Abstract
Purpose
To evaluate visual function variations in eyes with age-related macular degeneration (AMD) compared to normal eyes under different light/contrast conditions using a time-dependent visual acuity testing instrument, the Central Vision Analyzer (CVA).
Methods
Overall, 37 AMD eyes and 35 normal eyes were consecutively tested with the CVA after assessing best-corrected visual acuity (BCVA) using ETDRS charts. The CVA established visual thresholds for three mesopic environments (M1 (high contrast), M2 (medium contrast), and M3 (low contrast)) and three backlight-glare environments (G1 (high contrast, equivalent to ETDRS), G2 (medium contrast), and G3 (low contrast)) under timed conditions. Vision drop across environments was calculated, and repeatability of visual scores was determined.
Results
BCVA significantly reduced with decreasing contrast in all eyes. M1 scores for BCVA were greater than M2 and M3 (P<0.001); G1 scores were greater than G2 and G3 (P<0.01). BCVA dropped more in AMD eyes than in normal eyes between M1 and M2 (P=0.002) and between M1 and M3 (P=0.003). In AMD eyes, BCVA was better using ETDRS charts compared to G1 (P<0.001). The drop in visual function between ETDRS and G1 was greater in AMD eyes compared to normal eyes (P=0.004). Standard deviations of test–retest ranged from 0.100 to 0.139 logMAR.
Conclusion
The CVA allowed analysis of the visual complaints that AMD patients experience with different lighting/contrast time-dependent conditions. BCVA changed significantly under different lighting/contrast conditions in all eyes, however, AMD eyes were more affected by contrast reduction than normal eyes. In AMD eyes, timed conditions using the CVA led to worse BCVA compared to non-timed ETDRS charts.
Introduction
Age-related macular degeneration (AMD) is the leading cause of irreversible blindness in the developed world, and causes considerable morbidity despite recent advances in treatment.1 Patients with AMD now may be treated using a variety of preventive methods,2 and anti-vascular endothelial growth factor (VEGF) therapy for choroidal neovascularization has converted wet AMD from a disease often resulting in vision of 20/200 or worse to one where patients may enjoy vision of 20/40.3 However, despite the visual outcome may be better than it was before the anti-VEGF therapy, patients with treated wet AMD often complain about difficulties performing tasks of daily living.
The Snellen chart test is the most commonly used chart to evaluate vision in clinical setting. More recently, the ETDRS chart has become the gold-standard test for best-corrected visual acuity (BCVA) in clinical trials evaluating eyes with macular disease.4 However, these are non-timed tests and thus may not reflect real-life situations such as driving, where the patient has only a limited amount of time to recognize objects and read signs and make important visual-based decisions. This is important in patients with AMD because many of the vision tasks that they complain about involve visual difficulties in time-dependent experiences including reading, driving, and recognizing faces in fluid social situations.5 In addition, well-known issues of scoring the Snellen chart test and the ETDRS test are also present: means of calculating BCVA scores may vary between examiners, as patients are often able to read only some letters but from multiple lines.6, 7 Moreover, these chart tests use high-contrast black letters against an unchanging white background of one luminance in the moderate photopic environment of the examination office. For these reasons, other investigators have suggested that these chart tests are not a useful determination of vision in other contrast or luminance environments, and those measurements are poorly correlated with daily living activities8, 9 that require the patient to assess visual stimuli in multiple lighting, contrast, or glare conditions against a varied background color or luminance.7 Also, many common daily activities require an individual to react to a visual stimulus with limited time or information.7, 10
Testing of multiple aspects of patients' visual function (such as contrast sensitivity, luminance adaption, color perception, and reading speed) currently must be performed with separate tests and scoring methods. Recently, the Central Vision Analyzer (CVA; Visoptics, LLC, Mechanicsburg, PA, USA) has been introduced and approved by the Food and Drug Administration as a new interactive computer program to perform a comprehensive analysis of the central visual acuity under conditions that simulate environments of real-world activities that are often reported as visually stressful, such as those encountered under mesopic, lighting, or glare photopic conditions.11, 12, 13 It also simulates the fixation times that are required for those activities. Unlike the ETDRS chart test, the CVA presents tumbled Landolt C and thresholds for the smallest C for which the patient responds the correct position under the luminance, contrast and presentation times of the activity, watching and examining reaction times, and thus avoiding letter confusion with the consistent use of one symbol.10
The purpose of the present study was to evaluate how visual function varies in patients with AMD compared with normal subjects tested with this novel computerized test of time-dependent contrast sensitivity under conditions that simulate real-world environments.
