Abstract
The goal of the current manuscript was to replicate published data that show intrathecal infusions of Taxol ® (paclitaxel), an anti-neoplastic microtubule stabilizing agent, reduce fibrogliotic scarring caused by a dorsal spinal hemisection (DHx) injury and increase functional recovery and growth of serotonergic axons after moderate spinal contusion injury. These experiments were completed as part of an NIH-NINDS contract entitled “Facilities of Research Excellence – Spinal Cord Injury (FORE-SCI) – Replication”. Here, data are presented that confirm the anti-scarring effects of Taxol after DHx injury; however, Taxol did not confer neuroprotection or promote serotonergic axon growth nor did it improve functional recovery in a model of moderate spinal contusion injury. Thus, only partial replication was achieved. Possible explanations for disparate results in our studies and published data are discussed.
Keywords: scar formation, spinal cord injury, microtubule, hemisection, axon, serotonin, replication, regeneration, contusion, behavior
Introduction
Following traumatic spinal cord injury (SCI), microtubule stability is necessary for a wide range of responses, including cell survival, cell proliferation, migration of peripheral cells and glia, intracellular signaling and axon transport, and growth of injured and spared axons. Paclitaxel (Taxol®) is an FDA-approved anti-cancer drug that stabilizes microtubules and inhibits mitotic spindle assembly (Vyas and Kadow, 1995). Recent data indicate that this drug could also be a neuroregenerative therapy. Indeed, Taxol can prevent axon retraction, enhance axon growth, reduce leukocyte infiltration and migration and reduce fibrotic scarring, in part by inhibiting cell proliferation and secretion of extracellular matrix (ECM) molecules (Hellal et al., 2011; Erturk et al., 2007; Sengottuvel et al., 2011)
In a recent publication, Hellal et al. showed that infusion of low dose Taxol at the site of SCI reduced glial and mesenchymal scar formation and increased numbers of serotonergic (5HT) axons below the lesion. These anatomical changes were associated with improved behavioral recovery in a rat spinal cord contusion injury model (Hellal et al., 2011). In consideration of the promising translational potential for this drug, the present study was performed to provide independent replication of the original findings, under the guidelines of a Facilities of Research Excellence in Spinal Cord Injury (FORE-SCI) contract with the National Institute of Neurological Disorders and Stroke (NINDS). For this experiment, we attempted to replicate the primary histological and/or behavioral effects of Taxol infusion for 7 days following a mid-thoracic dorsal hemisection (DHx) or for 28 days following a moderate mid-thoracic spinal contusion injury. Here, partial replication was achieved. Specifically, the anti-scarring effects of Taxol were confirmed in both models of SCI; however, Taxol did not affect serotonergic axon density below the lesion, nor did it improve functional recovery in a model of spinal contusion injury.
Materials and methods
All methods and data were reported with consideration of guidelines provided by Animals in Research: Reporting in Vivo Experiments (ARRIVE) and Minimum Information About a Spinal Cord Injury Experiment (MIASCI) Kilkenny et al., 2010; Lemmon et al., 2014).
Animal group sizes, power calculations and group designations
The effort to replicate Hellal et al. was completed in two phases. Phase 1 targeted Figure 1 from Hellal et al. in which Taxol was shown to reduce scarring in a rat hemisection lesion model (Hellal et al., 2011). Phase 2 was designed to replicate Hellal's Fig. 4E&F, where Taxol was shown to increase 5HT axon labeling below the site of injury and improve behavioral recovery after spinal contusion injury. As the goal was to examine the potential for translation, we were not charged to replicate data in Fig. 4A-D, which show that Taxol improves axon growth following a peripheral conditioning lesion.
Figure 1.
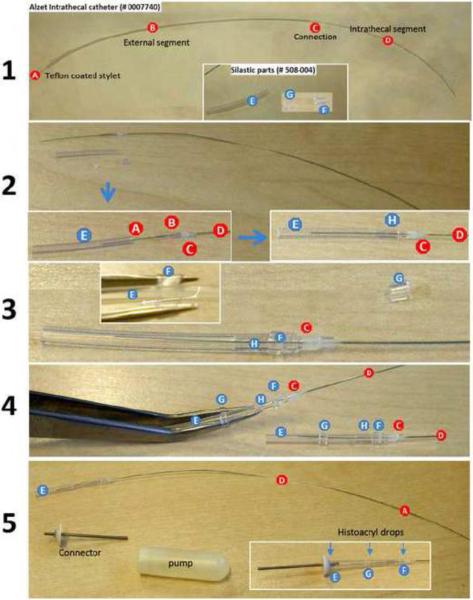
Custom designed catheters for intrathecal delivery of cremaphor vehicle and/or Taxol. A rat intrathecal catheter (Alzet #0007740) was modified by inserting a segment of silastic tubing at the junction between the osmotic pump and the remaining segment of intrathecal catheter. (1) Briefly, intrathecal catheters, comprised of a thin Teflon-coated wire stylet (A), an external catheter segment (B), a connector segment (C) and the intrathecal segment (D) were cut to a length of ~50 mm. Three separate pieces of silastic tubing (Dow Corning; #508-004) also were prepared for each catheter; two cuffs (4mm and 2mm) and a single 20 mm segment (E, F, G). (2) The external segment of catheter is cut to a length of ~8mm (B) then is inserted along with the stylet (A) into one end of the 20 mm segment of silastic tubing (E) until it abuts the connector (C) just proximal to the distal catheter segment (D). (3) A 4 mm silastic cuff (F) was slipped over this junction (H) then the external catheter segment was connected to the metal hub of the osmotic pump. (4) The remaining 2 mm silastic cuff (G) was placed mid way over the distal 20 mm segment of silastic tubing where it serves as an anchor point for securing sutures on each side of the cuff. (5) Finally, a few drops of Histoacryl® (TissueSeal, LLC; #TS1050071FP; Ann Arbor, MI) was applied to all cuffs (F,G) and at the interface between the long segment of silastic tubing (E) and the pump connector. This seals all connections and prevents leakage.
Figure 4.
Expanded anatomical analysis of spinal cord hemisection lesion after 7-day infusion with vehicle or Taxol. (A) Lesion centers from two vehicle and two Taxol specimens stained with EC/CV. Note the extended cap of connective tissue (black arrows) that is continuous with the lesion matrix in tissues from vehicle treatment group. These are absent in specimens from the Taxol-treated group (scale = 500 μm). (B) Taxol has no effect on the dorsal-ventral (DV) lesion length or total volume as measured from EC/CV stained sections. (C) Taxol reduces deposition of laminin, collagen IV and fibronectin (scale = 400 μm) and alters CS56 expression within the lesion (scale = 200 μm). Note how the diffuse pattern of fibronectin labeling in vehicle treated (white arrow) contrasts with the dense fibrillar staining pattern in Taxol-treated specimens (double white arrows). (D) GFAP- immunoreactivity lines the lesion border in both vehicle and taxol treated specimens. (F) Immunofluorescence intensity was measured in a constant region of interest (0.04 mm2 sample box) positioned at the rostral lesion border (scale = 200 μm). Both fibronectin and GFAP labeling were increased at this site after Taxol infusion. (F) Qualitative analyses reveal fewer neurofilament-positive axons and NG2+ profiles (asterisks) in Taxol-treated specimens (scale = 100 μm).
