Abstract
Hypoxic-ischemic (HI) brain injury is frequently associated with premature and/or full term birth related complications. HI injury often results in learning and processing deficits that reflect widespread damage to an extensive range of cortical and sub-cortical brain structures. Further, inflammation has been implicated in the long-term progression and severity of HI injury. Recently, Inter-alpha Inhibitor Proteins (IAIPs) have been shown to attenuate inflammation in models of systemic infection. Importantly, preclinical studies of neonatal HI injury and neuroprotection often focus on single time windows of assessment or single behavioral domains. This approach limits translational validity, given evidence for a diverse spectrum of neurobehavioral deficits that may change across developmental windows following neonatal brain injury. Therefore, the aims of this research were to assess the effects of human IAIPs on early neocortical cell death (72 hours post insult), adult regional brain volume measurements (cerebral cortex, hippocampus, striatum, corpus callosum) and long-term behavioral outcomes in juvenile (P38-50) and adult (P80+) periods across two independent learning domains (spatial and non-spatial learning), after postnatal day 7 HI injury in rats. Here, for the first time, we show that IAIPs reduce acute neocortical neuronal cell death and improve brain weight outcome 72 hours following HI injury in the neonatal rat. Further, these longitudinal studies are the first to show age, task and treatment dependent improvements in behavioral outcome for both spatial and non-spatial learning following systemic administration of IAIPs in neonatal HI injured rats. Finally, results also show sparing of brain regions critical for spatial and non-spatial learning in adult animals treated with IAIPs at the time of injury onset. These data support the proposal that Inter-alpha Inhibitor Proteins may serve as novel therapeutics for brain injury associated with premature birth and/or neonatal brain injury and highlight the importance of assessing multiple ages, brain regions and behavioral domains when investigating experimental treatment efficacy.
Keywords: neonatal brain injury, spatial and non-spatial learning, rodent model, brain volume estimation, neuronal cell death, water maze
Introduction
Premature and full term infants with perinatal complications including umbilical cord prolapse, uterine rupture, and abruptio placenta, etc., are at a high risk for cardiopulmonary perturbations that are associated with hypoxia and/or cerebral ischemia (Huang and Castillo, 2008, Volpe, 2008, Volpe, 2009). Hypoxic/ischemic (HI) injury can result in impaired neurological and behavioral outcomes as these insults can affect numerous brain structures (Hossain, 2008, Huang and Castillo, 2008, Volpe, 2009). HI-related behavioral deficits can include abnormalities in multiple learning domains including spatial, non-spatial reference learning and working memory (Conklin, et al., 2008, Hagberg, et al., 2002, Luu, et al., 2011, McClure, et al., 2007, Ortiz-Mantilla, et al., 2008, Rose and Feldman, 1996, Rose, et al., 2005, Woodward, et al., 2005). These potential deficits could contribute to the frequently observed learning disabilities later in life (Conklin, et al., 2008, Luu, et al., 2011, McClure, et al., 2007, Ortiz-Mantilla, et al., 2008, Woodward, et al., 2005). Long-term follow up studies suggest that more than 50% of infants at risk for HI injury develop some form of learning disability with more severe cases leading to cognitive delay and/or cerebral palsy (Marlow, et al., 2007, Volpe, 2008, Volpe, 2009, Wolke, et al., 2008, Wood, et al., 2005).
Preterm and full term infants can both develop brain injury that results in neurotoxicity (Ballabh, 2010, Ballabh, 2014, Volpe, 2001, Volpe, 2008, Volpe, 2009). Excitotoxicity and inflammation leading to neuronal cell death can extend from days to weeks following perinatal brain damage (Ferriero, 2004, Hagberg, et al., 2002, Rosen, et al., 2006, Volpe, 2008, Volpe, 2009). Elevated pro-inflammatory cytokines are major contributors to the onset of inflammation in neonatal HI injury, which may cause and/or accentuate neuronal pathology predisposing neonates to poor neurobehavioral outcomes (Grether, et al., 2003, Leviton, et al., 2011, Nelson, et al., 1998, Szaflarski, et al., 1995, Yoon, et al., 1997, Yoon, et al., 1997, Yoon, et al., 1997).
The only currently approved therapy to attenuate brain damage in infants is hypothermia (Gluckman, et al., 2006, Gunn, et al., 1997, Higgins, et al., 2006, Shankaran, et al., 2005). This therapeutic strategy is only approved for use in full term infants after hypoxic-ischemic encephalopathy (HIE) and is only partially protective (Gluckman, et al., 2006, Gunn, et al., 1997, Higgins, et al., 2011, Shankaran, et al., 2005, Shankaran, et al., 2012). Consequently, there is an urgent need for additional therapeutic strategies as potential adjunctive treatments to the currently available hypothermia regimen for full term infants with HIE and as a primary treatment strategy for preterm infants exposed to HI. Given the critical role of inflammation in prolonging cell death after neonatal HI, successful treatment strategies to improve neuronal survival and long-term behavioral outcomes will most likely require targeting some aspects of the inflammatory cascade (Ferriero, 2004).
Inter-alpha Inhibitor Proteins (IAIPs) are a family of naturally derived structurally related proteins found in high concentrations in human plasma that play an important role in inflammatory regulation and wound healing (Bost, et al., 1998, Businaro, et al., 1992, Fries and Blom, 2000, Kobayashi, 2006, Lim, 2013, Salier, et al., 1996, Zhuo and Kimata, 2008). The major forms found in plasma are Inter-alpha Inhibitor (IaI), which consists of two heavy chains (H1 & H2) and a single light chain (LC), and Pre-alpha Inhibitor (PaI) consisting of one heavy (H3) and one light chain (LC) (Fries and Blom, 2000). IAIPs are novel anti-inflammatory molecules that broadly inhibit destructive serine proteases, block pro-inflammatory cytokines, augment anti-inflammatory cytokine production, block complement activation during systemic inflammation and improve survival after sepsis in adults and neonates (Chaaban, et al., 2009, Fries and Blom, 2000, Fries and Kaczmarczyk, 2003, Kanayama, et al., 1996, Okroj, et al., 2012, Singh, et al., 2010). Recent findings show that IAIPs are reduced during sepsis (Chaaban, et al., 2009) and necrotizing enterocolitis (NEC) (Chaaban, et al., 2010 in premature infants (Chaaban, et al., 2010) suggesting that these proteins are consumed during inflammatory processes. In addition, both of these disorders are associated with increases in the incidence of brain injury in preterm infants (Shah, et al., 2008, Stoll, et al., 2004). Therefore, these findings suggest the interesting potential that sepsis and NEC related decreases in IAIPs (Chaaban, et al., 2010, Chaaban, et al., 2009) may be associated with the development of inflammation-related brain injury in preterm infants (Shah, et al., 2008, Stoll, et al., 2004).
In addition to the systemic link between IAIPs and inflammatory regulation following infection, IAIP related molecules and mRNA have been observed in neurons, astrocytes, and meningeal cells of the brain (Businaro, et al., 1992, Chan, et al., 1995, Daveau, et al., 1998, Kato, et al., 2001, Mizushima, et al., 1998, Sanchez, et al., 2002, Takano, et al., 1999, Werbowetski-Ogilvie, et al., 2006, Yoshida, et al., 1991). Recent evidence also demonstrates decreases in the Inter-alpha-Trypsin Inhibitor Heavy Chain 4 in the serum of human patients after acute ischemic stroke (Kashyap, et al., 2009). Although there is no information on the effects of the complex form of the IAIPs in brain injury, the light chain of IAIPs (also called bikunin), which are isolated from human urine, may have neuroprotective properties against ischemic stroke in adult rats (Yano, et al., 2003) and protect oligodendrocytes and promote re-myelination in an experimental autoimmune encephalitis model in adult rats (Shu, et al., 2011). Nonetheless, information is not available on the potential neuroprotective efficacy of the complex form of IAIPs on brain injury in any age group or species.
Importantly, much of the work evaluating effects of neuroprotective strategies on neuroanatomical and behavioral outcomes after brain injury has focused primarily on single maturational time points or cognitive/motor domains to determine treatment efficacy (Fisher, et al., 2009). Such an approach restricts the translational validity of experimental studies during development. For example, clinical behavioral profiles frequently reflect pathology in multiple processing and learning domains that vary depending upon the stage of development (Conklin, et al., 2008, Hagberg, et al., 2002, Luu, et al., 2011, McClure, et al., 2006, Ortiz-Mantilla, et al., 2008, Woodward, et al., 2005). In addition, neurobehavioral outcomes after brain injury in humans and animal models can vary depending on injury progression, timing of behavioral assessment after initial injury, and the behavioral domains examined (e.g., motor, working memory, spatial learning, non-spatial learning and/or sensory processing (Back, et al., 2002, Ferriero, 2004, Fitch, et al., 2013, Friedman, et al., 2004, Threlkeld, et al., 2009, Threlkeld, et al., 2006)). In order to understand the extent of possible neuroprotection afforded by IAIPs, comprehensive assessments across multiple learning domains, brain regions and ages post-insult are essential for understanding the short and long-term effects of these molecules following developmental brain injury (Fitch, et al., 2013, Friedman, et al., 2004, Threlkeld, et al., 2009, Threlkeld, et al., 2006). Based upon the above considerations, we hypothesized that human IAIPs would attenuate early neocortical cell death, preserve adult regional brain volumes, and improve longitudinal behavioral outcomes in juvenile (postnatal day (P) 38–50) and adult (P80+) subjects across two independent learning domains (spatial and non-spatial learning), following HI exposure in neonatal rats.
Material and methods
Animals and Surgical Treatment
Subjects were 67 male Wistar rats born to time-mated dams (Charles River Laboratories; Wilmington, MA) at Rhode Island College. Animals were housed using a 12-hour light/dark cycle with food and water available ad libitum. On postnatal day one (P1), pups were culled into litters of eight males and two females to control for litter size and sex ratio. On P7, male subjects were randomly assigned to one of three groups: Sham, hypoxia-ischemic vehicle treated and hypoxia-ischemic IAIP treated, hereafter designated as sham, HI+Vehicle and HI+IAIP, respectively. Male subjects were assessed, given prior evidence of behavioral deficits in male but not female rodents with neonatal brain injury (Hill, et al., 2011, Peiffer, et al., 2004) – findings that parallel higher diagnostic rates of neurodevelopmental disorders (e.g., dyslexia, epilepsy, autism and intellectual disability) in human males as compared to females (Liederman, et al., 2005, Raz, et al., 1995, Rutter, 2003).
