Abstract
It is not well described the pathophysiology of renal injuries caused by a high salt intake in humans. The authors analyzed the relationship between the 24-hr urine sodium-to-creatinine ratio (24HUna/cr) and renal injury parameters such as urine angiotensinogen (uAGT/cr), monocyte chemoattractant peptide-1 (uMCP1/cr), and malondialdehyde-to-creatinine ratio (uMDA/cr) by using the data derived from 226 hypertensive chronic kidney disease patients. At baseline, the 24HUna/cr group or levels had a positive correlation with uAGT/cr and uMDA/cr adjusted for related factors (P<0.001 for each analysis). When we estimated uAGT/cr in the 24HUna/cr groups by ANCOVA, the uAGT/cr in patients with ≥200 mEq/g cr was higher than in patients with <100 mEq/g cr (708 [95% CI, 448-967] vs. 334 [95% CI, 184-483] pg/mg cr, P=0.014). Similarly, uMDA/cr was estimated as 0.17 (95% CI, 0.14-0.21) pM/mg cr in patients with <100 mEq/g cr and 0.27 (95% CI, 0.20-0.33) pM/mg cr in patients with ≥200 mEq/g cr (P=0.016). During the 16-week follow-up period, an increase in urinary sodium excretion predicted an increase in urinary angiotensinogen excretion. In conclusion, high salt intake increases renal renin-angiotensin-system (RAS) activation, primarily, and directly or indirectly affects the production of reactive oxygen species through renal RAS activation.
Keywords: Chronic Renal Insufficiency, Sodium Chloride, Renin, Angiotensinogen
INTRODUCTION
Chronic kidney disease (CKD) is present in 13.7% of Korean adults and caused by chronic diseases, such as hypertension, diabetes mellitus, and obesity (1). The chief aim of CKD treatment is to prevent renal function deterioration and cardiovascular complications. The key of CKD treatment is to control blood pressure and lessen proteinuria (2). Sodium intake is one of the modifiable risk factors for control of blood pressure and proteinuria. Animal and human studies have showed that reduced salt intake results in decreased cardiovascular and renal events (2) and that high salt intake affects the benefits of CKD treatment modalities such as the renin angiotensin system inhibitors (RASI) (3, 4). Dietary education for 18 months decreased salt intake by 30% in participants with a high risk for hypertension and resulted in a 25% reduction in cardiovascular events at 13 yr after the original study (5). With a double-blind randomized controlled study design for CKD patients, dietary sodium restriction decreased ambulatory blood pressure, proteinuria, and albuminuria (6). The post hoc analysis of the Ramipril Efficacy In Nephrology (REIN) or Irbesartan Diabetic Nephropathy Trial (IDNT) and The Reduction of Endpoints in type II diabetes mellitus with the Angiotensin II Antagonist Losartan (RENAAL) trials showed that high salt intake reduced the antiproteinuric effect of RASI and increased renal and cardiovascular events among patients with CKD with (4) or without diabetes (3). However, it is difficult to follow the current guidelines on salt intake (7), especially in populations at high risk for cardio-renal events. As reported by Krikken (2), the baseline amount of daily urine sodium was >170 mEq/day in non-diabetic CKD patients (8, 9). Furthermore, the CKD cohort in Italy (10) also showed an amount of urinary sodium (nearly 150 mEq/day) similar to the general population cohort.
High sodium intake affects blood pressure, proteinuria, and glomerular hemodynamic and causes renal damage (11). To date, the results about the mechanism of renal injury by salt were as follows. A series of animal studies have demonstrated that the direct effect of high salt intake on the promotion of TGF-β1 (12, 13), RAS activation (14), inflammation (15, 16), production of reactive oxygen species (ROS) (16-18), and activation of mineralocorticoid receptors (19) in the endothelium and renal tissues. Although the pathophysiology of the harmful effects of high salt intake seems to have been well described in animal studies, it is not well described in human studies. In patients with diabetic nephropathy, urinary excretion of TGF-β1 was higher than in non-diabetic patients (20) or normoalbuminuric diabetes patients (21); however, salt restriction did not reduce urinary TGF-β1 excretion in the CKD cohort (22). Urinary MCP-1 as an inflammatory marker was increased with high salt intake (15 g NaCl/day) compared to low salt intake (5 g NaCl/day) in healthy volunteers (23). However, previous human studies included a small number of participants and had a cross-sectional study design (20, 21) or short-term follow-up period of 7 days (23).
We designed a prospective open-label randomized clinical trial of hypertensive CKD patients to verify the proteinuria-lowering effect of an intensive low-salt diet education for patients taking a RASI, with an observation period of 16 weeks. Here, we aim to determine the relationship between salt intake-estimated by measuring 24-hr urine sodium-and urinary biomarkers of the RAS, inflammation, ROS, and the changes in urinary sodium and biomarkers, from the urine samples collected in the study.
MATERIALS AND METHODS
Study population
The patients were selected from the outpatient renal clinics in 7 centers in Korea between March 2012 and March 2013. All patients fulfilled the following inclusion criteria: age of 19-75 yr; use of antihypertensive medication or a diagnosis of hypertension; Modification of Diet in Renal Disease (MDRD) estimated glomerular filtration rate (GFR) ≥30 mL/min/1.73 m2; random urine albumin to creatinine ratio ≥30 mg/g Cr in the last 6 months; and an ability and willingness to provide written informed consent. The exclusion criteria was as follows: uncontrolled hypertension (BP >160/100 mmHg) at the time of screening; pregnancy; serum potassium >5.5 mEq/L; malignancy; diagnosis of cardiovascular disease (cerebral infarction, hemorrhagic infarction, acute myocardial infarction or unstable angina, coronary angioplasty, or coronary artery bypass surgery) within the last 6 months; contraindication to angiotensin II receptor blockers (ARBs); diabetes mellitus; and use of steroids or other immunosuppressive agents at the time of registration.