Prior to starting phase 1, n=13 animals were used in pilot studies to establish consistent injury technique and catheter placement. No quantitative data were generated from these animals. For replication of Fig. 1, animal group sizes were matched to those described in the original manuscript (n=14/grp). Before attempting to replicate Fig. 4E&F, optimal group sizes were calculated via power analyses. Using raw data from Fig. 4E only (anatomical analysis of 5-HT+ fibers) it was determined that a 50% increase in the number of 5HT+ fibers would yield power of 0.8 with α=0.05 using n=7 rats/group. However, using raw data from Fig. 4F (provided by the original authors), power calculations for a 2 way ANOVA indicated that hundreds of animals/group were necessary to sufficiently power a replication of the effects of Taxol on behavioral recovery. This was impractical under the auspices of the replication contract, so logistics (e.g., timing, expense) and past experience with this type of injury and behavioral analyses were used as criteria to determine group size. To minimize surgical, cohort, and dayto-day variations, all experiments were designed such that control and Taxol-treated animals could be prepared on the same day and surgeries for each phase were completed over a 2 day period.
For each phase of replication, GraphPad's QuickCalcs software was used to randomly assign animals into one of two groups: (1) control/vehicle or (2) Taxol. In phase 1, a total of 28 animals received laminectomy or bilateral dorsal spinal hemisection injuries and 100% survived for the 7 day study period. For phase 2, 24 animals received a laminectomy or spinal contusion injury and 100% survived for the duration of the 8 week study period. A total of five animals were excluded from data analyses; one animal died before injury but was replaced and an additional four animals were excluded from phase 2 analysis based on post-mortem inspection of their intrathecal catheters. Specifically, catheters were found on top of the dura in two vehicle and one Taxol-treated contusion animal. Thus, we could not be certain that drug or vehicle was delivered consistently to the injury site throughout the duration of the study. In one other animal (vehicle group), the catheter tip had penetrated into and damaged the spinal cord.
Drug preparation
Taxol (Paclitaxel; cat #9600 LC Laboratories; Woburn, MA) was purchased just prior to the start of the first experiments and stored as a lyophilized powder at −20°C until use. Hellal et al. used 5 different lots of Taxol and confirmed efficacy of each lot by quantifying Taxol-induced changes in axon formation in embryonic neurons (personal communication). For replication, a single lot of Taxol (#ASM-114) was used. A stock solution of Taxol was prepared by adding 2.34 ml of DMSO (#D-2438 Sigma; St. Louis, MO) to 100 mg of Taxol. Stock vials (25μl aliquots, 42.7 mg/ml) were prepared and stored at −20°C. Working solutions for use in osmotic pumps were diluted with a 1:1 mixture of Cremophor Oil (#C-5135 Sigma; St. Louis, MO) and absolute ethanol (EtOH; #E-7023 Sigma Aldrich). The Cremophor Oil/EtOH vehicle was prepared by combining 100 ml of Cremophor Oil with 100 ml of EtOH and mixing until a homogenous solution (no visible phases) was achieved.
Alzet mini-osmotic pumps model #2004 (200 μl, 0.25 μl/hr; Durect Corp; Cupertino, CA) were used to infuse Taxol or vehicle (Cremaphor Oil/ETOH). To fill the pumps, the Taxol stock vial solution was diluted in the Cremophor Oil /EtOH mix (50 μM to achieve a rate of delivery of 256 ng Taxol/24 hrs). After mixing, the solution was allowed to settle to remove bubbles. To avoid interfering with the osmotic function of the pumps, care was taken to prevent Taxol or vehicle from contacting external surfaces of the pump during loading. If solution came into contact with the external pump surface, the pump was discarded. Pumps were primed before use in animals by submerging in sterile 0.9% saline in 50 ml cell culture tubes at 37°C for 48 hours.
Preparing custom intrathecal catheters
To ensure consistent intrathecal infusion of Taxol or vehicle, catheters were prepared according detailed designs provided by Dr. Hellal who developed this design through an iterative process over several years. Through discussion with her, we learned that conventional intrathecal catheters did not provide optimal delivery of Taxol solution or EtOH/Cremophor vehicle. All preparation details and materials are described in the legend that accompanies Fig. 1.
Spinal cord injury modeling and intrathecal delivery of drug or vehicle
To minimize variability between groups and different experiments, a single individual performed all laminectomies and spinal cord injuries. Immediately post-injury a second laminectomy was performed at T12/13 to allow insertion of the intrathecal catheter. Two expert surgeons performed catheter insertions and pump placements with equal distribution between surgeons and experimental groups. Surgeons were blinded to experimental groups. Female Sprague–Dawley (SD) rats (Harlan, Indianapolis, IN), ~10 weeks old and ~215 g were anesthetized with a ketamine/xylazine cocktail (80/10 mg/kg, i.p.). Hellal et al. used Dormitor (medetomidine hydrochloride) instead of xylazine, but both drugs are alpha-2 adrenergic agonists (analgesics and muscle relaxants). The skin overlying the thoracic spinal cord was shaved then swabbed with a sequence of betadine scrub, 80% ethanol then betadine solution. Body temperature was maintained at ~37 °C using a homeothermic blanket (World Precision Instruments; Model ATC1000). After surgery (laminectomy with or without SCI) and catheter placement, the muscle was closed in layers then the skin incision was closed with wound clips. Animals recovered from surgery in cages maintained at 37°C and received daily injections of Gentocin (5 mg/kg; s.c.) and 2–5 ml 0.9% saline (s.c.) for 7 days. Bladders were expressed manually 2x/day until spontaneous voiding returned.
Dorsal spinal hemisection (DHx) injury
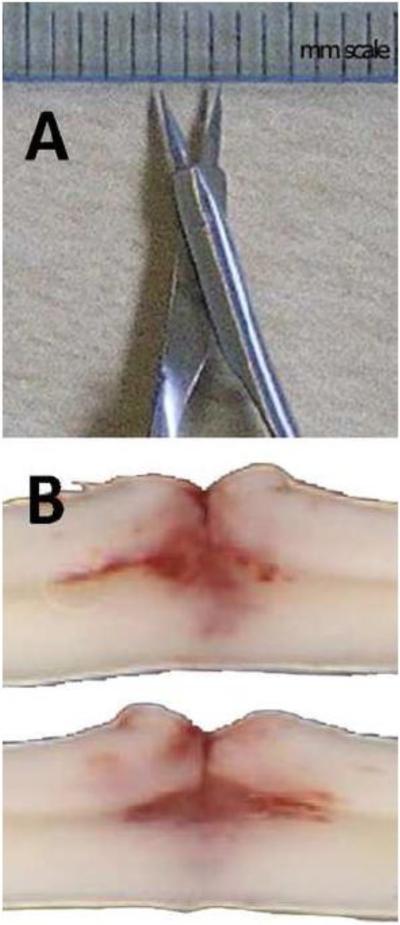
To create DHx lesions similar to those described in Hellal et al., iridectomy scissors (Fine Science Tools: cat# 15000-03; Foster City, CA) were modified based on instructions provided by the original authors. Scissor handles were bent so that the blades started in an open position with a gap of ~2.5-3 mm (Fig. 2A). When placed over the exposed dorsal surface of the spinal cord, closing the scissor handles provided a smooth single action cut of defined depth and width in each animal. Thoracic vertebrae (T6–T13) were exposed via incision and a laminectomy was performed at T9. In order to accommodate the modified iridectomy scissors, a slightly larger laminectomy site was created for DHx as compared to contusion injury (~3.5-4 mm vs 3 mm). The open scissor blades were carefully placed over the mid-line of the dorsal surface of the spinal cord between the intact dura and bone then were quickly closed and immediately released. A single closure of the scissors was used to completely transect the dorsal spinal cord to the depth of the central canal (Fig. 2B).
