Abstract
Background
Diarrhea, abdominal pain and fever are common among patients undergoing hematopoietic cell transplant (HCT), but such symptoms are also typical with foodborne infections. The burden of disease caused by foodborne infections in patients undergoing HCT is unknown. We sought to describe bacterial foodborne infection incidence post-transplant within a single-center population of HCT recipients.
Methods
All HCT recipients transplanted from 2001 through 2011 at the Fred Hutchinson Cancer Research Center in Seattle, WA were followed for one year post-transplant. Data were collected retrospectively using center databases, which include information from transplant, on-site examinations, outside records, and collected laboratory data. Patients were considered to have a bacterial foodborne infection if Campylobacter jejuni/coli, Listeria monocytogenes, E. coli 0157:H7, Salmonella species, Shigella species, Vibrio species or Yersinia species were isolated in culture within one-year post-transplant. Non-foodborne infections with these agents and patients with preexisting bacterial foodborne infection (within 30 days of transplant) were excluded from analyses.
Results
A total of 12/4069 (0.3%) patients developed a bacterial foodborne infection within one year post-transplant. Patients with infections had a median age at transplant of 50.5 years (interquartile range [IQR]: 35–57), and the majority were adults ≥18 years of age (9/12 [75%]), male gender (8/12 [67%]) and post-allogeneic transplant (8/12 [67%]). Infectious episodes occurred at an incidence rate of 1.0 per 100,000 patient-days (95% CI: 0.5–1.7) and at a median of 50.5 days after transplant (IQR: 26–58.5). The most frequent pathogen detected was Campylobacter jejuni/coli (5/12 [42%]) followed by Yersinia (3/12 [25%]), while Salmonella (2/12 [17%]) and Listeria (2/12 [17%]) showed equal frequencies; no cases of Shigella, Vibrio, or E. coli 0157:H7 were detected. Most patients were diagnosed via stool (8/12 [67%]), fewer through blood (2/12 [17%]), one via both stool and blood simultaneously, and one through urine. Mortality due to bacterial foodborne infection was not observed during follow-up.
Conclusions
Our large single-center study indicates that common bacterial foodborne infections were a rare complication following HCT, and the few cases that did occur resolved without complications. These data provide important baseline incidence for future studies evaluating dietary interventions for HCT patients.
INTRODUCTION
Immunocompromised patients are known to be vulnerable to foodborne pathogens.1–6 Hematopoietic cell transplant (HCT) recipients have multiple factors that increase risk for foodborne infections, including profound deficits in innate and adaptive immunity, and disruption of gastrointestinal (GI) mucosa from transplant-associated radiation therapy, chemotherapy and graft-versus-host disease (GVHD). While such alterations provide the ideal milieu for microbial invasion/dissemination, many patients have additional risk factors for bacterial infections such as transfusion-associated iron overload, enteric acid suppression, and GI microbiota perturbations from antibiotic use.7–10 Furthermore, diagnosis and treatment may be delayed as symptoms of foodborne infections, notably diarrhea and fever, are nearly universal amongst HCT recipients.11,12
Most transplant centers follow guidelines and implement specific dietary strategies to reduce the risk of exposure to foodborne pathogens. Particular emphasis has been placed on restricting the consumption of foods more likely to harbor high-risk bacteria with the use of various low-microbial diets.13 However, these commonly applied guidelines have not been evaluated in randomized prospective clinical trials.13,14 Credence for such recommendations is further stunted by a lack of studies addressing the burden of bacterial foodborne infections in HCT recipients.3,15 More recent data suggests that restrictive nutritional strategies intended to prevent the consumption of pathogenic organisms may in fact increase the risk of infection.16
We set out to determine the burden of common bacterial foodborne infections in a large comprehensive HCT center. Through retrospective chart review, we aimed to describe the incidence of bacterial foodborne pathogens within our HCT patient population during the first year post-transplant and to assess associated morbidity and mortality. These data are important for determining incidence of bacterial foodborne infections, and provide a baseline for future studies evaluating nutritional strategies in this high-risk population.
