Abstract
Purpose
We sought to evaluate the clinical outcomes of 3-dimensional conformal radiation therapy (3D-CRT) for portal vein tumor thrombosis (PVTT) alone in patients with advanced hepatocellular carcinoma.
Materials and Methods
We retrospectively analyzed data on 46 patients who received 3D-CRT for PVTT alone between June 2002 and December 2011. Response was evaluated following the Response Evaluation Criteria in Solid Tumors. Prognostic factors and 1-year survival rates were compared between responders and non-responders.
Results
Thirty-seven patients (80.4%) had category B Child-Pugh scores. The Eastern Cooperative Oncology Group performance status score was 2 in 20 patients. Thirty patients (65.2%) had main or bilateral PVTT. The median irradiation dose was 50 Gy (range, 35 to 60 Gy) and the daily median dose was 2 Gy (range, 2.0 to 2.5 Gy). PVTT response was classified as complete response in 3 patients (6.5%), partial response in 12 (26.1%), stable disease in 19 (41.3%), and progressive disease in 12 (26.1%). There were 2 cases of grade 3 toxicities during or 3 months after radiotherapy. Twelve patients in the responder group (15 patients) received at least 50 Gy irradiation, but about 84% of patients in the non-responder group received less than 50 Gy. The 1-year survival rate was 66.8% in responders and 27.4% in non-responders constituting a statistically significant difference (p = 0.008).
Conclusion
Conformal radiotherapy for PVTT alone could be chosen as a palliative treatment modality in patients with unfavorable conditions (liver, patient, or tumor factors). However, more than 50 Gy of radiation may be required.
Keywords: Radiotherapy, Hepatocellular carcinoma, Portal vein
Introduction
Portal vein tumor thrombosis (PVTT) in advanced hepatocellular carcinoma (HCC) can result in both intrahepatic metastasis through the portal pathway and a reduced hepatic blood stream. PVTT also leads to portal hypertension and can cause ascites, variceal rupture, and hepatic dysfunction [1]. Therefore, transarterial chemoembolization (TACE), transarterial chemotherapy, targeted drug therapy using sorafenib, and other conventional treatments can have limited applicability in patients with PVTT. In a previous study, the mean survival time was approximately 2.7 months without treatment of PVTT [2].
There is no definite treatment guideline for patients with PVTT [3,4]. Although the American Association for the Study of Liver Disease has recommended sorafenib as a palliative systemic therapy in advanced HCC with PVTT, it is not widely applied because 1) there is no appropriate consensus regarding dosage and 2) there is some controversy regarding safety in Child-Pugh class B patients.
The development of advanced radiation techniques, such as 3-dimensional conformal radiation therapy (3D-CRT), intensity-modulated radiation therapy, and image-guided radiation therapy, has allowed the precise delivery of a higher ablative dose of radiation to the target lesion [5,6,7]. Recent studies have shown that irradiation with 23.4-79.3 Gy10 to the PVTT including part of or the entire HCC lesions, resulted in complete or partial PVTT response rates of 44.7%-62.3% [8,9,10]. Another report demonstrated TACE contribute to control intrahepatic metastasis beyond the radiation treatment field, so the combination of radiotherapy after TACE within 2 weeks can theoretically maximize the synergic effect of two modalities without aggravating liver function [11].
However, wide-field irradiation (i.e., radiotherapy for PVTT including the whole primary HCC lesion) can lead to severe side effects, such as radiation-induced liver disease. Wide-field irradiation can especially harmful in patients with an extremely large HCC masses because these patients have comparatively small volumes of normal liver. In addition, the widened target field can exacerbate issues with liver function in unfavorable conditions, such as severe liver cirrhotic state or poor performance status. Therefore, it could lead to the interruption of additional treatment for the primary HCC after radiotherapy.
Most previous studies have focused PVTT treatments that include the entire primary HCC. In this retrospective study, we analyzed data on patients who underwent radiotherapy for the PVTT alone (for various underlying reasons). We sought to investigate treatment response, toxicity, and prognostic factors. Further, the results of local field radiotherapy and conventional treatment were compared.
Materials and Methods
1. Patients
From June 2002 to December 2011, 96 patients underwent 3D-CRT for HCC. We retrospectively analyzed data on only those patients in whom 3D-CRT had been conducted to treat PVTT alone. During the study period, patients who presented various unfavorable conditions had received palliative conformal radiotherapy for PVTT alone. Unfavorable conditions could be divided into several categories, such as patient factors, liver factors, and tumor factors. Patient factors included age, performance status, and the existence of severe duodenal ulceration. Liver factors included the severity of cirrhosis, the coexistence of active hepatitis, and normal liver volume. Tumor factors included adverse features due to the extension of HCC, distant or lymph node metastases, multifocal hepatic lesions, wide-raging venous invasion, advanced tumor stage, large tumor size, and other adverse factors.