Materials and methods
Subjects
We recruited 35 normal eyes and 37 eyes with an ophthalmoscopic diagnosis of wet or dry AMD, confirmed with retinal imaging, from 53 consecutive patients. Eyes with dry AMD had drusen, pigment changes, and/or drusenoid pigment epithelial detachment; all patients with wet AMD were under treatment with an anti-VEGF agent. Eyes with a concomitant ocular abnormality (eg, corneal opacity, cataract, or glaucoma) were not tested. Prior to CVA testing, all participants underwent refraction and BCVA assessment using a standard ETDRS chart. A full optical correction was given to each patient, if necessary. Written informed consent for non-invasive diagnostic procedure was obtained from each subject before examination. The study was approved by the Institutional Review Board at Shiley Eye Center at the University of California, San Diego (UCSD) and was conducted in adherence to the tenets of the Declaration of Helsinki. All applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research.
Visual function testing
Automated visual acuity testing was performed using the CVA. Participants were placed in a dark room of luminance <5 Cd/m2, sitting at a distance of 5 m from the testing 20-inch LED monitor, as calculated by the combined distance from the participant to the mirror and from the mirror to the testing monitor.12 First, an instruction video with a practice test was given: participants were shown a fixation cross on the monitor and asked to indicate the orientation of the tumbled Landolt C by pressing the corresponding arrow button ‘right,' ‘left,' ‘up', or ‘down' on a keypad on their lap. If they pressed the correct button, the response would be counted as correct. Any other button pressing, including no pressing within the maximum time period (up to 4 s), would be counted as incorrect. After the instruction video, patients performed the CVA testing twice using a Landolt C presentation of 900 ms. Our initial experience suggested that the 900-ms stimulus duration is well tolerated and fast enough to simulate a real-world, time-dependent situation, while patients expressed concern when tested with shorter presentations. Participants were allowed 15 min of rest in a dimly lit room between the two sessions.
As previously described by our group,13 the CVA thresholds acuity under three mesopic and three photopic (backlight glare) environments in sequential manner, automatically changing contrast and luminance levels. Contrasts and luminance levels of the six modules are presented in Table 1. Specifically, they were 99% Michelson contrast (MC) module with white letters against a 3 Cd/m2 background (‘M1' module), followed by lower-contrast mesopic module of 64% MC against a 3 Cd/m2 background (‘M2' module, simulating an environment similar to a dimly lit restaurant), and then an even lower-contrast module of 43% MC against a 3 Cd/m2 background (‘M3' module, simulating visual activities encountered while driving at dusk). Immediately following the mesopic conditions, backlight-glare conditions were then tested in the following order: 10% MC module with black letters against a 200 Cd/m2 background (‘G2' module, simulating playing golf or tennis outside with the sun over head), followed by an even lower-contrast module of 8% MC against a 200 Cd/m2 background (‘G3' module that simulates playing golf or tennis outside under the sun 15° off-axis), and finally ending with the ‘G1' module, a full-contrast module of black letters presented at 99% MC against a 200 Cd/m2 background. During the test, the CVA software automatically adjusts the size of the C depending upon the responses in a reversing staircase with progressively diminishing steps (6 : 4 : 2 : 1 of logMAR 0.05 steps) until a threshold is reached, determined by two correct responses at the lowest logMAR 0.05 presentation with two incorrect responses at one logMAR 0.05 step smaller. At the end of the test, results were automatically presented to the examiner in a report as six separate visual acuity threshold scores, one for each of the simulated lighting and contrast conditions.
Table 1. Central Vision Analyzer modules' characteristics.
| CVA modules | Contrast (MC, %) | Landolt C color | Landolt C luminance (Cd/m2) | Background luminance (Cd/m2) | Test simulates |
|---|---|---|---|---|---|
| M1 | 99 | White | 220 | 3 | Mesopic—full contrast |
| M2 | 64 | Gray | 4.8 | 3 | Mesopic—dim restaurant |
| M3 | 43 | Gray | 8.4 | 3 | Mesopic—driving at dusk |
| G1 | 99 | Black | 1.6 | 200 | Glare—full contrast |
| G2 | 10 | Gray | 180 | 200 | Glare—playing sports with sun over head |
| G3 | 8 | Gray | 186 | 200 | Glare—playing sports under the sun 15° off-axis |
Abbreviations: CVA, Central Vision Analyzer; Cd/m2, Candelas per meter squared; MC, Michelson contrast.
