Abstract
Neuroplasticity following spinal cord injury contributes to spontaneous recovery over time. Recent studies highlight the important role of brain-derived neurotrophic factor (BDNF) signaling via the high-affinity tropomyosin-related kinase (Trk) receptor subtype B (TrkB) in recovery of rhythmic diaphragm activity following unilateral spinal hemisection at C2 (C2SH). We hypothesized that TrkB kinase activity is necessary for spontaneous recovery of diaphragm activity post-C2SH. A chemical-genetic approach employing adult male TrkBF616A mice (n=49) was used to determine the impact of inhibiting TrkB kinase activity by the phosphoprotein phosphatase 1 inhibitor derivative 1NMPP1 on recovery of ipsilateral hemidiaphragm EMG activity. In mice, C2SH was localized primarily to white matter tracts comprising the lateral funiculus. The extent of damaged spinal cord (∼27%) was similar regardless of the presence of functional recovery, consistent with spontaneous recovery reflecting neuroplasticity primarily of contralateral spared descending pathways to the phrenic motor pools. Ipsilateral hemidiaphragm EMG activity was verified as absent in all mice at 3 days post-C2SH. By 2 weeks after C2SH, ipsilateral hemidiaphragm EMG activity was present in 39% of vehicle-treated mice compared to 7% of 1NMPP1-treated mice (P=0.03). These data support the hypothesis that BDNF/TrkB signaling involving TrkB kinase activity plays a critical role in spontaneous recovery of diaphragm activity following cervical spinal cord injury.
Keywords: brain-derived neurotrophic factor, diaphragm muscle, EMG, spinal cord injury
Introduction
Injuries to the spinal cord are usually incomplete and thus spare spinal cord pathways that can be exploited to restore function. Unilateral hemisection of the spinal cord at the C2 level (C2SH) is a commonly used model of spinal cord injury in animals including rodents (Fuller, et al., 2002, Fuller, et al., 2009, Golder, et al., 2001, Goshgarian, 2003, Mantilla, et al., 2013, Mantilla and Sieck, 2009, Minor, et al., 2006, Miyata, et al., 1995, Nantwi, et al., 1999, Seeds, et al., 2011, Zhan, et al., 1997). Following C2SH there is removal of the descending premotor drive to phrenic motor neurons and paralysis of the ipsilateral diaphragm muscle. Over time there is gradual and spontaneous recovery of rhythmic diaphragm muscle activity (Fuller, et al., 2008, Nantwi, et al., 1999). Indeed, ∼40% of rats display recovery of rhythmic ipsilateral diaphragm activity by 2-6 weeks after C2SH (Gransee, et al., 2013, Mantilla, et al., 2012, Mantilla, et al., 2013). The utility of the C2SH model in evaluating the mechanisms underlying functional recovery after spinal cord injury has been validated across a number of studies (Alilain and Goshgarian, 2008, Alilain, et al., 2011, Golder and Mitchell, 2005).
Brain-derived neurotrophic factor (BDNF) signaling via the high-affinity tropomyosin-related kinase (Trk) receptor subtype B (TrkB) is an important mediator of neuroplasticity following spinal cord injury (Weishaupt, et al., 2012). Neurotrophic BDNF/TrkB signaling in the phrenic motor neuron pool is critical for functional recovery of diaphragm activity following C2SH (Gransee, et al., 2013, Mantilla, et al., 2012). We recently determined that expression of the full-length TrkB receptor specifically at the phrenic motor neurons is sufficient for spontaneous recovery of diaphragm muscle activity following C2SH in rats (Gransee, et al., 2013). However, the role of BDNF/TrkB signaling involving TrkB kinase activity is currently unclear.
We hypothesized that inhibition of TrkB kinase activity will impair recovery of rhythmic ipsilateral hemidiaphragm activity after C2SH. A chemical-genetic approach to inhibition of TrkB kinase activity signaling using TrkBF616A mice has previously been used in a variety of physiological systems (Chen, et al., 2005, Mantilla and Ermilov, 2012). These mice express knockin alleles permitting rapid, selective and reversible inhibition of TrkB kinase activity by phosphoprotein phosphatase 1 inhibitor (PP1) derivative 1NMPP1, thus allowing for normal development and inhibition of TrkB kinase activity only following 1NMPP1 administration.
Materials and methods
Animals
Adult male TrkBF616A mice (n=49; 31.8 ± 0.7 g) were bred and maintained in a colony at Mayo Clinic. TrkBF616A mice carry a phenylalanine-to-alanine mutation in the ATP binding domain of the TrkB receptor (Chen, et al., 2005). All mice were genotyped by PCR analysis of DNA isolated from tail snips. The PCR amplification was conducted with the following primers (5′-GGGCTTGAGAAGAGGGCAAAAGGGTTGCTCAG-3′) and (5′-GTTGGTCACCAGCAGAACACTCGACTCAC-3′) obtained from Integrated DNA Technologies (Coralville IA). TrkBF616A mice were used to selectively and rapidly inhibit TrkB kinase activity following 1NMPP1 treatment (Chen, et al., 2005, Mantilla and Ermilov, 2012). All study and animal care guidelines were approved by the Institutional Animal Care and Use Committee (IACUC) at the Mayo Clinic in accordance with National Institute of Health Guidelines. Mice were given free access to food and water, while maintained on a 12 hour light-dark schedule.
For the primary analyses, two groups of TrkBF616A mice underwent C2SH and were randomized 3 days afterwards to receive either 11 days of oral 1NMPP1 treatment (n=14; 25 μM in drinking water; Calbiochem #529581) or vehicle treatment (n=18; 0.3% DMSO in drinking water). Thus, mice in both groups were studied at 2 weeks following C2SH. In additional analyses, TrkBF616A mice underwent C2SH (n=10) and were studied 3 days following surgery in order to verify that C2SH completely inactivates the ipsilateral hemidiaphragm muscle. A group of uninjured TrkBF616A mice received 11 days of 1NMPP1 treatment (n=7) in order to verify persistent diaphragm activity. In vivo inhibition of TrkB kinase activity in TrkBF616A mice was verified in protein extracted from brain tissue following systemic treatment with 1NMPP1 or vehicle. TrkB protein was immunoprecipitated using anti-TrkB (Neuromics # GT15080).