Subjects were weighed and anesthetized using 3% isoflurane and maintained with 1% during the surgical procedure. Methods for HI brain injury in neonatal rodents have been extensively described elsewhere (McClure, et al., 2006, Rice, et al., 1981). Briefly, following a 1 cm midline incision of the neck, the right common carotid artery (RCCA) was located and completely cauterized [see (McClure, et al., 2006) for further details)]. The pups were sutured and labeled with paw ink injections for identification. Sham subjects underwent identical surgical procedures without cauterization of the RCCA. Body temperature was maintained at 37°C preoperatively, during surgery and during postoperative recovery. After surgery, the pups were returned to their dams and allowed to feed for 2–3 hours before exposure to hypoxia. All procedures were performed according to the National Institutes of Health guide for the care and use of laboratory animals and approved by the Rhode Island College Institutional Animal Care and Use Committee.
Production and purification of IAIPs
IAIPs were extracted from fresh frozen human plasma (Rhode Island Blood Center, RI). The purification process used a monolithic anion-exchange chromatographic method (CIMmultus, BIA Separation, Villach, Austria) that resulted in highly (>90%) pure biologically active IAIPs (US Patent #7,932,365, 2011) (Lim, 2013, Opal, et al., 2011, Spasova, et al., 2014). Following binding, the column was washed sequentially with a buffer containing 200 mM NaCl and 200 mM acetate buffer, pH 3.0. IAIPs were eluted from the column using a buffer containing 750 mM NaCl. Eluted IAIPs were concentrated and buffer exchanged using a tangential flow filtration system (Labscale, Millipore). Purity analysis was performed by SDS-PAGE, Western immunoblot, competitive IAIP ELISA and standardized in-vitro trypsin inhibition assay (Lim, 2013, Opal, et al., 2011, Spasova, et al., 2014). The biological activity is based on the ability of IAIPs to inhibit the hydrolysis of the substrate N-Benzoyl-L-arginine)-p-nitroaniline HCl (BAPNA, Sigma St. Louis, MO) by trypsin.
Experiments
This study consists of two separate experiments as described below.
Experiment 1: Short-term survival for cortical cell death and brain weight analyses
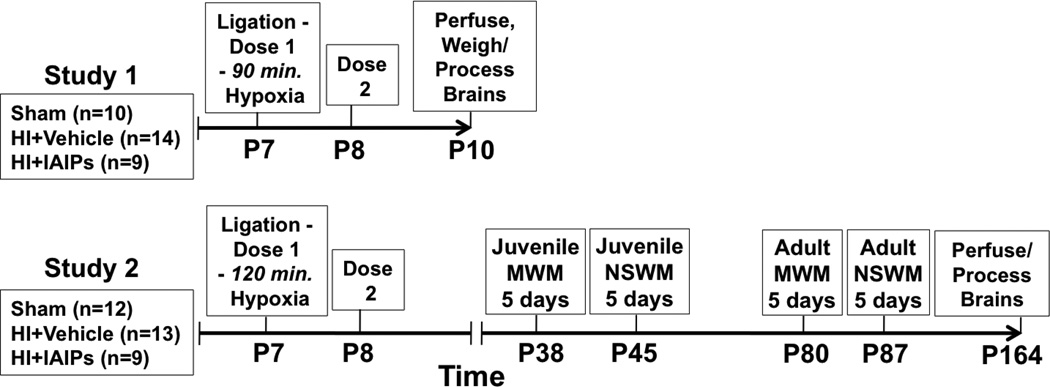
After recovery from surgery, as described above (Animals), subjects were removed from their home cage and received an intraperitoneal (IP) injection of either 30 mg/kg of human IAIP (HI+IAIP, ProThera biologics, East Providence, RI) or placebo (0.9% NaCl vehicle; sham and HI). This dose of IAIP was selected based upon studies showing that the same dose of IAIP reduced the incidence of death from sepsis in neonatal and adult rats (Opal, et al., 2011, Singh, et al., 2010). After the IP injections, the HI groups were placed into an acrylic hypoxia chamber and exposed to humidified 8% O2 and 92 % N2 for 90 minutes as shown in Fig.1. Sham subjects received identical treatment but were maintained in a separate container exposed to room air for 90 minutes and received 0.9% NaCl vehicle injections. A second dose of treatment (IAIP) or vehicle was administered 24 hours after hypoxia. The total number of neonatal rats examined in experiment 1 was: Sham, n=10, HI+Vehicle, n=14, HI+IAIPs, n=9.
Fig. 1.
Study 1 timeline showing age of injury, treatment timing and age at sacrifice. Study 2 timeline showing age of injury, treatment timing and order of juvenile spatial (MWM) and non-spatial (NSWM) water maze testing and age at sacrifice.
Experiment 2: Long-term survival/behavioral assessment and adult histopathology
The surgical procedures, doses, and timing of IAIP or vehicle administration were identical to those described above for experiment 1. However, subjects in experiment 2 were exposed to hypoxia for 120 minutes based upon our previous findings showing robust learning deficits after this duration of HI in neonatal rats (Hill, et al., 2011, McClure, et al., 2006). The total number of neonatal rats examined in experiment 2 was: Sham, n=12, HI+Vehicle, n=13, HI+IAIPs, n=9. The study timeline for experiment 2 is shown in Fig. 1.
Experiment 1: Histopathology on neonatal rats
Seventy-two hours after the induction of HI at P7, subjects were weighed, deeply anesthetized with pentobarbital 100 mg/kg, perfused with 5 mL phosphate buffered saline (PBS) at 4°C, and fixed with 5 mL of 4% paraformaldehyde. Brains were carefully extracted and weighed prior to paraffin embedding. Embedded brains were sectioned at 30 µm in the coronal plane and mounted on gelatin coated glass slides. One series of every 20th section was stained with Fluro jade B (FJB). FJB is an anionic fluorescent stain used to identify degenerating neurons (Rosen, et al., 2006, Schmued and Hopkins, 2000). Our protocol followed methods described by Rosen et al. (Rosen, et al., 2006). Paraffin embedded samples were submerged in three-15 minute changes of xylene followed by a 5 minute bath of 100% ethanol. Samples were then moved to 1% sodium hydroxide/ethanol solution for 5 minutes, followed by 2 minutes in 70% ethanol. Samples were rinsed using deionized water (dH2O) for 2 minutes and then agitated on a shaker in 0.06% KMnO4 for 10 minutes. Sections were once again rinsed with dH2O before staining in FJB working solution (0.004%) for 20 minutes. Tissue was then rinsed three times for 2 minute each in dH2O, allowed to dry on a slide warmer, cleaned in xylene, mounted and cover slipped using DPX mounting media.
The number of FJB labeled profiles in the right cerebral cortices was assessed in a treatment blind manner using digital image analysis software (Fractionator probe, Stereo Investigator, Burlington, VT). Three serial sections of right cerebral cortex (ipsilateral to RCC ligation) from each subject, ~600µm apart, were traced under 10x magnification. An automated stage paired with Stereoinvestigator software was used to systematically step through adjacent counting frames (Fractionator probe) at 40x magnification. Every FJB labeled profile was marked within each counting frame with a circular digital counting tool set to a scaled 2 µm diameter. Profiles smaller than 2 µm in diameter were not included as they were considered cell fragments. FJB also stains vascular elements, which also were excluded from analysis.
Experiment 2: Water escape, long-term spatial and non-spatial learning
Methods used to assess spatial and non-spatial learning have been extensively reviewed elsewhere [for review see, (Hyde, et al., 2002, Threlkeld, et al., 2012)]. All subjects were first tested on a water escape task as a motor, visual, and motivation control. The water escape task involved the use of a visible platform (20.3 cm diameter) placed at one end of a circular tub (122 cm) filled with water (20 cm) at room temperature, 22 °C. Subjects were released in the opposite end of the tub from the platform, and the latency to reach the platform was recorded. Behavioral testing began on P38, which is considered the juvenile period. Subjects received spatial testing first, for five days, followed by a two-day break and five days of non-spatial testing. Subjects were retested at P84+ in adulthood first on the spatial task and then on the non-spatial task in the following week to assess age related changes in learning performance in the presence of HI injury after having received IAIP or vehicle treatment as neonates on P7. Briefly, testing was conducted in a round 122 cm diameter tub filled with 22 °C water with a 20.3 cm diameter invisible submerged platform, consistently placed in the southeast (SE) quadrant, 2 cm below the water surface. Fixed, extra-maze cues were abundant (computer, sink, door, table), while precaution was taken to eliminate intra-maze cues. On each of five testing days, subjects underwent four trials, with each trial starting from a different randomly selected compass point (N, S, E, W). On day one, trial one, each subject was placed on the platform for 10 seconds, removed from the platform and then released from one of the starting locations. Each trial had a maximum duration of 45 seconds. Subjects unable to reach the platform within this time were guided to the target and remained there for 5 seconds. The latency to reach the platform for each trial was recorded.
The non-spatial Morris water maze (MWM) has been used to test non-spatial reference learning (e.g., the ability to consistently locate a hidden platform, using intra-maze visual cues that are independent of extra-maze space) (Hyde, et al., 2002, McClure, et al., 2006, Stavnezer, et al., 2002, Threlkeld, et al., 2012). Testing took place in the same 122 cm diameter tub as the spatial MWM task, with the submerged 20.3 cm diameter platform located 2 cm below the water surface. However, the non-spatial maze included four vinyl inserts characterized by four black/white complex visual stimuli, which acted as intra-maze cues for each quadrant of the outer maze wall; See (Threlkeld, et al., 2012) for details). Further, the non-spatial maze was isolated from external cues using black curtains, which eliminated interference from familiar extra-maze spatial cues used for navigation in the MWM. The intra-maze patterns consisted of black/white vertical stripes; black/white horizontal stripes; white panel with 10 cm black dots and a black panel with 10cm white dots. The cues were secured to the inside wall of the maze [for more details, see (Stavnezer, et al., 2002, Threlkeld, et al., 2012)]. The platform location was always paired with the vertical lines for each subject, such that learning required an association between the target intra-maze stimulus (i.e., vertical lines) and the platform, irrespective of extra-maze space. Therefore, on the four trials presented each day the platform and its associated cue (vertical stripes) were located in a new quadrant. Subjects were released from the same location on all trials, across five days of testing. Latency to reach the platform was recorded for each trial. All other testing parameters were similar to the spatial version of the MWM (number of trials, testing days, and length of time each subject was left on the platform).
