Abstract
Rationale
The neural mechanisms mediating the ontogeny of behavioral sensitization are poorly understood.
Objective
The purpose of the present study was to determine the role of the D1 receptor for the induction of dopamine agonist-induced behavioral sensitization during the preweanling period.
Methods
In the first experiment, the early ontogeny of NPA-induced behavioral sensitization was examined by pretreating male and female rats with saline or NPA (0.5, 1, or 2 mg/kg, IP) before placement in activity chambers on postnatal day (PD) 12, 16, 20, or 24. One day later, rats were tested with lower doses of NPA and the occurrence of locomotor sensitization was determined. In subsequent experiments, rats were injected with saline or the D1 receptor antagonist SCH23390 (0.1, 0.5, 1, or 5 mg/kg, IP) 0, 15, 30, or 60 min before cocaine, methamphetamine (METH), or NPA pretreatment. The next day, rats were tested with the same dopamine agonist again and sensitized responding was assessed.
Results
NPA produced one-trial behavioral sensitization at all ages tested. In preweanling rats, SCH23390, regardless of dose, was ineffective at preventing the induction of cocaine-, METH-, or NPA-induced one-trial behavioral sensitization.
Conclusions
The present results are in partial contrast to adult rodent studies, in which SCH23390 blocks the induction of METH- and apomorphine-induced behavioral sensitization, but not cocaine sensitization. When these findings are considered together, it appears that D1 receptor stimulation is not necessary for the induction of behavioral sensitization during the preweanling period, although D1 receptors may play a more important role in adulthood.
Keywords: Behavioral sensitization, Cocaine, Methamphetamine, R-propylnorapomorphine, SCH23390, Ontogeny
Introduction
The neural changes responsible for drug-induced behavioral sensitization may be an important component underlying addiction (for reviews, see Robinson and Berridge 1993, 2008; Wolf and Ferrario 2010). Behavioral sensitization occurs when rats pretreated with certain psychoactive compounds (e.g., cocaine, apomorphine, nicotine, or alcohol) show an augmented behavioral response after a challenge injection with the same drug (Kalivas and Stewart 1991; Vezina et al. 2007; Faria et al. 2008). Although sensitized responding is typically assessed after multiple pretreatment exposures to a psychostimulant, adult rats and mice show robust behavioral sensitization after a single pretreatment administration of cocaine or amphetamine (Weiss et al. 1989; Jackson and Nutt 1993; Battisti et al. 2000; Kameda et al. 2011). The number of pretreatment exposures does not appear to affect the longevity of the sensitized response in adult animals, because one-trial and multi-trial behavioral sensitization is detectible for months after initial drug exposure (Leith and Kuczenski 1982; Robinson et al. 1982; Paulson et al. 1991; Valjent et al. 2010).
Although both cocaine and amphetamine-like compounds are capable of inducing behavioral sensitization in adult rats and mice, there is accumulating evidence that the neural mechanisms underlying sensitized responding differ according to psychostimulant (for reviews, see White et al. 1998; Vanderschuren and Kalivas 2000). For example, cocaine- but not amphetamine-induced behavioral sensitization is dependent on prefrontal glutamatergic transmission (Li and Wolf 1997; Pierce et al. 1998). Also, D1 antagonists block the induction of amphetamine-, methamphetamine- (METH), and apomorphine-induced behavioral sensitization (Vezina and Stewart 1989; Mattingly et al. 1991; Kuribara and Uchihashi 1994; Dias et al. 2010); whereas, D1 receptor antagonism does not impair multi-trial cocaine sensitization (Mattingly et al. 1994; Kuribara and Uchihashi 1993; Kuribara 1995a). The major exception to the latter result is provided by studies using the one-trial paradigm, in which the D1 antagonist SCH23390 was injected 15 min before a single pretreatment administration of cocaine (Fontana et al. 1993; Valijent et al. 2010). When tested with cocaine 1 or 7 days later, adult rats did not exhibit a sensitized locomotor response. Thus, pretreatment with a D1 antagonist differentially affects the cocaine-induced behavioral sensitization of adult rats depending on whether a one- or multi-trial procedure is used. Because the one-trial behavioral sensitization of adult rats and mice is under complete contextual control (Drew and Glick 1989; Weiss et al. 1989; Battisti et al. 1999; Valjent et al. 2010), it is uncertain if SCH23390 prevented induction by disrupting associative learning processes or by altering the nonassociative neural mechanisms underlying behavioral sensitization (Fontana et al. 1993; see also White et al. 1998).
Preweanling rats also exhibit behavioral sensitization after one or more psychostimulant exposures; however, the sensitized responding of preweanling animals differs from adolescent and adult rats in some important respects (for a review, see Tirelli et al. 2003). For example, psychostimulant-induced behavioral sensitization appears to be weaker and less persistent in preweanling rats than adults (Smith and Morrell 2008; McDougall et al. 2009a). Similar to adults, the multi-trial behavioral sensitization of preweanling rats is influenced by associative processes, because sensitized responding is more persistent if drug pretreatment and testing occur in the same environmental context (Wood et al. 1998; Zavala et al. 2000). In contrast to adult rats, however, the one-trial behavioral sensitization of preweanling rats is context-independent, since the sensitized responding of young rats is equally robust if drug pretreatment occurs in the test chamber, home cage, or a separate novel compartment (McDougall et al. 2009b, 2011b; Herbert et al. 2010). In preweanling rats, the context-independent nature of one-trial behavioral sensitization has been shown using cocaine, amphetamine, and METH (Kozanian et al. 2012; McDougall et al. 2013). The neural mechanisms mediating behavioral sensitization during early ontogeny, including the role of the D1 receptor, have seldom been studied in the preweanling rat (for an exception, see Duke et al. 1997).
The purpose of the present study was to determine the importance of the D1 receptor for the one-trial behavioral sensitization of preweanling rats. SCH23390 was administered prior to a single pretreatment injection of cocaine, METH, or the direct dopamine (DA) receptor agonist R-propylnorapomorphine (NPA). One day later, rats were tested with the same DA agonist again and locomotor sensitization was determined. Although cocaine and METH are indirect agonists, both drugs were tested in the present study because they have different mechanisms of action (i.e., METH increases DA release through actions at the plasma membrane transporter and VMAT2, while cocaine is a DA and serotonin transport inhibitor). NPA has not been tested using this methodology, so a multi-dose parametric experiment was conducted to determine the early ontogeny of one-trial NPA-induced behavioral sensitization. The doses of cocaine and METH used in the present study were chosen because of their ability to produce strong one-trial behavioral sensitization in preweanling rats (McDougall et al. 2007, 2011a; Herbert et al. 2010). In all cases, lower doses of DA agonists were administered on the test day, relative to the pretreatment day, in order to preferentially induce locomotor activity rather than stereotypy (for a fuller discussion, see Robinson and Becker 1986).