Figure 2.
(A) Modified iridectomy scissors (see methods for details) used to create dorsal spinal hemisection (DHx) lesions. (B) Examples of two spinal cords that were “quick-frozen” then scanned to analyze depth and consistency of DHx.
After injury, blunt dissection was used to create a subcutaneous pocket proximal to the base of the tail. Primed osmotic mini-pumps with catheter attached were placed into the pocket then, a second laminectomy was performed at vertebral level T12. The catheter was inserted into a hole made in the dura then was advanced rostrally until the tip could be seen centered on the dorsal surface of the spinal cord at T9. To secure the catheter tip and prevent rostro-caudal movement, the exposed portion of the catheter at T12 was sutured on both sides of the 2 and 4 mm cuffs (see Fig. 1). Visible portions of the catheter were covered with surrounding fascia and muscle then the muscle overlying the T9 laminectomy site was sutured.
Spinal contusion injury
Thoracic vertebrae (T6–T10) were exposed via incision and a laminectomy was performed at T8. Injuries were performed using an Infinite Horizons impactor (Precision Systems, Kentucky, IL) equipped with a 2.5 mm tip. The target injury force was 150kDyn. Injury variability was again minimized by designating a single individual for performing all contusions and was monitored by recording the actual force and cord displacement at time of injury. Moving averages and standard deviations for these parameters were reviewed during the day of surgery to ensure that variations in primary trauma were equally distributed between the two experimental groups. After injury, the second laminectomy and pump placement and catheter insertion was the same as for the dorsal hemisection model except that the dura was not opened over the contusion site and the catheter tip was placed just caudal to the visible bruise.
After 28 days the animals were anesthetized via brief (<20 min) inhalation of an isoflurane/O2 mixture and a small incision was made to reveal the pump and its catheter connection. Care was taken to not disturb the catheter. To prevent leakage of CSF as pumps were removed, catheters were sealed by ligation with 4-0 suture before excising the pumps from the catheter. To confirm that excised pumps were functional, they were removed with some tubing attached then placed in 15ml tubes containing 0.9% saline. Any catheters that were found outside of the dura or sutures or that were disconnected from the pump were noted and were used as exclusion criteria (see above). Ligated catheters (no pump) remained in place for the duration of the study then were removed one day after all behavioral tasks were completed.
Behavioral outcome measures
A grid/ladder walking task was the sole behavioral assay used by Hellal et al. An identical assay was performed in this replication effort; however, this task was supplemented with periodic analysis of open-field locomotion using the Basso–Beattie–Bresnahan (BBB) locomotor rating scale (Basso et al., 1995). Two individuals, who were blind to group assignment, performed all behavioral analyses. BBB scoring was completed at 1, 3, 5 and 7dpi and then weekly thereafter for 8 weeks. Contusion injured rats also were evaluated on a ladder/grid walk task at 2, 4, 6 and 8 weeks post-injury. For the grid task, rats crossed a 1 m long horizontal runway with walls 10 cm high outfitted with rungs elevated approximately 15 cm from the ground. Rungs were placed at irregular intervals (1-4 cm spacing) to prevent habituation and were changed for every testing session. Rung distance interval was randomly determined for each time point using GraphPad's QuickCalcs software. Each animal crossed the grid twice (2 trials). All trials were recorded using a Sony HDR-SR11 HD 1080 video camera. An individual rater, unaware of treatment or group designation, evaluated each hind limb for mistakes (combination of complete misses and/or slips from the rungs) from videos via slow motion playback. All analyses were performed over a defined sector (60 cm) containing a fixed number of rungs.
Animals also were evaluated for spontaneous activity for 15 different parameters, using an activity monitoring device (Opto-M3, Columbus Instruments, Columbus, OH). Animals were placed in activity boxes for 30’. Data for each 30’ period was divided into three 10’ segments. Animals were tested pre-injury and then at 1, 3, 5 and 7 weeks post injury. If BBB testing occurred on a day when other behavioral testing was scheduled (activity box or ladder/grid) then a minimum of 2 hours elapsed before the second test. BBB open field scores were always evaluated first.
Histological outcomes
At 7 (phase 1) or 56 (phase 2) days post-injury (dpi), rats were anesthetized with ketamine (150 mg/kg) and xylazine (15 mg/kg) then were euthanized via intracardiac perfusion with 0.1 M phosphate buffered saline (PBS) followed by 4% paraformaldehyde in PBS. A 15 mm segment of spinal cord centered on the injury site was removed, post-fixed for 2h in 4% PFA, rinsed overnight in 0.2M PBS, equilibrated in 30% sucrose then embedded in optimal cutting temperature (OCT) Tissue-Tek™ media. Longitudinal sections of 25 μm thickness were cut in the sagittal plane and mounted on glass slides in adjacent series such that each specimen had 10 adjacent series of equally spaced sections ~250 μm apart. Slides with sections from all specimens were stained at the same time with the same solutions. An investigator with no knowledge of treatment group did all staining and quantification of histology. Spinal cords from rats that were excluded based on pump or catheter anomalies were not analyzed.
To identify matrix and cellular markers associated with scar formation, sections through the lesion site were stained with primary antibodies listed in Table 1. AlexaFluor-conjugated secondary antibodies were used for detection. Replicating the approach used by Hellal et al., for quantitative analysis, wide-field fluorescent images containing the full lesion site were collected from three sections using a 2.5× objective and Sony 970 CCD integrating camera. For each specimen, 3 images were measured including the center-most section (area of greatest damage) and sections 250 μm left and right of center. An MCID Elite 7.0 image analysis system (Imaging Research) was used to capture and analyze digital images.
Table 1.
