Abstract
Purpose
To examine the role of body mass index (BMI) in assessment of prostate cancer (PCa) risk.
Materials and Methods
3,258 participants who underwent biopsy (including 1,902 men with a diagnosis of PCa) were identified from the Selenium and Vitamin E Cancer Prevention Trial. The associations of BMI with PCa and high-grade PCa (HGPCa) were examined using logistic regression, adjusting for age, race, BMI-adjusted prostate-specific antigen, digital rectal exam, family history of PCa, previous biopsy history, PSA velocity and time between study entry and the last biopsy. The prediction models were compared with our previously-developed BMI-adjusted Prostate Cancer Prevention Trial prostate cancer Risk Calculator (bmiPCPTRC).
Results
Of the study subjects, 49.1% were overweight and 29.3% were obese. After adjustment, among men without a known family history of PCa, increased BMI was not associated with higher risk of PCa (per one-unit increase in logBMI: OR=0.83, p=0.54) but was significantly associated with higher risk of HGPCa (i.e., Gleason score≥7 prostate cancer) (OR=2.31, p=0.03). For men with a known family history of PCa, the risks of PCa and HGPCa increased rapidly as BMI increased (PCa: OR=3.73, p=0.02; HGPCa: OR=7.95, p=0.002). The bmiPCPTRC generally underestimated the risks of PCa and HGPCa.
Conclusions
BMI provided independently predictive information regarding risks of PCa and HGPCa, after adjusting for other risk factors. BMI, especially among men with a known family history of PCa, should be considered for inclusion in any clinical assessment of PCa risk and recommendations regarding prostate biopsy.
Keywords: prostate cancer, PSA, BMI, BMI-adjusted Prostate Cancer Prevention Trial prostate cancer Risk Calculator (bmiPCPTRC), high-grade prostate cancer
Introduction
The relationship between obesity, measured by body mass index (BMI), and prostate cancer (PCa) has been studied extensively. 1-3 Obesity has been consistently linked to higher PCa mortality. 4-5 However, the relationship between obesity and risk of PCa is unclear, with individual studies showing conflicting results. 2, 5-7 The inconsistency between individual studies might be due to differential effects of obesity on different tumor subtypes (localized/non-aggressive vs. advanced/aggressive). 8 In particular, obese men have been observed to have lower concentrations of free testosterone, which in turn was observed to be associated with a decreased risk of localized/non-aggressive PCa and with an increased risk of advanced/aggressive PCa. 9-13 A recent meta-analysis involving prospective studies on BMI and risk of PCa separately by subtype of the disease, confirmed a decreased risk for localized PCa and increased risk for advanced PCa. 14 Confounding these conclusions, several studies have shown that higher BMI levels are associated with decreased serum levels of prostate-specific antigen (PSA), potentially masking PCa detection including detection of high-grade PCa.15-19 Therefore, the observed protective effects of BMI on risk of PCa may be an artifact of hemodilution of PSA concentrations in obese men. 2, 20 Recently, we developed a BMI-adjusted Prostate Cancer Prevention Trial prostate cancer Risk Calculator (bmiPCPTRC), that predicts all PCa risk as well as high-grade PCa risk (HGPCa, Gleason score≥7) while accounting for the effect of BMI on PSA using BMI-adjusted PSA.18 However, the utility of this bmiPCPTRC has not been externally validated.
Herein, we report on a study of PCa detection using the Selenium and Vitamin E Cancer Prevention Trial (SELECT). 21 Our study has two goals: 1) to conduct the first external validation study for the bmiPCPTRC among a large cohort of healthy PSA-screened biopsy- confirmed men in North America; and 2) to examine the associations of BMI with screen- detected PCa as well as with HGPCa after adjusting for other risk factors.
Materials and Methods
Subjects
SELECT is the largest PCa prevention trial ever performed, with 35,534 participants recruited and randomized between 08/22/2001 and 06/24/2004 from more than 400 sites throughout the United States, Puerto Rico, and Canada.21 Men eligible to join the study were 1) age 55 or older or, in the case of African-American men, age 50 or older; 2) did not have a DRE suspicious for cancer; and 3) had a PSA≤4 ng/mL. Participants were recommended during annual clinic visits to undergo a PSA test and DRE according to the standard of care at their study sites and the participant's preferences. Study supplementation ended on 10/23/2008 at which point the median overall follow-up was 5.46 years (range, 4.17-7.33 years).
