Abstract
The success of hematopoietic stem-cell transplantation (HSCT) with reduced-intensity conditioning (RIC) is limited by a high rate of disease relapse. Early risk assessment could potentially improve outcomes by identifying appropriate patients for pre-emptive strategies that may ameliorate this high risk. Using a series of landmark analyses, we investigated the predictive value of early (day-30) donor chimerism measurements on disease relapse, graft-versus-host disease and survival in a cohort of 121 patients who were allografted with a uniform RIC regimen. Chimerism levels were analyzed as continuous variables. In multivariate analysis, day-30 whole blood chimerism levels were significantly associated with relapse (HR=0.90, p<0.001), relapse-free survival (HR=0.89, p<0.001) and overall survival (HR=0.94, p=0.01). Day-30 T-cell chimerism levels were also significantly associated with relapse (HR=0.97, p=0.002), relapse-free survival (HR=0.97, p<0.001) and overall survival (HR=0.99, p=0.05). Multivariate models that included T-cell chimerism provided a better prediction for these outcomes compared to whole blood chimerism. Day-30 chimerism levels were not associated with acute or chronic graft-versus-host disease. We found that high donor chimerism levels were significantly associated with a low lymphocyte count in the recipient prior to transplant, highlighting the impact of pre-transplant lymphopenia on the kinetics of engraftment after RIC HSCT. In summary, low donor chimerism levels are associated with relapse and mortality and can potentially be used as an early predictive and prognostic marker. These findings can be used to design novel approaches to prevent relapse and to improve survival after RIC HSCT.
INTRODUCTION
Reduced intensity conditioning (RIC) regimens are associated with decreased treatment-related mortality and make allogeneic hematopoietic stem-cell transplantation (HSCT) feasible in older patients and those with comorbidities. The primary barrier to the success of RIC HSCT is disease relapse [1]. The risk of relapse after RIC is 25–60% [2–7], and the median time to disease relapse is 3–7 months [8–11], implying that identification of patients at high-risk for relapse should be done very early, optimally within the first few weeks after transplant. The ability to detect relapse early in the post-transplant period is fundamental to the design of interventions that can potentially prevent disease recurrence and improve survival such as maintenance regimens or pre-emptive donor lymphocyte infusions (DLI).
The level of donor-recipient chimerism is an established method to document donor engraftment [12], and can be conducted in whole blood, bone marrow and in cellular subsets such as T-cells, myeloid cells and CD34+ cells [13, 14]. The kinetics of donor chimerism after myeloablative transplants have been characterized, but associations between attainment of complete donor chimerism and disease relapse or survival have not been consistently demonstrated [15–18].
In contrast to myeloablative transplants, RIC HSCT frequently results in varying degrees of mixed chimerism that may persist for months [19, 20], but the underlying biological features that determine this heterogeneity among patients are not well characterized. In addition, previous studies of RIC HSCT have shown conflicting results regarding the correlation between early chimerism levels and disease relapse [19–22]. As a result, there is uncertainty in how to interpret chimerism measurements in this setting, therefore limiting their clinical utility.
Our goal was to examine the utility of early chimerism measurement for prediction of disease relapse, graft-versus-host disease (GvHD) and survival. We therefore used a landmark analysis to investigate the predictive power of day-30 whole blood (WB) and T-cell chimerism levels for subsequent outcomes of patients undergoing RIC HSCT with a uniform and commonly used conditioning regimen.
METHODS
Patients and treatment
We reviewed data on adult recipients of a first allogeneic peripheral blood HSCT who were allografted with a uniform RIC regimen (fludarabine + busulfan) for a malignant hematological disorder between August 2006 and April 2013 at the University of Pennsylvania. We excluded patients who were transplanted for primary myelofibrosis where it is difficult to accurately define relapse and patients who did not have available results of day-30 chimerism levels. Since graft rejection was rare in this cohort (n=3), we excluded these patients. Our study population included 121 patients. To account for the heterogeneity of the cohort in disease type and disease burden, we reviewed relevant disease characteristics (i.e., cytogenetics in AML and MDS, disease subtype in MDS, disease stage and status in all diseases) and calculated the Disease Risk Index (DRI), a stratification system that predicts overall survival based on disease parameters. We used the 3-group version of the DRI that was recently validated using a large dataset from the Center for International Blood and Marrow Transplant Research (CIBMTR) [23]. Additional variables that were collected were the Karnofsky performance status and the hematopoietic cell transplantation-specific comorbidity index (HCT-CI) [24]. The Institutional Review Board of the University of Pennsylvania approved the study. All participants provided written informed consent for data collection at the time of their transplant.
All participants received a uniform conditioning regimen of fludarabine i.v. 120 mg/m2 and busulfan i.v. 6.4 mg/kg, followed by the infusion of granulocyte colony-stimulating factor-mobilized peripheral blood stem cells from either a related or an unrelated donor. T-cell depletion was not used. Participants received standard GvHD prophylaxis with oral tacrolimus 0.06 mg/kg/d or cyclosporine 5 mg/kg/d in 2 divided doses starting on day −3 and intravenous methotrexate 15 mg/m2 on day 1 and 10 mg/m2 on days 3, 6 and 11. Tacrolimus and cyclosporine doses were adjusted to attain trough levels between 5–15 ng/ml and 200–400 ng/ml respectively. Some patients (n=29) received maraviroc, a CCR5 antagonist, as part of a clinical trial in GvHD prophylaxis at doses of 150 mg or 300 mg twice daily between day −2 and day +30 [25]. All participants received standard antimicrobial prophylaxis. Patients did not receive prophylactic DLI.
Donor-recipient chimerism levels were measured in the peripheral blood on day 30 using short tandem repeat analysis [26, 27]. Chimerism levels were measured in WB samples and in the T-cell subset after immunomagnetic positive selection of CD3+ cells. (StemCell Technologies, Vancouver, Canada). The graft composition, including the nucleated cell dose and the CD34+, CD3+, CD4+ and CD8+ cell doses were determined using standard procedures [28]. Absolute lymphocyte counts (ALC) were measured on routine complete blood counts on day-6 prior to starting the conditioning regimen, and again on day 0, prior to the stem-cell infusion.
Clinical outcomes
The clinical outcomes of interest were time to disease relapse, grade II-IV acute GvHD, moderate-severe chronic GvHD, relapse-free survival and overall survival. Disease relapse was defined as morphologic, cytogenetic or radiologic evidence of disease demonstrating pre-transplant characteristics. Bone marrow biopsies and appropriate imaging studies were routinely performed at day 100 or earlier in patients with signs indicating early relapse. The consensus conference criteria and NIH criteria were used for acute and chronic GvHD grading respectively [29, 30].
Statistical analysis
Descriptive statistics were employed to characterize distributions of variables. Linear correlations between WB and T-cell donor chimerism at day 30 and other continuous variables were assessed by Pearson’s correlation coefficient and differences between groups defined by categorical variables were assessed by either Wilcoxon rank sum or Kruskal-Wallis tests. No adjustment for multiple testing was performed in the analysis of predictors of chimerism levels. A landmark approach was used for time-to-event outcomes by measuring the time from chimerism measurement (approximately day 30) to the event, which allowed us to evaluate day-30 WB and T-cell donor chimerism as predictors. Time to relapse was defined as the time from day-30 chimerism measurement to first documented relapse or last patient contact without relapse. Other outcomes were similarly defined. Patients were censored at the time of a second transplant in all analyses and at the time of DLI for GvHD analyses. Competing risks regression analyses were conducted to identify predictors of time to relapse and time to GvHD outcomes, allowing for death without the event as a competing risk. Cox regression was used to identify predictors of survival and relapse-free survival. Univariate and multivariate analyses were performed to identify significant independent predictors and the primary variables of interest, day-30 WB and T-cell chimerism, were entered into all models separately. The GvHD prophylaxis regimen was entered as a fixed covariate in the models for adjustment only since patients were not randomized to these treatments. Additional variables considered for model building exhibited univariate significance of p≤0.10 and a step-wise elimination method was then used. Statistical significance of predictors in the models was assessed by the Wald test. The Akaike Information Criterion (AIC) was used to assess the relative goodness of fit of the models built for WB and T-cell chimerism. Analyses were conducted in STATA v13.1 (STATA Corp, College Station, TX) and R using the cmprsk package (The R Project for Statistical Computing, http://www.rproject.org).
