Sir,
Collagen cross-linking by UVA light augmented by riboflavin was proposed to improve the biomechanical properties of keratoconic corneas.1 As an alternative to the standard technique (riboflavin saturation of stroma through denuded corneal surface, followed by UVA irradiation2, 3, 4) we developed a femtosecond-assisted intrastromal pocket for riboflavin induction.
Case report
Twelve eyes of 9 patients (mean age 29.75±9.3 years) with early progressive keratoconus (K-readings>48D, skewed steepest radial axis >22°, superior–inferior difference on the 5 mm circle >2.5D, inferior–superior difference >1.5 D, minimum corneal thickness >380 μm) were included. Progression was confirmed by K-reading increase of ≥1 D, or thickness decrease ≥5 μm in two consecutive Orbscan corneal tomographies.
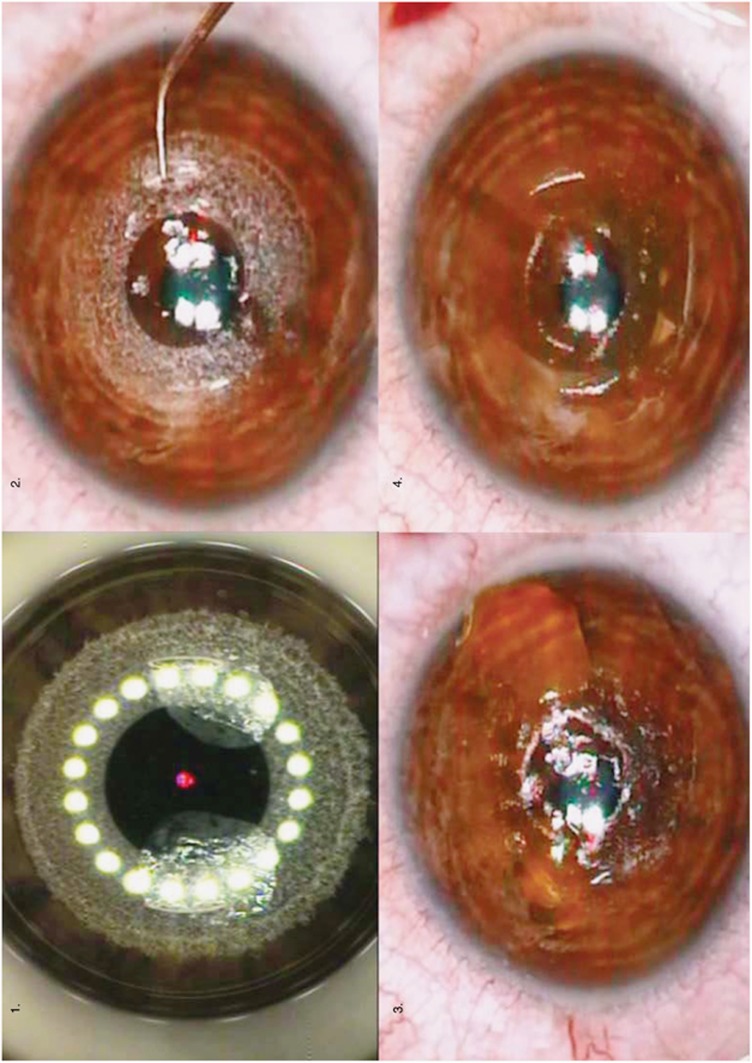
A 6-mm-diameter doughnut-shaped intrastromal pocket was created at 200 μm depth by the Technolas Femtec 520 (Technolas Perfect Vision GmbH, Munich, Germany), leaving a 3 mm clear central optical zone (Figure 1, 1). Two 0.5 × 0.5 mm entry channels 180° apart were created, for riboflavin infusion and depressurization; a tapered Intacs spatula hook was used to bluntly dissect the pocket (Figure 1, 2). In all, 0.3 ml of 0.1% riboflavin in 20% dextran solution was introduced into the pocket using Intacs stromal channel irrigation cannula, until the entire pocket was coloured bright yellow (Figure 1, 3). Cornea was irradiated with UVA 365–375 nm light (3 mW/cm2 irradiance) for 30 min. Total fluency at the corneal plane was 5.4 J/cm2.
Figure 1.
(1) Creation of a 6-mm-diameter circular intrastromal pocket, by means of the femtosecond laser, leaving a clear optical zone of 3 mm. (2) Creation of two 0.5-mm-width entry channels 180° apart. Following the pocket creation, a tapered Intacs spatula mall Jameson muscle hook was used to enter and bluntly dissect the pocket. (3) Infusion of 0.3 ml of 0.1% riboflavin solution into the pocket using an Intacs stromal channel irrigation cannula. Infusion continued until the entire pocket was colored bright yellow due to the presence of the riboflavin solution. (4) A UVA irradiation source of ∼370 nm wavelength (365–375 nm) was used for corneal surface irradiation.