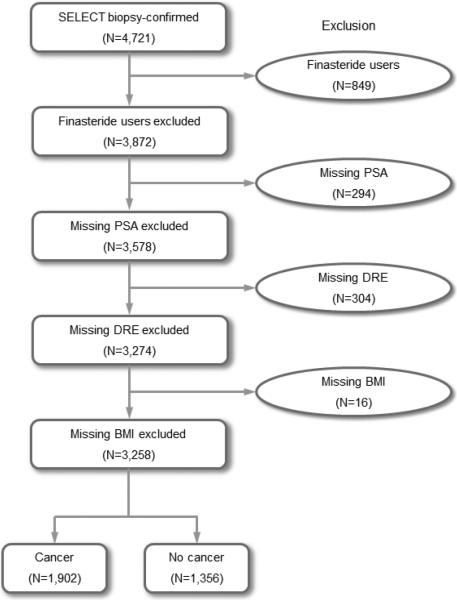
From 35,534 SELECT participants, we identified 4,721 who had undergone prostate biopsy. For patients undergoing more than one biopsy, the results of the most recent biopsy were used to assess the effect of prior negative biopsy findings. PSA and DRE were measured at or within one-year prior to the date of the most recent biopsy. For those with multiple PSA measurements longitudinally, PSA velocity was calculated by linear regression using all available PSA values measured from the study entry to the date of the last biopsy and dichotomized as 1 if PSA velocity was greater than 0.35 ng/mL per year and 0 otherwise as recommended by the clinical guidelines by the National Comprehensive Cancer Network and the American Urology Association and used by other researchers. 22 Age and BMI were collected at the date of the most recent biopsy. BMI-adjusted PSA was calculated by multiplying the most recent PSA by 1.09, 1.20, 1.50 and 1.71 for men in overweight (BMI 25-29.9), obese I (BMI 30-34.9), obese II (BMI 35-39.9) and obese III (BMI≥40) categories, respectively. 18 Information on race/ethnicity and first-degree family history of PCa were collected at study entry. We also evaluated the duration of observation based on the time from study entry to the date of the last biopsy. Patients were excluded if they were current or past finasteride users (n=849), or had missing PSA, DRE or BMI at or within one-year prior to the date of the most recent biopsy (n=614). The final sample size was 3,258, including 1,902 with a diagnosis of PCa and 1,356 without cancer (Figure 1). Compared with the 3,258 men included in the final analysis, the 614 men who were excluded due to missing PSA, DRE or BMI had similar distributions in age, race, family history and HGPCa rate, although they had higher prior negative biopsy rate (30.1% vs. 20.9%, p<0.001) and lower PCa rate (13.4% vs. 58.4%, p<0.001). This study was approved by the IRB at the University of Texas Health Science Center at San Antonio.
Figure 1.
Flow chart
Statistical analyses
Patient characteristics for those with a confirmed diagnosis of PCa were compared to the subjects without a PCa diagnosis using Fisher's exact test for categorical variables and Mann-Whitney U test for continuous variables. Calibration of the bmiPCPTRC was assessed using calibration plots. In addition, the average PCa and HGPCa risks based on the bmiPCPTRC were compared to the observed PCa and HGPCa rates, respectively, for the sample as a whole and among subgroups: PSA (<4 ng/mL vs. ≥4 ng/mL), DRE (normal vs. abnormal), age (≥65 yr vs. <65 yr), family history of PCa (yes vs. no), BMI category (<25 vs. 25-30 vs. ≥30), and race (White vs. African-American [AA] vs. non-AA Hispanic). Diagnostic performance of the bmiPCPTRC was evaluated using area underneath the receiver operating characteristic (ROC) curve (AUC). The difference between two AUCs was tested using Z-statistic for comparing the utility of bmiPCPTRC between two independent subgroups. 23-24
To assess the independent predictive effect of BMI on PCa and HGPCa, multivariable logistic regression was performed adjusting for other potential risk factors. A clinical judgment guided forward model selection procedure was used to fit a set of risk prediction models sequentially. We first fit a prediction model including only the risk factors that are included in the bmiPCPTRC. We then added other clinically relevant variables not related to BMI. Finally, in the full model, we added BMI-related variables to examine the independent predictive effect of BMI on PCa and HGPCa, respectively. Covariates considered in the full model included BMI-adjusted PSA, age, race, DRE, previous biopsy history, family history, BMI, PSA velocity, time between study entry and the date of the last biopsy, and the interaction between BMI and family history. Log transformation was applied to BMI and BMI-adjusted PSA due to skewness of the distribution. Diagnostic performance of risk prediction models was evaluated using AUC and the difference between two prediction models in terms of AUC was tested using nonparametric U-statistic. 23-24 Two additional definitions for HGPCa were explored: 1) Gleason score 4+3 and above prostate cancer; and 2) Gleason score≥8 prostate cancer. The effect of BMI did not change; therefore we only report the results for Gleason score≥7 in this paper. Statistical analyses were performed in SAS (Version 9.3). Graphs were produced using R (Version 2.15.0).
Results
The characteristics of the 3,258 SELECT participants who underwent biopsy are shown in Table 1. In this cohort, 49.1% were overweight and 29.3% were obese. Of the 1,513 patients with a diagnosis of PCa and a valid Gleason score, 34.7% had Gleason score≥7 cancer and 6.9% had Gleason score≥8 cancer. Compared to their counterparts, the patients with a diagnosis of PCa were significantly older (66.2 yr vs. 65.8 yr), heavier (BMI=27.9 vs. 27.3), had higher PSA levels (4.5 vs. 3.3) and BMI-adjusted PSA levels (5 vs. 3.7), and more patients had a family history of PCa (27.8% vs. 20.4%), no prior negative biopsy (82.9% vs. 73.8%) and a rapid increase in PSA (PSA velocity > 0.35 ng/mL/yr: 67.8% vs. 44.6%).
