Abstract
Several studies have shown comparable survival outcomes among different graft sources, but the relative resource needs of hematopoietic cell transplantation (HCT) by graft source have not been well studied. We compared total hospital length of stay in the first 100 days after HCT in 1577 patients with acute leukemia in remission receiving umbilical cord blood (UCB), matched unrelated donor (MUD) or mismatched unrelated donors (MMUD) HCT from 2008–2011. To ensure a relatively homogenous study population, the analysis was limited to patients with acute myeloid leukemia and acute lymphoblastic leukemia in first or second complete remission who received HCT in the United States. To account for early deaths, we compared the number of days alive and out of hospital in the first 100 days. For children receiving myeloablative conditioning, median days alive and out of hospital in the first 100 days were 50, 54 and 60 days for single UCB, double UCB and MUD bone marrow (BM) recipients, respectively. In multivariate analysis, use of UCB was significantly associated with fewer days alive and out of the hospital compared to MUD BM. For adults receiving HCT using myeloablative conditioning, median days alive and out of hospital in first 100 days were 52 for single UCB, 55 for double UCB, 69 for MUD BM, 75 for MUD peripheral blood stem cells (PBSC), 63 for MMUD BM and 67 days MMUD PBSC recipients. In multivariate analysis, UCB and MMUD BM recipients had fewer days alive and out of the hospital compared to other graft sources. For adults receiving a reduced intensity preparative regimen, median days alive and out of hospital during the first 100 days for single UCB, double UCB, MUD PBSC and MMUD PBSC were 65, 63, 79, and 79, respectively. Similar to the other two groups, use of UCB was associated with a fewer days alive and out of the hospital. In conclusion, length of stay in the first 100 days varies by graft source and is greater for UCB HCT recipients. These data provide insight into the resource needs of transplant patients receiving these graft sources.
Keywords: Hematopoietic cell transplantation, Umbilical cord blood, Leukemia, Length of stay, Resource utilization
INTRODUCTION
Use of alternative donors such as unrelated umbilical cord blood (UCB), haploidentical family members, and mismatched unrelated donors (MMUD) allows patients without HLA-matched sibling or matched unrelated donors (MUD) to proceed to hematopoietic cell transplantation (HCT). Several studies have shown comparable survival outcomes among different graft sources.1–7 However, limited data address the costs and resource needs of HCT using different graft sources.
Allogeneic hematopoietic cell transplantation (HCT) is a resource intense procedure, and health care resource allocation is now being analyzed closely. Khera et al and Preussler et al have recently summarized the trends in costs of HCT.8,9 In a study using a national claims database of commercially insured population in the United States, Majhail et al showed that the median cost for allogeneic HCT in the first 100 days was $203,026.10 The median total number of days hospitalized was 31 days with the initial transplant hospitalization contributing >75% of these early costs. Costs and resource needs by graft source could not be described as these data were not available. The Minnesota group compared costs in the first 100 days among recipients of UCB and matched related donor HCT transplanted using a myeloablative conditioning (MAC) or reduced intensity conditioning (RIC) regimen.11,12 The median cost per day survived (not including graft acquisition) was $1016 for MAC matched related donor, $2082 for MAC UCB recipients, $612 for RIC matched related donor recipients, and $1156 for RIC UCB recipients. In a separate study, they reported greater blood product usage in patients receiving UCB transplantation and in patients receiving a MAC regimen.13
An understanding of the resource needs of different alternative graft sources through a multicenter study has important policy implications for estimating costs and need for resources, infrastructure and personnel. Studies of costs of HCT have been limited to single center analyses and reflect institutional practices specific to that institution. Furthermore, resource utilization in this population has not been well described. Although the Center for International Blood and Marrow Transplant Research (CIBMTR) does not collect data on resource utilization and costs of HCT, it does capture information on the total hospital length of stay (LOS) in the first 100 days. Since hospitalization is the largest contributor to early post-transplant resource utilization, we compared LOS in the first 100 days among different graft sources in a multicenter cohort. This information will assist transplant physicians and centers in planning for resource allocation and utilization such as hospital beds and admissions.
MATERIALS AND METHODS
Data Source and Patients
The CIBMTR comprises a voluntary working group of more than 500 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous HCT to a statistical center at the Medical College of Wisconsin in Milwaukee and the NMDP Coordinating Center in Minneapolis. Participating centers are required to report all transplants consecutively; compliance is monitored by on-site audits. Patients are followed longitudinally, with yearly follow-up. Computerized checks for errors, physicians’ review of submitted data and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed in compliance with the Privacy Rule (HIPAA) as a Public Health Authority and in compliance with all applicable federal regulations pertaining to the protection of human research participants as determined by continuous review of the Institutional Review Board of the National Marrow Donor Program.
The study population consisted of patients with acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL) in first or second complete remission (CR) who received their first allogeneic HCT in the United States and were reported to the CIBMTR between 2008 and 2011. All age groups and recipients of both MAC and RIC regimens were considered. Graft sources included UCB and 7/8 HLA-MMUD and 8/8 HLA-MUD transplanted using bone marrow (BM) or peripheral blood stem cells (PBSC); HLA-matched sibling donors were excluded. Due to the small number of patients, haploidentical HCT recipients were not included in this analysis. To obtain a relatively homogenous group for comparison, we restricted our study population to commonly used conditioning regimens (for MAC regimens: busulfan (Bu) + cyclophosphamide (Cy) ± other or Cy + total body irradiation (TBI) ± other; for RIC regimens: TBI + Cy + fludarabine (Flu) ± other, TBI + Flu ± other (no Cy), Bu + Flu ± other or melphalan (Mel) + Flu ± other). For the same reason, we excluded patients who had received ex vivo T-cell depletion as part of GVHD prophylaxis.
Outcomes and Study Definitions
The primary objective of this study was to compare LOS among different graft sources. LOS is captured by the CIBMTR as total number of hospital days (initial admission and any readmissions) between day 0 (day of transplant) and day 100 post-transplant. Patients who die early post-transplant have less time at risk for hospitalization and a shorter LOS than those who survive to day 100. To account for this association of early mortality with shorter hospitalization, we used the number of days alive and out of the hospital as the metric to compare LOS in first 100 days for our analysis. For patients who died within 100 days, we evaluated the number of days that patient survived out of the hospital (i.e., the number of days alive and out of the hospital would be 0 days for a patient who died on day +20 and had spent all 20 days in the hospital, versus 20 days for a patient who died on day +40 and spent 20 days in the hospital). For patients who survived through day 100, we censored followup at that time point (e.g., the number of days alive and out of the hospital would be 80 days for a patient who survived through day 100 and spent 20 days in the hospital). We also evaluated the proportion of days alive and out of the hospital; the results for both analyses were similar and only data on number of days alive and out of the hospital are presented in this manuscript. In addition, we performed a subset analysis in patients who had survived through day 100. We also describe 100-day overall survival among the graft sources. All outcomes were assessed from the date of transplantation.