Following electrophoresis, protein was transferred to a PVDF membrane and probed using 1:500 phosphorylated TrkB (Cell Signaling #4621S) and 1:1000 TrkB (Neuromics #GT15080). Following 1NMPP1 treatment of TrkBF616A mice, there is a 13 fold reduction in the ratio of phosphorylated to total TrkB (P=0.04).
At the terminal experiment, mice were anesthetized with intramuscular ketamine (90 mg/kg) and xylazine (10 mg/kg) and diaphragm muscle EMG recordings were collected. Following EMG recordings, mice were euthanized by exsanguination and transcardially perfused with 4% paraformaldehyde solution in 0.1 M phosphate buffer, and the cervical spinal cord was removed and preserved for further analyses.
Spinal cord hemisection (C2SH)
The surgical procedure for C2SH was based on previously published techniques in the rat (Mantilla, et al., 2012, Mantilla, et al., 2013, Mantilla, et al., 2007, Miyata, et al., 1995, Prakash, et al., 1999). Briefly, mice were anesthetized with intramuscular ketamine (90 mg/kg) and xylazine (10 mg/kg). A bilateral dorsal laminectomy at C2 exposed the cervical spinal cord and a lateralized section of the right half of the spinal cord was performed at C2 anteriorly to the dorsal fissure (see Fig. 1). This lateral hemisection resulted in functional loss of ipsilateral diaphragm muscle activity evident by a change in the movement of the chest wall and abdomen. All animals were observed closely following surgery and intramuscular buprenorphine (0.1 mg/kg) and acetaminophen in drinking water (2 mg/ml) were administered following surgery, and as needed thereafter. Overall, 42 TrkBF616A mice underwent C2SH and were allocated to experimental treatment groups examined at 2 weeks following C2SH (n=32) and to a functional verification group examined at 3 days following C2SH (n=10) in order to ascertain the absence of ipsilateral diaphragm EMG activity at this time point. An additional 14 mice underwent C2SH but were not allocated to experimental groups due to death during surgery or prior to 3 days post-C2SH.
Figure 1.
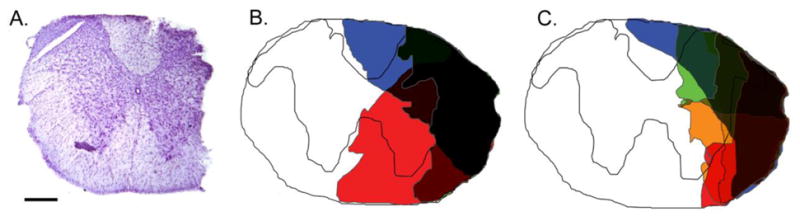
Histological evidence of spinal cord trauma following cervical spinal hemisection at the C2 level (C2SH) in adult TrkBF616A mice. Serial, 50 μm transverse sections of the spinal cord from C1 – C4 were stained with cresyl violet in order to visualize spinal cord anatomy and identify the area of injury. A representative image is displayed in panel A. The maximal area of damage for each mouse was determined from all images exhibiting injury by rendering injury areas onto a spinal cord section outline. The extent of C2SH for each animal is represented as a different color in the composite image in panels B and C, for mice that did display recovery of ipsilateral rhythmic hemidiaphragm EMG activity (panel B) and those that did not (panel C). Scale bar, 250 μm.
Histological assessment of spinal cord hemisection (C2SH)
The extent of C2SH was evaluated by histological examination of spinal cord sections from C1 – C4. Transverse sections (50 μm) were cut using a cryostat (Reichert-Jung 2800E) kept at -20 °C, mounted on Cell-Tak-coated slides (BD Biosciences #354241) and allowed to dry overnight. Sections were then placed in 1:1 alcohol:chloroform, 95% and 100% alcohol followed by distilled water for 5 minutes in each step. Sections were then stained with 0.1% cresyl violet in glacial acetic acid. After ∼10 minutes, slides were rinsed in water and dehydrated through graded alcohol steps followed by xylene. Images were obtained on an upright Nikon Optiphot-2 microscope equipped with an 8-bit CCD camera. All images were saved using as 24-bit multi-TIFF RGB color files.
In order to visualize the extent of C2SH for an individual animal, every spinal cord image exhibiting any evidence of injury was identified and exported into Adobe Photoshop (Adobe Systems Inc., San Jose CA). The area of injury in each image was then rendered onto a standard spinal cord section outline. The extent of C2SH was determined by planimetry from the maximal area of injury encompassing all images from that animal (Fig. 1).
Diaphragm EMG recordings
As previously described (Dow, et al., 2006, Dow, et al., 2009, Mantilla, et al., 2012, Mantilla, et al., 2013, Mantilla, et al., 2011, Trelease, et al., 1982), a pair of electrode wires (0.28 mm diameter - model AS631, Cooner Wire Inc., Chatsworth, CA) were stripped about 1 mm at the tip and implanted bilaterally into the midcostal diaphragm muscle regions. The uninsulated portion was embedded within the diaphragm muscle and EMG activity was recorded during spontaneous breathing in anesthetized mice.
The EMG signal from the diaphragm muscle was differentially amplified (2000x), bandpass filtered (20-1000 Hz), and analog-to-digital converted at 2 kHz using a LabView data acquisition board and software (National Instruments, Austin, TX). The diaphragm muscle EMG was used to determine evidence of functional recovery as well as parameters of respiratory function (i.e. inspiratory and expiratory durations, duty cycle, and respiratory frequency). The following criteria were used to establish evidence of recovery of ipsilateral hemidiaphragm EMG activity: 1) rhythmic (i.e., in phase with contralateral side), 2) periodic (i.e., occurring across >90% of eupneic bursts over at least 2 min period), and 3) signal comprising at least two motor units. In all cases parameters of respiratory function were determined from the side contralateral to C2SH injury, regardless of evidence of functional recovery. Diaphragm EMG recordings were not used for quantitative analyses of EMG amplitude since comparisons across animals or across sides are not meaningful given expected variability in electrode position, inter-electrode distance, and local fiber type composition. Indeed, chronic EMG recordings in the same animal are necessary for such quantitative analyses, and even then only when referenced to some maximal or near-maximal level of activation (c.f., Mantilla, et al., 2011). Chronic placement of EMG electrodes was attempted in the diaphragm muscle unsuccessfully (n=10; see Results).