Validation of the CVA
The validation study of the CVA is currently under review and therefore is not part of the present study. Validation data presented at the Association for Research in Vision and Ophthalmology meetings (Gutstein W et al IOVS 2006, 47, ARVO E-Abstract 92; Sinclair SH et al IOVS 2009, 50, ARVO E-Abstract 3977; Gutstein W et al IOVS 2009, 50, ARVO E-Abstract 3212; and Gutstein W et al IOVS 2012, 53, ARVO E-Abstract 3056), in brief, demonstrated 0.50 to 0.96 correlation with ETDRS charts presenting the same contrast and luminance with Bland and Altman statistics that demonstrate similar reproducibility and comparative visual acuities as acuity measurements with charts. One may question the ability of the CVA to measure sun glare environments on an LCD or LED monitor in a darkened room. The CVA reduces the contrast of the visualized targets against the background according to the research of Vos14 that predicted the resultant decrease in contrast for a 30-year-old individual visualizing the targets with the sun off-axis. This is defined by the off-axis distance of glare source and the ratio of the luminance of that source compared with the average luminance of the discriminated targets, not the absolute luminance (whether the source is the sun in daylight glare environments or headlight glare in mesopic environments).
For each activity, vision tasks were identified (eg, facial recognition and menu reading, among others for the activity restaurant dining) with their respective discriminated targets and backgrounds that were measured from electronic RAW images taken of each activity. Each element and the respective background represent point sources in the International Commission on Illumination (CIE) color space defined by their L × a × b × values (where ‘L' represents luminance, ‘a' represents the color hue, and ‘b' represents the saturation of the color from white light occupying the center of the space). The color contrast between the two points is then represented as ΔE/L, the calculated Euclidean distance between the two points in the CIE color space.15, 16 To define the black and white contrast of the Landolt C presented against the pedestal and background, the ΔE/L contrasts were converted to the equivalent MCs for the averages of the most important vision tasks defined by participants of the activity (in each case not <10 persons), and then examined to define those that required the finest resolution among the higher ranked.
Because the system does not utilize anti-aliasing, careful consideration if given of the monitor to subject's face viewing distances such that, given the pixel step sizes, the gap openings of the smallest Landolt C must represent no greater than a 10% variance from the correct size. The test monitor chart background luminance and contrast of the presented figures were controlled by calibration measurements that were repeated at required monthly intervals using the Pantone Huey colorimeter (Pantone LLC, Carlstadt, NJ, USA). In addition, the colorimeter is set facing toward the mirror in order to measure and control the luminance of the wall (and hence room luminance viewed) by the patient during the testing.
Statistical analysis
Statistical analyses were performed using SAS statistical software version 9.2 (SAS Inc., Cary, NC, USA). Main outcome measures in this study were the threshold logMAR BCVA scores for each CVA module. The repeatability of the BCVA scores was evaluated by the standard deviation (SD) of repeated measurements (test–retest) described by Bland and Altman.17 Generalized estimating equations, adjusted for age, were used to test for the overall significance of BCVA measurements across categories. Bonferroni-adjusted P-values were used for multiple pairwise comparisons. Pearson's correlation analysis was completed using subject-averaged logMAR BCVA measurements. A P-value <0.05 was considered to be statistically significant.
Results
The mean age of the 53 subjects (72 eyes) enrolled in the study was 39 years for normal patients (range 20–69 years, 35 eyes) and 79 years for AMD patients (range 46–83 years, 37 eyes; P<0.001). The median BCVA was 20/20 and 20/32 for normal and pathological groups, respectively. The average duration for the CVA testing was 2.1 min per eye in normal eyes (range 1.5–3.0 min), and 3.5 min per eye in eyes with AMD (range 2.0–4.0 min).
