Abstract
Background
±3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) produces “prosocial” effects, such as feelings of empathy and closeness, thought to be important to its abuse and its value in psychotherapy. However, it is not fully understood how MDMA alters basic emotional processes to produce these effects, or whether it produces corresponding changes in actual social behavior. Here we examined how MDMA affects perceptions of and responses to emotional expressions, and tested its effects on behavior during a social interaction. We also examined whether MDMA’s prosocial effects related to a measure of abuse liability.
Methods
Over three sessions 36 healthy volunteers with previous ecstasy use received MDMA (0.75mg/kg, 1.5mg/kg) and placebo under double-blind conditions. We measured i) mood and cardiovascular effects, ii) perception of and psychophysiological responses to emotional expressions iii) use of positive and negative words in a social interaction and iv) perceptions of an interaction partner. We then tested whether these effects predicted desire to take the drug again.
Results
MDMA slowed perception of angry expressions, increased psychophysiological responses to happy expressions, and increased positive word use and perceptions of partner empathy and regard in a social interaction. These effects were not strongly related to desire to take the drug again.
Conclusions
MDMA alters basic emotional processes by slowing identification of negative emotions and increasing responses to positive emotions in others. Further, it positively affects behavior and perceptions during actual social interaction. These effects may contribute to the efficacy of MDMA in psychotherapy, but appear less closely related to its abuse potential.
Keywords: Ecstasy, MDMA, emotion perception, social cognition, social interaction, psychophysiology
Introduction
±3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) is a popular recreational drug, which in addition to typical stimulant effects, also produces unique “prosocial” effects (broadly defined here as effects likely to promote positive social interactions), such as feelings of closeness and empathy with others, and increased desire to socialize (Bedi et al. 2010; Harris et al. 2002; Kirkpatrick et al. in press; Liechti et al. 2001; Sumnall et al. 2006; Vollenweider et al. 1998). Users report that these prosocial effects motivate MDMA use (Sumnall et al. 2006; Ter Bogt and Engels 2005), and recent evidence suggests these prosocial effects may have clinical value in psychotherapy for post-traumatic stress disorder (PTSD; Mithoefer et al. 2011; Mithoefer et al. 2013; Oehen et al. 2013).
Despite clinical interest in MDMA, we have only limited understanding of the basic emotional processes involved in its prosocial effects. Previous research has shown that MDMA reduces perception of negative emotional expressions (Bedi et al. 2010; Hysek et al. 2012; Hysek et al. 2013; Hysek et al. 2014; Kirkpatrick et al. in press), and reduces amygdala responses to angry facial expressions (Bedi et al. 2009). MDMA may also improve recognition of positive expressions, particularly ambiguous positive expressions (Hysek et al. 2012, although c.f. Bedi et al., 2010; Hysek et al., 2013; Kirkpatrick et al., in press; Hysek et al. 2014), and increase striatal responses to happy expressions (Bedi et al. 2009). However, these previous studies focused primarily on emotion perception. Psychoactive drugs may also alter responses to emotional stimuli once they are perceived, amplifying responses to positive stimuli or blunting responses to negative ones (Gospic et al. 2008; Harmer et al. 2004; Wardle and de Wit 2012). It has been suggested that such changes in emotional response are related to the therapeutic effects of selective serotonin reuptake inhibitors (SSRIs; Harmer 2008). Further, it has been proposed that MDMA contributes to therapy in part by reducing responses to negative material (Johansen and Krebs 2009), yet its effects on responses to emotional expressions have not been examined. Thus, in the current study we examined the effects of MDMA on both perceptions of emotional expressions and emotional responses to these expressions.
We used a novel and sensitive measure to assess emotion perception and elicit emotional responses. This consisted of full color video displays of dynamically developing emotional expressions, which are more ecologically valid than the static pictures used previously with MDMA, and more effectively activate emotional brain areas (LaBar et al. 2003; Platt et al. 2010; Walter et al. 2011). We used objective measures of electromyographic (EMG) activity in facial muscles to assess emotional responses to these displays. Negative stimuli (including negative faces) increase activity in the corrugator (“frown”) muscle, whereas positive stimuli (including positive faces) decrease corrugator activity and increase zygomatic (“smile”) muscle activity (Dimberg and Karlsson 1997; Dimberg et al. 2000; Larsen et al. 2003; Moody et al. 2007). We hypothesized that MDMA would slow perception of negative emotions and facilitate perception of positive emotions in these dynamic displays, consistent with previous reports using static stimuli. Further, we predicted that MDMA would decrease negative EMG responses to negative expressions, and increase positive EMG responses to positive expressions, indicating change in both perception and response.
In addition to the need to better understand the basic emotional processes involved in the putative prosocial effects of MDMA, it has also not yet been shown that MDMA produces measureable changes in behavior and perceptions in actual (as opposed to simulated) social interactions. Thus, we also measured use of positive and negative emotion words, and participants’ perceptions of their partner during a semi-structured social interaction. We predicted that MDMA would increase use of positive emotional words, and perceptions of the interaction partner as empathic and responsive.
Last, based on user reports of the desirability of these prosocial effects, we examined on a preliminary basis whether the prosocial effects of MDMA (broadly defined again as changes in subjective feelings, perceptions of others, and responses to others that seem likely to promote more positive social interactions) related to desire to use the drug again, a rough measure of abuse liability.
Methods and Materials
Study design
The study used a three-session within-subjects design in which healthy occasional MDMA users received placebo, 0.75mg/kg MDMA and 1.5mg/kg MDMA in counterbalanced order under double-blind conditions. Sessions were separated by a minimum of 7 days (M = 26 days, SD = 29.58). At each session, participants completed measures of self-reported and cardiovascular drug effects, and completed the emotional expression identification task while psychophysiological responses were recorded. Participants also engaged in a controlled social interaction, and rated their perceptions of that interaction. At the end of each session, participants completed a measure of desire to take the drug again.
Participants
Healthy participants (18 male, 18 female), ages 18-35 were recruited through flyers and online advertisements. Screening included a physical examination, electrocardiogram, modified structured clinical interview for DSM-IV (First et al. 1996), and self-reported drug and health history. Inclusion criteria were: self-reported ecstasy use 4-40 times with no adverse responses; high school English fluency; body mass index >19 and <30; no regular medication (except birth control); no medical conditions contraindicating MDMA; no past year DSM-IV Axis I diagnosis, excluding non-treatment seeking drug abuse; no history of stimulant drug dependence; no women who were pregnant or planning a pregnancy; no one smoking >25 cigarettes per week. See Table 1 for participant demographics.
