Abstract
Objective:
While transient ischemic attack (TIA) is a well-known harbinger of ischemic stroke, the mechanisms that link TIA to subsequent strokes remain poorly understood. The overall aim of this study was to determine whether: 1) brief periods of transient cerebral ischemia render this tissue more vulnerable to thrombus development and 2) antiplatelet agents used in TIA patients alter ischemia-induced thrombogenesis.
Approach & Results:
The middle cerebral artery of C57BL/6 mice was occluded for 2.5 – 10 minutes, followed by reperfusion periods of 1 – 28 days. Intravital microscopy was used to monitor thrombus development in cerebral microvessels induced by light/dye photoactivation. Thrombosis was quantified as the time to platelet aggregation on the vessel wall and the time for complete blood flow cessation. While brief periods of cerebral ischemia were not associated with neurological deficits or brain infarction (evaluated after 1 day), it yielded a pronounced and prolonged (up to 28 days) acceleration of thrombus formation, compared to control (sham) mice. This prothrombotic phenotype was not altered by pre- and/or post-treatment of mice with either aspirin (A), clopidogrel (C), dipyridamole (D), or atorvastatin (S), or with A + D + S.
Conclusions:
The increased vulnerability of the cerebral vasculature to thrombus development after a brief period of transient ischemia can be recapitulated in a murine model. Antiplatelet or antithrombotic agents used in patients with TIA show no benefit in this mouse model of brief transient ischemia.
Keywords: Transient ischemic attack, thrombosis, aspirin, statin, dipyridamole, clopidogrel
Introduction
Stroke remains a leading cause of mortality and disability worldwide (O’Donnell et al 2010), with approximately 800,000 people suffering a stroke each year in the US (Roger et al 2011). There are a number of underlying causes of stroke, including thrombosis, embolism, stenosis, and intracerebral hemorrhage. Ischemic stroke, which accounts for approximately 85% of all strokes, are more commonly characterized as either thrombotic or embolic, depending on the origin of the clot that causes vessel blockage. Embolic strokes are usually of extracranial origin and commonly result from clots formed in the heart due to atrial fibrillation. Thrombotic strokes, on the other hand, are typically of intracranial origin and result from the rupture of an atheroma and subsequent injury of a downstream vessel, with the ensuing development of a thrombus in either a large (middle cerebral artery) or small (lacunar) artery in the brain (Li et al 2010). Stroke recurrence occurs at a rate ranging between 4 and 14% and patients with risk factors, such as hypertension, are at higher risk for stroke recurrence. Up to 60-70% of recurrent strokes are of the same subtype as the initial stroke (Lovett et al 2004).
Transient ischemic attack (TIA), also known as a mini-stroke, has traditionally been defined as an ischemic stroke of short duration that produces focal neurological dysfunction (without objective evidence of brain infarction) that generally last less than 24 hrs (Easton et al 2009; Sacco et al, 2013). TIA is considered a warning sign of increased risk for a subsequent serious and debilitating stroke. About 30% of people experiencing a TIA later suffer a serious stroke (Dennis et al 1990). Little is known about the mechanism(s) that account for the ability of a TIA to render the brain more vulnerable to a subsequent severe ischemic episode. It is generally thought that TIA patients have an elevated risk for suffering a more profound adverse vascular event than produced by the initial TIA, such as clot production caused by atrial fibrillation or rupture of a large vessel atheroma due to instability in the underlying embolic or thrombotic process (Johnston 2005). It is also possible that TIA renders the cerebral vasculature hyper-responsive to thrombus formation in response to vessel wall injury, such as that induced by released atheroma fragments.
The increased likelihood for a second ischemic insult after TIA occurs in conjunction with the phenomenon of ischemic preconditioning (IPC) or tolerance, in which brain tissue is rendered resistant to the deleterious effects of prolonged ischemia and reperfusion by prior exposure to very brief periods of ischemia (Kirino 2002, Gidday 2006). The robust brain protection that is afforded by ischemic tolerance has been attributed to the induction of different survival mechanisms in neurons, rather than an improvement of the cerebral blood flow response after exposure to the second ischemic insult (Kirino 2002, Gidday 2006). Nonetheless, some elements (endothelial cells) of the cerebral vasculature do exhibit a protective phenotype after IPC and this is manifested as improved blood brain barrier integrity and an attenuated leukocyte-endothelial cell adhesion response (Gidday 2006). Although brain IPC is associated with prolonged coagulation and bleeding times (Kim et al 2008, Chen et al 2005), it remains unclear whether platelet dynamics at the blood-endothelial cell interface as well as the potential for microvascular thrombosis is altered in response to brief transient episodes of cerebral ischemia. Hence, a major objective of this study was to determine whether the phenomenon of IPC (or tolerance) in the brain includes a protective (diminished) response to thrombus development in the cerebral microvasculature or whether transient brain ischemia leads to an acceleration of thrombus development that may be relevant to the pathophysiology of TIA.
The results of this study revealed that a brief period (2.5 – 10 min) of transient focal cerebral ischemia elicits a pronounced and prolonged (up to 28 days) acceleration of injury-induced thrombus formation in the cerebral microvasculature. Given the potential relevance of these findings to TIA, additional studies were undertaken to assess the effectiveness of different antiplatelet agents (aspirin, clopidogrel, dipyridamole) and atorvastatin, either alone or in combination, in reversing the ischemia-induced enhancement of thrombus development in the cerebral microcirculation. These agents were selected for study because they represent the first line of therapeutic intervention for prevention of a secondary stroke after TIA (Johnston 2005).
Materials and Methods
Animals
Male C57BL/6 mice (25-35 g body weight, 6-10 weeks old) were purchased from Jackson Laboratory (Bar Harbor, ME). The experimental procedures were approved by the Institutional Animal Care and Use Committee at LSU Health Sciences Center and are in compliance with National Institutes of Health guidelines. All efforts were made to minimize animal distress and the number of animals used.