Table 1.
Characteristics of SELECT subjects
| Characteristic | Cancer N=1,902 | No Cancer N=1,356 | All Subjects N=3,258 | P-value |
|---|---|---|---|---|
| Age at the last biopsy (years) | 0.03a | |||
| Mean (SD) | 66.2 (6.1) | 65.8 (5.8) | 66 (6) | |
| Range | 51, 88 | 51, 89 | 51, 89 | |
| Age category, N (%) | 0.23b | |||
| < 55 | 21 (1.1) | 8 (0.6) | 29 (0.9) | |
| 55 to <65 | 807 (42.4) | 603 (44.5) | 1410 (43.3) | |
| 65 to <75 | 887 (46.6) | 628 (46.3) | 1515 (46.5) | |
| ≥ 75 | 187 (9.8) | 117 (8.6) | 304 (9.3) | |
| Race, N (%) | 0.02b | |||
| White | 1510 (79.4) | 1133 (83.6) | 2643 (81.1) | |
| African-American (AA) | 286 (15) | 167 (12.3) | 453 (13.9) | |
| Hispanic (non-AA) | 71 (3.7) | 35 (2.6) | 106 (3.3) | |
| Other | 35 (1.8) | 21 (1.5) | 56 (1.7) | |
| Biopsy history, N (%) | <0.001b | |||
| ≥1 prior negative biopsy | 326 (17.1) | 355 (26.2) | 681 (20.9) | |
| 0 prior negative biopsy | 1576 (82.9) | 1001 (73.8) | 2577 (79.1) | |
| Digital rectal exam, N (%) | 0.23b | |||
| Abnormal | 491 (25.8) | 325 (24) | 816 (25) | |
| Normal | 1411 (74.2) | 1031 (76) | 2442 (75) | |
| Family history, N (%) | <0.001b | |||
| Yesc | 528 (27.8) | 276 (20.4) | 804 (24.7) | |
| No | 1374 (72.2) | 1080 (79.6) | 2454 (75.3) | |
| BMI at the last biopsy (kg/m2) | <0.001a | |||
| Median | 27.9 | 27.3 | 27.7 | |
| Interquartile range | 25.6, 30.8 | 25, 30.4 | 25.4, 30.6 | |
| BMI category, N (%) | 0.002a | |||
| < 25 | 364 (19.1) | 338 (24.9) | 702 (21.5) | |
| 25 to < 30 | 954 (50.2) | 646 (47.6) | 1600 (49.1) | |
| 30 to < 35 | 430 (22.6) | 281 (20.7) | 711 (21.8) | |
| 35 to < 40 | 111 (5.8) | 69 (5.1) | 180 (5.5) | |
| ≥ 40 | 43 (2.3) | 22 (1.6) | 65 (2) | |
| PSA at the last biopsy (ng/mL) | <0.001a | |||
| Median | 4.5 | 3.3 | 4.1 | |
| Interquartile range | 3.5, 5.5 | 1.7, 4.7 | 2.8, 5.3 | |
| PSA category, N (%) | ||||
| < 1 | 32 (1.7) | 186 (13.7) | 218 (6.7) | |
| 1 to <2.5 | 175 (9.2) | 301 (22.2) | 476 (14.6) | |
| 2.5 to <4 | 444 (23.3) | 321 (23.7) | 765 (23.5) | |
| 4 to <10 | 1188 (62.5) | 515 (38) | 1703 (52.3) | |
| ≥10 | 63 (3.3) | 33 (2.4) | 96 (2.9) | |
| BMI-adjusted PSA (ng/mL)d | <0.001a | |||
| Median | 5 | 3.7 | 4.6 | |
| Interquartile range | 3.9, 6.3 | 1.8, 5.3 | 3, 6 | |
| BMI-adjusted PSA category, N (%) | <0.001b | |||
| < 1 | 24 (1.3) | 166 (12.2) | 190 (5.8) | |
| 1 to <2.5 | 150 (7.9) | 269 (19.8) | 419 (12.9) | |
| 2.5 to <4 | 340 (17.9) | 303 (22.3) | 643 (19.7) | |
| 4 to <10 | 1288 (67.7) | 575 (42.4) | 1863 (57.2) | |
| ≥10 | 100 (5.3) | 43 (3.2) | 143 (4.4) | |
| Time between baseline and the la | st biopsy (years) | <0.001a | ||
| Mean (SD) | 3.7 (2) | 4.1 (1.9) | 3.9 (1.9) | |
| Range | 0.1, 8.8 | 0.3, 8.8 | 0.1, 8.8 | |
| PSA velocitye, N (%) | <0.001b | |||
| > 0.35 ng/mL per year | 1278 (67.8) | 602 (44.6) | 1880 (58.1) | |
| ≥ 0.35 ng/mL per year | 606 (32.2) | 749 (55.4) | 1355 (41.9) | |
| Gleason score, N (%) | NA | |||
| 4 | 1 (0.1) | |||
| 5 | 6 (0.3) | |||
| 6 | 981 (51.6) | |||
| 7 (3+4) | 319 (16.8) | |||
| 7 (4+3) | 101 (5.3) | |||
| 8 | 64 (3.4) | |||
| 9 | 40 (2.1) | |||
| 10 | 1 (0.1) | |||
| Missing | 389 (20.5) |
P value for test of difference between cancer and no cancer using Mann-Whitney U Test
P value for test of difference between cancer and no cancer using Fisher's Exact Test
Self-reported known family history of prostate cancer among first-degree relatives
BMI-adjusted PSA equaled to unadjusted PSA multiplying 1.09, 1.20, 1.50, and 1.71 for men in overweight, obese I, obese II, and obese III categories, respectively. 19
PSA velocity was computed as the linear slope between PSA and time of measurement (i.e., change in PSA per one-year increase)
Risk prediction for total prostate cancer
The average bmiPCPTRC PCa risk for the entire cohort was significantly lower than the observed PCa rate (Table 2, Column A). In all subgroups, the estimated average risk of PCa calculated by the bmiPCPTRC was lower than the observed PCa rate, as confirmed by the calibration plot (Figure 2A). The bmiPCPTRC had an AUC of 0.71 for the detection of PCa and it worked better among those with lower PSA values (<4 ng/mL) (p <.001).