Three patients who showed PVTT combined with hepatic vein tumor thrombosis (HVTT) and inferior vena cava tumor thrombosis (IVCTT) were also included in this study. They received radiotherapy for PVTT, HVTT, and IVCTT simultaneously. This clinical study was approved by the Institutional Review Board of our hospital, and informed consent was obtained from all patients.
2. Radiation treatment
Three-dimensional computed tomography (CT) simulation was performed for all patients. For radiation planning and treatment, patients were positioned supine with their arms above their head. Patients were trained to hold their breath on the expiratory phase to minimize variation of the target location. Patients who were unable to hold their breath for a given time were allowed to breathe as shallowly as possible. In addition, the variations that resulted from respiration-induced hepatic movement were evaluated by 4D CT simulation to determine the boundary of the treatment target.
The gross tumor volume (GTV) was defined as the intravenous filling defect lesion on 3 phase CT images. The planning target volume (PTV) was contoured to include a safety margin of 1-2 cm from the GTV in consideration of set-up variation and diaphragmatic movement. When HCC lesions were adjacent to the area of the PVTT, some portions of the HCC could be added within the PTV but no entire HCC was included in the treatment field.
The 3D-CRT was designed according to provisional guidelines. The percentage of normal liver volume that was irradiated with more than 30 Gy (V30) was required to be 40% or below. Exposure to the stomach and duodenum was required to be 45-50 Gy or less. The normal liver was delineated excluding viable HCC portions and TACE lesions. X-rays (10-15 MV) were used to deliver precise radiation in five fractions per week. Multiple beams combined with 3-5 ports were adopted. In some cases, 1 or 2 non-coplanar beams were added to coplanar beams to improve the opportunity for highly conformal radiation delivery. Portal (verification) images were obtained regularly to minimize set-up errors. Patients who had gastric or duodenal ulcers at pre-irradiation endoscopic studies were provided antiulcer drugs and their radiotherapy was delayed for 2 weeks.
3. Evaluation and statistical analysis
Response to radiotherapy was evaluated by measuring the largest perpendicular diameters of the PVTT on 3 phase CT images, which were taken before irradiation and 2-3 months after the completion of radiotherapy. When the PVTT and the adjacent HCC were too close to be distinguished, the diameter of the PVTT was measured by estimating the vessel outline.
The Response Evaluation Criteria in Solid Tumors (RECIST) were used to classify the degree of response. According to these criteria, the complete disappearance of the PVTT was defined as a complete response (CR), a 30% or greater decrease in the thrombus diameter was defined as a partial response (PR), a less than 30% decrease in the diameter or a 20% or less increase in the thrombus diameter was defined as stable disease (SD), and a greater than 20% increase in the thrombus diameter was defined as progressive disease (PD). The cases that showed CR or PR were assigned to the responder group and those showing SD or PD were assigned to the non-responder group. Toxicities during or after radiation treatment were evaluated according to the Common Terminology Criteria for Adverse Events (v4.03) [12].
Survival statistics were estimated from the date of beginning of radiotherapy to the date of death or last follow-up. One-year survival was calculated with the Kaplan-Meier method. A univariate analysis was performed using the log-rank test to assess the factors affecting survival. The multivariate analysis was executed using Cox proportional hazard model and the backward stepwise variable selection method was used to control for multicollinearity among the covariates. Fisher exact test was used to compare patient characteristics between the responder and non-responder groups and to investigate correlations between total irradiation dose, PVTT site, and toxicity. A p-value of less than 0.05 was considered statistically significant. The statistical analyses were performed using SPSS software ver. 12.0 (SPSS Inc., Chicago, IL, USA).
Results
Of the 96 patients, 46 received irradiation of the PVTT alone in the study period. The median follow-up duration was 26 months (range, 1 to 46 months). The median patient age was 56 years (range, 40 to 81 years). Of the patients, 39 (84.8%) were male, and the male-to-female ratio was 5.58:1. According to the Child-Pugh classification for cirrhosis of the liver, 7 patients (15.2%) were in class A, 37 patients (80.4%) were in class B, and 2 patients (4.4%) had a non-cirrhotic condition. The Eastern Cooperative Oncology Group (ECOG) performance status score was 0 in 15 patients (32.6%), 1 in 11 patients (23.9%), and 2 in 20 patients (43.5%). Serum hepatitis antigen markers were positive for type B in 31 patients (67.4%), type A in 3 patients (6.5%), and type C in 8 patients (17.4%). The remaining 4 patients were not hepatitis carriers (8.7%). According to the American Joint Committee on Cancer staging system, 30 patients (65.2%) had stage IIIB disease, 8 patients (17.4%) had stage IVA disease, and 8 patients (17.4%) had stage IVB disease. The mean maximal diameter of the primary HCC was 9.6 cm (range, 5.1 to 18.5 cm). Unifocal HCC was observed in 20 patients (43.5%), while 26 patients (56.5%) had multifocal HCC. Five patients (10.9%) had confirmed duodenal ulcer or erosion at the screening endoscopic examination before the initiation of radiotherapy. Three of these patients (6.5% of the total cohort) had gastric ulcer or erosion. Thirty-one patients received at least one cycle of TACE first (average, 3.2 cycles; range, 1 to 9 cycles).