Induction of transient cerebral ischemia
Transient focal cerebral ischemia was induced in anesthetized (150 mg/kg ketamine and 7.5 mg/kg xylazine) mice using the intraluminal filament method, as previously described (Ishikawa et al 2005). The MCA was occluded for a period of 2.5 to 10 min, followed by a recovery (reperfusion) period of 1 - 28 days. Sham mice were subjected to the same procedure without arteriotomy and monofilament insertion.
Intravital videomicroscopy
Following 1 to 28 days of reperfusion, the mice were anesthetized and craniectomy (3 mm diameter; 1 mm posterior, 4 mm lateral from the bregma) was made and covered with a glass coverslip. The space between the glass and dura mater was filled with artificial cerebrospinal fluid (Gavins et al 2007). The mouse was placed to the stage of an upright fluorescent microscope (BX51WI; Olympus, Japan) and allowed to equilibrate for 30 minutes. Visualization of individual cerebral microvessels and the induction of thrombus formation were achieved using a 40× water immersion objective lens (LUMPlan FI/IR 40×/0.80×; Olympus). The images were recorded on a DVD player (SR-MV50; JVC, Wayne, NJ). The diameters of the selected microvessels were measured using video analysis software (Image J software; NIH, US) on a personal computer (G4 Macintosh; Apple, CA).
Light/dye induced thrombosis
A 10 ml/kg body weight dose of 5% fluorescein isothiocyanate dextran (FITC; 150,000 molecular weight; Sigma-Aldrich, US) was injected into the femoral vein and allowed to circulate for 10 minutes. Photoactivation of FITC (excitation, 495 nm; emission, 519 nm) was initiated by exposing 100 μm vessel length to epi-illumination with a 175-W xenon lamp (Lamda LS, Sutter, US) coupled with a fluorescein filter cube (HQ-FITC; Chroma Technology, US). The excitation power density was calibrated daily and maintained within 1% of 0.17 W/cm2, as previously described (Senchenkova et al 2013). During continuous epi-illumination, thrombus formation was visualized and quantified in both venules and arterioles (diameters: 20–40μm) by determining the time of onset of platelet deposition/aggregation (visualized as the initial accumulation of 3+ platelets) on the vessel wall (onset time) and the time to complete blood flow cessation (cessation time). Onset time and cessation time were averaged from 2–4 induced thrombi in each vessel type (i.e., venules and arterioles) of the same mouse. The internal diameters of arterioles and venules were measured by offline analysis for the period immediately preceding the determination of onset time and just prior to complete flow cessation. In order to address whether the light/dye method elicited vasoconstriction, the diameters measured at the time of flow cessation was normalized (expressed as %) to the diameter determined prior to onset time.
Neurological assessment
The functional consequences of the transient ischemic insult were evaluated in mice following 1-24 hrs of reperfusion using a 5-point scale neurological deficit score where: 0 = no deficit; 1 = failure to extend right paw; 2 = circling to the right; 3 = falling to the right; and 4 = unable to walk spontaneously, as previously described (Ishikawa et al 2005).
Quantification of infarction volume
After 24 hr reperfusion, mice were euthanized, the brain immediately removed, and placed into 4°C PBS for 15 minutes. Coronal sections (2-mm) were stained with 2% 2,3,5-triphenyltetrazolium chloride (TTC) solution for 15 minutes at 37°C and fixed by immersion in 10% formaldehyde (Terao et al 2008). The total areas of each brain section and the infarcted region were quantified using a computerized image analysis program (NIH Image Software).
Anti-platelet therapy
Aspirin, clopidogrel and dipyridamole were obtained from Sigma Chemicals (St. Louis, MO) and atorvastatin from AK Scientific Inc (Union City, CA). In these mice, the brain was subjected to 5 min of transient focal cerebral ischemia followed by 1 day of reperfusion. The following treatment (prior to MCA occlusion) regimens were used: aspirin (50mg/kg/day, 5 days pretreatment plus one dose 2 hrs after ischemia, by gavage) (Tailor et al 2007), dipyridamole (60mg/kg by gavage, 2 hrs after ischemia) (Kim et al 2008) and atorvastatin (10mg/kg/day, 5 days pretreatment plus one dose immediately after ischemia) (Chen et al 2005). Clopidogrel (3mg/kg by gavage)(Bird et al 2012) was administered 2 hrs following release of the MCA occlusion. For the combination treatment experiments, the following schedule was used: 1) atorvastatin (14 day pretreatment plus one dose after ischemia), 5 day dipyridamole pretreatment plus one dose after ischemia), or 2) atorvastatin and dipyridamole (as described in #1) plus 5 day aspirin pretreatment and one dose 2 hrs after ischemia.
Tail bleeding time
Anesthetized mice were placed on a warm pad and the tails were transected 5 mm from the tip with a razor blade and immediately immersed into a 15 ml falcon tube filled with 14 ml saline (37°C). The total time (including re-bleeding time) for cessation of bleeding was recorded, and after 10 minutes the bleeding was stopped by cauterization.
Immunohistochemistry
Spontaneous thrombus development in cerebral microvessels was evaluated in mice subjected to either 5 min or 60 min of MCAo followed by 24 hrs of reperfusion. For these experiments, mice were transcardially perfused with 1x phosphate buffered saline (PBS) followed by 10% formalin. Brains were collected, embedded in paraffin, and sectioned into 5μm sections. The sections were deparaffinized followed by antigen retrieval using 1x antigen decloaker (Biocare Medical, Concord, CA). Sections were then stained for fibrinogen and glycoprotein 1 beta (GP1b). The primary antibodies used were rabbit anti-mouse fibrinogen (1:500; GeneTex, Irvine, CA) and rat anti-mouse GP1b (1:100; emfret, Eibelstadt, Germany). Secondary antibodies used were Alexa Fluor® 647 goat anti-rabbit (1:600; Life Technologies, Grand Island, NY) and Alexa Fluor® 488 goat anti-rat (1:500; Life Technologies). Images of the double stained tissue sections were captured on an Olympus BX51WI upright microscope (Olympus, Center Valley, PA) with a 20X (LUCPlanFLN) objective and equipped with a 3i LaserStack laser launch (3i; Denver, CO), Yokogawa CSU-X1-A1N-E spinning disk confocal unit (Yokogawa Electric Corporation, Tokyo, Japan) and electron multiplier CCD camera (C9100-13; Hamamatsu, Bridgewater, NJ). 488-, and 640-nm laser excitation were used in rapid sequence and visualized with the appropriate filters (Semrock, Rochester, NY). Slidebook software (3i) was used to drive the confocal system and capture images for offline analysis. Merged images were used to determine areas of co-localization.