Primary antibodies and dilutions used for immunohistochemistry
| Antigen | Antibody | Recognizes | Species and Isotype | Source | Cat # Lot # | Dilution |
|---|---|---|---|---|---|---|
| Collagen IV | Purified polyclonal | Mouse tumor collagen IV | Rabbit polyclonal | Millipore | AB756P-NG1853860 | 1:400 |
| Laminin | Affinity purified polyclonal | EHS mouse sarcoma | Rabbit polyclonal | Sigma-Aldrich | L9393-041M4799 | 1:400 |
| Fibronectin | Affinity purified polyclonal | Human fibonectin | Rabbit polyclonal | Sigma-Aldrich | F3648-051M4777 | 1:200 |
| CSPG-GAGs | Clone CS56 ascites | Chicken fibroblast CSPGs | Mouse IgM | Sigma-Aldrich | C8035-071M4864 | 1:200 |
| GFAP | Chicken purified polyclonal | Recombinant GFAP | Chicken IgY | Aves Labs | GFAP-NA | 1:200 |
| GFAP | Rabbit polyclonal | Cow spinal cord GFAP | Rabbit polyclonal | Dako | Z0334-096 | 1:2000 |
| 5-HT | Goat polyclonal | 5-HT coupled to BSA and PFA | Goat IgG polyclonal | Immunostar | 20079-947001 | 1:2000 |
| Neurofilament | Chicken purified polyclonal | Bovine NF-200 kDa | Chicken IgY | Aves Labs | NFH-HS0506 | 1:200 |
| NG-2 | Immunopurified polyclonal | Rat NG2 | Rabbit polyclonal | Millipore | AB5320-2000807 | 1:200 |
| Secondary Antibodies | |
|---|---|
| Alexafluor goat anti-rabbit 488 | 1:200; 1:400; 1:500; 1:1000 |
| Alexafluor goat anti-rabbit 546 | 1:200 |
| Alexafluor goat anti-chicken 568 | 1:400; 1:500 |
| Alexafluor goat anti-mouse IgM 488 | 1:200 |
| Alexafluor donkey anti-goat 546 | 1:200 |
Lesion size was determined from a series of equally spaced sections spanning the full length and width of the spinal cord and centered on the injury site. Sections were stained with modified Eriochrome Cyanine and Cresyl Violet (EC/CV). A digital image of each section was collected using a Zeiss Axiophot microscope and 10x objective equipped with Sony CCD970 camera. Lesion area and maximal dorso-ventral height of the hemisection lesions were measured on digital images of the center most section using MCID. Lesion volumes were then calculated using a modification of the Cavalieri principle, where Volume = sum of (lesion area (pixels converted to μm2) * slice thickness (distance between sections in μm)) for ~10 evenly spaced sections with a random start, and spanning the full left-right extent of the spinal cord. Collagen IV, fibronectin and laminin labeling was quantified within DHx lesions using methods as described by Hellal et al (Hellal et al., 2011). A sample box of 3.2 mm2 was placed in the center of the injury on each image, and the proportional area of positive staining was determined for each section. Values for 3 sections per specimen (from the center of the lesion and 250 μm on either side of center) were averaged to obtain 1 value per animal. The treatment code was then broken and each value was expressed as a percentage of the mean value for the vehicle treatment group. For analysis of these features in a contusion lesion (not included in Hellal et al.), the approach was similar, but due to variations in the topography of the central necrotic region, the target stain area was measured using a 5× objective and expressed as a proportion of the total area of the lesion bounded by reactive astrocytes.
Antibodies specific for chondroitin sulfate (clone CS-56) were used to label chondroitin sulfate proteoglycans (CSPGs), i.e., inhibitory molecules that increase at the lesion site following SCI. The area of CS-56-staining was measured within a sample box of 1.28 mm2 (1.4 mm × 0.9 mm) that spanned the dorsal-ventral extent at the rostral and caudal borders of the contusion lesion site. Data are expressed as a proportional area, i.e., CS-56+ labeling/area of sample box.
Hellal et al also used an integer rating scale to score glial fibrillary acidic protein (GFAP)-positive astrocyte reactivity in hemisection lesions (phase 1). Three sections per animal were viewed in a wide-field fluorescence microscope and each 200 μm distance from the lesion edge was qualitatively scored with a 0, 1, 2, or 3 where 0 = not different from naïve GFAP staining; 1 = evidence of astrocyte morphological change or orientation; 2 = obvious hypertrophy and some overlap of thick GFAP+ processes; 3 = dense meshwork of GFAP+ processes and presence of a basal lamina (Hellal et al., 2011; Hsu et al., 2006). The average fluorescence intensity of GFAP+ staining adjacent to the lesion edge also was measured using MCID density analysis software. For this measure, a sample box of 0.04 mm2 was positioned directly over the rostral lesion border and the recorded intensity expressed in arbitrary units. Fluorescence intensity for fibronectin staining also was recorded. All intensity measures were performed during a single analysis session with identical light, filter, camera and software settings.
Anti-5HT antibodies were used to label serotonergic fibers at 56 dpi in contusion lesion specimens. 5HT+ axons were quantified using the method of Hellal et al. A sample field including only the dorsal half of the spinal cord caudal to the lesion site was examined with a wide-field fluorescent microscope and 40× objective. All 5-HT positive axon profiles within the sample region were tallied for each of 3 sections per specimen.
For illustrations, bright-field images were collected using the MCID analysis system and saved in .TIF format. Fluorescence images for illustrations were obtained by confocal microscopy using an Olympus FV1000 spectral scanning laser microscope with appropriate filters. For illustration of the large contusion lesion sections, multiple confocal images were collected using a 10× air objective. Final images were assembled using Photoshop Photomerge® (Adobe, Inc.). In some cases, brightness and/or contrast settings were adjusted for illustrations only; when these were applied, identical settings were used for representative vehicle and Taxol specimen images.
Statistical analysis
Investigators without knowledge of treatment acquired all data. After breaking the treatment code, placeholder group designations were assigned (X or Y) during data analysis. Data analyses were performed using Prism 5.0 (Graphpad Software, Inc.). All comparisons between treatment groups were made using Student's t-tests (two-sided). In some instances, the calculated group variances were heterogeneous, so Welch's correction was applied to the comparison test. For comparisons where multiple measures were made from the same specimen (e.g., CS-56 staining rostral and caudal to the lesion), 2-way ANOVA with repeated measures was used to determine main effects of site and treatment. Differences were considered significant when p was ≤0.05. To be consistent with data presentation in Hellal et al., all data are presented as mean values ± SEM.
Results
Pilot studies
Before initiating the phase 1 experiments, a pilot study was completed to ensure that the modified scissors and catheters worked as described by Hellal at el. A total of eleven animals were used to verify lesion consistency and proper placement and method for securing catheters. As an example, animals receiving a dorsal spinal hemisection injury were euthanized immediately after injury via intracardiac perfusion with 0.9% saline and 4% paraformaldehyde. The spinal cords were removed then were frozen with dry ice powder to a semi-solid consistency. Spinal cords were sliced in the sagittal plane through the middle of the injury site then were imaged on a flat bed scanner (Fig. 2). Using this approach, the surgeon received feedback regarding depth and width of the injury and any necessary adjustments were made before another animal was used.
Even though we have experience making and implanting intrathecal catheters, FH cautioned us that sustained delivery to the lesion site of the Taxol/Cremophor mixture is not trivial and that she spent years perfecting the design and optimal placement of intrathecal catheters. Because we did not want to introduce additional variables that could confound the replication effort, we built catheters using her specifications (Fig. 1). These catheters were implanted into rats then digital images were sent to FH for feedback. This feedback resulted in slight modifications being made to our method for securing catheters and their placement relative to the lesion site before we initiated formal Phase 1 replication experiments.
Phase 1 - Evaluating the effects of Taxol on ECM deposition and lesion pathology after dorsal spinal hemisection injury
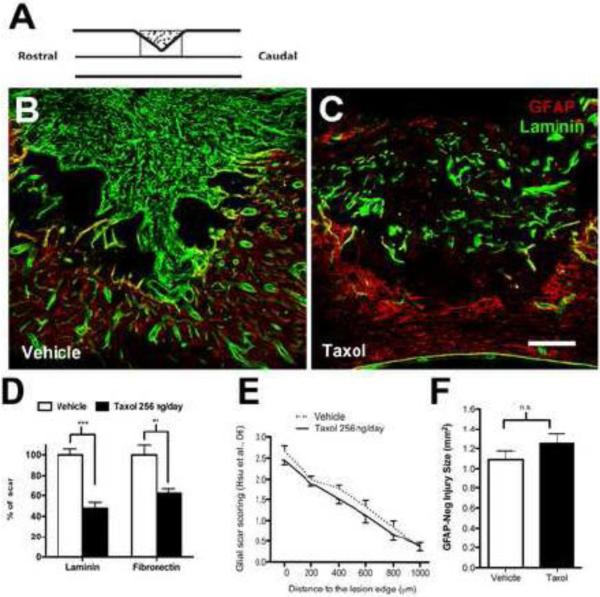
Consistent with data reported by Hellal et al., we found that Taxol significantly reduced scarring at the injury site (Fig. 3A-D). Specifically, laminin or fibronectin staining was reduced ~50% in DHx lesions infused with Taxol as compared to lesions receiving vehicle infusions. Also, in accordance with Hellal et al, Taxol-mediated inhibition of lesion fibrosis did not alter the general features of the astrogliotic scar; the overall pattern of GFAP staining (glial scar score; Fig. 3E) and the GFAP-negative lesion areas (Fig. 3F) were unaffected by Taxol.