Thirty patients (65.2%) presented invasion of the main or bilateral portal vein. Three patients showed PVTT combined with HVTT and IVCTT simultaneously. Abdominal lymph node metastasis was observed in 8 patients. Distant metastasis to the lung or bone was found in 8 patients.
The median irradiation dose was 50 Gy (range, 35 to 60 Gy), and the daily median dose was 2 Gy (range, 2.0 to 2.5 Gy). The mean PTV was 243.1 cm3 (range, 11.7 to 669.3 cm3). The mean percentage of normal liver volume that had been irradiated with 30 Gy or more (V30) was 21.93% (range, 3.6% to 41.5%). The mean volume of the normal liver was 886 cm3 (range, 121 to 1,543 cm3).
Of the 46 patients, 3 experienced CR (6.5%), 12 experienced PR (26.1%), 19 experienced SD (41.3%), and 12 experienced PD (26.1%). The objective response rate (CR + PR) on CT images was 32.6% (15 patients), and the progression-free rate (CR + PR + SD) was 73.9% (34 patients). A univariate analysis was performed to assess the factors affecting survivals. ECOG performance status, total dose, other additional treatment after RT and PVTT response were the statistically significant prognostic factors for overall survival (Table 1). However, in the multivariate analysis, there were no significant differences between prognostic factors. Table 2 compares the characteristics of the responder and non-responder groups. Using Fisher exact test, no statistically significant differences were observed in terms of patient or tumor factors. However, a significant difference was found between the groups of patients irradiated with over 50 Gy and less than 50 Gy (p = 0.033).
Table 1.
Univariate and multivariate analysis for prognostic factors
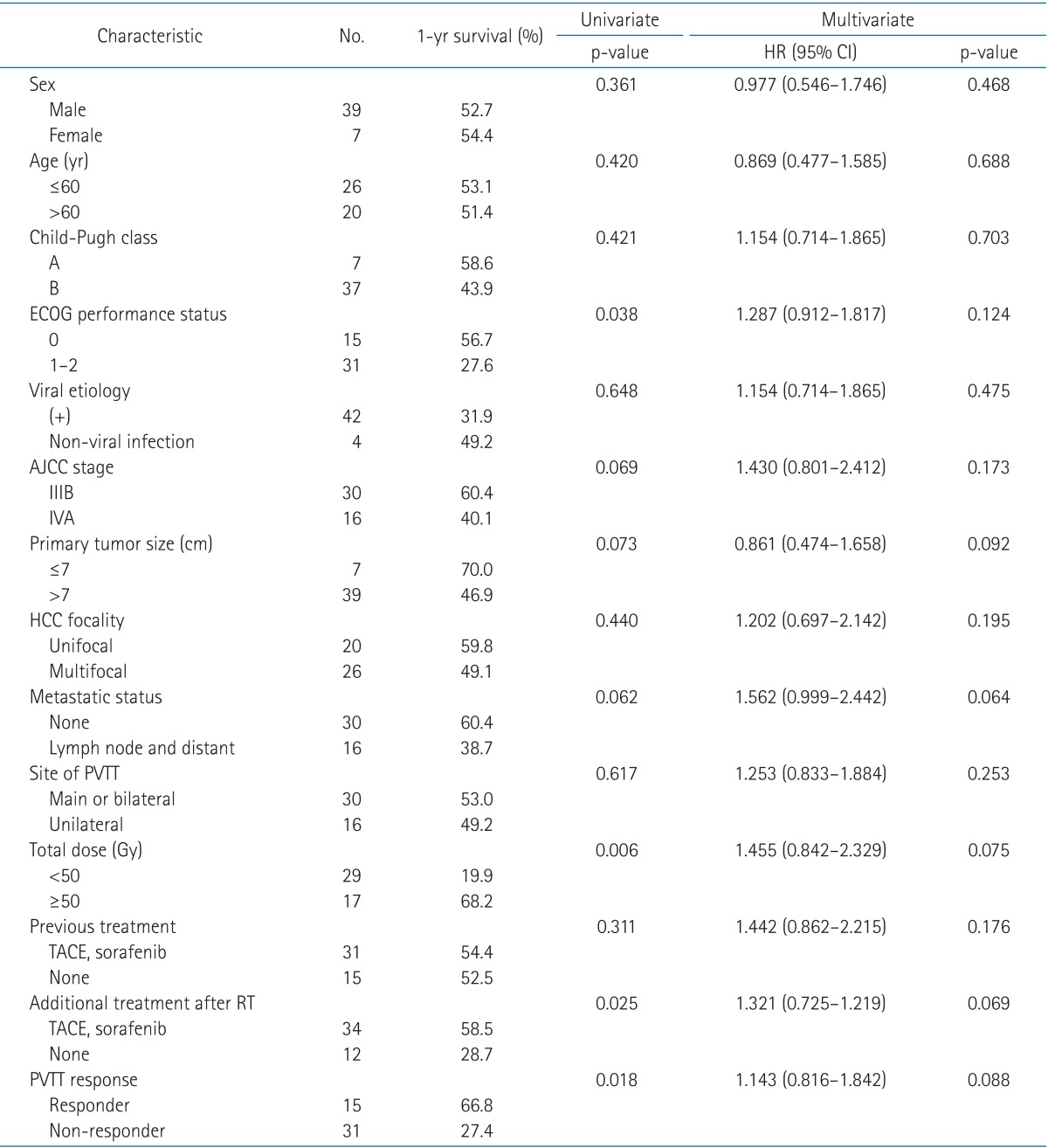
HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; AJCC, American Joint Committee on Cancer; HCC, hepatocellular carcinoma; PVTT, portal vein tumor thrombosis; TACE, transarterial chemoembolization; RT, radiotherapy.