Experiment 2: Histopathology for adult rats
On P164, adult subjects, used for in behavioral testing, were weighed anesthetized with 100 mg/kg of pentobarbital, transcardially perfused with 200 ml of PBS at 4 °C, and 200 ml of 10% formalin. All brains were removed after sacrifice and submerged in 10% formalin until tissue sectioning. Brains were then embedded in 3% agarose gel using a standard agarose embedding protocol. Each brain was sectioned using a vibrating microtome (Leica Microsystems, Richmond, Illinois) at 70 µm in the coronal plane. Every fifth section was mounted on glass slides in preparation for staining and volume analysis. Sections were stained with 1% thionine following rehydration in a series of alcohol gradients using a standard thionine staining protocol. Samples were then cover-slipped using DPX mounting media in preparation for analysis.
Images of each of the right hemispheres of the hippocampus, cerebral cortex, striatum, and corpus callosum were captured using a Q-imaging camera on an Olympus BX-53 bright-field microscope (Olympus Corporation, Central Valley, PA). Volumes of the hippocampus, striatum, cerebral cortex, and corpus callosum were measured with ImageJ using a grid overlay and point counting procedure (Rosen and Harry, 1990, Threlkeld, et al., 2009). The boundaries of each structure were defined using a stereotaxic rat brain atlas (Paxinos, 2004). Cavalieri’s estimator of volume equation was used to calculate volume estimates of each brain region. Cavaleiri’s rule is widely recognized as an unbiased estimator of volume derived from serial section reconstruction (Rosen and Harry, 1990).
Statistical analysis
Experiment 1: One-way analysis of variance (ANOVA) was used to assess group differences in brain weight. Repeated measures analysis of variance (RMANOVA) was used to compare FJB labeled profiles for three serial sections of cerebral cortex ipsilateral to ligation across treatment groups. In experiment 1, we used a one-tail test to evaluate changes in the FJB counts based upon the fact that HI injury has a unidirectional effect on brain injury consistent with our previous work (Hill, et al., 2011).
Experiment 2: Main effects were analyzed using repeated measures and oneway ANOVAs. Tukey’s Honest Significant Difference (HSD) test was used to examine simple effects between treatment groups. Correlation analysis was also performed between composite performance on juvenile/adult learning measures and adult histological volume measures. All results were expressed as means ± standard error of the mean (SEM). P<0.05 was considered statistically significant.
Results
IAIP reduces cell death and spares brain weight in neonatal HI rats
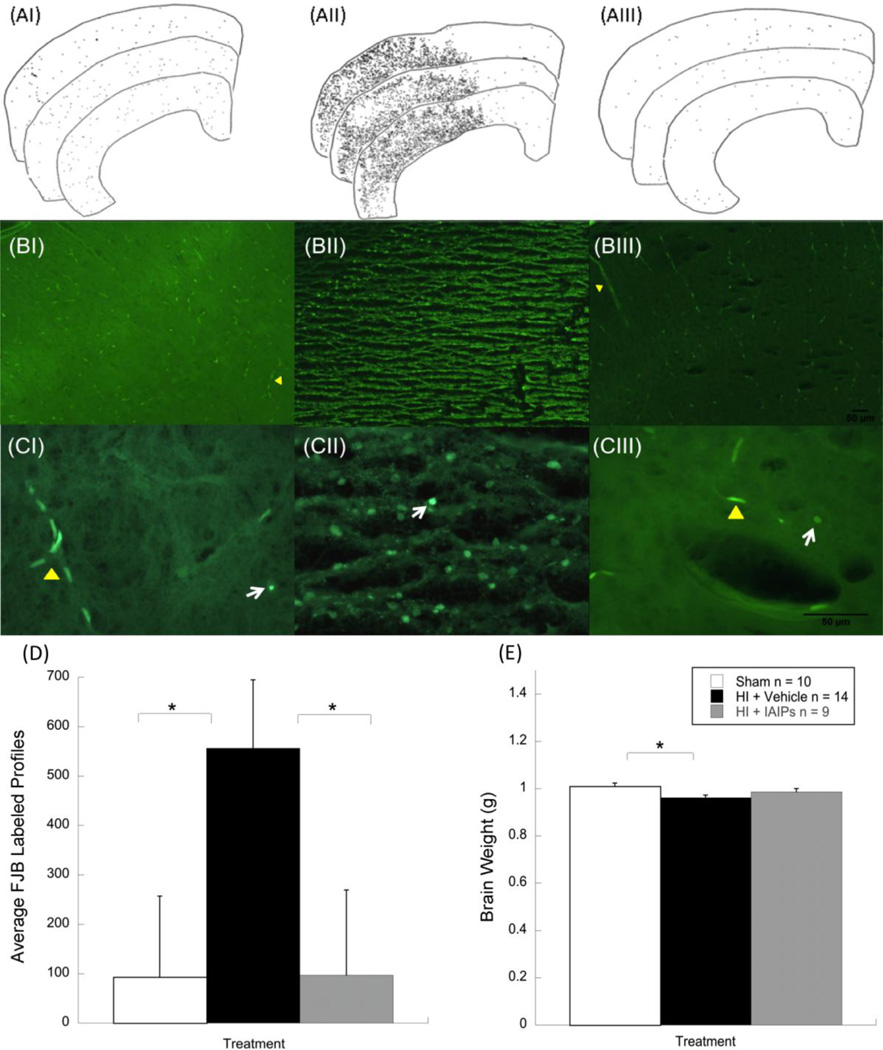
Results for cell death analysis 72 hours after HI injury revealed a significant effect of treatment (Sham n = 10, HI+Vehicle n = 14, HI+IAIP n = 9) on the number of FJB positive cells labeled in sections of cerebral cortex ipsilateral to the ligation [F (2, 30) = 3.209, p = 0.0275, one tail]. Results indicate that HI injury leads to a marked increase in FJB positive cells as compared to sham subjects (p = 0.019, one tail). In contrast, animals treated with 30 mg/kg IAIP prior to hypoxia and 24 hours after injury showed significantly fewer FJB labeled profiles as compared to untreated HI subjects (p = 0.023, one tail). In fact, IAIP treated subjects showed similar labeling to sham subjects suggesting a robust ability of IAIP to reduce cell death in cerebral cortex 72 hours post HI injury (see Fig. 2).
Fig. 2.
Line tracings from right cerebral cortices showing FJB positive cell distributions across three serial sections from representative sham (AI) HI+vehicle (AII) and HI+IAIP (AIII) subjects. Representative photomicrographs showing FJB positive cells from sham (BI) HI+vehicle (BII) and HI+IAIP (BIII) subjects at 10x and 40x (C) magnification. White arrows indicate FJB positive cells. Yellow markers indicate vascular elements. Histograms showing FJB labeled cells (D) and brain weight (E) differences across the three treatment groups. Comparisons show an effect of HI injury between HI+vehicle and sham subjects and an effect of treatment between HI+vehicle and HI+IAIP subjects (*P < 0.05, one tail). Brain weight comparisons show an effect of injury between sham and HI+vehicle animals (*P < 0.05).
Results for brain weight, using one-way analysis of variance (ANOVA), revealed a significant effect of treatment [F (2, 27) = 3.66, p < 0.05]. Post hoc analysis showed that neonatal HI injury resulted in significant reductions in brain weight 72 hours after exposure to the HI insult as compared to sham control treated subjects (p < 0.05). No significant brain weight differences were observed between sham control treated and IAIP treated neonatal HI rats.
IAIP improves spatial and non-spatial water maze performance in an age dependent manner after neonatal HI injury
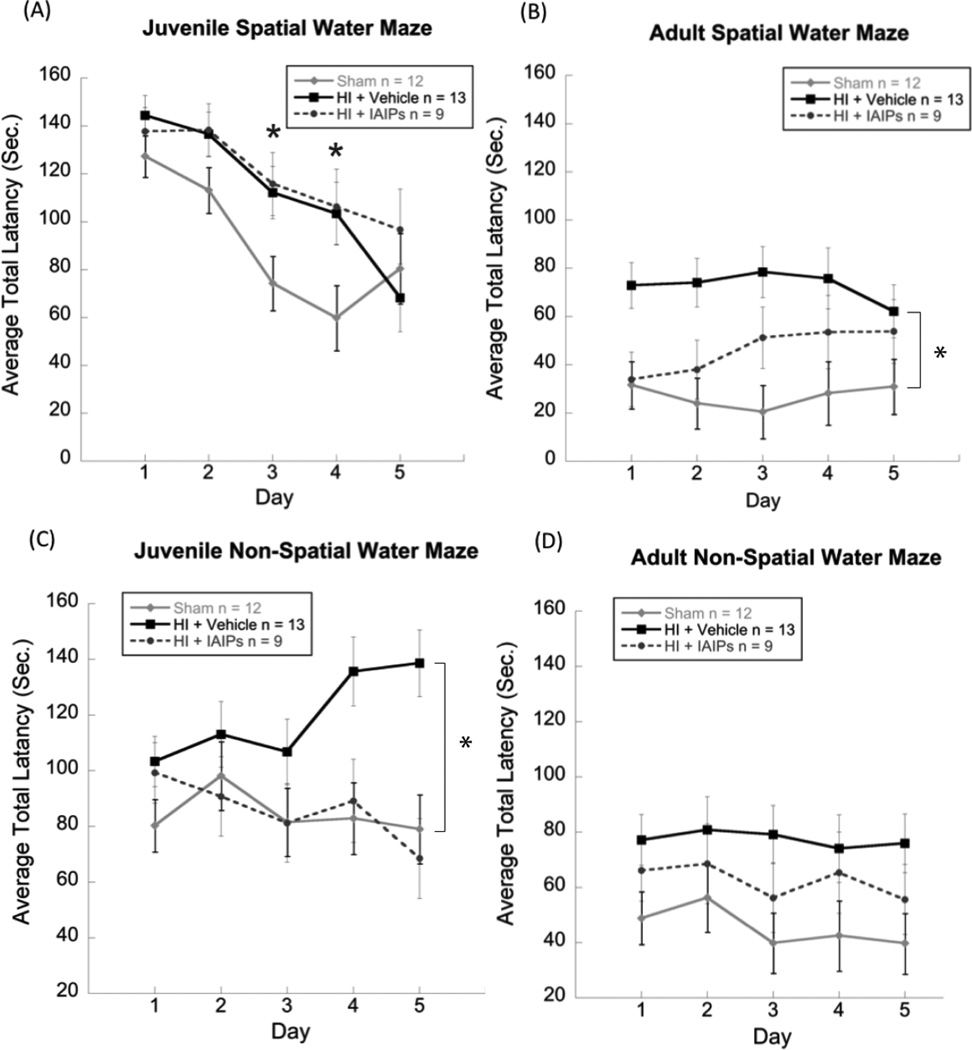
No group differences were observed for the water escape task, indicating comparable performance in swimming skill and finding a visible platform. For the MWM (spatial) task an overall 3 [(Treatment; Sham n=12, HI + IAIP n=9 and HI + Vehicle n=13) × 2 (Age; Juvenile (P38) and Adult (P80+) × 5 (Day) repeated measures ANOVA revealed a main effect of Treatment [F (2,31)= 4.69, p< 0.05], with HI subjects taking longer to locate the platform as compared to sham treated controls across age. Post hoc analyses of treatment across the two age conditions using Tukey’s HSD test revealed a significant difference between HI + Vehicle and sham subjects (p < 0.05). No overall across age difference was seen between HI + IAIP subjects and sham treated controls on the MWM. Further, overall main effects of Age [F (1, 31) = 122.76, p < 0.001] and Day [F (4, 124) = 11.259, p <0.001] were observed, indicating improved performance for adult as compared to juvenile subjects and improved performance from day one to day five of testing across both testing ages, respectively. Simple effects analyses using one-way ANOVAs for each day of juvenile testing revealed effects of treatment on day three [F (2, 31) = 3.901, p < 0.05 and day four [F (2, 31) = 3.509, p < 0.05] indicating worse performance for both injury groups as compared to sham treated controls. In contrast, an overall repeated measures ANOVA for adult MWM testing revealed a main effect of treatment [F (2, 31) = 5.532, p < 0.01] with Tukey’s HSD analysis showing a significant difference only between HI + Vehicle and sham treated control subjects (p < 0.05). These results indicate that spatial learning deficits seen in juvenile subjects persist into adulthood only in the untreated HI injured group (HI + vehicle), suggesting that IAIP treatment protects against spatial learning deficits in an age dependent manner.