Figure 3.
Taxol infusion reduces fibrotic scarring in acute dorsal spinal hemisection lesions (cf. Fig. 1 in Hellal et al). (A) Schematic illustrating location (box) where images were captured for analysis. (B,C) Mid-sagittal sections through boxed region in (A) showing distinct staining patterns for laminin (green) and GFAP (red) within the lesion site following vehicle or Taxol infusion. (D) Taxol decreased laminin and fibronectin staining in the lesion, expressed as % of the average value from vehicle group (2- tailed unpaired t-test, ***p<0.001; fibronectin staining t-test with Welch's correction **p<0.01). (E) GFAP+ stain at the lesion border was scored using the 0-3 scale used by Hellal. Two-way ANOVA with repeated measures (distance) revealed significant effects of distance and subject matching (p<0.0001), but no effect of treatment. (F) Taxol did not affect lesion size (2 tailed t-test, p=0.225). (n=14/group for all measures; Scale in A =200 μ)
Additional staining with EC/CV was used to facilitate quantitative analysis of dorsal-ventral lesion depth and lesion volume (Fig. 4A&B). Lesion size (depth and volume) did not differ between Taxol- and vehicle-treated groups indicating that Taxol was not neuroprotective nor did it accelerate lesion repair at this acute post-injury time point. Lesions in both groups extended ~1-2 mm in length with complete disruption of the dorsal columns and the entire dorsal gray matter in all animals. Group differences were observed in the extent and density of scar tissue capping the lesion site; notably 57% (n=8/14) of lesions treated with vehicle had a dense connective tissue scar that extended over the surface of the dorsal spinal cord. None of the specimens from Taxol-treated rats had a connective tissue cap (arrows, Figure 4A).
Antibody labeling of five distinct components of the ECM (laminin, fibronectin, collagen IV, CSPG-GAG sugar chains revealed with anti-CS56 antibodies, and NG2 proteoglycan) further revealed the anti-fibrotic effects of Taxol. The dense matrix of in vehicle-treated specimens included patches of densely stained elements upon a background matrix that appeared lacy and filled the lesion site. Although labeling was visible in Taxol-treated specimens, it was condensed and appeared in broken rope-like strands without the appearance of any underlying matrix, suggesting that ECM was present but assembly of scar tissue was impaired (Figure 4C-F). Qualitative observations of the scar were confirmed by assessing group differences in fibronectin immunoreactivity (Fig 4E). A subtle, but significant increase in GFAP+ immunofluorescence was observed at the edges of the less fibrotic Taxol-treated lesions (Fig 4D). Despite the absence of a dense fibrotic scar after Taxol infusion, there was no obvious increase in neurofilament-positive axons in or around the lesion at this early post-injury time period (7 dpi; Fig. 4F). These staining pattern comparisons are nearly identical to those described by Hellal et al. in their Fig 2G and supplementary Figure S1.
Phase 2 – Evaluating the effects of Taxol on recovery of locomotor function and serotonergic axon density after contusive SCI
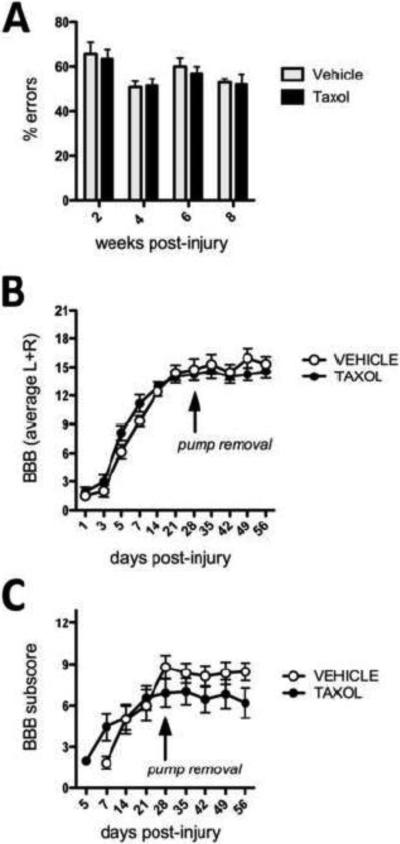
Having successfully replicated data showing that acute Taxol infusion (256 ng/d for 7d) limits fibrosis caused by DHx injury, we next attempted to replicate the efficacy of intrathecal Taxol infusion on functional recovery after a T9 spinal contusion injury. Rats were subjected to a dorsal midline contusion of 150 kDyn force using contusion parameters that were identical to those described by Hellal et al (Hellal et al., 2011). Hellal et al. evaluated the ability of rats to cross an elevated horizontal runway (1 meter in length; ~15 cm off the ground) comprised of round irregularly spaced (1-4 cm apart) metal bars. Bar spacing was changed for every testing session to eliminate effects of habituation. The number of footfalls (missed steps or slips) was quantified from videotape at 2, 4, 6 and 8 weeks post-SCI. They described equivalent performance in Taxol and vehicle-treated groups at 2 and 4 weeks but with progressive recovery only in Taxol-treated rats. Specifically, by 6-8 weeks post-injury, Taxol-treated rats had approximately half as many missed steps as vehicle-treated rats.Although all rats were tested and data analyzed using an approach identical to that described by Hellal et al., in our hands Taxol did not enhance recovery of function (Fig. 5A).
Figure 5.
Taxol infusion does not improve locomotor function after contusive SCI. Recovery of motor function as measured on an elevated ladder (A) was not different between Taxol and vehicle-treated rats. Spontaneous recovery of locomotor function, as measured in the open-field using Basso-Beattie-Bresnahan (BBB) locomotor rating scale (B) or BBB sub-scoring (C) also was unchanged by Taxol.
Hellal et al. used only the ladder task to measure recovery of function. Here, in addition to the ladder task, spontaneous recovery of locomotor function and overall activity were monitored using the BBB locomotor rating scale and automated activity boxes. On days when both BBB and ladder data were obtained, rats were allowed to rest in their home cages for at least 2 hours between tasks. All behavioral tests were completed in the morning at approximately the same time each day. Although subtle benefits were obvious in Taxol-treated rats during the first week post-injury, these differences were not sustained at later times post-injury. In fact, after removing the osmotic pumps, there was a slight but obvious decline in locomotor function (Fig. 5B). This change was most obvious using BBB subscoring (Fig. 5C). Monitoring of spontaneous locomotor activity in activity boxes revealed no differences (data not shown).
Anatomical effects of Taxol after moderate spinal contusion injury
Serotonergic (5HT) raphespinal axons are important for control of normal locomotion (Schmidt and Jordan, 2000) and the number or density of 5HT+ fibers is often used as an anatomical correlate to explain changes in locomotor function caused by spinal cord injury (Engesser-Cesar et al., 2007; Wang et al., 2011). Accordingly, Hellal et al. manually counted the total number of 5HT+ axon profiles in the dorsal half of 3 sagittal spinal cord sections caudal to the lesion site: one through the center of the lesion and one each 250 μm left and right of center. In their study, 50-300 5HT+ axons were tallied across sections with significantly more 5HT+ axons found in the Taxol-treated group (see Fig. 4E in Hellal et al.). In our hands, although robust 5HT labeling was achieved rostral to the lesion and throughout the ventral spinal cord, none of the specimens contained >100 5HT+ axons and there was no effect of Taxol treatment (Fig. 6).
