Abstract
Early brain injury (EBI) which comprises of vasogenic edema and apoptotic cell death is an important component of subarachnoid hemorrhage (SAH) pathophysiology. This study evaluated whether Cannabinoid receptor type 2 (CB2R) agonist, JWH133, attenuates EBI after SAH and whether CB2R stimulation reduces pro-apoptotic caspase-3 via up-regulation of cAMP response element-binding protein (CREB)-Bcl-2 signaling pathway. Male Sprague Dawley rats (n=123) were subjected to SAH by endovascular perforation. Rats received vehicle or JWH133 at 1 hour after SAH. Neurological deficits and brain water content were evaluated at 24 hours after SAH. Western blot was performed to quantify phosphorylated CREB (pCREB), Bcl-2, and cleaved caspase-3 levels. Neuronal cell death was evaluated with terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-biotin nick end-labeling staining. Additionally, CREB siRNA was administered to manipulate the proposed pathway. JWH133 (1.0 mg/kg) improved neurological deficits and reduced brain water content in left hemisphere 24 hours after SAH. JWH133 significantly increased activated CREB (pCREB) and Bcl-2 levels and significantly decreased cleaved caspase-3 levels in left hemisphere 24 hours after SAH. CREB siRNA reversed the effects of treatment. TUNEL positive neurons in the cortex were reduced with JWH133 treatment. Thus, CB2R stimulation attenuated EBI after SAH possibly through activation of pCREB-Bcl-2 pathway.
Keywords: Cannabinoid receptor type 2, JWH133, Subarachnoid hemorrhage, Apoptosis, Brain edema, CREB
Introduction
Subarachnoid hemorrhage (SAH), with a case fatality rate of around 50%, occurs in approximately 85% cases due to rupture of an intracranial aneurysm (van Gijn et al., 2007). Aneurysmal rupture initiates global ischemia due to elevated intracranial pressure and reduced cerebral blood flow which leads to early brain injury (EBI). EBI is compounded by inflammation, oxidative stress, ionic disturbances, which leads to blood brain barrier (BBB) disruption and subsequent vasogenic edema as well as neuronal apoptosis resulting in neurological deterioration after SAH (Fujii et al., 2013). Therefore, therapies that decrease BBB disruption and apoptosis may provide beneficial effects for EBI following SAH (Park et al., 2004; Zhang et al., 2012).
Recent studies have demonstrated neuroprotective properties of drugs based on marijuana-derived cannabinoids (Cohen-Yeshurun et al., 2011). Cannabinoid receptors include the Cannabinoid receptor type 1 (CB1R) and Cannabinoid receptor type 2 (CB2R), which are G protein-coupled receptors expressed by neurons, vascular endothelial cells, and immune cells (Glass and Northup, 1999; Ramirez et al., 2012). While CB1R activation accounts for the psychoactive effects of cannabinoids (Glass and Northup, 1999), CB2R agonist showed neuroprotective effects against experimental ischemic stroke (Murikinati et al., 2010; Zarruk et al., 2012), encephalitis (Ramirez et al., 2012), traumatic brain injury (Amenta et al., 2012), and in a model of remote cell death through its anti-inflammatory and anti-apoptotic properties (Viscomi et al., 2009).
Previous studies show that CB2R activation enhances the phosphorylation of extracellular signal-regulated kinases (ERK) 1/2 (Cohen-Yeshurun et al., 2011; Ofek et al., 2011) and AMP-activated protein kinase (AMPK) (Choi et al., 2013). Once phosphorylated, ERK and AMPK can in turn phosphorylate and thereby activate cAMP response element-binding protein (CREB) (Choi et al., 2013; Ofek et al., 2011). CREB is a transcription factor that promotes cell survival by up-regulating Bcl-2 expression and inhibiting caspase-3 activation (Meller et al., 2005; Tokudome et al., 2004; Wilson et al., 1996). Although apoptosis plays an important role in the pathophysiology of EBI after SAH, the specific anti-apoptotic role of CB2R after SAH has not been explored.
In this study we tested two hypotheses: (A) CB2R agonist, JWH133, attenuates EBI and improves neurological function after SAH. (B) CB2R stimulation activates CREB-Bcl-2 signaling pathway, which decreases pro-apoptotic caspase activation.
Materials and Methods
Animals and Pharmacological Interventions
This experiment was a controlled in vivo laboratory study conducted in an animal research laboratory. All protocols were approved by the Institutional Animal Care and Use Committee at Loma Linda University in accordance with National Institute of Health guidelines. Adult male Sprague Dawley rats (Harlan, IN) weighing 226–338 g were housed with a 12/12 hour light/dark cycle in a temperature and humidity controlled environment.
SAH rats received vehicle (0.2 ml of ethanol with 1.8 ml of 0.9% saline) or the selective CB2R agonist JWH133 (0.3, 1.0, or 3.0 mg/kg, Tocris Bioscience, MN; Ki value= 3.4 nM), which was dissolved in the vehicle, by intraperitoneal injection at 1 hour after SAH. The dose of JWH133 was selected based on previous publication (Murikinati et al., 2010). To ensure that the anti-apoptotic effects of CB2R activation was mediated by phosphorylation of CREB, we injected CREB small interfering ribonucleic acid (siRNA) via intracerebroventricular route to deactivate CREB 24 hours before SAH-induction surgery. SAH rats were injected with control siRNA or CREB siRNA and were divided into cont-JWH and siRNA-JWH groups.