Study protocol
Patients were screened 8 weeks prior to commencement of the study, and the protocol included a "run-in period" for antihypertensive medication adjustment. All participants had to stop any RAS blocking agent or diuretic therapy and switch to antihypertensive agents of different categories during this period. After an 8-week run-in period (0-week), the investigators conducted baseline laboratory investigations. From 0-week, all the enrolled patients were prescribed olmesartan medoxomil (Daewoong Pharmaceutical Co./Daiichi Sankyo Korea Co., Seoul, Korea) with a 40 mg once-a-day fixed dose until the end of the study. After another 8 weeks (8-week), participants were randomly assigned to receive a low salt diet (LSD) intervention after the second laboratory investigation. The participants in the conventional education group received routine LSD education at an outpatient clinic. Otherwise, the participants in the intensive education group received ongoing support from a dietary consultant and feedback by telephone for 30 minutes, once a week during the study period. The target amount of daily sodium intake was <100 mEq/day in the intensive education group and a reduction of salt intake of ≥25% was recommended in both groups. After randomization, participants with poor medication adherence to olmesartan (used ≤60% of the prescribed medication) were removed from the study. After the 8-week study (16-week), participants underwent a third laboratory investigation. At week 0, 8, and 16, on the day before each visit, patients were asked to collect 24-hr urine samples to assess proteinuria and urinary sodium, urea, potassium, and creatinine excretion. Some of the urine from the 24-hr urine collection was stored in the refrigerator at -70℃ for future urine cytokines measurements. Safety assessments included adverse events, self-reported hypotension, and selected hematological and biochemical measures.
Outcome measurement
We analyzed the relationship between 24HUna/cr levels and the uAGT/cr, uMCP1/cr, and uMDA/cr at 0-week as continuous variables and categorical variables and the change in 24HUna/cr levels and increase in urine cytokines during 16 weeks.
Statistical analysis
We assigned patients to the following 3 groups according to 24HUna/cr levels: 1) <100 mEq/g; 2) 100-199 mEq/g cr; and 3) ≥200 mEq/g. Furthermore, we divided patients into 3 subgroups according to the change in 24HUna/cr during 16 weeks-ratio of 24HUna/cr at 16-week compared to that at 0-week-: 1) decreased, <-25%; 2) unchanged, -24.9%-24.9%; and 3) increased, ≥25%. Finally, we divided patients into 2 subgroups according to the change in uAGT/cr, uMCP1/cr, and uMDA/cr with the ratio of urine cytokines to creatinine ratios at 16-week compared to those at 0-week: 1) increased,≥25%; and 2) unchanged, others. We defined the decrease of 24HUalb/cr as the ratio of 24HUalb/cr at 16-week to that at 0-week <-25%.
All analyses and calculations were performed with SPSS Statistics V21.0 (IBM Corporation, Armonk, NY, USA). Continuous variables were expressed as mean±standard deviation (SD) or as a percentage for categorical variables. The independent t-test or one-way ANOVA test was used to compare continuous variables between the groups according to the number of subgroups, and the Pearson's Chi-square test was used to analyze the categorical variables. The relationship between variables was estimated with Pearson's correlation coefficient and tested with multiple linear regression analysis for continuous variables and multiple logistic regression analysis for dichotomized variables by adjusting for related factors. We also calculated the estimated level of continuous variables according to the 24HUna/cr group by using co-variate analysis (ANCOVA) with adjustment for independently related factors. Two-tailed values of P<0.05 were considered statistically significant.
Ethics Statement
This study was approved by institutional review board of Seoul National University Bundang Hospital (IRB number: B-1112-142-008). The institutional review board of each participating hospital approved the treatment protocol before the initiation of investigation. Written informed consent was obtained from each patient before inclusion.
RESULTS
Basal characteristics of participants
A total of 312 subjects were screened, and 269 subjects were enrolled. Of the 269 subjects, 34 dropped out during the study period, 235 completed the trial. Among those who completed the trial, 9 did not provide urine samples for urine cytokines; therefore, data from 226 subjects were analyzed.
The mean age was 50.3±13.0 yr at enrollment and 50% of the participants were male (Table 1). Seventy-five subjects (33.2%) were assigned to group 1 (24HUna/cr <100 mEq/g), 125 subjects (55.3%) to group 2 (24HUna/cr 100-199 mEq/g), and 26 subjects (11.5%) to group 3 (24HUna/cr ≥200 mEq/g). Group 3 had the highest mean age, lowest proportion of men, highest BMI, and lowest levels of hemoglobin and 24-hr urine creatinine. The 24HUalb/cr ratio in group 3 was higher than in group 1 (post hoc analysis with Fisher's least significance difference (FLSD) method, P=0.028). Blood pressures (BPs) and GFR were not different among groups. Other characteristics including medical history, drinking or smoking habits, medications, and serum cholesterol were not correlated with the amount of 24HUna/cr.
Table 1.