For non-spatial water maze (NSWM) testing, an overall 3 (Treatment; Sham n=12, HI + IAIP n=9 and HI + Vehicle n=13) × 2 (Age; Juvenile (P38) and Adult (80+)) × 5 (Day) repeated measures ANOVA revealed a main effect of Treatment [F (2, 31) = 4.13, p <0.05], with HI subjects taking longer to locate the platform as compared to sham treated control subjects across age. Post hoc analyses of treatment across the two age conditions using Tukey’s HSD test revealed a significant difference between HI + Vehicle and sham treated control subjects (p < 0.05). Similar to findings on the spatial MWM, no overall across age difference was seen between HI + IAIP subjects and shams on the NSWM. Further, an overall main effect of Age [F (1, 31) = 55.422, p < 0.001] was observed indicating improved performance for adult as compared to juvenile subjects. For juvenile NSWM testing alone, an overall 3 (Treatment; Sham n=12, HI + IAIP n=9 and HI + Vehicle n=13) x 5 (Day) repeated measures ANOVA revealed a main effect of Treatment [F (2, 31) = 4.936, p < 0.05], with HI subjects taking longer to locate the platform as compared to shams. Post hoc analyses of treatment in the juvenile period using Tukey’s HSD test revealed a significant difference between HI + Vehicle and sham treated control subjects (p < 0.05) and HI + Vehicle and HI + IAIP subjects (p< 0.05), indicating impaired performance in HI + Vehicle subjects as compared to the other groups. In contrast, no effects of treatment were observed in the adult NSWM task (p was not significant). These results indicate that IAIP administration in neonatal HI subjects protects against juvenile non-spatial learning impairments and that juvenile deficits seen in HI + Vehicle subjects may be exhibited in an age or experience dependent manner.
IAIP improves regionally specific adult histological outcome after neonatal HI injury
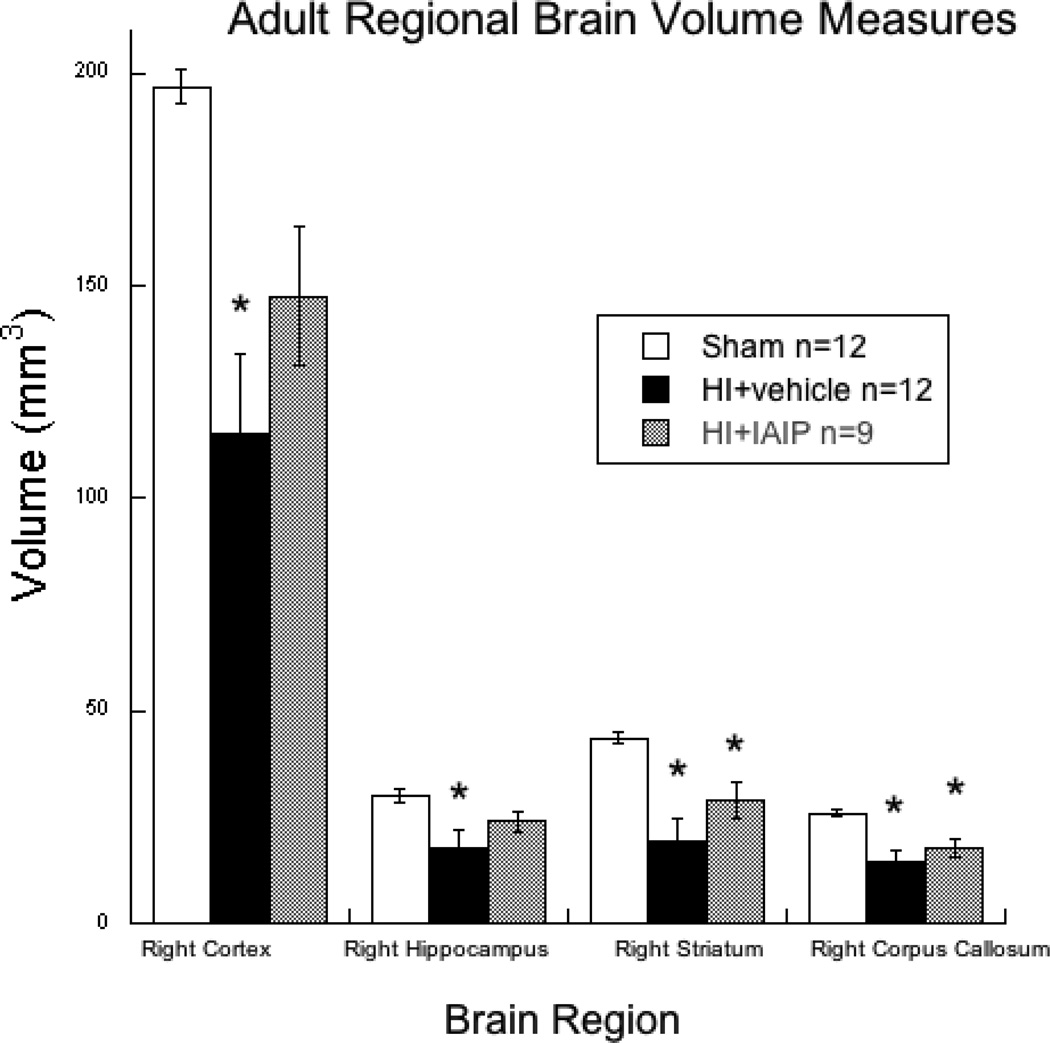
Regional volume analyses were performed on 33 brains (Sham n=12, HI + Vehicle n=12, HI+IAIP n=9) including right hemisphere (primary infarct hemisphere) cerebral cortex, hippocampus, striatum and corpus callosum from subjects that underwent behavioral testing. Repeated measures analysis of variance (RM-ANOVA) revealed a significant overall effect of Treatment [F (2, 30) = 8.991, p < 0.01] across the four regions of interest. Post hoc analyses using Tukey’s HSD test showed a significant overall reduction in volume across the four regions in HI + Vehicle subjects as compared to sham subjects (p < 0.01). No overall difference was seen between HI+IAIP and sham subjects. Simple effects analysis using a one-way analysis of variance (ANOVA) revealed an effect of Treatment for right cortical volume [F (2, 30) = 9.086, p <0.01]. Post hoc analysis showed a significant reduction in right cortical volume for HI + Vehicle subjects as compared to sham subjects (p <0.01). No difference was observed for cortical volume between sham and HI+IAIP subjects suggesting neuroprotection for this region. A one-way ANOVA revealed an effect of Treatment for right hippocampal volume [F (2, 30) = 4.4, p < 0.05]. Post hoc analysis showed a significant reduction in right hippocampal volume for HI + Vehicle subjects as compared to the sham subjects (p < 0.05). Similar to the finding in the cortex, no difference was observed between HI + IAIP and the sham subjects for hippocampal volume suggesting neuroprotective effects of IAIP on this structure. In addition, a one-way ANOVA showed a significant effect of Treatment for right striatal volume [F (2, 30) = 9.917, p < 0.01]. In contrast to cortical and hippocampal volumes, Post hoc analyses revealed significant differences in right striatal volumes between sham and HI + Vehicle subjects and HI + IAIP subjects (p < 0.05). Further, a one-way ANOVA revealed a significant effect of Treatment on right corpus callosum (CC) volume [F (2, 30) = 8.653, p < 0.05]. Similar to the findings in the striatum, post hoc analyses revealed significant differences in right CC volumes between the sham and HI + Vehicle and sham and HI + IAIP subjects (p < 0.05). These results suggest that IAIPs may exert regionally specific protective effects in the neocortex and hippocampus following HI brain injury.
Regional brain volume measures correlate with behavioral performance
Combined performance on the juvenile and adult spatial and non-spatial water mazes as well as anatomical measures were converted to z- scores for behaviorally assessed animals, and Pearson product–moment correlations were performed between anatomical and behavioral measures. Significant negative correlations were observed between scores on the spatial water maze task and the following anatomical measures: right cortex, right hippocampus, right striatum, and right corpus callosum (see Table 1). Further, significant negative correlations were seen between scores on the non-spatial water maze and right hippocampus, right striatum, and right corpus callosum. No significant correlation was observed on the non-spatial task for right cerebral cortex. These findings indicate that overall HI related reductions in the neuroanatomical structures predict worse performance for tasks assessing both spatial and non-spatial learning.
Table 1.