Table 2.
Comparison of observed prostate cancer incidence rates to average prostate cancer risks based on bmiPCPTRC
| (A) Total prostate cancer (PCa) | (B) Gleason grade ≥7 prostate cancer (HGPCa) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subgroup | N | Obs. PCa n (%) | Ave. Risk (%) | AUC | P Valuea | Nd | Obs. HGPCa n (%) | Ave. Risk (%) | AUC | P Valuea |
| All | 3258 | 1902 (58.4) | 40.4 | 0.713 | 2869 | 525 (18.3) | 13.3 | 0.705 | ||
| PSA <4.0 ng/mL | 1459 | 651 (44.6) | 33.4 | 0.748 | <0.001 | 1335 | 143 (10.7) | 7.8 | 0.708 | 0.01 |
| PSA ≥4.0 ng/mL | 1799 | 1251 (69.5) | 46.1 | 0.598 | 1534 | 382 (24.9) | 18.0 | 0.642 | ||
| Normal DRE | 2442 | 1411 (57.8) | 37.6 | 0.711 | 0.15 | 2139 | 381 (17.8) | 12.1 | 0.700 | 0.58 |
| Abnormal DRE | 816 | 491 (60.2) | 48.8 | 0.741 | 730 | 144 (19.7) | 16.6 | 0.714 | ||
| Age: ≥ 65 yrs | 1819 | 1074 (59.0) | 41.1 | 0.725 | 0.15 | 1598 | 333 (20.8) | 14.7 | 0.696 | 0.75 |
| Age: < 65 yrs | 1439 | 828 (57.5) | 39.6 | 0.698 | 1271 | 192 (15.1) | 11.5 | 0.703 | ||
| No Family History | 804 | 528 (65.7) | 45.1 | 0.730 | 0.21 | 694 | 127 (18.3) | 12.8 | 0.682 | 0.26 |
| Family History | 2454 | 1374 (56.0) | 38.9 | 0.702 | 2175 | 398 (18.3) | 13.4 | 0.712 | ||
| BMI<25 | 702 | 364 (51.9) | 38.2 | 0.697 | 0.56 | 632 | 89 (14.1) | 11.9 | 0.694 | 0.28 |
| 25≤BMI<30 | 1600 | 954 (59.6) | 40.2 | 0.711 | 0.53 | 1399 | 249 (17.8) | 12.7 | 0.729 | 0.008 |
| BMI≥30 | 956 | 584 (61.1) | 42.6 | 0.724 | 0.30b | 838 | 187 (22.3) | 15.2 | 0.658 | 0.29b |
| White | 2643 | 1510 (57.1) | 40.6 | 0.718 | 0.86 | 2359 | 422 (17.9) | 11.7 | 0.716 | 0.32 |
| AA | 453 | 286 (63.1) | 40.1 | 0.713 | 0.38 | 388 | 82 (21.1) | 23.2 | 0.684 | 0.37 |
| Hispanic (non-AA) | 106 | 71 (67.0) | 38.8 | 0.655 | 0.31c | 71 | 11 (15.5) | 11.4 | 0.605 | 0.19c |
AA=African American
Difference in AUC between two independent subgroups (current row vs. the row below it) using a Z statistic
Difference in AUC between BMI>30 and BMI<25
Difference in AUC between Hispanic and White
389 men were excluded due to missing Gleason score
Figure 2.