Table 2.
Comparison of factors associated with PVTT response
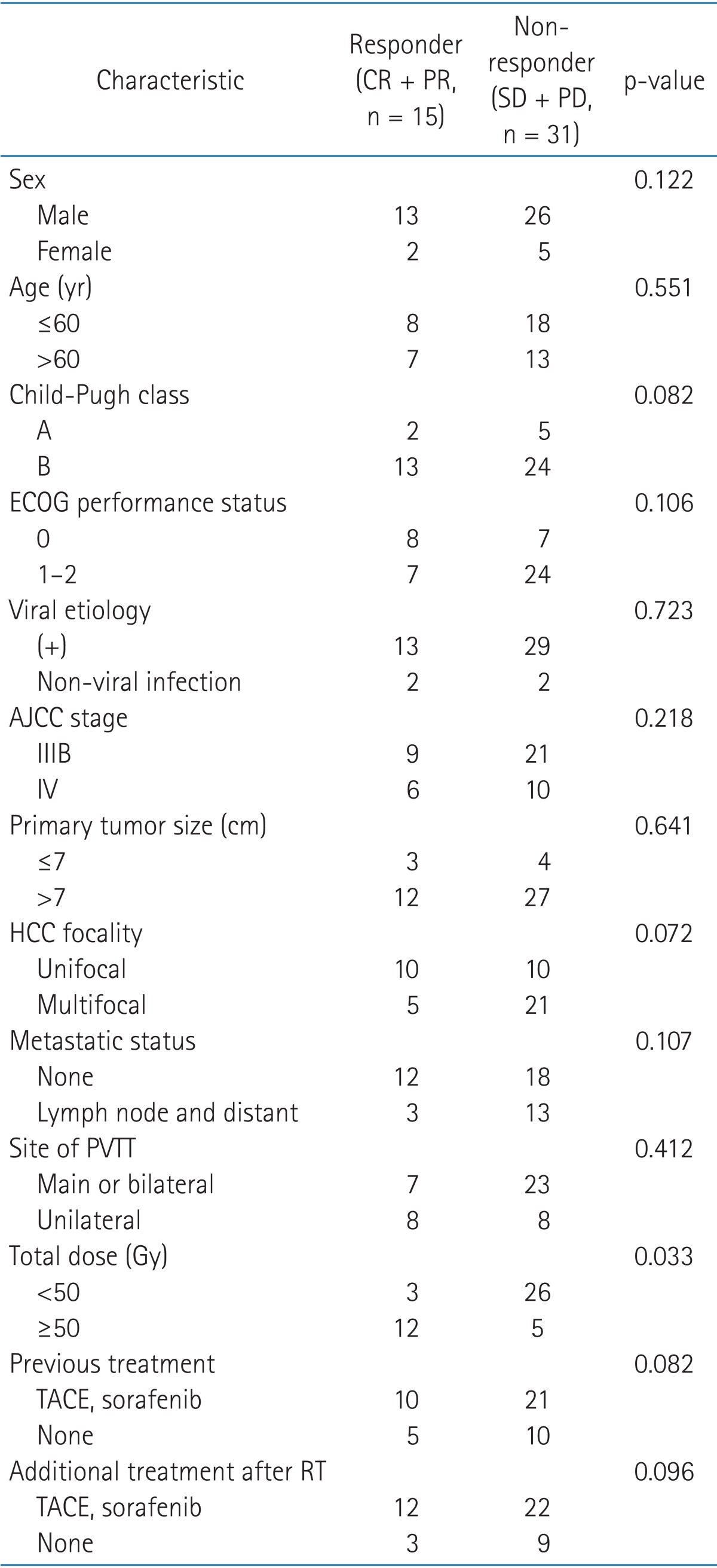
PVTT, portal vein tumor thrombosis; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ECOG, Eastern Cooperative Oncology Group; AJCC, American Joint Committee on Cancer; HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization; RT, radiotherapy.