Correlational matrix of z-scores from combined juvenile and adult performance on the spatial (ZMWM) and non-spatial water maze (ZNSWM) and from adult histology.
| R Cortex | R Hippocampus | R Striatum | R Corpus callosum | |
|---|---|---|---|---|
| ZMWM | −.609** | −.551** | −.656** | −.646** |
| ZNSWM | −.339 | −.365* | −.371* | −.406* |
p <0.05,
p < 0.01
Discussion
The primary objectives of this series of studies were to determine the effects of IAIPs on neurobehavioral and anatomical outcomes across distinct developmental time widows ((72 hours post injury, juvenile (P38) and adult (P80+)) and learning domains (spatial and non-spatial) following neonatal HI brain injury in rats. Historically, studies examining neuroprotection following brain injury have focused on either cellular markers of outcome or single behavioral measures within a constrained time window (Fisher, et al., 2009, Fitch, et al., 2013). However, research into the effects of maturation on long-term performance in learning disabled populations and in children with birth related complications has revealed complex relationships between treatment, age of assessment and long-term functional, anatomical and behavioral outcomes (Hautus, et al., 2003, Threlkeld, et al., 2009, Threlkeld, et al., 2006).
Although information is not available on the neuroprotective properties of the blood plasma derived IAIP complex (Lim, 2013), the light chain of IAIP exhibits neuroprotective properties and blocks production of inflammatory cytokines after ischemic injury in somatic organs (Cao, et al., 2000, El Maradny, et al., 1996, Kaga, et al., 1996, Kakinuma, et al., 1997, Koga, et al., 2010, Nakahama, et al., 1996, Shu, et al., 2011, Wang, et al., 2013, Yano, et al., 2003). Therefore, findings of the present study are the first to demonstrate that the blood plasma derived complex form of IAIP exhibits important neuroprotective properties. In addition, our results highlight the complex interactions among developmental brain injury, experimental treatment with IAIPs, behavioral domain, and age of both behavioral and neuroanatomical assessment. Here, we have shown that neocortical cell death was markedly reduced 72 hours after HI injury following administration of two doses of IAIP, as evidenced by fewer FJB positive cells in sham and IAIP treated HI injured subjects as compared to vehicle treated HI animals. The profile of reduced neocortical cell death ipsilateral to the ligation was complemented by results showing brain weight sparing in IAIP treated subjects 72 hours after the HI insult. In contrast, HI animals had significantly reduced brain weights as compared to sham control subjects 72 hours after the injury. Analyses of regional brain volume measures, ipsilateral to the ligation, in the adult subjects paralleled the results from the short-term survival study showing overall larger regional brain volume measures across the right cerebral cortex, hippocampus, striatum and corpus callosum (CC) in shams as compared to HI Vehicle subjects. However, independent analyses for each of these regions showed significant reductions in right striatal and CC volumes for both HI injured and IAIP treated HI subjects as compared to sham subjects. There were no differences in regional volumes between sham and IAIP treated animals in the neocortex and hippocampus. Even though histopathology findings in adult subjects suggest that IAIP treatment appears to produce both short and long-term protection against brain injury, some structures, such as the striatum and corpus callosum, could be more vulnerable after exposure of neonates to HI injury. A number of studies indicate that differences in developmental time course across brain regions and/or unique cellular properties (e.g., oligodendrocytes versus neurons) may contribute to differences in vulnerability that could account for the observed volume profiles (Andersen, 2003, Back, et al., 2002, Lodygensky, et al., 2011, Riddle, et al., 2012, Volpe, 2009). Furthermore, mRNA of bikunin, the light chain of IAIP, was localized to hippocampi and cerebral cortices in rats but not the striatum suggesting differential distribution across distinct brain regions (Takano, et al., 1999). How brain regional vulnerabilities or variations in the endogenous distribution of IAIPs, or related molecules, influence the effects of IAIP treatment is beyond the scope of the current study. However, future research with neonatal HI injury will explore possible differences in survival across various brain cell types (e.g., neurons, oligodendrocytes) and brain regions (e.g., cortex, hippocampus, striatum, corpus callosum) as a function of age at sacrifice.
In addition to our findings of improved histological outcome following early (72 hours) and late (Adult) assessment of HI injury, behavioral results indicated robust improvements in both spatial and non-spatial learning that were mediated by the age of assessment and task type. Specifically, we showed that spatial learning deficits seen in juvenile subjects persisted into adulthood only in the vehicle treated HI injured group, suggesting that IAIPs protect against spatial learning deficits in an age dependent manner. In contrast, IAIP treatment prevented non-spatial learning impairments in juvenile HI as compared to sham control subjects. However, when all groups were retested as adults, HI + Vehicle subjects no longer showed non-spatial learning deficits relative to the sham control or IAIP administered HI subjects. These findings provide support for the concept that HI injuries exert differential effects on distinct learning domains, which are likely mediated by differing brain regional vulnerabilities (Stavnezer, et al., 2002);(Threlkeld, et al., 2012); (Fitch, et al., 2013).
These results also highlight the potential roles of early experience and/or maturation, which likely contributed to some component of improvement seen for both IAIP and vehicle treated HI injured subjects. Previous studies from our group and others have assessed both spatial and non-spatial learning across multiple models of developmental brain injury, including models of neocortical microgyria and neonatal hypoxia-ischemia (Alexander, et al., 2012, Hill, et al., 2011, Hyde, et al., 2002, McClure, et al., 2007, Threlkeld, et al., 2012). Specifically, McClure et al. (McClure, et al., 2007) showed that P7 neonatal HI rats, but not HI animals treated with erythropoietin (Epo), were impaired in both spatial and non-spatial learning as compared to sham treated control counterparts, when tested as adults (P60+). Similarly, others have observed deficits in neonatal HI injured animals on spatial and non-spatial water maze paradigms (similar to those used in the present studies; (Alexander, et al., 2012, Hill, et al., 2011)). However, these studies assessed performance only in adult subjects (P83+), which resulted in residual questions regarding the influence of age at assessment on task performance. Recently, we showed a disassociation between spatial and non-spatial learning, in which rats with neocortical microgyria (postnatal day 1 induced neocortical lesions) showed significant impairments in spatial but not in non-spatial learning as compared to sham control subjects, regardless of which task was initially presented (Threlkeld, et al., 2012). In addition, Stavnezer and associates (Stavnezer, et al., 2002) described differential learning strategies in C57BL/6J mice for spatial and non-spatial versions of the MWM, similar to the tasks employed in the present studies. These findings support the contention that spatial and non-spatial water maze paradigms reflect distinct learning domains and that performance on these tasks is likely to provide important clues to long-term outcome that parallel behavioral systems influenced by HI injury in humans (Conklin, et al., 2008, Hagberg, et al., 2002, Luu, et al., 2011, McClure, et al., 2007, Ortiz-Mantilla, et al., 2008, Woodward, et al., 2005). However, the present study is the first to our knowledge to evaluate these learning domains across two distinct developmental windows (juvenile/adult) following neonatal HI injury, highlighting both age and IAIP dependent improvements in task performance.
Behavioral assessments in preterm and very low birth weight infants (<1250g), and in full term infants exposed to perinatal HIE also exhibit a diverse deficit profile, which correspond to findings in animal studies including impairments in non-spatial recognition memory (Rose and Feldman, 1996, Rose, et al., 2005), spatial learning (Curtis, et al., 2002) and working memory (Conklin, et al., 2008, Luu, et al., 2011, Woodward, et al., 2005). Several longitudinal studies of preterm infants and children with risk factors for learning impairments have shown age dependent changes in deficit severity across multiple cognitive domains (de Vries and Jongmans, 2010, Hautus, et al., 2003, Tideman, 2000). Both maturation and experience dependent factors are very likely to modify such changes, which are difficult to isolate in studies of human development. In contrast, we have previously shown that, early life auditory training after neonatal cortical injury can ameliorate auditory impairments compared with lesioned subjects that were not exposed to such early life training (Threlkeld, et al., 2009). Although the present study did not directly address the specific roles of maturation or experience on performance outcomes following neonatal brain injury, our assessments during both the juvenile and adult periods revealed the influence of developmental test timing on long-term behavioral performance. We found that age of assessment differentially influenced observed spatial and non-spatial learning profiles in both IAIP and vehicle treated neonatal HI injured rats as compared to sham subjects.
The present findings also emphasize that even after robust evidence of treatment related neuronal cell survival during the early phase of brain injury (72 hours), longer-term metrics including histological and behavioral outcomes are essential to consider because developmental brain injury has an extended time course (weeks-months), which is likely to interact with changing behavioral and environmental conditions (Allan and Rothwell, 2001, Andersen, 2003, Becker, 1998, Ferriero, 2004). Future studies will specifically isolate these variables in order to clarify the possible combinatorial benefits of early life experience/training and experimental treatments following neonatal HI injury.
Given the importance of inflammation in prematurity and HI related brain injury (Brochu, et al., 2011), novel treatment strategies (Lim, 2013) designed to target core inflammatory mediators common to these pathologies offer great advantages to improve long-term neurobehavioral outcome in neonates (Brochu, et al., 2011, Dammann, et al., 2001, Shah, et al., 2008, Stoll, et al., 2004). The present results along with increasing evidence suggesting the efficacy of the IAIP derived light chain (e.g. urinary trypsin inhibitor (UTI)) also known as ulinastatin or bikunin, to attenuate inflammation and reduce brain injury, emphasize the great potential for the blood plasma derived IAIP to attenuate neonatal brain injury (Baek, et al., 2003, Chaaban, et al., 2010, Chaaban, et al., 2009, Koga, et al., 2010, Singh, et al., 2010, Wang, et al., 2013, Yano, et al., 2003). We have previously demonstrated high levels of IAIPs in the brain of fetal, newborn, and adult sheep and in cerebral spinal fluid of fetal sheep (Spasova, et al., 2014). Based upon these findings, we speculated that IAIPs represent endogenous immunomodulators that could be important in brain development and represent endogenous neuroprotective agents (Spasova, et al., 2014). The present study taken together with our previous work (Spasova, et al., 2014) suggests that IAIPs could have an important role in brain development and in the evolution of brain injury after neonatal HI injury.
Several previous studies have suggested that the light chain derivative of IAIPs, (urinary trypsin inhibitor or UTI), could have important neuroprotective properties (Abe, et al., 1996, Shu, et al., 2011, Wang, et al., 2013, Yano, et al., 2003). Treatment with UTI attenuated brain damage and reduced inflammatory markers in young pigs after exposure to hypothermic low-flow cardiopulmonary bypass induced brain injury (Wang, et al., 2013). Similarly, pretreatment with UTI before the induction of ischemic stroke in adult rats reduced neutrophil infiltration and infarct size in the ipsilateral hemisphere (Yano, et al., 2003). UTI also suppressed jugular venous superoxide radical generation, oxidative stress, early inflammation, and endothelial activation after exposure to forebrain ischemia induced by bilateral common carotid artery occlusion and hemorrhagic hypotension followed by reperfusion (Koga, et al., 2010). Likewise, the light chain of IAIPs also has been shown to reduce neuronal apoptosis in the hippocampal CA1 region of adult gerbils 6 days after exposure to bilateral common carotid artery occlusion (Abe, et al., 1996) and to protect oligodendrocytes from apoptosis and promote re-myelination in an experimental autoimmune encephalitis model in adult rats (Shu, et al., 2011). Taken together with the findings in the current study, these studies suggest that urine derived light chain molecules and the plasma-derived complex IAIPs could have important anti-inflammatory, anti-oxidant neuroprotective, anti-apoptotic and robust neuroprotective properties that improve neurobehavioral outcomes.