Statistical Analysis
All statistical evaluations were performed using JMP (version 9.0.1, SAS Institute, Inc., Cary, North Carolina). Data were analyzed by one-way ANOVA comparing across groups for EMG analysis of recovery rate, time inspiratory, time expiratory, duty cycle, and respiratory frequency. Data are reported as mean ± SE, unless otherwise specified, significance was accepted at P<0.05.
Results
Animals
Following the C2SH, all TrkBF616A mice maintained spontaneous ventilation and none required ventilatory support, despite loss of function of the ipsilateral diaphragm muscle. Mice recovered promptly and were able to ambulate unassisted while displaying limited deficits in ipsilateral forelimb movement. No mice required bladder expression or assistance with ocular hygiene. Mice treated with 1NMPP1 did not show gross behavioral or other adverse effects.
Assessment of spinal cord hemisection (C2SH)
Histological analyses were conducted in a subset of mice across all C2SH groups to determine the maximum extent of spinal cord injury in the mouse model of C2SH (Fig 1). Composite images displaying the extent of injury for each animal were generated for animals displaying recovery of ipsilateral hemidiaphragm EMG activity (Fig 1B) and for those that did not display recovery (Fig 1C) at 2 weeks following C2SH. Overall, the C2SH injury to the cervical spinal cord was localized laterally and primarily to white matter tracts comprising the lateral funiculus, regardless of the presence of spontaneous recovery of rhythmic diaphragm muscle activity. The area of damage to white matter tracts was 31.1 ± 3.8% of the total white matter area at C2 compared to 22.2 ± 3.5% of gray matter. There was no difference between C2SH groups in either white or gray matter damage depending on whether animals displayed recovery or not (P≥0.11). Indeed the total area of spinal cord damage was slightly larger in mice showing recovery (34.5 ± 5.2%) than those that did not (22.5 ± 2.2%; P=0.05).
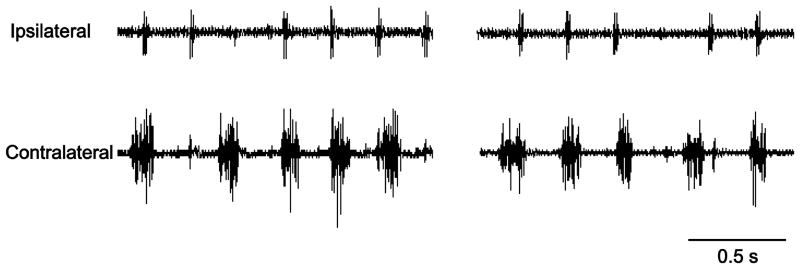
Preliminary studies attempted to validate chronic diaphragm EMG recordings (n=10 mice) in order to determine the loss of ipsilateral hemidiaphragm EMG activity following C2SH. Unfortunately, performing chronic EMG recordings with implantable electrodes in mouse diaphragm muscle proved technically very challenging due to the minimal thickness of the diaphragm muscle (∼350 μm). Most animals implanted with these electrodes succumbed to respiratory complications including pneumothorax. Thus, the assessment of functional impairment following C2SH was conducted in a group of mice examined 3 days after surgery (n=10) using electrode pairs implanted at the terminal experiment. No mouse displayed ipsilateral hemidiaphragm EMG activity at 3 days post-C2SH (Fig 2), verifying functional diaphragm muscle paralysis and thus complete interruption of descending drive to phrenic motor neurons ipsilateral to C2SH.
Figure 2.
Representative raw diaphragm EMG tracings from the beginning and end of the 2 minute recording period, obtained 3 days following C2SH surgery in an adult TrkBF616A mouse. Notice lack of rhythmic hemidiaphragm EMG activity ipsilateral to C2SH. Electrocardiographic spikes are visible in both traces. None out of ten C2SH mice displayed ipsilateral hemidiaphragm EMG activity, verifying the completeness of the C2SH surgery which interrupts ipsilateral descending drive to phrenic motor neurons causing hemidiaphragm muscle paralysis.
Recovery of ipsilateral hemidiaphragm activity after C2SH
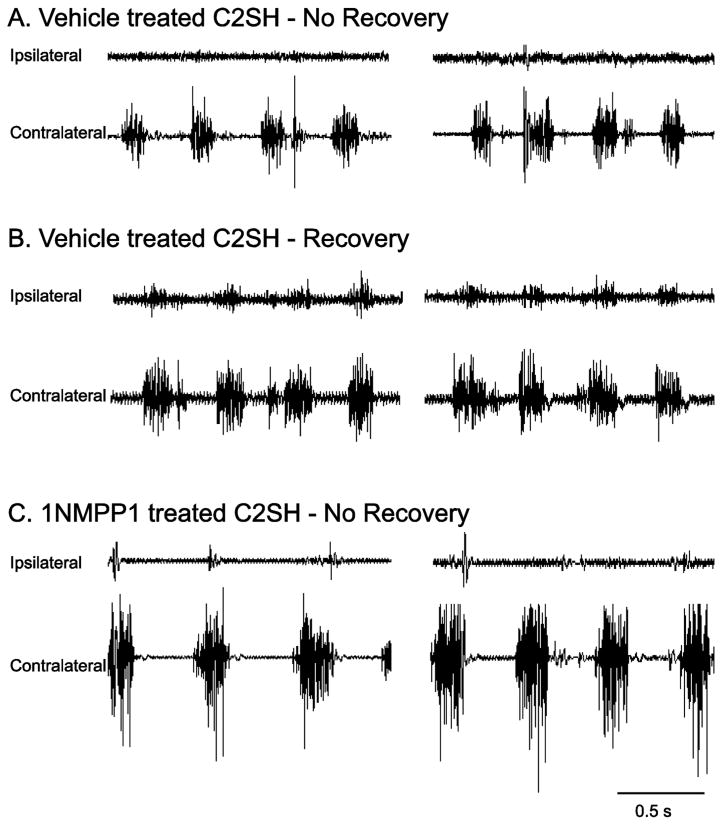
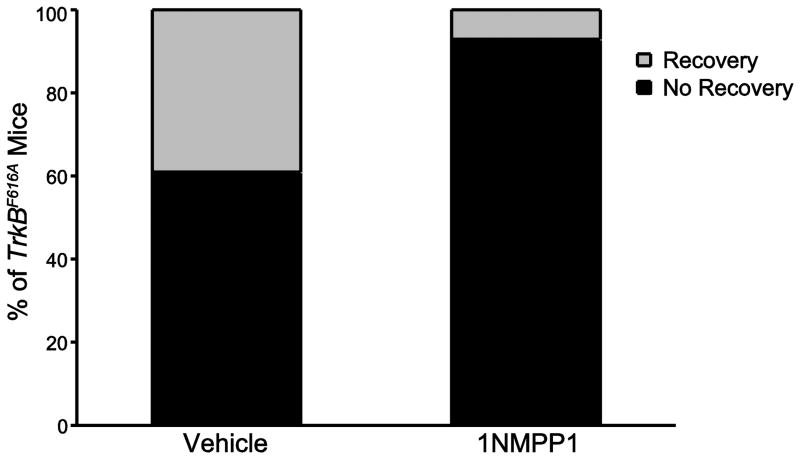
Spontaneous recovery was evident in a subset of C2SH animals. Diaphragm EMG analyses were used to determine presence of rhythmic and periodic activity of the ipsilateral diaphragm in anesthetized mice. Additionally, diaphragm EMG signals had to consistently display multiple motor unit spike morphologies as a criteria to represent functional recovery. Representative diaphragm EMG tracings for C2SH TrkBF616A mice treated with vehicle or 1NMPP1 are shown in Figure 3. In total, 39% (7 of 18) of mice in the vehicle-treated C2SH group showed spontaneous recovery of rhythmic ipsilateral diaphragm muscle activity by 2 weeks after C2SH. In contrast, only 7% (1 of 14) of mice displayed recovery when TrkB kinase activity was inhibited by oral 1NMPP1 treatment in TrkBF616A mice (P=0.03; Fig 4).