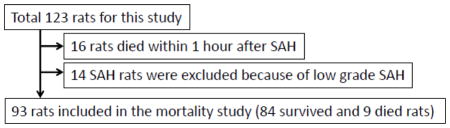
We used 123 animals in this study. The rats were randomly assigned to the following groups: SAH + vehicle in physiological parameter study (n=5), SAH + JWH133 (1.0) in physiological parameter study (n=5), sham-operated (sham group: n=15), SAH + vehicle (vehicle group: n=23), SAH + JWH133 (0.3) (low-JWH group: n=10), SAH + JWH133 (1.0) (JWH group: n=25), SAH + JWH133 (3.0) (high-JWH group: n=10), control siRNA with SAH + JWH133 (1.0) (cont-JWH group: n=7), and CREB siRNA with SAH + JWH133 (1.0) (siRNA-JWH group: n=7). Sixteen rats died within 1 hour after SAH during which time the rats had not received either the drug or vehicle yet.
SAH Rat Model
SAH was induced by the endovascular perforation method as previously described (Bederson et al., 1995; Suzuki et al., 2010). Briefly, rats were anesthetized, intubated and kept on artificial ventilation during surgery with 3% isoflurane in 70%/30% medical-air/oxygen. Normothermia was maintained by a heating lamp. A sharpened 4-0 nylon suture was introduced into the left internal carotid artery until resistance was felt (approximately 18 mm from the common carotid bifurcation). The suture was advanced to perforate the bifurcation of the anterior cerebral artery and middle cerebral artery until resistance was overcome after which the suture was immediately withdrawn. In sham-operated animals, the suture was inserted into the left internal carotid artery and then removed without perforating the artery. The skin incision was closed, and rats were individually housed in cages with temperature maintained at 98 degrees Fahrenheit by a heating pad until recovery. Buprenorphine (0.01 mg/kg) was administered subcutaneously for post-operative analgesia.
Physiological Parameters
The right femoral artery was cannulated for continuous measurement of mean arterial blood pressure, heart rate and for blood sampling as previously described (Fujii et al., 2012). Arterial blood gases, pH and serum glucose was measured 15 minutes before, immediately after, and every 30 or 60 minutes after SAH. The monitoring was continued for 120 minutes after SAH induction.
SAH Grade
The SAH severity was evaluated in a blinded manner as previously described (Sugawara et al., 2008). Briefly, picture of the base of the brain was taken and divided into six segments. Each segment was sub-scored (0 to 3) depending on the amount of blood in the subarachnoid space and a total score was calculated as the sum of all sub-scores. The score ranged from 0 (no SAH) to 18 (severe SAH). Animals with SAH score of 7 or less were excluded from the study as previously described (Sugawara et al., 2008).
Neurological Score
Neurological deficits were evaluated blindly using modified Garcia test (Garcia et al., 1995; Sugawara et al., 2008). The test consisted of six parameters which included spontaneous activity; symmetry in the movement of all four limbs; forepaw outstretching; climbing; body proprioception; and response to vibrissae stimulation. The total score ranged from 2 to 18 (Fujii et al., 2012). Neurological score was evaluated at 24 hours after sham or SAH-induction surgery. Additionally, neurological test was performed before the SAH-induction surgery and at 24 hours after the surgery in rats allocated to intracerebroventricular injection groups (cont-JWH and siRNA-JWH group).
Brain Water Content Measurement
Brain water content was measured 24 hours after SAH. Rats were sacrificed under lethal isoflurane anesthesia, and brains were quickly removed and then separated into left hemisphere, right hemisphere, cerebellum, and brain stem to measure brain edema as previously described (Suzuki et al., 2010). Each part was weighed immediately after removal (wet weight) and after drying at 100 °C for 72 hours (dry weight). The percentage of brain water content (BWC) was calculated as [(wet weight − dry weight)/wet weight] ×100%.
Intracerebroventricular Infusion
Under 3% isoflurane anesthesia, the needle of a 10-μL Hamilton syringe (Microliter No. 701; Hamilton Company, NV) was inserted through a burr hole on the skull into the left lateral ventricle according to the following coordinates relative to bregma: 1.5 mm posterior, 0.8 mm lateral, and 4.2 mm below the horizontal plane as previously described with modification (Peters et al., 2009; Suzuki et al., 2010). CREB-1 siRNA or an irrelevant control siRNA, 500 pmol each in 1μL siRNA dilution buffer (Santa Cruz Biotechnology, CA) was injected by a microinfusion pump (Harvard Apparatus, MA) at a rate 0.5 μL/min at 24 hours before SAH-induction. The needle was removed 20 minutes after the end of infusion, and the burr hole was plugged with bone wax. All the rats received JWH133 (1.0 mg/kg) intraperitoneally 1 hour after SAH.