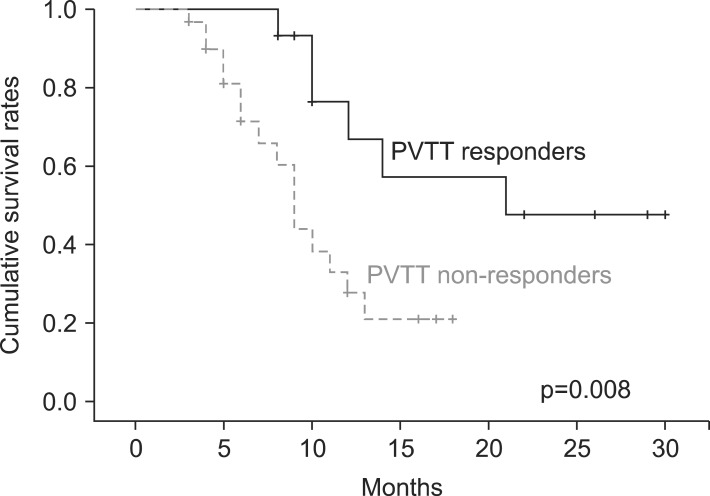
The 1-year survival rate was 66.8% in the responders group and 27.4% in the non-responders group (Fig. 1), which constituted a significant difference in survival (p = 0.008). There were 2 cases of duodenitis or cholangitis during or within 3 months after treatment. In another case, ascites was induced 3 months after the completion of radiotherapy. Radiation-related abnormality was observed in a liver function test without the progression of intrahepatic HCC in 3 patients (grade 1, 2 patients; grade 2, 1 patient). Most adverse effects were grade 2 or lower, except for 2 cases of grade 3 duodenal ulcer or hematologic abnormality. There was no grade 4 toxicity. We performed a comparative analysis of PVTT site and toxicities according to total radiation dose, finding no statistically significant differences in terms of site of PVTT or toxicity grade (Table 3).
Fig. 1.
Overall survival curves according to portal vein tumor thrombosis (PVTT) response. The 1-year survival rate was 66.8% and 27.4%, respectively (p = 0.008).
Table 3.
Comparison of PVTT site and toxicities according to total radiation dose
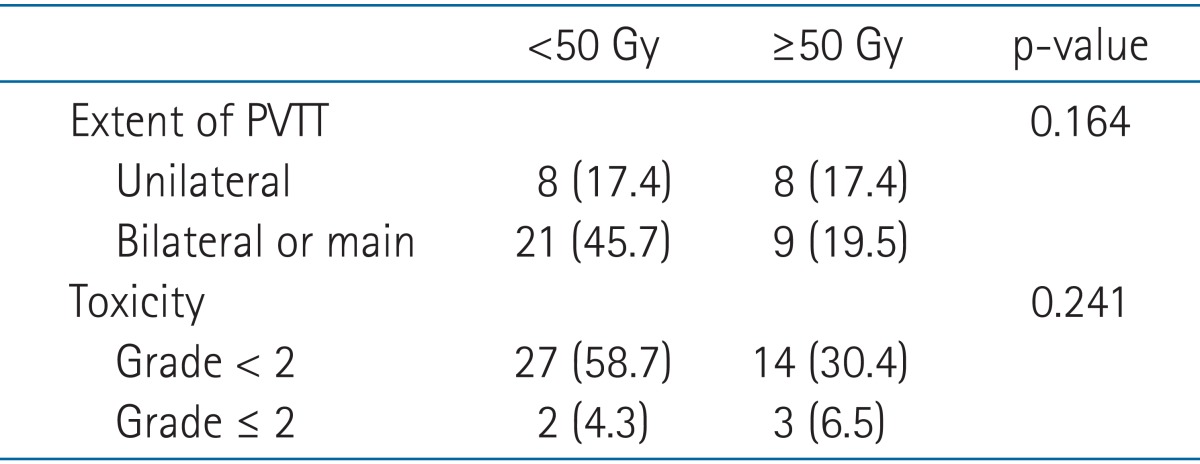
Values are presented as number (%).
PVTT, portal vein tumor thrombosis.
Discussion and Conclusion
Hepatic tolerance dose depends significantly on the volume of liver that is irradiated. Small volumes of liver tissue can tolerate higher dose of radiation without subsequent severe hepatic toxicity [10,13,14]. Therefore, the irradiated volume of the PVTT should be as small as possible.
Most of the patients in the present study had a poor general condition as indicated by Child-Pugh score B (37 patients, 80.4%) or ECOG performance status 1-2 (31 patients, 67.4%). In addition, the majority of patients presented with a comparatively severe tumor invasion status, such as bilateral or main portal vein invasion (30 patients, 65.2%) or huge primary masses that were over 7 cm in diameter and larger than one-third of the normal liver volume (25 patients, 54.3%). Five patients (10.8%) had duodenal ulcer or erosions at screening esophagogastroduodenoscopy before radiotherapy. In some cases, it was very difficult to irradiate the targets with higher doses and a wide safety margin because the target lesions were in close proximity to the duodenum.