Both in normal subjects and in AMD patients, visual function significantly reduced with decreasing contrast and luminance in all mesopic and glare environments (Table 2). M1 scores (high-contrast environment) were significantly better than M2 and M3 (low-contrast environments; both P<0.001). Similarly, G1 scores (high-contrast environment) were significantly better than G2 and G3 (low-contrast environments; both P<0.01). In AMD eyes, the visual function dropped to a greater extent than seen in normal eyes with decreasing contrast and luminance in mesopic environments (P=0.002 between M1 and M2, and P=0.003 between M1 and M3; Table 2 and Figure 1). In addition, the drop between M1 and M3 was significantly greater in eyes with wet AMD compared to eyes with dry AMD (P=0.003); no different visual drops were found in glare environments between wet and dry AMD eyes. The greater drop of visual function for AMD patients compared to normal subjects was not found in glare environments (P=0.957 between G1 and G2, and P=0.791 between G1 and G3; Table 2 and Figure 1).
Table 2. Comparison between visual acuity results for each module by disease group.
| Eyes | LogMAR (mean±SE) | Difference | P-valuea | |
|---|---|---|---|---|
| M2 | M1 | |||
| AMD | 0.763±0.055 | 0.346±0.037 | 0.417±0.062 | <0.001 |
| Normal | 0.169±0.047 | −0.015±0.058 | 0.185±0.036 | <0.001 |
| P-value | 0.002 | |||
| M3 | M1 | |||
| AMD | 0.908±0.071 | 0.346±0.037 | 0.562±0.076 | <0.001 |
| Normal | 0.261±0.050 | −0.015±0.058 | 0.277±0.045 | <0.001 |
| P-value | 0.003 | |||
| G2 | G1 | |||
| AMD | 0.560±0.056 | 0.417±0.034 | 0.143±0.049 | 0.007 |
| Normal | 0.168±0.077 | −0.0004±0.050 | 0.168±0.050 | 0.003 |
| P-value | 0.957 | |||
| G3 | G1 | |||
| AMD | 0.565±0.056 | 0.417±0.034 | 0.148±0.048 | 0.004 |
| Normal | 0.187±0.080 | −0.0004±0.050 | 0.187±0.062 | 0.007 |
| P-value | 0.791 | |||
| ETDRS | G1 | |||
| AMD | 0.184±0.028 | 0.417±0.034 | −0.233±0.033 | <0.001 |
| Normal | −0.076±0.025 | −0.0004±0.050 | −0.076±0.045 | 0.100 |
| P-value | 0.004 | |||
Abbreviations: AMD, age-related macular degeneration; SE, standard error. The bold values are P-values, obtained from the comparison between the logMAR differences among AMD and normal eyes.
Using paired t-test.
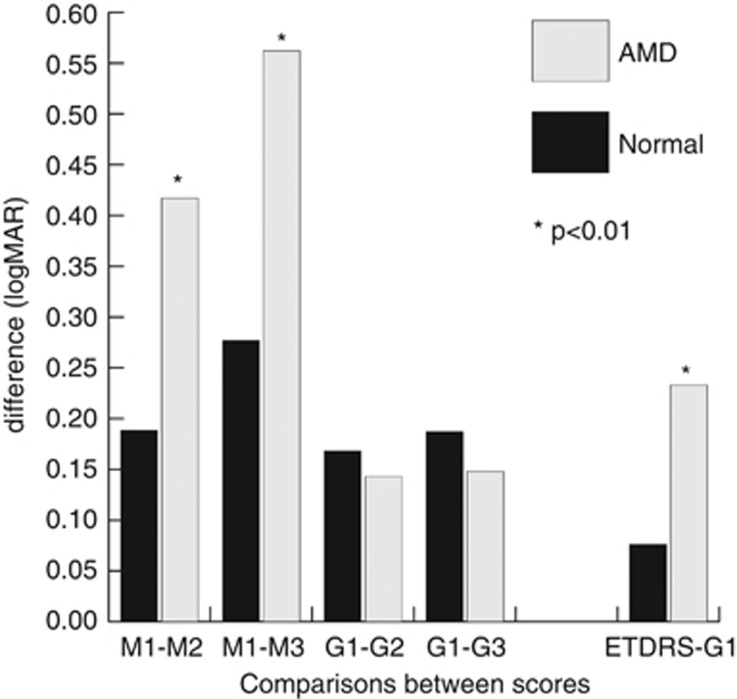
Figure 1.