Given the differences in patient characteristics and transplant practices among adult and pediatric transplant centers, pediatric (age ≤18 years) and adult (age >18 years) transplant recipients were analyzed separately. Also, MAC and RIC regimen recipients were analyzed separately as the time to neutrophil engraftment and consequently LOS varies. We excluded patient groups where the sample size was <30 patients. Specifically, we excluded: (1) pediatric patients receiving RIC regimens, (2) recipients of haploidentical transplantation, and (2) some graft source subcategories (e.g., pediatric MUD PBSC, pediatric MMUD BM and PBSC, and adult RIC MUD and MMUD BM). Hence, our final study population consisted of 1577 patients who were analyzed in three separate groups: (1) pediatric MAC HCT recipients (single UCB, double UCB and MUD BM), (2) adult MAC HCT recipients (single UCB, double UCB, MUD BM, MUD PBSC, MMUD BM and MMUD PBSC), and (3) adult RIC HCT recipients (single UCB, double UCB, MUD PBSC, MMUD PBSC).
Patient ethnicity (Hispanic or non-Hispanic) and race (White, Black, Asian, American Indian/Alaska Native, Native Hawaiian/Other Pacific Islander) are reported to the CIBMTR by transplant centers according to the US Office of Management and Budget classification.14–16 Preparative regimens were classified as MAC or RIC according to CIBMTR criteria.17,18 HLA matching was performed at low resolution for Class I and high resolution for Class II for UCB HCT, as per the majority of UCB transplants performed in this era. In patients receiving a double unit UCB transplant, the worst match to the patient between the two units was considered in categorizing the degree of recipient-UCB unit match. For MMUD and MUD HCT, high-resolution typing at HLA-A, -B, -C and -DRB1 was considered.
Statistical Analysis
Summaries of patient-, disease-, and treatment-related characteristics were generated for the graft source groups. The chi-square test was used to compare categorical variables, and the Kruskal-Wallis test was used for continuous variables. Univariate probabilities of overall survival were calculated using the Kaplan-Meier estimator.19
For multivariate analysis, we utilized Poisson regression. The overall survival and LOS were similar among recipients of single and double UCB HCT in all three groups analyzed, so we combined both into one category in multivariate analyses. Two models were built. The first considered the mean number of days patients were alive and out of the hospital within the first 100 days after transplant. Results are summarized as means ratio for comparing groups; a means ratio >1 indicates more days alive and out of the hospital. The second model looked at proportion of days alive in the first 100 days that were spent out of the hospital. Results for both models were similar and only the former are presented in this paper. Logistic regression models were used to assess 100-day mortality, as there was no censoring prior to 100 days (deaths occurring at day 100 were treated as events). In addition to graft source, the patient and disease characteristic covariates considered in the multivariable models included age, gender, recipient race, Karnofsky performance status prior to transplant, cytomegalovirus (CMV) serological status, HCT comorbidity index (HCT-CI) score, median household income (imputed by patient ZIP code of residence based on the 2011 US Census American Community Survey data), diagnosis, and disease status at transplant. Information about patient insurance coverage was not available and was therefore not considered in the analysis.
All computations were performed using the SAS statistical package (SAS Institute, Cary, NC). All P-values are two sided; a statistical significance level (alpha) of 0.05 was used throughout.
RESULTS
Patient Characteristics
Patient characteristics are outlined in Table 1. Among pediatric MAC HCT recipients, there were differences among single UCB, double UCB and MUD BM recipients in age, gender, race, diagnosis, CMV serostatus, conditioning regimen and exposure to anti-thymocyte globulin. Double UCB HCT recipients were older and were less likely to receive anti-thymocyte globulin. Compared with MUD BM transplant recipients, single and double UCB recipients more frequently belonged to non-White racial groups and received TBI + Cy based conditioning regimens. A greater proportion of MUD BM recipients as compared to UCB recipients had AML. Differences in patient characteristics were seen in the same variables in adult MAC and adult RIC recipients. For adults receiving MAC HCT, UCB recipients were again less frequently White and had more commonly received TBI + Cy based regimens. Similarly, in the adult RIC group, a smaller proportion of UCB recipients were White. UCB HCT patients were also younger compared to recipients of MUD PBSC and MMUD PBSC. There were notable differences in the conditioning regimen used, with UCB recipients more likely to have received TBI + Cy + Flu regimen. We observed no significant differences among the graft sources considered in the three cohorts with respect to HCT-CI score or patient socioeconomic status.
Table 1.
Patient characteristics
| Characteristics | Pediatric, MAC Regimen | Adult, MAC Regimen | Adult, RIC Regimen | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Single UCB |
Double UCB |
MUD BM |
P-value | Single UCB |
Double UCB |
MUD BM |
MUD PBSC |
MMUD BM |
MMUD PBSC |
P-value | Single UCB |
Double UCB |
MUD PBSC |
MMUD PBSC |
P-value | |
| Number of patients | 219 | 80 | 69 | 65 | 146 | 92 | 297 | 42 | 126 | 16 | 188 | 160 | 77 | |||
| Number of centers | 54 | 32 | 30 | 19 | 45 | 38 | 68 | 25 | 53 | 9 | 43 | 48 | 36 | |||
| Age at transplant, years, median (range) |
6 (<1– 18) |
12 (1– 18) |
9 (<1– 18) |
<.001 | 45 (18– 72) |
33 (18– 68) |
