Abstract
Autologous hematopoietic cell transplantation (AHCT) for plasma cell myeloma is performed less often in people >70 years old than in people ≤70 years old. We analyzed 11,430 AHCT recipients for plasma cell myeloma prospectively reported to the Center for International Blood and Marrow Transplant Research between 2008 and 2011, representing the majority of US AHCT activity during this period. Survival (OS) was compared in 3 cohorts: ages 18 to 59 years (n = 5818), 60 to 69 years (n = 4666), and >70 years (n = 946). Median OS was not reached for any cohort. In multivariate analysis, increasing age was associated with mortality (P = .0006). Myeloma-specific mortality was similar among cohorts at 12%, indicating an age-related effect on nonmyeloma mortality. Analyses were performed in a representative subgroup comparing relapse rate, progression-free survival (PFS), and nonrelapse mortality (NRM). One-year NRM was 0% for age >70 years and 2% for other ages (P = not significant). The three-year relapse rate was 56% in age 18 to 59 years, 61% in age 60 to 69 years, and 63% age >70 (P = not significant). Three-year PFS was similar at 42% in age 18 to 59 years, 38% in age 60 to 69 years, and 33% in age >70 years (P = not significant). Postrelapse survival was significantly worse for the older cohort (P = .03). Older subjects selected for AHCT derived similar antimyeloma benefit without worse NRM, relapse rate, or PFS.
Keywords: Myeloma, Older patients, Autologous transplantation
INTRODUCTION
Plasma cell myeloma is the most common indication for autologous hematopoietic cell transplantation (AHCT); however, a large number of eligible subjects are not offered a transplantation because of advanced age [1–3]. Randomized studies confirm the benefit of autologous transplantation in subjects ≤65 years of age, but these studies typically excluded older subjects [4,5]. The median age at diagnosis of patients with myeloma is 69 years and prospective transplantation studies in these older subjects are limited or use lower doses of conditioning [6]. Single-institution retrospective studies suggest that older persons with myeloma may receive an autologous transplantation with low risk of nonrelapse mortality (NRM) [7–18]. It is also well established that the recent dramatic improvements in survival have accrued disproportionately to younger patients, with relatively minor improvement in survival of those above age 60 [19].
We analyzed the effects of age on outcomes in persons with myeloma receiving upfront autologous transplantation.
SUBJECTS AND METHODS
Data Source
The Center for International Blood and Marrow Transplant Research (CIBMTR) is a voluntary group of more than 450 transplantation centers worldwide that contribute data on allogeneic and autologous transplantations to a statistical center at the Medical College of Wisconsin in Milwaukee or the National Marrow Donor Program Coordinating Center in Minneapolis, Minnesota. Participating centers are required to register all transplantations done consecutively in a prospective fashion. Subjects are followed longitudinally, with yearly data update. Computerized checks for errors, physicians’ review of submitted data, and on-site audits of participating centers are used to ensure data quality and compliance. Studies conducted by the CIBMTR are performed with a waiver of informed consent and in compliance with Health Insurance Portability and Accountability Act regulations as determined by the institutional review board and the privacy officer of the Medical College of Wisconsin. All CIBMTR centers contribute to the registration or transplant essential data. Detailed data are collected on the comprehensive report form (CRF) level on a subset of registered subjects and include detailed disease and pretransplantation and post-transplantation clinical information. Statistical methods (weighted randomization schema) are used to ensure that the CRF subset are representative of the transplant essential data cohort.
Study Population
Outcomes of 11,430 AHCT recipients with plasma cell myeloma between 2008 and 2011 (n = 11,430) reported from 148 transplantation centers in the United States and Canada were analyzed. During this period, the CIBMTR collected 60% of AHCT activity performed in the United States [20]. The study population included only those receiving a single AHCT within 24 months of diagnosis and receiving high-dose melphalan alone as conditioning.
Statistical Plan
The objective of this study was to analyze the effect of age on survival, NRM, relapse rates, and progression-free survival (PFS) after transplantation. Survival after AHCT was compared and subject to multivariate analyses in 3 age-dependent cohorts: ages 18 to 59 years (cohort 1, n = 5818), 60 to 69 years (cohort 2, n = 4666), and ≥70 years (cohort 3, n = 946). Relapse, PFS, and NRM were compared in a representative subset of 1279 subjects with CRF data after ensuring that survival was identical to the larger sample (Supplemental Figure 1). NRM was defined as mortality after AHCT in the absence of disease relapse or progression. Cumulative incidence probabilities for NRM were calculated accounting for relapse/progression as a competing risk. Point-wise comparison and log-rank analysis were used to analyze the NRM and survival of different groups.
Subject characteristics in study cohorts were compared using the Mann-Whitney-Wilcoxon test for continuous variables and chi-square test for discrete variables. Survival probabilities (overall survival [OS] and PFS) were calculated by using the Kaplan-Meier estimator with the variance estimated by Greenwood’s formula. Multivariate analysis was performed using Cox proportional hazard regression model to adjust for potentially confounding effects of other risk factors. The variables considered in multivariate analysis for survival included age, HCT-specific comorbidity index (HCTCI) [21,22], Karnofsky performance score (KPS), time from diagnosis to transplantation, year of transplantation, disease status at the time of transplantation, and the dose of melphalan conditioning regimen (in mg/m2). The variables considered in multivariate analyses for NRM, progression/relapse, and PFS included age, gender, KPS, HCTCI, disease status at the time of transplantation, melphalan dose (mg/m2), time from diagnosis to transplantation, and the year of transplantation. Stepwise variable selection at a .05 significance level was used to identify significant covariates. In the model, the assumption of proportional hazards was tested for each variable using a time-dependent covariate and graphical methods. All variables considered in the multivariate analysis satisfied the proportionality assumption. All computations were made using the statistical package SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
Subject Characteristics
Subject characteristics are summarized in Table 1, which compares 3 cohorts: patients from 18 to 59 years old (cohort 1, n = 5818), 60 to 69 years old (cohort 2, n = 4666), and ≥70 years old (cohort 3, n = 946). Median age at transplantation in cohorts 1, 2, and 3 was 53, 64, and 72 years, respectively. Subjects in cohort 3 were more likely to be male, have their transplantation in the United States, have a lower Karnofsky score (KPS <90), a worse comorbidity score (HCTCI >2), and have IgA myeloma as compared with those in cohorts 1 and 2. Older subjects in cohorts 2 and 3 were less likely to receive transplantation within the first year of diagnosis and more likely to have melphalan dose (MEL) reduction (MEL <180 mg/m2 in 42%).