Baseline characteristics of participants at 0-week*
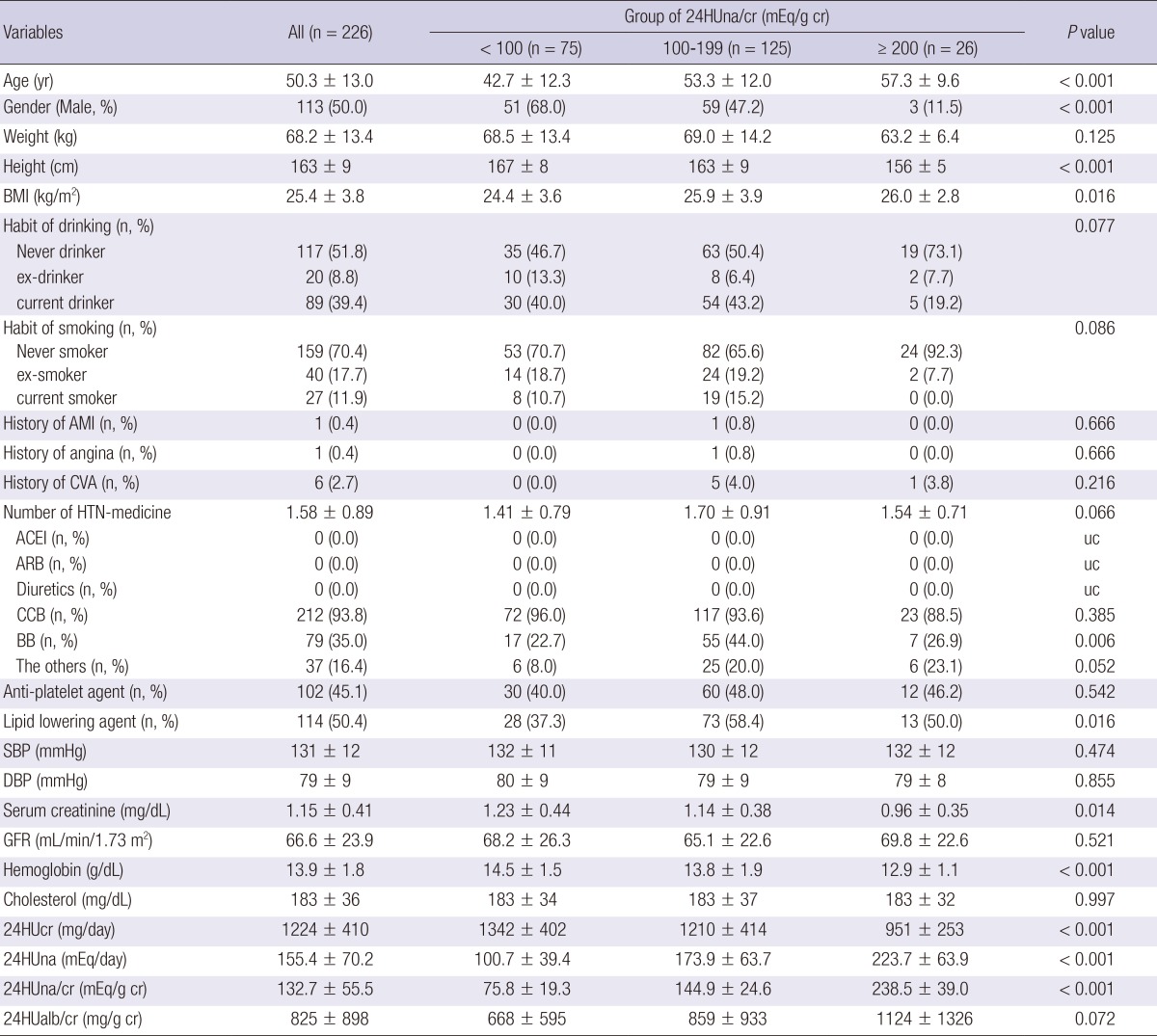
*0-week, the period starting Olmesartan (40 mg per day) after a 8-wash out period of any ARB, ACEI, or aldosterone blocker. BMI, body mass index; AMI, acute myocardial infarction; CVA, cerebrovascular accident including thrombotic cerebral infarction and intracranial hemorrhage; Number of HTN medications, number of medications to control hypertension; ACEI, angiotensin II converting enzyme inhibitor; ARB, angiotensin type I receptor blocker; CCB, calcium channel blocker; BB, beta-blocker; Anti-platelet agent, low dose aspirin, cilostazole, and clopidogrel; Lipid lowering agent, statins and fibrates; SBP, systolic blood pressure, DBP, diastolic blood pressure; GFR, glomerular filtration rate estimated using the original Modification of Diet in Renal Diease equation using serum creatinine values measured by the modified Jaffe reaction; 24HUcr, amount of daily urinary creatinine; 24HUna, amount of daily urinary sodium; 24HUna/cr, the ratio of 24-hr urine sodium to creatinine (mg/g creatinine); 24HUalb, amount of daily urinary albumin; 24HUalb/cr, the ratio of 24-hr urine albumin to creatinine (mg/g creatinine); uc, unable to calculate. P values were calculated using the One-way ANOVA test among 24-hr urinary sodium/cr groups.
Relationship between baseline urinary sodium and albumin excretion
After univariate analysis, we found that systolic blood pressure, diastolic blood pressure, height, serum cholesterol, hemoglobin, GFR, uAGT/cr, uMCP1/cr showed the relationship with baseline 24HUalb/cr. Multiple linear regression analysis adjusted for these parameters and age showed that 24HUna/cr levels or group of 24HUna/cr had a positive correlation with 24HUalb/cr levels (Table 2).
Table 2.
The factors related to the 24-hr urine albumin to creatinine ratio at 0-week
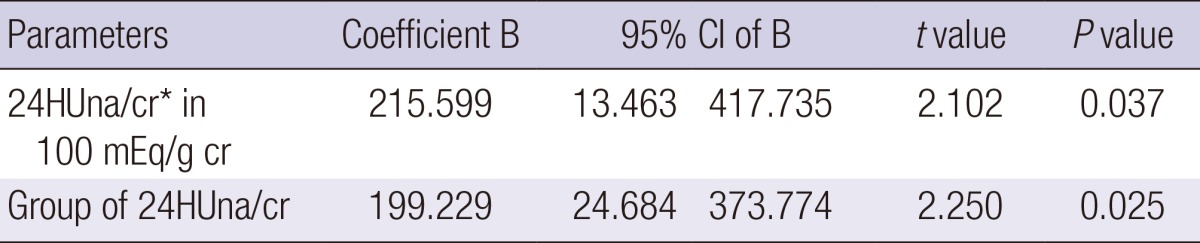
*24HUna/cr, 24-hr urine sodium to creatinine ratio. Each parameter was used in the multiple linear regression model adjusted with age and gender, systolic blood pressure, diastolic blood pressure, height, serum cholesterol, hemoglobin, GFR, urine MCP-1 to creatinine ratio, and urine angiotensinogen to creatinine ratio, which were the factors related to the 24-hr albumin to creatinine ratio.