Figure 3.
Representative raw diaphragm EMG tracings from the beginning and end of the 2 minute recording period, obtained 2 weeks following C2SH are shown for TrkBF616A mice randomly assigned to treatment with oral 1NMPP1 to block TrkB kinase activity (n=14) or not (n=18). Most C2SH mice show persistent paralysis of the ipsilateral diaphragm evidenced by lack of diaphragm EMG activity (panel A). A subset of animals display functional recovery as evidenced by ipsilateral diaphragm EMG activity (panel B). Following oral 1NMPP1 treatment, absence of diaphragm EMG activity ipsilateral to C2SH was observed in nearly all TrkBF616A mice (panel C).
Figure 4.
Proportion of TrkBF616A mice displaying functional recovery of ipsilateral hemidiaphragm EMG activity following C2SH. The proportion of TrkBF616A mice displaying recovery was significantly reduced in C2SH mice treated with 1NMPP1 (n=14) compared to vehicle-treated C2SH mice (n=18; p=0.03).
Respiratory patterns measured from contralateral diaphragm EMG recordings during eupnea in anesthetized C2SH mice were used to compare ventilatory impairment across treatment groups, and the presence of spontaneous recovery of rhythmic diaphragm muscle activity was also considered. Respiratory pattern was compared 2 weeks after C2SH in TrkBF616A mice treated with vehicle or 1NMPP1. There were no statistically significant differences in respiratory frequency, inspiratory or expiratory duration and duty cycle between C2SH mice with or without inhibition of TrkB kinase activity (Table 1). Overall, average respiratory rate was 125 ± 7 min-1, and average inspiratory and expiratory times were 153 ± 10 and 296 ± 18 ms, respectively, for a duty cycle of 35 ± 2 %. In addition, there was no difference across any respiratory pattern variable between vehicle-treated C2SH mice that recovered and those that did not (P≥0.22). In addition, respiratory patterns were compared across hemidiaphragm sides in animals displaying spontaneous recovery of rhythmic diaphragm muscle activity. There was no difference between the ipsilateral hemidiaphragm sides of mice for any of the respiratory pattern variables assessed (P≥0.68).
Table 1.
Respiratory pattern of TrkBF616A mice following 2 weeks of unilateral cervical spinal hemisection at C2 (C2SH) measured from diaphragm EMG
| Vehicle-treated C2SH mice (n=14) |
1NMPP1-treated C2SH mice (n=8) |
One-way ANOVA P-value |
|
|---|---|---|---|
| Respiratory frequency (min-1) | 123 ± 6 | 128 ± 17 | 0.72 |
| Time inspiratory (ms) | 144 ± 12 | 171 ± 20 | 0.22 |
| Time expiratory (ms) | 293 ± 17 | 301 ± 40 | 0.84 |
| Duty cycle (%) | 34 ± 2 | 38 ± 3 | 0.26 |
Mean±SE
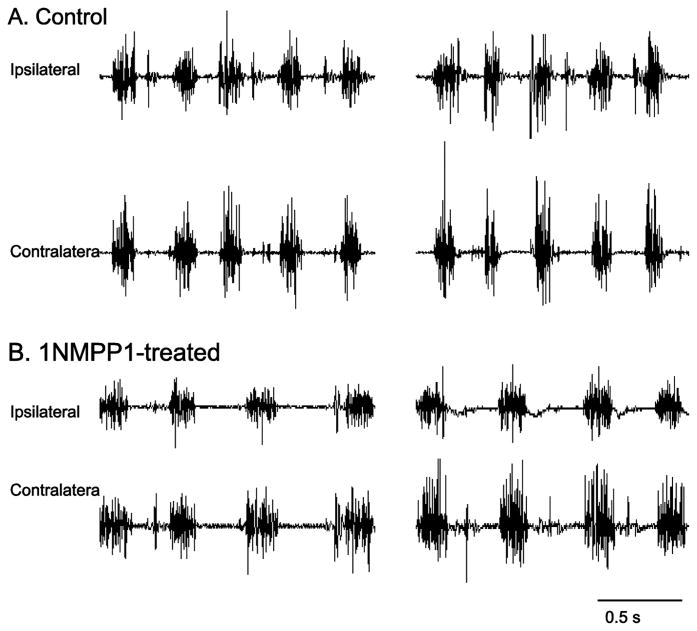
To evaluate a possible effect of inhibiting TrkB kinase activity on the ability to record diaphragm EMG activity, uninjured TrkBF616A mice were treated with 1NMPP1for 11 days, consistent with the duration of treatment in C2SH mice. There were no apparent differences in the diaphragm EMG (Fig 5). Uninjured TrkBF616A mice treated with 1NMPP1 had a respiratory rate of 115 ± 12 min-1, inspiratory time of 162 ± 30 ms, expiratory time of 335 ± 30 ms, and a duty cycle of 35 ± 5 % (no significant difference than other groups one-way ANOVA P≥0.50).