MATERIALS AND METHODS
Study Design / Participant Eligibility
All HCT recipients who underwent an autologous or allogeneic HCT at the Fred Hutchinson Cancer Research Center (FHCRC) in Seattle, WA between January 1, 2001 and December 31, 2011 were eligible for inclusion in this retrospective cohort. Patients with evidence of bacterial foodborne infection 30 days prior to transplant were excluded. All study activities were approved by the FHCRC institutional review board, and all participants provided written informed consent according to the principles of the Declaration of Helsinki.
Data Collection
Retrospective data were retrieved from a prospectively collected database of patients undergoing HCT at the FHCRC in Seattle, WA. Pre- and post-transplant demographic and outcome data were available from clinical databases and medical records. Clinical and laboratory data after discharge from the center were also available in long-term follow-up databases.
Nutrition, Transplant Procedures and Infection Prophylaxis
Patients undergoing transplantation were encouraged to follow an immunosuppressed patient diet13 until three months post-transplant (autologous recipients) or until cessation of immunosuppressive drugs (allogeneic recipients). Prior to transplantation, all patients and caregivers participated in a food safety training course that educated patients not only on what to foods to avoid, but also proper preparation, cleaning and storage of food and food products. Nutritional services were available for all patients to assist with questions regarding recommendations, to address post-transplant dietary issues and to assure and promote adequate nutrition.
HCT conditioning and GVHD prophylaxis/treatment were performed according to current standardization within the center.17 Patients who were neutropenic received prophylactic antibacterial therapy with either oral levofloxacin or intravenous ceftazidime. Post-transplant patients received antiviral prophylaxis with low-dose acyclovir,18 and all patients underwent cytomegalovirus screening and preemptive therapy;19,20 fungal and Pneumocystis jirovercii prophylaxis were also routine. To prevent late encapsulated bacterial infections in patients who developed chronic GVHD, long-term prophylaxis with trimethoprim-sulfamethoxazole, either daily or three times weekly, along with daily penicillin VK was administered to those with prior splenectomies.
Bacterial cultures from blood, stool and other sites were conducted at the discretion of the primary team, as center-based standard practice documents did not recommend routine testing for foodborne pathogens during initial episodes of diarrhea. All specimens submitted for stool culture were screened for the presence of Salmonella species (spp), Shigella spp, Campylobacter jejuni/coli, Yersinia spp, E. coli 0157-H7, Vibrio spp, Aeromonas spp and Plesiomonas. The following culture media were used: Hektoen Enteric (HE), blood (Trypticase soy agar with 5% sheep blood), MacConkey Lactose, MacConkey-Sorbitol, Yersinia and Campy CVA agars. All specimens were also inoculated into selenite broth and sub-cultured to HE agar following 12–18 hours of incubation. Microbial identification of potential stool pathogens present was performed using a combination of microbiological methods, including biochemical identification methods (e.g. Vitek 2 GN-ID) as well as agglutinating sera for Salmonella and Shigella spp.
Definitions and Statistical Analysis
All patient events were reviewed up to one year post-transplant for bacterial foodborne infections. An infectious event was defined as detection of Campylobacter jejuni/coli, Listeria monocytogenes, E. coli 0157:H7, Salmonella spp, Shigella spp, Vibrio spp or Yersinia spp from any clinical site (excluding the lung) from day 0 to day 365 posttransplant. Site of detection for all bacterial foodborne infections was defined as the site of first positive culture. Cultures epidemiologically linked to a non-foodborne exposure (e.g. zoonotic) and Campylobacter spp whose primary transmission is not epidemiologically established as foodborne, such as C. curvus and C. ureolyticus, were excluded from analyses21; non-speciated cases were included and noted as such.
In this study, an attributable cause of death was defined when death was documented as a direct result of the bacterial foodborne infection. Infections in patients who survived beyond 30 days, without recurrence, were considered resolved. All bacterial, viral, and fungal infections were identified as concomitant if they were documented within ± 7 days of foodborne event. The timing and severity of GVHD were reviewed and all episodes graded according to standard criteria.22 Neutropenia during bacterial foodborne infection was defined as an absolute neutrophil count (ANC) of <500 mm cells/mm3 within ± two days of infectious event.
Time at risk for bacterial foodborne infection was considered from the first day post-transplant until the bacterial foodborne event or occurrence of any of the following censoring events: lost to follow-up, death, re-transplant or 365 days. For patients with multiple transplant events, the at-risk period was considered only after the first transplant; the at-risk period of patients who underwent a planned tandem transplant began after the second transplant.