Figure 6.
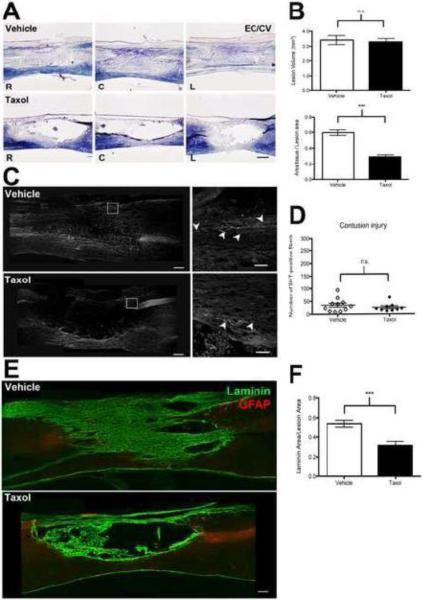
Taxol infusion does not affect contusion lesion size or number of serotonin (5HT+) axons but reduces intralesional matrix deposition. (A) Representative photomicrographs of EC/CV-stained sagittal sections 56 days after spinal contusion injury. Sections were cut through the lesion epicenter or 250 μm to the right or left of center (L= left of center, C=center, R= right of center; scale = 200 μm). (B) Both groups have similar mean lesion volume; however, Taxol reduced intralesional accumulation of tissue matrix (unpaired t-test; n=9-11/group; *** P<0.001). (C) Sagittal sections through the center of the contusion lesion stained with anti-5HT. Robust 5HT-positive axon labeling is evident in the dorsal region caudal to the lesion in both groups (boxed region enlarged in right panels, axons noted with arrowheads). Scale in left panels = 200 μm; right panels = 50 μm. (D) Taxol did not increase the number of 5-HT axons in the contused spinal cord. (E) The lesion site is filled with laminin-enriched ECM in vehicle-treated but not in Taxol-treated sections. Sections in (E) are cut through the lesion center. Scale = 200 μm. (F) The laminin-positive area within the lesion borders is reduced by Taxol (unpaired t-test; N=9-11/group; *** p<0.001).
Although Hellal et al. did not report contusion lesion area or volume, we expanded our analyses to see if Taxol affected these parameters. Similar to the results from the 7dpi DHx lesions (Fig. 3), Taxol did not affect total lesion volume measured 8 weeks after contusion injury (Fig 6A&B). However, gross evaluation of low-power digital EC/CV maps revealed obvious differences in the accumulation of cells and tissue/matrix within the lesion of a subset of animals. While still blind to the identity of the treatment groups, the area occupied by cells and tissue in the lesions was quantified (Fig. 6B). These analyzes revealed a marked effect of Taxol on reducing cellularity and/or matrix accumulation; 43-72 % of the lesion was filled with tissue (e.g., cells, axons, matrix, etc.) in specimens infused with vehicle while only 16-39% of the lesion area was filled by these tissue elements in Taxol-treated specimens. In several Taxol cases, there was no tissue present in the lesion cavity (Fig. 6).
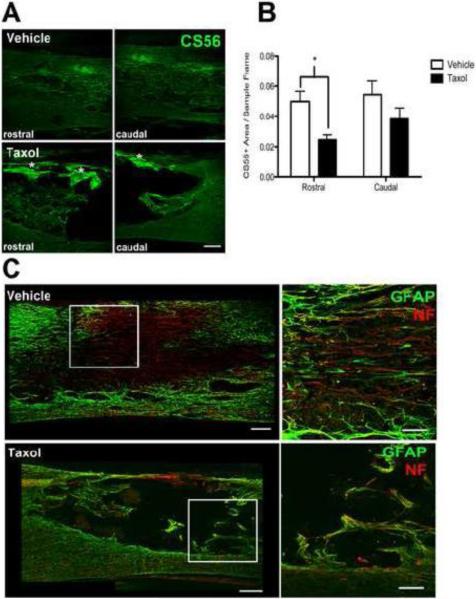
We suspected that the matrix occupying the lesion contained astrocytes, Schwann cells, mesenchymal cells and endothelia and therefore should be replete with a rich basal lamina composed of laminin, CSPGs, collagen and other matrix molecules. Accordingly, the composition of the lesion was evaluated by staining for laminin. As shown in Fig. 6E, a dense laminin matrix filled most of the lesion in vehicle-treated specimens. Although laminin staining also was evident in Taxol-treated specimens, it was reduced and morphologically distinct from that found in vehicle-treated lesions. Specifically, laminin strands in Taxol-treated specimens appeared to be “rope-like” and covered ~50% less of the lesion site than in controls (Fig. 6E&F). CSPGs (CS-56 immunoreactivity) were found along the lesion borders and throughout the lesion site of vehicle-infused spinal cords (Fig. 7A). By comparison, less CS-56 staining was evident in Taxol-treated specimens except within “hot-spots” located in the dorsal spinal cord beneath the meninges (Fig. 7A). Quantitative analyses at the rostral and caudal lesion borders revealed a significant reduction of CS-56 staining in Taxol-treated specimens, primarily because there was significantly less CSPG at the rostral lesion borders (Fig 7B). Even though less inhibitory CSPG accumulated in and surrounding the lesion site in Taxol-treated specimens, fewer axons grew/sprouted into or around the lesion cavity in these sections (Fig. 7C).
Figure 7.
Taxol infusion reduces CSPG accumulation but does not support axon growth/sprouting after contusive SCI. (A) Taxol infusion reduces the area of CS-56 labeling at the lesion borders, while dense staining is evident along the dorsal meningeal surface (*). Scale = 200 μm. (B) CS-56 immunoreactivity area as a proportion of the region of interest at the borders. 2-way ANOVA with repeated measures revealed significant overall effect of Taxol (p= 0.02) with a difference in staining at the rostral lesion border by post-hoc analysis (p<0.05; n=9-11/group). (C) Double staining with GFAP (green) and neurofilament (NF) (red) antibodies reveals dense penetration by axons into the laminin-rich lesion site in vehicle-treated subjects (see also Fig. 6E). Boxed area is enlarged to the right. Conversely, few axons penetrate the acellular void that defines the lesion site in Taxol treated specimens. Scale in left panels = 200 μm, scale in enlarged panels = 100 μm.
Discussion
Paclitaxel (Taxol) is an FDA-approved anti-cancer drug that stabilizes microtubules and as a result interferes with normal cell division and migration (Vyas and Kadow, 1995). Recent data indicate that low-dose Taxol alters microtubule dynamics without causing toxicity and may therefore be therapeutic for various diseases associated with excess fibrosis and inflammation (Zhang et al., 2014). In the central nervous system, microtubules and their dynamic rearrangements are essential for axonal outgrowth (Dent and Gertler, 2003; Forscher and Smith, 1988; Sabry et al., 1991; Tanaka and Kirschner, 1991) and the stability and organization of axon microtubules determines whether damaged axons develop dystrophic retraction bulbs or regenerative growth cones. Although Taxol does not enhance neuron survival or improve the intrinsic growth potential of injured axons, it limits the chaotic disruption of microtubules that culminates in “die-back” and formation of retraction bulbs after axotomy. Furthermore, Taxol can “desensitize” axons to growth cone collapse and reduce the formation of retraction bulbs caused by extrinsic inhibitory molecules including myelin and CSPGs.