Although evidence to support a protective role of these molecules has increased in recent years, studies supporting the potential neuroprotective role have utilized the light chain of IAIPs (UTI/bikunin/ulinastatin), but there are no previous studies examining the effects of the blood derived IAIP complex on brain injury. In typical blood plasma, the light chain (bikunin) is covalently linked to two polypeptide chains constituting IAIPs (Enghild, et al., 1989). Even though the light chain is thought to be responsible for the serine protease inhibitory activity of IAIPs, one important advantage of administering IAIPs is their longer latency to metabolism and physiological clearance (Lim, et al., 2003). Specifically, IAIPs have a reported half-life of 12 hours as compared to the much shorter (3–15 minute) half-life of the light chain because of rapid clearance by the kidney (Fries and Blom, 2000). In addition, recent evidence suggests that the heavy chains of IAIP have important biological functions that synergize with the anti-inflammatory and immunomodulatory activity of the light chain (Zhuo and Kimata, 2008). The increased half-life and the additional synergetic anti-inflammatory functions of the heavy chains of IAIPs, make it an especially robust candidate for clinical application given the already strong evidence supporting these molecules as anti-inflammatory mediators of infection and brain injury (Abe, et al., 1996, Koga, et al., 2010, Lim, et al., 2003, Shu, et al., 2011, Yano, et al., 2003). Taken together, our results reinforce and extend the emerging evidence supporting a neuroprotective role for IAIPs. Further, our findings emphasize the importance of assessing multiple behavioral domains and markers of neuronal injury, at varying time points during injury progression, in order to frame a more accurate picture of a therapeutic agent’s potential for long-term neurobehavioral protection.
Fig. 3.
Line graphs showing juvenile (A, P38) and Adult (B, P80+) spatial water maze performance and juvenile (C) and adult (D) non-spatial water maze performance. Results for juvenile spatial learning (MWM) showed effects of treatment for both injury groups on day three and four of testing (*p <0.05) as compared to shams (A). For adult MWM performance, results showed an effect of injury for HI+vehicle as compared to sham subjects (*p <0.05), indicating a persistent spatial learning impairment in the vehicle treated HI group (B). Results for juvenile non-spatial learning showed an effect of injury for HI+vehicle subjects as compared to both sham and HI+IAIP groups, with vehicle HI subjects taking significantly longer to locate the hidden platform across the five days of testing (C). As adults neither of the injury groups showed impairments in NSWM performance as compared to shams (D).
Fig. 4.
Histogram showing adult brain regional volume measures ipsilateral to the injury hemisphere. Brain volume sparing was seen for IAIP treated subjects in the right neocortex and hippocampus while reductions were observed in the striatum and corpus callosum as compared to shams (*p<0.05). For HI+vehicle subjects, significant volume reductions were observed as compared to shams, in all areas measured (*p<0.05).
Highlights.
-
-
IAIPs reduce cortical cell death and spare brain weight 72 hours after neonatal HI
-
-
IAIPs improve adult spatial learning after neonatal HI injury
-
-
IAIPs improve juvenile non-spatial learning after neonatal HI injury
-
-
IAIPs protect cortex and hippocampus from atrophy after neonatal HI injury
Acknowledgements
The authors would like to acknowledge and thank Keyshla and Zahra Melendez and Katrina Feyerherm for their assistance with adult histological processing. Research reported in this publication was supported by RI-INBRE and the NIH National Center for Research Resources (NCRR) under grant #5P20RR016457-12, by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under grant #R15HD077544 and by R01HD057100 from the National Institutes of Health, National Institutes for Child Health and Human Development (NICHD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe H, Sugino N, Matsuda T, Kanamaru T, Oyanagi S, Mori H. Effect of ulinastatin on delayed neuronal death in the gerbil hippocampus. Masui. 1996;45:38–43. [PubMed] [Google Scholar]
- Alexander ML, Hill CA, Rosenkrantz TS, Fitch RH. Evaluation of the therapeutic benefit of delayed administration of erythropoietin following early hypoxic-ischemic injury in rodents. Dev Neurosci. 2012;34:515–524. doi: 10.1159/000345645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Back SA, Han BH, Luo NL, Chricton CA, Xanthoudakis S, Tam J, Arvin KL, Holtzman DM. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci. 2002;22:455–463. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek YW, Brokat S, Padbury JF, Pinar H, Hixson DC, Lim YP. Inter-alpha inhibitor proteins in infants and decreased levels in neonatal sepsis. J Pediatr. 2003;143:11–15. doi: 10.1016/S0022-3476(03)00190-2. [DOI] [PubMed] [Google Scholar]
- Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. 2010;67:1–8. doi: 10.1203/PDR.0b013e3181c1b176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballabh P. Pathogenesis and prevention of intraventricular hemorrhage. Clin Perinatol. 2014;41:47–67. doi: 10.1016/j.clp.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker KJ. Inflammation and acute stroke. Curr Opin Neurol. 1998;11:45–49. doi: 10.1097/00019052-199802000-00008. [DOI] [PubMed] [Google Scholar]
- Bost F, Diarra-Mehrpour M, Martin JP. Inter-alpha-trypsin inhibitor proteoglycan family--a group of proteins binding and stabilizing the extracellular matrix. Eur J Biochem. 1998;252:339–346. doi: 10.1046/j.1432-1327.1998.2520339.x. [DOI] [PubMed] [Google Scholar]
- Brochu ME, Girard S, Lavoie K, Sebire G. Developmental regulation of the neuroinflammatory responses to LPS and/or hypoxia-ischemia between preterm and term neonates: An experimental study. J Neuroinflammation. 2011;8:55–69. doi: 10.1186/1742-2094-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Businaro R, Leali FM, De Renzis G, Pompili E, Pagliari G, Menghi G, Fumagalli L. Inter-alpha-trypsin inhibitor-related immunoreactivity in human tissues and body fluids. Cell Mol Biol. 1992;38:463–471. [PubMed] [Google Scholar]
- Cao ZL, Okazaki Y, Naito K, Ueno T, Natsuaki M, Itoh T. Ulinastatin attenuates reperfusion injury in the isolated blood-perfused rabbit heart. Ann Thorac Surg. 2000;69:1121–1126. doi: 10.1016/s0003-4975(99)01433-2. [DOI] [PubMed] [Google Scholar]
- Chaaban H, Shin M, Sirya E, Lim YP, Caplan M, Padbury JF. Inter-alpha inhibitor protein level in neonates predicts necrotizing enterocolitis. J Pediatr. 2010;157:757–761. doi: 10.1016/j.jpeds.2010.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaaban H, Singh K, Huang J, Siryaporn E, Lim YP, Padbury JF. The role of inter-alpha inhibitor proteins in the diagnosis of neonatal sepsis. J Pediatr. 2009;154:620–622. doi: 10.1016/j.jpeds.2008.10.008. e621. [DOI] [PubMed] [Google Scholar]
- Chan P, Risler JL, Raguenez G, Salier JP. The three heavy-chain precursors for the inter-alpha-inhibitor family in mouse: new members of the multicopper oxidase protein group with differential transcription in liver and brain. Biochem J. 1995;306(Pt 2):505–512. doi: 10.1042/bj3060505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin HM, Salorio CF, Slomine BS. Working memory performance following paediatric traumatic brain injury. Brain Inj. 2008;22:847–857. doi: 10.1080/02699050802403565. [DOI] [PubMed] [Google Scholar]
- Curtis WJ, Lindeke LL, Georgieff MK, Nelson CA. Neurobehavioural functioning in neonatal intensive care unit graduates in late childhood and early adolescence. Brain. 2002;125:1646–1659. doi: 10.1093/brain/awf159. [DOI] [PubMed] [Google Scholar]
- Dammann O, Phillips TM, Allred EN, O’Shea TM, Paneth N, Van Marter LJ, Bose C, Ehrenkranz RA, Bednarek FJ, Naples M, Leviton A. Mediators of fetal inflammation in extremely low gestational age newborns. Cytokine. 2001;13:234–239. doi: 10.1006/cyto.2000.0820. [DOI] [PubMed] [Google Scholar]
- Daveau M, Jean L, Soury E, Olivier E, Masson S, Lyoumi S, Chan P, Hiron M, Lebreton JP, Husson A, Jegou S, Vaudry H, Salier JP. Hepatic and extra-hepatic transcription of inter-alpha-inhibitor family genes under normal or acute inflammatory conditions in rat. Arch Biochem Biophys. 1998;350:315–323. doi: 10.1006/abbi.1997.0515. [DOI] [PubMed] [Google Scholar]
- de Vries LS, Jongmans MJ. Long-term outcome after neonatal hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2010;95:F220–F224. doi: 10.1136/adc.2008.148205. [DOI] [PubMed] [Google Scholar]
- El Maradny E, Kanayama N, Halim A, Maehara K, Kobayashi T, Terao T. Effects of urinary trypsin inhibitor on myometrial contraction in term and preterm deliveries. Gynecol Obstet Invest. 1996;41:96–102. doi: 10.1159/000292051. [DOI] [PubMed] [Google Scholar]
- Enghild JJ, Thogersen IB, Pizzo SV, Salvesen G. Analysis of inter-alpha-trypsin inhibitor and a novel trypsin inhibitor, pre-alpha-trypsin inhibitor, from human plasma. Polypeptide chain stoichiometry and assembly by glycan. J Biol Chem. 1989;264:15975–15981. [PubMed] [Google Scholar]
- Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351:1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch RH, Alexander ML, Threlkeld SW. Early neural disruption and auditory processing outcomes in rodent models: implications for developmental language disability. Front Syst Neurosci. 2013;7:58. doi: 10.3389/fnsys.2013.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JT, Peiffer AM, Clark MG, Benasich AA, Fitch RH. Age and experience-related improvements in gap detection in the rat. Brain Res Dev Brain Res. 2004;152:83–91. doi: 10.1016/j.devbrainres.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Fries E, Blom AM. Bikunin--not just a plasma proteinase inhibitor. Int J Biochem Cell Biol. 2000;32:125–137. doi: 10.1016/s1357-2725(99)00125-9. [DOI] [PubMed] [Google Scholar]
- Fries E, Kaczmarczyk A. Inter-alpha-inhibitor, hyaluronan and inflammation. Acta Biochim Pol. 2003;50:735–742. [PubMed] [Google Scholar]
- Gluckman PD, Gunn AJ, Wyatt JS. Hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2006;354:1643–1645. author reply 1643–1645. [PubMed] [Google Scholar]
- Grether JK, Nelson KB, Walsh E, Willoughby RE, Redline RW. Intrauterine exposure to infection and risk of cerebral palsy in very preterm infants. Arch Pediatr Adolesc Med. 2003;157:26–32. doi: 10.1001/archpedi.157.1.26. [DOI] [PubMed] [Google Scholar]
- Gunn AJ, Gunn TR, de Haan HH, Williams CE, Gluckman PD. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest. 1997;99:248–256. doi: 10.1172/JCI119153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg H, Ichord R, Palmer C, Yager JY, Vannucci SJ. Animal models of developmental brain injury: relevance to human disease. A summary of the panel discussion from the Third Hershey Conference on Developmental Cerebral Blood Flow and Metabolism. Dev Neurosci. 2002;24:364–366. doi: 10.1159/000069040. [DOI] [PubMed] [Google Scholar]
- Hautus MJ, Setchell GJ, Waldie KE, Kirk IJ. Age-related improvements in auditory temporal resolution in reading-impaired children. Dyslexia. 2003;9:37–45. doi: 10.1002/dys.234. [DOI] [PubMed] [Google Scholar]
- Higgins RD, Raju T, Edwards AD, Azzopardi DV, Bose CL, Clark RH, Ferriero DM, Guillet R, Gunn AJ, Hagberg H, Hirtz D, Inder TE, Jacobs SE, Jenkins D, Juul S, Laptook AR, Lucey JF, Maze M, Palmer C, Papile L, Pfister RH, Robertson NJ, Rutherford M, Shankaran S, Silverstein FS, Soll RF, Thoresen M, Walsh WF. Hypothermia and other treatment options for neonatal encephalopathy: an executive summary of the Eunice Kennedy Shriver NICHD workshop. J Pediatr. 2011;159:851–858. doi: 10.1016/j.jpeds.2011.08.004. e851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins RD, Raju TN, Perlman J, Azzopardi DV, Blackmon LR, Clark RH, Edwards AD, Ferriero DM, Gluckman PD, Gunn AJ, Jacobs SE, Eicher DJ, Jobe AH, Laptook AR, LeBlanc MH, Palmer C, Shankaran S, Soll RF, Stark AR, Thoresen M, Wyatt J. Hypothermia and perinatal asphyxia: executive summary of the National Institute of Child Health and Human Development workshop. J Pediatr. 2006;148:170–175. doi: 10.1016/j.jpeds.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Hill CA, Threlkeld SW, Fitch RH. Early testosterone modulated sex differences in behavioral outcome following neonatal hypoxia ischemia in rats. Int J Dev Neurosci. 2011;29:381–388. doi: 10.1016/j.ijdevneu.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MA. Hypoxic-ischemic injury in neonatal brain: involvement of a novel neuronal molecule in neuronal cell death and potential target for neuroprotection. Int J Dev Neurosci. 2008;26:93–101. doi: 10.1016/j.ijdevneu.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang BY, Castillo M. Hypoxic-ischemic brain injury: imaging findings from birth to adulthood. Radiographics. 2008;28:417–439. doi: 10.1148/rg.282075066. quiz 617. [DOI] [PubMed] [Google Scholar]
- Hyde LA, Stavnezer AJ, Bimonte HA, Sherman GF, Denenberg VH. Spatial and nonspatial Morris maze learning: impaired behavioral flexibility in mice with ectopias located in the prefrontal cortex. Behav Brain Res. 2002;133:247–259. doi: 10.1016/s0166-4328(02)00022-0. [DOI] [PubMed] [Google Scholar]
- Kaga N, Katsuki Y, Futamura Y, Obata M, Shibutani Y. Role of urinary trypsin inhibitor in the maintenance of pregnancy in mice. Obstet Gynecol. 1996;88:872–882. doi: 10.1016/0029-7844(96)00268-2. [DOI] [PubMed] [Google Scholar]
- Kakinuma C, Kuwayama C, Kaga N, Futamura Y, Katsuki Y, Shibutani Y. Trophoblastic apoptosis in mice with preterm delivery and its suppression by urinary trypsin inhibitor. Obstet Gynecol. 1997;90:117–124. doi: 10.1016/S0029-7844(97)00176-2. [DOI] [PubMed] [Google Scholar]
- Kanayama N, el Maradny E, Yamamoto N, Tokunaga N, Maehara K, Terao T. Urinary trypsin inhibitor: a new drug to treat preterm labor: a comparative study with ritodrine. Eur J Obstet Gynecol Reprod Biol. 1996;67:133–138. doi: 10.1016/0301-2115(96)02454-2. [DOI] [PubMed] [Google Scholar]
- Kashyap RS, Nayak AR, Deshpande PS, Kabra D, Purohit HJ, Taori GM, Daginawala HF. Inter-alpha-trypsin inhibitor heavy chain 4 is a novel marker of acute ischemic stroke. Clin Chim Acta. 2009;402:160–163. doi: 10.1016/j.cca.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Kato M, Seki N, Sugano S, Hashimoto K, Masuho Y, Muramatsu M, Kaibuchi K, Nakafuku M. Identification of sonic hedgehog-responsive genes using cDNA microarray. Biochem Biophys Res Commun. 2001;289:472–478. doi: 10.1006/bbrc.2001.5976. [DOI] [PubMed] [Google Scholar]
- Kobayashi H. Endogenous anti-inflammatory substances, inter-alpha-inhibitor and bikunin. Biol Chem. 2006;387:1545–1549. doi: 10.1515/BC.2006.192. [DOI] [PubMed] [Google Scholar]
- Koga Y, Fujita M, Tsuruta R, Koda Y, Nakahara T, Yagi T, Aoki T, Kobayashi C, Izumi T, Kasaoka S, Yuasa M, Maekawa T. Urinary trypsin inhibitor suppresses excessive superoxide anion radical generation in blood, oxidative stress, early inflammation, and endothelial injury in forebrain ischemia/reperfusion rats. Neurol Res. 2010;32:925–932. doi: 10.1179/016164110X12645013515133. [DOI] [PubMed] [Google Scholar]
- Leviton A, Kuban K, O’Shea TM, Paneth N, Fichorova R, Allred EN, Dammann O. The relationship between early concentrations of 25 blood proteins and cerebral white matter injury in preterm newborns: the ELGAN study. J Pediatr. 2011;158:897–903. doi: 10.1016/j.jpeds.2010.11.059. e891–895. [DOI] [PubMed] [Google Scholar]
- Liederman J, Kantrowitz L, Flannery K. Male vulnerability to reading disability is not likely to be a myth: a call for new data. J Learn Disabil. 2005;38:109–129. doi: 10.1177/00222194050380020201. [DOI] [PubMed] [Google Scholar]
- Lim YP. ProThera Biologics, Inc.: A Novel Immunomodulator and Biomarker for Life-Threatening Diseases. R I Med J. 2013;96:16–18. (2013) [PubMed] [Google Scholar]
- Lim YP, Bendelja K, Opal SM, Siryaporn E, Hixson DC, Palardy JE. Correlation between mortality and the levels of inter-alpha inhibitors in the plasma of patients with severe sepsis. J Infect Dis. 2003;188:919–926. doi: 10.1086/377642. Epub 2003 Aug 2026. [DOI] [PubMed] [Google Scholar]
- Lodygensky GA, West T, Moravec MD, Back SA, Dikranian K, Holtzman DM, Neil JJ. Diffusion characteristics associated with neuronal injury and glial activation following hypoxia-ischemia in the immature brain. Magn Reson Med. 2011;66:839–845. doi: 10.1002/mrm.22869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu TM, Ment L, Allan W, Schneider K, Vohr BR. Executive and memory function in adolescents born very preterm. Pediatrics. 2011;127:e639–e646. doi: 10.1542/peds.2010-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlow N, Hennessy EM, Bracewell MA, Wolke D. Motor and executive function at 6 years of age after extremely preterm birth. Pediatrics. 2007;120:793–804. doi: 10.1542/peds.2007-0440. [DOI] [PubMed] [Google Scholar]
- McClure MM, Threlkeld SW, Fitch RH. The effects of erythropoietin on auditory processing following neonatal hypoxic-ischemic injury. Brain Res. 2006;1087:190–195. doi: 10.1016/j.brainres.2006.03.016. [DOI] [PubMed] [Google Scholar]
- McClure MM, Threlkeld SW, Fitch RH. Auditory processing and learning/memory following erythropoietin administration in neonatally hypoxic-ischemic injured rats. Brain Res. 2007;1132:203–209. doi: 10.1016/j.brainres.2006.11.006. [DOI] [PubMed] [Google Scholar]
- McClure MM, Threlkeld SW, Rosen GD, Holly Fitch R. Rapid auditory processing and learning deficits in rats with P1 versus P7 neonatal hypoxic-ischemic injury. Behav Brain Res. 2006;172:114–121. doi: 10.1016/j.bbr.2006.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima S, Nii A, Kato K, Uemura A. Gene expression of the two heavy chains and one light chain forming the inter-alpha-trypsin-inhibitor in human tissues. Biol Pharm Bull. 1998;21:167–169. doi: 10.1248/bpb.21.167. [DOI] [PubMed] [Google Scholar]
- Nakahama H, Obata K, Sugita M. Ulinastatin ameliorates acute ischemic renal injury in rats. Ren Fail. 1996;18:893–898. doi: 10.3109/08860229609047715. [DOI] [PubMed] [Google Scholar]
- Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol. 1998;44:665–675. doi: 10.1002/ana.410440413. [DOI] [PubMed] [Google Scholar]
- Okroj M, Holmquist E, Sjolander J, Corrales L, Saxne T, Wisniewski HG, Blom AM. Heavy chains of inter alpha inhibitor (IalphaI) inhibit the human complement system at early stages of the cascade. J Biol Chem. 2012;287:20100–20110. doi: 10.1074/jbc.M111.324913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opal SM, Lim YP, Cristofaro P, Artenstein AW, Kessimian N, Delsesto D, Parejo N, Palardy JE, Siryaporn E. Inter-alpha inhibitor proteins: a novel therapeutic strategy for experimental anthrax infection. Shock. 2011;35:42–44. doi: 10.1097/SHK.0b013e3181e83204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Mantilla S, Choudhury N, Leevers H, Benasich AA. Understanding language and cognitive deficits in very low birth weight children. Dev Psychobiol. 2008;50:107–126. doi: 10.1002/dev.20278. [DOI] [PubMed] [Google Scholar]