Statistical analysis
Data are presented as means ± SEM unless otherwise indicated. One-way ANOVA was used with the Bonferroni post hoc test. Differences between two data sets were determined using the two-tailed unpaired t-test. Statistical significance was set at p < 0.05.
Results
Neurological deficit and injury response to transient cerebral ischemia
While 2.5 to 10 min of MCAo, followed by reperfusion, did not induce significant sustained neurological deficits or produce a detectable brain infarction at 24 hrs, exposure of the mouse brain to 120 min of ischemia (followed by the same durations of reperfusion) elicited significant sustained behavioral changes at 2-4 and 24 hrs after reperfusion, and yielded a large infarct volume at 24 hrs (Table I).
Table I.
Neurological deficit and infarct size after transient cerebral ischemia.
| Group | Post-MCAo ( 2-4hr R) |
Post-MCAo (24hr R) | Infarct Volume (%) |
|---|---|---|---|
| Sham | No deficit (0) | No deficit (0) | 0 |
|
MCAo
2.5 min |
No deficit (0) | No deficit (0) | 0 |
| 5 min | No deficit (0) | No deficit (0) | 0 |
| 10 min | Transient circling to the right (2.0 ± 0*) |
No deficit (0) | 0 |
| 120 min | - | Circling/falling to the right or unable to walk (2.67 ± 0.33*) |
38.25 ± 0.68* |
Enhanced thrombus formation after brief periods of MCAo and reperfusion
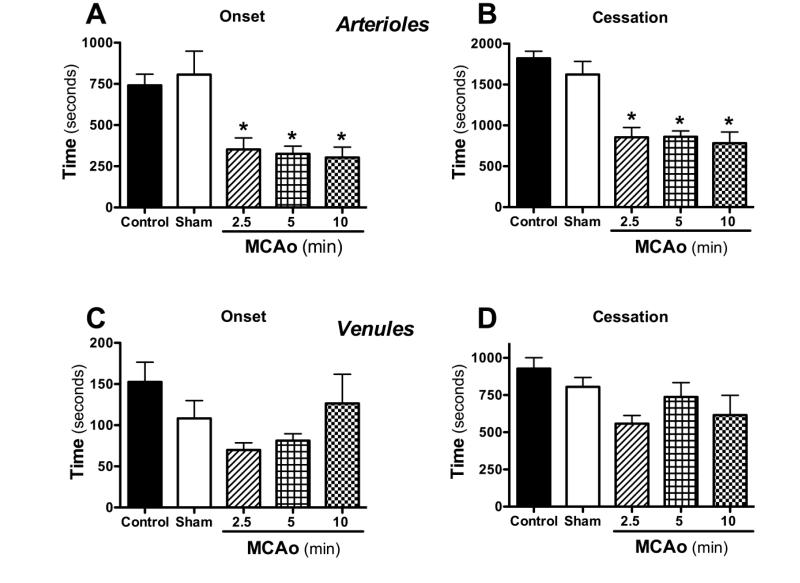
Thrombus formation in cerebral arterioles and venules was quantified in control and shamoperated mice, and in mice subjected to 2.5 – 10 min of MCAo, followed by 24 hr reperfusion (Figure 1). A significant enhancement of thrombus development (reflected in both onset and cessation times) was detected in cerebral arterioles of mice subjected to 2.5 – 10 min MCAo and 24 hr reperfusion (MCAo/R). While a similar trend was noted in venules, the response was more variable and did not achieve statistical significance. Because no differences in the thrombosis response were noted between 2.5, 5 or 10 min of MCAo, all subsequent experiments were performed using 5 min MCAo.
Figure 1. Transient ischemia accelerates thrombus development in cerebral arterioles.
Following middle cerebral artery occlusion (MCAo) for either 2.5 (n=6), 5 (n=8) or 10 minutes (n=9) and 24 hrs reperfusion, thrombosis was induced in arterioles and venules by light/dye injury. Onset indicates the time of onset of platelet deposition on the vessel wall, while cessation refers to the time required for complete flow cessation. * indicates p<0.01 compared to control (n=13) or sham (n=8) mice.
An assessment of the changes in vessel diameter elicited by the light/dye method was performed in sham control mice and in mice exposed to 5 min MCAo and 24 hrs reperfusion. Vessel diameters detected just prior to complete flow cessation and normalized to the diameter measured just prior to onset time were as follows: arteriolessham controls (97.6±0.8 %) and MCAo/R (97.8±0.6 %), and venules-sham controls (96.6±0.7%) & MCAo/R (96.1±1.0 %). The data suggest that the light/dye method elicited minimal vasoconstriction in both arterioles and venules of mice subjected to either a sham procedure or MCAo/R.
