporally controlled knockdown of ATPα in the adult stage excludes the involvement of obvious developmental problems. Second, the light response of photoreceptors was abolished in 1-day-old ATPα knockdown flies despite the overall normal shape of the rhabdomeres. Based on these findings, we conclude that loss of the light response is independent of degeneration in ATPα knockdown photoreceptors.
Potential mechanisms underlying the neurodegeneration of ATPα knockdown photoreceptors
Drosophila photoreceptors have been used as a genetic model for retinal degeneration studies. Photoreceptor degeneration in many mutants is caused by defects in the regulation of the visual transduction cascade and is light-dependent (Kiselev et al., 2000; Wang and Montell, 2007). In ATPα knockdown photoreceptors, however, the visual cascade may not mediate or regulate degeneration because light deprivation does not change the severity of neurodegeneration in 10-day-old flies. Instead, the progressive degeneration in ATPα knockdown photoreceptors has demonstrated characteristics of apoptotic/necrosis hybrid cell death that are reminiscent of those observed in Na+/K+-ATPase-inhibited mammalian cells (Yu, 2003a). When Na+/K+-ATPase is inhibited by ouabain, mammalian cells undergo hybrid cell death, which has been attributed to a loss of intracellular K+ ions. In vitro studies suggest that the depletion of intracellular K+ may induce apoptosis or act as a necessary cofactor to promote apoptosis (Yu, 2003a, b; Yu and Choi, 2000). We estimate that the intracellular levels of K+ in ATPα knockdown photoreceptors could be as low as 10 mM, which is comparable to the 50–80% decrease in K+ observed in ouabain-treated mammalian cells (Nobel et al., 2000; Xiao et al., 2002). Thus, the low levels of intracellular K+ could have contributed to the degeneration of ATPα knockdown photoreceptors. Additionally, Ca2+ overload may also have a role in photoreceptor degeneration. The depolarization of the membrane potential in ATPα knockdown photoreceptors may activate voltage-gated Ca2+ channels and a reversed operation of the Na+-Ca2+ exchanger (Archibald and White, 1974; DiPolo and Beauge, 1991). Both activities will increase the intracellular Ca2+ concentration and could promote necrotic cell death (Choi, 1988). Finally, accumulation of Na+ inside ATPα knockdown photoreceptors impairs the driving force of nutrient import through the secondary membrane transporters, which may also play a role in cell degeneration. Hybrid cell death, an intermediate form of cell death falling along an apoptosis-necrosis continuum, can also be found in the neurodegeneration caused by excitotoxicity and ischemia (Martin et al., 1998; Yu, 2003a). Therefore, further studies on the role Na+/K+-ATPase in hybrid cell death will elucidate the mechanism of the cell death bearing both apoptotic and necrotic features in different neuropathological conditions.
Drosophila photoreceptors as a genetic model for the study of neuronal Na+/K+-ATPase deficiency-mediated neurodegeneration
Until now, most in vivo studies on Na+/K+-ATPase-related neurodegeneration have relied on either pharmacological agents (Bignami and Palladini, 1966; Lees and Leong, 1994) or Drosophila ATPα mutants (Palladino et al., 2003). Those studies have suggested that both dysregulation and deficiency of Na+/K+-ATPase lead to extensive neurodegeneration. However, the degeneration observed in those studies could be partially derived from defects in non-neuronal tissues and cells in the brain. For example, Na+/K+-ATPase in the blood-brain-barrier participates in the maintenance of water and ion homeostasis in the central nervous system (CNS) (Harik, 1986; Keep et al., 1999), which is critical for neuronal function and survival. In the Drosophila auditory organ (Johnston’s organ), Roy et al. found that knocking down ATPα in scolopale cells, principal support cells that enclose neuronal dendrites, results in neuronal dysfunction and complete deafness (Roy et al., 2013). Additionally, a defect of Na+/K+-ATPase in astrocytes could be responsible for neonatal seizures and spongiform encephalopathy (Renkawek et al., 1992). In common neurodegenerative disorders such as AD, PD and RPD, however, Na+/K+-ATPase is only reduced in specific subgroups of neurons (Cannon, 2004; Chauhan et al., 1997; de Carvalho Aguiar et al., 2004; Kumar and Kurup, 2002; McGrail et al., 1991). To better mimic the neuropathological conditions of neurodegenerative diseases to study this degeneration mechanism, it is necessary to specifically downregulate Na+/K+-ATPase in particular neurons to avoid perturbations in other cells.