Materials and methods
Subjects
Subjects were young male and female rats of Sprague-Dawley descent (Charles River, Hollister, CA) that were born and raised at California State University, San Bernardino (CSUSB). Litters were culled to 10 pups on PD 3. In all cases, 8 subjects were used per group. All rats were housed on racks in large polycarbonate maternity cages (56 × 34 × 22 cm) with wire lids. Food and water were freely available. The colony room was maintained at 22–23°C and kept under a 12:12 light/dark cycle. Except during testing, rats were kept with the dam and littermates. Testing was done in a separate experimental room, maintained at 24–25°C, and was conducted during the light phase of the cycle. Subjects were cared for according to the “Guide for the Care and Use of Laboratory Animals” (National Research Council, 2010) under a research protocol approved by the Institutional Animal Care and Use Committee of CSUSB.
Apparatus
Behavioral testing was done in activity monitoring chambers (25.5 × 25.5 × 41 cm) that consisted of acrylic walls, a plastic floor, and an open top (Coulbourn Instruments, Allentown, PA). Each chamber included an X–Y photobeam array, with 16 photocells and detectors, that was used to determine distance traveled (a measure of locomotor activity).
Drugs
NPA hydrochloride was dissolved in saline containing 0.1% metabisulfite (an antioxidant), whereas (+)-METH hydrochloride, (−)-cocaine hydrochloride, and R(+)-SCH23390 hydrochloride were dissolved in saline. Drugs were purchased from Sigma-Aldrich (St. Louis, MO) and injected intraperitoneally (IP) at a volume of 5 ml/kg.
Procedure
Experiment 1: NPA dose response
Four different age groups (N = 72 at each age) were tested: PD 12–13, PD 16–17, and PD 20– 21 (early, middle, and late preweanling periods, respectively), as well as PD 24–25 (preadolescence). At each age, rats were randomly assigned to one of nine different treatment conditions (Table 1). On the test day (i.e., PD 13, PD 17, PD 21, or PD 25), rats were given an injection of NPA (0.25, 0.5, or 1 mg/kg) prior to behavioral assessment. On the pretreatment day, which occurred 24 h earlier, rats received a single injection of saline (i.e., the acute control group) or a greater dose of NPA (0.5, 1, or 2 mg/kg) than was administered on the test day. In other words, rats pretreated with 0.5 mg/kg NPA were tested with 0.25 mg/kg NPA; rats pretreated with 1 mg/kg NPA were tested with 0.25 or 0.5 mg/kg NPA; and rats pretreated with 2 mg/kg NPA were tested with 0.25, 0.5, or 1 mg/kg NPA. On both days, rats were placed in activity chambers immediately after being injected and distance traveled was measured for either 30 min (pretreatment day) or 120 min (test day).
Table 1.
Design of Experiment 1
| Groups | Pretreatment dose | Test day dose |
|---|---|---|
| Acute control group | Saline | 0.25 mg/kg NPA |
| Sensitization group | 0.5 mg/kg NPA | 0.25 mg/kg NPA |
| Sensitization group | 1 mg/kg NPA | 0.25 mg/kg NPA |
| Sensitization group | 2 mg/kg NPA | 0.25 mg/kg NPA |
| Acute control group | Saline | 0.5 mg/kg NPA |
| Sensitization group | 1 mg/kg NPA | 0.5 mg/kg NPA |
| Sensitization group | 2 mg/kg NPA | 0.5 mg/kg NPA |
| Acute control group | Saline | 1 mg/kg NPA |
| Sensitization group | 2 mg/kg NPA | 1 mg/kg NPA |
Note: Rats were pretreated on PD 12, PD 16, PD 20, or PD 24 and tested 24 h later.
Experiment 2: Effects of D1 receptor blockade on cocaine-, METH-, and NPA-induced behavioral sensitization
Ontogenetic studies examining the preweanling period have shown that cocaine-induced one-trial behavioral sensitization is strongest when assessed around PD 21, whereas METH- and amphetamine-induced one-trial sensitization is most robust at PD 17 (Kozanian et al. 2012; McDougall et al. 2013). Cocaine and METH sensitization was either weak or not evident at the opposing ages (Kozanian et al. 2012). In the present study, therefore, cocaine-induced behavioral sensitization was assessed on PD 20–21, while METH- and NPA-induced sensitization was assessed on PD 16–17 (N = 48 for each agonist condition). On the pretreatment day, rats were injected with SCH23390 (0, 0.1, 0.5, 1, or 5 mg/kg) followed, 15 min later, by an injection of 30 mg/kg cocaine. Rats in the acute control group were given two injections of saline. After the second injection, rats were placed in activity chambers and distance traveled was measured for 30 min. On the test day, all rats were injected with 20 mg/kg cocaine and placed in activity chambers for 120 min. To examine the effects of D1 receptor antagonism on other DA-acting drugs, separate groups of rats were treated as just described, except they were pretreated and tested with METH (pretreatment day, 4 mg/kg; test day, 2 mg/kg) or NPA (pretreatment day, 2 mg/kg; test day, 0.5 mg/kg). The general methodology employed in this experiment (i.e., administering SCH23390 15 min prior to DA agonist treatment and testing rats 24 hr later) was identical to a study conducted by Fontana et al. (1993), in which it was found that SCH23390 blocked the cocaine-induced one-trial behavioral sensitization of adult rats.
Experiment 3: Effects of varying the time of SCH23390 administration
As in Experiment 2, cocaine-induced behavioral sensitization was assessed on PD 20–21, while METH- and NPA-induced sensitization were assessed on PD 16–17 (N = 40 for each agonist condition). On the pretreatment day, rats were injected with 0.5 mg/kg SCH23390 either 0, 30, or 60 min before receiving a single injection of cocaine (30 mg/kg), METH (4 mg/kg), or NPA (2 mg/kg). Rats in the acute control groups were given two injections of saline. After the second injection, rats were placed in activity chambers and distance traveled was measured for 30 min. On the test day, rats were injected with cocaine (20 mg/kg), METH (2 mg/kg), or NPA (0.5 mg/kg) and placed in activity chambers for 120 min (rats were pretreated and tested with the same compound).
Data analysis
Litter effects were controlled through both experimental design and statistical procedures. In most experiments no more than one subject per litter was assigned to a particular group. In cases where this procedure was not possible (e.g., analysis of the pretreatment day), a single litter mean was calculated from multiple littermates assigned to the same group (Holson and Pearce 1992; Zorrilla 1997). When possible, litter was used as the unit of analysis for statistical purposes (Zorrilla 1997). With this statistical model each litter, rather than each rat, is treated as an independent observation (i.e., a within analysis using one value/condition/litter). Between-subjects statistical procedures were used when experiments included more than ten groups (i.e., when individual litters did not contain enough subjects to provide one subject per group). Unlike adults, prepubescent rats do not typically exhibit sex differences after treatment with DA agonists (Frantz et al. 1996; Bowman et al. 1997; Snyder et al. 1998; McDougall et al. 2013). Consistent with these past studies, preliminary analyses indicated that distance traveled data did not differ according to sex, so this variable was not included in the final statistical analyses.