41 (18– 63) |
42 (19– 66) |
35 (18– 61) |
41 (19– 65) |
<.01 | 58 (19– 70) |
58 (19– 72) |
63 (21– 78) |
61 (23– 73) |
<.01 |
| Male gender | 114 (52) | 54 (68) | 41 (59) | 0.05 | 25 (38) | 72 (49) | 48 (52) | 146 (49) | 21 (50) | 66 (52) | 0.57 | 8 (50) | 94 (50) | 104 (65) | 43 (56) | 0.04 |
| Recipient race | <.01 | <.01 | ||||||||||||||
| White | 172 (79) | 64 (80) | 57 (83) | 0.01 | 48 (74) | 106 (73) | 86 (93) | 276 (93) | 36 (86) | 110 (87) | 13 (81) | 153 (81) | 154 (96) | 68 (88) | ||
| Black | 26 (12) | 7 (9) | 2 (3) | 9 (14) | 24 (16) | 2 (2) | 6 (2) | 4 (10) | 8 (6) | 2 (13) | 13 (7) | 2 (1) | 5 (6) | |||
| Other | 11 (5) | 8 (10) | 2 (3) | 7 (11) | 13 (9) | 2 (2) | 11 (4) | 1 (2) | 7 (6) | 1 (6) | 18 (10) | 0 | 2 (3) | |||
| Unknown | 10 (5) | 1 (1) | 8 (12) | 1 (2) | 3 (2) | 2 (2) | 4 (1) | 1 (2) | 1 (1) | 0 | 4 (2) | 4 (3) | 2 (3) | |||
| Karnofsky/Lansky score ≥80 |
209 (95) | 79 (99) | 67 (97) | 0.34 | 57 (88) | 137 (94) | 86 (93) | 260 (88) | 42 | 116 (92) | 0.13 | 12 (75) | 176 (94) | 151 (94) | 63 (82) | <.01 |
| Diagnosis | <.01 | 0.02 | 0.08 | |||||||||||||
| AML | 88 (40) | 35 (44) | 46 (67) | 44 (68) | 94 (64) | 70 (76) | 236 (79) | 30 (71) | 94 (75) | 14 (88) | 160 (85) | 148 (93) | 72 (94) | |||
| ALL | 131 (60) | 45 (56) | 23 (33) | 21 (32) | 52 (36) | 22 (24) | 61 (21) | 12 (29) | 32 (25) | 2 (13) | 28 (15) | 12 (8) | 5 (6) | |||
| Disease status | 0.08 | 0.12 | 0.10 | |||||||||||||
| CR1 | 111 (51) | 34 (43) | 42 (61) | 45 (69) | 87 (60) | 61 (66) | 217 (73) | 27 (64) | 85 (67) | 13 (81) | 131 (70) | 129 (81) | 60 (78) | |||
| CR2 | 108 (49) | 46 (58) | 27 (39) | 20 (31) | 59 (40) | 31 (34) | 80 (27) | 15 (36) | 41 (33) | 3 (19) | 57 (30) | 31 (19) | 17 (22) | |||
| HCT-CI score | 0.07 | 0.29 | 0.09 | |||||||||||||
| 0 | 178 (81) | 61 (76) | 48 (70) | 22 (34) | 59 (40) | 30 (33) | 107 (36) | 21 (50) | 51 (40) | 8 (50) | 53 (28) | 55 (34) | 16 (21) | |||
| 1–2 | 29 (13) | 15 (19) | 11 (16) | 16 (25) | 49 (34) | 34 (37) | 94 (32) | 6 (14) | 45 (36) | 3 (19) | 68 (36) | 48 (30) | 23 (30) | |||
| ≥ 3 | 12 (5) | 4 (5) | 10 (14) | 27 (42) | 38 (26) | 28 (30) | 95 (32) | 15 (36) | 30 (24) | 5 (31) | 67 (36) | 57 (36) | 38 (49) | |||
| Unknown | 0 | 0 | 0 | 0 | 0 | 0 | 1 (<1) | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Time from diagnosis to HCT, months, median (range) |
8 (<1– 82) |
10 (3– 62) |
6 (2– 108) |
0.18 | 7 (<1– 135) |
7 (2–70) | 6 (3– 168) |
6 (<1– 177) |
7 (3–54) | 7 (2–86) | 0.06 | 5 (2–49) | 7 (2– 313) |
6 (2–58) | 7 (2–36) | 0.20 |
| Conditioning regimen | <.01 | <.01 | <.01 | |||||||||||||
| Bu+Cy±Other | 46 (21) | 7 (9) | 28 (41) | 13 (20) | 16 (11) | 34 (37) | 118 (40) | 15 (36) | 48 (38) | - | - | - | - | |||
| TBI+Cy±Other | 173 (79) | 73 (91) | 41 (59) | 52 (80) | 130 (89) | 58 (63) | 179 (60) | 27 (64) | 78 (62) | - | - | - | - | |||
| TBI+Cy+Flu±Other | - | - | - | - | - | - | - | - | - | 9 (56) | 152 (81) | 1 (1) | 0 | |||
| TBI+Flu±Other (no Cy) | - | - | - | - | - | - | - | - | - | 0 | 2 (1) | 30 (19) | 21 (27) | |||
| Bu+Flu±Other | - | - | - | - | - | - | - | - | - | 1 (6) | 1 (1) | 101 (63) | 42 (55) | |||
| Mel+Flu±Other | - | - | - | - | - | - | - | - | - | 6 (38) | 33 (18) | 28 (18) | 14 (18) | |||
| TBI conditioning | 173 (79) | 73 (91) | 41 (59) | <.01 | 52 (80) | 130 (89) | 58 (63) | 179 (60) | 27 (64) | 78 (62) | <.01 | 9 (56) | 154 (82) | 31 (19) | 21 (27) | <.01 |
| CMV status* | <.01 | <.01 | <.01 | |||||||||||||
| D and R negative | 51 (23) | 14 (18) | 23 (33) | 10 (15) | 26 (18) | 31 (34) | 90 (30) | 10 (24) | 41 (33) | 4 (25) | 25 (13) | 46 (29) | 12 (16) | |||
| D or R positive | 138 (63) | 48 (60) | 45 (65) | 49 (75) | 99 (68) | 58 (63) | 203 (68) | 32 (76) | 84 (67) | 10 (63) | 126 (67) | 112 (70) | 62 (81) | |||
| Unknown | 30 (14) | 18 (23) | 1 (1) | 6 (9) | 21 (14) | 3 (3) | 4 (1) | 0 | 1 (1) | 2 (13) | 37 (20) | 2 (1) | 3 (4) | |||
| ATG prior to transplant | 75 (34) | 11 (14) | 26 (38) | <0.01 | 47 (72) | 132 (90) | 79 (86) | 238 (80) | 27 (64) | 81 (64) | <.01 | 8 (50) | 131 (70) | 78 (49) | 39 (51) | <.01 |
| Median household income, $ (x1000), median (range)† |
52 (16– 150) |
51 (14– 166) |
48 (24– 155) |
0.47 | 61 (22– 157) |
52 (17– 141) |
53 (20– 134) |
57 (14– 143) |
53 (24– 151) |
54 (22– 181) |
0.11 | 65 (20– 106) |
55 (20– 184) |
60 (22– 166) |
55 (24– 127) |
0.42 |
MAC – myeloablative conditioning; RIC – reduced intensity conditioning; UCB – umbilical cord blood; MUD – HLA 8/8 matched unrelated donor; MMUD – HLA 7/8 mis-matched unrelated donor; BM – bone marrow; PBSC – peripheral blood stem cells; AML – acute myeloid leukemia; ALL – acute lymphoblastic leukemia; CR – complete remission; HCT-CI – hematopoietic cell transplantation comorbidity index; Bu – busulfan; Cy – cyclophosphamide; TBI – total body irradiation; Flu – fludarabine; Mel – melphalan; CMV – cytomegalovirus; D – donor; R – recipient; ATG – anti-thymocyte globulin; CI – confidence intervals
For double UCB recipients, donor is classified as CMV seropositive if either of the two units were CMV positive
Based on ZIP code of patient residence (from the 2011 US Census American Community Survey data)
For the pediatric MAC group, the median total nucleated cell dose (pre-freeze) was 7 × 107/kg recipient weight for single UCB and 8 × 107/kg for double UCB recipients. HLA 4/6 matched units were used in 29% of single and 46% of double UCB transplants. Among adult MAC patients receiving single and double UCB transplantation, the corresponding median cell doses were 3 × 107/kg and 5 × 107/kg and utilization of HLA 4/6 matched units occurred in 55% and 68% of patients, respectively. Among adult RIC patients, median pre-freeze total nucleated cell dose was 2 × 107/kg in both single and double UCB recipients. Forty-four percent of single UCB and 57% of double UCB transplants used HLA 4/6 matched units.