Because homozygous mutations in the mouse α3 subunit of Na+/K+-ATPase cause neonatal lethality, genetic studies on neuronal isoforms of Na+/K+-ATPase have so far been primarily based on the characterization of heterozygous α3 mutants. Those mouse studies, however, have not revealed direct evidence of neurodegeneration in the brain most likely due to the relatively moderate reduction of Na+/K+-ATPase activity in the heterozygous mutants (Clapcote et al., 2009). Our in vivo model using Drosophila photoreceptors, which mimics the neuropathological conditions of those neurodegenerative disorders, could be a valuable tool for further investigating the mechanism of neuronal Na+/K+-ATPase deficiency-mediated neurodegeneration. In addition to the genetic tools for gene modulation (Brand and Perrimon, 1993; Dietzl et al., 2007; Roy et al., 2013), Drosophila utilizes simple assays such as ERG, phototaxis and optomotor responses, and the optical neutralization assay to evaluate both the function and morphology of photoreceptor neurons. Loss of Na+/K+-ATPase in photoreceptors does not change the environmental K+ levels, allowing us to study the cell-autonomous effects of neuronal Na+/K+-ATPase deficiency. Taking advantage of this simple and convenient neuronal model may allow us to identify the key players in Na+/K+-ATPase deficiency-mediated neurodegeneration, which will thereby guide us in the design of new therapeutic strategies for neurodegenerative disorders.
Herein, we have provided evidence that either dysregulation or deficiency of neuronal Na+/K+-ATPase causes abnormal depolarization of neurons by disrupting the intracellular ion balance instead of extracellular ion homeostasis, which leads to neuronal dysfunction and behavioral abnormality. Furthermore, disrupted neuronal Na+/K+-ATPase activity triggers progressive neurodegeneration. Therefore, our study suggests that early intervention against dysregulation or deficiency of neuronal Na+/K+-ATPase may alleviate the progression of neurodegenerative disorders.
Supplementary Material
Highlights.
The loss of neuronal Na+/K+-ATPase causes abnormal depolarization of neurons by disrupting the intracellular ion balance instead of extracellular ion homeostasis in vivo.
The loss of neuronal Na+/K+-ATPase activity leads to neuronal dysfunction and behavioral abnormality in vivo.
The loss of neuronal Na+/K+-ATPase activity triggers progressive neurodegeneration in vivo.
We are providing in vivo evidence that links neuronal Na+/K+-ATPase deficiency to neurodegeneration for the first time.
Acknowledgments
We thank BDRC, VDRC, TRiP and Developmental Studies Hybridoma Bank for fly stocks and antibodies. This work is supported by NIH grants R01AG022508 and R01EY021796 awarded to H.-S. L.