Depending on experiment, data from the pretreatment day were analyzed using two- or three-way repeated measures (5-min time blocks) analyses of variance (ANOVAs). When the assumption of sphericity was violated, as determined by Mauchly’s test of sphericity, the Huynh-Feldt epsilon statistic was used to adjust degrees of freedom (Huynh and Feldt 1976). Test day data were collapsed across the time variable and analyzed using one- or two-way ANOVAs. Each experiment included distinct subsets of treatment groups (e.g., the cocaine-, METH-, and NPA-treated rats of Experiments 2 and 3), so these data were analyzed separately. Post hoc analysis of distance traveled data was done using Tukey tests. When further analyzing statistically significant higher order interactions, the mean square error terms (i.e., MSerror) used for the Tukey calculations were based on separate one-way ANOVAs at each time block.
Results
Experiment 1: NPA dose response
Pretreatment day
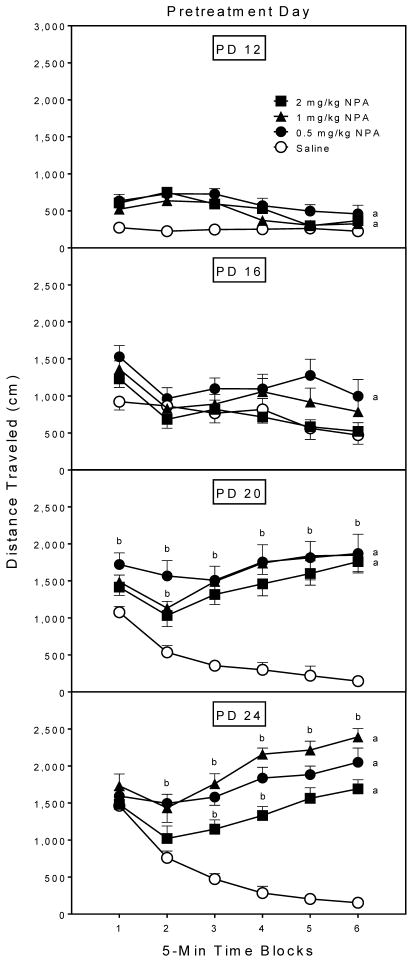
An omnibus Age × Dose × Time block ANOVA indicated that the age variable interacted with NPA dose to affect distance traveled on the pretreatment day (Fig. 1) [Age × Dose interaction, F9,112 =6.81, P<0.001; Age × Dose × Time block interaction, F36,449 =6.56, P<0.001]. Overall, rats tested on PD 16 exhibited more locomotor activity than rats tested on PD 12, with the PD 20 and PD 24 rats having greater distance traveled scores than either of the younger age groups [Age main effect, F3,112 =63.76, P<0.001; and Tukey tests, P<0.05]. To further assess the higher order interactions, separate Dose × Time block ANOVAs were used to analyze distance traveled at each age. On PD 12, all doses of NPA (0.5, 1, or 2 mg/kg) increased distance traveled scores (upper graph, Fig. 1) [Dose main effect, F3,21 =13.10, P<0.001; and Tukey tests, P<0.05]. On PD 16, however, only the lower dose of NPA (0.5 mg/kg) significantly enhanced locomotor activity relative to saline-treated rats [Dose main effect, F3,21 =3.67, P<0.05; and Tukey tests, P<0.05]. All three doses of NPA increased distance traveled scores on PD 20 [Dose main effect, F3,21 =18.63, P<0.001; and Tukey tests, P<0.05], with significant differences between the 2 mg/kg NPA group and saline controls occurring on time blocks 3–6 (subjects receiving 0.5 or 1 mg/kg NPA differed from saline controls on all six time blocks) [Dose × Time block interaction, F14,99 =9.17, P<0.001; and Tukey tests, P<0.05]. On PD 24, 0.5 and 1 mg/kg NPA induced the most distance traveled, with 2 mg/kg producing an intermediate level of locomotion that was significantly different from both the saline and 1 mg/kg NPA groups [Dose main effect, F3,21 =42.06, P<0.001; and Tukey tests, P<0.05]. Rats receiving 0.5 or 1 mg/kg NPA had greater distance traveled scores than saline controls on time blocks 2–6, while the 2 mg/kg NPA group differed from the saline group on time blocks 3–6 (lower graph, Fig. 1) [Dose × Time block interaction, F12,84 =22.70, P<0.001; and Tukey tests, P<0.05].
Fig. 1.
Mean (±SEM) distance traveled scores of rats (n = 8 per group) on the pretreatment day. Rats were injected with saline or NPA immediately before a 30-min placement in the activity chambers on PD 12, PD 16, PD 20, or PD 24.
a Significantly different from the saline control group when collapsed across time blocks 1–6.
b Significantly different from the saline control group on the same time block.
Test day
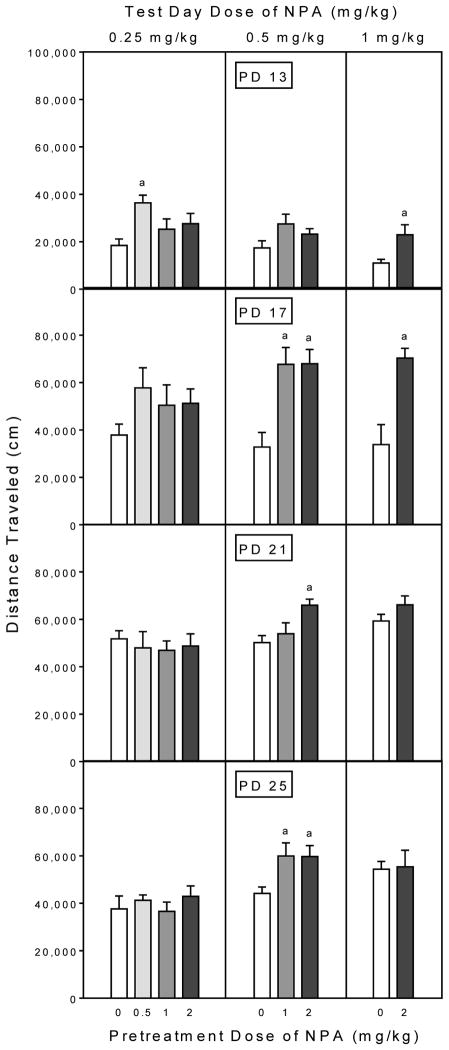
Sensitized responding to NPA was evident in all four age groups, but the pattern of effects often differed according to dose (Fig. 2). On PD 13, rats tested with 0.25 mg/kg NPA and pretreated with 0.5 mg/kg NPA exhibited greater distance traveled scores than rats given an acute injection of 0.25 mg/kg NPA on the test day (i.e., the acute control group; upper graph, Fig. 2) [Group main effect, F3,21 =5.35, P<0.01; and Tukey tests, P<0.05]. PD 13 rats pretreated and tested with the highest doses of NPA (2 mg/kg and 1 mg/kg, respectively) also exhibited a sensitized locomotor response [Group effect, t7 =3.42, P<0.01]. On PD 17, rats challenged with 0.5 mg/kg NPA [Group main effect, F2,14 =16.58, P<0.001; and Tukey tests, P<0.05] or 1 mg/kg NPA [Group effect, t7 =5.12, P<0.001] and pretreated with 1 or 2 mg/kg NPA had greater distance traveled scores than the acute control group.
Fig. 2.