Relationship between baseline urine sodium and cytokines
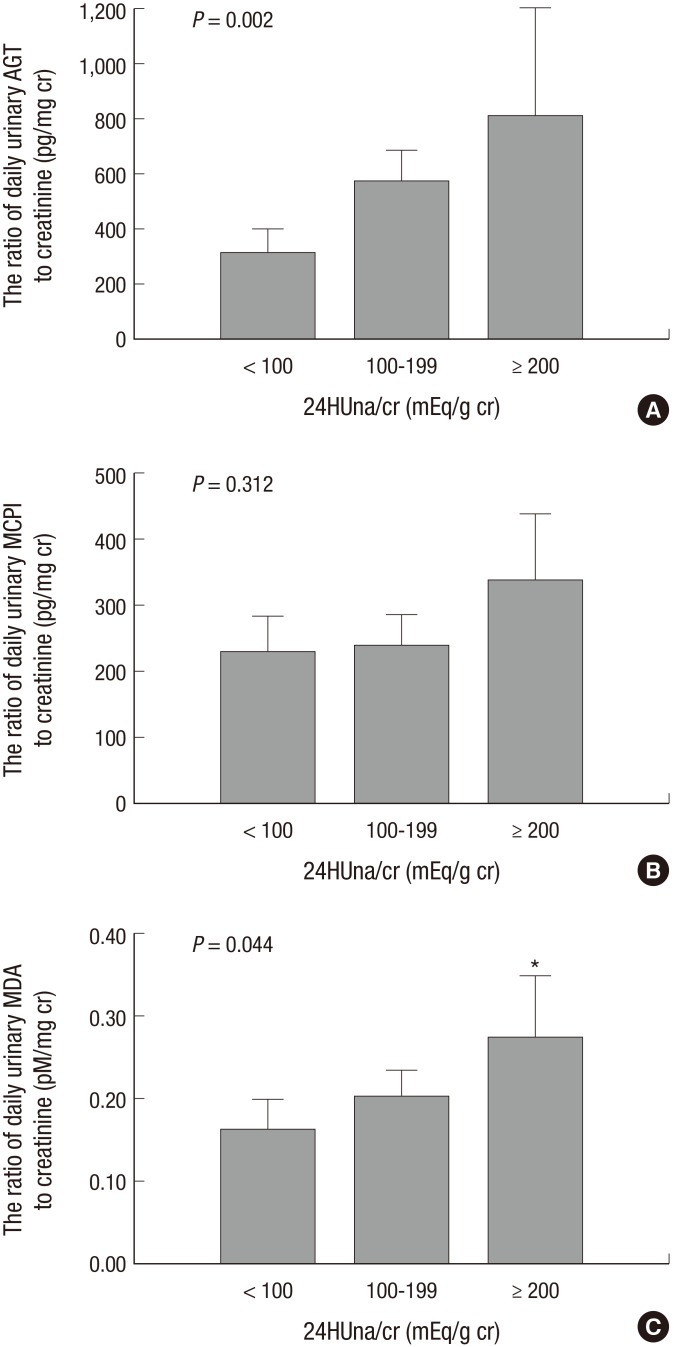
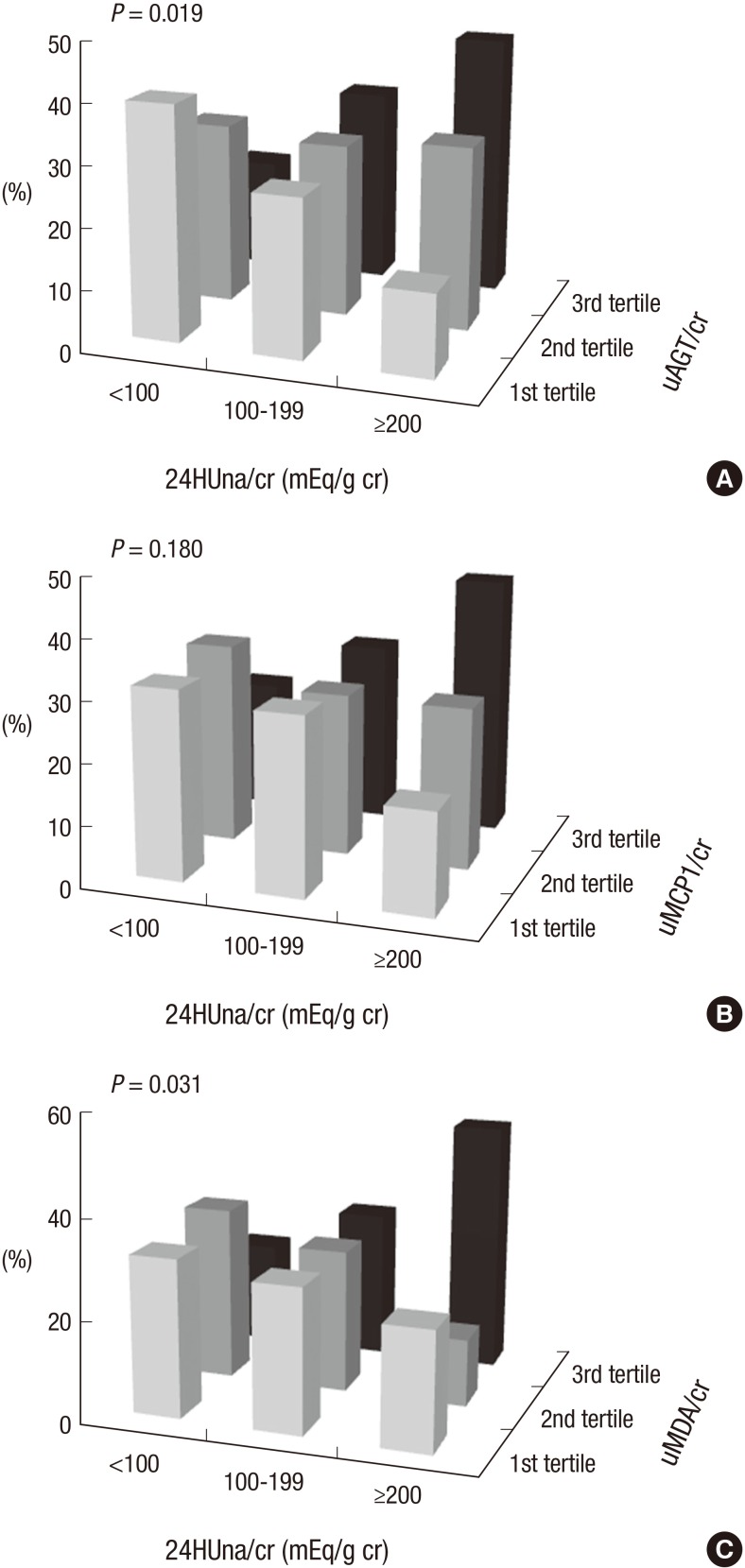
The levels of uAGT/cr and uMDA/cr were different between 24HUna/cr groups. Group 3 had the highest level of uAGT/cr and uMDA/cr and was different than group 1, although the uMCP1/cr was not different among groups (Fig. 1). The uAGT/cr was 316±284 pg/mg cr in group 1, 577±744 pg/mg cr in group 2, and 840±971 pg/mg cr in group 3 (P=0.002), and the uMDA/cr was 0.17±0.15 pM/mg cr in group 1, 0.20±0.18 pM/mg cr in group 2, and 0.26±0.19 pM/mg cr in group 3 (P=0.044). When we divided participants according to the tertile value of each urine cytokine, group 3 was assigned to the highest tertile group of uAGT/cr or uMDA/cr, more frequently than other groups (Fig. 2).
Fig. 1.
Urinary cytokines to creatinine ratio among 24HUna/cr groups. (A) AGT, angiotensinogen. (B) MCP1, monocyte chemoattractant protein-1. (C) MDA, malondialdehyde. 24HUna/cr, the ratio of 24-hr urine sodium to creatinine (mg/g creatinine). The P value was calculated by one-way ANOVA test. *Different from the group with 24HUna/cr<100 mEq/g cr. The bar means 95% confidence interval of each mean value.
Fig. 2.
The frequency of tertile groups of cytokines according to 24HUna/cr groups. The ratio of 24-hr urine sodium to creatinine (mg/g creatinine) by (A) uAGT/cr, urine angiotensinogen to creatinine ratio (pg/mg cr), (B) uMCP1/cr, urine monocyte chemoattractant protein-1 to creatinine ratio (pg/mg cr), (C) uMDA/cr: urine malondialdehyde to creatinine ratio (pM/mg cr).