In a rat model of static spinal cord compression injury, systemic treatment with Taxol immediately post-injury improved functional recovery; however, the precise mechanisms of action were not determined (Perez-Espejo et al., 1996). More recently, Hellal et al. described robust anti-fibrotic and axon growth promoting effects of Taxol in a model of dorsal spinal hemisection (DHx) injury (Hellal et al., 2011). In that report, Taxol also increased serotonin fiber density and recovery of motor function in a rat model of spinal contusion injury. As part of an NIH/NINDS contract, we chose to replicate a subset of data published by Hellal et al. Consistent with their data, we found that Taxol significantly inhibits scarring at and nearby the site of an acute DHx lesion (one week post-injury). A similar effect was observed in chronic spinal contusion lesions after infusion of Taxol for one month post-injury. However, despite the robust anatomical effects of Taxol in both SCI models, a corresponding improvement in motor function was not observed.
It is generally believed that successful regeneration of injured CNS axons can only be achieved by reducing astrogliotic scarring (Cregg et al., 2014). Astrocytes synthesize highly sulfated and glycosylated CSPGs, which are potent inhibitors of axon growth. As receptors on axonal growth cones bind to CSPGs, signaling cascades are initiated that cause growth cones to retract, forming dystrophic end bulbs or “retraction bulbs” that are characterized by disruption of microtubules (Ertürk et al., 2007). Myelin inhibitory proteins that accumulate within the lesion and penumbra induce a similar effect on growing axons (Xie and Zheng, 2008). Application of gelfoam soaked in Taxol at the site of optic nerve injury increased growth of retinal ganglion cell axons and delayed gliosis and inflammation (Sengottuvel et al., 2011). These effects were dose-dependent with maximal efficacy of Taxol at doses of 3 nM-1μM; higher doses (10-1000μM) were less effective. In contrast, Hellal et al. did not find an obvious reduction of GFAP+ astrogliosis in the injured spinal cord of rats treated with continuous infusion of Taxol (Hellal et al., 2011). We confirmed these results. If anything, by 7 dpi, the intensity of GFAP labeling was increased at the lesion border of Taxol-treated specimens (see Fig. 4D&E). Therefore, Taxol may reduce scarring in a lesion-dependent manner. Alternatively, Taxol may have a more significant effect on ECM secretion or assembly or exert more dramatic effects on other cell types present in the scar after SCI.
The glial limitans that forms around the lesion in all forms of SCI is comprised of glial processes in contact with other cell types. Astrocytes and other cells signal fibroblasts derived from the meninges and/or the perivascular niche to migrate into the injury site culminating in the formation of a basal lamina around blood vessels and the lesion border and a fibrotic scar within the lesion site (Bundesen et al., 2003; Soderblom et al., 2013). In Hellal et al. and the present replication experiments, Taxol dramatically reduced fibrotic scarring within the lesion site. In fact, Taxol eliminated the fibrotic cap that forms on the surface of the spinal cord after a DHx lesion. Our analyses of contusion lesions revealed a similar effect of Taxol. Specifically, consistent with published data showing that fibrosis extends into the contusion lesion core and is comprised of various ECM elements, blood vessels and growing axons (Beattie et al., 1997; Casella et al., 2002), we found that a dense laminin-enriched fibrotic matrix and numerous neurofilament-positive axons filled the contusion lesions of control rats. Only cysts, devoid of ECM or axons, were evident in Taxol-treated specimens. From these data we can conclude that Taxol could have conflicting effects on endogenous repair after SCI; Taxol can increase axon stability but it also limits the formation of a tissue matrix that can support axon growth. Also, even though spinal contusion lesions are often described simply as “fluid-filled” cavities, the present data illustrate that these lesions are more complex and possess many histologic features of the scarring phenomenon that is usually associated only with mouse SCI models.
Since we worked closely with the original authors to ensure that the technical details were replicated with fidelity, there are no obvious explanations for why we could replicate the effects of Taxol in the DHx lesion but not the primary anatomical and behavioral data produced in a spinal contusion injury model (see Hellal et al. original Figure 4E&F; compare with Fig. 5A, Fig. 6C&D in this report). One possibility is that the rats in our experiments received more severe contusion injuries than those produced by Hellal et al. Previously, we found that subtle differences in the location or severity of primary trauma can influence the timing and magnitude of secondary injury and confound interpretation of drug treatments (Popovich et al., 2010; 2012). In the current study, using the same injury device and an experienced technical staff, we defined the injury impact force (150 kDyn) to match exactly that used by Hellal et al. However, based both on measures of functional recovery and serotonergic axon labeling, our injuries were more severe. Compared to data from Hellal et al, our contused SCI rats made approximately three times as many errors on the elevated ladder task and had approximately 3-fold fewer 5-HT+ axons in the spinal cord caudal to the lesion. Although Hellal et al did not publish the biomechanical parameters of their contusion injuries, we obtained them from their collaborator (Dr. Andres Hurtado). A direct comparison of the biomechanics revealed no difference in injury force but a significantly lower displacement of the spinal cord in rats injured at Ohio State (Hellal et al: n=40 injuries; force=156.8±4.6 kDyn; displacement = 951.1±53.8 μm/Ohio State: n=24 injuries; force 155.6±3.7; displacement = 885.9±33.5 μm; p=0.2827 and p<0.0001, respectively via unpaired t-tests). This is intriguing data but it is counterintuitive because a lower displacement would be expected to cause less severe injury with better spontaneous recovery and greater preservation or sprouting of 5HT axons. There are other procedural variables that can affect injury biomechanics but that are not easily measured or compared between laboratories. For example, injury severity can be influenced by the amount of traction or stretch on vertebrae while animals are suspended during the contusion injury, the length of time and amount of prior manipulation of the cord during laminectomy and the size and dimensions of the laminectomy site (unpublished observations). If the lesions created in the replication study were larger and more severe than those described by Hellal et al., then the data could indicate that the effects of Taxol are not robust or that they are not universally applicable in all types or severities of spinal cord injury.
In summary, this study demonstrated a clear biological effect of Taxol infusion on fibrotic scar formation in two different rat SCI models. Differences in quantitative measures of ECM molecule deposition within and around the lesion site were accompanied by qualitative differences in the staining patterns, suggesting that Taxol may disrupt both the synthesis and assembly of the ECM. Taxol does not appear to be neuroprotective, as there were no effects on lesion size in either model. Despite evidence of a reproducible biological effect, we did not replicate findings of improved functional recovery in a contusion injury model, suggesting that further basic and preclinical mechanistic studies are needed before moving forward with translation of this anti-cancer drug for repair after SCI.
Highlights.
Partial replication of published data showing the therapeutic effects of Taxol in experimental model of spinal cord injury
Taxol limits scarring in model of dorsal spinal hemisection injury
Taxol does not improve recovery of locomotor function
Taxol does not improve growth or sprouting of serotonergic axons
Taxol limits extracellular matrix deposition in model of spinal contusion injury
Acknowledgments
Thanks to Drs. Farida Hellal, Andres Hurtado, Jörg Ruschel and Frank Bradke for discussions related to experimental design and for providing their original data to complete power analyses. Thank you also to Drs. Dana McTigue, Michele Basso, John Buford, Sandra Kostyk and the technical staff and trainees of OSU's Center for Brain and Spinal Cord Repair for discussions related to manuscript selection and experimental design. This research was supported by P30NS045758, NIH-NINDS contract HHSN271200800040C and the Ray W. Poppleton Endowment. Images presented in this report were generated using the instruments and services at the Campus Microscopy and Imaging Facility, The Ohio State University.