Pediatric MAC HCT Group
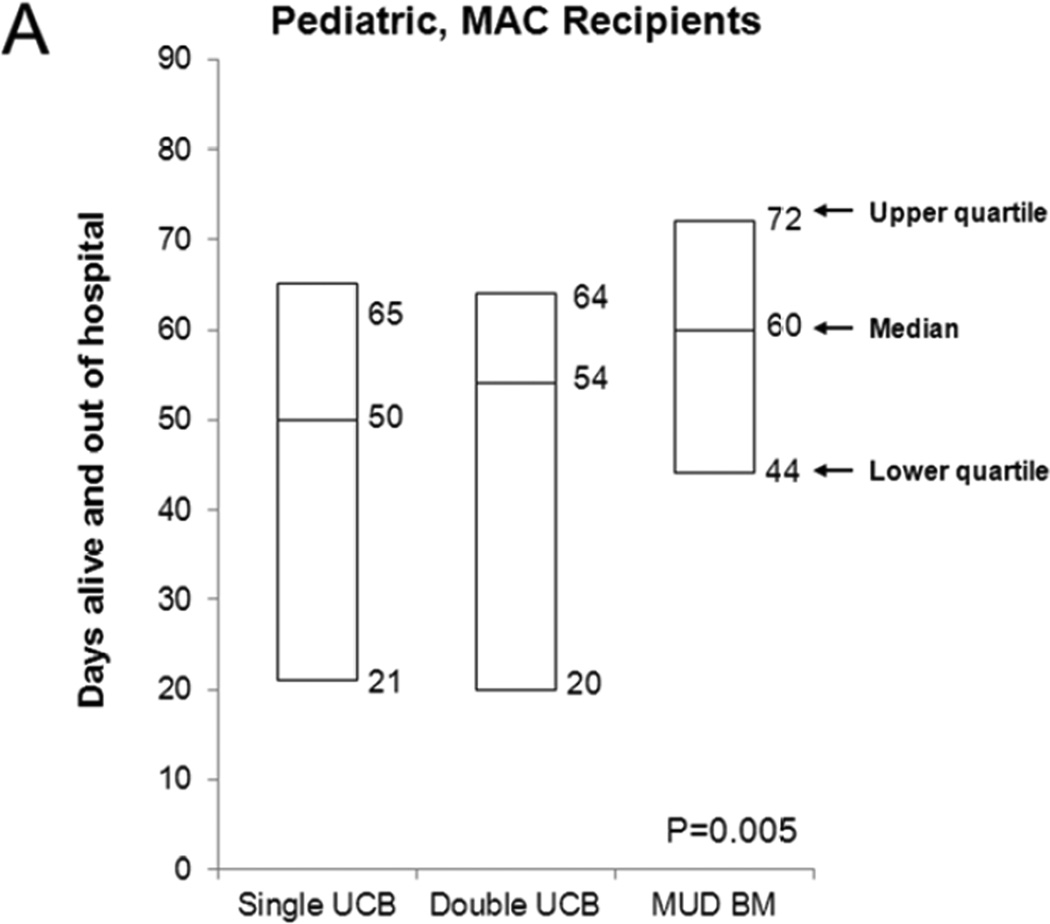
Figure 1A shows the number of days alive and out of the hospital in the first 100 days after transplantation among patients receiving each of the three graft sources compared in this cohort. The median values were 50 days for single UCB, 54 days for double UCB and 60 days for MUD BM (P=0.005). Survival at 100 days for single and double UCB HCT recipients was similar (88% [95% CI, 83–92%] and 85% [95% CI, 76–92%]). Because of comparable LOS, the two graft sources were combined for multivariate analysis.
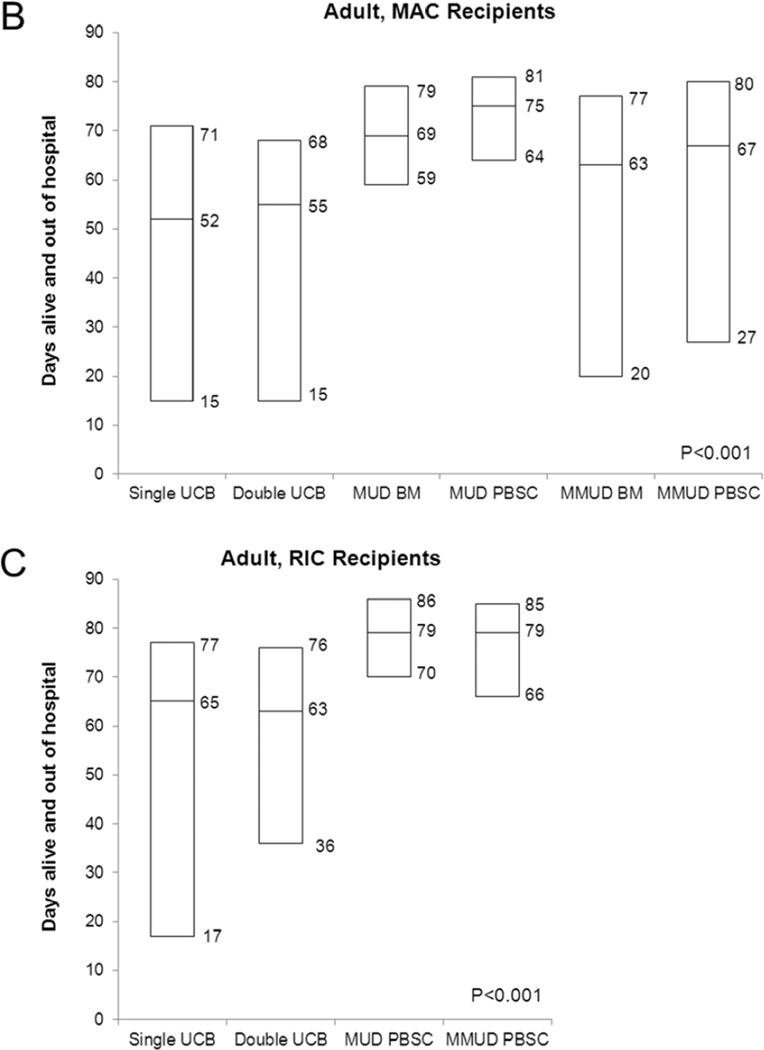
Figure 1.