Incidence rates of bacterial foodborne infection were estimated by dividing the number of incident cases developed in cohort subjects by the number of post-transplant at risk patient-days contributed by the overall cohort; 95% confidence intervals (CI) were estimated based on a Poisson distribution. Incidence rates were also stratified by age (pediatric/adult), with pediatric HCT recipients considered <18 years of age.
RESULTS
Of the 4,074 patients who underwent HCT at the FHCRC during the 2001–2011 study period, 5 were excluded from primary analysis due to a preexisting foodborne event (3 Yersinia spp, one Campylobacter jejuni and one Salmonella spp). Among the remaining HCT recipients, a total of 12/4,069 (0.3%) of patients developed a post-transplant bacterial foodborne infection; none experienced multiple events. Patients with these infections had a median age at transplant of 50.5 years (interquartile range [IQR]: 35–57), and were primarily adults (9/12 [75%]) and male gender (8/12 [67%]) (Table 1). The majority of infections also occurred following allogeneic (8/12 [67%]) rather than autologous transplant, although cumulative incidence estimates were similar between the two transplant types (8/2540 [0.3%] among allogeneic vs. 4/1529 [0.3%] among autologous). Clinical circumstances surrounding the foodborne infectious event can be found in Table 1.
Table 1.
Case demographics and clinical characteristics (n=12)
| Patient Demographics | Patient Demographics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Gender /Age* |
Disease/ HCT type |
Conditioning regimen |
GVHD prophylaxis |
Organism | Primary site/ Days post-HCT |
Hospitalized† | Gut GVHD |
Concomitant infections‡ |
Neutropenic§ | Antibacterial regimen at time of diagnosis‖ |
| 1 | M/35 | MM/Auto | L-PAM | None | Yersinia, NOS | Feces/4 | No | No | None | Yes | None |
| 2 | M/67 | MM/Auto | L-PAM | None |
Campylobacter jejuni/coli |
Feces/6 | No | No | None | Yes | Levofloxacin1 |
| 3 | F/46 | AML/Allo | BU, CY | CSP, MTX | Yersinia, NOS | Urine/15 | No | No | None | Yes | Levofloxacin |
| 4 | M/57 | ML/Auto | CY, VP-16, I-131 |
None | Yersinia, NOS | Feces/15 | Yes | No | CoNS; C. difficile |
No | Clindamycin Vancomycin Imipenem2 |
| 5 | M/60 | RA/Allo | TREO, FLU, TBI (200 Gy) |
MTX, FK-506 |
Campylobacter jejuni/coli |
Feces/19 | Yes | Yes | RSV/Rhinovirus | No | None |
| 6 | F/55 | AMM/Allo | BU, CY, ATG | CSP, MTX, FK-506 |
Listeria monocytogenes |
Blood/46 | No | Yes | None | No | Dapsone |
| 7 | F/4 | CIMMDIS/ Allo |
FLU, TBI (400 Gy) |
CSP, MMF |
Campylobacter jejuni |
Feces/55 | Yes | Yes | Parainfluenza 3; Rhinovirus; C. difficile |
No | Bactrim |
| 8 | M/68 | MDS/Allo | FLU, TBI (300 Gy) |
CSP, MMF |
Campylobacter jejuni |
Feces/71 | Yes | No | None | No | Dapsone, Bactrim3 |
| 9 | M/55 | MYLFI/Allo | BU, CY | MTX, FK-506 |
Campylobacter, NOS |
Feces/74 | No | No | None | No | Bactrim |
| 10 | M/45 | RAEB/Allo | BU, CY | MTX, FK-506 |
Listeria monocytogenes |
Blood/135 | Yes | Yes | None | Yes4 | Dapsone |
| 11 | F/17 | CML/Allo | BU, CY | CSP, MTX |
Salmonella, NOS |
Feces/175 | Yes | Yes | C. difficile | No | Dapsone, Cephalexin |
| 12 | M/3 | NBL/Auto | L-PAM, VP-16, CARBO |
None |
Salmonella, NOS |
Blood & Feces/ 351 |
Yes | No | None | Unk | Bactrim |
at time of HCT
Hospitalized during the course of foodborne infection
Documented within ± 7 days of foodborne infection diagnosis
Absolute neutrophil count (ANC) <500 mm cells/mm3 within ± two days of foodborne infection diagnosis
Antibiotics at time of diagnosis, not those used for treatment 1-Levofloxacin started 12 hours prior to diagnosis; 2-Multiple antibiotics changed throughout presentation; 3-On low dose Bactrim during desensitization protocol at time of diagnosis; 4-Presented with neutropenia during acute episode.