Clustered column graph showing comparisons between visual acuity drops for normal eyes and eyes with age-related macular degeneration (AMD) while changing lighting/contrast conditions using the Central Vision Analyzer. The visual function dropped more in AMD eyes than normal eyes while decreasing contrast and Landolt C luminance in mesopic environments (P=0.002 between M1 and M2, and P=0.003 between M1 and M3). This greater drop for AMD patients compared to normal patients was not found in glare environments (P=0.957 between G1 and G2, and P=0.791 between G1 and G3). The drop in visual function between ETDRS and G1 was significantly greater in AMD eyes compared to normal eyes (P=0.004).
We also compared results of the logMAR BCVA of the G1 module (the closest one to lighting of the ETDRS chart—high-contrast black type over a bright white background) to the logMAR ETDRS BCVA (Table 2). In AMD eyes, we found that BCVA score was significantly different between ETDRS and G1 (P<0.001); more specifically, the ETDRS score was better than the G1 score. On the contrary, ETDRS and G1 scores for BCVA did not differ for normal patients (P=0.100). The drop in visual function between ETDRS and G1 was significantly greater in AMD eyes compared to normal eyes (P=0.004; Table 2 and Figure 1).
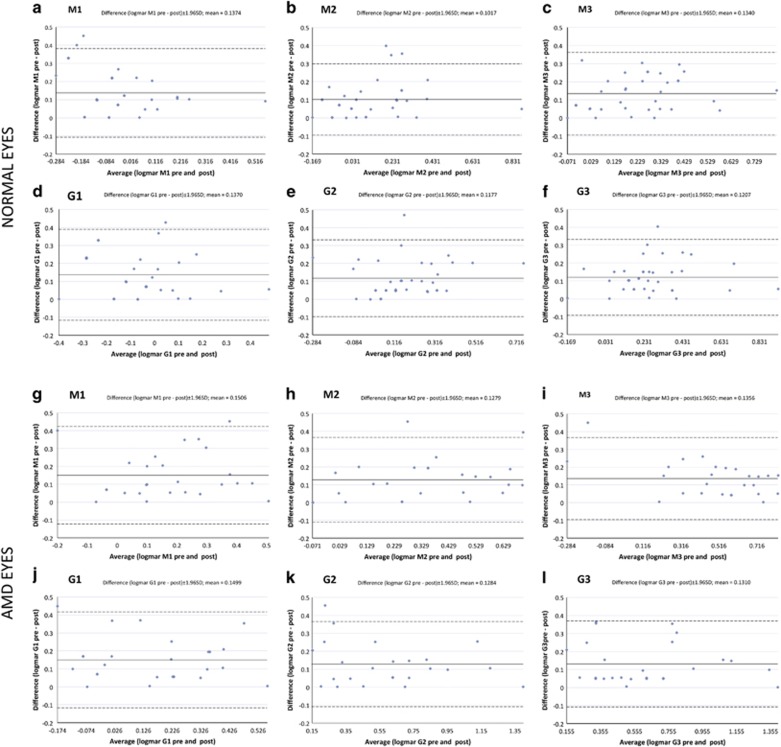
Table 3 summarizes results of the test–retest coefficients of repeatability (equal to 1.96 × standard error sigma) for each of the CVA modules. The SDs of test–retest ranged from 0.100 to 0.129 logMAR in normal eyes, from 0.118 to 0.139 logMAR in eyes with AMD, and from 0.100 to 0.132 in the entire sample. Independent t-test demonstrated no significant difference in repeatability scores between normal and AMD eyes. Bland–Altman plots for visual function measurements in each CVA environment are shown in Figure 2, both for normal eyes and AMD eyes. Scrutiny of Bland–Altman charts did not demonstrate any untoward relationship between agreement and magnitude of any measures, with random scatter above and below zero and no significant bias.
Table 3. Results of repeatability of visual acuity scores using Central Vision Analyzer (logMAR units).
|
Normal eyes (N=35) |
AMD eyes (N=37) |
All eyes (N=72) |
||||
|---|---|---|---|---|---|---|
| SD test–retest | 95% LOA | SD test–retest | 95% LOA | SD test–retest | 95% LOA | |
| M1 | 0.125 | 0.244 | 0.139 | 0.273 | 0.131 | 0.257 |
| M2 | 0.100 | 0.197 | 0.121 | 0.238 | 0.110 | 0.215 |
| M3 | 0.116 | 0.228 | 0.118 | 0.231 | 0.117 | 0.230 |
| G1 | 0.129 | 0.253 | 0.136 | 0.267 | 0.132 | 0.259 |
| G2 | 0.110 | 0.215 | 0.121 | 0.237 | 0.114 | 0.224 |
| G3 | 0.108 | 0.212 | 0.122 | 0.239 | 0.114 | 0.224 |
Abbreviations: AMD, age-related macular pathology; LOA, limits of agreement; SD, standard deviation.