Abbreviations
- SCI
spinal cord injury
- dpi
days post-injury
- ECM
extracellular matrix
- CSPG
chondroitin sulfate proteoglycan
- DHx
dorsal hemisection
- 5HT
5-hydroxytryptamine (serotonin)
- NIH
National Institutes of Health
- NINDS
National Institute of Neurological Disorders and Stroke
- FORE-SCI
Facilities of Research Excellence in Spinal Cord Injury
- EC/CV
Eriochrome® Cyanine/Cresyl Violet
- MCID
Microcomputer Imaging Device
- BBB
Basso-Beattie-Bresnahan locomotor rating scale
- GFAP
glial fibrillary acidic protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Beattie MS, Bresnahan JC, Komon J, Tovar CA, Van Meter M, Anderson DK, Faden AI, Hsu CY, Noble LJ, Salzman S, Young W. Endogenous repair after spinal cord contusion injuries in the rat. Experimental Neurology. 1997;148:453–463. doi: 10.1006/exnr.1997.6695. [DOI] [PubMed] [Google Scholar]
- Bundesen LQ, Scheel TA, Bregman BS, Kromer LF. Ephrin-B2 and EphB2 regulation of astrocyte-meningeal fibroblast interactions in response to spinal cord lesions in adult rats. J. Neurosci. 2003;23:7789–7800. doi: 10.1523/JNEUROSCI.23-21-07789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casella GTB, Marcillo A, Bunge MB, Wood PM. New vascular tissue rapidly replaces neural parenchyma and vessels destroyed by a contusion injury to the rat spinal cord. Experimental Neurology. 2002;173:63–76. doi: 10.1006/exnr.2001.7827. [DOI] [PubMed] [Google Scholar]
- Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, Silver J. Functional regeneration beyond the glial scar. Experimental Neurology. 2014;253:197–207. doi: 10.1016/j.expneurol.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW, Gertler FB. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron. 2003;40:209–227. doi: 10.1016/s0896-6273(03)00633-0. [DOI] [PubMed] [Google Scholar]
- Engesser-Cesar C, Ichiyama RM, Nefas AL, Hill MA, Edgerton VR, Cotman CW, Anderson AJ. Wheel running following spinal cord injury improves locomotor recovery and stimulates serotonergic fiber growth. Eur J Neurosci. 2007;25:1931–1939. doi: 10.1111/j.1460-9568.2007.05469.x. [DOI] [PubMed] [Google Scholar]
- Ertürk A, Hellal F, Enes J, Bradke F. Disorganized Microtubules Underlie the Formation of Retraction Bulbs and the Failure of Axonal Regeneration. J. Neurosci. 2007;27:9169–9180. doi: 10.1523/JNEUROSCI.0612-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forscher P, Smith SJ. Actions of cytochalasins on the organization of actin filaments and microtubules in a neuronal growth cone. J Cell Biol. 1988;107:1505–1516. doi: 10.1083/jcb.107.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellal F, Hurtado A, Ruschel J, Flynn KC, Laskowski CJ, Umlauf M, Kapitein LC, Strikis D, Lemmon V, Bixby J, Hoogenraad CC, Bradke F. Microtubule Stabilization Reduces Scarring and Causes Axon Regeneration After Spinal Cord Injury. Science. 2011;331:928–931. doi: 10.1126/science.1201148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu J-YC, McKeon R, Goussev S, Werb Z, Lee J-U, Trivedi A, Noble-Haeusslein LJ. Matrix metalloproteinase-2 facilitates wound healing events that promote functional recovery after spinal cord injury. J. Neurosci. 2006;26:9841–9850. doi: 10.1523/JNEUROSCI.1993-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010;8(6):e1000412. doi: 10.1371/journal.pbio.1000412. doi:10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon VP, Ferguson AR, Popovich PG, Xu XM, Snow DM, Igarashi M, Beattie CE, Bixby JL, et al. Minimum Information About a Spinal Cord Injury Experiment (MIASCI) - a proposed reporting standard for spinal cord injury experiments. J. Neurotrauma. epub ahead of print. PMID. 2014:24870067. doi: 10.1089/neu.2014.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Espejo MA, Haghighi SS, Adelstein EH, Madsen R. The effects of taxol, methylprednisolone, and 4-aminopyridine in compressive spinal cord injury: a qualitative experimental study. Surg Neurol. 1996;46:350–357. doi: 10.1016/s0090-3019(96)00200-5. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Lemeshow S, Gensel JC, Tovar CA. Independent evaluation of the effects of glibenclamide on reducing progressive hemorrhagic necrosis after cervical spinal cord injury. Experimental Neurology. 2010 doi: 10.1016/j.expneurol.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovich PG, Tovar CA, Wei P, Fisher L, Jakeman LB, Basso DM. A reassessment of a classic neuroprotective combination therapy for spinal cord injured rats: LPS/pregnenolone/indomethacin. Experimental Neurology. 2012:1–9. doi: 10.1016/j.expneurol.2011.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabry JH, O'Connor TP, Evans L, Toroian-Raymond A, Kirschner M, Bentley D. Microtubule behavior during guidance of pioneer neuron growth cones in situ. J Cell Biol. 1991;115:381–395. doi: 10.1083/jcb.115.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Research Bulletin. 2000;53:689–710. doi: 10.1016/s0361-9230(00)00402-0. [DOI] [PubMed] [Google Scholar]
- Sengottuvel V, Leibinger M, Pfreimer M, Andreadaki A, Fischer D. Taxol Facilitates Axon Regeneration in the Mature CNS. J. Neurosci. 2011;31:2688–2699. doi: 10.1523/JNEUROSCI.4885-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderblom C, Luo X, Blumenthal E, Bray E, Lyapichev K, Ramos J, Krishnan V, Lai-Hsu C, Park KK, Tsoulfas P, Lee JK. Perivascular Fibroblasts Form the Fibrotic Scar after Contusive Spinal Cord Injury. J. Neurosci. 2013;33:13882–13887. doi: 10.1523/JNEUROSCI.2524-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka EM, Kirschner MW. Microtubule behavior in the growth cones of living neurons during axon elongation. J Cell Biol. 1991;115:345–363. doi: 10.1083/jcb.115.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas DM, Kadow JF. Paclitaxel: a unique tubulin interacting anticancer agent. Prog Med Chem. 1995;32:289–337. doi: 10.1016/s0079-6468(08)70456-9. [DOI] [PubMed] [Google Scholar]
- Wang X, Duffy P, McGee AW, Hasan O, Gould G, Tu N, Harel NY, Huang Y, Carson RE, Weinzimmer D, Ropchan J, Benowitz LI, Cafferty WBJ, Strittmatter SM. Recovery from chronic spinal cord contusion after nogo receptor intervention. Ann Neurol. 2011;70:805–821. doi: 10.1002/ana.22527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F, Zheng B. White matter inhibitors in CNS axon regeneration failure. Experimental Neurology. 2008;209:302–312. doi: 10.1016/j.expneurol.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Yang R, Wang S, Dong Z. Paclitaxel: new uses for an old drug. Drug Des Devel Ther. 2014;8:279–284. doi: 10.2147/DDDT.S56801. [DOI] [PMC free article] [PubMed] [Google Scholar]