Abbreviations: F = female; M = male; HCT = hematopoietic cell transplantation; Auto = autologous transplant; Allo = allogeneic transplant; NOS = not otherwise specified; MM = multiple myeloma; AML = acute myeloid leukemia; ML = malignant lymphoma, follicular, NOS; RA = refractory anemia, NOS; AMM = agnogenic myeloid metaplasia; CIMMDIS = immune deficiency disorder; MDS = myelodysplastic syndrome; MYLFI = myelofibrosis; RAEB = refractory anemia with excess blasts; CML = chronic myeloid leukemia; NBL = neuroblastoma; BU = busulfan; CY = cyclophosphamide; I-131 = monoclonal antibody I-131 infusion; TREO = treosulfan; FLU = fludarabine; TBI = total-body irradiation; ATG = anti-thymocyte globulin; CARBO = carboplatin; CSP = cyclosporine; MTX = methotrexate; MMF = mycophenolate mofetil; C. difficile = Clostridium difficile; CoNS = coagulase negative Staphyloccocus; Unk = unknown
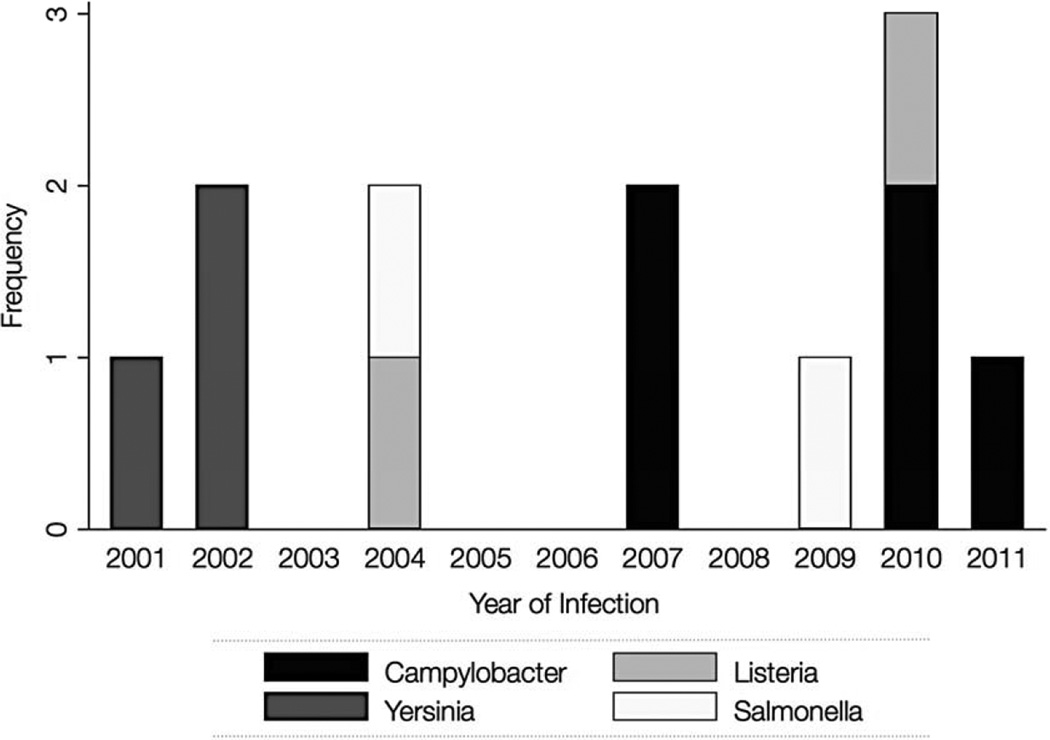
Documented infections were noted throughout the entire study period at a median of 50.5 days post-transplant (IQR: 26–58.5). The incidence rate of bacterial foodborne infection was 1.0 per 100,000 patient-days (95% CI: 0.5–1.7) for all patients, 0.8 (95% CI: 0.4–1.5) for adults, and 2.2 (95% CI: 0.5–6.4) for pediatric patients. There were no apparent associations between incidence and calendar year of transplant (Figure 1). The most frequently detected pathogen was Campylobacter jejuni/coli (5/12 [42%]), followed by Yersinia spp (3/12 [25%]), and equal distributions of Salmonella and Listeria spp (2/12 [17%], respectively); no Shigella spp, Vibrio spp, or E. coli O157:H7 were detected. Diagnoses were made in most patients through stool culture (8/12 [67%]), while a smaller proportion were first positive through blood cultures (2/12 [17%]); one patient was positive simultaneously at both sites (blood and stool) and another was first positive in the urine (Table 1).