Table 1.
Characteristics of Subjects who Underwent First PBSC AHCT within Two Years of Diagnosis for Plasma Cell Myeloma in the United States and Canada, Registered to CIBMTR between 2008 and 2011 (Transplant Essential Data)
| Characteristics of Subjects | Cohort 1 (ages 18–59 yr) | Cohort 2 (ages 60–69 yr) | Cohort 3 (70+ yr) | P Value |
|---|---|---|---|---|
| No. of patients | 5818 | 4666 | 946 | |
| Age at transplantation, median (range), yr | 53(18–59) | 64 (60–69) | 72 (70–89) | |
| Age at transplantation, yr | ||||
| 18–49 yr | 1728 (30) | – | – | <.0001 |
| 50–59 yr | 4090(70) | – | – | |
| 60–64 yr | – | 2617 (56) | – | |
| 65–69 yr | – | 2049 (44) | – | |
| 70–74 yr | – | – | 794 (83) | |
| 75–79 yr | – | – | 146(15) | |
| 80+ | – | – | 6(<1) | |
| Male sex | 3278 (56) | 2689 (58) | 608 (64) | <.0001 |
| Region | ||||
| United States | 5488 (94) | 4424 (95) | 938 (99) | <.0001 |
| Canada | 330 (6) | 242 (5) | 8(<1) | |
| KPS before transplantation | ||||
| >90 | 766(13) | 500(11) | 88(9) | <.0001 |
| 80–90 | 4213(72) | 3444 (74) | 716(76) | |
| <80 | 515(9) | 515(11) | 106(11) | |
| Missing | 324 (6) | 207 (4) | 8(<1) | |
| HCTCI score | ||||
| 0–1 | 3277(56) | 2373(51) | 454 (48) | <.0001 |
| 2–3 | 1551(27) | 1295(28) | 307(32) | |
| >4 | 725(12) | 795 (17) | 117(19) | |
| Missing | 265 (5) | 203 (4) | 8(<1) | |
| Serum creatinine ≥ 1.5 at diagnosis | 164 (23) | 121 (24) | 17(24) | |
| Immunochemical subtype of plasma cell myeloma | ||||
| IgG | 3312(57) | 2690(58) | 535(57) | <.0001 |
| IgA | 1127(19) | 1028 (22) | 234(25) | |
| Light chain | 1170(20) | 807 (17) | 152(16) | |
| Others (Ig M, D, or E) | 82(1) | 45(1) | 11(1) | |
| Nonsecretory | 127(2) | 96(2) | 14(1) | |
| Disease status at transplantation | ||||
| CR | 839(14) | 662 (14) | 117(12) | .1209 |
| VGPR | 1628(28) | 1384 (30) | 278 (29) | |
| PR | 2798(48) | 2186 (47) | 462 (49) | |
| SD | 341 (6) | 248 (5) | 57(6) | |
| REL/PROG | 180(3) | 170(4) | 31 (3) | |
| Missing | 32 (<1) | 16 (<1) | 1(<1) | |
| MEL | 200 (100–220) | 200 (100–220) | 200 (100–200) | |
| <140 | 140 (2) | 132 (3) | 44(5) | <.0001 |
| 140–180 | 336 (6) | 552 (12) | 354 (37) | |
| ≥180 | 4982 (86) | 3715 (80) | 480(51) | |
| Missing | 330 (6) | 267 (6) | 68(7) | |
| Time from diagnosis to transplantation, median (range), mo | 7 (<l–24) | 8 (<l–24) | 8 (<l–24) | |
| <12 mo | 4739(81) | 3632 (78) | 745 (79) | <.0001 |
| 12–18 mo | 760(13) | 698 (15) | 136(14) | |
| 18–24 mo | 319(6) | 336 (7) | 65(7) | |
| Yr of transplantation | ||||
| 2008 | 1177(20) | 877 (19) | 149(16) | |
| 2009 | 1392(24) | 1077(23) | 195(21) | |
| 2010 | 1566(27) | 1273 (27) | 286 (30) | |
| 2011 | 1683 (29) | 1439(31) | 316(33) |
VGPR indicates very good partial response; PR, partial response; SD, stable disease; REL, relapse; PROG, progression.
Table 2 summarizes data in subset of subjects (n = 1279) analyzed for relapse and NRM, specifically. Survival curves for this subset were identical to those of the larger set (P = .41, Supplemental Figure 1). There were 710 subjects in cohort 1, 498 in cohort 2, and 71 in cohort 3 (Table 2). Age distribution in the subset was similar to the total cohort of 11,430 subjects. Gender, KPS, HCTCI, immunochemical subtype, and time from diagnosis to AHCT showed similar distribution trends but did not reach statistical significance, primarily because of smaller cohort size. Higher international staging system stage, serum creatinine at diagnosis, and increased frequency of MEL reduction was noted in subjects ≥70 years of age. The median time in the hospital was 14 days for all cohorts. Median follow-up of survivors was 3 years.
Table 2.