With regard to the factors related to the level of each urine cytokine, independent factors for each cytokine were determined by an adjusted multiple linear regression analysis. 24HUna/cr levels had a positive correlation with uAGT/cr and uMDA/cr (Table 3). When we estimate uAGT/cr in the 24HUna/cr group by ANCOVA adjusted for 24HUalb/cr-which was another independent factor for uAGT/cr-the uAGT/cr was 334 (95% CI, 184-483) pg/mg cr in group 1, 573 (95% CI, 457-689) pg/mg cr in group 2, and 708 (95% CI, 448-967) pg/mg cr in group 3 (P=0.014) (data not shown). Furthermore, differences between group 1 and group 2 (P=0.013) or group 3 (P=0.015) were identified. Similarly, uMDA/cr was estimated as 0.17 (95% CI, 0.14-0.21) pM/mg cr in group 1, 0.20 (95% CI, 0.17-0.23) pM/mg cr in group 2, and 0.27 (95% CI, 0.20-0.33) pM/mg cr in group 3 adjusted for beta-blocker medication (P=0.054). There was a difference between group 1 and 2 or 3 (P=0.016) based on the LSD method (data not shown).
Table 3.
The group of 24HUna/cr as an independent factor to the level of urine cytokines at 0-week* by multiple linear regression
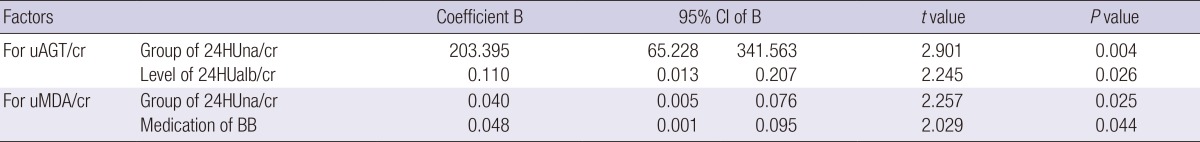
*0 week, starting week for Olmesartan (40 mg per day) after a 8-wash out period of any ARB, ACEI, or aldosterone blocker; 24HUna/cr group: group according to the ratio of 24-hr urine sodium to creatinine with the criteria of 100 and 200 mEq/g cr; 24HUalb/cr: the ratio of 24-hr urine albumin to creatinine (mg/g creatinine); uAGT/cr: urine angiotensinogen to creatinine ratio (pg/mg cr); uMDA/cr: urine malondialdehyde to creatinine ratio (pM/mg cr); BB medication: beta-blocker medication; Linear regression for uAGT/cr was adjusted for age, gender, hemoglobin, 24-hr urine albumin to creatinine ratio, GFR, and group of 24HUna/cr, which were the factors related to the urine angiotensinogen to creatinine ratio at 0-week. Linear regression for uMDA/cr was adjusted for age, gender, beta-blocker, serum total CO2, and group of 24HUna/cr.