At initial diagnosis, 26 patients (56.5%) had multifocal HCC. The effectiveness of radiotherapy for PVTT may certainly be doubted in cases that already involve intrahepatic seeding. However, we suggest that it may have been helpful to irradiate the PVTT even though intrahepatic seeding had already been observed; irradiation could delay the exacerbation of liver function by improving hepatic blood supply.
Table 4 shows the outcome of the present study as compared with the results of previous investigations that included wide-field (PVTT + HCC) irradiated cases. The objective PVTT response rates were 32.6% and 39%-62.3% in this and the previous studies, respectively. However, the present study's 1-year survival rate was approximately equal to or greater than the survival rates found in previous studies. Kim et al. [15] performed radiotherapy for unresectable HCC with PVTT at a total dose from 44 to 54 Gy. The objective response rate was 54.3% for primary tumor and a 39.0% for PVTT. They concluded that primary tumor and PVTT responses were closely correlated and that each of them affected overall survival. The present study appears to have a lower response rate that has been observed in some previous studies. In this study, the primary HCC lesions were not fully covered by the localized PVTT fields simultaneously. Instead, only some parts of the HCC area adjacent to the PVTT were included within the PTV. This could lead to re-expansion of the thrombus to the portal vein (due to the primary HCC) during or after the radiotherapy schedule. Differences between the objective response rates of this study and previous investigations probably resulted from differing numbers of patients, the general condition of the patients, total radiation doses, additional treatments after radiation, or criteria of evaluation for PVTT response.
Table 4.
Recent reports of radiotherapy treatment outcomes for HCC with PVTT
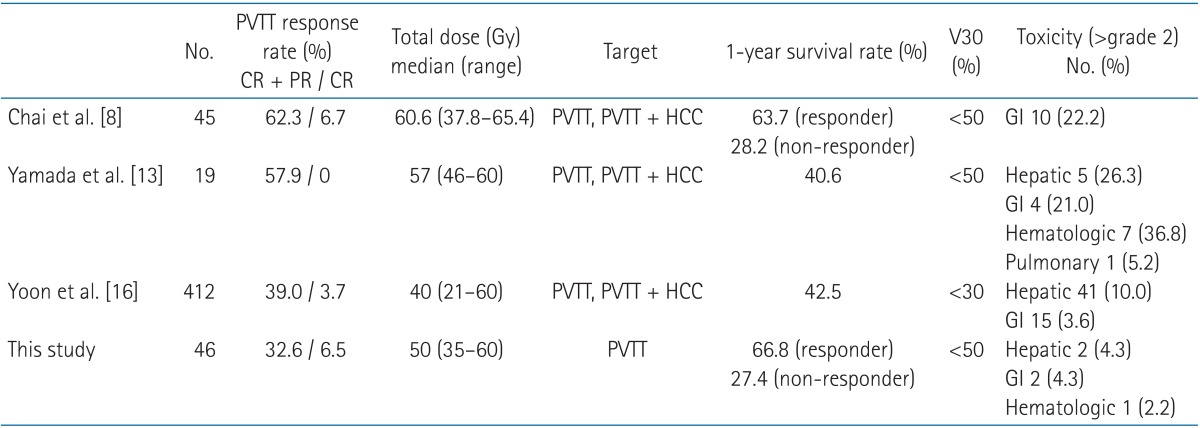
HCC, hepatocellular carcinoma; PVTT, portal vein tumor thrombosis; V30, percentage of normal liver volume that was irradiated with more than 30 Gy; CR, complete response; PR, partial response; GI, gastrointestinal.
At baseline, there were no statistically significant differences between the responder and non-responder groups' patient and tumor characteristics. However, a significant difference was observed between the PVTT responses of the group irradiated with over 50 Gy and the group irradiated with less than 50 Gy (p = 0.033). Rim et al. [10] reported a 62.3% PVTT response rate in a study with a mean total dose of 60.6, while Yoon et al. [16] reported a 39.0% PVTT response rate in a study with a mean dose of 40 Gy. In contrast, Kamiyama et al. [17] investigated 6 patients whose PVTT lesions were treated with a radiation dose of 30-36 Gy in 10-12 fractions. Within the 2 weeks following radiotherapy, surgical resection (hepatectomy) was performed in all patients. The pathologic reports of the surgical specimens revealed complete necrosis of the PVTT lesions in 5 patients (83.3%).
Several studies have suggested that biologic effective dose (BED) is the most important prognostic factor for localized hepatic irradiation. Table 5 shows that response rate increases significantly with irradiation equaling or exceeding 58 Gy10. Therefore, the response of PVTT depends on total dose of irradiation, particularly BED. However, Huang et al. [19] suggested that good performance status might allow patients to receive definite treatment after radiotherapy, which could have a positive effect on survival rates. Therefore, localized irradiation of the PVTT should be followed by other treatment modalities to control the primary HCC. Tazawa et al. [20] performed a combined therapy (localized radiotherapy followed by TACE) for 24 patients with HCC including PVTT. The PVTT response rate was 50%, and the responders had a significantly higher survival rate than the non-responders.