Duration of accelerated arteriolar thrombosis after transient focal cerebral ischemia
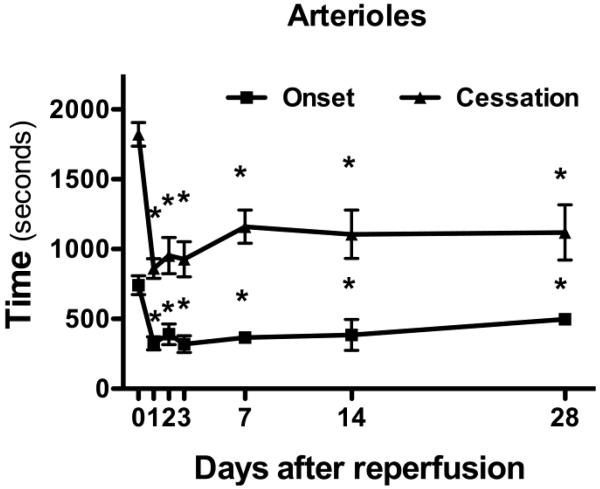
The time-course of the 5 min MCAo-induced enhancement of cerebral arteriolar thrombosis was assessed over a period of 28 days following reperfusion (Figure 2). The accelerated onset time and time to flow cessation induced in cerebral arterioles by 5 min MCAo were detected up to 28 days following the transient ischemic insult.
Figure 2. The accelerated arteriolar thrombosis following transient focal ischemia (5 min) is sustained for 28 days following reperfusion.
Thrombus development was evaluated in control (day-0; n=13) mice and at 1- (n=8), 2- (n=4), 3- (n=4), 7- (n=3), 14- (n=3) and 28- (n=2) days of reperfusion. Onset indicates the time of onset of platelet deposition on the vessel wall, while cessation refers to the time required for complete flow cessation. *denotes p<0.05 compared to control (day-0) response.
Spontaneous thrombus formation following transient ischemia
Fluorescent staining was performed on the brains of mice that were subjected to either 60 min or 5 min of MCAo followed by 24 hrs of reperfusion. The brains were stained for fibrinogen and GP1b (platelet marker) to identify thrombi. As previously reported (Kleinschnitz et al, 2006), mice exposed to 60 min ischemia and 24 hrs reperfusion had evidence of spontaneous thrombus formation in arterioles in the ipsilateral hemisphere, as indicated by areas of co-localization of fibrinogen and GP1b (Supplementary Figure S1A); no staining was observed in the contralateral hemisphere (Supplementary Figure S1B). Spontaneous thrombus formation was not observed in cerebral arterioles of mice subjected to 5 min ischemia and 24 h reperfusion. However, the shorter duration of ischemia did result in fibrinogen and GP1b positive staining within capillary sized vessels in the ipsilateral hemisphere, as evidenced by activated platelets surrounded by lower levels of fibrinogen staining (Supplementary Figure S1C). This pattern was not detected in the contralateral hemisphere (Supplementary Figure S1D).
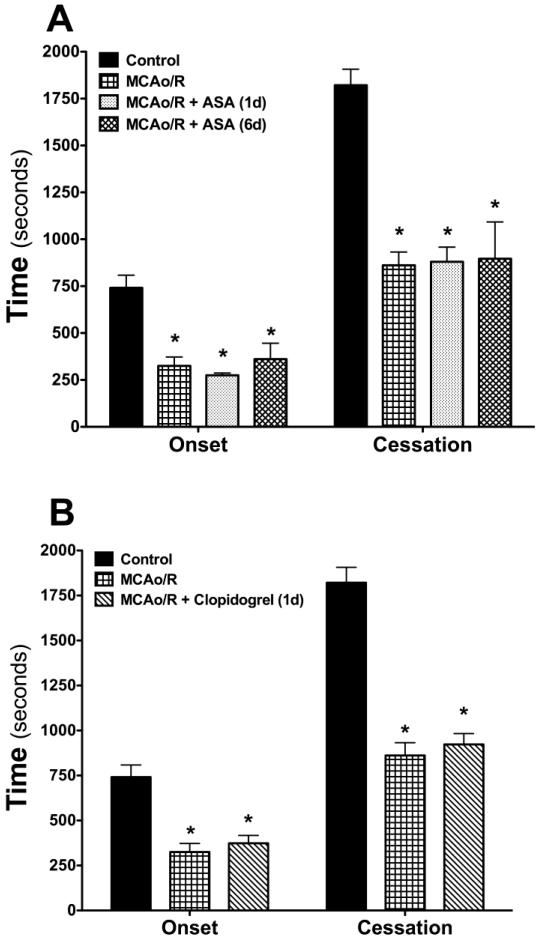
Effects of aspirin (ASA) treatment on the thrombosis response to focal cerebral ischemia
Mice treated with ASA for either 1 or 6 consecutive days prior to measurement of thrombus formation following MCAo/R did not reveal a significant change in either the time of onset or time to flow cessation in cerebral arterioles after light/dye injury (Figure 3A). We have previously reported that the dose of ASA used in these studies completely inhibits cyclooxygenase activity in mouse platelets (Tailor et al, 2007). The anti-platelet effect of the ASA treatment regimen is supported by the significant increase in tail bleeding time (Table II).
Figure 3. Effects of treatment with either aspirin (ASA) or clopidogrel on the accelerated thrombus development in arterioles induced by transient (5 min) ischemia and 24 hrs reperfusion.
The following numbers of mice were included in each group: Controls (n=13), MCAo/R (n=8), MCAo/R + ASA (1d) (n=7), (6d) (n=6), MCAo/R + clopidogrel (1d) (n=5). Onset indicates the time of onset of platelet deposition on the vessel wall, while cessation refers to the time required for complete flow cessation. *denotes p<0.05 compared to control (day-0) response.
Table II.