Figure 1.
Frequency of common bacterial foodborne infections among HCT patients per year of infection (n=12)
Four cases had one or more concomitant infectious event, including the following: Clostridium difficile infection (3/12 [25%]), rhinovirus upper respiratory infection (2/12 [17%]), and single events of parainfluenza (type 3) upper respiratory infection and coagulase-negative Staphylococcus bacteremia. Of the 8 cases in allogeneic HCT recipients, 5/8 (62%) were diagnosed with gut GVHD; four had GVHD onset before the bacterial foodborne infection diagnosis and one after. Among the 11 cases with ANC measurements within ± 2 days of infection, 4/11 (36%) were found to be neutropenic. Nearly half (5/12 [42%]) of cases were admitted for treatment of their infection and/or for symptom management. No death was found to be attributable or associated with bacterial foodborne infection during follow-up, and no patients developed septic shock or required admission to intensive care.
DISCUSSION
In this retrospective cohort study, we sought to describe the incidence of bacterial foodborne pathogens after HCT. Incidence rates in the first year following transplant were very low, with just 12 cases identified over an eleven-year period. Overall, Campylobacter were the most frequently identified pathogens, followed by Yersinia, Salmonella and Listeria monocytogenes. No events, even those with documented bacteremia, were associated with major complications or mortality in this high risk population.
Foodborne illness remains an important cause of morbidity and mortality in the US. The Centers for Disease Control and Prevention estimates that 1 in 6 Americans will develop a foodborne infection each year,23 and the most common of these microbial agents are expected to cause greater than 9 million illnesses and over 50,000 hospitalizations in the US annually.24 Given underreporting, limitations to current diagnostic methods, and emerging pathogens, rates are likely an underestimate of the true burden of these infections.24,25
Bacterial foodborne events in our HCT cohort were infrequent, with approximately one event per 100,000 patient-days. Comparing rates observed in our cohort to other populations is difficult, particularly when considering the multiple risks associated with transplantation, including immunosuppression, mucosal injury, and the higher frequency of testing and healthcare engagement. Estimating the general population's rate of those foodborne illnesses described in this study using the Foodborne Diseases Active Surveillance Network (FoodNet) data from 2001–2012,26 and extrapolating incidence rates to 100,000 patient-days, suggests that our HCT recipients experienced a rate approximately ten-fold that of the general population (1.0 in HCT vs. 0.1 in the general population). However, it should be noted that these rough estimates do not account for significant underreporting in the general population24, and may therefore overestimate rate differences.
These data must also be taken in context particularly when considering factors known to modify infection risk in our patient population, such as the routine use of prophylactic antibiotics, which would be expected to provide a level of protection against foodborne pathogens during the post-transplant period. Alterations in oral intake that occur following HCT may further limit exposure, particularly those who receive total parenteral or peripheral nutrition.27 Since unregulated foodborne exposures in outpatient environments likely make up the majority of foodborne risk, and hospital-based bacterial foodborne infectious events are rarely observed,28,29 expanded inpatient time would also be expected to limit risk in these patients. Standardized food safety education and nutritional support are also likely to decrease exposures.