Nonstandard abbreviations
- AD
Alzheimer’s disease
- PD
Parkinson’s disease
- RDP
Rapid-onset dystonia Parkinsonism
- ERG
Electroretinograms
- GFS
Giant fiber system
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Archibald JT, White TD. Rapid reversal of internal Na+ and K+ contents of synaptosomes by ouabain. Nature. 1974;252:595–596. doi: 10.1038/252595a0. [DOI] [PubMed] [Google Scholar]
- Ashmore LJ, Hrizo SL, Paul SM, Van Voorhies WA, Beitel GJ, Palladino MJ. Novel mutations affecting the Na, K ATPase alpha model complex neurological diseases and implicate the sodium pump in increased longevity. Hum Genet. 2009;126:431–447. doi: 10.1007/s00439-009-0673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasaki T, Ito K. Engulfing action of glial cells is required for programmed axon pruning during Drosophila metamorphosis. Curr Biol. 2004;14:668–677. doi: 10.1016/j.cub.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Baumann O, Salvaterra PM, Takeyasu K. Developmental changes in beta-subunit composition of Na,K-ATPase in the Drosophila eye. Cell Tissue Res. 2010;340:215–228. doi: 10.1007/s00441-010-0948-x. [DOI] [PubMed] [Google Scholar]
- Benzer S. BEHAVIORAL MUTANTS OF Drosophila ISOLATED BY COUNTERCURRENT DISTRIBUTION. Proc Natl Acad Sci U S A. 1967;58:1112–1119. doi: 10.1073/pnas.58.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignami A, Palladini G. Experimentally produced cerebral status spongiosus and continuous pseudorhythmic electroencephalographic discharges with a membrane-ATPase inhibitor in the rat. Nature. 1966;209:413–414. doi: 10.1038/209413a0. [DOI] [PubMed] [Google Scholar]
- Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol. 1998;275:F633–650. doi: 10.1152/ajprenal.1998.275.5.F633. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Cagan RL, Ready DF. The emergence of order in the Drosophila pupal retina. Dev Biol. 1989;136:346–362. doi: 10.1016/0012-1606(89)90261-3. [DOI] [PubMed] [Google Scholar]
- Cannon SC. Paying the price at the pump: dystonia from mutations in a Na+/K+-ATPase. Neuron. 2004;43:153–154. doi: 10.1016/j.neuron.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Chauhan NB, Lee JM, Siegel GJ. Na,K-ATPase mRNA levels and plaque load in Alzheimer’s disease. J Mol Neurosci. 1997;9:151–166. doi: 10.1007/BF02800498. [DOI] [PubMed] [Google Scholar]
- Chen Y, Akin O, Nern A, Tsui CY, Pecot MY, Zipursky SL. Cell-type-specific labeling of synapses in vivo through synaptic tagging with recombination. Neuron. 2014;81:280–293. doi: 10.1016/j.neuron.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintapalli VR, Wang J, Dow JA. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nature Genetics. 2007;39:715–720. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- Choi DW. Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci. 1988;11:465–469. doi: 10.1016/0166-2236(88)90200-7. [DOI] [PubMed] [Google Scholar]
- Clapcote SJ, Duffy S, Xie G, Kirshenbaum G, Bechard AR, Schack VR, Petersen J, Sinai L, Saab BJ, Lerch JP. Mutation I810N in the α3 isoform of Na+, K+-ATPase causes impairments in the sodium pump and hyperexcitability in the CNS. Proceedings of the National Academy of Sciences. 2009;106:14085–14090. doi: 10.1073/pnas.0904817106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho Aguiar P, Sweadner KJ, Penniston JT, Zaremba J, Liu L, Caton M, Linazasoro G, Borg M, Tijssen MA, Bressman SB, Dobyns WB, Brashear A, Ozelius LJ. Mutations in the Na+/K+-ATPase alpha3 gene ATP1A3 are associated with rapid-onset dystonia parkinsonism. Neuron. 2004;43:169–175. doi: 10.1016/j.neuron.2004.06.028. [DOI] [PubMed] [Google Scholar]