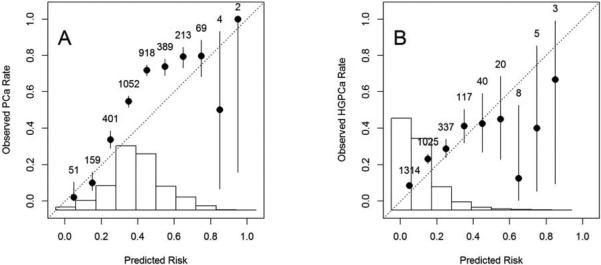
Calibration plots for bmiPCPTRC
(A) bmiPCPTRC PCa risk vs. observed PCa rate; (B) bmiPCPTRC HGPCa (Gleason scores≥7) risk vs. observed HGPCa rate. The histogram of predicted risk and the number of participants within each risk interval are displayed as well.
After calibration, the effect of BMI on risk of PCa was dependent on family history of PCa (Table 3A, Model 3). For those without a known family history of PCa, an increase in BMI was not associated with an increased risk of PCa (OR=0.83, p=0.54). By comparison, for men with a known family history of PCa, the risk of PCa increased rapidly as BMI increased (OR=3.73, p=0.02). In terms of AUC, the model with BMI-related predictors was significantly better than BMI-adjusted PSA alone (p<0.001) and the calibrated bmiPCPTRC (p=0.006).
Table 3.
Summary of risk prediction models for total, high-grade prostate cancer among men undergoing biopsy
| (A) Total prostate cancer | |||||||
|---|---|---|---|---|---|---|---|
| Risk factor | Model 1a | Model 2b | Model 3c | ||||
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | ||
| log BMI-adjusted PSA | 3.79 (3.29, 4.37) | <0.001 | 3.74 (3.15, 4.44) | <0.001 | 3.69 (3.10, 4.40) | <0.001 | |
| Family history | Yes | 1.57 (1.31, 1.88) | <0.001 | 1.62 (1.35, 1.94) | <0.001 | 0.01 (0.0002, 0.71) | 0.03 |
| DRE | Abnormal | 2.26 (1.85, 2.75) | <0.001 | 2.22 (1.81, 2.72) | <0.001 | 2.20 (1.80, 2.70) | <0.001 |
| Prior negative biopsy | Yes | 0.43 (0.35, 0.51) | <0.001 | 0.44 (0.36, 0.53) | <0.001 | 0.44 (0.36, 0.53) | <0.001 |
| Race | AA | 1.32 (1.05, 1.66) | 0.02 | 1.33 (1.06, 1.67) | 0.01 | ||
| Age | 1.01 (1.00, 1.03) | 0.08 | 1.01 (1.00, 1.03) | 0.08 | |||
| PSA velocity >0.35 | Yes | 1.11 (0.91, 1.35) | 0.32 | 1.11 (0.91, 1.36) | 0.30 | ||
| Time between baseline and last biopsy | 0.90 (0.86, 0.94) | <0.001 | 0.90 (0.86, 0.94) | <0.001 | |||
| logBMI | 0.83 (0.45, 1.51) | 0.54 | |||||
| logBMI x Family history | Yes | 4.51 (1.28, 15.87) | 0.02 | ||||
| AUC | 0.722 | <0.001d | 0.723 | <0.001d | 0.723 | <0.001d | |
| 0.006e | 0.006e | ||||||
| 0.706f | |||||||
| (B) Gleason grade ≥7 prostate cancer | |||||||
|---|---|---|---|---|---|---|---|
| Risk factor | Model 1a | Model 2b | Model 3c | ||||
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | ||
| log BMI-adjusted PSA | 3.43 (2.82, 4.17) | <0.001 | 2.99 (2.37, 3.77) | <0.001 | 2.75 (2.18, 3.48) | <0.001 | |
| Age | 1.04 (1.03, 1.06) | <0.001 | 1.05 (1.03, 1.07) | <0.001 | 1.05 (1.03, 1.07) | <0.001 | |
| DRE . | Abnormal | 1.88 (1.48, 2.38) | <0.001 | 1.85 (1.45, 2.36) | <0.001 | 1.85 (1.45, 2.36) | <0.001 |
| Prior negative biopsy | Yes | 0.42 (0.32, 0.56) | <0.001 | 0.45 (0.34, 0.60) | <0.001 | 0.46 (0.35, 0.61) | <0.001 |
| Race | AA | 1.38 (1.04, 1.83) | 0.03 | 1.38 (1.03, 1.83) | 0.03 | 1.33 (0.99, 1.77) | 0.06 |
| Family history | Yes | 1.08 (0.85, 1.36) | 0.54 | 0.02 (<.001, 2.75) | 0.12 | ||
| PSA velocity >0.35 | Yes | 1.38 (1.04, 1.82) | 0.02 | 1.45 (1.09, 1.92) | 0.010 | ||
| Time between baseline and last biopsy | 0.95 (0.90, 1.01) | 0.12 | 0.95 (0.90, 1.01) | 0.12 | |||
| logBMI | 2.31 (1.08, 4.93) | 0.03 | |||||
| logBMI x Family history | Yes | 3.45 (0.76, 15.69) | 0.11 | ||||
| AUC | 0.716 | <0.001d | 0.718 | <0.001d | 0.724 | <0.001d | |
| 0.282e | 0.036e | ||||||
| 0.061f |
OR=odds ratio; AA = African American; AUC=area under ROC curve
Model 1 includes all risk factors included in the bmiPCPTRC for PCa
Model 2 includes all risk factors in Model 1 and four additional risk factors (race, age, PSA velocity and time between baseline and last biopsy)
Model 3 includes all risk factors in Model 2 and logBMI and the interaction between family history and logBMI.
p value for comparing the AUC with the AUC of 0.673 for BMI-adjusted PSA alone.
p value for comparing the AUC with the AUC for Model 1.
p value for comparing the AUC with the AUC for Model 2.