Three different CREB siRNA duplexes were pooled and the sequences were as follows:
(a) sense, 5′-CUGCAGACAUUAACCAUGA [dT][dT]-3′ and
antisense, 5′-UCAUGGUUAAUGUCUGCAG [dT][dT]-3′;
(b) sense, 5′-CAACCAAGUUGUUGUUCAA[dT][dT]-3′ and
antisense, 5′-UUGAACAACAACUUGGUUG [dT][dT]-3′;
(c) sense, 5′-GCAAGAGAAUGUCGUAGAA[dT][dT]-3′ and
antisense, 5′-UUCUACGACAUUCUCUUGC [dT][dT]-3′
The sequence of control siRNA used was as follows:
sense, 5′-UUCUCCGAACGUGUCACGU[dT][dT]-3′ and
antisense, 5′-ACGUGACACGUUCGGAGAA [dT][dT]-3′
Western Blot Analysis
Animals were euthanized at 24 hours after surgery, and left hemisphere was processed as previously described (Suzuki et al., 2010). Equal amounts (40 μg) of protein were separated on an SDS-PAGE gel and transferred to a nitrocellulose membrane. The membranes were incubated overnight at 4°C with the following primary antibodies: anti-phospho-CREB (Ser133) and anti-Bcl-2 (1:200, Santa Cruz Biotechnology, CA), and anti-cleaved caspase-3 (1:1000, Cell Signaling, MA). Appropriate secondary antibodies (1:2000, Santa Cruz Biotechnology, CA) were incubated for 1 hour at room temperature. Immunoblots were then probed with an ECL Plus chemiluminescence reagent kit (Amersham Bioscience, IL) and bands were quantified by densitometry with Image J software (National Institutes of Health, MD). Alpha tubulin (1:2000, Santa Cruz Biotechnology, CA) was blotted on the same membrane as a loading control. Results are expressed as relative density to alpha tubulin and subsequently normalized to mean value of cont-JWH.
Terminal Deoxynucleotidyl Transferase-Mediated Uridine 5′-Triphosphate-Biotin Nick End-Labeling (TUNEL) Staining
At 24 hours after sham or SAH-induction surgery, the rats were perfused with phosphate-buffered saline and 10% paraformaldehyde at the time of sacrifice. The brains were harvested and postfixed in 10% paraformaldehyde followed by 30% sucrose (weight/volume) for 3 days. Ten-micron-thick coronal sections were cut at the level of bregma-2 mm using a cryostat (LM3050S; Leica Microsystems) and mounted on poly-L-lysine-coated slides. Sections were stained with anti-neuronal nuclei antibody (1:100; Chemicon International, MA) and then subjected to terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) staining with an in situ cell death detection kit (Roche Inc., Germany). TUNEL-positive neurons were evaluated in the left pyriform cortex.
Statistical Analysis
Data for SAH grades and neurological scores were expressed as median and 25th to 75th percentiles and were analyzed by Mann-Whitney U test or Kruskal-Wallis one-way Analysis of Variance (ANOVA) on Ranks followed by Dunn’s or Tukey post hoc analysis. Other values were expressed as mean ± standard deviation (SD) and were analyzed by one-way ANOVA followed by Tukey post hoc analysis. T-test was used for statistical analysis in the intracerebroventricular siRNA injection groups. Mortality was analyzed by Fischer exact test. The data for physiological parameters were analyzed using two-way repeated measures ANOVA. P <0.05 was considered statistically significant.
Results
Animal Number and Mortality
We performed 123 surgeries (Table 1.). Fourteen animals were excluded because of low SAH grade (6 animals in vehicle and 8 in JWH groups, respectively). Sixteen animals with severe SAH died within 1 hour after SAH, and 9 animals died after 1 hour (2 animals in vehicle, 2 in low-JWH, 1 in JWH, 2 in high-JWH, 1 in cont-JWH, and 1 in siRNA-JWH groups). The mortality rate was: sham 0.0% (0 of 15 rats), vehicle 11.8% (2 of 17), low-JWH 20.0% (2 of 10), JWH 5.9% (1 of 17), and high-JWH 20.0% (2 of 10). There was no significant difference in mortality rates between the groups.
Table 1.
The animal numbers and mortality used in each group and analysis. There was no significant difference in mortality rates between the groups.
| |||||
|---|---|---|---|---|---|
| Mortality rate | |||||
| Sham | SAH
|
||||
| vehicle | low-JWH | JWH | high-JWH | ||
| Physiological parameter study | 0.0% (0/5) | 0.0% (0/5) | |||
|
| |||||
| Studies from BWC, WB, and IHC | 0.0% (0/15) | 11.8% (2/17) | 20.0% (2/10) | 5.9% (1/17) | 20.0% (2/10) |
|
| |||||
| Cont-JWH | siRNA-JWH | ||||
|
|
|||||
| ICVstudy | 14.3% (1/7) | 14.3% (1/7) | |||
Abbreviations: SAH, subarachnoid hemorrhage; BWC, brain water content; WB, western blot analysis; IHC, immunohistochemistry; ICV, intracerebroventricular infusion.
Subarachnoid Hemorrhage Grade and Physiological Parameters
SAH grade was similar among vehicle (n=15), low-JWH (n=8), JWH (n=16), and high-JWH (n=8) groups (Fig. 1A). There was no significant difference in the mean arterial blood pressure, heart rate, arterial blood gases (paO2, paCO2), pH levels, and blood glucose levels between the vehicle and JWH group (n=5, each) (Figs. 1B–D).
Fig. 1.
Subarachnoid hemorrhage (SAH) grading and physiological parameters in vehicle and JWH133 treated SAH rats. SAH grade was comparable between vehicle and JWH groups (A). Physiological parameters including mean arterial blood pressure (BP) and heart rate (HR) (B), PaO2 and PaCO2 (C), pH and blood glucose (Glu) (D) in vehicle and JWH groups was not significantly different. The group box depicted in B applies to C and D. Values are expressed as mean ± SD. NS=not significant.
Outcome Study
JWH133 Attenuates Neurological Deficits and Brain Edema at 24 Hours After SAH
Neurological deficits and brain water content were evaluated at 24 hours after surgery. For the 24 hours outcome study we used sham, vehicle, low-JWH, JWH, and high-JWH groups. Neurological score was significantly lower in vehicle group (n=15) compared to sham (n=15) (P<0.05, Fig. 2A). JWH group (n=16) had significantly higher neurological score compared to vehicle group (P<0.05).