Characteristics of Subjects with High-Level Data Reporting
| Characteristics of Subjects | Cohort 1 (18–59 yr) | Cohort 2 (60–69 yr) | Cohort 3 (70+ yr) | P Value |
|---|---|---|---|---|
| No. of subjects | 710 | 498 | 71 | |
| Age at transplantation, median (range), yr | 53 (22–59) | 64 (60–69) | 71(70–78) | |
| Age at transplantation, yr | ||||
| 18–49 yr | 222(31) | – | <.0001 | |
| 50–59 yr | 488 (69) | – | – | |
| 60–64 yr | – | 303 (61) | – | |
| 65–69 yr | – | 195 (39) | – | |
| 70–74 yr | – | – | 64 (90) | |
| 75–79 yr | – | – | 7(10) | |
| Male sex | 408 (57) | 275 (55) | 49 (69) | .0878 |
| Region | ||||
| United States | 693 (98) | 484 (97) | 70 (99) | .7494 |
| KPS before transplantation | ||||
| ≥80 | 394 (55) | 248 (50) | 35 (49) | .3293 |
| <80 | 281 (40) | 219 (44) | 31(44) | |
| Missing | 35(5) | 31 (6) | 5(7) | |
| HCTCI score | ||||
| 0–1 | 423 (60) | 280 (56) | 36(51) | .6178 |
| 2–3 | 195 (27) | 138(28) | 23 (32) | |
| >3 | 79(11) | 69 (14) | 11(15) | |
| Missing | 13(2) | 11 (2) | 1(1) | |
| Immunochemical subtype of plasma cell myeloma | ||||
| IgG | 410(58) | 297 (60) | 40 (56) | .7742 |
| IgA | 138(19) | 114(23) | 15(21) | |
| Light chain | 135(19) | 71 (14) | 14 (20) | |
| Others (Ig D/M/E) | 12(2) | 5(1) | 1(1) | |
| Nonsecretory | 15(2) | 11 (2) | 1(1) | |
| Serum creatinine at transplantation ≥1.5 | 70(10) | 71 (14) | 13(18) | .0245 |
| Lines of chemotherapy before transplantation | ||||
| 1 | 551 (78) | 375 (75) | 55(77) | .6394 |
| 2 | 159(22) | 123(25) | 16(23) | |
| Induction chemotherapy | ||||
| Thalidomide + bortezomib | 58(8) | 43(9) | 6(8) | .1158 |
| Lenalidomide + bortezomib | 160(23) | 79(16) | 12(17) | |
| Thalidomide-based | 116(16) | 103(21) | 20 (28) | |
| Lenalidomide-based | 175 (25) | 134(27) | 16(23) | |
| Bortezomib-based | 161 (23) | 116(23) | 14 (20) | |
| Steroids/cytoxan | 40(6) | 23(5) | 3(4) | |
| Disease status before AHCT | ||||
| CR | 114(16) | 85(17) | 12(17) | .8670 |
| VGPR | 199(28) | 143 (29) | 22(31) | |
| PR | 326 (46) | 230 (46) | 30 (42) | |
| SD | 56(8) | 32(6) | 4(6) | |
| REL/PROG | 15(2) | 8(2) | 3(4) | |
| Sensitivity to chemotherapy before transplantation | 639 (90) | 458 (92) | 64 (90) | .4992 |
| MEL, median (range), mg/m2 | 200(116–214) | 200 (137–220) | 200 (108–200) | |
| <140 mg/m2 | 5(<1) | 2(3) | 6(8) | <.0001 |
| 140–180 mg/m2 | 68(10) | 86(17) | 22(31) | |
| 180- ≥200 mg/m2 | 631 (89) | 406(82) | 43(61) | |
| Unknown | 6(<1) | 4(<1) | 0(0) | |
| Cytogenetic before transplantation | ||||
| Abnormal | 311(44) | 220 (43) | 33 (46) | .2932 |
| Normal | 294(41) | 191 (38) | 32 (45) | |
| Untested/unknown | 105(15) | 87(17) | 6(8) | |
| Time from diagnosis to transplantation, median (range), mo | 7(2–23) | 8 (3–24) | 8 (4–23) | |
| <12 mo | 605 (85) | 411(83) | 61 (86) | .5835 |
| 12–18 mo | 77(11) | 66(13) | 6(8) | |
| 19–24 mo | 28(4) | 21 (4) | 4(6) | |
| Type of transplantation | ||||
| Single | 632 (89) | 454 (91) | 70 (99) | .0249 |
| Tandem | 78(11) | 44(9) | 1(1) | |
| Year of transplantation | ||||
| 2008 | 364(51) | 252(51) | 35 (49) | .0068 |
| 2009 | 92(13) | 98 (20) | 16(23) | |
| 2010 | 102 (14) | 53(11) | 12(17) | |
| 2011 | 152(21) | 95 (19) | 8(11) | |
| In-hospital days, median (range) | 14(0–71) | 14 (0–60) | 14 (0–42) | .0012 |
| Evaluable | 597 (84) | 442 (89) | 64 (90) | |
| Median follow-up of survivors, mo | 35(3–61) | 37 (3–60) | 36(5–52) |
Follow-up completeness index: at 1 year (99%), at 3 years (90%), and at 5 years (82%).
OS (n = 11430)
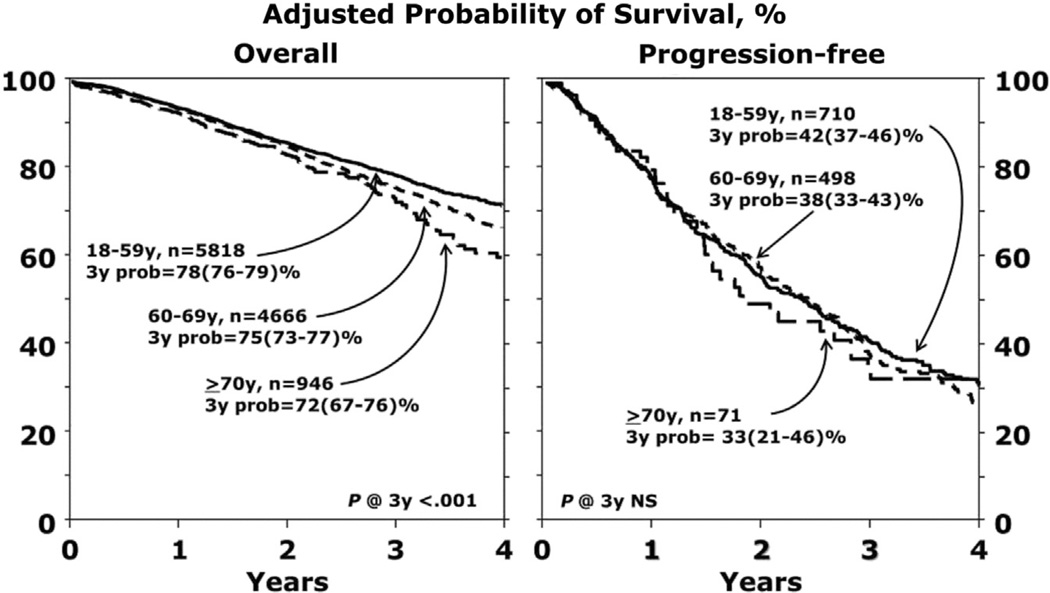
Median OS is not yet reached for any cohort. Survival data at 1, 2, and 3 years are summarized in Table 3 and Figure 1. In multivariate analysis, increasing age was associated with worse survival (P = .0006) (Table 4). Hazard ratios for death were 1.12 (95% confidence interval [CI], 1.02 to 1.24) for cohort 1 versus cohort 2, 1.35 (95% CI, 1.15 to 1.59) for cohort 1 versus cohort 3, and 1.2 (95% CI, 1.02 to 1.42) for cohort 2 versus cohort 3. The primary cause of death was myeloma in all 3 cohorts, with a similar myeloma-specific mortality rate (Table 5). In multivariate analysis, significant predictors of worse survival were higher HCTCI score, lower KPS, longer interval from diagnosis to transplantation, and inferior disease status (not in complete remission [CR]) at transplantation.