Concerns for increased susceptibility for infection have led most programs to limit exposure to foodborne pathogens through use of a low-microbial diet, which aims to prevent consumption of these organisms by restricting certain higher-risk foods. Despite implementation of low-microbial diets across US transplant centers,13 empirical evidence supporting such recommendations is lacking.30 While this study cannot directly address the value of our center’s immunosuppressed diet, the safety and efficacy of such low-microbial diets during HCT have been questioned by other studies,31,32 with one transplant center even noting an increased risk of infection with the use of their neutropenic diet.32 Further evaluation of low-microbial diets in HCT is complicated by the variety of dietary restrictions and decisions regarding timing of diet implementation across centers.30,33
These data could be interpreted as evidence of our immunosuppressed patient diet’s effectiveness, but it is also important to note that bacterial foodborne illnesses did occur in this cohort, regardless of our center’s dietary restrictions. Infectious events occurred even during periods of neutropenia. Case reports in similar populations and one cohort study of non-typhoidal Salmonella spp from a large transplant center2,3,34,35 also suggest that these findings are not likely isolated to our center.
The foodborne bacteria evaluated in this study are not considered normal flora, have been epidemiologically defined as foodborne pathogens, and as such, can serve as a proxy for assessing risk of foodborne exposures in future studies. It is important to note that while HCT dietary recommendations are organized to prevent exposure to these foodborne pathogens, such recommendations also aim to prevent exposure to other organisms that might occur through improper processing or food preparation practices.36,37 Any studies addressing dietary recommendations in this population will need to evaluate how these changes affect C. difficile, E. coli (non-0157:H7), Staphylococcus aureus and other infections that are not exclusively foodborne.
As with all retrospective studies, our data were limited by available records and reporting. Dietary compliance was not evaluated in this study, so it is unknown if the observed events resulted from failures of dietary guidelines or from non-adherence. Stool cultures were not standardized and were dependent on the clinician directing care, so it is possible that we underestimated the true incidence. Direct associations with specific foods or exposures could not be addressed in this study, and these infections may not have been acquired through foodborne pathways. Finally, this is a single-center study, which may limit the generalizability to other centers. Regardless, this study is the largest to date that addresses the incidence of these pathogens in this population.
In conclusion, common bacterial foodborne infections were infrequently observed after HCT at our center, and these pathogens were not associated with significant morbidity or mortality. These results raise additional questions about low-microbial dietary recommendations in HCT and indicate a need for additional studies to determine the value of such practices. These data provide important baseline incidence for future studies addressing dietary interventions among HCT recipients.
Acknowledgments
Financial Support: This research was supported by NIH grants CA-18029 and CA- 15704. Dr. Pergam is supported by NIH grant K23HL096831 and an ASBMT/Viropharma New Investigator Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Conflicts of Interest: S.A.P. has received research support from Chimerix, Merck, and has been a consultant from Merck and Optimer/Cubist Pharmaceuticals.
Presentation of material in submitted manuscript: Data from this manuscript have been presented in part at the ASBMT/CIBMTR Tandem Meeting in Salt Lake City, Utah February 2013.
REFERENCES
- 1.Allerberger F, Wagner M. Listeriosis: a resurgent foodborne infection. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2010 Jan;16(1):16–23. doi: 10.1111/j.1469-0691.2009.03109.x. [DOI] [PubMed] [Google Scholar]
- 2.Barton JC, Ratard RC. Vibrio vulnificus bacteremia associated with chronic lymphocytic leukemia, hypogammaglobulinemia, and hepatic cirrhosis: relation to host and exposure factors in 252 V. vulnificus infections reported in Louisiana. The American journal of the medical sciences. 2006 Oct;332(4):216–220. doi: 10.1097/00000441-200610000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Dadwal SS, Tegtmeier B, Nakamura R, et al. Nontyphoidal Salmonella infection among recipients of hematopoietic SCT. Bone marrow transplantation. 2011 Jun;46(6):880–883. doi: 10.1038/bmt.2010.204. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez-Cruz A, Munoz P, Mohedano R, et al. Campylobacter bacteremia: clinical characteristics, incidence, and outcome over 23 years. Medicine. 2010 Sep;89(5):319–330. doi: 10.1097/MD.0b013e3181f2638d. [DOI] [PubMed] [Google Scholar]