RESULTS
Patient and transplant characteristics are summarized in Table 1. The median follow up was 22.5 months (range 1.4–57.9 months). The median day-30 WB chimerism level was 96% (range 77–100%). T-cell chimerism levels were available in 103 of 121 patients; the median day-30 T-cell chimerism was 65% (range 18–100%).
TABLE 1.
SUBJECT CHARACTERISTICS
| N=121 | ||
|---|---|---|
|
| ||
| Recipient age, median (range) | 63 (21–76) | |
|
| ||
| Donor age, median (range) | 42 (18–73) | |
|
| ||
| Recipient sex: M/F, % | 60/40 | |
|
| ||
| Donor sex: M/F, % | 55/45 | |
|
| ||
| Sex mismatch, % | 40 | |
|
| ||
| Diagnosis, n (%) | Myeloid Disease: | 84 (69) |
| AML | 52 (43) | |
| CR1 | 34 (28) | |
| CR2 | 12 (10) | |
| Not in CR | 6 (5) | |
| Favorable cytog.* | 1 (1) | |
| Intermediate/unknown cytog.* | 37 (31) | |
| Adverse cytog.* | 14 (12) | |
| MDS | 29 (24) | |
| Low risk* | 13 (11) | |
| High risk* | 16 (13) | |
| Intermediate/unknown cytog.* | 17 (14) | |
| Adverse cytog.* | 12 (10) | |
| CML (chronic phase 2) | 3 (2) | |
| Lymphoid Disease: | 37 (31) | |
| NHL | 22 (18) | |
| Indolent B-NHL | 3 (2) | |
| MCL | 3 (2) | |
| Aggressive B-NHL | 3 (2) | |
| T cell lymphoma | 13 (11) | |
| CLL | 5 (4) | |
| CR | 2 (2) | |
| PR | 1 (1) | |
| Active relapse | 1 (1) | |
| MM | 3 (2) | |
| VGPR | 1 (1) | |
| PR | 2 (2) | |
| Hodgkin (in PR) | 2 (2) | |
| ALL (in CR1) | 5 (4) | |
|
| ||
| Disease Risk Index, n (%)* | Low | 9 (7) |
| Intermediate | 82 (68) | |
| High/Very High | 30 (25) | |
|
| ||
| Donor, n (%) | Sibling | 53 (44) |
| Unrelated | 68 (56) | |
|
| ||
| HLA compatibility, n (%) | 8/8 match | 102 (84) |
| Single-antigen mismatch | 19 (16) | |
|
| ||
| GvHD prophylaxis*, n (%) | Csa + MTX or MMF | 13 (11) |
| Tac + MTX | 79 (65) | |
| Tac + MTX + MVC | 29 (24) | |
|
| ||
| Nucleated cell dose, cells/kg *108 median (range) | 7.9 (1.3–19.0) | |
|
| ||
| CD34+ cell dose, cells/kg *106 median (range) | 5.5 (1.4–21.4) | |
|
| ||
| CD3+ cell dose, cells/kg *108 median (range) | 2.2 (0.4–5.5) | |
|
| ||
| CD4+ cell dose, cells/kg *108 Median (range) | 1.3 (0.2–4.8) | |
|
| ||
| CD8+ cell dose, cells/kg *108 Median (range) | 0.4 (0.1–1.8) | |
AML denotes acute myeloid leukemia; MDS myelodysplastic syndrome; CML chronic myeloid leukemia; NHL non-Hodgkin lymphoma; MCL mantle cell lymphoma; PTCL peripheral T cell lymphoma; CTCL cutaneous T cell lymphoma; CLL chronic lymphocytic leukemia; MM multiple myeloma; ALL acute lymphoblastic leukemia; HLA human leukocyte antigen; GvHD graft versus host disease; Tac tacrolimus; Csa cyclosporine; MTX methotrexate; MMF mycophenolate mofetil; MVC maraviroc
Disease categories and Disease Risk Index summarized in [23].
Predictors of day-30 chimerism levels
Our goal was to assess the associations between day-30 chimerism levels and RIC HSCT outcomes. We first examined whether day-30 chimerism levels were associated with various patient, disease and transplant characteristics (Table 2).
TABLE 2.
PREDICTORS OF DAY-30 CHIMERISM LEVELS
| Whole blood chimerism | T-cell chimerism | |||
|---|---|---|---|---|
| Variable | Pearson r | p-value | Pearson r | p-value |
| ALC pre-conditioning+ | −0.34 | 0.0001 | −0.45 | <0.0001 |
| ALC day 0+ | −0.42 | <0.0001 | −0.41 | <0.0001 |
| Nucleated cell dose | 0.19 | 0.04 | 0.24 | 0.01 |
| CD34 cell dose | 0.10 | 0.27 | 0.08 | 0.42 |
| CD3 cell dose | 0.10 | 0.30 | 0.15 | 0.13 |
| CD4 cell dose | 0.02 | 0.86 | 0.10 | 0.35 |
| CD8 cell dose | 0.12 | 0.24 | 0.15 | 0.14 |
| CD4/CD8 ratio | −0.11 | 0.25 | −0.17 | 0.09 |
| Recipient age | −0.05 | 0.59 | −0.07 | 0.47 |
| Donor age | −0.09 | 0.34 | −0.14 | 0.17 |
| Variable | Median (range) | p-value# | Median (range) | p-value# |
|---|---|---|---|---|
|
| ||||
| Disease type | 0.32 | 0.23 | ||
| Myeloid | 96.0% (77–100%) | 66.0% (18–98%) | ||
| Lymphoid | 97.0% (82–100%) | 82.5% (19–99%) | ||
|
| ||||
| Disease Risk Index | 0.007^ | 0.07^ | ||
| Low | 98.0% (95–100%) | 66.0%(59–100%) | ||
| Intermediate | 96.0% (77–100%) | 88.0% (18–99%) | ||
| High/Very High | 95.0% (82–100%) | 70.0%(35–94%) | ||
|
| ||||
| GvHD prophylaxis | 0.30^ | 0.04^ | ||
| Csa/MTX or MMF | 98.0% (85–100) | 87.0% (65–97%) | ||
| Tac/MTX | 96.0% (82–100) | 65.0% (18–100%) | ||
| Tac/MTX/MVC | 97.0% (77–100) | 74.5% (33–99%) | ||
|
| ||||
| Donor Source | 0.57 | 0.60 | ||
| Sibling | 96.0 (77–100%) | 66.0% (18–100%) | ||
| Unrelated | 96.0 (82–100%) | 71.5% (19–99%) | ||
|
| ||||
| HLA Matching | 0.99 | 0.05 | ||
| 8/8 | 96.0% (77–100) | 66.0% (18–100) | ||
| 7/8 | 96.0% (82–100) | 77.0% (47–97) | ||
|
| ||||
| Recipient Sex | 0.02 | 0.56 | ||
| Male | 95.0% (82–100) | 66.0% (18–99) | ||
| Female | 96.0% (77–100) | 70.5% (19–100) | ||
|
| ||||
| Donor Sex | 0.33 | 0.89 | ||
| Male | 96.0% (82–100) | 67.5% (18–100) | ||
| Female | 96.0% (77–100) | 71.0% (26–99) | ||
| Recipient CMV Serostatus | 0.30 | 0.24 | ||
| Positive | 95.0% (82–100) | 66.0% (19–100) | ||
| Negative | 96.0% (77–100) | 70.0% (18–99) | ||
| Donor CMV Serostatus | 0.88 | 0.81 | ||
| Positive | 96.0%(83–100) | 70.0% (19–99) | ||
| Negative | 96.0% (77–100) | 67.0% (18–100) | ||
|
| ||||
| ABO Compatibility | 0.26 | 0.36 | ||
| No | 96.0% (77–100%) | 65.5% (35–99) | ||
| Yes | 96.0% (82–100%) | 71.0% (18–100) | ||
ALC denotes absolute lymphocyte count; AUC area under the curve; GvHD graft versus host disease; Csa cyclosporine; Tac tacrolimus; MVC maraviroc; MMF mycophenolate mofetil