Fig. 2.

Effects of JWH133 treatment on neurological function and brain water content at 24 hours after SAH. JWH133 (1.0 mg/kg) significantly improved neurological score compared to vehicle after SAH (A). JWH133 (1.0 mg/kg) significantly reduced brain water content in the left hemisphere compared to vehicle at 24 hours after SAH (B). Values in (A) are expressed as median and 25th to 75th percentiles and values in (B) are mean ± SD. *P <0.05 compared to sham group, #P <0.05 compared to vehicle group, †P <0.01 compared to sham group, ‡P <0.05 compared to JWH group. LH, left hemisphere; RH, right hemisphere; Ce, cerebellum; BS, brain stem.
At 24 hours after SAH, BWC in the left and right hemispheres was significantly higher in vehicle group compared to sham (P<0.01, Fig. 2B). JWH group had significantly lower BWC in the left hemisphere compared to vehicle group (P<0.05, Fig. 2B). However, there was no significant difference in BWC between vehicle and low-JWH or high-JWH groups. Since JWH133 (1.0 mg/kg) significantly improved neurobehavioral function and reduced brain edema in the left hemisphere, we chose this dose and the left hemisphere for rest of the experiments.
Mechanism Study
JWH133 Increases pCREB-Bcl2 Expression and Decreases Cleaved Caspase-3 at 24 Hours After SAH
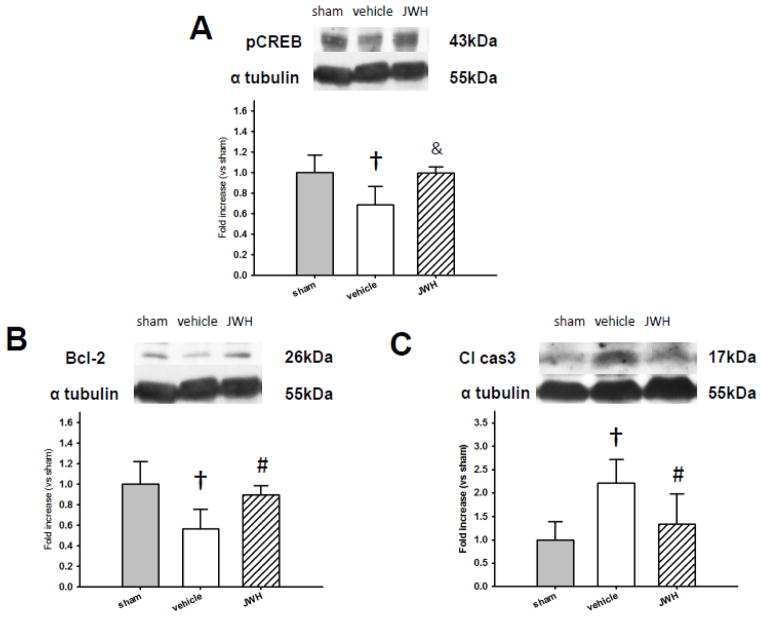
Western blot analysis was used to quantify phosphorylated CREB (pCREB), Bcl-2, and cleaved caspase-3 levels in the left hemisphere 24 hours after surgery in sham, vehicle, and JWH groups (n=6, each). pCREB and Bcl-2 was significantly lower in vehicle compared to sham (P<0.01), and JWH group had significantly higher pCREB (P<0.01) and Bcl-2 (P<0.05) levels compared to vehicle (Figs. 3A and 3B). In addition, cleaved caspase-3 expression was significantly higher in vehicle group compared to sham (P<0.01) and was significantly reduced in the JWH group compared to vehicle (P<0.05) (Fig. 3C).
Fig. 3.
Anti-apoptotic effect of CB2R stimulation at 24 hours after SAH. Representative Western blots and quantitative analysis of phosphorylated cAMP response element-binding protein (pCREB) (A), Bcl-2 (B), and cleaved caspase-3 (cl cas-3) (C) in the left hemisphere. JWH133 treatment significantly increased pCREB and Bcl-2 expression, and decreased cleaved caspase-3 significantly compared to vehicle group after SAH. Values are expressed as mean ± SD. †P <0.01 compared to sham group, &P <0.01 compared to vehicle group, #P <0.05 compared to vehicle group.
CREB siRNA Reverses the Anti-Apoptotic Effect of JWH133 at 24 Hours After SAH
SAH grade was similar between cont-JWH and siRNA-JWH groups (P=NS, Fig. 4A). The difference in performance in neurological test conducted before SAH-induction surgery and 24 hours after SAH showed that the siRNA-JWH group performed worse compared to cont-JWH group, though it did not reach statistical significance (Fig. 4B).
Fig. 4.
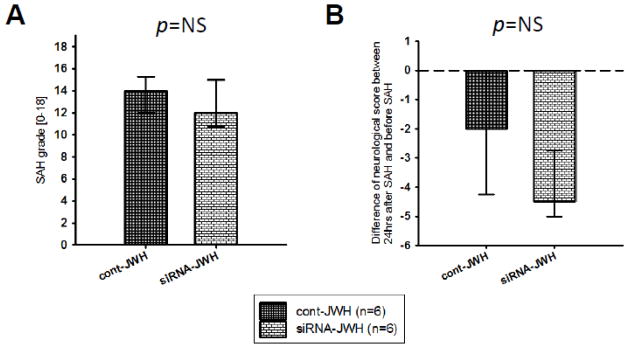
Effect of CREB siRNA administration on neurological function at 24 hours after SAH in rats treated with JWH133. SAH grade was similar between groups that received control siRNA or CREB siRNA administered intracerebroventricularly 24 hours before SAH (A). SAH rats that received CREB siRNA showed a tendency to perform worse in the neurological test compared to SAH rats that received control siRNA (B). Values are expressed as median and 25th to 75th percentiles. NS=not significant.