- DeAndrade MP, Yokoi F, van Groen T, Lingrel JB, Li Y. Characterization of Atp1a3 mutant mice as a model of rapid-onset dystonia with parkinsonism. Behav Brain Res. 2011;216:659–665. doi: 10.1016/j.bbr.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, Couto A, Marra V, Keleman K, Dickson BJ. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- DiPolo R, Beauge L. Regulation of Na-Ca exchange. An overview. Ann N Y Acad Sci. 1991;639:100–111. doi: 10.1111/j.1749-6632.1991.tb17294.x. [DOI] [PubMed] [Google Scholar]
- Franceschini N, Kirschfeld K. Pseudopupil phenomena in the compound eye of drosophila. Kybernetik. 1971;9:159–182. doi: 10.1007/BF02215177. [DOI] [PubMed] [Google Scholar]
- Geering K. Na,K-ATPase. Curr Opin Nephrol Hypertens. 1997;6:434–439. doi: 10.1097/00041552-199709000-00005. [DOI] [PubMed] [Google Scholar]
- Gloor S, Antonicek H, Sweadner KJ, Pagliusi S, Frank R, Moos M, Schachner M. The adhesion molecule on glia (AMOG) is a homologue of the beta subunit of the Na,K-ATPase. J Cell Biol. 1990;110:165–174. doi: 10.1083/jcb.110.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie RC. Phototransduction in Drosophila melanogaster. J Exp Biol. 2001;204:3403–3409. doi: 10.1242/jeb.204.20.3403. [DOI] [PubMed] [Google Scholar]
- Harik SI. Blood--brain barrier sodium/potassium pump: modulation by central noradrenergic innervation. Proc Natl Acad Sci U S A. 1986;83:4067–4070. doi: 10.1073/pnas.83.11.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisberger JD. Recent insights into the structure and mechanism of the sodium pump. Physiology (Bethesda) 2004;19:377–387. doi: 10.1152/physiol.00013.2004. [DOI] [PubMed] [Google Scholar]
- Johnson EC, Pak WL. Electrophysiological study of Drosophila rhodopsin mutants. J Gen Physiol. 1986;88:651–673. doi: 10.1085/jgp.88.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JH. Biochemistry of Na,K-ATPase. Annu Rev Biochem. 2002;71:511–535. doi: 10.1146/annurev.biochem.71.102201.141218. [DOI] [PubMed] [Google Scholar]
- Keep RF, Ulanski LJ, 2nd, Xiang J, Ennis SR, Lorris Betz A. Blood-brain barrier mechanisms involved in brain calcium and potassium homeostasis. Brain research. 1999;815:200–205. doi: 10.1016/s0006-8993(98)01155-x. [DOI] [PubMed] [Google Scholar]
- Kiselev A, Socolich M, Vinos J, Hardy RW, Zuker CS, Ranganathan R. A molecular pathway for light-dependent photoreceptor apoptosis in Drosophila. Neuron. 2000;28:139–152. doi: 10.1016/s0896-6273(00)00092-1. [DOI] [PubMed] [Google Scholar]
- Kita H, Armstrong W. A biotin-containing compound N-(2-aminoethyl)biotinamide for intracellular labeling and neuronal tracing studies: comparison with biocytin. J Neurosci Methods. 1991;37:141–150. doi: 10.1016/0165-0270(91)90124-i. [DOI] [PubMed] [Google Scholar]
- Knust E. Photoreceptor morphogenesis and retinal degeneration: lessons from Drosophila. Curr Opin Neurobiol. 2007;17:541–547. doi: 10.1016/j.conb.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Kumar AR, Kurup PA. Endogenous sodium-potassium ATPase inhibition related biochemical cascade in trisomy 21 and Huntington’s disease: neural regulation of genomic function. Neurol India. 2002;50:174–180. [PubMed] [Google Scholar]
- Lebovitz RM, Takeyasu K, Fambrough DM. Molecular characterization and expression of the (Na+ + K+)-ATPase alpha-subunit in Drosophila melanogaster. Embo J. 1989;8:193–202. doi: 10.1002/j.1460-2075.1989.tb03364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees GJ, Leong W. Brain lesions induced by specific and non-specific inhibitors of sodium-potassium ATPase. Brain research. 1994;649:225–233. doi: 10.1016/0006-8993(94)91068-5. [DOI] [PubMed] [Google Scholar]