P-values≤0.05 highlighted in bold
Natural log transformation applied
Wilcoxon rank sum test
Kruskal-Wallis test
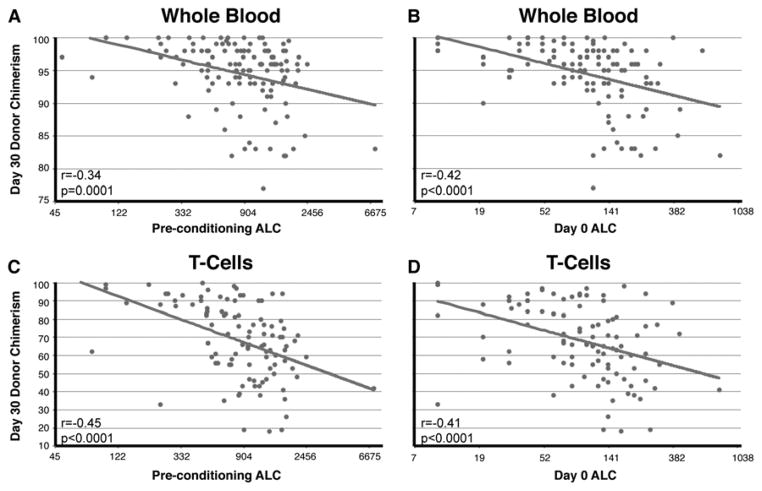
The primary variable that was associated with day-30 chimerism levels was the recipient’s ALC prior to transplant. Low ALC, both pre-conditioning (day minus 6) and on day-0, was strongly associated with higher levels of WB and T-cell chimerism levels (Figure 1, p≤0.0001 for all associations). The DRI showed a significant association with day-30 WB chimerism and a trend (p=0.07) with day-30 T-cell chimerism with higher disease risk correlating with lower chimerism levels. In addition, the total nucleated cell dose demonstrated a positive association with WB and T-cell chimerism, female recipients had slightly higher WB chimerism and HLA-mismatching resulted in higher T-cell chimerism levels. The use of tacrolimus vs. cyclosporine was associated with lower T-cell chimerism, and maraviroc did not seem to affect chimerism levels.
FIGURE 1. Pearson correlations between day-30 chimerism levels and pre-conditioning or day 0 absolute lymphocyte counts (ALC).
Donor-recipient chimerism levels were measured on day 30 post-transplant in whole blood (A,B; n=121) and in the CD3+ T-cell fraction (C,D; n=103) of peripheral blood samples. Chimerism values are plotted against each patient’s ALC prior to starting the conditioning regimen (A,C; day -6) and on the day of transplant (B,D; day 0). The x axis intervals represent natural log transformation. The Pearson correlation coefficient (r) and p-value are presented in each plot.
We wanted to check whether the association between pre-transplant ALC and day-30 chimerism was driven primarily by patients with lymphoid malignancies who are more likely to receive lymphodepleting therapies prior to transplant. Surprisingly, we found that recipients’ ALC was associated with day-30 chimerism levels regardless of disease type (Table 3). Both pre-conditioning and day-0 ALC were highly correlated with WB and T-cell chimerism in both lymphoid and myeloid diseases (p<0.005). The only association that was strong but did not reach statistical significance was between pre-conditioning ALC and WB chimerism in myeloid disease (p=0.08). These results demonstrate that pre-transplant lymphopenia may support early engraftment regardless of disease.
TABLE 3.
PREDICTORS OF DAY-30 CHIMERISM LEVELS WITH PRETRANSPLANT LYMPHOCYTE COUNTS IN DISEASE SUBSETS
| MYELOID | Whole blood chimerism | T-cell chimerism | ||
|---|---|---|---|---|
| Variable | Pearson r | p-value | Pearson r | p-value |
| ALC pre-conditioning+ | −0.19 | 0.08 | −0.34 | 0.003 |
| ALC day 0+ | −0.32 | 0.004 | −0.33 | 0.004 |
| LYMPHOID | Whole blood chimerism | T-cell chimerism | ||
|---|---|---|---|---|
| Variable | Pearson r | p-value | Pearson r | p-value |
| ALC pre-conditioning+ | −0.59 | 0.0001 | −0.61 | 0.0006 |
| ALC day 0+ | −0.59 | 0.0001 | −0.51 | 0.005 |
P-values≤0.05 highlighted in bold
ALC denotes absolute lymphocyte count
Relapse
Disease relapse, a major cause of mortality following RIC HSCT, was our primary focus. The cumulative incidence of relapse was 37.7% (95% CI [29.7–47.1]) at day 180 and 46.3% (95% CI [37.7–55.9]) at 1 year. The 1-year incidence of relapse in AML, MDS and NHL, the most common diseases in our cohort, was 39.4%, 46.3% and 56.1% respectively (p>0.05 for all comparisons).
To assess the predictive power of day-30 chimerism on relapse, we used a landmark analysis approach starting on the day of chimerism measurement (approximately day 30). Three patients who relapsed prior to the landmark date were excluded. We first assessed the effect of each covariate independently of others (Table 4) and then built a multivariate model for prediction of time to relapse (Table 5). Importantly, chimerism levels were analyzed as continuous variables and therefore the hazard ratios reflect the increased or decreased risk for the outcome for each 1% difference in chimerism levels.
TABLE 4.
UNIVARIATE ASSOCIATIONS WITH DISEASE RELAPSE, RELAPSE-FREE SURVIVAL AND OVERALL SURVIVAL
| Univariate associations | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Relapse | Relapse-free survival | Overall survival | |||||||
|
| |||||||||
| Variable | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value |
|
| |||||||||
| Day 30 WB chimerism (per 1%) | 0.90 | 0.86 – 0.94 | <0.001 | 0.90 | 0.86 – 0.94 | <0.001 | 0.95 | 0.91 – 1.00 | 0.04 |
|
| |||||||||
| Day 30 T-cell chimerism (per 1%) | 0.98 | 0.97 – 1.00 | 0.02 | 0.98 | 0.97 – 0.99 | 0.001 | 0.99 | 0.98 – 1.00 | 0.09 |
|
| |||||||||
| Disease, Lymphoid vs. Myeloid | 1.25 | 0.72 – 2.19 | 0.43 | 1.09 | 0.61 – 1.79 | 0.73 | 0.75 | 0.44 – 1.30 | 0.31 |
|
| |||||||||
| Disease Risk Index | |||||||||
| Low vs. Intermediate | 0.76 | 0.25 – 2.38 | 0.64 | 1.00 | 0.40 – 2.51 | 0.99 | 1.36 | 0.54 – 3.46 | 0.52 |
| High/Very High vs. Intermediate | 2.56 | 1.44 – 2.53 | 0.001 | 2.36 | 1.44 – 3.85 | 0.001 | 2.02 | 1.17 – 3.47 | 0.01 |
|
| |||||||||
| Donor source, URD vs. Sib | 0.92 | 0.54 – 1.57 | 0.77 | 0.90 | 0.57 – 1.41 | 0.63 | 0.90 | 0.55 – 1.47 | 0.68 |
|
| |||||||||
| GvHD prophylaxis | |||||||||
| Tac/MTX vs. Csa/MTX or MMF | 0.38 | 0.18 – 0.80 | 0.01 | 0.30 | 0.15 – 0.59 | 0.001 | 0.42 | 0.21 – 0.83 | 0.01 |
| Tac/MTX/MVC vs. Csa/MTX or MMF | 0.52 | 0.23 – 1.16 | 0.11 | 0.39 | 0.19 – 0.83 | 0.01 | 0.53 | 0.25 – 1.13 | 0.10 |