Change of 24HUna/cr and increase of urine cytokines
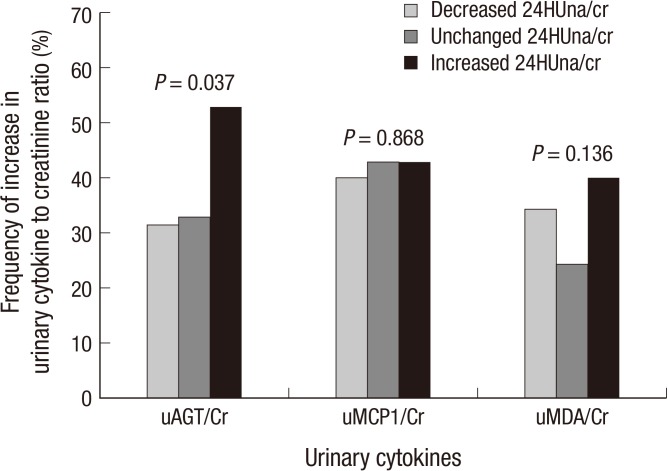
The factors related to an increase in urine cytokine were determined for each cytokine. The clinical parameters at baseline examination were not related to an increase in all urinary cytokines. The patients with an increase in 24HUna/cr levels showed a higher frequency of increase in uAGT/cr levels during 16 weeks (P=0.037, Fig. 3). The patients with increased 24HUna/cr showed 2.441-fold (95% CI, 1.182-5.042) higher risk for increase in uAGT/cr during 16 weeks by multiple logistic regression adjusted for age and gender (P=0.040). The group of change in 24HUna/cr was not related to an increase in uMCP1/cr or uMDA/cr.
Fig. 3.
The relationship between the changes of 24-hr urine cytokines to creatinine ratio and urine sodium to creatinine ratio during 16 weeks after ARB medication. Frequency of increase in cytokine level: Frequency of increase in 24-hr urinary cytokine to creatinine ratio at 16-week compared to 0-week 25% or more. Increased 24HUna/cr defined as increase of 24HUna/cr ratio 25% or more, Decreased 24HUna/cr defined as decrease of 24HUna/cr ratio 25% or more, and unchanged 24HUna/cr defined as the ratio of 24HUna/cr between -24.9% and 24.9%, at 16-week compared to 0-week. uAGT/Cr: 24-hr urine angiotensinogen to creatinine ratio (pg/mg cr), uMCP1/Cr: 24-hr urine monocyte chemoattractant protein-1 to creatinine ratio (pg/mg cr), uMDA/Cr: 24-hr urine malondialdehyde to creatinine ratio (pM/mg cr).
DISCUSSION
In this prospective, open-label, randomized trial, we confirmed that the 24-hr urine sodium to creatinine ratio had a positive correlation to the amount of albuminuria. A high urinary excretion of sodium was related to high excretion of urinary angiotensinogen or MDA, and an increase of urinary sodium excretion predicted an increase in urinary angiotensinogen excretion.
This study reported the difference in urinary cytokines according to the level of urinary sodium excretion, to confirm the pathophysiology of high salt diet related to renal injuries previously reported in animal experiments. Urinary excretion of angiotensinogen was reported to reflect the status of the intrarenal renin-angiotensin system in a previous animal experiment (24) and in patients with hypertension or glomerulonephritis (25, 26). High sodium intake increased renal angiotensin II levels and systolic blood pressure (SBP) in normal rats, salt sensitive hypertensive rats (27), and obese Zucker rats (28). Furthermore, it increased angiotensin converting enzyme (ACE) activity in obese rats (28), and activated angiotensin II type I receptor in the renal tissue of spontaneously hypertensive rats associated with a rise in proteinuria and renal injury (16, 29), compared to animals on low salt diets. Increased RAS activity is associated with an increased risk of chronic kidney disease in rodent models (30). However, Krikken reviewed that there were few reports about the direct effect of salt intake on renal RAS activation in patients and the available human data only suggests that a high sodium intake increases vascular ACE activity (2). In this study, we showed the direct relationship between the amount of salt intake estimated by the 24-hr urine sodium levels and renal RAS activity with a marker of urinary angiotensinogen in hypertensive CKD patients without RASI or diuretic medication. An increase in urinary sodium excretion could predict an increase in urinary angiotensinogen excretion and a decreased anti-proteinuric effect of ARB during 16 weeks. Recently, a randomized controlled trial and post hoc analysis of large trials studying the RAS effect on renal outcomes showed similar findings with regard to the relationship between salt intake and proteinuria (3, 4, 6). A post hoc analysis including non-diabetic CKD patients enrolled in the REIN study (3) showed that baseline urinary sodium/creatinine excretion was positively correlated with the urinary protein/creatinine ratio at baseline and follow-up, independent from blood pressure. The antiproteinuric effect of RASI was significantly higher in low salt diet (LSD) patients compared with medium-salt diet (MSD) or high salt diet (HSD), after 3 months of treatment (3). Three months after the RASI treatment, the urinary protein/creatinine ratio was reduced by 31%, 25%, and 20% in LSD, MSD, and HSD patients, respectively (3). The incidence of ESRD was also the highest in HSD patients (32.1%); however, in MSD patients (17.0%), the rate was not different from that of LSD (16.2%). In diabetic CKD patients, the effects of ARB treatment on reducing renal and cardiovascular events compared to the non-RASI treatment were lower with higher sodium intake (4). Taken together, proteinuria induced by a high salt diet was closely related to an increase of RAS activity in the kidney tissue, which also decreased the antiproteinuric effect of the RASI treatment in hypertensive CKD patients.