Figure 5.
Representative raw diaphragm EMG tracings from the beginning and end of the 2 minute recording period, obtained from a control untreated TrkBF616A mouse (panel A) and one treated with 1NMPP1 via drinking water to inhibit TrkB kinase activity (panel B). There was no evidence of impaired diaphragm EMG activity in any mice treated with 1NMPP1 that did not undergo C2SH surgery (n=7).
Discussion
Using a chemical-genetic approach that permits selective inhibition of TrkB kinase activity (Chen, et al., 2005), we show that TrkB kinase activity plays a critical role in spontaneous recovery of rhythmic diaphragm muscle activity following unilateral cervical spinal cord injury. TrkBF616A mice develop normally and only display inhibition of TrkB kinase activity following 1NMPP1 administration. Ipsilateral hemidiaphragm EMG activity was present in 39% of vehicle-treated mice 2 weeks following functional C2SH. In contrast, following 1NMPP1-induced inhibition of TrkB kinase activity, only 7% of TrkBF616A mice displayed any ipsilateral hemidiaphragm EMG activity. This substantial blunting of spontaneous recovery of diaphragm activity is consistent with the role of BDNF/TrkB signaling in neuroplasticity (Gransee, et al., 2013, Mantilla, et al., 2013, Weishaupt, et al., 2012), emphasizing the importance of TrkB kinase activity in mediating neurotrophin-dependent neuroplasticity and functional recovery.
Mouse models of spinal cord hemisection are frequently used in the lumbar and thoracic regions of the spinal cord. Spinal cord hemisection models to the cervical region have been limited (Anderson, et al., 2004, Blanco, et al., 2007, Harel, et al., 2010), and only a few previous studies report spinal cord hemisection to the C2 level in mice (Minor, et al., 2006, Seeds, et al., 2011). The C2SH model has been used extensively and validated across a range of species (c.f., Goshgarian, 2003). Importantly, cervical segments are the most commonly affected by spinal cord injury in humans (2013). Use of mouse models to examine genetic manipulations offers the potential for gaining unique insight into the mechanisms underlying recovery from spinal cord injury.
The present C2SH mouse model of functional hemisection resulted in predominant lateral neuroanatomical injury to white matter tracts comprising the lateral funiculus. Complete injury of descending excitatory premotor drive to phrenic motor neurons was verified functionally 3 days following C2SH by the absence of ipsilateral hemidiaphragm EMG activity. At this time no mice displayed any ipsilateral activity of the diaphragm muscle. A rat model of C2SH has been used in a number of previous studies by our group (Mantilla, et al., 2012, Mantilla, et al., 2013, Mantilla, et al., 2007, Miyata, et al., 1995, Prakash, et al., 1999) and others (Fuller, et al., 2002, Fuller, et al., 2009, Golder, et al., 2001, Goshgarian, et al., 1991, Nantwi, et al., 1999). In general agreement with the mouse model reported in the present study, the rat C2SH model exhibits histological evidence of injury to the cervical spinal cord that is localized to both the lateral and ventral funiculi with sparing of the dorsal and dorsolateral funiculi (Prakash, et al., 1999). Of note, descending excitatory pathways to phrenic motor neurons predominantly emanate from premotor interneurons located in the medulla including the ventral respiratory group (Dobbins and Feldman, 1994, Ellenberger and Feldman, 1988, Feldman, et al., 1985). In the mouse, reticulospinal projections appear to be located more laterally within white matter tracts (Barrette, et al., 2007) than in rats (Fuller, et al., 2009, Lane, et al., 2008, Vinit, et al., 2006). However, detailed neuroanatomical tracing studies (e.g., using pseudorabies virus to trans-synaptically identify phrenic premotor projections) are lacking in mice. Regardless, in the present study, absence of ipsilateral hemidiaphragm EMG activity was verified 3 days after C2SH indicating complete transection of ipsilateral descending projections to phrenic motor neurons. Interestingly, the extent of involvement of other white matter tracts (Fig 1) did not impact whether mice displayed spontaneous recovery of rhythmic ipsilateral hemidiaphragm EMG activity or not.
Rhythmic hemidiaphragm EMG activity was present ipsilaterally in a subset of mice following C2SH. In previous studies, functional recovery after C2SH has been reported anywhere from a few hours (Hadley, et al., 1999, Sperry and Goshgarian, 1993) to several weeks or more (Fuller, et al., 2006, Golder, et al., 2003, Golder and Mitchell, 2005, Nantwi, et al., 1999, Sieck and Mantilla, 2009, Vinit, et al., 2006). In the present study, 39% of vehicle-treated C2SH mice displayed spontaneous recovery of hemidiaphragm EMG activity by 2 weeks post-injury. Of note, this proportion of animals displaying recovery is similar to that in rats where we have previously reported that 38% of rats exhibit spontaneous recovery by 2 weeks post-C2SH (Gransee, et al., 2013, Mantilla, et al., 2012). This proportion remained fairly constant over time with 42% of rats displaying ipsilateral hemidiaphragm EMG activity 6 weeks post-C2SH (Mantilla, et al., 2013). Taken together these results support the utility of the C2SH model to evaluate the impact of inhibiting TrkB kinase activity on the recovery of ipsilateral diaphragm activity. Indeed, the present study documents a robust blunting of recovery when TrkB kinase activity was inhibited.
In previous rat studies, chronic diaphragm EMG recordings were employed to examine diaphragm EMG activity longitudinally (Gransee, et al., 2013, Mantilla, et al., 2013, Mantilla, et al., 2011, Nantwi, et al., 1999, Trelease, et al., 1982). Unfortunately, chronic EMG recordings from mouse diaphragm muscle proved technically very challenging with implantable electrodes, likely due to the minimal thickness of the diaphragm muscle. Since chronic EMGs were not performed in this study, quantitative analyses (e.g., using root mean squared EMG) cannot be reliably conducted. It is important to emphasize that quantitative analyses of diaphragm EMG amplitude across animals or across sides are not meaningful given expected variability in electrode position, inter-electrode distance, and local fiber type composition. Indeed, chronic EMG recordings in the same animal are necessary for such quantitative analyses, and even then only when referenced to some maximal or near-maximal level of activation (Mantilla, et al., 2011). In the present study, no baseline pre-injury measurement is available; accordingly, there was no attempt at quantifying the EMG signal.