- Lessing D, Bonini NM. Maintaining the brain: insight into human neurodegeneration from Drosophila melanogaster mutants. Nat Rev Genet. 2009;10:359–370. doi: 10.1038/nrg2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HS, Montell C. TRP and the PDZ protein, INAD, form the core complex required for retention of the signalplex in Drosophila photoreceptor cells. J Cell Biol. 2000;150:1411–1422. doi: 10.1083/jcb.150.6.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Al-Abdulla NA, Brambrink AM, Kirsch JR, Sieber FE, Portera-Cailliau C. Neurodegeneration in excitotoxicity, global cerebral ischemia, and target deprivation: A perspective on the contributions of apoptosis and necrosis. Brain Res Bull. 1998;46:281–309. doi: 10.1016/s0361-9230(98)00024-0. [DOI] [PubMed] [Google Scholar]
- McGrail KM, Phillips JM, Sweadner KJ. Immunofluorescent localization of three Na,K-ATPase isozymes in the rat central nervous system: both neurons and glia can express more than one Na,K-ATPase. J Neurosci. 1991;11:381–391. doi: 10.1523/JNEUROSCI.11-02-00381.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire SE, Mao Z, Davis RL. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE. 2004;2004:pl6. doi: 10.1126/stke.2202004pl6. [DOI] [PubMed] [Google Scholar]
- McQuilton P, St Pierre SE, Thurmond J. FlyBase 101--the basics of navigating FlyBase. Nucleic Acids Res. 2012;40:D706–714. doi: 10.1093/nar/gkr1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinertzhagen IA, O’Neil SD. Synaptic organization of columnar elements in the lamina of the wild type in Drosophila melanogaster. J Comp Neurol. 1991;305:232–263. doi: 10.1002/cne.903050206. [DOI] [PubMed] [Google Scholar]
- Mobasheri A, Avila J, Cozar-Castellano I, Brownleader MD, Trevan M, Francis MJ, Lamb JF, Martin-Vasallo P. Na+, K+-ATPase isozyme diversity; comparative biochemistry and physiological implications of novel functional interactions. Biosci Rep. 2000;20:51–91. doi: 10.1023/a:1005580332144. [DOI] [PubMed] [Google Scholar]
- Moseley AE, Williams MT, Schaefer TL, Bohanan CS, Neumann JC, Behbehani MM, Vorhees CV, Lingrel JB. Deficiency in Na, K-ATPase α isoform genes alters spatial learning, motor activity, and anxiety in mice. The Journal of neuroscience. 2007;27:616–626. doi: 10.1523/JNEUROSCI.4464-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobel CS, Aronson JK, van den Dobbelsteen DJ, Slater AF. Inhibition of Na+/K(+)-ATPase may be one mechanism contributing to potassium efflux and cell shrinkage in CD95-induced apoptosis. Apoptosis. 2000;5:153–163. doi: 10.1023/a:1009684713784. [DOI] [PubMed] [Google Scholar]
- Okamura H, Yasuhara JC, Fambrough DM, Takeyasu K. P-type ATPases in Caenorhabditis and Drosophila: implications for evolution of the P-type ATPase subunit families with special reference to the Na,K-ATPase and H,K-ATPase subgroup. J Membr Biol. 2003;191:13–24. doi: 10.1007/s00232-002-1041-5. [DOI] [PubMed] [Google Scholar]
- Palladino MJ, Bower JE, Kreber R, Ganetzky B. Neural dysfunction and neurodegeneration in Drosophila Na+/K+ ATPase alpha subunit mutants. J Neurosci. 2003;23:1276–1286. doi: 10.1523/JNEUROSCI.23-04-01276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul SM, Palladino MJ, Beitel GJ. A pump-independent function of the Na,K-ATPase is required for epithelial junction function and tracheal tube-size control. Development. 2007;134:147–155. doi: 10.1242/dev.02710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov KV, Sokolov VS. Electrogenic ion transport by Na+,K+-ATPase. Membr Cell Biol. 2000;13:745–788. [PubMed] [Google Scholar]