Table 1.
Demographic and Substance Use Characteristics.
| n(%) or M(SD), Total N = 36 | |
|---|---|
| Demographic Variables | |
| Sex (Male/Female) | 18/18 (50%/50%) |
| Ethnicity | 29 (81%) Non-Hispanic |
| Race | 24 (67%) Caucasian |
| 4 (11%) African-American | |
| 1 (3%) Asian | |
| 7 (19%) Other/Mixed Race | |
| Age | 24.6 (4.7) |
| Education in years | 15.1 (1.5) |
| Current Substance Use | |
| Typical alcoholic drinks/week | 9.9 (10.6) |
| Smoking at all in past month | 8 (22%) |
| Lifetime Occasions Recreational Use | |
| MDMA | 10.2 (8.2) |
| Cannabis 1-10x | 5 (14%) |
| 11-50x etc | 3 (8%) |
| 5 (14%) 51-100x | |
| 23 (64%) > 100x | |
| Tranquilizers | 21 (58%) never |
| 11 (31%) 1-10x | |
| 4 (11%) 11-50x | |
| Stimulants | 7 (19%) never |
| 14 (39%) 1-10x | |
| 11 (31%) 11-50x | |
| 2 (6%) 51-100x | |
| 2 (6%) >100x | |
| Opiates | 17 (47%) never |
| 14 (39%) 1-10x | |
| 4 (11%) 11-50x | |
| 1 (3%) >100x | |
| Hallucinogens | 2 (6%) never |
| 24 (67%) 1-10x | |
| 9 (25%) 11-50x | |
| 1 (3%) 51-100x | |
| Other Drugs | 26 (72%) never |
| 6 (17%) 1-10x | |
| 3 (8%) 11-50x | |
| 1 (3%) 51-100x |
Participants were instructed to refrain from alcohol and over-the-counter drugs for 24hrs before and 12hrs after the session, from marijuana for 7 days before and 24hrs after the session, and from all other recreational drugs for 48hrs before and 24hrs after the session. The minimum reported time since last illicit drug use was 4 days, with only 3 participants reporting any illicit drug use in the week preceding the session. Compliance was verified using breath (Alcosensor III, Intoximeters Inc., St. Louis, MO) and urine tests (ToxCup, Branan Medical Corporation, Irvine, CA). Participants were instructed to consume normal amounts of caffeine and nicotine, and to fast for 2hrs prior to the session. Women not on hormonal contraceptives were scheduled only during the follicular phase (White et al. 2002).
Participants were told that the purpose of the study was to investigate individual differences in drug responses, and that they might receive a stimulant, a sedative, a cannabinoid, or a placebo. All participants provided written informed consent, and all procedures were approved by the University of Chicago Institutional Review Board.
Procedure
Sessions were conducted from 9:00am to 2:00pm in a “living room” style laboratory. At arrival, participants provided breath and urine samples for drug and pregnancy testing. At 9:15am, they completed baseline subjective and cardiovascular measures. At 9:30am participants ingested a size 00 opaque capsule containing MDMA powder (0.75 or 1.5 mg/kg, maximum of 125mg) with lactose filler, or a placebo capsule containing only lactose. When no measures were scheduled, participants relaxed, watched a movie from a selection available or read. At 10:00am, subjective and cardiovascular measures were collected. At 10:15am, psychophysiological sensors were attached. At 10:30am participants completed subjective and cardiovascular measures and at 10:40am they began computerized tasks including the measure of emotional expression identification and response. Tasks were conducted in counterbalanced order from 10:40 to 11:20, at which time psychophysiological sensors were removed. At 11:30am subjective and cardiovascular measures were collected. At 11:50am subjects completed a semi-structured social interaction task with the research assistant (RA), and rated their perceptions of the RA. Further subjective and cardiovascular measures were collected at 12:30pm, 1:00pm and 1:30pm. At 2:00pm participants completed the end of session questionnaire, and were discharged provided subjective and cardiovascular measures had returned to baseline.
Mood
Mood was assessed using the Profile of Mood States (POMS), a 72-adjective list rated on 5-point Likert scales from 0 (“not at all”) to 4 (“extremely”), which contains 8 subscales. For this study we examined the Elation and Arousal subscales, which are sensitive to stimulant effects (Gabbay 2003; Tancer and Johanson 2007).
Cardiovascular Measures
Blood pressure and heart rate were measured using portable monitors (Life Source, A&D Company, Tokyo, Japan). Heart rate results were similar in time course and dose-dependence to blood pressure, so we used mean arterial pressure (MAP; [Systolic BP + 2 * Diastolic BP]/3) as our measure of cardiovascular effects.
Subjective Effects on Social Emotions
A Visual Analog Scale (VAS) scale was used to assess specific social emotions thought to be related to MDMA. It was comprised of 13 adjectives such as “Insightful”, “Playful”, “Loving” and “Lonely”, each rated on a 1-100 (not at all – extremely) line. Based on Bedi et al., (2010) we selected “Playful” and “Loving” as likely to be most sensitive to the effects of MDMA on social emotions.
Emotional Expression Identification and Response
To measure emotional perception and responses, we used the Dynamic Emotional Identification Task (DEIT) adapted by our laboratory (Wardle et al. 2012) from Benton et al (2007). Five female and 5 male actors performed angry, fearful, sad and happy expressions, for a total of 40 sequences, which were presented in random order. Each sequence consisted of 50 “frames” progressing from 0-100% emotional intensity at 2% steps, each presented for an average of 250ms (within a random range of 100-400ms), producing a color video of an emotional expression developing. Participants were instructed to “press the space bar as soon as you know what expression is being displayed.” This ended the sequence, and presented options of “Angry,” “Fearful,” “Sad,” and “Happy.”
Perception of expressions was quantified as the intensity (0-100%) of the face when the participant pressed the space bar for correctly identified sequences. Accuracy was high (M = 93%, SD = 4), and not sufficiently variable for analysis. Responses to emotional expressions were quantified as mean electromyographic activity (EMG) in the corrugator and zygomatic muscles during the final 1s of face presentation for correctly identified sequences, minus mean EMG of a 1s pre-picture baseline. EMG was measured using the same procedures and equipment as previously reported with this task (Wardle et al. 2012).