Contralateral diaphragm EMG recordings were used to determine respiratory pattern at the terminal experiment (2 weeks post-C2SH) in anesthetized vehicle- and 1NMPP1-treated TrkBF616A mice during eupnea. Importantly, there was no difference in the respiratory frequency, inspiratory or expiratory duration and duty cycle between C2SH mice with or without inhibition of TrkB kinase activity. All of these mice were examined under identical experimental conditions with ketamine/xylazine anesthesia. This anesthetic agent produces modest effects on ventilation (Taie, et al., 1999), which would be expected to be identical across treatment groups regardless of the presence (or not) of functional recovery. Accordingly, there were no differences in any of the respiratory patterns between mice displaying or not ipsilateral hemidiaphragm EMG activity post-C2SH (i.e., recovery vs. no recovery), indicating that the lack of differences in ventilation (and thus arterial blood gases) between experimental groups. In a previous study, we documented the lack of differences in arterial blood gases between anesthetized control and C2SH rats (Miyata, et al., 1995).
The use of a chemical genetic approach to selectively inhibit TrkB kinase activity in TrkBF616A mice allowed for specific examination of the mechanisms underlying the effect of neurotrophins such as BDNF on recovery after spinal cord injury. Following 1NMPP1 administration, we validated the inhibition of TrkB kinase activity in TrkBF616A mice by the extensive reduction in phosphorylated TrkB. Although evidence of TrkB phosphorylation was still present despite 1NMPP1 treatment, methods for assessment of phosphorylated TrkB may permit detection of endogenous phosphorylation sites outside of the autocatalytic sites (Reichardt, 2006). Thus, this chemical-genetic approach to the inhibition of TrkB kinase activity provides for robust and rapid pharmacological inhibition without causing developmental abnormalities related to the lack of TrkB receptor (Klein, et al., 1993).
Previously, we showed that BDNF signaling via phrenic motor neuron TrkB receptors promotes recovery of ipsilateral hemidiaphragm EMG activity following C2SH (Mantilla, et al., 2013). In addition, when BDNF availability in cervical spinal cord segments containing phrenic motor neurons (C3-C5) was reduced by intrathecal TrkB-Fc administration, recovery was impaired. Importantly, TrkB knockdown in phrenic motor neurons was achieved using targeted delivery of either TrkB or non-sense siRNA via intrapleural injection (Mantilla, et al., 2009). Whereas intrapleural TrkB siRNA completely blunted recovery after C2SH, no effect on recovery was evident with intrapleural non-sense siRNA treatment. Furthermore, selectively targeting delivery of full-length TrkB receptor to phrenic motor neurons using an AAV7 vector promotes recovery of ipsilateral hemidiaphragm EMG activity (Gransee, et al., 2013). The selective retrograde transduction of phrenic motor neurons was validated when AAV7 is administered intrapleurally and increased gene expression of full-length TrkB was confirmed in phrenic motor neurons.
Signaling via TrkB receptors involves receptor dimerization upon neurotrophin binding and transphosphorylation by the kinase domain of Trk receptors (Mantilla and Sieck, 2008, Reichardt, 2006). Subsequently, phosphatidyl inositol-3 kinase, phospholipase C-γ1 and mitogen activated protein kinase (MAPK) pathways are activated (Huang and Reichardt, 2003). In phrenic motor neurons, nuclear translocation of the transcription factor cAMP response element-binding protein (CREB) is evident following intrathecal BDNF treatment (Mantilla, et al., 2013), consistent with activation of MAPK pathways (Pizzorusso, et al., 2000). In addition to the catalytically-active full-length TrkB receptor, truncated TrkB receptors lacking the intracellular tyrosine kinase domain can be expressed via alternative mRNA splicing (Barbacid, 1994). Intracellular, neurotrophin-independent transactivation of immature TrkB receptors may result from interaction with Gs-coupled receptor signaling (Rajagopal, et al., 2004) and contribute to neuroplasticity in phrenic motor output (Golder, et al., 2008). Although such alternative mechanisms cannot be discarded, the results of the present study support the hypothesis that BDNF/TrkB signaling involving TrkB kinase activity plays a critical role in spontaneous recovery of diaphragm activity following C2SH. Furthermore, inhibition of TrkB kinase activity in phrenic motor neurons may be sufficient for blunting recovery in the C2SH model of incomplete cervical spinal cord injury.
Highlights.
Unilateral C2 spinal hemisection causes ipsilateral loss of diaphragm EMG activity
Spontaneous recovery of diaphragm activity is evident 14 days after C2 injury
A chemical-genetic approach permits in vivo inhibition of TrkB kinase activity
Inhibiting TrkB kinase activity blunts spontaneous recovery after C2 hemisection
TrkB kinase activity is critical for recovery following spinal cord injury
Acknowledgments
We thank Dr. David D. Ginty (Johns Hopkins University) who kindly provided the original breeder pair of TrkBF616A mice. We would also like to thank Ms. Yun-Hua Fang for technical assistance with the performance of immunohistochemical studies. This work was supported by NIH grants HL096750 (CBM & GCS), HL105355 (SMG) and the Mayo Clinic.
Footnotes
The authors declare no competing financial interests.