Number of days alive and out of the hospital in the first 100 days after allogeneic transplantation: (A) Pediatric MAC HCT recipients, (B) Adult MAC HCT recipients, and (C) Adult RIC HCT recipients. The lower and upper bars represent the interquartile range (25th–75th percentile) and the middle bar represents the median.
Table 2 shows results of multivariate analysis. Compared to UCB recipients, patients receiving MUD BM were alive and stayed out of the hospital for a significantly longer duration in the first 100 days after transplantation (means ratio 1.18, P=0.03). We also identified other factors that were significantly associated with total hospital LOS. On average, patients had fewer number of days alive and out of the hospital if they were Black (means ratio 0.75 compared to Whites, P=0.01), had performance score of <80 at HCT (means ratio 0.63 compared to score ≥ 80, P=0.03) and were CMV seropositive (means ratio 0.85, P=0.006).
Table 2.
Overall survival at 100 days, results of multivariate analysis for overall mortality and results of multivariate analysis comparing the number of days alive and out of the hospital among graft sources
| Category | N | 100-day survival (95% CI) |
P-value† | Odds ratio for 100-day mortality (95% CI) |
P-value‡ | Means ratio for days alive and out of hospital£ (95% CI) |
P-value¥ |
|---|---|---|---|---|---|---|---|
| Pediatric, MAC recipients | 0.09 | 0.28 | 0.03 | ||||
| UCB* | 295 | 87% (83–91%) | 1.00 | 1.00 | |||
| MUD BM | 69 | 95% (89–100%) | 0.55 (0.18–1.63) | 1.18 (1.02–1.36) | |||
| Adult, MAC recipients | <0.001 | <0.001€ | <0.001€ | ||||
| UCB* | 210 | 77% (73–83%) | 1.00 | 1.00 | |||
| MUD BM | 92 | 91% (86–97%) | 0.33 (0.15–0.73) | 0.006 | 1.36 (1.21–1.54) | <0.001 | |
| MUD PBSC | 296 | 90% (87–93%) | 0.37 (0.22–0.61) | <0.001 | 1.45 (1.32–1.59) | <0.001 | |
| MMUD BM | 42 | 76% (64–90%) | 1.08 (0.49–2.36) | 0.85 | 1.06 (0.89–1.26) | 0.49 | |
| MMUD PBSC | 126 | 77% (70–85%) | 1.04 (0.61–1.77) | 0.88 | 1.19 (1.06–1.33) | 0.003 | |
| Adult, RIC recipients | 0.002 | <0.001€ | <0.001€ | ||||
| UCB* | 204 | 79% (74–85%) | 1.00 | 1.00 | |||
| MUD PBSC | 160 | 93% (89–97%) | 0.25 (0.12–0.50) | <0.001 | 1.38 (1.26–1.52) | <0.001 | |
| MMUD PBSC | 77 | 87% (80–95%) | 0.43 (0.19–0.94) | 0.03 | 1.33 (1.19–1.48) | <0.001 |
CI – Confidence intervals; MAC – myeloablative conditioning; RIC – reduced-intensity conditioning; UCB – umbilical cord blood; MUD – matched unrelated donor; MMUD – mis-matched unrelated donor; BM – bone marrow; PBSC – peripheral blood stem cells
Single and double UCB were combined as one category as the 100-day survival and days alive and out of the hospital were comparable
Log-rank P-value
Logistic regression P-value
Means ratio compares the mean number of days patients were alive and stayed out of the hospital in the first 100 days after transplant among the graft sources considered and is adjusted for important patient characteristics; means ratio >1 indicates a better outcome and longer duration alive and out of the hospital (for example, in the pediatric MAC transplant group, recipients of MUD BM on an average, stayed 18% more days alive and out of the hospital compared to UCB recipients)
Poisson regression P-value
Overall P-value
Table 3 describes days alive and out of hospital by selected patient characteristics such as recipient race, Lansky score at transplantation, HCT-CI score, diagnosis and median household income.
Table 3.
Number of days alive and out of the hospital in the first 100 days after allogeneic transplantation by selected patient demographic factors. Data by graft source is presented in Figure 1.
| Characteristic | N | Median days alive and out of hospital* |
Interquartile range |
P-value‡ |
|---|---|---|---|---|
| Pediatric, MAC HCT recipients | ||||
| Recipient race | 0.05 | |||
| White | 293 | 54 | 32–67 | |
| Black | 35 | 38 | 0–61 | |
| Other | 21 | 42 | 17–58 | |
| Unknown | 19 | 53 | 29–66 | |
| Lansky score at transplant | 0.17 | |||
| ≥ 80 | 355 | 53 | 28–66 | |
| < 80 | 11 | 19 | 7–48 | |
| Unknown | 2 | 37 | 22–52 | |
| HCT-CI score | 0.99 | |||
| 0 | 287 | 52 | 27–66 | |
| 1–2 | 55 | 54 | 16–65 | |
| ≥ 3 | 26 | 49 | 34–63 | |
| Diagnosis | 0.35 | |||
| Acute myeloid leukemia | 169 | 54 | 28–67 | |
| Acute lymphoblastic leukemia | 199 | 51 | 22–65 | |
| Median household income† | .98 | |||
| < $50,000 | 165 | 53 | 31–65 | |
| $50,000–100,000 | 167 | 51 | 21–67 | |
| ≥ $100,000 | 20 | 53 | 40–61 | |
| Unknown | 16 | 46 | 34–67 | |
| Adult, MAC HCT recipients | ||||
| Recipient race | <0.001 | |||
| White | 662 | 70 | 47–79 | |
| Black | 53 | 48 | 21–68 | |
| Other | 41 | 66 | 45–73 | |
| Unknown | 12 | 64 | 54–80 | |
| Karnofsky score at transplant | 0.05 | |||
| ≥ 80 | 698 | 69 | 44–78 | |
| < 80 | 59 | 54 | 38–80 | |
| Unknown | 11 | 80 | 67–86 | |
| HCT-CI score | 0.47 | |||
| 0 | 290 | 69 | 41–79 | |
| 1–2 | 244 | 69 | 45–79 | |
| ≥ 3 | 233 | 68 | 47–78 | |
| Diagnosis | <0.001 | |||
| Acute myeloid leukemia | 568 | 70 | 52–79 | |
| Acute lymphoblastic leukemia | 200 | 60 | 29–76 | |
| Median household income† | 0.18 | |||
| < $50,000 | 294 | 68 | 36–77 | |
| $50,000–100,000 | 410 | 70 | 52–79 | |
| ≥ $100,000 | 45 | 67 | 32–77 | |
| Unknown | 19 | 68 | 44–80 | |
| Adult, RIC HCT recipients | ||||
| Recipient race | 0.05 | |||
| White | 388 | 74 | 54–83 | |
| Black | 22 | 70 | 43–78 | |
| Other | 21 | 55 | 21–71 | |
| Unknown | 10 | 72 | 24–78 | |
| Karnofsky score at transplant | 0.008 | |||
| ≥ 80 | 402 | 73 | 51–82 | |
| < 80 | 33 | 74 | 57–85 | |
| Unknown | 6 | 50 | 48–81 | |
| HCT-CI score | 0.52 | |||
| 0 | 132 | 75 | 54–83 | |
| 1–2 | 142 | 73 | 51–84 | |
| ≥ 3 | 167 | 73 | 50–81 | |
| Diagnosis | 0.43 | |||
| Acute myeloid leukemia | 394 | 74 | 53–83 | |
| Acute lymphoblastic leukemia | 47 | 70 | 43–81 | |
| Median household income† | 0.86 | |||
| < $50,000 | 159 | 74 | 48–85 | |
| $50,000–100,000 | 237 | 71 | 53–81 | |
| ≥ $100,000 | 33 | 76 | 59–82 | |
| Unknown | 12 | 74 | 43–84 |
HCT – hematopoietic cell transplantation; MAC – myeloablative conditioning; RIC – reduced intensity conditioning; HCT-CI – hematopoietic cell transplant comorbidity index
Larger number indicates more days alive and out of hospital in the first 100 days after transplantation
Based on Zip code of patient residence (from the 2011 US Census American Community Survey data)
Univariate P-value
Overall 43 (12%) patients died before day 100 (39 UCB, 4 MUD BM) and 31 (72%) of these patients stayed in the hospital the entire time. Results of logistic regression analysis showed that graft source did not have any association with mortality at 100 days (Table 2). We also performed multivariate analysis for hospital LOS after excluding these 43 patients. In this subset analysis of 100-day survivors, UCB was still associated with fewer days alive and out of the hospital.