- Pichaud F, Desplan C. A new visualization approach for identifying mutations that affect differentiation and organization of the Drosophila ommatidia. Development. 2001;128:815–826. doi: 10.1242/dev.128.6.815. [DOI] [PubMed] [Google Scholar]
- Rendahl KG, Jones KR, Kulkarni SJ, Bagully SH, Hall JC. The dissonance mutation at the no-on-transient-A locus of D. melanogaster: genetic control of courtship song and visual behaviors by a protein with putative RNA-binding motifs. J Neurosci. 1992;12:390–407. doi: 10.1523/JNEUROSCI.12-02-00390.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkawek K, Renier WO, de Pont JJ, Vogels OJ, Gabreels FJ. Neonatal status convulsivus, spongiform encephalopathy, and low activity of Na+/K(+)-ATPase in the brain. Epilepsia. 1992;33:58–64. doi: 10.1111/j.1528-1157.1992.tb02283.x. [DOI] [PubMed] [Google Scholar]
- Roy M, Sivan-Loukianova E, Eberl DF. Cell-type-specific roles of Na+/K+ ATPase subunits in Drosophila auditory mechanosensation. Proc Natl Acad Sci U S A. 2013;110:181–186. doi: 10.1073/pnas.1208866110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler C, Kirschfeld K. Light-Induced Extracellular Calcium and Sodium Concentration Changes in the Retina of Calliphora - Involvement in the Mechanism of Light Adaptation. Journal of Comparative Physiology a-Sensory Neural and Behavioral Physiology. 1991;169:299–311. [Google Scholar]
- Schnell B, Joesch M, Forstner F, Raghu SV, Otsuna H, Ito K, Borst A, Reiff DF. Processing of horizontal optic flow in three visual interneurons of the Drosophila brain. J Neurophysiol. 2010;103:1646–1657. doi: 10.1152/jn.00950.2009. [DOI] [PubMed] [Google Scholar]
- Semb SO, Amundsen B, Sejersted OM. A new improved way of making double-barrelled ion-selective micro-electrodes. Acta Physiol Scand. 1997;161:1–5. doi: 10.1046/j.1365-201X.1997.00178.x. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Barber TR, Xia H, Ready DF, Hardie RC. Depletion of PtdIns(4,5)P2 underlies retinal degeneration in Drosophila trp mutants. J Cell Sci. 2013;126:1247–1259. doi: 10.1242/jcs.120592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh BH. Molecular genetics of retinal degeneration: A Drosophila perspective. Fly (Austin) 2011;5:356–368. doi: 10.4161/fly.5.4.17809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiina N, Yamaguchi K, Tokunaga M. RNG105 deficiency impairs the dendritic localization of mRNAs for Na+/K+ ATPase subunit isoforms and leads to the degeneration of neuronal networks. The Journal of neuroscience. 2010;30:12816–12830. doi: 10.1523/JNEUROSCI.6386-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoshani L, Contreras RG, Roldan ML, Moreno J, Lazaro A, Balda MS, Matter K, Cereijido M. The polarized expression of Na+,K+-ATPase in epithelia depends on the association between beta-subunits located in neighboring cells. Mol Biol Cell. 2005;16:1071–1081. doi: 10.1091/mbc.E04-03-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeyasu K, Okamura H, Yasuhara JC, Ogita Y, Yoshimura SH. P-type ATPase diversity and evolution: the origins of ouabain sensitivity and subunit assembly. Cell Mol Biol (Noisy-le-grand) 2001;47:325–333. [PubMed] [Google Scholar]
- Takeyasu K, Tamkun MM, Renaud KJ, Fambrough DM. Ouabain-sensitive (Na+ + K+)-ATPase activity expressed in mouse L cells by transfection with DNA encoding the alpha-subunit of an avian sodium pump. J Biol Chem. 1988;263:4347–4354. [PubMed] [Google Scholar]