Mean (±SEM) distance traveled scores of rats (n = 8 per group) on the test day. Rats were challenged with NPA (0.25, 0.5, or 1 mg/kg) immediately before behavioral testing on PD 13, PD 17, PD 21, or PD 25. On the pretreatment day (i.e., 24 h earlier), rats had received a single injection of saline or NPA (0.5, 1, or 2 mg/kg).
a Significantly different from rats given 0 mg/kg NPA on the pretreatment day.
Sensitized responding was more difficult to detect on PD 21, since only rats challenged with 0.5 mg/kg NPA and pretreated with 2 mg/kg NPA had significantly greater distance traveled scores than the appropriate acute control group [Group main effect, F3,14 =4.73, P<0.05; and Tukey tests, P<0.05]. A similar pattern of effects occurred on PD 25, because only rats tested with 0.5 mg/kg NPA exhibited a sensitized locomotor response (lower graph, Fig. 2) [Group main effect, F2,14 =7.86, P<0.01; and Tukey tests, P<0.05].
Experiment 2: Effects of D1 receptor blockade
Pretreatment day
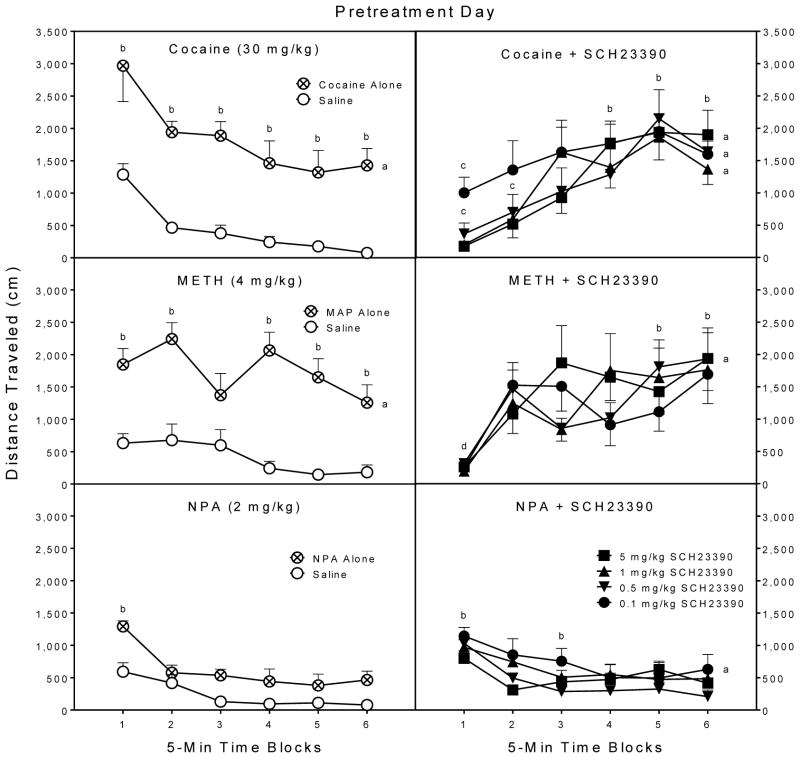
When collapsed across the pretreatment session, rats treated with cocaine alone or cocaine plus SCH23390 (0.1, 0.5, 1, or 5 mg/kg) had greater distance traveled scores than saline controls (upper graphs, Fig. 3) [Group main effect, F5,42 =8.29, P<0.001; and Tukey tests, P<0.05]. Overall, rats injected with cocaine plus SCH23390 did not differ from rats given cocaine alone. These effects varied across the testing session, because rats treated with cocaine plus SCH23390 had smaller distance traveled scores than the cocaine alone group on time block 1 [Group × Time block interaction, F22,188 =5.39, P<0.001; and Tukey tests, P<0.05]. On time block 2, the three higher doses of SCH23390 (0.5, 1, or 5 mg/kg) decreased locomotor activity relative to the cocaine alone group (Tukey tests, P<0.05). On subsequent time blocks, SCH23390 did not reduce the distance traveled scores of cocaine-treated rats. Consistent with this finding, rats treated with cocaine plus SCH23390 had greater distance traveled scores than saline controls on time blocks 4–6 (Tukey tests, P<0.05).
Fig. 3.
Mean distance traveled scores (±SEM) of rats (n = 8 per group) on the pretreatment day. Rats were injected with saline, cocaine, METH, or NPA immediately before a 30-min placement in the activity chambers (left panels). Additional groups of rats were injected with SCH23390 (0.1, 0.5, 1, or 5 mg/kg) 15 min before DA agonist pretreatment (right panels).
a Significantly different from the saline control group (left panels) when collapsed across time blocks 1–6.
b Significantly different from the saline control group (left panels) on the same time block.
c Significantly different from the cocaine alone group (top left panel) on the same time block.
d Significantly different from the METH alone group (middle left panel) on the same time block.
A somewhat similar pattern of effects was evident after METH treatment (middle graphs, Fig. 3), since rats given METH alone or METH plus 5 mg/kg SCH23390 exhibited more locomotion on the pretreatment day than saline controls [Group main effect, F5,42 =4.66, P<0.01; and Tukey tests, P<0.05]. Differences between the METH alone and saline groups were apparent on all time blocks except for time block 3, while rats given METH plus SCH23390 had greater distance traveled scores than saline controls on time blocks 5 and 6 [Group × Time block interaction, F21,181 =3.21, P<0.001; and Tukey tests, P<0.05]. Relative to the METH alone group, SCH23390 (0.1, 0.5, 1, or 5 mg/kg) decreased distance traveled scores on only time block 1 (Tukey tests, P<0.05).
When collapsed across the pretreatment session, rats treated with NPA plus 0.1 mg/kg SCH23390 had greater distance traveled scores than saline controls (lower graphs, Fig. 3) [Group main effect, F5,42 =2.72, P<0.05; and Tukey tests, P<0.05]. Analysis of the individual time blocks showed that the NPA alone group exhibited more locomotion than the saline controls on time block 1, while rats given NPA plus 0.1 mg/kg SCH23390 had greater distance traveled scores than the saline controls on time blocks 1 and 3 [Group × Time block interaction, F24,204 =1.71, P<0.05; and Tukey tests, P<0.05]. At no point did SCH23390 reduce distance traveled scores relative to the NPA alone group.