|
| |||||||||
| HLA matching, 7/8 vs. 8/8 | 1.01 | 0.50 – 2.02 | 0.99 | 0.98 | 0.53 – 1.82 | 0.96 | 1.10 | 0.56 – 2.17 | 0.78 |
|
| |||||||||
| Recipient sex, Female vs. Male | 0.56 | 0.32 – 0.97 | 0.04 | 0.68 | 0.43 – 1.09 | 0.11 | 0.60 | 0.35 – 1.01 | 0.05 |
|
| |||||||||
| Donor sex, Female vs. Male | 0.63 | 0.36 – 1.10 | 0.10 | 1.06 | 0.68 – 1.68 | 0.79 | 1.59 | 0.97 – 2.60 | 0.07 |
|
| |||||||||
| Recipient age | 1.02 | 0.98 – 1.06 | 0.26 | 1.02 | 0.99 – 1.05 | 0.31 | 1.02 | 0.99 – 1.06 | 0.18 |
|
| |||||||||
| Donor age | 1.00 | 0.98 – 1.01 | 0.90 | 1.01 | 0.99 – 1.02 | 0.37 | 1.01 | 1.00 – 1.03 | 0.13 |
|
| |||||||||
| CD4 dose | 0.96 | 0.60 – 1.53 | 0.87 | 0.98 | 0.69 – 1.39 | 0.91 | 0.95 | 0.66 – 1.36 | 0.76 |
|
| |||||||||
| CD8 dose | 0.44 | 0.18 – 1.09 | 0.08 | 0.38 | 0.18 – 0.80 | 0.01 | 0.36 | 0.16 – 0.84 | 0.02 |
|
| |||||||||
| CD4/CD8 dose ratio | 1.14 | 1.04 – 1.24 | 0.007 | 1.15 | 1.06 – 1.26 | 0.002 | 1.15 | 1.04 – 1.27 | 0.005 |
|
| |||||||||
| Nucleated cell dose | 0.97 | 0.89 – 1.06 | 0.53 | 0.98 | 0.91 – 1.05 | 0.52 | 0.98 | 0.91 – 1.05 | 0.59 |
|
| |||||||||
| CD34 dose | 1.04 | 0.94 – 1.16 | 0.42 | 1.01 | 0.93 – 1.09 | 0.84 | 0.94 | 0.86 – 1.02 | 0.15 |
|
| |||||||||
| CD3 dose | 0.83 | 0.60 – 1.15 | 0.26 | 0.87 | 0.68 – 1.11 | 0.27 | 0.85 | 0.65 – 1.11 | 0.23 |
|
| |||||||||
| ALC pre+ | 1.22 | 0.85 – 1.73 | 0.28 | 1.23 | 0.90 – 1.68 | 0.20 | 1.11 | 0.80 – 1.54 | 0.52 |
|
| |||||||||
| ALC day 0 | 1.18 | 0.84 – 1.66 | 0.33 | 1.21 | 0.91 – 1.60 | 0.19 | 0.98 | 0.74 – 1.30 | 0.90 |
URD denotes unrelated donor; Sib sibling; ALC absolute lymphocyte count; WB whole blood; HLA human leukocyte antigen; CMV cytomegalovirus
Additional variables that lacked significant associations and are not presented in the table include donor and recipient CMV serostatus, ABO compatibility, Karnofsky performance status and HCT-CI.
P-values≤0.05 highlighted in bold.
Natural log transformation applied.
TABLE 5.
MULTIVARIATE MODELS FOR DISEASE RELAPSE, RELAPSE-FREE SURVIVAL AND OVERALL SURVIVAL
| Relapse | |||
|
| |||
| Multivariate model for WB chimerism* | |||
|
| |||
| Day 30 WB chimerism (per 1%) | 0.90 | 0.86 – 0.94 | <0.001 |
|
| |||
| Disease Risk Index | |||
| Low vs. Intermediate | 1.52 | 0.45 – 5.15 | 0.50 |
| High/Very High vs. Intermediate | 2.68 | 1.47 – 4.88 | 0.001 |
|
| |||
| CD4/CD8 Ratio | 1.20 | 1.10 – 1.31 | <0.001 |
|
| |||
| Donor sex, Female vs. Male | 0.35 | 0.18 – 0.69 | 0.002 |
|
| |||
| Multivariate model for T- cell chimerism* | |||
|
| |||
| Day 30 T-cell chimerism (per 1%) | 0.97 | 0.96 – 0.99 | 0.002 |
|
| |||
| Disease Risk Index | |||
| Low vs. Intermediate | 1.73 | 0.49 – 6.09 | 0.39 |
| High/Very High vs. Intermediate | 4.30 | 2.30 – 8.02 | <0.001 |
|
| |||
| CD4/CD8 Ratio | 1.18 | 1.08 – 1.29 | <0.001 |
|
| |||
| Donor sex, Female vs. Male | 0.31 | 0.16 – 0.61 | 0.001 |
|
| |||
| Relapse-free survival | |||
|
| |||
| Multivariate model for WB chimerism* | |||
|
| |||
| Day 30 WB chimerism (per 1%) | 0.89 | 0.85 – 0.94 | <0.001 |
|
| |||
| Disease Risk Index | |||
| Low vs. Intermediate | 1.66 | 0.62 – 4.43 | 0.31 |
| High/Very High vs. Intermediate | 2.37 | 1.36 – 4.16 | 0.002 |
|
| |||
| CD4/CD8 Ratio | 1.14 | 1.04 – 1.24 | 0.006 |
|
| |||
| Multivariate model for T- cell chimerism* | |||
|
| |||
| Day 30 T-cell chimerism (per 1%) | 0.97 | 0.96 – 0.99 | <0.001 |
|
| |||
| Disease Risk Index | |||
| Low vs. Intermediate | 1.60 | 0.54 – 4.74 | 0.40 |
| High/Very High vs. Intermediate | 3.01 | 1.65 – 5.50 | <0.001 |
|
| |||
| CD4/CD8 Ratio | 1.11 | 1.01 – 1.22 | 0.03 |
|
| |||
| Overall survival | |||
|
| |||
| Multivariate model for WB chimerism* | |||
|
| |||
| Day 30 WB chimerism (per 1%) | 0.94 | 0.89 – 0.99 | 0.01 |
|
| |||
| Disease Risk Index | |||
| Low vs. Intermediate | 1.78 | 0.64 – 4.92 | 0.27 |
| High/Very High vs. Intermediate | 1.68 | 0.89 – 3.18 | 0.11 |
|
| |||
| CD4/CD8 ratio | 1.14 | 1.04 – 1.26 | 0.008 |
|
| |||
| Multivariate model for T- cell chimerism* | |||
|
| |||
| Day 30 T-cell chimerism (per 1%) | 0.99 | 0.97 – 1.00 | 0.05 |
|
| |||
| Disease Risk Index | |||
| Low vs. Intermediate | 1.56 | 0.49 – 4.91 | 0.45 |
| High/Very High vs. Intermediate | 1.89 | 0.95 – 3.72 | 0.07 |
|
| |||
| CD4/CD8 ratio | 1.12 | 1.01 – 1.25 | 0.03 |
WB denotes whole blood. P-values≤0.05 highlighted in bold.
Two separate models were constructed for each outcome. The GvHD prophylaxis regimen was entered into all multivariate models as a confounder variable for adjustment only (multivariate results are not shown for this variable).
In univariate analysis, the day-30 WB chimerism level was significantly associated with a reduction in relapse (HR=0.90, 95% CI [0.86–0.94]; p<0.001). This strong association reflects a 10% decrease in the relapse risk for every 1% increase in WB chimerism levels. Day-30 T-cell chimerism levels also demonstrated an inverse association with relapse (HR=0.98, 95% CI [0.97–1.00], p=0.02). Other variables that were associated with relapse and met our threshold for modeling included donor or recipient sex and the GvHD prophylaxis regimen (cyclosporine vs. tacrolimus). Because the GvHD prophylaxis regimen was not selected by randomization, it was treated as a confounder and used for adjustment only. In addition, low CD8 cell doses and a high graft CD4/CD8 ratio correlated with a higher risk for relapse.