Table 3.
Univariate Results
| Outcome | At Risk, n | Cohort 1 (18–59 yr) |
Cohort 2 (60–69 yr) |
Cohort 3 (70+ yr) |
P Value Cohort Comparison |
||||
|---|---|---|---|---|---|---|---|---|---|
| Prob. % (95% CI) | At Risk, n | Prob. % (95% CI) | At Risk, n | Prob. % (95% CI) | 1 versus 2 | 1 versus 3 | 2 versus 3 | ||
| OS (n = 11430) | |||||||||
| At 1 yr | 4430 | 94 (93–94) | 3511 | 94 (93–94) | 693 | 93 (91–94) | .7490 | .2123 | .2874 |
| At 2yr | 2505 | 86 (84–87) | 1927 | 85 (83–86) | 362 | 83 (80–85) | .3003 | .0737 | .2272 |
| At 3yr | 1168 | 78 (76–79) | 904 | 75 (73–77) | 133 | 72 (67–76) | .0136 | .0071 | .1603 |
| PFS (n = 1279) | |||||||||
| At 1 yr | 492 | 77(74–80) | 340 | 77(73–81) | 52 | 80 (69–88) | .8655 | .5626 | .5160 |
| At 2yr | 267 | 56 (52–60) | 198 | 57 (52–62) | 25 | 50 (37–62) | .7581 | .3805 | .3173 |
| At 3yr | 136 | 42 (37–46) | 101 | 38 (33–43) | 12 | 33 (21–46) | .2829 | .2178 | .4934 |
| NRM (n = 1279) | |||||||||
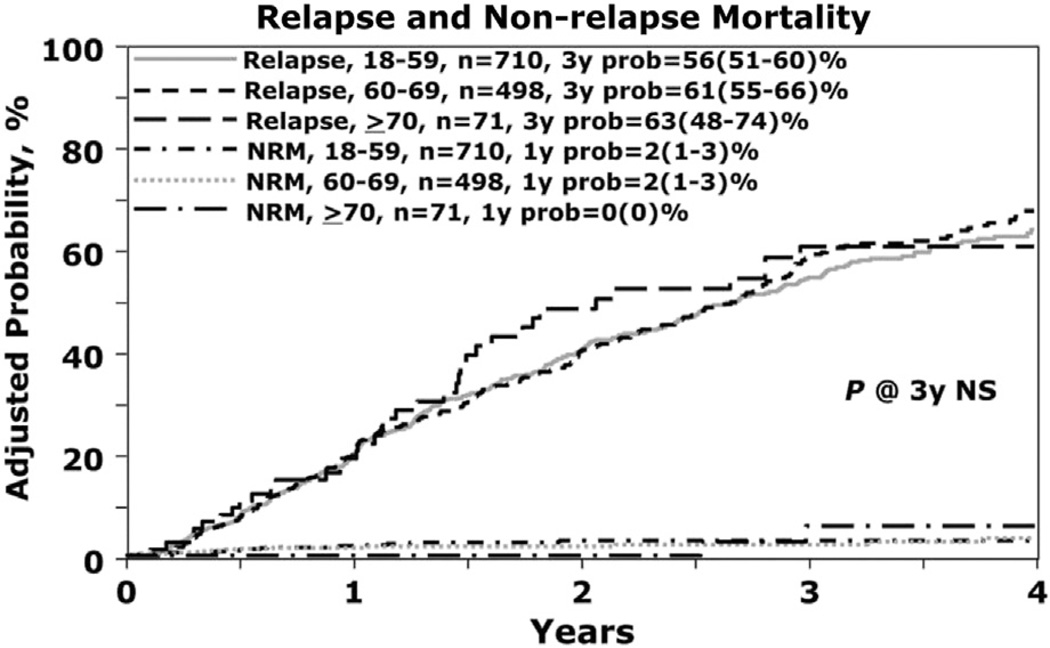
| At 1 yr | 492 | 2(1–3) | 340 | 2(1–3) | 52 | 0 | .6264 | .0003 | .0048 |
| At 2yr | 267 | 3(2–5) | 198 | 2(1–3) | 25 | 0 | .1980 | .0001 | .0027 |
| At 3yr | 136 | 3(2–5) | 101 | 2 (1–4) | 12 | 6(1–16) | .3898 | .4663 | .3406 |
| Progression (n = 1279) | |||||||||
| At 1 yr | 492 | 21 (18–24) | 340 | 21(18–25) | 52 | 20(11–30) | .7525 | .8481 | .7359 |
| At 2yr | 267 | 41(37–45) | 198 | 42 (37–46) | 25 | 50 (37––62) | .9546 | .2075 | .2264 |
| At 3yr | 136 | 56(51–60) | 101 | 61 (55–66) | 12 | 63 (48–74) | .1827 | .3570 | .7900 |
Prob indicates probability.