Adult MAC HCT Group
We observed significant differences in hospital LOS among patients receiving each of the graft sources compared in this cohort (Figure 1B). The median number of days alive and out of the hospital in the first 100 days was 52 days for single UCB, 55 days for double UCB, 69 days for MUD BM, 75 days for MUD PBSC, 63 days for MMUD BM and 67 days for MMUD PBSC (P<0.001). Single and double UCB recipients had comparable survival at 100 days (74% [95% CI, 63–84%] and 79% [95% CI, 72–85%]); similar to the pediatric analysis, the two graft sources were combined as one category in multivariate analysis given the similar hospital LOS.
Table 2 shows results of multivariate analysis for this cohort. There was no difference in the number of days alive and out of the hospital for recipients of UCB and MMUD BM (means ratio 1.06, P=0.49 compared to UCB). However, compared to UCB, the number of days alive and out of the hospital in the first 100 days was significantly greater for recipients of MUD BM (means ratio 1.36, P<0.001), MUD PBSC (means ratio 1.45, P<0.001) and MMUD PBSC (means ratio 1.19, P=0.003). On other pairwise comparisons, patients receiving MMUD BM had a shorter time alive and out of the hospital than patients receiving either MUD BM (means ratio 0.78, P=0.007) or MUD PBSC (means ratio 0.73, P<0.001). MMUD PBSC recipients also had fewer days alive and out of the hospital compared to MUD BM (means ratio 0.87, P=0.03) and MUD PBSC (means ratio 0.82, P<0.001). There was no significant difference in the 100 day LOS between MMUD BM and MMUD PBSC recipients (means ratio 1.12, P=0.22) and between MUD BM and MUD PBSC recipients (means ratio 1.06, P=0.27). Shorter duration of days alive and out of the hospital was seen in Black patients (means ratio 0.72 compared to Whites, P=0.03) and in patients with ALL (means ratio 0.90 compared to AML, P=0.01). Patients ≥ 25 years of age at HCT had a shorter LOS compared to patients 18–25 years of age (means ratio 1.18, P=0.001). Table 3 describes LOS by selected patient characteristics.
Sixteen percent of patients (N=125) died within the first 100 days, 48% (N=60) of whom remained hospitalized the entire time. In logistic regression analysis of 100-day mortality, UCB recipients were significantly more likely than MUD BM and MUD PBSC recipients to die during the first 100 days (Table 2). In multivariate analysis for hospital LOS within the subgroup of patients surviving >100 days, results were similar to the whole cohort, with the number of days alive and out of the hospital for UCB recipients being comparable with MMUD BM recipients, but significantly shorter compared to MUD BM, MUD PBSC and MMUD PBSC recipients.
Adult RIC HCT Group
Figure 1C shows the days alive and out of the hospital for this cohort (median 65 days for single UCB, 63 days for double UCB, and 79 days for MUD PBSC and for MMUD PBSC [P<0.001]). Four patients had no reported inpatient days, presumably because they received their transplant as an outpatient procedure and did not require subsequent hospitalization in the first 100 days. Single and double UCB HCT were again combined into one category for multivariate analysis because of similar LOS and 100-day survival (75% [95% CI, 52–92%] and 80% [95% CI, 74–85%]).
In multivariate analysis, graft source was the only variable associated with number of days alive and out of the hospital (Table 2). Compared to UCB recipients, number of days alive and out of the hospital was greater for recipients of MUD PBSC (means ratio 1.38, P<0.001) and MMUD PBSC (means ratio 1.33, P<0.001). There was no difference in hospitalization duration when comparing MUD PBSC and MMUD PBSC (means ratio 1.04, P=0.47). Table 3 describes days alive and out of hospital by selected patient characteristics for this cohort.
Fifteen percent of patients (N=64) died within the first 100 days, 38% (N=24) of whom remained hospitalized the entire time. In logistic regression analysis, we observed an association between graft source and 100-day mortality, with UCB recipients less likely to survive to 100 days compared to MUD PBSC and MMUD PBSC recipients (Table 2). Results of multivariate analysis of number of days alive and out of the hospital, restricted to the subgroup of 100-day survivors, were similar to those seen for the whole cohort.
DISCUSSION
In patients who do not have an HLA-matched related donor, the decision to use one alternative graft source over another is complex. Transplant physicians take several factors into consideration in selecting a graft source, including the patient’s underlying disease, urgency to proceed with transplantation, and donor availability. Little is known about how resource needs compare across alternative graft sources. Using a nationally representative and contemporary cohort of patients, we show that the total length of hospital stay in the first 100 days is significantly greater among pediatric and adult recipients of UCB and MMUD transplantation compared with recipients of MUD HCT. Although resources do not generally figure directly in decisions about choice of graft source, our data have important policy implications and will inform multiple stakeholders, especially transplant providers and centers, about the resources needed to care for patients receiving HCT from alternative graft sources.