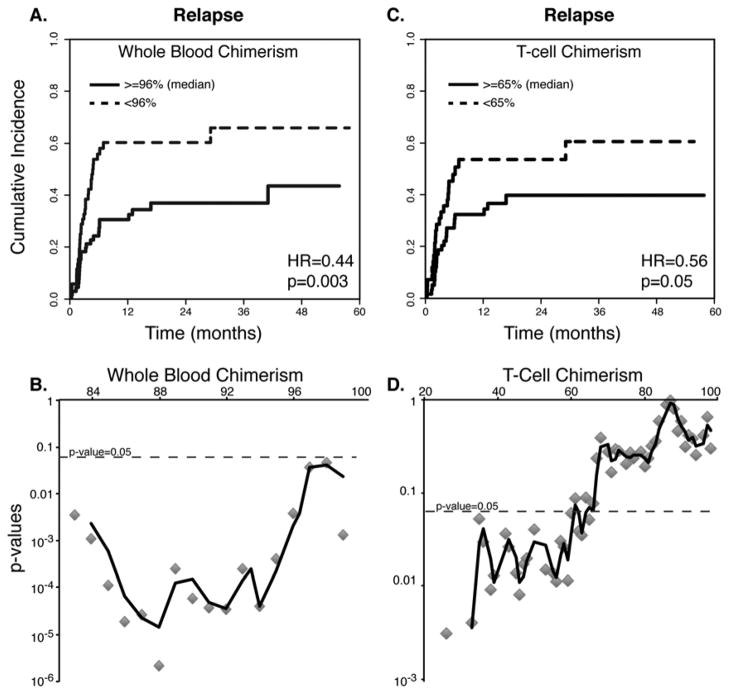
To further characterize the association between day-30 chimerism and disease relapse and identify a chimerism threshold that optimally predicts relapse, we examined all possible cutoffs. For WB chimerism, there was a strong association at any relevant cutoff, precluding a choice of an optimal cutoff for prediction of relapse. For example, cutoffs of 90%, 93% and 96% resulted in hazard ratios of 0.26, 0.25 and 0.44, respectively (all three cutoffs with p<0.01). As a representative example, cumulative incidence plots that compare disease relapse in patients grouped according to the median WB chimerism level (96%) are displayed in Figure 2A. We plotted the p-values for this association against all cutoff levels to demonstrate that this association was continuous, highly significant and consistent across multiple cutoff levels (Figure 2B).
FIGURE 2. Univariate associations of disease relapse with day-30 donor chimerism levels.
Cumulative incidence plots of disease relapse are shown, starting from the time of day-30 chimerism testing (defined here as “0” for a landmark analysis). Comparative plots for groups of patients who had higher or lower than median chimerism levels are presented for whole blood (A) and T-cell chimerism (C). Hazard ratios and p-values represent a univariate cumulative incidence analysis. All possible cutoffs for whole blood (B) and T-cell chimerism (D) were examined and plotted against the cutoffs, showing significant correlations at multiple cutoffs. The plot lines represent a moving 3-point average.
T-cell chimerism levels also predicted disease relapse at multiple cutoffs. For example, a comparison of relapse rates in patients with day-30 T-cell chimerism above and below the median (65%) showed a hazard ratio of 0.56 (p=0.05; Figure 2C). We assessed different cutoff levels for T-cell chimerism and found that the distribution of p-values was asymmetric. Cutoffs that were at the median level or lower provided a better prediction than higher cutoffs (Figure 2D). Still, we could not identify a single optimal cutoff.
We then wanted to assess whether day-30 chimerism levels were prognostic as opposed to diagnostic because it is possible that patients with low chimerism levels had already relapsed on day 30. To ascertain that the associations that we found were not driven by patients with very early relapse, we repeated our analysis after excluding 6 patients who experienced relapse between day 30 – 60. We found that the ability to predict relapse was unchanged after excluding these early relapse patients (WB: HR=0.90, p<0.001; T-cell: HR=0.98, p=0.02). This confirms that there was a window of opportunity for intervention in the majority of patients with low chimerism levels.
Finally, both WB and T-cell chimerism levels were strong predictors of relapse in multivariate models that were constructed separately for each predictor (Table 5). High DRI, male donors and grafts with a high CD4/CD8 cell dose ratio remained significant predictors for a high relapse rate. No differential effect (i.e. statistical interaction) was noted between subsets of patients with myeloid and lymphoid disease (p=0.66), acute leukemia and other diseases (p=0.58) or unrelated and sibling donors (p=0.71).
Relapse-free survival and overall survival
Due to their highly significant correlation with relapse, we hypothesized that day-30 chimerism levels might predict relapse-free survival (RFS) and overall survival (OS). The 2-year estimated rates of RFS and OS were 33.9% (95% CI [25.1–42.9]) and 45.1% (95%CI [35.1–54.6]) respectively.
We conducted a landmark analysis to determine the associations between day-30 chimerism levels and RFS or OS (Table 4). Univariate analyses showed that both WB and T-cell chimerism strongly correlated with RFS (HR=0.90; p<0.001 for WB chimerism and HR=0.98; p=0.001 for T-cell chimerism), in addition to other factors (DRI, GvHD prophylaxis, CD8 dose and CD4/8 ratio). Multivariate models confirmed the predictive value of either WB chimerism or T-cell chimerism for RFS (Table 5).
A similar approach revealed that WB and T-cell chimerism predicted OS in multivariate models that adjusted for the DRI, graft CD4/8 ratio and GvHD prophylaxis (HR=0.94; p=0.01 for WB chimerism, HR=0.99; p=0.05 for T-cell chimerism). Additional variables did not improve the OS model.
Graft-versus-host disease
The day-180 cumulative incidence rate of acute grade II-IV GvHD was 40.1% (95% CI [31.9–48.7]), and the 2-year incidence rate of moderate-severe chronic GvHD was 23.8% (95% CI [15.0–33.3]).
We conducted a landmark analysis to determine the associations between day-30 chimerism levels and grade II-IV acute GvHD. Patients (n=8) who had GvHD prior to day 30 were excluded. In univariate and multivariate analyses, day-30 WB and T-cell chimerism levels had no significant association with time to acute grade II-IV GvHD (Table S1). We also examined more immediate GvHD incidence rates (day-60 and day-100) and found no associations between chimerism levels on day-30 and the occurrence of acute GvHD at these time points (data not shown). A similar analysis for moderate-severe chronic GvHD (Table S2) also showed no significant associations. Subset analyses of GvHD outcomes in patients who received different GvHD prophylaxis regimens also revealed no significant associations (data not shown).
Whole blood versus T-cell chimerism
All multivariate models were constructed with either WB or T-cell chimerism. Since these two variables were highly correlated (Figure 3, r=0.61; p<0.0001), models that include both factors together arbitrarily choose one as significant and remove the other. To compare the predictive models that included WB and T-cell chimerism, we used the Akaike Information Criterion (AIC) that measures the relative goodness of fit of a statistical model and allows comparison between models, with the lower AIC reflecting a better model. We found that the AIC was lower (better) for T-cell chimerism compared to WB chimerism in prediction of all 3 outcomes – relapse (312 vs. 365), RFS (437 vs. 504) and OS (381 vs. 440), implying that day-30 T-cell chimerism may perform better in prediction of RIC HSCT outcomes.
FIGURE 3. Pearson correlation between day-30 whole blood and T-cell chimerism levels.
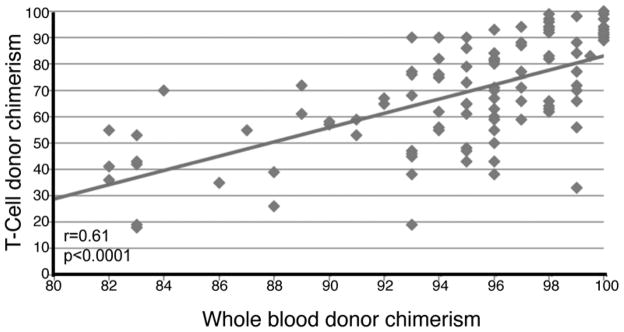
Day-30 whole blood and T-cell chimerism levels are plotted against each other (n=103), demonstrating a significant correlation by Pearson.