Figure 1.
Adjusted probability of survival. (Left) Shows OS and (Right) shows PFS.
Table 4.
Multivariate Analyses
| Risk Factors | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| For mortality: | ||
| Age group (main effect) | Overall test | .0006* |
| Reference group : 18–59 yr | 1 | NA |
| 60–69 versus 18–59 yr | 1.123(1.018–1.239) | .0204* |
| 70+ versus 18–59 yr | 1.353 (1.150–1.593) | .0003 |
| 70+ versus 60–69 yr | 1.205 (1.022–1.420) | .0261* |
| HCTCI | Overall test | <.0001* |
| ≥ 2 versus <2 | 1.279(1.163–1.407) | <.0001* |
| KPS | Overall test | <.0001* |
| 80–90 versus 100 | 1.242 (1.058–1.407) | .0082* |
| <80 versus 100 | 1.640 (1.344–2.002) | <.0001* |
| Time from diagnosis to transplantation | ||
| 12–24 versus 0–12 mo | 1.321 (1.188–1.469) | <.0001* |
| Disease status at transplantation | Overall test | <.0001* |
| VGPR/PR versus CR | 1.313(1.127–1.530) | .0005* |
| SD versus CR | 1.693(1.353–2.119) | <.0001* |
| REL/PROG versus CR | 3.301 (2.647–4.116) | <.0001* |
| For treatment failure/PFS: | ||
| Age group (main effect) | Overall test | .7014 |
| 60–69 versus 18–59 yr | 1.046 (.892–1.226) | .5832 |
| 70+ versus 18–59 yr | 1.131 (.816–1.567) | .4598 |
| 70+ versus 60–69 yr | 1.082 (.776–1.507) | .6433 |
| KPS | Overall test | .0029* |
| 80–90 versus 100 | 1.069 (.817–1.398) | .6279 |
| <80 versus 100 | 1.594 (1.128–2.253) | .0082* |
| Time from diagnosis to transplantation | ||
| 12–24 versus 0–12 mo | 1.294(1.066–1.571) | .0092* |
| Disease status at transplantation | Overall test | <.0001* |
| VGPR/PR versus CR | 1.327 (1.062–1.658) | .0129* |
| SD versus CR | 1.963 (1.423–2.708) | <.0001* |
| REL/PROG versus CR | 3.076 (1.882–5.028) | <.0001* |
| For NRM: | ||
| Age group (main effect) | Overall test | .9734 |
| 60–69 versus 18–59 yr | .921 (.450–1.884) | .8214 |
| 70+ versus 18–59 yr | 1.006 (.232–4.365) | .9941 |
| 70+ versus 60–69 yr | 1.092 (.245–4.866) | .9082 |
| KPS | Overall test | |
| 80–90 versus 100 | 2.890 (.389–21.485) | .2998 |
| <80 versus 100 | 9.554(1.170–78.039) | .0352* |
| Time from diagnosis to transplantation | ||
| 12–24 versus 0–12 mo | 2.158 (.997– 4.668) | .0508 |
| For progression: | ||
| Age group (main effect) | Overall test | .6811 |
| 60–69 versus 18–59 yr | 1.052 (.893–1.238) | .5465 |
| 70+ versus 18–59 yr | 1.136 (.813–1.587) | .4548 |
| 70+ versus 60–69 yr | 1.080 (.769–1.518) | .6561 |
| KPS | Overall test | .0209* |
| 80–90 versus 100 | 1.039 (.792–1.363) | .7847 |
| <80 versus 100 | 1.456 (1.020–2.079) | .0385* |
| Time from diagnosis to transplantation | ||
| 12–24 versus 0–12 mo | 1.259(1.031–1.539) | .0241* |
| Disease status at transplantation | Overall test | <.0001* |
| VGPR/PR versus CR | 1.299 (1.036–1.630) | .0237 |
| SD versus CR | 1.935 (1.939–2.688) | <.0001* |
| REL/PROG versus CR | 3.074 (1.858–5.085) | <.0001* |
Significant at level of .05.
Table 5.
Causes of Death
| Characteristics of Patients | Cohort 1 (18–59 yr) |
Cohort 2 (60–69 yr) |
Cohort 3 (70+ yr) |
|---|---|---|---|
| No. of patients | 5818 | 4666 | 946 |
| No. of deaths | 843 | 770 | 176 |
| Myeloma | 655(11) | 552(12) | 118(12) |
| Infection | 23 (<1) | 18 (<1) | 3(<1) |
| Pulmonary | 3(<1) | 3(<1) | 1(<1) |
| Organ failure | 14 (<1) | 20 (<1) | 6(<1) |
| Secondary malignancy | 11(<1) | 16 (<1) | 5(<1) |
| Hemorrhage | 1(<1) | 0(0) | 0(0) |
| Vascular/thrombotic/other | 136(3) | 161 (3) | 43(5) |
Data presented are n (%) unless otherwise indicated.
Relapse (n = 1279)
The 3-year rate of relapse was 56% (95% CI, 51% to 60%), 61% (95% CI, 55% to 66%), and 63% (95% CI, 48% to 74%) in cohorts 1, 2, and 3, respectively, which was not statistically significant (Table 3, Figure 2). On multivariate analysis, a lower KPS (<80), longer interval from diagnosis to AHCT (>12 months), and inferior disease status before AHCT (not in CR) were predictive factors for relapse. Increasing age was not associated with greater incidence of relapse.
Figure 2.
Relapse and NRM.
PFS (n = 1279)
The 3-year PFS in cohorts 1, 2, and 3 was similar at 42% (95% CI, 37% to 46%), 38% (95% CI, 33% to 43%), and 33% (95% CI, 21% to 46%), respectively, which was not statistically significant (Figure 1). Age was not a significant risk factor for PFS in multivariate analysis; however, KPS, longer interval from diagnosis to transplantation, and more advanced disease (not in CR) at transplantation were significant predictors of treatment failure (and worse PFS).