- Thomas JB, Wyman RJ. Mutations altering synaptic connectivity between identified neurons in Drosophila. J Neurosci. 1984;4:530–538. doi: 10.1523/JNEUROSCI.04-02-00530.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev K, Joly W, Hadjieconomou D, Salecker I. Localized netrins act as positional cues to control layer-specific targeting of photoreceptor axons in Drosophila. Neuron. 2012;75:80–93. doi: 10.1016/j.neuron.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagin O, Turdikulova S, Sachs G. Recombinant addition of N-glycosylation sites to the basolateral Na,K-ATPase beta1 subunit results in its clustering in caveolae and apical sorting in HGT-1 cells. J Biol Chem. 2005;280:43159–43167. doi: 10.1074/jbc.M508262200. [DOI] [PubMed] [Google Scholar]
- Velentzas PD, Velentzas AD, Pantazi AD, Mpakou VE, Zervas CG, Papassideri IS, Stravopodis DJ. Proteasome, but not autophagy, disruption results in severe eye and wing dysmorphia: a subunit- and regulator-dependent process in Drosophila. PLoS ONE. 2013;8:e80530. doi: 10.1371/journal.pone.0080530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Smith NA, Xu Q, Fujita T, Baba A, Matsuda T, Takano T, Bekar L, Nedergaard M. Astrocytes modulate neural network activity by Ca(2)+-dependent uptake of extracellular K+ Sci Signal. 2012;5:ra26. doi: 10.1126/scisignal.2002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Montell C. Phototransduction and retinal degeneration in Drosophila. Pflugers Arch. 2007;454:821–847. doi: 10.1007/s00424-007-0251-1. [DOI] [PubMed] [Google Scholar]
- Watts AG, Sanchez-Watts G, Emanuel JR, Levenson R. Cell-specific expression of mRNAs encoding Na+, K (+)-ATPase alpha-and beta-subunit isoforms within the rat central nervous system. Proceedings of the National Academy of Sciences. 1991;88:7425–7429. doi: 10.1073/pnas.88.16.7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernet MF, Labhart T, Baumann F, Mazzoni EO, Pichaud F, Desplan C. Homothorax switches function of Drosophila photoreceptors from color to polarized light sensors. Cell. 2003;115:267–279. doi: 10.1016/s0092-8674(03)00848-1. [DOI] [PubMed] [Google Scholar]
- Xiao AY, Wei L, Xia S, Rothman S, Yu SP. Ionic mechanism of ouabain-induced concurrent apoptosis and necrosis in individual cultured cortical neurons. J Neurosci. 2002;22:1350–1362. doi: 10.1523/JNEUROSCI.22-04-01350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuhara JC, Baumann O, Takeyasu K. Localization of Na/K-ATPase in developing and adult Drosophila melanogaster photoreceptors. Cell Tissue Res. 2000;300:239–249. doi: 10.1007/s004410000195. [DOI] [PubMed] [Google Scholar]
- Yu SP. Na(+), K(+)-ATPase: the new face of an old player in pathogenesis and apoptotic/hybrid cell death. Biochem Pharmacol. 2003a;66:1601–1609. doi: 10.1016/s0006-2952(03)00531-8. [DOI] [PubMed] [Google Scholar]
- Yu SP. Regulation and critical role of potassium homeostasis in apoptosis. Prog Neurobiol. 2003b;70:363–386. doi: 10.1016/s0301-0082(03)00090-x. [DOI] [PubMed] [Google Scholar]
- Yu SP, Choi DW. Ions, cell volume, and apoptosis. Proc Natl Acad Sci U S A. 2000;97:9360–9362. doi: 10.1073/pnas.97.17.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan XL, Clemens JC, Neves G, Hattori D, Flanagan JJ, Hummel T, Vasconcelos ML, Chess A, Zipursky SL. Analysis of Dscam diversity in regulating axon guidance in Drosophila mushroom bodies. Neuron. 2004;43:673–686. doi: 10.1016/j.neuron.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Nern A, Zipursky SL, Frye MA. Peripheral visual circuits functionally segregate motion and phototaxis behaviors in the fly. Curr Biol. 2009;19:613–619. doi: 10.1016/j.cub.2009.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.