References
- 1.Spinal cord injury facts and figures at a glance. J Spinal Cord Med. 2013;36:1–2. doi: 10.1179/1079026813Z.000000000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alilain WJ, Goshgarian HG. Glutamate receptor plasticity and activity-regulated cytoskeletal associated protein regulation in the phrenic motor nucleus may mediate spontaneous recovery of the hemidiaphragm following chronic cervical spinal cord injury. Exp Neurol. 2008;212:348–357. doi: 10.1016/j.expneurol.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alilain WJ, Horn KP, Hu H, Dick TE, Silver J. Functional regeneration of respiratory pathways after spinal cord injury. Nature. 2011;475:196–200. doi: 10.1038/nature10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson KD, Abdul M, Steward O. Quantitative assessment of deficits and recovery of forelimb motor function after cervical spinal cord injury in mice. Exp Neurol. 2004;190:184–191. doi: 10.1016/j.expneurol.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 5.Barbacid M. The Trk family of neurotrophin receptors. J Neurobiol. 1994;25:1386–1403. doi: 10.1002/neu.480251107. [DOI] [PubMed] [Google Scholar]
- 6.Barrette B, Vallieres N, Dube M, Lacroix S. Expression profile of receptors for myelin-associated inhibitors of axonal regeneration in the intact and injured mouse central nervous system. Mol Cell Neurosci. 2007;34:519–538. doi: 10.1016/j.mcn.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Blanco JE, Anderson KD, Steward O. Recovery of forepaw gripping ability and reorganization of cortical motor control following cervical spinal cord injuries in mice. Exp Neurol. 2007;203:333–348. doi: 10.1016/j.expneurol.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Ye H, Kuruvilla R, Ramanan N, Scangos KW, Zhang C, Johnson NM, England PM, Shokat KM, Ginty DD. A chemical-genetic approach to studying neurotrophin signaling. Neuron. 2005;46:13–21. doi: 10.1016/j.neuron.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- 10.Dow DE, Mantilla CB, Zhan WZ, Sieck GC. EMG-based detection of inspiration in the rat diaphragm muscle. Conf Proc IEEE Eng Med Biol Soc. 2006;1:1204–1207. doi: 10.1109/IEMBS.2006.260688. [DOI] [PubMed] [Google Scholar]
- 11.Dow DE, Zhan WZ, Sieck GC, Mantilla CB. Correlation of respiratory activity of contralateral diaphragm muscles for evaluation of recovery following hemiparesis. Conf Proc IEEE Eng Med Biol Soc. 2009;1:404–407. doi: 10.1109/IEMBS.2009.5334892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellenberger HH, Feldman JL. Monosynaptic transmission of respiratory drive to phrenic motoneurons from brainstem bulbospinal neurons in rats. J Comp Neurol. 1988;269:47–57. doi: 10.1002/cne.902690104. [DOI] [PubMed] [Google Scholar]
- 13.Feldman JL, Loewy AD, Speck DF. Projections from the ventral respiratory group to phrenic and intercostal motoneurons in cat: an autoradiographic study. J Neurosci. 1985;5:1993–2000. doi: 10.1523/JNEUROSCI.05-08-01993.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuller DD, Doperalski NJ, Dougherty BJ, Sandhu MS, Bolser DC, Reier PJ. Modest spontaneous recovery of ventilation following chronic high cervical hemisection in rats. Exp Neurol. 2008;211:97–106. doi: 10.1016/j.expneurol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuller DD, Golder FJ, Olson EB, Jr, Mitchell GS. Recovery of phrenic activity and ventilation after cervical spinal hemisection in rats. J Appl Physiol. 2006;100:800–806. doi: 10.1152/japplphysiol.00960.2005. [DOI] [PubMed] [Google Scholar]
- 16.Fuller DD, Johnson SM, Johnson RA, Mitchell GS. Chronic cervical spinal sensory denervation reveals ineffective spinal pathways to phrenic motoneurons in the rat. Neurosci Lett. 2002;323:25–28. doi: 10.1016/s0304-3940(02)00121-0. [DOI] [PubMed] [Google Scholar]
- 17.Fuller DD, Sandhu MS, Doperalski NJ, Lane MA, White TE, Bishop MD, Reier PJ. Graded unilateral cervical spinal cord injury and respiratory motor recovery. Respir Physiol Neurobiol. 2009;165:245–253. doi: 10.1016/j.resp.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golder FJ, Fuller DD, Davenport PW, Johnson RD, Reier PJ, Bolser DC. Respiratory motor recovery after unilateral spinal cord injury: eliminating crossed phrenic activity decreases tidal volume and increases contralateral respiratory motor output. J Neurosci. 2003;23:2494–2501. doi: 10.1523/JNEUROSCI.23-06-02494.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci. 2005;25:2925–2932. doi: 10.1523/JNEUROSCI.0148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golder FJ, Ranganathan L, Satriotomo I, Hoffman M, Lovett-Barr MR, Watters JJ, Baker-Herman TL, Mitchell GS. Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. J Neurosci. 2008;28:2033–2042. doi: 10.1523/JNEUROSCI.3570-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golder FJ, Reier PJ, Bolser DC. Altered respiratory motor drive after spinal cord injury: supraspinal and bilateral effects of a unilateral lesion. J Neurosci. 2001;21:8680–8689. doi: 10.1523/JNEUROSCI.21-21-08680.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goshgarian HG. Plasticity in Respiratory Motor Control: Invited Review: The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J Appl Physiol. 2003;94:795–810. doi: 10.1152/japplphysiol.00847.2002. [DOI] [PubMed] [Google Scholar]
- 23.Goshgarian HG, Ellenberger HH, Feldman JL. Decussation of bulbospinal respiratory axons at the level of the phrenic nuclei: a possible substrate for the crossed-phrenic phenomenon. Exp Neurol. 1991;111:135–139. doi: 10.1016/0014-4886(91)90061-g. [DOI] [PubMed] [Google Scholar]
- 24.Gransee HM, Zhan WZ, Sieck GC, Mantilla CB. Targeted Delivery of TrkB Receptor to Phrenic Motoneurons Enhances Functional Recovery of Rhythmic Phrenic Activity after Cervical Spinal Hemisection. PloS One. 2013;8:e64755. doi: 10.1371/journal.pone.0064755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hadley SD, Walker PD, Goshgarian HG. Effects of serotonin inhibition on neuronal and astrocyte plasticity in the phrenic nucleus 4 h following C2 spinal cord hemisection. Exp Neurol. 1999;160:433–445. doi: 10.1006/exnr.1999.7238. [DOI] [PubMed] [Google Scholar]