NRM (n = 1279)
One-year NRM was 0% for cohort 3 and 2% (95% CI, 1% to 3%) for cohorts 1 and 2, respectively (Figure 2). Age was not significantly associated with NRM in multivariate analysis but KPS <80% was predictive in multivariate analysis.
Postrelapse Survival
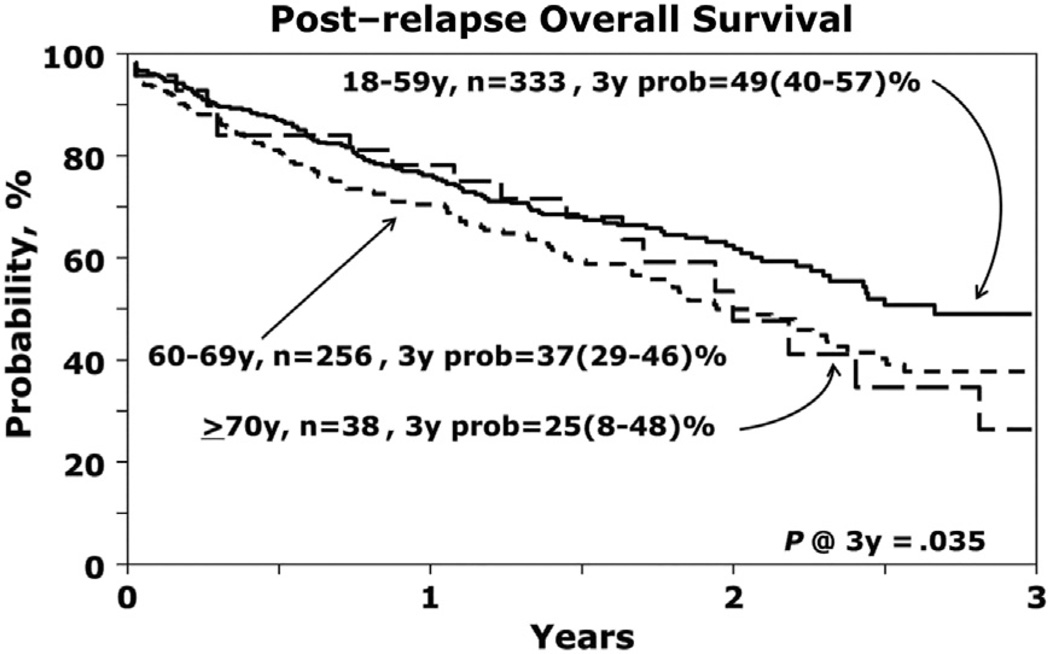
Survival after myeloma relapse was significantly worse for cohort 3 (P = .03, Figure 3). The 2-year postrelapse survivals were 63% (95% CI, 56% to 69%), 50% (95% CI, 42% to 57%), and 54% (95% CI, 32% to 71%) for cohorts 1, 2, and 3, respectively.
Figure 3.
Postrelapse OS.
Causes of Death
The primary cause of death was myeloma in all 3 cohorts (Table 5). Incidence of second malignancy was similar and low at <1%. A higher proportion of deaths in the older cohort were attributed to vascular and unknown causes.
Outcomes in Those >75 Years Old
There were 146 patients 75 to 79 years old and 8 who were ≥ 80 years old. The use of reduced MEL (<180 mg/m2) was 57% among those 75 to 79 years old and 67% in those ≥80 years old. Two-year survival in the 75 to 79–year-old cohort and ≥80-years-old cohort was 91% (83% to 95%) and 100%, respectively.
DISCUSSION
Our data indicate that freedom from progression of myeloma is similar regardless of age in persons who receive autologous transplantations. Expectedly, survival was worse in older persons. Interestingly, the increased mortality was not correlated with NRM or early progression but rather with worse postrelapse survival. The cause of death in these older subjects was mainly myeloma (as it was in younger cohorts) and not from transplantation-related causes, as might be speculated. Lack of clinical studies specifically applicable to this subject population may contribute to limited options after relapse and postrelapse survival [23]. The role of post- AHCT maintenance therapy was not analyzed in this study and, as such, we are unable to determine if differential use of maintenance therapy contributed to the results.
The survival analysis presented involves >11,000 subjects and represents approximately prospectively collected data at the time of transplantation, and longitudinally thereafter, in 60% of all AHCT for multiple myeloma (MM) in the United States. The characteristics of the elderly population receiving AHCT and the practice of AHCT are, thus, indicative of actual practice. In the older cohort, we observed significant MEL reductions (MEL <180 mg/m2 in 42%), worse HCTCI and KPS, and approximately 40% in > very good partial response (VGPR) disease state before AHCT. Multivariate analysis revealed that the risk factors for earlier relapse and shorter PFS included a lower KPS, longer time from diagnosis to transplantation, and a less than very good partial response before transplantation. NRM was 0% in subjects >70 years old, which indicates that careful subject selection and dose adjustment of melphalan were highly successful at controlling treatment toxicity without compromising benefit.
The perception of advanced age being an indicator of inferior outcomes after an autologous transplantation is a barrier to using high-dose melphalan as a therapeutic modality. Although autologous transplantation is now being offered more often to older patients, there still remains a large population of eligible patients who can benefit from this treatment modality [24]. The inclusion of older patients eligible for an autologous transplantation has been, in part, due to the improvement in supportive care and improved understanding of patient selection, as well as several studies showing that biologic fitness, rather than chronological age, is crucial in patient selection for transplantation in general [25,26]. The alternatives to autologous transplantation for older subjects include the continuation of the induction antimyeloma regimen or oral melphalan and prednisone (MP)–based induction regimens. Until recently, MP in combination with thalidomide, lenalidomide, or bortezomib was believed to be the optimal treatment strategy for transplantation-ineligible subjects. In a recent study, ongoing lenalidomide plus dexamethasone was shown to be superior to oral melphalan, prednisone, and thalidomide in a phase III randomized study [27].