Western blot analysis showed that pCREB expression in the left hemisphere was significantly lower in siRNA-JWH compared to cont-JWH groups (P<0.01, Fig. 5A). Further, Bcl-2 was significantly reduced in siRNA-JWH compared to cont-JWH group (P<0.01, Fig. 5B), and cleaved caspase-3 was significantly increased in siRNA-JWH compared to cont-JWH group (P<0.01, Fig. 5C).
Fig. 5.
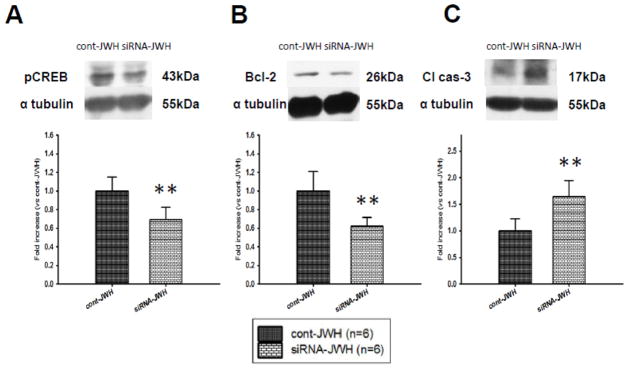
Effect of CREB siRNA administration on the anti-apoptotic effect of CB2R stimulation at 24 hours after SAH in rats treated with JWH133. Representative Western blots and quantitative analysis of pCREB (A), Bcl-2 (B) and cleaved caspase-3 (cl cas-3) (C). SAH rats that received CREB siRNA showed significantly lower pCREB expression compared to the control siRNA administered SAH rats (A). Bcl-2 expression was significantly down-regulated (B) and cleaved caspase-3 level was significantly up-regulated (C) in the CREB siRNA administered SAH rats compared to the control siRNA group. Values are expressed as mean ± SD. **P <0.01 compared to cont-JWH group.
JWH133 Reduces TUNEL Positive Neurons at 24 Hours After SAH
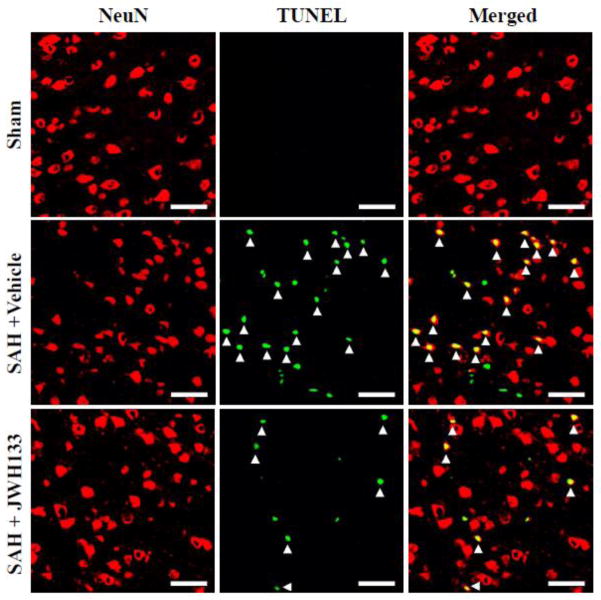
Immunofluorescence staining showed TUNEL-positive neurons in the left pyriform cortex at 24 hours after SAH (Fig. 6). The vehicle group had increased TUNEL-positive neurons compared to JWH group. The SAH grade was 15 in both the vehicle and JWH133 treated rats depicted in the pictographs.
Fig. 6.
Evaluation of neuronal cell death in the pyriform cortex at 24 hours after SAH. Representative picture shows increased colocalization of neuronal nuclei (NeuN) and TUNEL-positive cells (arrowhead) in SAH rat that received vehicle compared to JWH133 treatment. Sham did not have any TUNEL-positive cells in the pyriform cortex. Scale bars: 50μm.
Discussion
This study showed that CB2R activation with JWH133 administered 1 hour after SAH reduced neurological deficits and brain edema. Further, CB2R activation increased the phosphorylation of CREB and Bcl-2 expression. The anti-apoptotic effects of JWH 133 were reversed by CREB siRNA.
First, we evaluated the effects of three different doses of JWH133 to determine the neuroprotective dose. SAH rats treated with JWH133 1.0 mg/kg had significantly reduced BWC at 24 hours after SAH compared to vehicle rats. JWH133 1.0 mg/kg also significantly improved neurobehavioral function. Our results are in accordance with previous studies which have reported that JWH133 between 1.0 to 1.5 mg/kg reduced infarct area after MCA occlusion, but doses lower or higher were less effective (Zarruk et al., 2012; Zhang et al., 2009a). The bell-shaped dose-response curve of JWH133 may be due to non-specific effects that can occur at higher doses (Murikinati et al., 2010). Although, JWH133 has 40-fold more selectivity for CB2R over CB1R (Yamamoto et al., 2008) it is possible that higher doses may activate the CB1R (Murikinati et al., 2010), which has been shown to play a detrimental role after cerebral ischemia-reperfusion injury (Zhang et al., 2009b).