Behavior and Perceptions in a Social Interaction
The semi-structured social interaction was a validated modification (Wardle et al. 2011) of the Interpersonal Perception Task used by Janowsky (2003) to study effects of psychoactive drugs on speech. At orientation, participants nominated three “important people in your life.” At each session, the participant talked with the RA about one of these individuals for 5min. RAs were trained in reflective listening. We recorded and transcribed the participant’s speech, then scored the transcriptions for percentage of positive and negative emotional words using Linguistic Inquiry and Word Count Software (Pennebaker et al. 2007). Participant’s perceptions of the RA were measured immediately after the interaction using a brief Barrett-Lennard Relationship Inventory previously used to assess effects of marijuana on interpersonal interactions (Janowsky et al. 1979). This includes six-item scales for Regard (“S/he was truly interested in me”), Empathy (e.g. “S/he understood me”), and Congruence (“I felt that s/he was real and genuine with me”) with each item rated on a -3 (strongly disagree) to +3 (strongly agree) scale.
Desire to Take the Drug Again
Desire to take the drug again was assessed using a single VAS “If you had the opportunity to take this drug again, how much would you want to?” rated on a line from 0-100 (“not at all” to “would want to very much”), administered at the end of each session. Self-reported desire to take the drug again is a proxy of abuse liability (Griffiths et al. 2003)
Statistical Analyses
We used linear mixed effect modeling (LME) in the lme4 package (v 0.999999-0; Bates et al. 2011) of the R statistical computing environment (v. 2.15.2; R Development Core Team 2011) as our primary statistical approach.
For POMS Elation, POMS Arousal, MAP, VAS Playful, and VAS Loving, we first calculated an Area Under the Curve (AUC) score for each session relative to the participant’s session baseline. A small number of missing time points (<8) were imputed as the average of the two time points on either side to produce complete data. We then conducted LME models on these AUC scores with dose as an independent (fixed) factor, and subject as a random effect. In all analyses, dose was examined using orthogonal polynomial contrasts, with significant linear contrasts followed up with paired t-tests comparing each dose to placebo.
For intensity at identification, corrugator EMG and zygomatic EMG we conducted LME models with dose, emotion displayed and stimulus sex as independent (fixed) factors and subject and actor as random factors, following our previous strategy (Wardle et al. 2012). Emotion effects were examined using Helmert contrasts: 1. Happy vs. all negative emotions, 2. Anger vs. other negative emotions (fear and sadness), 3. Fear vs. Sadness. EMG analyses included grand-mean-centered intensity at display termination as a covariate, because display intensity might influence EMG and might in turn be systematically affected by drug.
For percentage of emotional words we conducted an LME with word type (positive vs. negative) and dose as fixed factors and subject as a random factor. For ratings of Regard, Empathy and Congruence we conducted LME models with drug as a fixed factor and subject as a random factor.
In all models, we tested effects of participant sex and session order. For simplicity these were only included in final models when significant effects were observed. For all models, effect sizes are reported as unstandardized coefficients (B) with standard errors (SE). We calculated p-values using the t distribution with n-1 degrees of freedom (see Wardle et al. 2012 for rationale).
On an exploratory basis, we examined how MDMA’s social effects related to desire to take the drug again. To estimate the effects of MDMA on our social variables (our predictors of interest), on POMS Elation, POMS Arousal and MAP (which we used to examine the general sensitivity to the drug) and on Desire to Take Again (our dependent variable) for each participant, we conducted individual linear regressions on each participant’s data and extracted the non-standardized estimates of the linear drug effect on each variable of interest (if the effect took the form of an interaction, we used the estimate of the interaction). We first entered these per-participant estimates of MDMA’s social and typical stimulant effects into individual linear regressions to see whether any of these predicted desire to take the drug again. Then we entered any individually significant effects simultaneously into a linear regression predicting desire to take the drug again, to examine whether the social of MDMA contributed uniquely to desire to take the drug again above and beyond its typical stimulant effects.
Results
Mood, Subjective and Cardiovascular Drug Effects
POMS
MDMA (1.5mg/kg and 0.75 mg/kg) increased both Elation and Arousal (linear drug effect on Elation: B = 422.5, SE = 159.3, t(35) = 2.65, p = 0.01; linear drug effect on Arousal: B = 1636.0, SE = 404.9, t(35) = 4.04, p < 0.001). The effect of MDMA on Arousal was slightly stronger in women, although significant in both sexes (linear drug × sex interaction: B = -1678.8, SE = 809.9, t(35) = 2.07, p = 0.05). Results for Elation are shown in Panel A of Fig 1. Results for Arousal were similar in dose dependence and time course.
Figure 1.
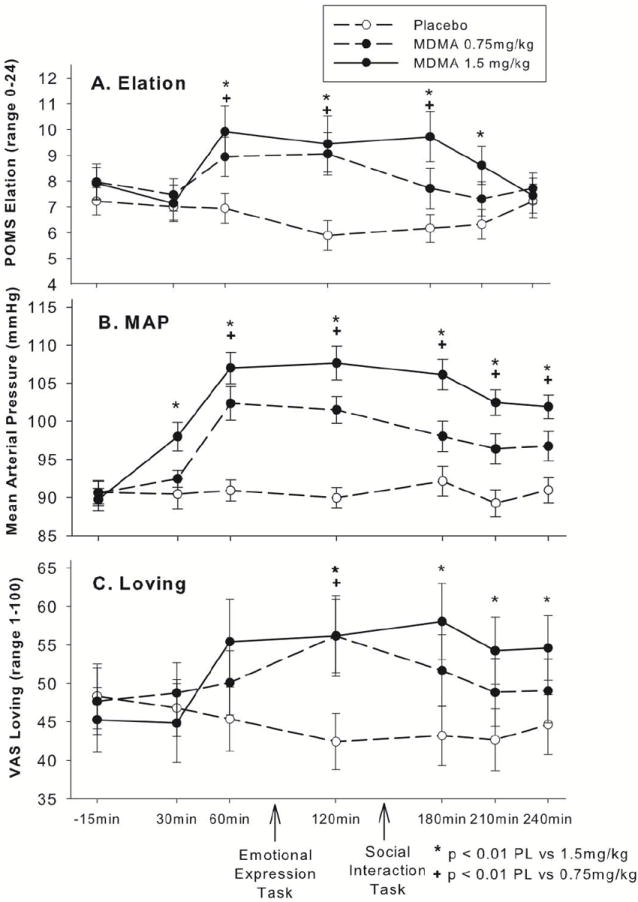
MDMA dose dependently increased self-reports of Elation on the Profile of Mood States (POMS; Panel A), mean arterial pressure (MAP; Panel B) and self-reports of the social emotion “Loving” on a visual analog scale (VAS; Panel C). Subjective and cardiovascular effects of MDMA were significantly evident both before and after the emotional expression task and the social interaction task.