Table 5.
Comparison of BED and response rate to HCC with PVTT
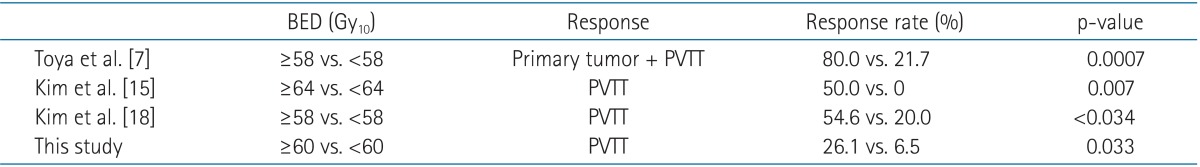
BED, biologic effective dose; HCC, hepatocellular carcinoma; PVTT, portal vein tumor thrombosis.
As shown in Table 3, there was no significant difference in radiation-induced toxicities between the patients receiving over 50 Gy and less than 50 Gy in the present study. The unusual severe toxicity results may have been a consequence of the small number of patients with higher irradiation dose. In several cases with PVTT located in close proximity to the stomach or duodenum, pre-irradiation endoscopic studies showed multiple erosive or ulcerative lesions in the stomach or duodenum. In addition, some of the patients had normal liver volumes that were less than one-third of whole liver volume due to a huge HCC.
Recently, several investigations have attempted to deliver higher effective radiation doses at minimum field using high-precision irradiation techniques or proton beam therapy. Kim et al. [21] introduced hypofractionated radiotherapy with helical tomotherapy in 35 patients with HCC including PVTT. The total radiation dose was 45-60 Gy (median, 50 Gy) in 10 fractions and capecitabine was administered concurrently during radiation. The PVTT response rate was 42.9%. Sugahara et al. [22] performed proton beam therapy for 32 patients with advanced HCC and PVTT. Patients received 55.0-77.0 GyE (median, 72.6 GyE) in 10-35 fractions. The response rate was 89% and CR was observed in 54% of cases.
There were several limitations to the present study. First, the functional aspects of PVTT, for example, evaluation of liver functional parameters or portal flow restoration by Doppler ultrasonography was deemphasized for assessing the PVTT response. Second, the small sample size may have obscured some differences between the responder and non-responder groups. Further, the retrospective nature of the data could conceal selection biases. Additional prospective studies that include larger numbers of cases and parameters of liver function will be needed to obtain more accurate results.
In the treatment of advanced HCC combined with PVTT, localized field radiotherapy for PVTT using 3D-conformal techniques can decrease irradiated volumes of the liver or surrounding organs, such as the stomach and the duodenum. Consequentially, this approach can reduce adverse events developing during or after radiation therapy in patients with unfavorable conditions. However, this reduced-field approach may carry a risk of re-expansion or re-invasion from the untreated HCC into portal vein, which consequentially reduces the local control and survival rates.
Irradiation of the primary HCC and PVTT is recommended for patients with normal liver function, relatively fair general condition, and primary HCC that is small enough to be included in the target volume of the PVTT lesion. However, radiotherapy for PVTT alone is preferred in patients with unfavorable conditions, such as a huge primary mass (or a small normal liver volume), poor general condition or the possibility of exacerbated liver function, intestinal toxicities, and other negative consequences of high-dose wide-field irradiation. In this situation, however, there is an outstanding need to increase the BED to the targets. Further, it remains necessary to consider additional treatment for the HCC (for example, TACE), which could provide better results.