- 5.Lund BM, O'Brien SJ. The occurrence and prevention of foodborne disease in vulnerable people. Foodborne pathogens and disease. 2011 Sep;8(9):961–973. doi: 10.1089/fpd.2011.0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz S, Vergoulidou M, Schreier E, et al. Norovirus gastroenteritis causes severe and lethal complications after chemotherapy and hematopoietic stem cell transplantation. Blood. 2011 Jun 2;117(22):5850–5856. doi: 10.1182/blood-2010-12-325886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammer MJ, Casper C, Gooley TA, O'Donnell PV, Boeckh M, Hirsch IB. The contribution of malglycemia to mortality among allogeneic hematopoietic cell transplant recipients. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009 Mar;15(3):344–351. doi: 10.1016/j.bbmt.2008.12.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer SC, O'Meara A, Buser AS, Tichelli A, Passweg JR, Stern M. Prognostic impact of posttransplantation iron overload after allogeneic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013 Mar;19(3):440–444. doi: 10.1016/j.bbmt.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Murphy S, Nguyen VH. Role of gut microbiota in graft-versus-host disease. Leukemia & lymphoma. 2011 Oct;52(10):1844–1856. doi: 10.3109/10428194.2011.580476. [DOI] [PubMed] [Google Scholar]
- 10.Ubeda C, Taur Y, Jenq RR, et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. The Journal of clinical investigation. 2010 Dec;120(12):4332–4341. doi: 10.1172/JCI43918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker CC, Anderson RA, Sauve RS, Butzner JD. GI complications in pediatric patients post-BMT. Bone marrow transplantation. 2005 Jul;36(1):51–58. doi: 10.1038/sj.bmt.1705004. [DOI] [PubMed] [Google Scholar]
- 12.Holmberg L, Kikuchi K, Gooley TA, et al. Gastrointestinal graft-versus-host disease in recipients of autologous hematopoietic stem cells: incidence, risk factors, and outcome. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2006 Feb;12(2):226–234. doi: 10.1016/j.bbmt.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009 Oct;15(10):1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boeckh M. Neutropenic diet--good practice or myth? Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012 Sep;18(9):1318–1319. doi: 10.1016/j.bbmt.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Kamboj M, Mihu CN, Sepkowitz K, Kernan NA, Papanicolaou GA. Work-up for infectious diarrhea after allogeneic hematopoietic stem cell transplantation: single specimen testing results in cost savings without compromising diagnostic yield. Transplant infectious disease : an official journal of the Transplantation Society. 2007 Dec;9(4):265–269. doi: 10.1111/j.1399-3062.2007.00230.x. [DOI] [PubMed] [Google Scholar]
- 16.Trifilio S, Helenowski I, Giel M, et al. Questioning the role of a neutropenic diet following hematopoetic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012 Sep;18(9):1385–1390. doi: 10.1016/j.bbmt.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 17.Nakamae H, Kirby KA, Sandmaier BM, et al. Effect of conditioning regimen intensity on CMV infection in allogeneic hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009 Jun;15(6):694–703. doi: 10.1016/j.bbmt.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erard V, Guthrie KA, Varley C, et al. One-year acyclovir prophylaxis for preventing varicella-zoster virus disease after hematopoietic cell transplantation: no evidence of rebound varicella-zoster virus disease after drug discontinuation. Blood. 2007 Oct 15;110(8):3071–3077. doi: 10.1182/blood-2007-03-077644. [DOI] [PubMed] [Google Scholar]
- 19.Green ML, Leisenring W, Stachel D, et al. Efficacy of a viral load-based, riskadapted, preemptive treatment strategy for prevention of cytomegalovirus disease after hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012 Nov;18(11):1687–1699. doi: 10.1016/j.bbmt.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milano F, Pergam SA, Xie H, et al. Intensive strategy to prevent CMV disease in seropositive umbilical cord blood transplant recipients. Blood. 2011 Nov 17;118(20):5689–5696. doi: 10.1182/blood-2011-06-361618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Man SM. The clinical importance of emerging Campylobacter species. Nature reviews. Gastroenterology & hepatology. 2011 Dec;8(12):669–685. doi: 10.1038/nrgastro.2011.191. [DOI] [PubMed] [Google Scholar]
- 22.Thomas ED, Storb R, Clift RA, et al. Bone-marrow transplantation (second of two parts) The New England journal of medicine. 1975 Apr 24;292(17):895–902. doi: 10.1056/NEJM197504242921706. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. CDC Estimates of Foodborne Illness in the United States. 2013 Available at: http://www.cdc.gov/foodborneburden/estimates-overview.html.