MP has been studied in combination with thalidomide (MPT) [28–33], lenalidomide [34], and bortezomib [35–38] in randomized, phase III trials in Europe. The NRM using these regimens is 5% to 7% in patients selected for clinical trials, and OS has been shown to be higher in patients receiving either MPT or MP with bortezomib, compared with MP. Facon et al. compared the outcomes in older subjects randomized to either MP, MPT, or to an autologous transplantation using lower intensity conditioning with melphalan 100 mg/m2 [30]. The incidence of death in the first 3 months of therapy in the MP, MPT, and transplantation group was 7%, 2%, and 9%, respectively. The MPT arm was associated with a longer PFS and OS, compared with transplantation or MP, which had similar outcomes.
Palumbo et al. compared MP versus 2 courses of melphalan 100 mg/m2 followed by stem cell transplantation and were able to show an improvement in event-free survival and OS in patients receiving stem cell transplantation [39]. Whether a single melphalan 140 mg/m2 is equivalent to a single melphalan 200 mg/m2 is currently unknown. In our study, melphalan dose reduction did not impact myeloma-related outcomes, although the majority of >70-year-old patients still received melphalan 200 mg/m2. However, it seems that the optimal manner in which to administer melphalan is at higher doses with autologous stem cell support, rather than as a part of MP-based regimens, as NRM is lower in the transplantation strategy in the modern era. Notably, our subjects underwent transplantation more recently compared with the studies mentioned above, and 97% received a novel agent (lenalidomide or bortezomib) – based induction regimen.
In conclusion, advanced subject age was not associated with a worse NRM, relapse rate, or PFS after AHCT for MM. Post-relapse survival and OS of older subjects were inferior compared with younger subjects, which is likely multifactorial. Subjects with MM and an adequate performance status or acceptable HCTCI scores should not be considered for AHCTon the basis of age alone. Strategies offered to younger patients, such as post-AHCT maintenance, aggressive therapy at relapse, and clinical trial enrollment, may improve overall and post-relapse outcomes further for older persons with myeloma.
Supplementary Material
ACKNOWLEDGMENTS
The CIBMTR is supported by Public Health Service Grant/ Cooperative Agreement U24-CA076518 from the National Cancer Institute, the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases; a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration; two Grants N00014-12-1-0142 and N00014-13-1-0039 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals; Alios Therapeutics, Inc.; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; *Blue Cross and Blue Shield Association; *Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; *Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.; *Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc. ROSWELL PARK CANCER INSTITUTE; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children’s Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co., Inc.; Millennium: The Takeda Oncology Co.; *Milliman USA, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; *Remedy Informatics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick’s Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; *Tarix Pharmaceuticals; *Terumo BCT; *Teva Neuroscience, Inc.; *Texas Instruments Inc.; University of Minnesota; University of Utah; and *WellPoint , Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration or any other agency of the US Government.
Corporate Members.
Footnotes
Conflict of interest statement: There are no conflicts of interest to report.
Financial disclosure: The authors have nothing to disclose.
SUPPLEMENTARY DATA
Supplementary data related to this article can be found online at http://dx.doi.org/10.1016/j.bbmt.2014.07.013
REFERENCES
- 1.Costa LJ, Zhang MJ, Zhong X, et al. Trends in utilization and outcomes of autologous transplantation as early therapy for multiple myeloma. Biol Blood Marrow Transplant. 2013;19:1615–1624. doi: 10.1016/j.bbmt.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 4.Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 5.Attal M, Harousseau JL, Facon T, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495–2502. doi: 10.1056/NEJMoa032290. [DOI] [PubMed] [Google Scholar]
- 6.Anderson KC, Alsina M, Bensinger W, et al. Multiple myeloma, version 1.2013. J Natl Compr Canc Netw. 2013;11:11–17. doi: 10.6004/jnccn.2013.0004. [DOI] [PubMed] [Google Scholar]
- 7.Badros A, Barlogie B, Siegel E, et al. Autologous stem cell transplantation in elderly multiple myeloma patients over the age of 70 years. Br J Haematol. 2001;114:600–607. doi: 10.1046/j.1365-2141.2001.02976.x. [DOI] [PubMed] [Google Scholar]
- 8.Bashir Q, Shah N, Parmar S, et al. Feasibility of autologous hematopoietic stem cell transplant in patients aged ≥ 70 years with multiple myeloma. Leuk Lymphoma. 2012;53:118–122. doi: 10.3109/10428194.2011.606942. [DOI] [PubMed] [Google Scholar]
- 9.El Cheikh J, Kfoury E, Calmels B, et al. Age at transplantation and outcome after autologous stem cell transplantation in elderly patients with multiple myeloma. Hematol Oncol Stem Cell Ther. 2011;4:30–36. doi: 10.5144/1658-3876.2011.30. [DOI] [PubMed] [Google Scholar]
- 10.Kumar SK, Dingli D, Lacy MQ, et al. Autologous stem cell transplantation in patients of 70 years and older with multiple myeloma: results from a matched pair analysis. Am J Hematol. 2008;83:614–617. doi: 10.1002/ajh.21191. [DOI] [PubMed] [Google Scholar]
- 11.Kusnierz-Glaz CR, Schlegel PG, Wong RM, et al. Influence of age on the outcome of 500 autologous bone marrow transplant procedures for hematologic malignancies. J Clin Oncol. 1997;15:18–25. doi: 10.1200/JCO.1997.15.1.18. [DOI] [PubMed] [Google Scholar]
- 12.O’Shea D, Giles C, Terpos E, et al. Predictive factors for survival in myeloma patients who undergo autologous stem cell transplantation: a single-centre experience in 211 patients. Bone Marrow Transplant. 2006;37:731–737. doi: 10.1038/sj.bmt.1705307. [DOI] [PubMed] [Google Scholar]
- 13.Powles R, Raje N, Milan S, et al. Outcome assessment of a population-based group of 195 unselected myeloma patients under 70 years of age offered intensive treatment. Bone Marrow Transplant. 1997;20:435–443. doi: 10.1038/sj.bmt.1700917. [DOI] [PubMed] [Google Scholar]
- 14.Qazilbash MH, Saliba RM, Hosing C, et al. Autologous stem cell transplantation is safe and feasible in elderly patients with multiple myeloma. Bone Marrow Transplant. 2007;39:279–283. doi: 10.1038/sj.bmt.1705580. [DOI] [PubMed] [Google Scholar]
- 15.Schaapveld M, Visser O, Siesling S, et al. Improved survival among younger but not among older patients with multiple myeloma in the Netherlands, a population-based study since 1989. Eur J Cancer. 2010;46:160–169. doi: 10.1016/j.ejca.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Siegel DS, Desikan KR, Mehta J, et al. Age is not a prognostic variable with autotransplants for multiple myeloma. Blood. 1999;93:51–54. [PubMed] [Google Scholar]
- 17.Sirohi B, Powles R, Treleaven J, et al. The role of autologous transplantation in patients with multiple myeloma aged 65 years and over. Bone Marrow Transplant. 2000;25:533–539. doi: 10.1038/sj.bmt.1702188. [DOI] [PubMed] [Google Scholar]
- 18.Terpos E, Apperley JF, Samson D, et al. Autologous stem cell transplantation in multiple myeloma: improved survival in nonsecretory multiple myeloma but lack of influence of age, status at transplant, previous treatment and conditioning regimen. A single-centre experience in 127 patients. Bone Marrow Transplant. 2003;31:163–170. doi: 10.1038/sj.bmt.1703818. [DOI] [PubMed] [Google Scholar]
- 19.Brenner H, Gondos A, Pulte D. Recent major improvement in long-term survival of younger patients with multiple myeloma. Blood. 2008;111:2521–2526. doi: 10.1182/blood-2007-08-104984. [DOI] [PubMed] [Google Scholar]
- 20.Pasquini MC, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR summary slides. 2013 Available at: http://www.cibmtr.org.