In animal experiments, high salt intake increased the concentration of renal cortical hydrogen peroxide (17), renal cortical NADH and NADPH oxidase activity, expression of gp91phox, p47phox (18), p22phox (16), and decreased renal expression of Mn-superoxide dismutase (SOD) (18) or Cu/Zn-SOD (16). Furthermore, it increased urinary 8-isoprostane prostaglandin F2α and MDA, the end products of lipid oxidation (16). In human studies, we could not find any direct evidence of the relationship between salt intake and these ROS production in the literature. The positive relationship between urinary salt and MDA excretion was evident in patients without RASI or diuretics; however, the relationship was weaker than that between urinary salt and angiotensinogen excretion. In fact, a linear regression model including the urine angiotensinogen to creatinine ratio to estimate independent factors related to the urine MDA to creatinine ratio revealed that the 24-hr urine sodium had no effect on continuous or categorical variables. However, the relationship between urinary sodium and angiotensinogen excretion was not affected by urinary MDA levels in the regression model. This finding suggested that the relationship between urinary ROS and urinary sodium might be confounded by urinary angiotensinogen. A prolonged angiotensin II infusion in Sprague-Dawley rats increased the expression of gp91phox and p22phox in the renal cortex and increased urinary excretion of 8-isoprostane prostaglandin F2α and MDA (18, 31). Therefore, high salt intake increases renal RAS activation, primarily, and affects the production of ROS in a direct or indirect manner through renal RAS activation.
This study had some limitations affecting generalization to all hypertensive CKD patients. The study did not intend to confirm the relationship between salt intake and urinary cytokines; hence, there might be confounding factors that were not considered, regardless of the multivariate analysis. We used only one parameter for each signal of RAS, inflammation, and ROS, which reduces the accuracy of detecting the signal in the kidney. Although we standardized the urinary cytokines and sodium to urine creatinine levels to remove an error introduced by inappropriate urine collection, their accuracy could be affected by this error. Finally, urine samples were stored until the end of the study and the cytokines were measured 6 months after the first period of urine collection. During this period, the urine contents could have been altered in each sample, introducing an accuracy error.
In conclusion, our study confirms that high salt intake in hypertensive CKD patients is related to a high level of urine albumin, based on the activation of the intrarenal RAS.
Footnotes
This research was supported by a 2013 grant (13162MFDS104) from Ministry of Food and Drug Safety.
The authors have no conflicts of interest to disclose.
References
- 1.Kim S, Lim CS, Han DC, Kim GS, Chin HJ, Kim SJ, Cho WY, Kim YH, Kim YS. The prevalence of chronic kidney disease (CKD) and the associated factors to CKD in urban Korea: a population-based cross-sectional epidemiologic study. J Korean Med Sci. 2009;24:S11–S21. doi: 10.3346/jkms.2009.24.S1.S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krikken JA, Laverman GD, Navis G. Benefits of dietary sodium restriction in the management of chronic kidney disease. Curr Opin Nephrol Hypertens. 2009;18:531–538. doi: 10.1097/MNH.0b013e3283312fc8. [DOI] [PubMed] [Google Scholar]
- 3.Vegter S, Perna A, Postma MJ, Navis G, Remuzzi G, Ruggenenti P. Sodium intake, ACE inhibition, and progression to ESRD. J Am Soc Nephrol. 2012;23:165–173. doi: 10.1681/ASN.2011040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambers Heerspink HJ, Holtkamp FA, Parving HH, Navis GJ, Lewis JB, Ritz E, de Graeff PA, de Zeeuw D. Moderation of dietary sodium potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers. Kidney Int. 2012;82:330–337. doi: 10.1038/ki.2012.74. [DOI] [PubMed] [Google Scholar]
- 5.Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP) BMJ. 2007;334:885–888. doi: 10.1136/bmj.39147.604896.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMahon EJ, Bauer JD, Hawley CM, Isbel NM, Stowasser M, Johnson DW, Campbell KL. A randomized trial of dietary sodium restriction in CKD. J Am Soc Nephrol. 2013;24:2096–2103. doi: 10.1681/ASN.2013030285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drüeke TB, Parfrey PS. Summary of the KDIGO guideline on anemia and comment: reading between the (guide)line(s) Kidney Int. 2012;82:952–960. doi: 10.1038/ki.2012.270. [DOI] [PubMed] [Google Scholar]
- 8.The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia) Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet. 1997;349:1857–1863. [PubMed] [Google Scholar]
- 9.Ruggenenti P, Perna A, Loriga G, Ganeva M, Ene-Iordache B, Turturro M, Lesti M, Perticucci E, Chakarski IN, Leonardis D, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365:939–946. doi: 10.1016/S0140-6736(05)71082-5. [DOI] [PubMed] [Google Scholar]
- 10.De Nicola L, Minutolo R, Chiodini P, Zoccali C, Castellino P, Donadio C, Strippoli M, Casino F, Giannattasio M, Petrarulo F, et al. Global approach to cardiovascular risk in chronic kidney disease: reality and opportunities for intervention. Kidney Int. 2006;69:538–545. doi: 10.1038/sj.ki.5000085. [DOI] [PubMed] [Google Scholar]
- 11.Sanders PW. Salt intake, endothelial cell signaling, and progression of kidney disease. Hypertension. 2004;43:142–146. doi: 10.1161/01.HYP.0000114022.20424.22. [DOI] [PubMed] [Google Scholar]