DISCUSSION
In this study, we found that early donor-recipient chimerism levels, both in WB and in the T-cell subset, predicted subsequent relapse in HSCT recipients who received a peripheral blood stem-cell graft after a uniform RIC regimen. For each 1% difference in chimerism level, there was a difference of 10% (for WB chimerism) or 2% (for T-cell chimerism) in the relative risk for subsequent relapse. Based on our model, the projected risk for relapse at 1 year in patients who have WB chimerism levels of 90, 95 and 100% are 52, 34 and 21% respectively. Early chimerism levels also correlated with RFS and OS but not with acute or chronic GvHD. These associations did not differ between patients with myeloid and lymphoid diseases or acute leukemia versus other diseases. To the best of our knowledge, this study is the first to characterize early chimerism levels as a continuous variable that accurately predicts relapse and survival, and can be used to identify high-risk patients after RIC HSCT. These findings suggest that chimerism levels can be used to guide early post-transplant interventions in prospective clinical trials and possibly in standard clinical practice.
In our study, more than half of the relapsing patients experienced disease relapse by day 100. The outcome of relapsing patients was poor with a median survival of 4.9 months from the time of relapse and a 3-year survival of 18%. Similar outcomes have been previously reported [8, 9, 31–33], highlighting the importance of identifying patients at high risk for relapse at a very early time point after transplant.
The associations of day-30 chimerism with relapse, RFS and OS were significant, continuous and clinically meaningful. In assessing the relative goodness of fit of the models, we found an advantage to T-cell chimerism in prediction of all outcomes. These findings strengthen the rationale for the use of both WB and T-cell chimerism early after RIC HSCT.
The predictive value of peripheral blood chimerism testing after RIC HSCT has been previously examined. It was commonly, but not universally, observed that low T-cell chimerism predicts graft rejection whereas high T-cell chimerism is associated with GvHD [20–22, 34–36]. For relapse and survival outcomes, previous studies have demonstrated conflicting results. Early studies of RIC HSCT noted that complete T-cell donor chimerism seemed to precede malignant disease responses [21, 37]. These findings were not confirmed by other studies possibly due to heterogeneity in conditioning regimens and inclusion of T-cell depleting antibodies in some studies [19, 20, 38, 39]. Several studies attempted to use CD34 cell-specific chimerism and found that either a low level or a decline in the CD34-cell chimerism level predicted relapse, however this test is not always feasible early after transplant and is likely limited to patients with acute leukemia and myelodysplastic syndromes [14, 40].
These conflicting results regarding the predictive value of chimerism testing possibly reflect the heterogeneity in studied populations in previous studies, inclusion of multiple conditioning regimens and graft sources, and inconsistent timing of testing. In our study, we aimed to overcome some of these barriers by studying patients who received a uniform, widely used conditioning regimen, and received peripheral blood stem cells only. We also focused on a single early time point (day 30), which we feel is the most relevant one after RIC HSCT due to the high incidence of early relapse. Another major difference is that most previous studies focused on achievement of complete donor chimerism using a historical threshold of 95% (or similar) whereas we handled chimerism levels as continuous variables. The rationale for our approach is that the analytic sensitivity of this assay has improved due to advances in technology and standardization. The EuroChimerism Consortium reported a detection limit of 0.8–1.6% and confidence intervals of 1.6–1.8% for donor chimerism levels >94% [41]. Our lab’s internal validation is in line with these results, which allows us to take advantage of this molecular test with high precision. Our results show that the predictive value of both WB and T-cell chimerism levels is sustained across a wide range of values and not just at the historical threshold of 95%.
The findings of this study can be immediately applied. The primary advantage of chimerism measurement is that it can be done in all patients even in the absence of disease-specific information. This stands in contrast to other types of minimal residual disease (MRD) testing such as mutiparameter flow cytometry or quantitative PCR, which are limited to patients with a known immunophenotype or molecular abnormality [42]. These novel MRD assays are emerging as useful prognostic markers prior to transplant [43, 44], but their clinical utility in post-transplant monitoring has been questioned, because the detection of leukemic cells early after transplant is not always predictive of relapse [45]. After RIC in particular, positive MRD is common and residual malignant cells can still be eradicated by the potent graft-versus-tumor response. A strategy that combines chimerism testing with other MRD methods can be envisioned to further increase the sensitivity of MRD detection and ensure that all patients benefit from early prognostication regardless of disease.
We did not identify any correlation between day-30 chimerism and GvHD. Previous studies have shown conflicting results on this association [19, 20, 22, 37]. In our cohort, acute GvHD was often delayed, with a median time to onset of 4.7 months post-transplant, which could explain why early chimerism measurement on day 30 failed to predict this outcome. It is possible that chimerism measurements at later time points, or trends between serial measurements, have a better predictive value for this outcome.
In our study, the early (day-30) achievement of high chimerism levels was strongly associated with a lymphopenic state prior to transplant. Lymphopenic individuals may have better homeostatic expansion of donor T-cells [46] and retrospective studies have noted that a higher number of anti-tumor therapies prior to transplant predicted early complete donor chimerism, which can be mechanistically linked to lymphopenia [19, 20, 22]. This was also shown prospectively in a study in which accelerated donor engraftment was achieved with lymphodepleting chemotherapy prior to RIC HSCT for lymphoma [47]. Our results demonstrate that lower pre-transplant lymphocyte counts are associated with faster engraftment in any disease, not just in lymphomas, suggesting that aggressive lymphodepletion prior to transplant can be used broadly to accelerate donor cell engraftment. Ultimately, randomized studies will be required to demonstrate the effect of lymphodepletion on transplant outcomes.
Certain limitations to our study should be noted. For uniformity, we focused on a single RIC regimen (Flu/Bu2), which is the most commonly used RIC regimen according to 2011 CIBMTR data. Whether the kinetics of engraftment differs among RIC regimens is unknown, but it has been suggested that melphalan-based regimens achieve complete T-cell chimerism more rapidly, implying that the predictive value of chimerism should be validated for other regimens [19]. Our study also analyzed the outcomes of a heterogeneous patient population in terms of disease characteristics (e.g., disease type, cytogenetic risk). The DRI that was recently validated in more than 13,000 patients was used to adjust our analyses to overcome this barrier. In addition, we analyzed broad disease categories (myeloid vs. lymphoid, acute leukemia vs. others) and found no significant impact on any of the outcomes or interactions with any of the important covariates. However, the small number of patients in some of the disease categories precluded meaningful disease-specific analyses.
The clinical utility of any prognostic biomarker is limited if not tied with a strategy that prevents overt relapse. It is known that reduction of immunosuppression and DLI can convert mixed chimerism to complete donor chimerism and even eliminate measurable residual disease [45, 48–50]. Whether this can be safely, rapidly, and meaningfully achieved without excessive toxicity as early as 30 days after HSCT is unknown. More recently, maintenance regimens such as methyltransferase inhibitors and targeted therapies (e.g., Flt3 inhibitors) have entered clinical trials in the early post-transplant setting [51–54], but similarly, whether these interventions can be safely initiated very early after transplant remains to be determined. The findings of our study suggest that low WB or T-cell chimerism levels on day 30 after RIC HSCT indicate a higher risk for relapse independent of any other indicators. This may help inform patient selection for approaches such as enhanced tapering or withdrawal of immunosuppression, pre-emptive DLI or experimental therapy. The safety and efficacy of these approaches will need to be examined in prospective clinical trials.
Supplementary Material
Acknowledgments
Funding sources: Amy Strelzer Manasevit Award from the National Marrow Donor Program (R.R.), Career Development Award from the Conquer Cancer Foundation (R.R.); CURE grant from the Commonwealth of Pennsylvania (D.L.P., R.R. & P.V.); National Institutes of Health grants P30-CA16520 (E.A.S. & R.M.), K23-CA178202 (R.R.) & U01-HL069286 (E.A.S. and D.L.P.). We wish to thank Amy Gao for assistance with data collection and Oren Litvin (Columbia University) for assistance with preparation of the figures.