Figure 2.
Bland–Altman plots for visual function measures for normal eyes (a–f) and for eyes with age-related macular degeneration (g–l) in the six modules of the Central Vision Analyzer. (a,g) ‘M1' module (full-contrast module of white letters presented at 99% contrast against a 3 Cd/m2 background). (b,h): ‘M2' module (lower-contrast module of 64% contrast against a 3 Cd/m2 background, simulating an environment similar to a dimly lit restaurant). (c,i) ‘M3' module and (lower-contrast module of 43% contrast against a 3 Cd/m2 background, simulating an environment similar to driving at dusk). (d,j) ‘G1' module (full-contrast module of black letters presented at 99% contrast against a 200 Cd/m2 background). (e,k) ‘G2' module (lower contract module of 10% contrast against a 200 Cd/m2 background, simulating playing golf or tennis outside with the sun over head). (f,l) ‘G3' module (lower-contrast module of 8% contrast against a 200 Cd/m2 background, simulating an environment similar to playing golf or tennis outside under the sun 15° off-axis).
Discussion
In the present study, we used the CVA to evaluate visual function in different mesopic- and backlight-glare environments in eyes with AMD compared to normal eyes, using time-dependent settings to simulate real-life conditions. BCVA scores reduced significantly with decreasing contrast and luminance conditions in both normal and AMD eyes. However, the visual function in AMD eyes was more affected than in normal eyes by the decreasing contrast in mesopic conditions; that is, the magnitude of vision drop was larger for AMD eyes than for normal eyes. In addition, we found that ETDRS scores and G1 scores for BCVA were equal only for normal eyes, while the ETDRS charts measured a better acuity compared to the G1 module (timed test) for AMD eyes. This means that, under timed conditions, AMD eyes (and not normals) have significantly lower acuity than expected. As discussed further below, CVA testing was generally a repeatable method, with repeatability within 0.10 and 0.14 logMAR for both normal and AMD eyes.
In an era of important technological treatment advances in AMD and other ophthalmic disorders, it is important to understand the real-world effects on vision of such interventions. For example, a patient with treated wet AMD may achieve a BCVA of 20/40 but the quality of that vision may not be good in everyday situations where speed-related vision tasks at varying contrast or background illumination are common. The Snellen chart test and the ETDRS chart test are the current standard methods for testing BCVA in clinical and research settings,4 and Pelli–Robson charts are one of the most common methods for testing contrast sensitivity. However, these tests allow the patient to take an indefinite amount of time to recognize the target; this is not reproducing real-world experiences, which are time-dependent. The Pelli–Robson contrast chart requires different test cards than the ETDRS, has a different illumination, and tests only contrast at large spatial frequencies. On the contrary, the CVA tests contrast at both high and low spatial frequencies (ie, high and low visual acuity). Moreover, the Snellen testing is less accurate than ETDRS in patients with AMD probably because of its lower contrast and brightness, resulting in lower acuity measurements than ETDRS.18 With the use of the timed CVA testing we demonstrated that lighting and contrast variations significantly reduce the visual performance for both normal and AMD subjects. However, contrast reduction affects AMD patients more adversely than normal subjects; in addition, eyes with wet AMD experience more trouble in low-contrast environments than dry AMD eyes. This is consistent with results of previous studies that reported delayed light adaptation and reduced contrast sensitivity in patients with AMD.19, 20 We did not find differences in drops of visual function between normal and AMD eyes in backlight-glare environments. The presence of a smaller pupil size in elderly population may account for the lack of effect of backlight glare, and therefore explain this finding.
The luminance and contrast of the CVA's G1 module is most similar to that of the ETDRS chart test (eg, high-contrast black type over a bright white background). We demonstrated that in eyes with AMD, testing BCVA with conventional non-timed ETDRS charts lead to better visual scores compared to the timed G1 testing; this finding is likely due to the time-dependent nature of the automated CVA testing. It is known that patients with macular pathology have a slower reading speed than normal patients21 and thus may fail to recognize letters if projected only for a short period of time. However, we emphasize that in real-life situations one does not have all the time he wants to read something, such as reading a road sign while driving, or reading a sign on a moving object. Therefore, we believe that a time-dependent test for BCVA, such as the CVA, may reproduce real-life situations better than a static ETDRS chart.