Cardiovascular
As shown in Panel B of Fig. 1, MDMA (1.5mg/kg and 0.75mg/kg) increased mean arterial pressure (linear drug effect on MAP: B = 3467.1, SE = 344.2, t(35) = 10.07, p < 0.001).
Social Variables
Subjective Social Emotions
MDMA (1.5mg/kg and 0.75mg/kg) increased VAS scores for Loving, and marginally increased scores for Playful, linear drug effect on Loving: B = 3079.8, SE = 1001.8, t(35) = 3.07, p = 0.004; linear drug effect on Playful: B = 1948.5, SE = 1196.2, t(35) = 1.63, p = 0.10. Results for Loving are shown in Panel C of Fig. 1. Results for Playful were similar in dose dependence and time course.
Emotional Expression Identification and Responses
Four participants did not correctly complete the DEIT, waiting until the end of every sequence to identify the emotion. Their data were excluded, leaving n = 32 for the intensity analyses. There was a significant effect of session, such that participants identified emotions more quickly across sessions, so the session covariate was included. There were no effects of subject or stimulus sex. As shown in Fig. 2, MDMA (1.5mg/kg) increased the intensity required to identify anger compared to other negative emotions; linear effect of drug × anger vs. other negative emotions: B = 2.83, SE = 1.16, t(31) = 2.44, p = 0.03. Importantly, anger was not the most difficult to identify, as intensity at identification for anger was lower than for other negative emotions (B = -5.03, SE = 0.49, t[31] = 10.36, p < 0.001). MDMA did not affect identification of any other emotions.
Figure 2.
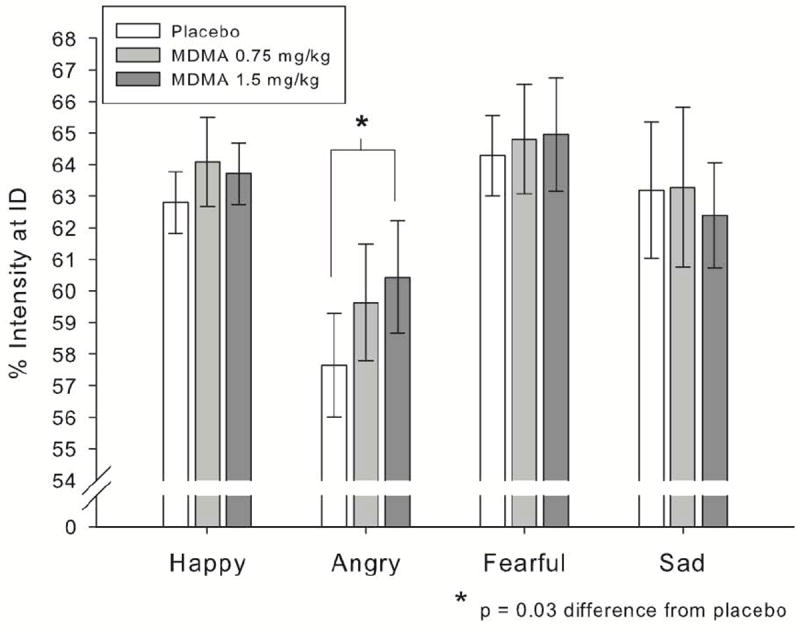
MDMA (1.5mg/kg) increased the intensity required for identification (ID) of angry facial expressions, without affecting identification (ID) of other emotions.
Corrugator and zygomatic responses were lost for 5 participants due to contamination from jaw clenching, a known MDMA side effect. One participant’s corrugator data was lost due to technical difficulties, and one outlier on zygomatic activity (+3 SD from the mean on zygomatic responses at 1.5mg/kg MDMA) was removed, leaving 30 participants in each EMG analysis.
Corrugator activity decreased across sessions, so the session covariate was included. MDMA (1.5mg/kg) decreased corrugator activity to happy faces relative to negative faces, indicative of more positive responses to happy faces while on the drug, linear drug effect × emotion interaction: B = -0.76, SE = 0.35, t(29) = 2.20, p = 0.04. Further, this effect was moderated by participant sex, such that it was primarily evident in female participants, sex × linear drug × emotion interaction: B = 1.92, SE = 0.69, t(29) = 2.78, p = 0.009. Fig. 3 shows corrugator results for males and females by drug and emotion condition.
Figure 3.
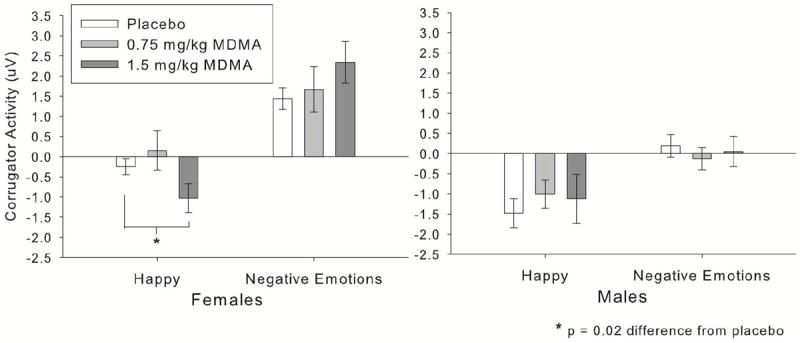
MDMA (1.5mg/kg) reduced corrugator (“frown”) muscle activity to happy facial expressions, indicative of more positive responses to happy expressions. This effect was evident in female participants only (significant drug × expression × sex interaction). Negative expressions are collapsed across, as MDMA had no differential effects on specific negative emotional expressions.
Zygomatic activity decreased across sessions, so the session covariate was included. MDMA (1.5mg/kg) increased zygomatic responses to happy expressions relative to negative expressions, indicative of more positive responses to happy expressions while on the drug, linear drug × emotion contrast interaction: B = 0.82, SE = 0.32, t(29) = 2.58, p = 0.02. Participant sex did not moderate zygomatic responses. Fig. 4 shows zygomatic results by drug and emotion condition.
Figure 4.