Tail bleeding times in control and treated mice.
| Treatment | Bleeding Time |
|---|---|
| Control (n=5) | 62 ± 4.1 seconds |
| Aspirin (50mg/kg) (n=5) | >10 minutes |
| Clopidogrel (3mg/kg) (n=4) | >10 minutes |
| Dipyridamole (60mg/kg) (n=4) | >10 minutes |
| Atorvastatin (10mg/kg) (n=5) | >10 minutes |
| Atorvastatin + Dipyridamole (n=5) | >10 minutes |
| Atorvastatin + Dipyridamole + Aspirin (n=5) |
>10 minutes |
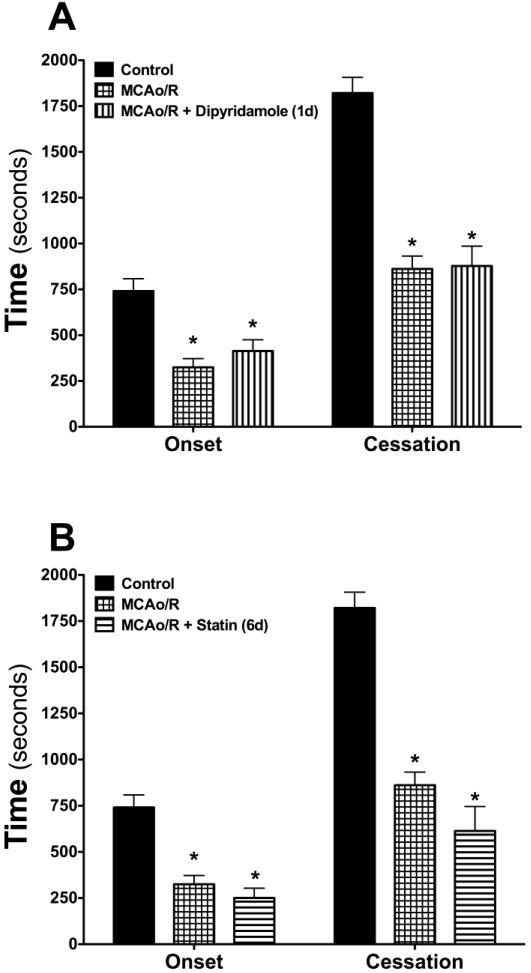
Effects of clopidogrel, dipyridamole and statin monotherapy on the thrombosis response
Studies were also undertaken to examine the influence of treatment with either clopidogrel (Figure 3B), dipyridamole (Figure 4A) or atorvastatin (Figure 4B) on the accelerated arteriolar thrombosis elicited by transient cerebral ischemia and reperfusion. With all three agents, either short term (1 day) or long-term (6 day) pretreatment did not alter the MCAo/R-induced enhancement of cerebral arteriolar thrombosis, despite the efficacy of all three agents (individually) in prolonging tail bleeding time (Table II).
Figure 4. Effects of dipyridamole or atorvastatin treatment on focal ischemia-reperfusion induced thrombogenesis in cerebral arterioles.
The following numbers of mice were included in each group: Controls (n=13), MCAo/R (n=8), MCAo/R + dipyridamole (n=5), MCAo/R + statin (n=5). Onset indicates the time of onset of platelet deposition on the vessel wall, while cessation refers to the time required for complete flow cessation. *denotes p<0.05 compared to control (day-0) response.
Effects of anti-platelet + statin polytherapy on microvascular thrombosis response
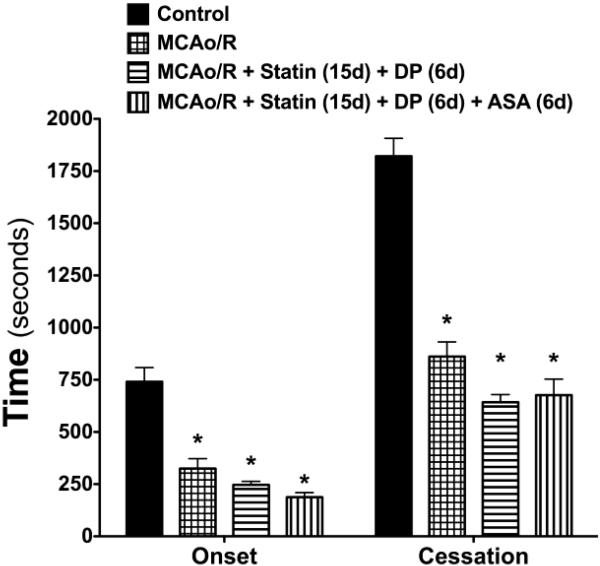
Given the absence of effect of the above-mentioned drug treatments on the accelerated thrombosis response to MCAo/R, we evaluated the effects of combinations of these drugs (Figure 5). Treatment with either a combination of atorvastatin (15 days) + dipyridamole (6 days), or atorvastatin (15 days) + dipyridamole (6 days) + aspirin (6 days) did not alter the thrombosis response to MCAo/R. Both polytherapy regimens were effective in prolonging the tail bleeding time (Table II).
Figure 5. Effects of combined treatment with atorvastatin, dipyridamole, and/or aspirin on the enhanced cerebral arteriolar thrombosis induced by MCAo/R.
The following numbers of mice were included in each group: Controls (n=13), MCAo/R (n=8), MCAo/R + statin + dipyridamole (DP) (n=7), MCAo/R + statin + dipyridamole (DP) + aspirin (ASA) (n=6). Onset indicates the time of onset of platelet deposition on the vessel wall, while cessation refers to the time required for complete flow cessation. *denotes p<0.05 compared to control (day-0) response.
Discussion
Animal models of ischemic stroke have revealed that exposure of the brain to a brief period (5-10 min) of sublethal ischemia protects the tissue and improves outcome following a more severe ischemic insult (Kirino 2002, Gidday 2006). This preconditioning response, which has been attributed to the upregulation of neuroprotective genes, is seemingly at odds with clinical evidence that TIA patients are at greater risk for a subsequent more severe ischemic stroke. It has been suggested that the divergent effects of antecedent sublethal ischemia on stroke risk versus outcome may reflect differential responses of the cerebral vasculature and neuronal tissue to the initial insult, with the former (e.g., endothelial cells) exhibiting damage and the latter (neurons, glia) showing protection (Danton et al 2002). Nonetheless, there are some reports that describe an improvement of cerebral vascular function (e.g., blood brain barrier permeability, endothelium-dependent vasodilation) in response to IPC (Gidday 2006). However, vascular wall thrombogenicity may be of greater relevance to the increased risk for stroke following brief periods of transient ischemia (or TIA). In the present study, we examined whether brief periods of sublethal cerebral ischemia followed by reperfusion alter the potential for thrombus development in the cerebral microvasculature. Our study revealed that 2.5 – 10 min of brain ischemia elicits a pronounced and prolonged (up to 28 days) acceleration of thrombus formation in cerebral arterioles, findings consistent with an increased risk for thrombotic stroke after TIA.