Test day
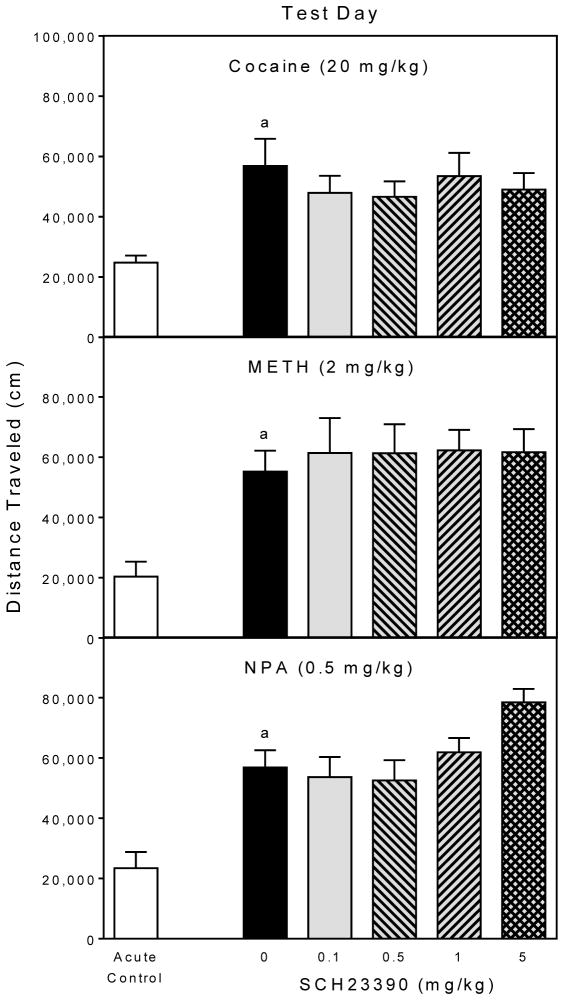
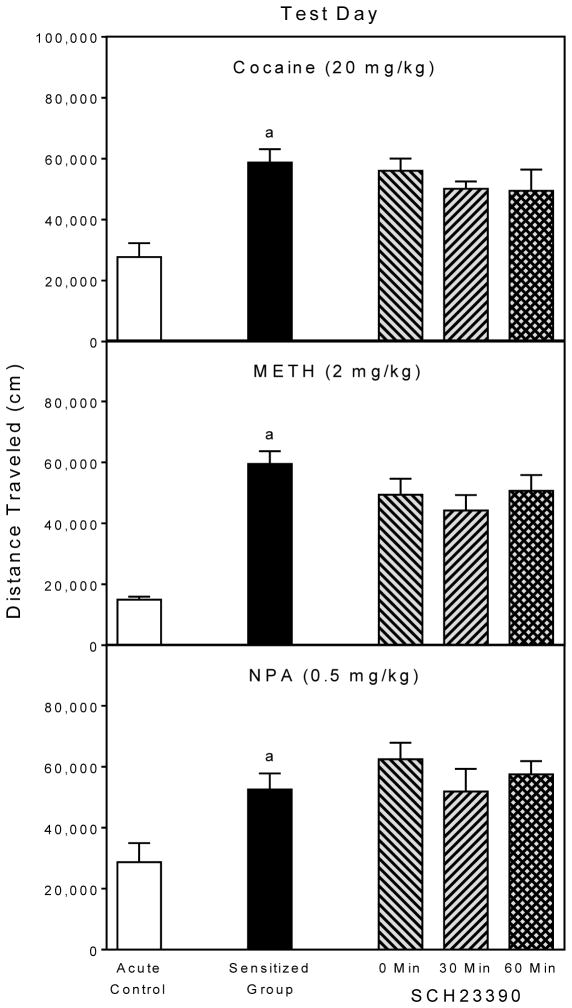
Cocaine produced behavioral sensitization in nonSCH23390-treated animals (upper graph, Fig. 4), because rats pretreated and tested with cocaine (black bars) had greater distance traveled scores than rats given their first injection of cocaine on the test day (open bars) [Group main effect, F5,42 =3.27, P<0.05; and Tukey tests, P<0.05]. Importantly, a pretreatment day injection of SCH23390 (0.1, 0.5, 1, or 5 mg/kg) did not attenuate distance traveled scores on the test day when compared to cocaine-pretreated rats given 0 mg/kg SCH23390.
Fig. 4.
Mean distance traveled scores (±SEM) of rats (n = 8 per group) on the test day. Rats were challenged with cocaine, METH, or NPA immediately before behavioral testing. On the pretreatment day (i.e., 24 h earlier), rats were injected with SCH23390 (0, 0.1, 0.5, 1, or 5 mg/kg) 15 min before DA agonist pretreatment.
a Significantly different from the acute control group.
A similar pattern of results was apparent in the METH groups (middle graph, Fig. 4), as rats pretreated and tested with METH had greater distance traveled scores than the acute controls [Group main effect, F5,42 =4.07, P<0.01; and Tukey tests, P<0.05], with SCH23390 not influencing test day locomotor activity. Repeated treatment with NPA also produced a sensitized locomotor response (lower graph, Fig. 4) [Group main effect, F5,42 =9.92, P<0.001; and Tukey tests, P<0.05], which SCH23390 pretreatment did not significantly modify. Rats pretreated with NPA plus 5 mg/kg SCH23390 exhibited somewhat greater distance traveled scores than rats pretreated with NPA plus 0 mg/kg SCH23390, but this effect was not statistically significant (P=0.10).
Experiment 3: Effects of varying the time of SCH23390 administration
Performance on the pretreatment day was similar to Experiment 2 (data not shown). On the test day, a single pretreatment injection of cocaine, METH, or NPA was sufficient to produce behavioral sensitization, because preweanling rats given a pretreatment and test day injection of these compounds had greater distance traveled scores than their acute control groups (Fig. 5) [Group main effects, F4,28 =7.21, P<0.001; F4,28 =15.65, P<0.001; F4,28 =5.60, P<0.01, respectively; and Tukey tests, P<0.05]. SCH23390 did not attenuate sensitized responding, regardless of whether the D1 antagonist was administered 0, 30, or 60 min prior to DA agonist treatment.
Fig. 5.
Mean distance traveled scores (±SEM) of rats (n = 8 per group) on the test day. Rats were challenged with cocaine, METH, or NPA immediately before behavioral testing. On the pretreatment day (i.e., 24 h earlier), rats were injected with saline or SCH23390 (0.5 mg/kg) 0, 30, or 60 min before DA agonist pretreatment.
a Significantly different from the acute control group.
Discussion
In a series of studies, we previously reported that young rats show strong one-trial behavioral sensitization, but the age at which sensitized responding was exhibited depended on the psychostimulant being administered (McDougall et al. 2011a, 2013; Kozanian et al. 2012). For example, amphetamine and METH caused the most robust sensitized responding at PD 13 and PD 17, while cocaine only produced behavioral sensitization in older animals (i.e., at PD 21). In the present study, the direct DA agonist NPA also produced one-trial behavioral sensitization in young rats (see Fig. 2), but age did not serve as a constraining influence. More specifically, NPA-induced sensitized responding was apparent at all age tested (i.e., PD 13, PD 17, PD 21, and PD 25), with the occurrence of behavioral sensitization varying according to drug dose. In the three older age groups, a pretreatment injection of 2 mg/kg NPA and a test day injection of 0.5 mg/kg NPA produced the strongest behavioral sensitization; whereas, PD 13 rats expressed a sensitized locomotor response after a test day injection of 0.25 or 1 mg/kg NPA. It is curious that NPA supported one-trial behavioral sensitization at all ages tested, while the sensitized responding of METH- and cocaine-treated rats was restricted to specific ages. Whether these differences are a consequence of each drug’s mode of action (i.e., direct vs. indirect agonism) or some other combination of factors (e.g., drug pharmacokinetics, neurotransmitter systems affected, doses used, etc.) is uncertain.