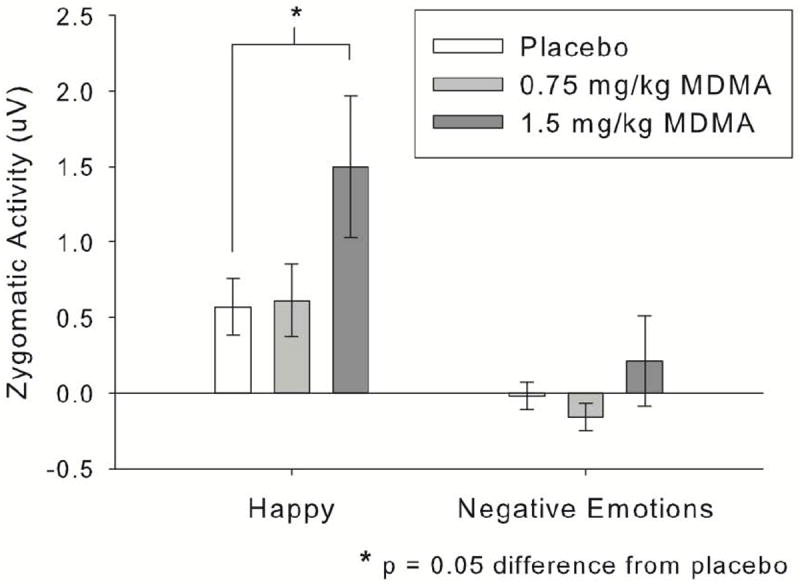
MDMA (1.5mg/kg) increased zygomatic (“smile”) muscle activity to happy facial expressions, indicative of more positive responses to happy expressions. Negative expressions are collapsed across, as MDMA had no differential effects on specific negative emotional expressions.
Behavior and Perceptions in a Social Interaction
One individual had incomplete word count data, leaving 35 participants. As shown in Fig. 5, MDMA (0.75mg/kg and 1.5mg/kg) increased use of positive emotion words, without affecting negative emotion words, linear effect of drug × word type interaction: B = 0.66, SE = 0.32, t(34) = 2.08, p = 0.05.
Figure 5.
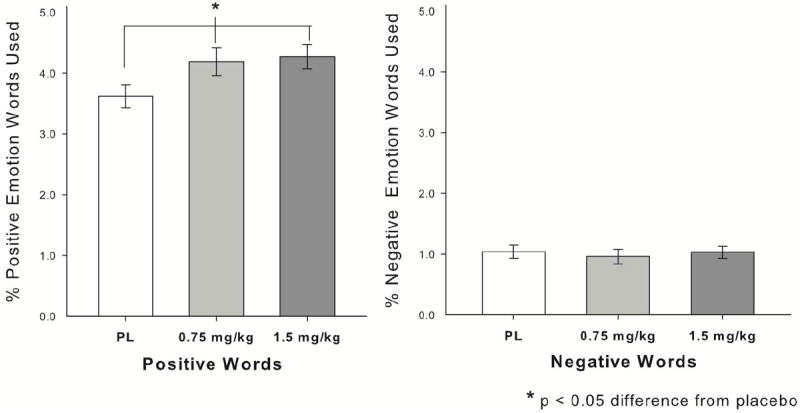
MDMA (0.75mg/kg and 1.5mg/kg) increased the percentage of positive emotional words that participants used in a semi-structured 5min. interaction, without affecting percentage of negative words used.
MDMA (1.5 mg/kg) marginally increased ratings of perceived Regard during the social interaction, and slightly but significantly increased ratings of Empathy; Regard linear drug effect: B = 0.18, SE = 0.10, t(35) = 1.82, p = 0.08; Empathy linear drug effect: B = 0.18, SE = 0.09, t(34) = 2.06, p = 0.05. Follow up t-tests indicated a significant effect of 1.5mg/kg MDMA vs. placebo on Regard (t[35] = 2.04, p = 0.05, Panel A of Fig 6), and a marginal effect on Empathy (t[35] = 1.91, p = 0.07, Panel B of Fig. 6). There was no significant effect on Congruence.
Figure 6.
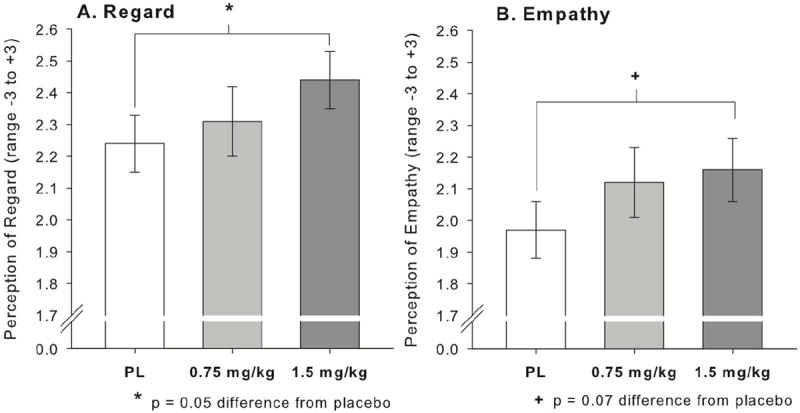
MDMA (1.5mg/kg) increased participants’ perceptions of regard in their interaction partner (Panel A), and marginally increased participants’ perceptions of empathy in their interaction partner (Panel B).
Contribution of Social Effects to Desire to Take the Drug Again
MDMA significantly increased desire to take the drug again, linear drug effect B = 28.88, SE = 6.81, t(35) = 4.24, p < 0.001. Column 2 of Table 2 shows the individual linear regressions, with only VAS Playful and POMS Elation predicting desire to take the drug again. When these were jointly entered into a regression, the overall regression was significant, F(2, 33) = 3.80, p = 0.03, but not the individual beta weights (column 3 of Table 2), suggesting the explanatory power of these variables overlapped.
Table 2.