Next, we evaluated whether CB2R stimulation decreases apoptosis by CREB activation. CB2R can couple to Gi proteins (Glass and Northup, 1999), and CB2R activation has been shown to activate cAMP/protein kinase A (PKA) (Börner et al., 2009), phosphorylate ERK1/2 (Cohen-Yeshurun et al., 2011; Ofek et al., 2011), and activate phosphatidylinositol 3-kinase (PI3K)/Akt pathway (Viscomi et al., 2009). Recently it has been demonstrated that CB2R stimulation enhanced CREB activation after cerebral ischemia through phosphorylation of AMPK (Choi et al., 2013). Furthermore, CREB can also be activated by various signaling pathways, including cAMP/PKA, ERK1/2, and PI3K/Akt (Ichiki, 2006; Ofek et al., 2011). Moreover, CREB phosphorylation at Ser133 is indispensable for its transcriptional activation (Chrivia et al., 1993), and activated CREB can increase Bcl-2 expression and inhibit caspase-3 activation (Meller et al., 2005; Tokudome et al., 2004; Wilson et al., 1996). In this study, CB2R agonist JWH133 significantly enhanced the expression of CREB phosphorylated at Ser133, up-regulated Bcl-2, and reduced caspase-dependent apoptosis after SAH. Additionally, we used TUNEL staining, a maker for cells undergoing DNA fragmentation in the final phase of apoptotic cell death (Negoescu et al., 1996), and observed TUNEL positive neurons in the left pyriform cortex 24 hours after SAH, and JWH133 administration reduced neuronal cell death after SAH.
We then wanted to ensure that activated CREB was essential for Bcl-2 up-regulation and reduction of caspase-dependent apoptosis by the CB2R agonist JWH133. Previous study by Liu et al. showed that CREB-1 siRNA can suppress the phosphorylation of CREB-1 at ser133 (Liu et al., 2006). We administered CREB siRNA to determine whether it would abolish the protective anti-apoptotic effects of JWH133. Control or CREB siRNA was administered 24 hours before SAH induction to rats that also received JWH133 treatment after SAH. We administered CREB siRNA at 24 hours before SAH induction based on published literature. Chen et al. reported that siRNA of P2X purinoceptor 7 receptors administered intracerebroventricularly 24 hours before SAH induction in a rat model was effective in eliciting gene knockdown (Chen et al., 2013). We observed that CREB siRNA significantly decreased pCREB expression compared to control siRNA in SAH rats despite JWH133 administration. Further, SAH rats treated with JWH133 that received also CREB siRNA had significantly reduced Bcl-2 expression and increased cleaved caspase-3 levels compared to JWH133 treated SAH rats that received control siRNA. This suggests that CREB activation is essential for the anti-apoptotic effects of CB2R stimulation after SAH by the CB2R agonist JWH133.
This study has some limitations. First, we did not investigate how CB2R stimulation inhibits neuronal apoptotic cell death after SAH. In the brain CB2R is expressed in neurons, activated astrocytes, endothelial cells, and microglias (Onaivi et al, 2011). CB2R activation has been reported to attenuate microglial activation and prevent neurodegeneration (Amenta et al., 2012; Palazuelos et al., 2009). Since inflammatory response in the central nervous system is mediated by activated microglia (Dheen et al., 2007), it is possible that CB2R activation on the pro-inflammatory microglia might play a pivotal role to ameliorate neuronal cell death after SAH. Second, we focused on the anti-apoptotic mechanisms of CB2R stimulation. However, other potential mechanisms of CB2R stimulation could play a role in providing beneficial effect after SAH, which we did not explore in this study (Simard et al., 2012). This might be a possible explanation as to why we did not observe statistically significant neurological deterioration in SAH rats that received CREB siRNA injection as opposed to control siRNA, since anti-apotosis may be one of the many protective mechanisms of CB2R agonist induced neuroprotection. Third, we did not quantify the number of TUNEL positive neurons in the cortex after SAH to demonstrate the difference between the vehicle and JWH133 treated groups and also for the cont-JWH and siRNA-JWH groups. Since, we primarily wanted to demonstrate the cell type that undergoes apoptosis after SAH we did not perform cell quantification. We provide immunohistochemistry pictures that show apoptosis occurs in the neurons after SAH and that JWH133 treated group had relatively fewer TUNEL positive neurons. Further, western blot quantification for cleaved caspase-3 and Bcl-2 levels provide support to our hypothesis that CB2R stimulation decreases apoptosis after SAH. However, quantification of TUNEL positive neurons would have certainly strengthened our hypothesis. Further studies are necessary for clinical translation (Bahjat et al., 2013; Lapchak, 2013; Tajiri et al., 2013).
Conclusion
CB2R stimulation reduced brain edema and improved neurological function in a rat model of SAH. CB2R stimulation was associated with reduced neuronal apoptosis via activation of phosphorylated CREB/Bcl-2 pathway after SAH.
Highlights.
JWH133 (1.0 mg/kg) ameliorated neurological deficits and brain edema after SAH.
JWH133 ameliorated apoptosis through phosphorylated CREB and Bcl-2 pathway.
CREB siRNA administration increased cleaved caspase-3 despite JWH133 treatment.
Apoptotic cell death occurred in the neurons after SAH.
Acknowledgments
Sources of Funding
This research was supported by NIH grant to Dr. Zhang.