Footnotes
Presented in part at the 2011 American Society of Hematology Annual Meeting, San Diego, CA
Financial Disclosures: The authors have no relevant disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Porter DL, Alyea EP, Antin JH, DeLima M, Estey E, Falkenburg JH, et al. NCI First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: Report from the Committee on Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2010;16:1467–503. doi: 10.1016/j.bbmt.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmid C, Labopin M, Nagler A, Niederwieser D, Castagna L, Tabrizi R, et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood. 2012;119:1599–606. doi: 10.1182/blood-2011-08-375840. [DOI] [PubMed] [Google Scholar]
- 3.Storb R, Gyurkocza B, Storer BE, Sorror ML, Blume K, Niederwieser D, et al. Graft-versus-host disease and graft-versus-tumor effects after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2013;31:1530–8. doi: 10.1200/JCO.2012.45.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen YB, Coughlin E, Kennedy KF, Alyea EP, Armand P, Attar EC, et al. Busulfan dose intensity and outcomes in reduced-intensity allogeneic peripheral blood stem cell transplantation for myelodysplastic syndrome or acute myeloid leukemia. Biol Blood Marrow Transplant. 2013;19:981–7. doi: 10.1016/j.bbmt.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Luger SM, Ringden O, Zhang MJ, Perez WS, Bishop MR, Bornhauser M, et al. Similar outcomes using myeloablative vs reduced-intensity allogeneic transplant preparative regimens for AML or MDS. Bone Marrow Transpl. 2012;47:203–11. doi: 10.1038/bmt.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunstein CG, Fuchs EJ, Carter SL, Karanes C, Costa LJ, Wu J, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118:282–8. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sureda A, Robinson S, Canals C, Carella AM, Boogaerts MA, Caballero D, et al. Reduced-intensity conditioning compared with conventional allogeneic stem-cell transplantation in relapsed or refractory Hodgkin’s lymphoma: an analysis from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2008;26:455–62. doi: 10.1200/JCO.2007.13.2415. [DOI] [PubMed] [Google Scholar]
- 8.Gyurkocza B, Storb R, Storer BE, Chauncey TR, Lange T, Shizuru JA, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia. J Clin Oncol. 2010;28:2859–67. doi: 10.1200/JCO.2009.27.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bethge WA, Storer BE, Maris MB, Flowers ME, Maloney DG, Chauncey TR, et al. Relapse or progression after hematopoietic cell transplantation using nonmyeloablative conditioning: effect of interventions on outcome. Exp Hematol. 2003;31:974–80. doi: 10.1016/s0301-472x(03)00225-x. [DOI] [PubMed] [Google Scholar]
- 10.Tauro S, Craddock C, Peggs K, Begum G, Mahendra P, Cook G, et al. Allogeneic stem-cell transplantation using a reduced-intensity conditioning regimen has the capacity to produce durable remissions and long-term disease-free survival in patients with high-risk acute myeloid leukemia and myelodysplasia. J Clin Oncol. 2005;23:9387–93. doi: 10.1200/JCO.2005.02.0057. [DOI] [PubMed] [Google Scholar]
- 11.Sureda A, Canals C, Arranz R, Caballero D, Ribera JM, Brune M, et al. Allogeneic stem cell transplantation after reduced intensity conditioning in patients with relapsed or refractory Hodgkin’s lymphoma. Results of the HDR-ALLO study - a prospective clinical trial by the Grupo Espanol de Linfomas/Trasplante de Medula Osea (GEL/TAMO) and the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2012;97:310–7. doi: 10.3324/haematol.2011.045757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antin JH, Childs R, Filipovich AH, Giralt S, Mackinnon S, Spitzer T, et al. Establishment of complete and mixed donor chimerism after allogeneic lymphohematopoietic transplantation: recommendations from a workshop at the 2001 Tandem Meetings of the International Bone Marrow Transplant Registry and the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2001;7:473–85. doi: 10.1053/bbmt.2001.v7.pm11669214. [DOI] [PubMed] [Google Scholar]
- 13.van Leeuwen JE, van Tol MJ, Bodzinga BG, Wijnen JT, van der Keur M, Joosten AM, et al. Detection of mixed chimaerism in flow-sorted cell subpopulations by PCR-amplified VNTR markers after allogeneic bone marrow transplantation. Br J Haematol. 1991;79:218–25. doi: 10.1111/j.1365-2141.1991.tb04525.x. [DOI] [PubMed] [Google Scholar]
- 14.Bornhauser M, Oelschlaegel U, Platzbecker U, Bug G, Lutterbeck K, Kiehl MG, et al. Monitoring of donor chimerism in sorted CD34+ peripheral blood cells allows the sensitive detection of imminent relapse after allogeneic stem cell transplantation. Haematologica. 2009;94:1613–7. doi: 10.3324/haematol.2009.007765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackinnon S, Barnett L, Bourhis JH, Black P, Heller G, O’Reilly RJ. Myeloid and lymphoid chimerism after T-cell-depleted bone marrow transplantation: evaluation of conditioning regimens using the polymerase chain reaction to amplify human minisatellite regions of genomic DNA. Blood. 1992;80:3235–41. [PubMed] [Google Scholar]
- 16.Dubovsky J, Daxberger H, Fritsch G, Printz D, Peters C, Matthes S, et al. Kinetics of chimerism during the early post-transplant period in pediatric patients with malignant and non-malignant hematologic disorders: implications for timely detection of engraftment, graft failure and rejection. Leukemia. 1999;13:2059, 60–9. [PubMed] [Google Scholar]
- 17.Frassoni F, Strada P, Sessarego M, Miceli S, Corvo R, Scarpati D, et al. Mixed chimerism after allogeneic marrow transplantation for leukaemia: correlation with dose of total body irradiation and graft-versus-host disease. Bone Marrow Transpl. 1990;5:235–40. [PubMed] [Google Scholar]
- 18.van Leeuwen JE, van Tol MJ, Joosten AM, Wijnen JT, Verweij PJ, Khan PM, et al. Persistence of host-type hematopoiesis after allogeneic bone marrow transplantation for leukemia is significantly related to the recipient’s age and/or the conditioning regimen, but it is not associated with an increased risk of relapse. Blood. 1994;83:3059–67. [PubMed] [Google Scholar]
- 19.Valcarcel D, Martino R, Caballero D, Mateos MV, Perez-Simon JA, Canals C, et al. Chimerism analysis following allogeneic peripheral blood stem cell transplantation with reduced-intensity conditioning. Bone Marrow Transpl. 2003;31:387–92. doi: 10.1038/sj.bmt.1703846. [DOI] [PubMed] [Google Scholar]
- 20.Baron F, Baker JE, Storb R, Gooley TA, Sandmaier BM, Maris MB, et al. Kinetics of engraftment in patients with hematologic malignancies given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2004;104:2254–62. doi: 10.1182/blood-2004-04-1506. [DOI] [PubMed] [Google Scholar]
- 21.Childs R, Clave E, Contentin N, Jayasekera D, Hensel N, Leitman S, et al. Engraftment kinetics after nonmyeloablative allogeneic peripheral blood stem cell transplantation: full donor T-cell chimerism precedes alloimmune responses. Blood. 1999;94:3234–41. [PubMed] [Google Scholar]
- 22.Saito B, Fukuda T, Yokoyama H, Kurosawa S, Takahashi T, Fuji S, et al. Impact of T cell chimerism on clinical outcome in 117 patients who underwent allogeneic stem cell transplantation with a busulfan-containing reduced-intensity conditioning regimen. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2008;14:1148–55. doi: 10.1016/j.bbmt.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the disease risk index for allogeneic stem cell transplantation: a study from the CIBMTR. Blood. 2014 doi: 10.1182/blood-2014-01-552984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reshef R, Luger SM, Hexner EO, Loren AW, Frey NV, Nasta SD, et al. Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease. N Engl J Med. 2012;367:135–45. doi: 10.1056/NEJMoa1201248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Deerlin VM, Reshef R. Chimerism Testing in Allogeneic Hematopoietic Stem Cell Transplantation. In: Leonard DG, editor. Molecular Pathology in Clinical Practice. 2. Springer; 2014. [Google Scholar]