Although thrombus formation following IPC in the brain has not been previously addressed, there are studies of other tissues that bear on this issue. For example, an examination of thrombus formation in large arteries (coronary and femoral) subjected to either local or remote (distant tissue) IPC have revealed improved vessel patency, the generation of small thrombi and fewer emboli after a subsequent insult (Hata et al 1998, Ropcke et al 2012). The anti-thrombogenic response of larger arteries to IPC is supported by a report describing attenuation of different molecular indices of platelet activation, including fibrinogen binding and aggregation with neutrophils (Linden et al 2006). Studies of microvascular thrombogenesis after preconditioning have been more limited. However, one report (Rucker et al 2009) describes a significant acceleration of thrombus formation in microvessels (both arterioles and venules) of muscle and subcutis of rat hindlimb preconditioned by thermal stress (either hypothermia or hyperthermia). The latter study, when considered with the findings in our study of the cerebral microvasculature and published data from large arteries, suggest that, unlike large arteries, arterioles respond to preconditioning stimuli by exhibiting an exaggerated thrombogenic response. Our findings of an enhancement of thrombogenicity in cerebral arterioles after brief transient ischemia may have relevance to the role of small vessel disease in the pathogenesis of TIA and to the increased risk for silent strokes after TIA (Laloux et al 1994, Wang et al 2011).
It has been previously reported that 45-60 min of focal brain ischemia followed by 23-24 hrs of reperfusion is associated with spontaneous development of microvascular thrombosis, as evidenced by fibrin deposition in the ipsilaterl cerebral hemisphere (Choudhri et al, 1998; Kleinschnitz et al, 2006). While we were able to confirm this observation in this study using fibrinogen and platelet immunostaining, we found no evidence for spontaneous thrombus development in arterioles or venules following a brief period (5 min) of ischemia and 24 hrs reperfusion. This observation, coupled to our intravital microscopic findings, suggest that the deleterious effect of the brief transient ischemic episode is to render the cerebral microvasculature more vulnerable to a subsequent stress condition that promotes thrombogenesis.
A variety of drugs have been used to reduce the risk of recurrent stroke following TIA or ischemic stroke due to thrombosis. These include antiplatelet therapies such as aspirin, clopidogrel, and dipyridamole, as well as statins (Phillips 2008, Sudlow 2008). These agents, when used alone, have generally shown some efficacy in reducing stroke recurrence. Since the recurrence rate following treatment with an antiplatelet agent or statin remains high, emphasis has also been given to using combination therapy, which has proven to be more effective for secondary prevention of stroke than single drug therapy (Phillips 2008). Hence, another major objective of our study was to determine whether aspirin, clopidogrel, dipyridamole, and atorvastatin attenuate the accelerated cerebral microvascular thrombus formation that follows a brief period of transient focal ischemia. Our findings indicate that none of these drugs, when used alone or in combination, significantly blunt the accelerated cerebral microvascular thrombus development elicited by a brief ischemic episode. This lack of protection was noted despite evidence for a significant prolongation of bleeding time with each treatment regimen.
While a definitive explanation for the insensitivity of thrombus formation in cerebral arterioles to antiplatelet and/or statin therapy is not readily available, this may reflect species-related differences in coagulation profiles and/or efficacy of anti-platelet drugs between rodents and human, and/or differences in the contribution of platelets vs the vessel wall to thrombus development in microscopic vs large arteries. Consistent with the latter notion is a report describing a greater dependency of occlusive thrombus formation on platelet count in the carotid artery than in arterioles (Morowski et al 2013). It was noted that while thrombus development in damaged arterioles occurs even when platelet count is reduced by 97.5%, thrombosis in the carotid artery requires a platelet count that is greater than 20% of control (Morowski et al 2013). Macroscopic and microscopic arteries also exhibit significant differences in physical forces (e.g., shear rate), expression of coagulation factors (e.g., von Willbrand factor) and local production of platelet agonists (e.g., ADP, thrombin, thromboxane) and antagonists (e.g., adenosine, prostacyclin) that could account for the insensitivity of thrombus formation in cerebral arterioles to antiplatelet agents and statins. Finally, it should be noted that anti-platelet and anticoagulant agents other than the ones used in this study have shown some efficacy in murine models of ischemic stroke. For example, it has been reported that targeting coagulation factor XII effectively reduces vessel-occluding fibrin formation in ischemic cerebral microvessels (Kleinschnitz et al, 2006). Furthermore, it has been shown that interfering with either von Willbrand factor (Kleinschnitz et al, 2009) or GPIb-alpha (Kleinschnitz et al, 2007) protects mice against ischemic stroke. Consequently, these targets may be more appropriate for interference with TIA-enhanced thrombogenesis.
The known antithrombotic actions of statins appear to differ from that of antiplatelet drugs. While statins can inhibit components of the coagulation cascade (e.g., tissue factor), and blunt the activation, adhesion and aggregation of platelets, these drugs also diminish the adhesivity of microvascular endothelium for both platelets and leukocytes (Violi et al 2013, Stokes et al 2002). The anti-adhesive effects of statins have been attributed to the activation of endothelial cell nitric oxide synthase and generation of nitric oxide (Stokes et al 2002). Manipulation of nitric oxide (NO) production alters thrombus development in the microvasculature, with studies showing that endothelial cell-derived NO protects against thromboembolism in venules, but not in arterioles (Broeders et al 1998). While the effects of statin treatment on thrombus development in cerebral arterioles have not been previously addressed, rosuvastatin-treated mice have been shown to exhibit an attenuation of ferric chloride-induced thrombus formation in the carotid artery (Schafer et al 2005).