Acknowledgments
This work was supported by a 2-year Research Grant from Pusan National University.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Zhang XB, Wang JH, Yan ZP, Qian S, Du SS, Zeng ZC. Hepatocellular carcinoma with main portal vein tumor thrombus: treatment with 3-dimensional conformal radiotherapy after portal vein stenting and transarterial chemoembolization. Cancer. 2009;115:1245–1252. doi: 10.1002/cncr.24139. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–67. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 3.Kumada K, Ozawa K, Okamoto R, et al. Hepatic resection for advanced hepatocellular carcinoma with removal of portal vein tumor thrombi. Surgery. 1990;108:821–827. [PubMed] [Google Scholar]
- 4.Tanaka A, Morimoto T, Yamaoka Y. Implications of surgical treatment for advanced hepatocellular carcinoma with tumor thrombi in the portal vein. Hepatogastroenterology. 1996;43:637–643. [PubMed] [Google Scholar]
- 5.Liang SX, Zhu XD, Lu HJ, et al. Hypofractionated three-dimensional conformal radiation therapy for primary liver carcinoma. Cancer. 2005;103:2181–2188. doi: 10.1002/cncr.21012. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh CH, Liu CY, Shueng PW, et al. Comparison of coplanar and noncoplanar intensity-modulated radiation therapy and helical tomotherapy for hepatocellular carcinoma. Radiat Oncol. 2010;5:40. doi: 10.1186/1748-717X-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chuma M, Taguchi H, Yamamoto Y, et al. Efficacy of therapy for advanced hepatocellular carcinoma: intra-arterial 5-fluorouracil and subcutaneous interferon with image-guided radiation. J Gastroenterol Hepatol. 2011;26:1123–1132. doi: 10.1111/j.1440-1746.2011.06745.x. [DOI] [PubMed] [Google Scholar]
- 8.Zeng ZC, Fan J, Tang ZY, et al. A comparison of treatment combinations with and without radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombus. Int J Radiat Oncol Biol Phys. 2005;61:432–443. doi: 10.1016/j.ijrobp.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 9.Toya R, Murakami R, Baba Y, et al. Conformal radiation therapy for portal vein tumor thrombosis of hepatocellular carcinoma. Radiother Oncol. 2007;84:266–271. doi: 10.1016/j.radonc.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Rim CH, Yang DS, Park YJ, Yoon WS, Lee JA, Kim CY. Effectiveness of high-dose three-dimensional conformal radiotherapy in hepatocellular carcinoma with portal vein thrombosis. Jpn J Clin Oncol. 2012;42:721–729. doi: 10.1093/jjco/hys082. [DOI] [PubMed] [Google Scholar]
- 11.Kim SW, Oh D, Park HC, et al. Transcatheter arterial chemoembolization and radiation therapy for treatment-naïve patients with locally advanced hepatocellular carcinoma. Radiat Oncol J. 2014;32:14–22. doi: 10.3857/roj.2014.32.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.European Organisation for Research and Treatment of Cancer. Common Toxicity Criteria for Adverse Events (CTCAE) version 4.0 [Internet] Brussels, Belgium: European Organisation for Research and Treatment of Cancer; c2013. [cited 2014 Aug 20]. Available from: http://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. [Google Scholar]
- 13.Yamada K, Izaki K, Sugimoto K, et al. Prospective trial of combined transcatheter arterial chemoembolization and three-dimensional conformal radiotherapy for portal vein tumor thrombus in patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2003;57:113–119. doi: 10.1016/s0360-3016(03)00434-6. [DOI] [PubMed] [Google Scholar]
- 14.Keum KC, Park HC, Seong JS, et al. Preliminary results of 3-dimensional conformal radiotherapy for primary unresectable hepatocellular carcinoma. J Korean Soc Ther Radiol Oncol. 2002;20:123–129. [Google Scholar]
- 15.Kim TH, Kim DY, Park JW, et al. Three-dimensional conformal radiotherapy of unresectable hepatocellular carcinoma patients for whom transcatheter arterial chemoembolization was ineffective or unsuitable. Am J Clin Oncol. 2006;29:568–575. doi: 10.1097/01.coc.0000239147.60196.11. [DOI] [PubMed] [Google Scholar]
- 16.Yoon SM, Lim YS, Won HJ, et al. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomes. Int J Radiat Oncol Biol Phys. 2012;82:2004–2011. doi: 10.1016/j.ijrobp.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Kamiyama T, Nakanishi K, Yokoo H, et al. Efficacy of preoperative radiotherapy to portal vein tumor thrombus in the main trunk or first branch in patients with hepatocellular carcinoma. Int J Clin Oncol. 2007;12:363–368. doi: 10.1007/s10147-007-0701-y. [DOI] [PubMed] [Google Scholar]
- 18.Kim DY, Park W, Lim DH, et al. Three-dimensional conformal radiotherapy for portal vein thrombosis of hepatocellular carcinoma. Cancer. 2005;103:2419–2426. doi: 10.1002/cncr.21043. [DOI] [PubMed] [Google Scholar]
- 19.Huang YJ, Hsu HC, Wang CY, et al. The treatment responses in cases of radiation therapy to portal vein thrombosis in advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2009;73:1155–1163. doi: 10.1016/j.ijrobp.2008.06.1486. [DOI] [PubMed] [Google Scholar]
- 20.Tazawa J, Maeda M, Sakai Y, et al. Radiation therapy in combination with transcatheter arterial chemoembolization for hepatocellular carcinoma with extensive portal vein involvement. J Gastroenterol Hepatol. 2001;16:660–665. doi: 10.1046/j.1440-1746.2001.02496.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim JY, Yoo EJ, Jang JW, Kwon JH, Kim KJ, Kay CS. Hypofractionated radiotheapy using helical tomotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Radiat Oncol. 2013;8:15. doi: 10.1186/1748-717X-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugahara S, Nakayama H, Fukuda K, et al. Proton-beam therapy for hepatocellular carcinoma associated with portal vein tumor thrombosis. Strahlenther Onkol. 2009;185:782–788. doi: 10.1007/s00066-009-2020-x. [DOI] [PubMed] [Google Scholar]