OR=odds ratio; AA = African American; AUC=area under ROC curve
Model 1 includes all risk factors included in the bmiPCPTRC for HGPCa
Model 2 includes all risk factors in Model 1 and three additional risk factors (family history, PSA velocity and time between baseline and last biopsy)
Model 3 includes all risk factors in Model 2 and logBMI and the interaction between family history and logBMI.
p value for comparing the AUC with the AUC of 0.673 for BMI-adjusted PSA alone.
p value for comparing the AUC with the AUC for Model 1.
p value for comparing the AUC with the AUC for Model 2.
Risk prediction for high-grade prostate cancer
The average bmiPCPTRC HGPCa risk for the entire cohort was significantly lower than the observed HGPCa rate; and in all subgroups except for African-Americans, the estimated HGPCa risk was lower than the observed HGPCa rate (Table 2, Column B). The calibration plot confirmed that the bmiPCPTRC generally underestimated the risk of HGPCa in this cohort (Figure 2B). The bmiPCPTRC had an AUC of 0.71 for the detection of HGPCa and it worked better among men with lower PSA values (<4 ng/mL, p=0.01) and among overweight men (p=0.008).
After calibration, risk of HGPCa increased rapidly as BMI increased (OR=2.31, p=0.03 for men without a known family history of PCa; OR=7.95, p=0.002 for men with a known family history of PCa; Table 3B, Model 3). In terms of AUC, the model with BMI-related predictors was significantly better than BMI-adjusted PSA alone (p<0.001) and the calibrated bmiPCPTRC (p=0.036), and it was moderately better than the model without BMI-related predictors (p=0.06).
Discussion
Both PCa and obesity affect substantial proportions of the male population. PCa is the second most commonly diagnosed cancer and the 6th most common cause of cancer-related mortality among men worldwide. 25 Among adult men in the U.S., 40% are overweight and 32% are obese. 26 There is increasing evidence that obesity is associated with elevated risk of HGPCa and increased PCa specific mortality.3 In this cohort from SELECT, we found that, after adjusting for other risk factors, BMI provided independently predictive information regarding risk of PCa and, more importantly, risk of HGPCa, especially among men with a known family history of PCa. For men without a known family history of PCa, BMI was not associated with risk of PCa but was significantly associated with elevated risk of HGPCa. For men with a known PCa family history, PCa and HGPCa risks all increased significantly with increases in BMI. Although these observed associations do not necessarily imply a causal role for BMI in PCa, our study showed that BMI was one of the factors that predict PCa on biopsy. The finding of this interaction between BMI and family history suggests that biological or environmental factors associated with obesity may amplify inherited genetic risk factors. One possible link could be in obesity related to diabetes and the metabolic syndrome. 27 In addition, overweight and obese men may have less healthy behaviors and more reluctant to screen for PCa than those of normal weight. However, for those overweight and obese men with a known family history of PCa, they may be more apt to get screened and thus have cancer detected. Another challenge, clinically, is that DRE can be difficult to perform in an obese man, masking the presence of prostate nodules, especially at the prostatic base. Studies have shown that obese men are less likely to have abnormal DREs diagnosed than non-obese men, and the predictive value of DRE is dependent upon obesity. 28 Therefore, BMI should be included in any clinical assessment of PCa risk and recommendations regarding prostate biopsy, especially among men with a family history of PCa.
This study is the first to externally validate the bmiPCPTRC in a healthy PSA screened contemporary population and, in so doing, we found that the bmiPCPTRC generally underestimated the risk of both PCa and HGPCa. There are several potential explanations for this phenomenon. First, higher PSA levels were allowed at study entry in SELECT compared to the PCPT study and larger percent of AA men were enrolled in SELECT than PCPT. Therefore, the SELECT population was inherently at higher risk of PCa than the PCPT population as evident in Table 2 that AA men had higher PCa/HGPCa rates than non-AA men and men with PSA≥4 ng/mL had higher PCa/HGPCa rates than men with PSA<4 ng/mL. Second, this study involved men who had for-cause biopsy as part of their regular care, and a major indicator for biopsy was elevated PSA. This selection bias would result in a study population with more cancers. Third, the bmiPCPTRC was built upon the original PCPTRC which relied on 6-core biopsies, and may lead to lower rates of PCa or HGPCa detection compared with the current standard of minimum 6-core biopsies. 29-30 Fourth, 614 men were excluded from the final analysis due to missing PSA, DRE, or BMI, and the PCa rate among these excluded men was significantly lower than those included in the analysis. This contributes to the higher PCa risk in the current cohort.