Many researchers, including ourselves, have shown that SCH23390 blocks the induction of amphetamine- and METH-induced behavioral sensitization in adult rats and mice (Vezina and Stewart 1989; Kuribara 1995b; Karper et al. 2002; Kelly et al. 2008). In contrast, an extremely broad dose range of SCH23390 (0.1–5 mg/kg) did not attenuate the METH-induced sensitized responding of preweanling rats. At 5 mg/kg, SCH23390 has nonspecific actions, but this dose was included to highlight that D1 receptor antagonism does not eliminate or even significantly reduce the strength of the sensitized response in preweanling rats. Direct DA agonists produce a similar pattern of effects as METH, since SCH23390 blocks the induction of apomorphine-induced sensitization in adult rats (Mattingly et al. 1991; Dias et al. 2010); whereas, preweanling rats showed an undiminished sensitized response on the test day, despite the fact that one day earlier SCH23390 was injected 0–60 min before NPA pretreatment. The inability of a D1 receptor antagonist to block behavioral sensitization during the preweanling period does not extend to other reward-related behaviors, because SCH23390 attenuates the appetitive approach responding (McDougall et al. 1991) and sucrose ingestion (Tyrka and Smith 1991) of preweanling rats. Moreover, administering SCH23390 either systemically or directly into the prefrontal cortex blocks the cocaine-induced place preference conditioning of preweanling and adolescent rats, respectively (Pruitt et al. 1995; Brenhouse et al. 2008). Together, these results suggest that the role played by the D1 receptor in behavioral sensitization differs markedly from other reward-related behaviors.
In adult rodents, the impact of D1 receptor blockade on cocaine-induced behavioral sensitization is more complex than for other DA-acting drugs, because SCH23390 blocks one-trial, but not multi-trial, cocaine sensitization (Fontana et al. 1993; Kuribara 1995a; Steketee 1998; Valijent et al. 2010). It is uncertain why D1 receptor antagonism is only able to disrupt the one-trial cocaine sensitization of adult rodents, although the context-dependent nature of one-trial sensitization may be critical. Because drug-environment associations are unimportant for the one-trial behavioral sensitization of preweanling rats (McDougall et al. 2009b, 2011b; Herbert et al. 2010), we originally hypothesized that SCH23390 would not attenuate cocaine-induced sensitized responding on PD 21. This result was obtained. Even so, the inability of SCH23390 to block the sensitizing effects of all three DA agonists is noteworthy, especially considering the disparate actions of SCH23390 in adult rats. From this perspective, it is the inconsistent effects of SCH23390 in adult rats (vis-à-vis cocaine and amphetamine sensitization) that are puzzling. In contrast, the uniform pattern of SCH23390’s actions in younger animals supports the conclusion that D1 receptor stimulation is not necessary for the induction of one-trial behavioral sensitization in preweanling rats, regardless of the DA agonist being administered or its mechanism of action (e.g., DA releaser, transport blocker, or direct receptor agonist).
Another interesting finding is that SCH23390 (0.1–5 mg/kg) was unable to fully attenuate the cocaine-, METH-, and NPA-induced locomotor activity of preweanling rats on the pretreatment day. This does not imply that D1 receptor antagonism was without behavioral impact, because SCH23390 reduced the locomotor activity of cocaine- and METH-treated rats at the beginning of the 30-min pretreatment session. By the end of the session, however, distance traveled scores of preweanling rats treated with cocaine plus SCH23390 (or METH plus SCH23390) did not differ from rats given the same indirect agonist alone. These results vary from data gained using adult rodents, because comparatively low doses of SCH23390 (0.03–0.5 mg/kg) fully attenuate the acute locomotor activating effects of cocaine and METH on the pretreatment day (Ujike et al. 1989; Kuribara and Uchihashi 1994; Kuribara 1995b; White et al. 1998).
The pattern of results just discussed may imply that there is a relationship between the occurrence of psychostimulant-induced locomotion on the pretreatment day and the expression of behavioral sensitization on the test day. This relationship does not appear to be causative, however, because preweanling and adult rats anesthetized during the pretreatment phase (i.e., rats are incapable of locomotion) still exhibit behavioral sensitization on the test day (Wang and Hsiao 2003; Herbert et al. 2010). Furthermore, adult mice injected with SCH23390 up to 5 h after METH pretreatment do not exhibit behavioral sensitization on the test day (Kuribara 1995c). Thus, SCH23390 can block the induction of METH-induced behavioral sensitization even when animals are able to express a full locomotor response on the pretreatment day. When considered together, these results suggest that the inability of SCH23390 to block the sensitized responding of preweanling rats is not attributable to an incomplete inhibition of locomotor activity on the pretreatment day. A more parsimonious conclusion is that these SCH23390-induced effects are dissociable: (a) D1 receptor blockade reduces, but does not fully attenuate the acute locomotor activating properties of cocaine and METH in young rats; and (b) D1 receptor stimulation is unnecessary for the induction of cocaine-, METH-, or NPA-induced one-trial behavioral sensitization during the preweanling period.
Both White et al. (1998) and Valjent et al. (2010) have suggested that the mechanisms underlying one-trial and multi-trial behavioral sensitization differ. Accumulating evidence also indicates that the mechanisms mediating one-trial behavioral sensitization vary markedly between the preweanling period and adulthood. For example, there is abundant evidence that contextual conditioning does not influence the one-trial behavioral sensitization of preweanling rats (e.g., McDougall et al. 2009b), whereas drug-environment associations are necessary for the occurrence of one-trial behavioral sensitization in older animals (e.g., Weiss et al. 1989). In adult rats, the D1 antagonist SCH23390 blocks the induction of one-trial cocaine-induced behavioral sensitization, as well as METH- and apomorphine-induced sensitized responding (Mattingly et al. 1991; Kuribara 1995b; Valjent et al. 2010). In preweanling rats, on the other hand, SCH23390 does not block or significantly reduce cocaine-, METH-, or NPA-induced behavioral sensitization. Lastly, the persistence of one-trial behavioral sensitization differs dramatically according to age. During adulthood, a sensitized response can be detected for months after a single pretreatment injection of cocaine or amphetamine (Robinson et al. 1982; Valjent et al. 2010), whereas preweanling rats only exhibit one-trial behavioral sensitization for a few days after initial drug exposure (McDougall et al. 2009a). Together, these results suggest that the behavioral manifestations and underlying mechanisms mediating one-trial behavioral sensitization differ across ontogeny.
In summary, both indirect (cocaine and METH) and direct (NPA) DA agonists produce robust one-trial behavioral sensitization in preweanling rats. Regardless of the agonist being tested, D1 receptor stimulation is unnecessary for the induction of behavioral sensitization during the preweanling period. These results are contrary to what is observed in adult rats and suggest that the mechanisms mediating one-trial behavioral sensitization differ markedly across ontogeny.
Acknowledgments
Funding sources:
This research was supported by NIDA research grant DA027985 (SAM), NIGMS training grant GM083883 (AEG), and NIDA research training grants DA025319 (AMY) and DA033877 (AMY).
Footnotes
Conflict of Interest
All authors declare no conflict of interest.