Hospital stay is the major driver of early post-transplant resource use and costs, with an estimated 75–95% of total transplant costs in the first 100 days attributed to inpatient stay.9–12,20–25 We used total LOS stay in the first 100 days for our analysis (transplant admission and any subsequent admissions), as these data are captured by the CIBMTR. To account for different rates of early mortality, we used the number of days alive and out of the hospital in the first 100 days as the endpoint for our analysis. The finding of a longer LOS for UCB patients may reflect the fact that engraftment occurs later in patients receiving UCB and patients are generally hospitalized until neutrophil recovery. We were able to compare UCB with MMUD in the adult MAC HCT group, where MMUD BM recipients had a similar LOS, while MMUD PBSC recipients had a shorter LOS compared to UCB recipients. In the adult RIC HCT group, we were able to compare UCB with MMUD PBSC, and found that the former was associated with a longer LOS in the first 100 days.
LOS, days alive and out of the hospital, and 100-day survival were similar after single and double UCB transplantation in our cohort of patients transplanted in the recent era. This likely reflects our current understanding that has been developed over years of research where centers are able to appropriately select patients for single versus double UCB transplantation based on unit cell dose and HLA-match, with many centers selecting double UCB transplantation if no single UCB unit of adequate cell dose is available. Our study was not designed to compare outcomes of single versus double UCB transplantation. Our results differ from a recently published French study which showed a decreased relapse rate, improved survival and cost-effectiveness in favor of double over single UCB transplantation among adult patients with acute leukemia.26
There is considerable interest in comparing outcomes between UCB and haplo-identical transplantation. Results from parallel multicenter phase II studies from the Blood and Marrow Transplant Clinical Trials Network have shown 1-year overall and progression-free survival rates of 54% and 46% after UCB HCT and 62% and 48% after haplo-marrow transplantation, respectively.27 An ongoing multicenter randomized phase III study through the Network is comparing outcomes between these two graft sources. As part of our original study question, we were interested in comparing LOS between UCB and haploidentical transplantation. However, we were not able to include haploidentical HCT in our analysis because of the small number of patients (only 41 patients reported to the CIBMTR between 2008 and 2011 had received HCT using an ‘other relative’ donor source and met the other study selection criteria). As more experience is obtained with haploidentical transplantation, an assessment of its costs and resource needs as compared with other donor sources will be of paramount importance.
An interesting observation was the longer length of hospitalization in Black children and adults receiving MAC conditioning, even after adjusting for other patient and disease characteristics. Although our study was not designed specifically to study the association of race with outcomes, Black pediatric MAC HCT recipients (but not adult recipients) had significantly higher 100 day mortality compared to Whites on logistic multivariate regression analysis. It is well known that UCB increases access to transplantation and is more likely to be utilized in Black patients who frequently lack other suitable donor sources.28–30 Previous studies have also shown an association between race and allogeneic HCT outcomes, including those after single UCB transplantation.15,16,28 Black recipients of single UCB HCT are also more likely to receive UCB units that are smaller and less well matched than Whites,16 which may influence the time to engraftment and consequently their duration of hospitalization. Hence, factors such as health care disparities and availability of a suitable donor (e.g., an adequate UCB unit) may partly explain our finding of race-hospitalization association. The observation of racial/ethnic difference in LOS requires more detailed examination in future studies.
We considered patient socioeconomic status in our analysis using median household income based on ZIP code of residence. Socioeconomic status is a surrogate for several healthcare status indicators, including insurance status.31 We did not find an association between socioeconomic status and LOS or 100 day mortality. It is possible that biologic factors may contribute to the longer LOS seen in Black patients (e.g., lower cell dose for UCB units). However, patient numbers were too small to analyze the association of socioeconomic factors with LOS within each racial/ethnic subgroup.
This study has some limitations. Patients were treated at many centers using a variety of conditioning and GVHD prophylaxis regimens. We restricted our study to patients with acute leukemia in CR1 or CR2 and to commonly used conditioning and GVHD prophylaxis regimens to establish a relatively homogenous cohort. We were not able to account for variation in transplant center practices,32,33 which may influence the length of hospitalization for patients. In addition, the CIBMTR does not collect information on caregiver and community support, which may influence LOS. Only 4 patients received outpatient transplants without any need for hospital admission during the first 100 days. Our study was limited to patients transplanted in the United States. We were not able to consider patient payor type and quality of insurance coverage, which may impact LOS after transplantation. For example, duration of hospital stay has been shown to be longer in Medicaid patients in other medical situations.34 Medicare patients may have additional pressure to limit LOS. We were not able to account for important pre-transplant cost and resource factors (e.g., costs of graft acquisition). Also, our study focused on hospitalizations over the first 100 days and was not able to account for later hospitalizations and resource utilization. Chronic GVHD and relapsed disease have significant costs to the health care system but were beyond the scope of this study. For example, chronic GVHD may be less prevalent among UCB recipients compared to other graft sources,1,6 which may in turn be associated with lower long-term costs and resource needs.
We comprehensively describe the length of hospital stay in the first 100 days among patients undergoing HCT with alternative graft sources. LOS is long for all alternative graft sources and protocols to improve engraftment, decrease infection, and improve home monitoring need to be developed to decrease LOS. The use of two UCB units was not associated with shorter LOS. UCB was associated with a longer LOS than other alternative graft sources for both pediatric and adult patients. These data will assist transplant providers and centers to understand the expected resources needed to treat the individual patient, develop strategies to reduce LOS, and select the graft source that is likely to have the best outcome and to be most cost effective. Future studies are needed to elucidate factors that may account for differential LOS among alternative graft sources, such as rates of infections, GVHD and engraftment, and to address interventions to reduce hospitalization duration in general.
ACKNOWLEDGEMENTS
We would like to thank Yoshiko Atsuta, MD, PhD, for her review of the draft manuscript.