Linear regressions examining the extent to which each effect of MDMA predicts desire to take the drug again. Only effects that were significant individually were entered into the simultaneous regression.
| Effect of MDMA | Standardized beta and p entered individually | Standardized beta and p entered simultaneously |
|---|---|---|
| Mood | ||
| POMS Arousal | b = -0.19, p =.27 | |
| POMS Elation | b = 0.36, p =.03* | b = 0.26, p =.14 |
| Cardiovascular | ||
| MAP | b = -0.24, p =.82 | |
| Social Variables | ||
| VAS Loving | b = 0.28, p =.10 | |
| VAS Playful | b = 0.38, p =.02* | b = 0.24, p =.18 |
| Identification of Anger | b = -0.02, p =.93 | |
| CR response to Happiness | b = 0.33, p =.07 | |
| ZG response to Happiness | b = -0.06, p =.74 | |
| Use of Positive Words | b = -0.11, p =.55 | |
| Perceptions of Regard | b = 0.27, p =.13 | |
| Perceptions of Empathy | b = 0.06, p =.75 |
p < 0.05
Discussion
MDMA altered basic processes of emotional perception and response, increasing the intensity required to perceive angry expressions, and increasing positive psychophysiological responses to happy expressions, especially in women. It also impacted behavior and perceptions in an actual social interaction, increasing use of positive emotion words and perceptions of empathy and regard in an interaction partner. Exploratory analyses suggested that these “prosocial” effects were not closely related to desire to take the drug again, a rough index of abuse potential.
Our results extend previous findings, suggesting that MDMA not only affects emotion perception, but also impacts responses to emotional stimuli once they are perceived. Consistent with previous reports that MDMA reduces amygdala responsiveness to angry expressions and identification of anger (Bedi et al. 2009; Hysek et al. 2013; Kirkpatrick et al. in press), we found that MDMA slowed perception of anger. The drug did not reduce perception of fear as it did in other reports (Bedi et al. 2010; Hysek et al. 2013; Hysek et al. 2014; Kirkpatrick et al. in press), perhaps due to differences in stimuli (black and white static pictures vs. full color videos). MDMA also did not facilitate perception of happiness, consistent with some previous reports (Bedi et al. 2010; Hysek et al. 2013; Hysek et al. 2014; Kirkpatrick et al. in press), but not others (Hysek et al. 2012; of note, this study used more ambiguous positive expressions than the others). However, on our EMG measures of emotional response, MDMA increased positive responses to happiness, especially in women. This is consistent with previous research showing that MDMA enhances ventral striatal activity to happy expressions (Bedi et al. 2009).
These findings suggest that MDMA has distinct effects on basic emotional processing compared to related drugs, including other stimulants and drugs impacting serotonergic functioning. Amphetamine, a stimulant with a similar chemical structure, increases responses to positive emotional stimuli, similar to MDMA (Wardle and de Wit 2012), but rather than decreasing perception of negative emotions, it instead facilitated detection of all emotions (Wardle et al. 2012). Similarly, methylphenidate, another stimulant, facilitated identification of negative faces rather than hampering their identification as MDMA does (Hysek et al. 2014). Pharmacological differences may account for these distinctions, including a stronger serotonergic effect of MDMA compared to other stimulants (Liechti and Vollenweider 2001). However, MDMA also has divergent effects from SSRIs, which acutely increase serotonin levels (Harmer 2008; Liechti and Vollenweider 2001). SSRIs increase perception and responses to both positive and negative stimuli (Harmer 2008), in contrast to MDMA’s reduction of negative perceptions and amplification of positive responses. In summary, MDMA appears to have a distinct profile of effects on basic emotional processes, which may in turn allow it a unique niche in treatment of psychological disorders.
We also observed for the first time pro-social effects of MDMA on behavior and perceptions in an actual face-to-face social interaction (as opposed to simulated or pencil-and-paper mediated interactions; Frye et al. 2014; Hysek et al. 2013). We showed that MDMA increased use of positive emotion words in a real-time verbal interaction. Although a previous study found that MDMA did not increase observer-ratings of happiness or sociability during interactions (Marrone et al. 2010), we detected an increase in positive language using the more sensitive method of transcribing and scoring speech with a validated dictionary. MDMA also slightly increased participants’ perceptions of empathy and regard in their interaction partner; consistent with suggestions that MDMA improves therapist-client alliance (Bouso et al. 2008; Johansen and Krebs 2009; Mithoefer et al. 2011). This finding is distinct from previous studies on “empathogenic” effects of MDMA, which have shown that MDMA increases the extent to which participants “feel for” a person depicted in an emotionally charged situation (Hysek et al. 2013). Here, we examined the effects of MDMA on perceptions of an interaction partner, and found that MDMA also changes the extent to which individuals perceive themselves as “felt for” or “understood” by someone else. To our knowledge this is the first time such an effect has been demonstrated in a placebo-controlled double-blind study. However, our interaction task differed in important ways from therapy, including overall positive content, in contrast to most therapeutic interactions, and very brief duration. Additionally, our participants were carefully screened for psychiatric disorders, which might alter the effects of MDMA. For example, individuals with depression appear to derive stronger effects from d-amphetamine, a related stimulant (Tremblay et al. 2005). Thus, it remains to be determined whether the MDMA effects observed here generalize to therapy in individuals with psychiatric disorders.
Finally, we examined on a preliminary basis the relationship between social effects of MDMA and participants’ desire to take the drug again. Although MDMA users report that they use the drug for its social effects, we did not see strong evidence for this here. The only social effect that related to desire to take the drug again was self-reported playfulness, and this mostly overlapped with the typical stimulant effect of increased overall positive mood.
Limitations include the relatively small and homogeneous sample. Screening out DSM-IV disorders and heavy drug use may limit generalizability and our ability to detect abuse liability. For example, individuals with social anxiety, who are at risk for MDMA use (Lieb et al. 2002; Soar et al. 2006), may experience the social effects of MDMA as particularly beneficial. Thus, in at risk populations, the social effects of MDMA might produce greater abuse liability. Conversely, all of our participants had some previous use of MDMA, and were moderate recreational drug users, which might limit generalizability to effects of MDMA in a naïve population (e.g. in psychotherapy). However, similar effects on perception of emotion have been found in MDMA-naïve participants in other studies (Hysek et al. 2012; Hysek et al. 2013; Hysek et al. 2014). Second, some effects of this highly “social” drug may not be as detectable or desirable in the relatively isolated circumstances of the current study. It has been shown that other drugs produce different effects in isolated and social contexts (Kirkpatrick and de Wit 2013), so this may also be the case with MDMA. Third, our measure of abuse potential was a single self-report item. More detailed assessments including choice paradigms, monetary valuation or assessments of actual use might produce different results (Griffiths et al. 2003).