Abbreviations
- EBI
early brain injury
- SAH
subarachnoid hemorrhage
- CB2R
cannabinoid receptor type 2
- CREB
cAMP response element-binding protein
- siRNA
small interfering ribonucleic acid
- pCREB
phosphorylated CREB
- BBB
blood brain barrier
- CB1R
cannabinoid receptor type 1
- ERK
extracellular signal-regulated kinases
- AMPK
AMP-activated protein kinase
- BWC
brain water content
- TUNEL
terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-biotin nick end-labeling
- ANOVA
analysis of variance
- SD
standard deviation
- cAMP
cyclic adenosine monophosphate
- PKA
protein kinase A
- PI3K
phosphatidylinositol 3-kinase
- WB
western blot analysis
- IHC
immunohistochemistry
- ICV
intracerebroventricular infusion
Footnotes
Conflict of Interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amenta PS, Jallo JI, Tuma RF, Elliott MB. A cannabinoid type 2 receptor agonist attenuates blood-brain barrier damage and neurodegeneration in a murine model of traumatic brain injury. J Neurosci Res. 2012;90:2293–2305. doi: 10.1002/jnr.23114. [DOI] [PubMed] [Google Scholar]
- Bahjat FR, Gesuete R, Stenzel-Poore MP. Steps to translate preconditioning from basic research to the clinic. Transl Stroke Res. 2013;4:89–103. doi: 10.1007/s12975-012-0223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bederson JB, Germano IM, Guarino L. Cortical blood flow and cerebral perfusion pressure in a new noncraniotomy model of subarachnoid hemorrhage in the rat. Stroke. 1995;26:1086–1091. doi: 10.1161/01.str.26.6.1086. [DOI] [PubMed] [Google Scholar]
- Börner C, Smida M, Höllt V, Schraven B, Kraus J. Cannabinoid receptor type 1- and 2-mediated increase in cyclic AMP inhibits T cell receptor-triggered signaling. J Biol Chem. 2009;284:35450–35460. doi: 10.1074/jbc.M109.006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Ma Q, Krafft PR, Hu Q, Rolland W, 2nd, Sherchan P, Zhang J, Tang J, Zhang JH. P2X7R/cryopyrin inflammasome axis inhibition reduces neuroinflammation after SAH. Neurobiol Dis. 2013;58:296–307. doi: 10.1016/j.nbd.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi IY, Ju C, Jalin AMAA, da Lee I, Prather PL, Kim WK. Activation of cannabinoid CB2 receptor-mediated AMPK/CREB pathway reduces cerebral ischemic injury. Am J Pathol. 2013;182:928–939. doi: 10.1016/j.ajpath.2012.11.024. [DOI] [PubMed] [Google Scholar]
- Chrivia JC, Kwok RP, Lamb N, Hagiwara M, Montminy MR, Goodman RH. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- Cohen-Yeshurun A, Trembovler V, Alexandrovich A, Ryberg E, Greasley PJ, Mechoulam R, et al. N-arachidonoyl-L-serine is neuroprotective after traumatic brain injury by reducing apoptosis. J Cereb Blood Flow Metab. 2011;31:1768–1777. doi: 10.1038/jcbfm.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dheen ST, Kaur C, Ling EA. Microglial activation and its implications in the brain diseases. Curr Med Chem. 2007;14:1189–1197. doi: 10.2174/092986707780597961. [DOI] [PubMed] [Google Scholar]
- Fujii M, Duris K, Altay O, Soejima Y, Sherchan P, Zhang JH. Inhibition of Rho kinase by hydroxyfasudil attenuates brain edema after subarachnoid hemorrhage in rats. Neurochem Int. 2012;60:327–333. doi: 10.1016/j.neuint.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii M, Yan J, Rolland WB, Soejima Y, Caner B, Zhang JH. Early brain Injury, an evolving frontier in subarachnoid hemorrhage research. Transl Stroke Res. 2013;4:432–446. doi: 10.1007/s12975-013-0257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369:306–318. doi: 10.1016/S0140-6736(07)60153-6. [DOI] [PubMed] [Google Scholar]
- Glass M, Northup JK. Agonist selective regulation of G proteins by cannabinoid CB(1) and CB(2) receptors. Mol Pharmacol. 1999;56:1362–1369. doi: 10.1124/mol.56.6.1362. [DOI] [PubMed] [Google Scholar]
- Ichiki T. Role of cAMP response element binding protein in cardiovascular remodeling: good, bad, or both? Arterioscler Thromb Vasc Biol. 2006;26:449–455. doi: 10.1161/01.ATV.0000196747.79349.d1. [DOI] [PubMed] [Google Scholar]
- Lapchak PA. Drug-like property profiling of novel neuroprotective compounds to treat acute ischemic stroke: guidelines to develop pleiotropic molecules. Transl Stroke Res. 2013;4:328–342. doi: 10.1007/s12975-012-0200-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G1, Ding W, Neiman J, Mulder KM. Requirement of Smad3 and CREB-1 in mediating transforming growth factor-beta (TGF beta) induction of TGF beta 3 secretion. J Biol Chem. 2006;281:29479–29490. doi: 10.1074/jbc.M600579200. [DOI] [PubMed] [Google Scholar]
- Meller R, Minami M, Cameron JA, Impey S, Chen D, Lan JQ, et al. CREB-mediated Bcl-2 protein expression after ischemic preconditioning. J Cereb Blood Flow Metab. 2005;25:234–246. doi: 10.1038/sj.jcbfm.9600024. [DOI] [PubMed] [Google Scholar]
- Murikinati S, Jüttler E, Keinert T, Ridder DA, Muhammad S, Waibler Z, et al. Activation of cannabinoid 2 receptors protects against cerebral ischemia by inhibiting neutrophil recruitment. FASEB J. 2010;24:788–798. doi: 10.1096/fj.09-141275. [DOI] [PubMed] [Google Scholar]