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from AABB; Allos, Inc.; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US, Inc.; Be the Match Foundation; Biogen IDEC; BioMarin Pharmaceutical, Inc.; Biovitrum AB; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Children’s Leukemia Research Association; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Eisai, Inc.; Genentech, Inc.; Genzyme Corporation; Histogenetics, Inc.; HKS Medical Information Systems; Hospira, Inc.; Kirin Brewery Co., Ltd.; The Leukemia & Lymphoma Society; Merck & Company; The Medical College of Wisconsin; Millennium Pharmaceuticals, Inc.; Miller Pharmacal Group; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Nature Publishing Group; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Pall Life Sciences; Pfizer Inc; Schering Corporation; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; StemCyte, Inc.; StemSoft Software, Inc.; Sysmex America, Inc.; THERAKOS, Inc.; Vidacare Corporation; ViraCor Laboratories; ViroPharma, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure: None of the authors has any conflicts of interest to declare in relation to this manuscript
REFERENCES
- 1.Eapen M, Rocha V, Sanz G, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol. 2010;11:653–660. doi: 10.1016/S1470-2045(10)70127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laughlin MJ, Eapen M, Rubinstein P, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 3.Rocha V, Labopin M, Sanz G, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351:2276–2285. doi: 10.1056/NEJMoa041469. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi S, Ooi J, Tomonari A, et al. Comparative single-institute analysis of cord blood transplantation from unrelated donors with bone marrow or peripheral blood stem-cell transplants from related donors in adult patients with hematologic malignancies after myeloablative conditioning regimen. Blood. 2007;109:1322–1330. doi: 10.1182/blood-2006-04-020172. [DOI] [PubMed] [Google Scholar]
- 5.Peffault de Latour R, Brunstein CG, Porcher R, et al. Similar overall survival using sibling, unrelated donor, and cord blood grafts after reduced-intensity conditioning for older patients with acute myelogenous leukemia. Biol Blood Marrow Transplant. 2013;19:1355–1360. doi: 10.1016/j.bbmt.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Chen YB, Aldridge J, Kim HT, et al. Reduced-intensity conditioning stem cell transplantation: comparison of double umbilical cord blood and unrelated donor grafts. Biol Blood Marrow Transplant. 2012;18:805–812. doi: 10.1016/j.bbmt.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Eapen M, Rubinstein P, Zhang MJ, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369:1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 8.Khera N, Zeliadt SB, Lee SJ. Economics of hematopoietic cell transplantation. Blood. 2012;120:1545–1551. doi: 10.1182/blood-2012-05-426783. [DOI] [PubMed] [Google Scholar]
- 9.Preussler JM, Denzen EM, Majhail NS. Costs and cost-effectiveness of hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:1620–1628. doi: 10.1016/j.bbmt.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majhail NS, Mau LW, Denzen EM, et al. Costs of autologous and allogeneic hematopoietic cell transplantation in the United States: a study using a large national private claims database. Bone Marrow Transplant. 2013;48:294–300. doi: 10.1038/bmt.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majhail NS, Mothukuri JM, Brunstein CG, et al. Costs of hematopoietic cell transplantation: comparison of umbilical cord blood and matched related donor transplantation and the impact of posttransplant complications. Biol Blood Marrow Transplant. 2009;15:564–573. doi: 10.1016/j.bbmt.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Majhail NS, Mothukuri JM, Macmillan ML, et al. Costs of pediatric allogeneic hematopoietic-cell transplantation. Pediatr Blood Cancer. 2010;54:138–143. doi: 10.1002/pbc.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solh M, Brunstein C, Morgan S, et al. Platelet and red blood cell utilization and transfusion independence in umbilical cord blood and allogeneic peripheral blood hematopoietic cell transplants. Biol Blood Marrow Transplant. 2011;17:710–716. doi: 10.1016/j.bbmt.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasquini M, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides. 2013 Available at: http://www.cibmtr.org.
- 15.Baker KS, Davies SM, Majhail NS, et al. Race and socioeconomic status influence outcomes of unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15:1543–1554. doi: 10.1016/j.bbmt.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ballen KK, Klein JP, Pedersen TL, et al. Relationship of race/ethnicity and survival after single umbilical cord blood transplantation for adults and children with leukemia and myelodysplastic syndromes. Biol Blood Marrow Transplant. 2012;18:903–912. doi: 10.1016/j.bbmt.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15:367–369. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 20.Saito AM, Cutler C, Zahrieh D, et al. Costs of allogeneic hematopoietic cell transplantation with high-dose regimens. Biol Blood Marrow Transplant. 2008;14:197–207. doi: 10.1016/j.bbmt.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saito AM, Zahrieh D, Cutler C, et al. Lower costs associated with hematopoietic cell transplantation using reduced intensity vs high-dose regimens for hematological malignancy. Bone Marrow Transplant. 2007;40:209–217. doi: 10.1038/sj.bmt.1705733. [DOI] [PubMed] [Google Scholar]
- 22.Esperou H, Brunot A, Roudot-Thoraval F, et al. Predicting the costs of allogeneic sibling stem-cell transplantation: results from a prospective, multicenter, French study. Transplantation. 2004;77:1854–1858. doi: 10.1097/01.tp.0000129409.84087.62. [DOI] [PubMed] [Google Scholar]
- 23.Jaing TH, Tsay PK, Yang CP, et al. Evaluation of readmission in children receiving allogeneic hematopoietic stem cell transplantation: an institutional experience. Transplant Proc. 2008;40:3643–3645. doi: 10.1016/j.transproceed.2008.06.086. [DOI] [PubMed] [Google Scholar]
- 24.van Agthoven M, Groot MT, Verdonck LF, et al. Cost analysis of HLA-identical sibling and voluntary unrelated allogeneic bone marrow and peripheral blood stem cell transplantation in adults with acute myelocytic leukaemia or acute lymphoblastic leukaemia. Bone Marrow Transplant. 2002;30:243–251. doi: 10.1038/sj.bmt.1703641. [DOI] [PubMed] [Google Scholar]
- 25.Blommestein HM, Verelst SG, Huijgens PC, et al. Real-world costs of autologous and allogeneic stem cell transplantations for haematological diseases: a multicentre study. Ann Hematol. 2012;91:1945–1952. doi: 10.1007/s00277-012-1530-2. [DOI] [PubMed] [Google Scholar]
- 26.Labopin M, Ruggeri A, Gorin NC, et al. Cost-effectiveness and clinical outcomes of double versus single cord blood transplantation in adults with acute leukemia in France. Haematologica. 2014;99:535–540. doi: 10.3324/haematol.2013.092254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118:282–288. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Majhail NS, Nayyar S, Santibanez ME, et al. Racial disparities in hematopoietic cell transplantation in the United States. Bone Marrow Transplant. 2012;47:1385–1390. doi: 10.1038/bmt.2011.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Majhail NS, Omondi NA, Denzen E, et al. Access to hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2010;16:1070–1075. doi: 10.1016/j.bbmt.2009.12.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barker JN, Byam CE, Kernan NA, et al. Availability of cord blood extends allogeneic hematopoietic stem cell transplant access to racial and ethnic minorities. Biol Blood Marrow Transplant. 2010;16:1541–1548. doi: 10.1016/j.bbmt.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Center for Health Statistics. Health, United States, 2011: With special feature on socioeconomic status and health. Hyattsville, MD: 2012. [PubMed] [Google Scholar]
- 32.Loberiza FR, Jr, Zhang MJ, Lee SJ, et al. Association of transplant center and physician factors on mortality after hematopoietic stem cell transplantation in the United States. Blood. 2005;105:2979–2987. doi: 10.1182/blood-2004-10-3863. [DOI] [PubMed] [Google Scholar]
- 33.Majhail NS, Murphy EA, Omondi NA, et al. Allogeneic transplant physician and center capacity in the United States. Biol Blood Marrow Transplant. 2011;17:956–961. doi: 10.1016/j.bbmt.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allen LA, Smoyer Tomic KE, Wilson KL, et al. The inpatient experience and predictors of length of stay for patients hospitalized with systolic heart failure: comparison by commercial, Medicaid, and Medicare payer type. J Med Econ. 2013;16:43–54. doi: 10.3111/13696998.2012.726932. [DOI] [PMC free article] [PubMed] [Google Scholar]