In conclusion, MDMA alters emotional processing in ways that may encourage social interaction, and produces “prosocial” behavior and perceptions during actual social interactions. These effects may contribute to both recreational use of MDMA, and the efficacy of MDMA as a psychotherapy adjunct. We hope a better understanding of the form of these effects and their relationship to abuse potential will contribute to prevention and treatment of MDMA use and abuse, and efforts to adapt this drug for treatment of PTSD and other psychiatric conditions.
Acknowledgments
The authors would like to thank Celina Joos, Charles Frye, Lindsey Davis, Aoibhin Curran and Sarah Ellefson for help with data collection and scoring, and the University of Chicago Investigational Pharmacy service for preparing the drug capsules.
Funding
This work was supported by a grant from the National Institute on Drug Abuse (R01 DA002812) to HdW, and MCW was supported during the execution of this work by a National Institute on Drug Abuse Training Grant (T32 DA007255).
Footnotes
Disclosure
Dr. Wardle reports no biomedical financial interests or potential conflicts of interest. Dr. de Wit reports no biomedical financial interests or potential conflicts of interest.
References
- Bates DM, Meachler M, Bolker B. lme4: Linear mixed-effects model using S4 classes 2011 [Google Scholar]
- Bedi G, Hyman D, de Wit H. Is ecstasy an “empathogen”? Effects of ±3,4-methylenedioxymethamphetamine on prosocial feelings and identification of emotional states in others. Biol Psychiatry. 2010;68:1134–1140. doi: 10.1016/j.biopsych.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, Phan KL, Angstadt M, de Wit H. Effects of MDMA on sociability and neural response to social threat and social reward. Psychopharmacology (Berl) 2009;207:73–83. doi: 10.1007/s00213-009-1635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton CP, Etchells PJ, Porter G, Clark AP, Penton-Voak IS, Nikolov SG. Turning the other cheek: The viewpoint dependence of facial expression after-effects. Proceedings of the Royal Society B: Biological Sciences. 2007;274:2131–2137. doi: 10.1098/rspb.2007.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouso JC, Doblin R, Farré M, Alcázar MÁ, Gómez-Jarabo G. MDMA-Assisted Psychotherapy Using Low Doses in a Small Sample of Women with Chronic Posttraumatic Stress Disorder. J Psychoactive Drugs. 2008;40:225–236. doi: 10.1080/02791072.2008.10400637. [DOI] [PubMed] [Google Scholar]
- Dimberg U, Karlsson B. Facial reactions to different emotionally relevant stimuli. Scand J Psychol. 1997;38:297–303. [Google Scholar]
- Dimberg U, Thunberg M, Elmehed K. Unconscious facial reactions to emotional facial expressions. Psychological Science. 2000;11:86–89. doi: 10.1111/1467-9280.00221. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Strutured clinical interview for DSM-IV axis I disorders. Biometrics Research Department; New York: 1996. [Google Scholar]
- Frye CG, Wardle MC, Norman GJ, de Wit H. MDMA decreases the effects of simulated social rejection. Pharmacology Biochemistry and Behavior. 2014;117:1–6. doi: 10.1016/j.pbb.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay FH. Variations in affect following amphetamine and placebo: Markers of stimulant drug preference. Experimental and Clinical Psychopharmacology. 2003;11:91–101. doi: 10.1037//1064-1297.11.1.91. [DOI] [PubMed] [Google Scholar]
- Gospic K, Gunnarsson T, Fransson P, Ingvar M, Lindefors N, Petrovic P. Emotional perception modulated by an opioid and a cholecystokinin agonist. Psychopharmacology (Berl) 2008;197:295–307. doi: 10.1007/s00213-007-1032-4. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Ator NA. Principles of initial experimental drug abuse liability assessment in humans. Drug Alcohol Depend. 2003;70:S41–S54. doi: 10.1016/s0376-8716(03)00098-x. [DOI] [PubMed] [Google Scholar]
- Harmer CJ. Serotonin and emotional processing: Does it help explain antidepressant drug action? Neuropharmacology. 2008;55:1023–1028. doi: 10.1016/j.neuropharm.2008.06.036. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Shelley NC, Cowen PJ, Goodwin GM. Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. Am J Psychiatry. 2004;161:1256–1263. doi: 10.1176/appi.ajp.161.7.1256. [DOI] [PubMed] [Google Scholar]
- Harris D, Baggott M, Mendelson J, Mendelson J, Jones R. Subjective and hormonal effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology (Berl) 2002;162:396–405. doi: 10.1007/s00213-002-1131-1. [DOI] [PubMed] [Google Scholar]
- Hysek C, Domes G, Liechti M. MDMA enhances “mind reading” of positive emotions and impairs “mind reading” of negative emotions. Psychopharmacology (Berl) 2012:1–10. doi: 10.1007/s00213-012-2645-9. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Schmid Y, Simmler LD, Domes G, Heinrichs M, Eisenegger C, Preller KH, Quednow BB, Liechti ME. MDMA enhances emotional empathy and prosocial behavior. Soc Cogn Affect Neurosci. 2013 doi: 10.1093/scan/nst161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Simmler LD, Schillinger N, Meyer N, Schmid Y, Donzelli M, Grouzmann E, Liechti ME. Pharmacokinetic and pharmacodynamic effects of methylphenidate and MDMA administered alone or in combination. The International Journal of Neuropsychopharmacology. 2014;17:371–381. doi: 10.1017/S1461145713001132. [DOI] [PubMed] [Google Scholar]
- Janowsky DS. Depression and dysphoria effects on the interpersonal perception of negative and positive moods and caring relationships: Effects of antidepressants, amphetamine, and methylphenidate. Curr Psychiatry Rep. 2003;5:451–459. doi: 10.1007/s11920-003-0084-3. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, Clopton P, Leichner PP, Abrams AA, Judd LL, Pechnick R. Interpersonal effects of marijuana: A model for the study of interpersonal psychopharmacology. Arch Gen Psychiatry. 1979;36:781–785. doi: 10.1001/archpsyc.1979.01780070059006. [DOI] [PubMed] [Google Scholar]