- Negoescu A, Lorimier P, Labat-Moleur F, Drouet C, Robert C, Guillermet C, et al. In situ apoptotic cell labeling by the TUNEL method: improvement and evaluation on cell preparations. J Histochem Cytochem. 1996;44:959–968. doi: 10.1177/44.9.8773561. [DOI] [PubMed] [Google Scholar]
- Ofek O, Attar-Namdar M, Kram V, Dvir-Ginzberg M, Mechoulam R, Zimmer A, et al. CB2 cannabinoid receptor targets mitogenic Gi protein-cyclin D1 axis in osteoblasts. J Bone Miner Res. 2011;26:308–316. doi: 10.1002/jbmr.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gu S, Liu QR. CNS effects of CB2 cannabinoid receptors: beyond neuro-immuno-cannabinoid activity. J Psychopharmacol. 2011;26:92–103. doi: 10.1177/0269881111400652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazuelos J, Aguado T, Pazos MR, Julien B, Carrasco C, Resel E, et al. Microglial CB2 cannabinoid receptors are neuroprotective in Huntington’s disease excitotoxicity. Brain. 2009;132:3152–3164. doi: 10.1093/brain/awp239. [DOI] [PubMed] [Google Scholar]
- Park S, Yamaguchi M, Zhou C, Calvert JW, Tang J, Zhang JH. Neurovascular protection reduces early brain injury after subarachnoid hemorrhage. Stroke. 2004;35:2412–2417. doi: 10.1161/01.STR.0000141162.29864.e9. [DOI] [PubMed] [Google Scholar]
- Peters M, Bletsch M, Catapano R, Zhang X, Tully T, Bourtchouladze R. RNA interference in hippocampus demonstrates opposing roles for CREB and PP1alpha in contextual and temporal long-term memory. Genes Brain Behav. 2009;8:320–329. doi: 10.1111/j.1601-183X.2009.00474.x. [DOI] [PubMed] [Google Scholar]
- Ramirez SH, Haskó J, Skuba A, Fan S, Dykstra H, McCormick R, et al. Activation of cannabinoid receptor 2 attenuates leukocyte-endothelial cell interactions and blood-brain barrier dysfunction under inflammatory conditions. J Neurosci. 2012;32:4004–4016. doi: 10.1523/JNEUROSCI.4628-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Tosun C, Ivanova S, Kurland DB, Hong C, Radecki L, et al. Heparin reduces neuroinflammation and transsynaptic neuronal apoptosis in a model of subarachnoid hemorrhage. Transl Stroke Res. 2012;3 (Suppl 1):155–165. doi: 10.1007/s12975-012-0166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara T, Ayer R, Jadhav V, Zhang JH. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods. 2008;167:327–334. doi: 10.1016/j.jneumeth.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Hasegawa Y, Kanamaru K, Zhang JH. Mechanisms of osteopontin-induced stabilization of blood-brain barrier disruption after subarachnoid hemorrhage in rats. Stroke. 2010;41:1783–1790. doi: 10.1161/STROKEAHA.110.586537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajiri N, Dailey T, Metcalf C, Mosley YI, Lau T, Staples M, et al. In vivo animal stroke models: a rationale for rodent and non-human primate models. Transl Stroke Res. 2013;4:308–321. doi: 10.1007/s12975-012-0241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokudome T, Horio T, Fukunaga M, Okumura H, Hino J, Mori K, et al. Ventricular nonmyocytes inhibit doxorubicin-induced myocyte apoptosis: involvement of endogenous endothelin-1 as a paracrine factor. Endocrinology. 2004;145:2458–2466. doi: 10.1210/en.2003-1322. [DOI] [PubMed] [Google Scholar]
- Viscomi MT, Oddi S, Latini L, Pasquariello N, Florenzano F, Bernardi G, et al. Selective CB2 receptor agonism protects central neurons from remote axotomy-induced apoptosis through the PI3K/Akt pathway. J Neurosci. 2009;29:4564–4570. doi: 10.1523/JNEUROSCI.0786-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BE, Mochon E, Boxer LM. Induction of bcl-2 expression by phosphorylated CREB proteins during B-cell activation and rescue from apoptosis. Mol Cell Biol. 1996;16:5546–5556. doi: 10.1128/mcb.16.10.5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto W, Mikami T, Iwamura H. Involvement of central cannabinoid CB2 receptor in reducing mechanical allodynia in a mouse model of neuropathic pain. Eur J Pharmacol. 2008;583:56–61. doi: 10.1016/j.ejphar.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Zarruk JG, Fernández-López D, García-Yébenes I, García-Gutiérrez MS, Vivancos J, Nombela F, et al. Cannabinoid type 2 receptor activation downregulates stroke-induced classic and alternative brain macrophage/microglial activation concomitant to neuroprotection. Stroke. 2012;43:211–219. doi: 10.1161/STROKEAHA.111.631044. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Badaut J, Tang J, Obenaus A, Hartman R, Pearce WJ. The vascular neural network--a new paradigm in stroke pathophysiology. Nat Rev Neurol. 2012;8:711–716. doi: 10.1038/nrneurol.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Adler MW, Abood ME, Ganea D, Jallo J, Tuma RF. CB2 receptor activation attenuates microcirculatory dysfunction during cerebral ischemic/reperfusion injury. Microvasc Res. 2009a;78:86–94. doi: 10.1016/j.mvr.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Martin BR, Adler MW, Razdan RJ, Kong W, Ganea D, et al. Modulation of cannabinoid receptor activation as a neuroprotective strategy for EAE and stroke. J Neuroimmune Pharmacol. 2009b;4:249–259. doi: 10.1007/s11481-009-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]