- Paxinos G. The Rat Nervous System. San Diego, CA: Elsiever; 2004. [Google Scholar]
- Peiffer AM, Rosen GD, Fitch RH. Sex differences in rapid auditory processing deficits in microgyric rats. Brain Res Dev Brain Res. 2004;148:53–57. doi: 10.1016/j.devbrainres.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Raz N, Torres IJ, Acker JD. Age, gender, and hemispheric differences in human striatum: a quantitative review and new data from in vivo MRI morphometry. Neurobiol Learn Mem. 1995;63:133–142. doi: 10.1006/nlme.1995.1013. [DOI] [PubMed] [Google Scholar]
- Rice JE, 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9:131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- Riddle A, Maire J, Gong X, Chen KX, Kroenke CD, Hohimer AR, Back SA. Differential susceptibility to axonopathy in necrotic and non-necrotic perinatal white matter injury. Stroke. 2012;43:178–184. doi: 10.1161/STROKEAHA.111.632265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SA, Feldman JF. Memory and processing speed in preterm children at eleven years: a comparison with full-terms. Child Dev. 1996;67:2005–2021. [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Recall memory in the first three years of life: a longitudinal study of preterm and term children. Dev Med Child Neurol. 2005;47:653–659. doi: 10.1017/S0012162205001349. [DOI] [PubMed] [Google Scholar]
- Rosen GD, Harry JD. Brain volume estimation from serial section measurements: a comparison of methodologies. J Neurosci Methods. 1990;35:115–124. doi: 10.1016/0165-0270(90)90101-k. [DOI] [PubMed] [Google Scholar]
- Rosen GD, Mesples B, Hendriks M, Galaburda AM. Histometric changes and cell death in the thalamus after neonatal neocortical injury in the rat. Neuroscience. 2006;141:875–888. doi: 10.1016/j.neuroscience.2006.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M. Introduction: autism--the challenges ahead. Novartis Found Symp. 2003;251:1–9. discussion 109–111, 281–197. [PubMed] [Google Scholar]
- Salier JP, Rouet P, Raguenez G, Daveau M. The inter-alpha-inhibitor family: from structure to regulation. Biochem J. 1996;315(Pt 1):1–9. doi: 10.1042/bj3150001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez D, Martinez S, Lindqvist A, Akerstrom B, Falkenberg C. Expression of the AMBP gene transcript and its two protein products, alpha(1)-microglobulin and bikunin, in mouse embryogenesis. Mech Dev. 2002;117:293–298. doi: 10.1016/s0925-4773(02)00202-2. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Shah DK, Doyle LW, Anderson PJ, Bear M, Daley AJ, Hunt RW, Inder TE. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr. 2008;153:170–175. doi: 10.1016/j.jpeds.2008.02.033. 175 e171. [DOI] [PubMed] [Google Scholar]
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotten CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- Shankaran S, Pappas A, McDonald SA, Vohr BR, Hintz SR, Yolton K, Gustafson KE, Leach TM, Green C, Bara R, Petrie Huitema CM, Ehrenkranz RA, Tyson JE, Das A, Hammond J, Peralta-Carcelen M, Evans PW, Heyne RJ, Wilson-Costello DE, Vaucher YE, Bauer CR, Dusick AM, Adams-Chapman I, Goldstein RF, Guillet R, Papile LA, Higgins RD. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. 2012;366:2085–2092. doi: 10.1056/NEJMoa1112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Yang Y, Qiu W, Lu Z, Li Y, Bao J, Feng M, Hu X. Neuroprotection by ulinastatin in experimental autoimmune encephalomyelitis. Neurochem Res. 2011;36:1969–1977. doi: 10.1007/s11064-011-0520-4. [DOI] [PubMed] [Google Scholar]
- Singh K, Zhang LX, Bendelja K, Heath R, Murphy S, Sharma S, Padbury JF, Lim YP. Inter-alpha inhibitor protein administration improves survival from neonatal sepsis in mice. Pediatr Res. 2010;68:242–247. doi: 10.1203/PDR.0b013e3181e9fdf0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spasova MS, Sadowska GB, Threlkeld SW, Lim YP, Stonestreet BS. Ontogeny of inter-alpha inhibitor proteins in ovine brain and somatic tissues. Exp Biol Med (Maywood) 2014 doi: 10.1177/1535370213519195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavnezer AJ, Hyde LA, Bimonte HA, Armstrong CM, Denenberg VH. Differential learning strategies in spatial and nonspatial versions of the Morris water maze in the C57BL/6J inbred mouse strain. Behav Brain Res. 2002;133:261–270. doi: 10.1016/s0166-4328(02)00021-9. [DOI] [PubMed] [Google Scholar]
- Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, Higgins RD. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. Jama. 2004;292:2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- Szaflarski J, Burtrum D, Silverstein FS. Cerebral hypoxia-ischemia stimulates cytokine gene expression in perinatal rats. Stroke. 1995;26:1093–1100. doi: 10.1161/01.str.26.6.1093. [DOI] [PubMed] [Google Scholar]
- Takano M, Mori Y, Shiraki H, Horie M, Okamoto H, Narahara M, Miyake M, Shikimi T. Detection of bikunin mRNA in limited portions of rat brain. Life Sci. 1999;65:757–762. doi: 10.1016/s0024-3205(99)00302-1. [DOI] [PubMed] [Google Scholar]
- Threlkeld SW, Hill CA, Rosen GD, Fitch RH. Early acoustic discrimination experience ameliorates auditory processing deficits in male rats with cortical developmental disruption. Int J Dev Neurosci. 2009;27:321–328. doi: 10.1016/j.ijdevneu.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlkeld SW, Hill CA, Szalkowski CE, Truong DT, Rosen GD, Fitch RH. Effects of test experience and neocortical microgyria on spatial and non-spatial learning in rats. Behav Brain Res. 2012;235:130–135. doi: 10.1016/j.bbr.2012.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlkeld SW, McClure MM, Rosen GD, Fitch RH. Developmental timeframes for induction of microgyria and rapid auditory processing deficits in the rat. Brain Res. 2006;1109:22–31. doi: 10.1016/j.brainres.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Tideman E. Longitudinal follow-up of children born preterm: cognitive development at age 19. Early Hum Dev. 2000;58:81–90. doi: 10.1016/s0378-3782(00)00055-4. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Perinatal brain injury: from pathogenesis to neuroprotection. Ment Retard Dev Disabil Res Rev. 2001;7:56–64. doi: 10.1002/1098-2779(200102)7:1<56::AID-MRDD1008>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Neonatal encephalitis and white matter injury: more than just inflammation? Ann Neurol. 2008;64:232–236. doi: 10.1002/ana.21466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe JJ. The encephalopathy of prematurity--brain injury and impaired brain development inextricably intertwined. Semin Pediatr Neurol. 2009;16:167–178. doi: 10.1016/j.spen.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Xue Q, Yan F, Li L, Liu J, Li S, Hu S. Ulinastatin as a neuroprotective and anti-inflammatory agent in infant piglets model undergoing surgery on hypothermic low-flow cardiopulmonary bypass. Paediatr Anaesth. 2013;23:209–216. doi: 10.1111/pan.12073. [DOI] [PubMed] [Google Scholar]
- Werbowetski-Ogilvie TE, Agar NY, Waldkircher de Oliveira RM, Faury D, Antel JP, Jabado N, Del Maestro RF. Isolation of a natural inhibitor of human malignant glial cell invasion: inter alpha-trypsin inhibitor heavy chain 2. Cancer Res. 2006;66:1464–1472. doi: 10.1158/0008-5472.CAN-05-1913. [DOI] [PubMed] [Google Scholar]
- Wolke D, Samara M, Bracewell M, Marlow N. Specific language difficulties and school achievement in children born at 25 weeks of gestation or less. J Pediatr. 2008;152:256–262. doi: 10.1016/j.jpeds.2007.06.043. [DOI] [PubMed] [Google Scholar]
- Wood NS, Costeloe K, Gibson AT, Hennessy EM, Marlow N, Wilkinson AR. The EPICure study: associations and antecedents of neurological and developmental disability at 30 months of age following extremely preterm birth. Arch Dis Child Fetal Neonatal Ed. 2005;90:F134–F140. doi: 10.1136/adc.2004.052407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward LJ, Edgin JO, Thompson D, Inder TE. Object working memory deficits predicted by early brain injury and development in the preterm infant. Brain. 2005;128:2578–2587. doi: 10.1093/brain/awh618. [DOI] [PubMed] [Google Scholar]
- Yano T, Anraku S, Nakayama R, Ushijima K. Neuroprotective effect of urinary trypsin inhibitor against focal cerebral ischemia-reperfusion injury in rats. Anesthesiology. 2003;98:465–473. doi: 10.1097/00000542-200302000-00028. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, Kim IO. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin- 1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177:19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Kim CJ, Romero R, Jun JK, Park KH, Choi ST, Chi JG. Experimentally induced intrauterine infection causes fetal brain white matter lesions in rabbits. Am J Obstet Gynecol. 1997;177:797–802. doi: 10.1016/s0002-9378(97)70271-0. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Romero R, Kim CJ, Koo JN, Choe G, Syn HC, Chi JG. High expression of tumor necrosis factor-alpha and interleukin-6 in periventricular leukomalacia. Am J Obstet Gynecol. 1997;177:406–411. doi: 10.1016/s0002-9378(97)70206-0. [DOI] [PubMed] [Google Scholar]
- Yoshida E, Sumi H, Tsushima H, Maruyama M, Mihara H. Distribution and localization of inter-alpha-trypsin inhibitor and its active component acid-stable proteinase inhibitor: comparative immunohistochemical study. Inflammation. 1991;15:71–79. doi: 10.1007/BF00917911. [DOI] [PubMed] [Google Scholar]
- Zhuo L, Kimata K. Structure and function of inter-alpha-trypsin inhibitor heavy chains. Connect Tissue Res. 2008;49:311–320. doi: 10.1080/03008200802325458. [DOI] [PubMed] [Google Scholar]