- 24.Scallan E, Hoekstra RM, Angulo FJ, et al. Foodborne illness acquired in the United States--major pathogens. Emerging infectious diseases. 2011 Jan;17(1):7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris JG., Jr How safe is our food? Emerging infectious diseases. 2011 Jan;17(1):126–128. doi: 10.3201/eid1701.101821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. Table 2a FoodNet–Number of Laboratory–Confirmed Infections by Year 2012. 2013 Available at: http://www.cdc.gov/foodnet/data/trends/tables/2012/table2a-b.html#table-2b.
- 27.Rzepecki P, Barzal J, Sarosiek T, Oborska S, Szczylik C. Which parameters of nutritional status should we choose for nutritional assessment during hematopoietic stem cell transplantation? Transplantation proceedings. 2007 Nov;39(9):2902–2904. doi: 10.1016/j.transproceed.2007.08.067. [DOI] [PubMed] [Google Scholar]
- 28.Gaul LK, Farag NH, Shim T, Kingsley MA, Silk BJ, Hyytia-Trees E. Hospital-acquired listeriosis outbreak caused by contaminated diced celery--Texas, 2010. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013 Jan;56(1):20–26. doi: 10.1093/cid/cis817. [DOI] [PubMed] [Google Scholar]
- 29.Lee MB, Greig JD. A review of nosocomial Salmonella outbreaks: infection control interventions found effective. Public health. 2013 Mar;127(3):199–206. doi: 10.1016/j.puhe.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 30.Fox N, Freifeld AG. The neutropenic diet reviewed: moving toward a safe food handling approach. Oncology. 2012 Jun;26(6):572–575. 580, 582 passim. [PubMed] [Google Scholar]
- 31.Galati PC, Lataro RC, Souza VM, de Martinis EC, Chiarello PG. Microbiological profile and nutritional quality of raw foods for neutropenic patients under hospital care. Revista brasileira de hematologia e hemoterapia. 2013;35(2):94–98. doi: 10.5581/1516-8484.20130028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trifilio SM, Pi J, Mehta J. Changing epidemiology of Clostridium difficile-associated disease during stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013 Mar;19(3):405–409. doi: 10.1016/j.bbmt.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 33.Rasheed W, Ghavamzadeh A, Hamladji R, et al. Hematopoietic stem cell transplantation practice variation among centers in the Eastern Mediterranean Region (EMRO): Eastern Mediterranean Bone Marrow Transplantation (EMBMT) group survey. Hematology/oncology and stem cell therapy. 2013 Mar;6(1):14–19. doi: 10.1016/j.hemonc.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Bousquet A, Demoures T, Malfuson JV, Martinaud C, Soler C. Campylobacter jejuni cutaneous infection in a patient with graft versus host disease. Medecine et maladies infectieuses. 2012 May;42(5):235–236. doi: 10.1016/j.medmal.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Radice C, Munoz V, Castellares C, et al. Listeria monocytogenes meningitis in two allogeneic hematopoietic stem cell transplant recipients. Leukemia & lymphoma. 2006 Aug;47(8):1701–1703. doi: 10.1080/10428190600648135. [DOI] [PubMed] [Google Scholar]
- 36.Falomir MP, Rico H, Gozalbo D. Enterobacter and Klebsiella species isolated from fresh vegetables marketed in Valencia (Spain) and their clinically relevant resistances to chemotherapeutic agents. Foodborne pathogens and disease. 2013 Dec;10(12):1002–1007. doi: 10.1089/fpd.2013.1552. [DOI] [PubMed] [Google Scholar]
- 37.Jensen AN, Storm C, Forslund A, Baggesen DL, Dalsgaard A. Escherichia coli contamination of lettuce grown in soils amended with animal slurry. Journal of food protection. 2013 Jul;76(7):1137–1144. doi: 10.4315/0362-028X.JFP-13-011. [DOI] [PubMed] [Google Scholar]