- 12.Ying WZ, Aaron K, Sanders PW. Mechanism of dietary salt-mediated increase in intravascular production of TGF-beta1. Am J Physiol Renal Physiol. 2008;295:F406–F414. doi: 10.1152/ajprenal.90294.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ying WZ, Sanders PW. The interrelationship between TGF-beta1 and nitric oxide is altered in salt-sensitive hypertension. Am J Physiol Renal Physiol. 2003;285:F902–F908. doi: 10.1152/ajprenal.00177.2003. [DOI] [PubMed] [Google Scholar]
- 14.Franco M, Martínez F, Quiroz Y, Galicia O, Bautista R, Johnson RJ, Rodríguez-Iturbe B. Renal angiotensin II concentration and interstitial infiltration of immune cells are correlated with blood pressure levels in salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol. 2007;293:R251–R256. doi: 10.1152/ajpregu.00645.2006. [DOI] [PubMed] [Google Scholar]
- 15.Tian N, Gu JW, Jordan S, Rose RA, Hughson MD, Manning RD., Jr Immune suppression prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol. 2007;292:H1018–H1025. doi: 10.1152/ajpheart.00487.2006. [DOI] [PubMed] [Google Scholar]
- 16.Chandramohan G, Bai Y, Norris K, Rodriguez-Iturbe B, Vaziri ND. Effects of dietary salt on intrarenal angiotensin system, NAD(P)H oxidase, COX-2, MCP-1 and PAI-1 expressions and NF-kappaB activity in salt-sensitive and -resistant rat kidneys. Am J Nephrol. 2008;28:158–167. doi: 10.1159/000110021. [DOI] [PubMed] [Google Scholar]
- 17.Tian N, Moore RS, Braddy S, Rose RA, Gu JW, Hughson MD, Manning RD., Jr Interactions between oxidative stress and inflammation in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol. 2007;293:H3388–H3395. doi: 10.1152/ajpheart.00981.2007. [DOI] [PubMed] [Google Scholar]
- 18.Kitiyakara C, Chabrashvili T, Chen Y, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Salt intake, oxidative stress, and renal expression of NADPH oxidase and superoxide dismutase. J Am Soc Nephrol. 2003;14:2775–2782. doi: 10.1097/01.asn.0000092145.90389.65. [DOI] [PubMed] [Google Scholar]
- 19.Nagase M, Matsui H, Shibata S, Gotoda T, Fujita T. Salt-induced nephropathy in obese spontaneously hypertensive rats via paradoxical activation of the mineralocorticoid receptor: role of oxidative stress. Hypertension. 2007;50:877–883. doi: 10.1161/HYPERTENSIONAHA.107.091058. [DOI] [PubMed] [Google Scholar]
- 20.Sharma K, Ziyadeh FN, Alzahabi B, McGowan TA, Kapoor S, Kurnik BR, Kurnik PB, Weisberg LS. Increased renal production of transforming growth factor-beta1 in patients with type II diabetes. Diabetes. 1997;46:854–859. doi: 10.2337/diab.46.5.854. [DOI] [PubMed] [Google Scholar]
- 21.Ellis D, Forrest KY, Erbey J, Orchard TJ. Urinary measurement of transforming growth factor-beta and type IV collagen as new markers of renal injury: application in diabetic nephropathy. Clin Chem. 1998;44:950–956. [PubMed] [Google Scholar]
- 22.Yu W, Luying S, Haiyan W, Xiaomei L. Importance and benefits of dietary sodium restriction in the management of chronic kidney disease patients: experience from a single Chinese center. Int Urol Nephrol. 2012;44:549–556. doi: 10.1007/s11255-011-9986-x. [DOI] [PubMed] [Google Scholar]
- 23.Zhou X, Zhang L, Ji WJ, Yuan F, Guo ZZ, Pang B, Luo T, Liu X, Zhang WC, Jiang TM, et al. Variation in dietary salt intake induces coordinated dynamics of monocyte subsets and monocyte-platelet aggregates in humans: implications in end organ inflammation. PLoS One. 2013;8:e60332. doi: 10.1371/journal.pone.0060332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61:579–585. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urushihara M, Kondo S, Kagami S, Kobori H. Urinary angiotensinogen accurately reflects intrarenal Renin-Angiotensin system activity. Am J Nephrol. 2010;31:318–325. doi: 10.1159/000286037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobori H, Alper AB, Jr, Shenava R, Katsurada A, Saito T, Ohashi N, Urushihara M, Miyata K, Satou R, Hamm LL, et al. Urinary angiotensinogen as a novel biomarker of the intrarenal renin-angiotensin system status in hypertensive patients. Hypertension. 2009;53:344–350. doi: 10.1161/HYPERTENSIONAHA.108.123802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franco M, Martínez F, Quiroz Y, Galicia O, Bautista R, Johnson RJ, Rodríguez-Iturbe B. Renal angiotensin II concentration and interstitial infiltration of immune cells are correlated with blood pressure levels in salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol. 2007;293:R251–R256. doi: 10.1152/ajpregu.00645.2006. [DOI] [PubMed] [Google Scholar]
- 28.Samuel P, Ali Q, Sabuhi R, Wu Y, Hussain T. High Na intake increases renal angiotensin II levels and reduces expression of the ACE2-AT(2)R-MasR axis in obese Zucker rats. Am J Physiol Renal Physiol. 2012;303:F412–F419. doi: 10.1152/ajprenal.00097.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Susic D, Frohlich ED, Kobori H, Shao W, Seth D, Navar LG. Salt-induced renal injury in SHRs is mediated by AT1 receptor activation. J Hypertens. 2011;29:716–723. doi: 10.1097/HJH.0b013e3283440683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mills KT, Kobori H, Hamm LL, Alper AB, Khan IE, Rahman M, Navar LG, Liu Y, Browne GM, Batuman V, et al. Increased urinary excretion of angiotensinogen is associated with risk of chronic kidney disease. Nephrol Dial Transplant. 2012;27:3176–3181. doi: 10.1093/ndt/gfs011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chabrashvili T, Kitiyakara C, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Effects of ANG II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase, and SOD expression. Am J Physiol Regul Integr Comp Physiol. 2003;285:R117–R124. doi: 10.1152/ajpregu.00476.2002. [DOI] [PubMed] [Google Scholar]