- 21.Saad A, Mahindra A, Zhang M, et al. Hematopoietic cell transplant comorbidity index is predictive of survival after autologous hematopoietic cell transplantation in multiple myeloma. Biol Blood Marrow Transplant. 2014;20:402–408. doi: 10.1016/j.bbmt.2013.12.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorror ML, Giralt S, Sandmaier BM, et al. Hematopoietic cell transplantation specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: combined FHCRC and MDACC experiences. Blood. 2007;110:4606–4613. doi: 10.1182/blood-2007-06-096966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 24.Palumbo A, Bringhen S, Ludwig H, et al. Personalized therapy in multiple myeloma according to patient age and vulnerability: a report of the European Myeloma Network (EMN) Blood. 2011;118(17):4519–4529. doi: 10.1182/blood-2011-06-358812. [DOI] [PubMed] [Google Scholar]
- 25.McCarthy PL, Jr, Hahn T, Hassebroek A, et al. Trends in use of and survival after autologous hematopoietic cell transplantation in North America, 1995–2005: significant improvement in survival for lymphoma and myeloma during a period of increasing recipient age. Biol Blood Marrow Transplant. 2013;19:1116–1123. doi: 10.1016/j.bbmt.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sorror ML, Sandmaier BM, Storer BE, et al. Long-term outcomes among older patients following nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation for advanced hematologic malignancies. JAMA. 2011;306:1874–1883. doi: 10.1001/jama.2011.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Facon T. Initial phase 3 results of the First (frontline investigation of lenalidomide + dexamethasone versus standard thalidomide) Trial (MM-020/IFM 07 01) In newly diagnosed multiple myeloma (NDMM) patients (Pts) ineligible for stem cell transplantation (SCT). Am Soc Hematol Meeting 2013. Blood. 2013;122:2. [Abstract] [Google Scholar]
- 28.Palumbo A, Bringhen S, Caravita T, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet. 2006;367:825–831. doi: 10.1016/S0140-6736(06)68338-4. [DOI] [PubMed] [Google Scholar]
- 29.Palumbo A, Bringhen S, Liberati AM, et al. Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: updated results of a randomized controlled trial. Blood. 2008;112:3107–3114. doi: 10.1182/blood-2008-04-149427. [DOI] [PubMed] [Google Scholar]
- 30.Facon T, Mary JY, Hulin C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370:1209–1218. doi: 10.1016/S0140-6736(07)61537-2. [DOI] [PubMed] [Google Scholar]
- 31.Hulin C, Facon T, Rodon R, et al. Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 trial. J Clin Oncol. 2009;27:3664–3670. doi: 10.1200/JCO.2008.21.0948. [DOI] [PubMed] [Google Scholar]
- 32.Waage A, Gimsing P, Fayers P, et al. Melphalan and prednisone plus thalidomide or placebo in elderly patients with multiple myeloma. Blood. 2010;116:1405–1412. doi: 10.1182/blood-2009-08-237974. [DOI] [PubMed] [Google Scholar]
- 33.Wijermans P, Schaafsma M, Termorshuizen F, et al. Phase III study of the value of thalidomide added to melphalan plus prednisone in elderly patients with newly diagnosed multiple myeloma: the HOVON 49 Study. J Clin Oncol. 2010;28:3160–3166. doi: 10.1200/JCO.2009.26.1610. [DOI] [PubMed] [Google Scholar]
- 34.Palumbo A, Hajek R, Delforge M, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med. 2012;366:1759–1769. doi: 10.1056/NEJMoa1112704. [DOI] [PubMed] [Google Scholar]
- 35.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 36.Mateos MV, Oriol A, Martinez-Lopez J, et al. Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: a randomised trial. Lancet Oncol. 2010;11:934–941. doi: 10.1016/S1470-2045(10)70187-X. [DOI] [PubMed] [Google Scholar]
- 37.Mateos MV, Richardson PG, Schlag R, et al. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J Clin Oncol. 2010;28:2259–2266. doi: 10.1200/JCO.2009.26.0638. [DOI] [PubMed] [Google Scholar]
- 38.Palumbo A, Bringhen S, Rossi D, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: a randomized controlled trial. J Clin Oncol. 2010;28:5101–5109. doi: 10.1200/JCO.2010.29.8216. [DOI] [PubMed] [Google Scholar]
- 39.Palumbo A, Triolo S, Argentino C, et al. Dose-intensive melphalan with stem cell support (MEL100) is superior to standard treatment in elderly myeloma patients. Blood. 1999;94:1248–1253. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.