References
- Battisti JJ, Chang CH, Uretsky NJ, Wallace LJ. Sensitization of stereotyped behavior to amphetamine is context and response dependent. Pharmacol Biochem Behav. 1999;63:263–269. doi: 10.1016/s0091-3057(98)00259-7. [DOI] [PubMed] [Google Scholar]
- Battisti JJ, Uretsky NJ, Wallace LJ. Importance of environmental context in the development of amphetamine- or apomorphine-induced stereotyped behavior after single and multiple doses. Pharmacol Biochem Behav. 2000;66:671–677. doi: 10.1016/s0091-3057(00)00214-8. [DOI] [PubMed] [Google Scholar]
- Bowman BP, Blatt B, Kuhn CM. Ontogeny of the behavioral response to dopamine agonists after chronic cocaine. Psychopharmacology (Berl) 1997;129:121–127. doi: 10.1007/s002130050171. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J Neurosci. 2008;28:2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias FR, Carey RJ, Carrera MP. Apomorphine-induced context-specific behavioural sensitization is prevented by the D1 antagonist SCH-23390 but potentiated and uncoupled from contextual cues by the D2 antagonist sulpiride. Psychopharmacology (Berl) 2010;209:137–151. doi: 10.1007/s00213-009-1768-0. [DOI] [PubMed] [Google Scholar]
- Drew KL, Glick SD. Environment-dependent sensitization to amphetamine-induced circling behavior. Pharmacol Biochem Behav. 1989;31:705–708. doi: 10.1016/0091-3057(88)90251-1. [DOI] [PubMed] [Google Scholar]
- Duke MA, O’Neal J, McDougall SA. Ontogeny of dopamine agonist-induced sensitization: role of NMDA receptors. Psychopharmacology (Berl) 1997;129:153–160. doi: 10.1007/s002130050175. [DOI] [PubMed] [Google Scholar]
- Faria RR, Rueda AVL, Sayuri C, Soares SL, Malta MB, Carrara-Nascimento PF, da Silva Alves A, Marcourakis T, Yonamine M, Scavone C, Britto LRG, Camarini R. Environmental modulation of ethanol-induced locomotor activity: Correlation with neuronal activity in distinct brain regions of adolescent and adult Swiss mice. Brain Res. 2008;1239:127–140. doi: 10.1016/j.brainres.2008.08.056. [DOI] [PubMed] [Google Scholar]
- Fontana D, Post RM, Weiss SRB, Pert A. The role of D1 and D2 dopamine receptors in the acquisition and expression of cocaine-induced conditioned increases in locomotor activity. Behav Pharmacol. 1993;4:375–387. [PubMed] [Google Scholar]
- Frantz K, Babcock D, Van Hartesveldt C. The locomotor effects of a putative dopamine D3 receptor agonist in developing rats. Eur J Pharmacol. 1996;302:1–6. doi: 10.1016/0014-2999(96)00014-3. [DOI] [PubMed] [Google Scholar]
- Herbert MS, Der-Ghazarian T, Palmer AG, McDougall SA. One-trial cocaine-induced behavioral sensitization in preweanling rats: role of contextual stimuli. Exp Clin Psychopharm. 2010;18:284–295. doi: 10.1037/a0019142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Huynh H, Feldt LS. Estimation of the Box correction for degrees of freedom from sample data in randomized block and split-plot designs. J Educ Stat. 1976;1:69–82. [Google Scholar]
- Jackson HC, Nutt DJ. A single preexposure produces sensitization to the locomotor effects of cocaine in mice. Pharmacol Biochem Behav. 1993;45:733–735. doi: 10.1016/0091-3057(93)90533-y. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kameda SR, Fukushiro DF, Trombin TF, Procópio-Souza R, Patti CL, Hollais AW, Calzavara MB, Abílio VC, Ribeiro RA, Tufik S, D’Almeida V, Frussa-Filho R. Adolescent mice are more vulnerable than adults to single injection-induced behavioral sensitization to amphetamine. Pharmacol Biochem Behav. 2011;98:320–324. doi: 10.1016/j.pbb.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Karper PE, De la Rosa H, Newman ER, Krall CM, Nazarian A, McDougall SA, Crawford CA. Role of D1-like receptors in amphetamine-induced behavioral sensitization: a study using D1A receptor knockout mice. Psychopharmacology (Berl) 2002;159:407–414. doi: 10.1007/s00213-001-0936-7. [DOI] [PubMed] [Google Scholar]
- Kelly MA, Low MJ, Rubinstein M, Phillips TJ. Role of dopamine D1-like receptors in methamphetamine locomotor responses of D2 receptor knockout mice. Genes Brain Behav. 2008;7:568–577. doi: 10.1111/j.1601-183X.2008.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozanian OO, Gutierrez A, Mohd-Yusof A, McDougall SA. Ontogeny of methamphetamine- and cocaine-induced one-trial behavioral sensitization in preweanling and adolescent rats. Behav Pharmacol. 2012;23:367–379. doi: 10.1097/FBP.0b013e32835651c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuribara H. Modification of cocaine sensitization by dopamine D1 and D2 receptor antagonists in terms of ambulation in mice. Pharmacol Biochem Behav. 1995a;51:799–805. doi: 10.1016/0091-3057(95)00037-w. [DOI] [PubMed] [Google Scholar]
- Kuribara H. Dopamine D1 receptor antagonist SCH 23390 retards methamphetamine sensitization in both combined administration and early posttreatment schedules in mice. Pharmacol Biochem Behav. 1995b;52:759–763. doi: 10.1016/0091-3057(95)00173-t. [DOI] [PubMed] [Google Scholar]
- Kuribara H. Inhibition of methamphetamine sensitization by post-methamphetamine treatment with SCH 23390 or haloperidol. Psychopharmacology (Berl) 1995c;119:34–38. doi: 10.1007/BF02246051. [DOI] [PubMed] [Google Scholar]
- Kuribara H, Uchihashi Y. Dopamine antagonists can inhibit methamphetamine sensitization, but not cocaine sensitization, when assessed by ambulatory activity in mice. J Pharm Pharmacol. 1993;45:1042–1045. doi: 10.1111/j.2042-7158.1993.tb07177.x. [DOI] [PubMed] [Google Scholar]
- Kuribara H, Uchihashi Y. Effects of dopamine antagonism on methamphetamine sensitization: evaluation by ambulatory activity in mice. Pharmacol Biochem Behav. 1994;47:101–106. doi: 10.1016/0091-3057(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Leith NJ, Kuczenski R. Two dissociable components of behavioral sensitization following repeated amphetamine administration. Psychopharmacology (Berl) 1982;76:310–315. doi: 10.1007/BF00449116. [DOI] [PubMed] [Google Scholar]
- Li Y, Wolf ME. Ibotenic acid lesions of prefrontal cortex do not prevent expression of behavioral sensitization to amphetamine. Behav Brain Res. 1997;84:285–289. doi: 10.1016/s0166-4328(96)00158-1. [DOI] [PubMed] [Google Scholar]
- Mattingly BA, Rowlett JK, Graff JT, Hatton BJ. Effects of selective D1 and D2 dopamine antagonists on the development of behavioral sensitization to apomorphine. Psychopharmacology (Berl) 1991;105:501–507. doi: 10.1007/BF02244370. [DOI] [PubMed] [Google Scholar]