In conclusion, the findings of this study reveal an increased thrombogenicity of cerebral arterioles after exposure to a brief period of sub-lethal ischemia followed by reperfusion. This response, which persists for up to 28 days after the ischemic insult, suggests that the walls of cerebral arterioles do not exhibit the protective preconditioning that is manifested in neuronal tissue subjected to similar insults. The inability of different antiplatelet agents and/or a statin to blunt the ischemia-reperfusion induced acceleration of thrombus formation in cerebral arterioles indicates that alternative cellular and molecular mechanisms/targets contribute to this response. Additional work is needed to determine the pathophysiological relevance of these observations relative to the increased risk for stroke after TIA, and to define the mechanism(s) that underlie the enhanced thrombogenicity in cerebral arterioles following a brief period of sub-lethal ischemia.
Supplementary Material
Highlights.
Cerebral microvascular thrombus formation was evaluated after ischemia (2-10 min).
The rate of thrombus formation was accelerated following brain ischemia.
The accelerated thrombogenesis was evident up to 28 days after ischemia.
Antiplatelet and antithrombotic agents did not blunt this response to ischemia.
Acknowledgments
Supported by a grant from the National Heart Lung and Blood Institute (HL26441).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bird JE, Wang X, Smith PL, Barbera F, Huang C, Schumacher WA. A platelet target for venous thrombosis? P2Y1 deletion or antagonism protects mice from vena cava thrombosis. J Thromb Thrombolysis. 2012;34(2):199–207. doi: 10.1007/s11239-012-0745-3. [DOI] [PubMed] [Google Scholar]
- Broeders MA, Tangelder GJ, Slaaf DW, Reneman RS, oude Egbrink MG. Endogenous nitric oxide protects against thromboembolism in venules but not in arterioles. Arterioscler Thromb Vasc Biol. 1998;18(1):139–45. doi: 10.1161/01.atv.18.1.139. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, et al. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25(2):281–90. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhri TF, Hoh BL, Zerwes HG, Prestigiacomo CJ, Kim SC, Connolly ES, Jr., Kottirsch G, Pinsky DJ. Reduced microvascular thrombosis and improved outcome in acute murine stroke by inhibiting GP IIb/IIIa receptor-mediated platelet aggregation. J. Clin. Invest. 1998;102:1301–1310. doi: 10.1172/JCI3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danton GH, Prado R, Watson BD, Dietrich WD. Temporal profile of enhanced vulnerability of the post-thrombotic brain to secondary embolic events. Stroke. 2002;33(4):1113–9. doi: 10.1161/hs0402.105554. [DOI] [PubMed] [Google Scholar]
- Dennis M, Bamford J, Sandercock P, Warlow C. Prognosis of transient ischemic attacks in the Oxfordshire Community Stroke Project. Stroke. 1990;21(6):848–53. doi: 10.1161/01.str.21.6.848. [DOI] [PubMed] [Google Scholar]
- Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council. Stroke. 2009;40(6):2276–93. doi: 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- Gavins FN, Dalli J, Flower RJ, Granger DN, Perretti M. Activation of the annexin 1 counter-regulatory circuit affords protection in the mouse brain microcirculation. FASEB J. 2007;21(8):1751–8. doi: 10.1096/fj.06-7842com. [DOI] [PubMed] [Google Scholar]
- Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7(6):437–48. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- Hata K, Whittaker P, Kloner RA, Przyklenk K. Brief antecedent ischemia attenuates platelet-mediated thrombosis in damaged and stenotic canine coronary arteries: role of adenosine. Circulation. 1998;97(7):692–702. doi: 10.1161/01.cir.97.7.692. [DOI] [PubMed] [Google Scholar]
- He Y, Karabiyikoglu M, Hua Y, Keep RF, Xi G. Ischemic preconditioning attenuates brain edema after experimental intracerebral hemorrhage. Transl Stroke Res. 2012;3(Suppl 1):180–187. doi: 10.1007/s12975-012-0171-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Vowinkel T, Stokes KY, Arumugam TV, Yilmaz G, Nanda A, Granger DN. CD40/40 ligand signaling in mouse cerebral microvasculature after focal ischemia/reperfusion. Circulation. 2005;111:1690–1696. doi: 10.1161/01.CIR.0000160349.42665.0C. [DOI] [PubMed] [Google Scholar]
- Johnston SC. Transient ischemic attack: a dangerous harbinger and an opportunity to intervene. Semin Neurol. 2005;25(4):362–70. doi: 10.1055/s-2005-923530. [DOI] [PubMed] [Google Scholar]
- Kim HH, Sawada N, Soydan G, Lee HS, Zhou Z, Hwang SK, et al. Additive effects of statin and dipyridamole on cerebral blood flow and stroke protection. J Cereb Blood Flow Metab. 2008;28(7):1285–93. doi: 10.1038/jcbfm.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirino T. Ischemic tolerance. J Cereb Blood Flow Metab. 2002;22(11):1283–96. doi: 10.1097/01.WCB.0000040942.89393.88. [DOI] [PubMed] [Google Scholar]
- Kleinschnitz C, De Meyer SF, Schwarz T, Austinat M, Vanhoorelbeke K, Nieswandt B, Deckmyn H, Stoll G. Deficiency of von Willebrand factor protects mice from ischemic stroke. Blood. 2009;113(15):3600–3. doi: 10.1182/blood-2008-09-180695. [DOI] [PubMed] [Google Scholar]