- 26.Harel NY, Song KH, Tang X, Strittmatter SM. Nogo receptor deletion and multimodal exercise improve distinct aspects of recovery in cervical spinal cord injury. J Neurotrauma. 2010;27:2055–2066. doi: 10.1089/neu.2010.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 28.Klein R, Smeyne RJ, Wurst W, Long LK, Auerbach BA, Joyner AL, Barbacid M. Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neuronal death. Cell. 1993;75:113–122. [PubMed] [Google Scholar]
- 29.Lane MA, White TE, Coutts MA, Jones AL, Sandhu MS, Bloom DC, Bolser DC, Yates BJ, Fuller DD, Reier PJ. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J Comp Neurol. 2008;511:692–709. doi: 10.1002/cne.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mantilla CB, Bailey JP, Zhan WZ, Sieck GC. Phrenic motoneuron expression of serotonergic and glutamatergic receptors following upper cervical spinal cord injury. Exp Neurol. 2012;234:191–199. doi: 10.1016/j.expneurol.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mantilla CB, Ermilov LG. The novel TrkB receptor agonist 7,8-dihydroxyflavone enhances neuromuscular transmission. Muscle Nerve. 2012;45:274–276. doi: 10.1002/mus.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mantilla CB, Gransee HM, Zhan WZ, Sieck GC. Motoneuron BDNF/TrkB signaling enhances functional recovery after cervical spinal cord injury. Exp Neurol. 2013;247C:101–109. doi: 10.1016/j.expneurol.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mantilla CB, Greising SM, Zhan WZ, Seven YB, Sieck GC. Prolonged C2 spinal hemisection-induced inactivity reduces diaphragm muscle specific force with modest, selective atrophy of type IIx and/or IIb fibers. J Appl Physiol. 2013;114:380–386. doi: 10.1152/japplphysiol.01122.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mantilla CB, Rowley KL, Zhan WZ, Fahim MA, Sieck GC. Synaptic vesicle pools at diaphragm neuromuscular junctions vary with motoneuron soma, not axon terminal, inactivity. Neuroscience. 2007;146:178–189. doi: 10.1016/j.neuroscience.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 35.Mantilla CB, Seven YB, Hurtado-Palomino JN, Zhan WZ, Sieck GC. Chronic assessment of diaphragm muscle EMG activity across motor behaviors. Respir Physiol Neurobiol. 2011;177:176–182. doi: 10.1016/j.resp.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mantilla CB, Sieck GC. Trophic factor expression in phrenic motor neurons. Respir Physiol Neurobiol. 2008;164:252–262. doi: 10.1016/j.resp.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mantilla CB, Sieck GC. Neuromuscular adaptations to respiratory muscle inactivity. Respir Physiol Neurobiol. 2009;169:133–140. doi: 10.1016/j.resp.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mantilla CB, Zhan WZ, Sieck GC. Retrograde labeling of phrenic motoneurons by intrapleural injection. J Neurosci Methods. 2009;182:244–249. doi: 10.1016/j.jneumeth.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minor KH, Akison LK, Goshgarian HG, Seeds NW. Spinal cord injury-induced plasticity in the mouse--the crossed phrenic phenomenon. Exp Neurol. 2006;200:486–495. doi: 10.1016/j.expneurol.2006.02.125. [DOI] [PubMed] [Google Scholar]
- 40.Miyata H, Zhan WZ, Prakash YS, Sieck GC. Myoneural interactions affect diaphragm muscle adaptations to inactivity. J Appl Physiol. 1995;79:1640–1649. doi: 10.1152/jappl.1995.79.5.1640. [DOI] [PubMed] [Google Scholar]
- 41.Nantwi KD, El-Bohy A, Schrimsher GW, Reier PJ, Goshgarian HG. Spontaneous functional recovery in a paralyzed hemidiaphragm following upper cervical spinal cord injury in adult rats. Neurorehab Neural Repair. 1999;13:225–234. [Google Scholar]
- 42.Pizzorusso T, Ratto GM, Putignano E, Maffei L. Brain-derived neurotrophic factor causes cAMP response element-binding protein phosphorylation in absence of calcium increases in slices and cultured neurons from rat visual cortex. J Neurosci. 2000;20:2809–2816. doi: 10.1523/JNEUROSCI.20-08-02809.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prakash YS, Miyata H, Zhan WZ, Sieck GC. Inactivity-induced remodeling of neuromuscular junctions in rat diaphragmatic muscle. Muscle Nerve. 1999;22:307–319. doi: 10.1002/(sici)1097-4598(199903)22:3<307::aid-mus3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 44.Rajagopal R, Chen ZY, Lee FS, Chao MV. Transactivation of Trk neurotrophin receptors by G-protein-coupled receptor ligands occurs on intracellular membranes. J Neurosci. 2004;24:6650–6658. doi: 10.1523/JNEUROSCI.0010-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seeds N, Mikesell S, Vest R, Bugge T, Schaller K, Minor K. Plasminogen activator promotes recovery following spinal cord injury. Cell Mol Neurobiol. 2011;31:961–967. doi: 10.1007/s10571-011-9701-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sieck GC, Mantilla CB. Role of neurotrophins in recovery of phrenic motor function following spinal cord injury. Respir Physiol Neurobiol. 2009;169:218–225. doi: 10.1016/j.resp.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sperry MA, Goshgarian HG. Ultrastructural changes in the rat phrenic nucleus developing within 2 h after cervical spinal cord hemisection. Exp Neurol. 1993;120:233–244. doi: 10.1006/exnr.1993.1058. [DOI] [PubMed] [Google Scholar]
- 49.Taie S, Leichtweis SB, Liu KJ, Miyake M, Grinberg O, Demidenko E, Swartz HM. Effects of ketamine/xylazine and pentobarbital anesthesia on cerebral tissue oxygen tension, blood pressure, and arterial blood gas in rats. Advances in experimental medicine and biology. 1999;471:189–198. doi: 10.1007/978-1-4615-4717-4_23. [DOI] [PubMed] [Google Scholar]
- 50.Trelease RB, Sieck GC, Harper RM. A new technique for acute and chronic recording of crural diaphragm EMG in cats. Electroencephalogr Clin Neurophysiol. 1982;53:459–462. doi: 10.1016/0013-4694(82)90011-6. [DOI] [PubMed] [Google Scholar]
- 51.Vinit S, Gauthier P, Stamegna JC, Kastner A. High cervical lateral spinal cord injury results in long-term ipsilateral hemidiaphragm paralysis. J Neurotrauma. 2006;23:1137–1146. doi: 10.1089/neu.2006.23.1137. [DOI] [PubMed] [Google Scholar]
- 52.Weishaupt N, Blesch A, Fouad K. BDNF: the career of a multifaceted neurotrophin in spinal cord injury. Exp Neurol. 2012;238:254–264. doi: 10.1016/j.expneurol.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Zhan WZ, Miyata H, Prakash YS, Sieck GC. Metabolic and phenotypic adaptations of diaphragm muscle fibers with inactivation. J Appl Physiol. 1997;82:1145–1153. doi: 10.1152/jappl.1997.82.4.1145. [DOI] [PubMed] [Google Scholar]