In this paper, we developed new risk prediction models for biopsy-detectable PCa and HGPCa, using the SELECT participants who underwent biopsy. The overall diagnostic performance of the new models in terms of AUC is significantly better than the BMI-adjusted PSA alone and the calibrated bmiPCPTRC.
Several potential limitations should be acknowledged. First, unlike the PCPT study where all participants received an end of study biopsy, not all participants in SELECT were biopsied. We included only biopsied SELECT participants in the analysis, which may have introduced selection bias that will drive down the diagnostic performance of PSA and bmiPCPTRC because most men have biopsy performed due to increased PSA values. Second, participants with missing PSA, DRE or BMI at or within one-year prior to the date of the latest biopsy were excluded from the analysis, which may leave bias in data findings as well. Third, family history and obesity known by the participants themselves may have influenced their decision to biopsy and this could have altered the results compared with the setting in PCPT where an attempt was made to biopsy everyone regardless of risk factor status. Fourth, the sample size for higher grade tumors was relatively small (n=206 for Gleason score 4+3 and above; n=105 for Gleason score≥8) in this cohort. Continued efforts are needed to develop risk prediction models for high-grade/aggressive cancers in large prospective studies. Finally, there was strong evidence that BMI increased the risk of PCa and even HGPCa, especially among men with a family history of PCa. However, without mortality data, this study cannot examine the impact of BMI on the survival of men diagnosed with PCa.
Conclusions
BMI provided independently predictive information regarding risks of PCa and HGPCa, after adjusting for other risk factors. BMI, especially among men with a family history of PCa, should be considered for inclusion in clinical assessment of PCa risk and recommendations regarding prostate biopsy.
Acknowledgements
Data were provided by the Southwest Oncology Group (SWOG). SWOG is a clinical trials cooperative group supported by the National Cancer Institute (NCI). This manuscript was prepared using a limited access data set obtained from SWOG and does not necessarily reflect the opinions or views of SWOG or the NCI.
Grant Support: Funding was provided in part by the Cancer Center Support Grant for the Cancer Therapy & Research Center at the University of Texas Health Science Center at San Antonio [P30CA054174], and a grant from the Early Detection Research Network, National Cancer Institute [U01-CA086402], and by Public Health Service grant CA37429 from the National Cancer Institute, Division of Cancer Prevention.
Abbreviations
- AUC
Area Under the ROC Curve
- BMI
Body Mass Index
- DRE
Digital Rectal Exam
- bmiPCPTRC
BMI-adjusted PCPTRC
- HGPCa
High-Grade Prostate Cancer
- PCa
Prostate Cancer
- PCPT
Prostate Cancer Prevention Trial
- PCPTRC
Prostate Cancer Prevention Trial prostate cancer Risk Calculator
- PSA
Prostate Specific Antigen
- ROC
Receiver Operating Characteristic
- SELECT
Selenium and Vitamin E Cancer Prevention Trial
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our subscribers we are providing this early version of the article. The paper will be copy edited and typeset, and proof will be reviewed before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to The Journal pertain.
Conflicts of Interest: None
References
- 1.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 2.Dimitropoulou P, Martin RM, Turner EL, et al. Association of obesity with prostate cancer: a case-control study within the population-based PSA testing phase of the ProtecT study. Br J Cancer. 2011;104:875–81. doi: 10.1038/sj.bjc.6606066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emma HA, Elizabeth MM, Stephen JF. Obesity and Prostate Cancer: Weighing the Evidence. European urology. 2012;63:800–9. doi: 10.1016/j.eururo.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. New England Journal of Medicine. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 5.Wright ME, Chang SC, Schatzkin A, et al. Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer. 2007;109:675–84. doi: 10.1002/cncr.22443. [DOI] [PubMed] [Google Scholar]
- 6.Giovannucci E, Rimm EB, Liu Y, et al. Body mass index and risk of prostate cancer in U.S. health professionals. Journal of the National Cancer Institute. 2003;95:1240–4. doi: 10.1093/jnci/djg009. [DOI] [PubMed] [Google Scholar]
- 7.Gallina A, Karakiewicz PI, Hutterer GC, et al. Obesity does not predispose to more aggressive prostate cancer either at biopsy or radical prostatectomy in European men. International journal of cancer Journal international du cancer. 2007;121:791–5. doi: 10.1002/ijc.22730. [DOI] [PubMed] [Google Scholar]