- 27.Van Deerlin VM, Leonard DG. Bone marrow engraftment analysis after allogeneic bone marrow transplantation. Clin Lab Med. 2000;20:197–225. [PubMed] [Google Scholar]
- 28.Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996;5:213–26. doi: 10.1089/scd.1.1996.5.213. [DOI] [PubMed] [Google Scholar]
- 29.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–8. [PubMed] [Google Scholar]
- 30.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–56. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Oran B, Giralt S, Couriel D, Hosing C, Shpall EJ, de Meis E, et al. Treatment of AML and MDS relapsing after reduced-intensity conditioning and allogeneic hematopoietic stem cell transplantation. Leukemia. 2007;21:2540–4. doi: 10.1038/sj.leu.2404828. [DOI] [PubMed] [Google Scholar]
- 32.Craddock C, Nagra S, Peniket A, Brookes C, Buckley L, Nikolousis E, et al. Factors predicting long-term survival after T-cell depleted reduced intensity allogeneic stem cell transplantation for acute myeloid leukemia. Haematologica. 2010;95:989–95. doi: 10.3324/haematol.2009.013920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pollyea DA, Artz AS, Stock W, Daugherty C, Godley L, Odenike OM, et al. Outcomes of patients with AML and MDS who relapse or progress after reduced intensity allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2007;40:1027–32. doi: 10.1038/sj.bmt.1705852. [DOI] [PubMed] [Google Scholar]
- 34.McSweeney PA, Niederwieser D, Shizuru JA, Sandmaier BM, Molina AJ, Maloney DG, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 35.Matthes-Martin S, Lion T, Haas OA, Frommlet F, Daxberger H, Konig M, et al. Lineage-specific chimaerism after stem cell transplantation in children following reduced intensity conditioning: potential predictive value of NK cell chimaerism for late graft rejection. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2003;17:1934–42. doi: 10.1038/sj.leu.2403087. [DOI] [PubMed] [Google Scholar]
- 36.Bornhauser M, Thiede C, Platzbecker U, Jenke A, Helwig A, Plettig R, et al. Dose-reduced conditioning and allogeneic hematopoietic stem cell transplantation from unrelated donors in 42 patients. Clin Cancer Res. 2001;7:2254–62. [PubMed] [Google Scholar]
- 37.Mohty M, Avinens O, Faucher C, Viens P, Blaise D, Eliaou JF. Predictive factors and impact of full donor T-cell chimerism after reduced intensity conditioning allogeneic stem cell transplantation. Haematologica. 2007;92:1004–6. doi: 10.3324/haematol.10971. [DOI] [PubMed] [Google Scholar]
- 38.van Besien K, Dew A, Lin S, Joseph L, Godley LA, Larson RA, et al. Patterns and kinetics of T-cell chimerism after allo transplant with alemtuzumab-based conditioning: mixed chimerism protects from GVHD, but does not portend disease recurrence. Leuk Lymphoma. 2009;50:1809–17. doi: 10.3109/10428190903200790. [DOI] [PubMed] [Google Scholar]
- 39.El-Cheikh J, Vazquez A, Crocchiolo R, Furst S, Calmels B, Castagna L, et al. Acute GVHD is a strong predictor of full donor CD3+ T cell chimerism after reduced intensity conditioning allogeneic stem cell transplantation. Am J Hematol. 2012;87:1074–8. doi: 10.1002/ajh.23319. [DOI] [PubMed] [Google Scholar]
- 40.Rosenow F, Berkemeier A, Krug U, Muller-Tidow C, Gerss J, Silling G, et al. CD34(+) lineage specific donor cell chimerism for the diagnosis and treatment of impending relapse of AML or myelodysplastic syndrome after allo-SCT. Bone Marrow Transplant. 2013;48:1070–6. doi: 10.1038/bmt.2013.2. [DOI] [PubMed] [Google Scholar]
- 41.Lion T, Watzinger F, Preuner S, Kreyenberg H, Tilanus M, de Weger R, et al. The EuroChimerism concept for a standardized approach to chimerism analysis after allogeneic stem cell transplantation. Leukemia. 2012;26:1821–8. doi: 10.1038/leu.2012.66. [DOI] [PubMed] [Google Scholar]
- 42.Paietta E. Minimal residual disease in acute myeloid leukemia: coming of age. Hematology Am Soc Hematol Educ Program. 2012;2012:35–42. doi: 10.1182/asheducation-2012.1.35. [DOI] [PubMed] [Google Scholar]
- 43.Walter RB, Buckley SA, Pagel JM, Wood BL, Storer BE, Sandmaier BM, et al. Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood. 2013;122:1813–21. doi: 10.1182/blood-2013-06-506725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walter RB, Gooley TA, Wood BL, Milano F, Fang M, Sorror ML, et al. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J Clin Oncol. 2011;29:1190–7. doi: 10.1200/JCO.2010.31.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perez-Simon JA, Caballero D, Diez-Campelo M, Lopez-Perez R, Mateos G, Canizo C, et al. Chimerism and minimal residual disease monitoring after reduced intensity conditioning (RIC) allogeneic transplantation. Leukemia. 2002;16:1423–31. doi: 10.1038/sj.leu.2402550. [DOI] [PubMed] [Google Scholar]
- 46.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. The Journal of experimental medicine. 2005;202:907–12. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salit RB, Fowler DH, Wilson WH, Dean RM, Pavletic SZ, Dunleavy K, et al. Dose-Adjusted EPOCH-Rituximab Combined With Fludarabine Provides an Effective Bridge to Reduced-Intensity Allogeneic Hematopoietic Stem-Cell Transplantation in Patients With Lymphoid Malignancies. J Clin Oncol. 2012 doi: 10.1200/JCO.2011.37.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Massenkeil G, Nagy M, Lawang M, Rosen O, Genvresse I, Geserick G, et al. Reduced intensity conditioning and prophylactic DLI can cure patients with high-risk acute leukaemias if complete donor chimerism can be achieved. Bone Marrow Transpl. 2003;31:339–45. doi: 10.1038/sj.bmt.1703859. [DOI] [PubMed] [Google Scholar]
- 49.Liga M, Triantafyllou E, Tiniakou M, Lambropoulou P, Karakantza M, Zoumbos NC, et al. High alloreactivity of low-dose prophylactic donor lymphocyte infusion in patients with acute leukemia undergoing allogeneic hematopoietic cell transplantation with an alemtuzumab-containing conditioning regimen. Biol Blood Marrow Transplant. 2013;19:75–81. doi: 10.1016/j.bbmt.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 50.Mapara MY, Sykes M. Induction of mixed vs full chimerism to potentiate GVL effects after bone-marrow transplantation. Methods in molecular medicine. 2005;109:469–74. doi: 10.1385/1-59259-862-5:469. [DOI] [PubMed] [Google Scholar]
- 51.Platzbecker U, Wermke M, Radke J, Oelschlaegel U, Seltmann F, Kiani A, et al. Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: results of the RELAZA trial. Leukemia. 2012;26:381–9. doi: 10.1038/leu.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Lima M, Giralt S, Thall PF, de Padua Silva L, Jones RB, Komanduri K, et al. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer. 2010;116:5420–31. doi: 10.1002/cncr.25500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Metzelder SK, Schroeder T, Finck A, Scholl S, Fey M, Gotze K, et al. High activity of sorafenib in FLT3-ITD-positive acute myeloid leukemia synergizes with allo-immune effects to induce sustained responses. Leukemia. 2012;26:2353–9. doi: 10.1038/leu.2012.105. [DOI] [PubMed] [Google Scholar]
- 54.Sharma M, Ravandi F, Bayraktar UD, Chiattone A, Bashir Q, Giralt S, et al. Treatment of FLT3-ITD-positive acute myeloid leukemia relapsing after allogeneic stem cell transplantation with sorafenib. Biol Blood Marrow Transplant. 2011;17:1874–7. doi: 10.1016/j.bbmt.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.