We also tested the repeatability of the CVA testing. The test–retest coefficients of repeatability of the CVA were found to be within 0.10 and 0.14 logMAR for both normal and AMD eyes, without significant differences between the two groups. At first glance, the coefficients of repeatability of the CVA appear to be slightly larger than those that are reported for reading logMAR charts. For 0.1 logMAR chart reading, previous studies reported repeatability mean sigmas of ∼0.04 to 0.05 logMAR (95% confidence limits: 0.086 to 0.100 logMAR) among young subjects, and up to 0.08 logMAR (95% confidence limits: 0.130 logMAR) among older individuals for letter-by-letter scoring. Our repeatability scores using the CVA are slightly lower than that reported with ETDRS likely because our subjects were patients with clinical problems, while most ETDRS studies often tested young motivated optometry students without refractive errors.22, 23 Second, the ETDRS chart test and the CVA test are designed with different size steps; on an ETDRS-style chart each letter accounts for 0.02 logMAR in size, while the CVA uses 0.05 logMAR size steps. Random fluctuations in repeatability will lead to poorer repeatability of the CVA because of its coarser scale.22, 23 Third, letter-by-letter scoring (as measured using the ETDRS chart) is dependent upon the termination criteria of the examiner and whether the examined individual is forced to proceed through the entire chart to the end with encouraged guessing,6 as opposed to proceeding downward to a line in which none of the letters are guessed correctly.24 If the examiner knows the prior test result, this will influence his/her termination criteria. Therefore, in a clinical setting testing of real patients, in which the examiner cannot control or influence the automated CVA test, and in which guessing is allowed but not forced, the resultant mean test–retest coefficient of repeatability is elevated to levels of 0.10 to 0.14 logMAR with 95% confidence limits of 0.19 to 0.24 logMAR.
In conclusion, the automated CVA showed the problems that AMD patients have in a six-domain real-life vision simulation scenario and helped explaining the complaints that such individuals have under timed conditions. We suggest that testing vision using timed tests across different lighting/contrast situations will help ophthalmologists in better understanding the quality of vision in AMD patients compared to the standard ETDRS charts test.
Acknowledgments
This study was supported by NIH grants R01EY007366 and R01EY018589 (WRF), R01EY016323 (D-UB), and in part by an unrestricted fund from Research to Prevent Blindness to the Department of Ophthalmology, University of California, San Diego.
The authors declare no conflict of interest.
References
- Khandhadia S, Cherry J, Lotery AJ. Age-related macular degeneration. Adv Exp Med Biol. 2012;724:15–36. doi: 10.1007/978-1-4614-0653-2_2. [DOI] [PubMed] [Google Scholar]
- Age-Related Eye Disease Study 2 Research Group Lutein+zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309 (19:2005–2015. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355 (14:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- Lim LA, Frost NA, Powell RJ, Hewson P. Comparison of the ETDRS logMAR, ‘compact reduced logMar' and Snellen charts in routine clinical practice. Eye (Lond) 2010;24 (4:673–677. doi: 10.1038/eye.2009.147. [DOI] [PubMed] [Google Scholar]
- Mitchell J, Bradley C. Quality of life in age-related macular degeneration: a review of the literature. Health Qual Life Outcomes. 2006;4:97. doi: 10.1186/1477-7525-4-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris FL, 3rd, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94 (1:91–96. [PubMed] [Google Scholar]
- Yavuz GA, Unver YB, Bekiroglu N, Presti P, Sinclair SH. Central field perimetry of discriminated targets: I. Results for normal individuals using high-contrast targets. Eye (Lond) 2009;23 (11:2082–2089. doi: 10.1038/eye.2009.177. [DOI] [PubMed] [Google Scholar]