- Johansen P, Krebs T. How could MDMA (ecstasy) help anxiety disorders? A neurobiological rationale. J Psychopharmacol (Oxf) 2009;23:389–391. doi: 10.1177/0269881109102787. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick MG, de Wit H. In the company of others: Social factors alter acute alcohol effects. Psychopharmacology (Berl) 2013 doi: 10.1007/s00213-013-3147-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, Lee R, Wardle MC, Jacob S, de Wit H. Effects of MDMA and intranasal oxytocin on social and emotional processing. Neuropsychopharmacology. doi: 10.1038/npp.2014.12. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Crupain MJ, Voyvodic JT, McCarthy G. Dynamic perception of facial affect and identity in the human brain. Cereb Cortex. 2003;13:1023–1033. doi: 10.1093/cercor/13.10.1023. [DOI] [PubMed] [Google Scholar]
- Larsen JT, Norris CJ, Cacioppo JT. Effects of positive and negative affect on electromyographic activity over zygomaticus major and corrugator supercilii. Psychophysiology. 2003;40:776–785. doi: 10.1111/1469-8986.00078. [DOI] [PubMed] [Google Scholar]
- Lieb R, Schuetz CG, Pfister H, von Sydow K, Wittchen H-U. Mental disorders in ecstasy users: a prospective-longitudinal investigation. Drug Alcohol Depend. 2002;68:195–207. doi: 10.1016/s0376-8716(02)00190-4. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Gamma A, Vollenweider FX. Gender differences in the subjective effects of MDMA. Psychopharmacology (Berl) 2001;154:161–168. doi: 10.1007/s002130000648. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Vollenweider FX. Which neuroreceptors mediate the subjective effects of MDMA in humans? A summary of mechanistic studies Human Psychopharmacology: Clinical and Experimental. 2001;16:589–598. doi: 10.1002/hup.348. [DOI] [PubMed] [Google Scholar]
- Marrone GF, Pardo JS, Krauss RM, Hart CL. Amphetamine analogs methamphetamine and 3,4-methylenedioxymethamphetamine (MDMA) differentially affect speech. Psychopharmacology (Berl) 2010;208:169–177. doi: 10.1007/s00213-009-1715-0. [DOI] [PubMed] [Google Scholar]
- Mithoefer MC, Wagner MT, Mithoefer AT, Jerome L, Doblin R. The safety and efficacy of 3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: The first randomized controlled pilot study. J Psychopharmacol (Oxf) 2011;25:439–52. doi: 10.1177/0269881110378371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithoefer MC, Wagner MT, Mithoefer AT, Jerome L, Martin SF, Yazar-Klosinski B, Michel Y, Brewerton TD, Doblin R. Durability of improvement in post-traumatic stress disorder symptoms and absence of harmful effects or drug dependency after 3,4-methylenedioxymethamphetamine-assisted psychotherapy: A prospective long-term follow-up study. J Psychopharmacol (Oxf) 2013;27:28–39. doi: 10.1177/0269881112456611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody EJ, McIntosh DN, Mann LJ, Weisser KR. More than mere mimicry? The influence of emotion on rapid facial reactions to faces. Emotion. 2007;7:447–457. doi: 10.1037/1528-3542.7.2.447. [DOI] [PubMed] [Google Scholar]
- Oehen P, Traber R, Widmer V, Schnyder U. A randomized, controlled pilot study of MDMA (±3,4-Methylenedioxymethamphetamine)-assisted psychotherapy for treatment of resistant, chronic Post-Traumatic Stress Disorder (PTSD) J Psychopharmacol (Oxf) 2013;27:40–52. doi: 10.1177/0269881112464827. [DOI] [PubMed] [Google Scholar]
- Pennebaker JW, Booth RJ, Francis ME. Linguistic Inquiry and Word Count: LIWC 2007. LIWC; Austin, TX: 2007. [Google Scholar]
- Platt B, Kamboj S, Morgan CJA, Curran HV. Processing dynamic facial affect in frequent cannabis-users: Evidence of deficits in the speed of identifying emotional expressions. Drug Alcohol Depend. 2010;112:27–32. doi: 10.1016/j.drugalcdep.2010.05.004. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2011. [Google Scholar]
- Soar K, Turner JJD, Parrott AC. Problematic versus non-problematic ecstasy/MDMA use: the influence of drug usage patterns and pre-existing psychiatric factors. J Psychopharmacol (Oxf) 2006;20:417–424. doi: 10.1177/0269881106063274. [DOI] [PubMed] [Google Scholar]
- Sumnall HR, Cole JC, Jerome L. The varieties of ecstatic experience: an exploration of the subjective experiences of ecstasy. J Psychopharmacol (Oxf) 2006;20:670–682. doi: 10.1177/0269881106060764. [DOI] [PubMed] [Google Scholar]
- Tancer M, Johanson C-E. The effects of fluoxetine on the subjective and physiological effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology (Berl) 2007;189:565–573. doi: 10.1007/s00213-006-0576-z. [DOI] [PubMed] [Google Scholar]
- Ter Bogt TFM, Engels RCME. “Partying” hard: party style, motives for and effects of MDMA use at rave parties. Subst Use Misuse. 2005;40:1479–1502. doi: 10.1081/JA-200066822. [DOI] [PubMed] [Google Scholar]
- Tremblay LK, Naranjo CA, Graham SJ, et al. Functional neuroanatomical substrates of altered reward processing in major depressive disorder revealed by a dopaminergic probe. Arch Gen Psychiatry. 2005;62:1228–1236. doi: 10.1001/archpsyc.62.11.1228. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Gamma A, Liechti M, Huber T. Psychological and Cardiovascular Effects and Short-Term Sequelae of MDMA ([ldquo]Ecstasy[rdquo]) in MDMA-Naive Healthy Volunteers. Neuropsychopharmacology. 1998;19:241–251. doi: 10.1016/S0893-133X(98)00013-X. [DOI] [PubMed] [Google Scholar]
- Walter NT, Mutic S, Markett S, Montag C, Klein AM, Reuter M. The influence of alcohol intake and alcohol expectations on the recognition of emotions. Alcohol Alcohol. 2011 doi: 10.1093/alcalc/agr082. [DOI] [PubMed] [Google Scholar]
- Wardle M, Cederbaum K, de Wit H. Quantifying talk: Developing reliable measures of verbal productivity. Behavior Research Methods. 2011;43:168–178. doi: 10.3758/s13428-010-0019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle M, de Wit H. Effects of amphetamine on reactivity to emotional stimuli. Psychopharmacology (Berl) 2012;220:143–153. doi: 10.1007/s00213-011-2498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle M, Garner M, Munafò M, de Wit H. Amphetamine as a social drug: effects of d-amphetamine on social processing and behavior. Psychopharmacology (Berl) 2012:1–12. doi: 10.1007/s00213-012-2708-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TL, Justice AJH, de Wit H. Differential subjective effects of d-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav. 2002;73:729–741. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]