- 8.Freedland SJ, Giovannucci E, Platz EA. Are findings from studies of obesity and prostate cancer really in conflict? Cancer causes & control : CCC. 2006;17:5–9. doi: 10.1007/s10552-005-0378-3. [DOI] [PubMed] [Google Scholar]
- 9.Lima N CH, Knobel M, Halpern A, Medeiros-Neto G. Decreased androgen levels in massively obese men may be associated with impaired function of the gonadostat. Int J Obes Relat Metab Disord. 2000;24:1433–7. doi: 10.1038/sj.ijo.0801406. [DOI] [PubMed] [Google Scholar]
- 10.Platz EA, Leitzmann MF, Rifai N, et al. Sex Steroid Hormones and the Androgen Receptor Gene CAG Repeat and Subsequent Risk of Prostate Cancer in the Prostate-Specific Antigen Era. Cancer Epidemiology Biomarkers & Prevention. 2005;14:1262–9. doi: 10.1158/1055-9965.EPI-04-0371. [DOI] [PubMed] [Google Scholar]
- 11.Severi G, Morris HA, MacInnis RJ, et al. Circulating Steroid Hormones and the Risk of Prostate Cancer. Cancer Epidemiology Biomarkers & Prevention. 2006;15:86–91. doi: 10.1158/1055-9965.EPI-05-0633. [DOI] [PubMed] [Google Scholar]
- 12.Roddam AW, Allen NE, Appleby P, Key TJ. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst. 2008;100:170–83. doi: 10.1093/jnci/djm323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freedland SJ, Banez LL, Sun LL, Fitzsimons NJ, Moul JW. Obese men have higher-grade and larger tumors: an analysis of the duke prostate center database. Prostate Cancer Prostatic Dis. 2009;12:259–63. doi: 10.1038/pcan.2009.11. [DOI] [PubMed] [Google Scholar]
- 14.Discacciati A, Orsini N, Wolk A. Body mass index and incidence of localized and advanced prostate cancer—a dose–response meta-analysis of prospective studies. Annals of Oncology. 2012;23:1665–71. doi: 10.1093/annonc/mdr603. [DOI] [PubMed] [Google Scholar]
- 15.Baillargeon J, Pollock BH, Kristal AR, et al. The association of body mass index and prostate-specific antigen in a population-based study. Cancer. 2005;103:1092–5. doi: 10.1002/cncr.20856. [DOI] [PubMed] [Google Scholar]
- 16.Werny DM, Thompson T, Saraiya M, et al. Obesity Is Negatively Associated with Prostate-Specific Antigen in U.S. Men, 2001-2004. Cancer Epidemiology Biomarkers & Prevention. 2007;16:70–6. doi: 10.1158/1055-9965.EPI-06-0588. [DOI] [PubMed] [Google Scholar]
- 17.Beebe-Dimmer JL, Faerber GJ, Morgenstern H, et al. Body composition and serum prostate-specific antigen: review and findings from Flint Men's Health Study. Urology. 2008;71:554–60. doi: 10.1016/j.urology.2007.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang Y, Ankerst DP, Sanchez M, Leach RJ, Thompson IM. Body Mass Index Adjusted Prostate-specific Antigen and Its Application for Prostate Cancer Screening. Urology. 2010;76:1268, e1–6. doi: 10.1016/j.urology.2010.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grubb RL, Black A, Izmirlian G, et al. Serum Prostate-Specific Antigen Hemodilution Among Obese Men Undergoing Screening in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Epidemiology Biomarkers & Prevention. 2009;18:748–51. doi: 10.1158/1055-9965.EPI-08-0938. [DOI] [PubMed] [Google Scholar]
- 20.Bañez LL, Hamilton RJ, Partin AW, et al. OBesity-related plasma hemodilution and psa concentration among men with prostate cancer. JAMA. 2007;298:2275–80. doi: 10.1001/jama.298.19.2275. [DOI] [PubMed] [Google Scholar]
- 21.Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carter HB, Ferrucci L, Kettermann A, et al. Detection of life-threatening prostate cancer with prostate-specific antigen velocity during a window of curability. J Natl Cancer Inst. 2006;98:1521–7. doi: 10.1093/jnci/djj410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 24.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 25.Center MM, Jemal A, Lortet-Tieulent J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–92. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 26.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 27.Gong Z, Neuhouser ML, Goodman PJ, et al. Obesity, diabetes, and risk of prostate cancer: results from the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2006;15:1977–83. doi: 10.1158/1055-9965.EPI-06-0477. [DOI] [PubMed] [Google Scholar]
- 28.Chu DI, De Nunzio C, Gerber L, et al. Predictive value of digital rectal examination for prostate cancer detection is modified by obesity. Prostate Cancer Prostatic Dis. 2011;14:346–53. doi: 10.1038/pcan.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canto EI, Singh H, Shariat SF, et al. Effects of systematic 12-core biopsy on the performance of percent free prostate specific antigen for prostate cancer detection. The Journal of urology. 2004;172:900–4. doi: 10.1097/01.ju.0000134619.72675.8d. [DOI] [PubMed] [Google Scholar]
- 30.Motoi Tobiume YY, Nakamura Kogenta, Honda Nobuaki. Retrospective Study Comparing Six- and Twelve-Core Prostate Biopsy in Detection of Prostate Cancer. International Braz J Urol. 2008;34:9–14. doi: 10.1590/s1677-55382008000100003. [DOI] [PubMed] [Google Scholar]