- Kleinschnitz C, Pozgajova M, Pham M, Bendszus M, Nieswandt B, Stoll G. Targeting platelets in acute experimental stroke: impact of glycoprotein Ib, VI, and IIb/IIIa blockade on infarct size, functional outcome, and intracranial bleeding. Circulation. 2007;115(17):2323–30. doi: 10.1161/CIRCULATIONAHA.107.691279. [DOI] [PubMed] [Google Scholar]
- Kleinschnitz C, Stoll G, Bendszus M, Schuh K, Pauer HU, Burfeind P, Renne C, Gailani D, Nieswandt B, Renne T. Targeting coagulation factor XII provides protection from pathological thrombosis in cerebral ischemia without interfering with hemostasis. J Exp Med. 2006;203(3):513–518. doi: 10.1084/jem.20052458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laloux P, Ossemann M, Jamart J. Stroke subtypes and risk factors associated with silent infarctions in patients with first-ever ischemic stroke or transient ischemic attack. Acta Neurol Belg. 1994;94(1):17–23. [PubMed] [Google Scholar]
- Li HF, Zhang X, Zhang Y, Pan XD, Zhao HQ, Li H. Clinical and neuroradiological features of internal watershed infarction and the occlusive diseases of carotid artery system. Neurol Res. 2010;32(10):1090–6. doi: 10.1179/016164110X12681290831324. [DOI] [PubMed] [Google Scholar]
- Linden MD, Whittaker P, Frelinger AL, 3rd, Barnard MR, Michelson AD, Przyklenk K. Preconditioning ischemia attenuates molecular indices of platelet activation-aggregation. J Thromb Haemost. 2006;4(12):2670–7. doi: 10.1111/j.1538-7836.2006.02228.x. [DOI] [PubMed] [Google Scholar]
- Lovett JK, Coull AJ, Rothwell PM. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology. 2004;62(4):569–73. doi: 10.1212/01.wnl.0000110311.09970.83. [DOI] [PubMed] [Google Scholar]
- Morowski M, Vögtle T, Kraft P, Kleinschnitz C, Stoll G, Nieswandt B. Only severe thrombocytopenia results in bleeding and defective thrombus formation in mice. Blood. 2013;121(24):4938–47. doi: 10.1182/blood-2012-10-461459. [DOI] [PubMed] [Google Scholar]
- O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376(9735):112–23. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- Phillips RA. A review of therapeutic strategies for risk reduction of recurrent stroke. Prog Cardiovasc Dis. 2008;50(4):264–73. doi: 10.1016/j.pcad.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropcke DM, Hjortdal VE, Toft GE, Jensen MO, Kristensen SD. Remote ischemic preconditioning reduces thrombus formation in the rat. J Thromb Haemost. 2012;10:2405–6. doi: 10.1111/j.1538-7836.2012.04914.x. [DOI] [PubMed] [Google Scholar]
- Rücker M, Laschke MW, Stamm A, Harder Y, Vollmar B, Menger MD. Local preconditioning by thermal stress accelerates microvascular thrombus formation. Shock. 2009;31(6):627–33. doi: 10.1097/SHK.0b013e318188f815. [DOI] [PubMed] [Google Scholar]
- Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MS, George MG, Hamdan AD, Higashida RT, Hoh BL, Janis LS, Kase CS, Kleindorfer DO, Lee JM, Moseley ME, Peterson ED, Turan TN, Valderrama AL, Vinters HV. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(7):2064–89. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer K, Kaiser K, Konstantinides S. Rosuvastatin exerts favourable effects on thrombosis and neointimal growth in a mouse model of endothelial injury. Thromb Haemost. 2005;93(1):145–52. doi: 10.1160/TH04-07-0415. [DOI] [PubMed] [Google Scholar]
- Senchenkova EY, Komoto S, Russell J, Almeida-Paula LD, Zhang S, Granger DN. Interleukin-6 mediates the platelet abnormalities and thrombogenesis associated with experimental colitis. Am J Pathol. 2013;183(1):173–81. doi: 10.1016/j.ajpath.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Stevens SL, Xiong Z, Lessov NS, Harrington CA, Mori M, et al. Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: similarity to neuroprotective strategies in hibernation and hypoxia-tolerant states. Lancet. 2003;362:1028–37. doi: 10.1016/S0140-6736(03)14412-1. [DOI] [PubMed] [Google Scholar]
- Stokes KY, Cooper D, Tailor A, Granger DN. Hypercholesterolemia promotes inflammation and microvascular dysfunction: role of nitric oxide and superoxide. Free Radic Biol Med. 2002;33(8):1026–36. doi: 10.1016/s0891-5849(02)01015-8. [DOI] [PubMed] [Google Scholar]
- Sudlow C. Preventing further vascular events after a stroke or transient ischaemic attack: an update on medical management. Pract Neurol. 2008;8(3):141–57. doi: 10.1136/jnnp.2008.148064. [DOI] [PubMed] [Google Scholar]
- Tailor A, Wood KC, Wallace JL, Granger DN. Roles of platelet and endothelial cell COX-1 in hypercholesterolemia-induced microvascular dysfunction. Am J Physiol Heart Circ Physiol. 2007;293(6):H3636–42. doi: 10.1152/ajpheart.01105.2006. [DOI] [PubMed] [Google Scholar]
- Terao S, Yilmaz G, Stokes KY, Ishikawa M, Kawase T, Granger DN. Inflammatory and injury responses to ischemic stroke in obese mice. Stroke. 2008;39(3):943–50. doi: 10.1161/STROKEAHA.107.494542. [DOI] [PubMed] [Google Scholar]
- Violi F, Calvieri C, Ferro D, Pignatelli P. Statins as antithrombotic drugs. Circulation. 2013;127(2):251–7. doi: 10.1161/CIRCULATIONAHA.112.145334. [DOI] [PubMed] [Google Scholar]
- Wang JJ, Baker ML, Hand PJ, Hankey GJ, Lindley RI, Rochtchina E, et al. Transient ischemic attack and acute ischemic stroke: associations with retinal microvascular signs. Stroke. 2011;42(2):404–8. doi: 10.1161/STROKEAHA.110.598599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.