- Mattingly BA, Hart TC, Lim K, Perkins C. Selective antagonism of dopamine D1 and D2 receptors does not block the development of behavioral sensitization to cocaine. Psychopharmacology (Berl) 1994;114:239–242. doi: 10.1007/BF02244843. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Nonneman AJ, Crawford CA. Effects of SCH 23390 and sulpiride on the reinforced responding of the young rat. Behav Neurosci. 1991;105:744–754. doi: 10.1037//0735-7044.105.5.744. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Baella SA, Stuebner NM, Halladay LM, Crawford CA. Cocaine-induced behavioral sensitization in preweanling and adult rats: effects of a single drug-environment pairing. Psychopharmacology (Berl) 2007;193:323–332. doi: 10.1007/s00213-007-0788-x. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Charntikov S, Cortez AM, Amodeo DA, Martinez CE, Crawford CA. Persistence of one-trial cocaine-induced behavioral sensitization in young rats: regional differences in Fos immunoreactivity. Psychopharmacology (Berl) 2009a;203:617–628. doi: 10.1007/s00213-008-1407-1. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Cortez AM, Palmer AG, Herbert MS, Martinez CE, Charntikov S, Amodeo DA. Importance of environmental context for one- and three-trial cocaine-induced behavioral sensitization. Psychopharmacology (Berl) 2009b;206:377–388. doi: 10.1007/s00213-009-1616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall SA, Kozanian OO, Greenfield VY, Horn LR, Gutierrez A, Mohd-Yusof A, Castellanos KA. One-trial behavioral sensitization in preweanling rats: differential effects of cocaine, methamphetamine, methylphenidate, and D-amphetamine. Psychopharmacology (Berl) 2011a;217:559–571. doi: 10.1007/s00213-011-2316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall SA, Nuqui CM, Quiroz AT, Martinez CM. Early ontogeny of D-amphetamine-induced one-trial behavioral sensitization. Pharmacol Biochem Behav. 2013;104:154–162. doi: 10.1016/j.pbb.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall SA, Pothier AG, Der-Ghazarian T, Herbert MS, Kozanian OO, Castellanos KA, Flores AT. Importance of associative learning processes for the one-trial behavioral sensitization of preweanling rats. Behav Pharmacol. 2011b;22:693–702. doi: 10.1097/FBP.0b013e32834affb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. 8. Washington, DC: National Academies Press; 2010. [Google Scholar]
- Paulson PE, Camp DM, Robinson TE. Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology (Berl) 1991;103:480–492. doi: 10.1007/BF02244248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Reeder DC, Hicks J, Morgan ZR, Kalivas PW. Ibotenic acid lesions of the dorsal prefrontal cortex disrupt the expression of behavioral sensitization to cocaine. Neuroscience. 1998;82:1103–1114. doi: 10.1016/s0306-4522(97)00366-7. [DOI] [PubMed] [Google Scholar]
- Pruitt D, Bolanos CA, McDougall SA. Effects of dopamine D1 and D2 receptor antagonists on cocaine-induced place preference conditioning in preweanling rats. Eur J Pharmacol. 1995;283:125–131. doi: 10.1016/0014-2999(95)00309-9. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res Rev. 1986;11:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Becker JB, Presty SK. Long-term facilitation of amphetamine-induced rotational behavior and striatal dopamine release produced by a single exposure to amphetamine: sex differences. Brain Res. 1982;571:330–337. doi: 10.1016/0006-8993(82)90690-4. [DOI] [PubMed] [Google Scholar]
- Smith KS, Morrell JI. Behavioral responses during the initial exposures to a low dose of cocaine in late preweanling and adult rats. Neurotoxicol Teratol. 2008;30:202–212. doi: 10.1016/j.ntt.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder KJ, Katovic NM, Spear LP. Longevity of the expression of behavioral sensitization to cocaine in preweanling rats. Pharmacol Biochem Behav. 1998;60:909–914. doi: 10.1016/s0091-3057(98)00078-1. [DOI] [PubMed] [Google Scholar]
- Steketee JD. Injection of SCH 23390 into the ventral tegmental area blocks the development of neurochemical but not behavioral sensitization to cocaine. Behav Pharmacol. 1998;9:69–76. [PubMed] [Google Scholar]
- Tirelli E, Laviola G, Adriani W. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neurosci Biobehav Rev. 2003;27:163–178. doi: 10.1016/s0149-7634(03)00018-6. [DOI] [PubMed] [Google Scholar]
- Tyrka A, Smith GP. Potency of SCH 23390 for decreasing sucrose intake in rat pups depends on mode of ingestion. Pharmacol Biochem Behav. 1991;39:955–961. doi: 10.1016/0091-3057(91)90059-b. [DOI] [PubMed] [Google Scholar]
- Ujike H, Onoue T, Akiyama K, Hamamura T, Otsuki S. Effects of selective D-1 and D-2 dopamine antagonists on development of methamphetamine-induced behavioral sensitization. Psychopharmacology (Berl) 1989;98:89–92. doi: 10.1007/BF00442011. [DOI] [PubMed] [Google Scholar]
- Valjent E, Bertran-Gonzalez J, Aubier B, Greengard P, Hervé D, Girault JA. Mechanisms of locomotor sensitization to drugs of abuse in a two-injection protocol. Neuropsychopharmacology. 2010;35:401–415. doi: 10.1038/npp.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vezina P, Stewart J. The effect of dopamine receptor blockade on the development of sensitization to the locomotor activating effects of amphetamine and morphine. Brain Res. 1989;499:108–120. doi: 10.1016/0006-8993(89)91140-2. [DOI] [PubMed] [Google Scholar]
- Vezina P, McGehee DS, Green WN. Exposure to nicotine and sensitization of nicotine-induced behaviors. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1625–1638. doi: 10.1016/j.pnpbp.2007.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Hsiao S. Amphetamine sensitization: nonassociative and associative components. Behav Neurosci. 2003;117:961–969. doi: 10.1037/0735-7044.117.5.961. [DOI] [PubMed] [Google Scholar]
- Weiss SRB, Post RM, Pert A, Woodward R, Murman D. Context-dependent cocaine sensitization: differential effect of haloperidol on development versus expression. Pharmacol Biochem Behav. 1989;34:655–661. [PubMed] [Google Scholar]
- White FJ, Joshi A, Koeltzow TE, Hu X-T. Dopamine receptor antagonists fail to prevent induction of cocaine sensitization. Neuropsychopharmacology. 1998;18:26–40. doi: 10.1016/S0893-133X(97)00093-6. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev. 2010;35:185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RD, Tirelli E, Snyder KJ, Heyser CJ, LaRocca TM, Spear LP. Evidence for behavioral sensitization to cocaine in preweanling rat pups. Psychopharmacology (Berl) 1998;138:114–123. doi: 10.1007/s002130050653. [DOI] [PubMed] [Google Scholar]
- Zavala AR, Nazarian A, Crawford CA, McDougall SA. Cocaine-induced behavioral sensitization in the young rat. Psychopharmacology (Berl) 2000;151:291–298. doi: 10.1007/s002130000377. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol. 1997;30:141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]