- Bradley A, Thomas T, Kalaher M, Hoerres M. Effects of spherical and astigmatic defocus on acuity and contrast sensitivity: a comparison of three clinical charts. Optom Vis Sci. 1991;68 (6:418–426. doi: 10.1097/00006324-199106000-00003. [DOI] [PubMed] [Google Scholar]
- Pesudovs K, Hazel CA, Doran RM, Elliott DB. The usefulness of Vistech and FACT contrast sensitivity charts for cataract and refractive surgery outcomes research. Br J Ophthalmol. 2004;88 (1:11–16. doi: 10.1136/bjo.88.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruamviboonsuk P, Tiensuwan M, Kunawut C, Masayaanon P. Repeatability of an automated Landolt C test, compared with the early treatment of diabetic retinopathy study (ETDRS) chart testing. Am J Ophthalmol. 2003;136 (4:662–669. doi: 10.1016/s0002-9394(03)00394-5. [DOI] [PubMed] [Google Scholar]
- Gutstein W, Sinclair SH, North RV, Bekiroglu N. Screening athletes with Down syndrome for ocular disease. Optometry. 2010;81 (2:94–99. doi: 10.1016/j.optm.2009.09.017. [DOI] [PubMed] [Google Scholar]
- Gomez ML. Measuring the Quality of vision after cataract surgery. In “Cataract surgery and lens implantation”. Curr Opin Ophthalmol. 2014;25 (1:3–11. doi: 10.1097/ICU.0000000000000011. [DOI] [PubMed] [Google Scholar]
- Barteselli G, Chhablani J, Gomez ML, Doede AL, Dustin L, Kozak I, et al. Visual function assessment in simulated real-life situations in HIV-infected subjects. PLoS One. 2014;9 (5:e97023. doi: 10.1371/journal.pone.0097023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos JJ. On the cause of disability glare and its dependence on glare angle, age and ocular pigmentation. Clin Exp Optom. 2003;86 (6:363–370. doi: 10.1111/j.1444-0938.2003.tb03080.x. [DOI] [PubMed] [Google Scholar]
- Luo MR, Cui G, Rigg B. The development of the CIE 2000 colour-difference formula: CIEDE2000. Color Res Appl. 2001;26 (5:340–350. [Google Scholar]
- Peli E. Contrast in complex images. J Opt Soc Am A. 1990;7 (10:2032–2040. doi: 10.1364/josaa.7.002032. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Measurement error. BMJ. 1996;312 (7047:1654. doi: 10.1136/bmj.312.7047.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein IA, Cochran DE, Azen SP, Dustin L, Tammewar AM, Kozak I, et al. Comparison of visual acuity in macular degeneration patients measured with Snellen and early treatment diabetic retinopathy study charts. Ophthalmology. 2008;115 (2:319–323. doi: 10.1016/j.ophtha.2007.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher DC, Schuchard RA. Visual function in patients with choroidal neovascularization resulting from age-related macular degeneration: the importance of looking beyond visual acuity. Optom Vis Sci. 2006;83 (3:178–189. doi: 10.1097/01.opx.0000204510.08026.7f. [DOI] [PubMed] [Google Scholar]
- Bellmann C, Unnebrink K, Rubin GS, Miller D, Holz FG. Visual acuity and contrast sensitivity in patients with neovascular age-related macular degeneration. Results from the Radiation Therapy for Age-Related Macular Degeneration (RAD-) Study. Graefes Arch Clin Exp Ophthalmol. 2003;241 (12:968–974. doi: 10.1007/s00417-003-0689-6. [DOI] [PubMed] [Google Scholar]
- Nguyen NX, Weismann M, Trauzettel-Klosinski S. Improvement of reading speed after providing of low vision aids in patients with age-related macular degeneration. Acta Ophthalmol. 2009;87 (8:849–853. doi: 10.1111/j.1755-3768.2008.01423.x. [DOI] [PubMed] [Google Scholar]
- Raasch TW, Bailey IL, Bullimore MA. Repeatability of visual acuity measurement. Optom Vis Sci. 1998;75 (5:342–348. doi: 10.1097/00006324-199805000-00024. [DOI] [PubMed] [Google Scholar]
- Arditi A, Cagenello R. On the statistical reliability of letter-chart visual acuity measurements. Invest Ophthalmol Vis Sci. 1993;34 (1:120–129. [PubMed] [Google Scholar]
- Bailey IL, Lovie JE. New design principles for visual acuity letter charts. Am J Optom Physiol Opt. 1976;53 (11:740–745. doi: 10.1097/00006324-197611000-00006. [DOI] [PubMed] [Google Scholar]