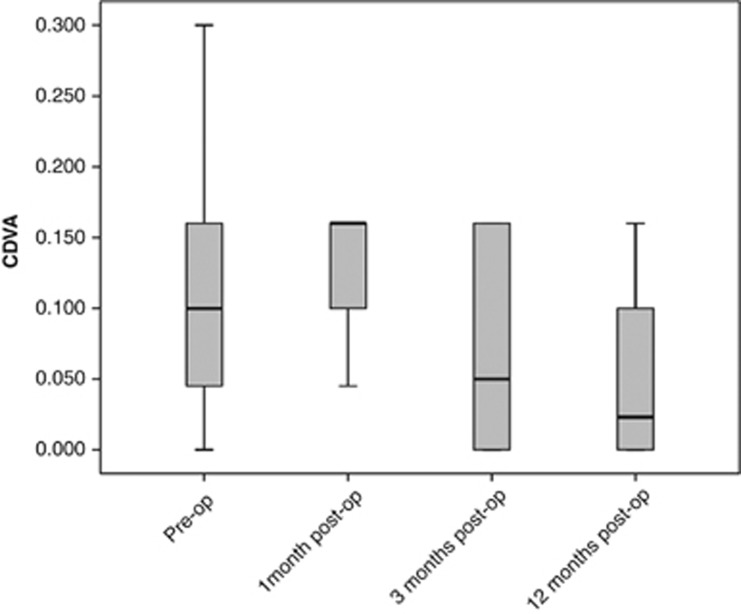
CDVA initially decreased at the first month (P=0.157), followed by marked improvement at the 3rd and 12th months postoperatively (P=0.042) (Figure 2). Significant reduction was observed in astigmatic power (P=0.016), eccentricity (P=0.044), and thinnest point corneal thickness 1 year postoperatively (P=0.043). Keratoconus remained stable 12 months postoperatively, Kmax remaining unchanged and Kmin increasing after the first postoperative month (P=0.034) (Table 1).
Figure 2.
Change in corrected distance visual acuity.
Table 1. Preoperative, 1st, 3rd and 12nd postoperative month follow-up mean and standard deviation for corrected distant visual acuity (CDVA) (log MAR), Kmax, Kmin, and eccentricity (Ecc) (Topolyser, Oculus Instruments), thinnest point (μm) (thin), and irregularity in 3 mm (Irr) (Corneal Topography System—Bausch & Lomb—ORBSCAN II).
| Pre-op | 1st | 3rd | 12nd | |
|---|---|---|---|---|
| CDVA | 0.1±0.09a | 0.13±0.05 | 0.07±0.08 | 0.05±0.06 |
| Kmax | 49.7±2.86 | 48.6±2.24 | 49±3.11 | 50±2.57 |
| Kmin | 45.6±2.36a | 45.7±2.46 | 46.2±2.59 | 46.4±2.28 |
| Ecc | 0.9±0.3a | b0.8±0.2 | c0.9±0.3 | d1±0.2 |
| Thin point | 417±31.4a | 317±23.3 | 357±18.7 | 357±30.8 |
| Irr | 4.7±2.2 | 5.6±0.2 | 4.6±3.2 | 4.4±2.7 |
Statistically important differences are marked as:
Preoperative vs 1 year (P<0.05).
1 month vs 1 year (P<0.05).
3 months vs 1 month (P<0.05).
3 months vs 1 year (P<0.05).
Comment
Riboflavin injected intrastromally into a precisely designed pocket is a painless procedure, with fast rehabilitation, reinforcing collagen at a selected location. The greatest effect of UVA light occurs at the area of maximal absorbance and its close vicinity.5 Riboflavin introduced at the mid-stromal ring 200 μm deep will maximize the cross-linking effect around the protrusion.
This procedure's safety was proven by the unchanged endothelial cell density and morphology. As long as the cornea treated has a minimum thickness of 380 μm, the corneal endothelium (and deeper structures) will not experience damage.6
There was a significant improvement in CDVA, with concomitant stabilization for 12 months. Concerns about biomechanical instability from the femto ring have been countered.7
This surgical approach merits additional exploration in a larger cohort to further confirm the safety of the technique.
The authors declare no conflict of interest.
References
- Kohlhaas M, Spoerl E, Schilde T, Unger G, Wittig C, Pillunat LE. Biomechanical evidence of the distribution of cross-links in corneas treated with riboflavin and ultraviolet a light. J Cataract Refract Surg. 2006;32:279–283. doi: 10.1016/j.jcrs.2005.12.092. [DOI] [PubMed] [Google Scholar]
- Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- Wollensak G. Crosslinking treatment of progressive keratoconus: new hope. Curr Opin Ophthalmol. 2006;17:356–360. doi: 10.1097/01.icu.0000233954.86723.25. [DOI] [PubMed] [Google Scholar]
- Tu KL, Aslanides IM. Orbscan II anterior elevation changes following corneal collagen cross-linking treatment for keratoconus. J Refract Surg. 2009;25:715–722. doi: 10.3928/1081597X-20090707-06. [DOI] [PubMed] [Google Scholar]
- Malhotra C, Shetty R, Kumar RS, Veluri H, Nagaraj H, Shetty KB. In vivo imaging of riboflavin penetration during collagen cross-linking with hand-held spectral domain optical coherence tomography. J Refract Surg. 28:776–780. doi: 10.3928/1081597X-20121011-05. [DOI] [PubMed] [Google Scholar]
- Spoerl E, Mrochen M, Sliney D, Trokel S, Seiler T. Safety of UVA-riboflavin cross-linking of the cornea. Cornea. 2007;26:385–389. doi: 10.1097/ICO.0b013e3180334f78. [DOI] [PubMed] [Google Scholar]
- Kanellopoulos AJ.Collagen cross-linking in early keratoconus with riboflavin in a Femtosecond laser-created pocket: initial clinical results J Refract Surg 2009251034–1037.86. [DOI] [PubMed] [Google Scholar]