Highlights
-
•
Integrin-mediated adhesion used by Drosophila blood cells to migrate in vivo.
-
•
SCAR/WAVE is required for lamellipodia but also for clearance of apoptotic cells.
-
•
The formins Fhos and Diaphanous regulate Drosophila macrophage migration and morphology.
-
•
Calcium waves drive hydrogen peroxide production to regulate inflammatory migrations.
-
•
The steroid hormone Ecdysone controls the onset of immune competence.
Abstract
Drosophila melanogaster contains a population of blood cells called hemocytes that represent the functional equivalent of vertebrate macrophages. These cells undergo directed migrations to disperse during development and reach sites of tissue damage or altered self. These chemotactic behaviors are controlled by the expression of PDGF/Vegf-related ligands in developing embryos and local production of hydrogen peroxide at wounds. Recent work reveals that many molecules important in vertebrate cell motility, including integrins, formins, Ena/VASP proteins and the SCAR/WAVE complex, have a conserved function in these innate immune cells. The use of this model organism has elucidated how damage signals are activated by calcium signaling during inflammation and that the steroid hormone ecdysone activates immune competence at key developmental stages.
Current Opinion in Cell Biology 2014, 30:1–8
This review comes from a themed issue on Cell adhesion and migration
Edited by Anna Huttenlocher and Erik Sahai
For a complete overview see the Issue and the Editorial
Available online 8th May 2014
0955-0674/$ – see front matter, © 2014 The Authors. Published by Elsevier Ltd. All rights reserved.
Introduction
Chemotaxis is the directed movement of cells (or an organism) towards or away from a chemical source. A classical example of chemotaxis is the movement of immune cells, such as neutrophils or macrophages, towards chemoattractants released at sites of infection or injury (e.g. fMLP and CSF-1) [1]. This process has been studied intensively in vitro, while the slime mould Dictyostelium discoideum has also proven vital in dissecting out the migration machinery and its regulation [2]. Whilst understanding regulation of cell migration represents a key biological problem, the fact that so many studies focus on immune cell motility reflects the diverse range of human diseases driven or exacerbated by inflammation.
Insects contain a population of blood cells, called hemocytes (Box 1), which make up the cellular component of their innate immune system [3, 4]. Given the genetic tractability and imaging capabilities of Drosophila melanogaster, the hemocytes of this organism have emerged as a prime cell type with which to study the regulation of migration and inflammation in vivo. Hemocytes are functionally equivalent to vertebrate macrophages and undergo chemotaxis to undergo developmental migrations and reach sites of tissue damage, while also detecting and removing apoptotic cells, debris and pathogens [4]. In this review we will discuss recent developments in our understanding of the machinery used by Drosophila hemocytes to chemotax during both developmental and pathological events occurring over the lifespan of a fruit fly. We will also focus on the latest work elucidating how damage signals are triggered and immune cell activation controlled.
Box 1. Blood cell lineages in Drosophila.
Drosophila fruit flies contain a population of blood cells called hemocytes that consists of at least three cell types: plasmatocytes, lamellocytes and crystal cells. Plasmatocytes are migratory, phagocytic and resemble vertebrate macrophages; lamellocytes are induced during immune responses to encapsulate invading parasites with their large lamellar processes [65]; crystal cells are non-motile and rupture during immune responses to activate the phenoloxidase pathway and the melanization cascade [66], a humoral form of host defense. Insect blood cells have been used extensively as a model for blood cell specification and proliferation, since many of the signaling pathways used during vertebrate hematopoiesis are conserved and related transcription factors employed [67, 68], such as the GATA factor Serpent [69] and the RUNX homologue Lozenge, which is specifically required for the production of crystal cells [70]. Embryonic hemocytes are derived from the head mesoderm [71], while a second wave of hematopoiesis occurs in the lymph gland, with cells released from this organ during larval stages [72]. Migration studies typically focus on the highly motile plasmatocytes, which disperse over the entire embryo during the course of development [71]. Plasmatocytes persist through to adult stages [72] and are often referred to simply as hemocytes (as we have done in this review) or macrophages.
Hemocytes use an evolutionarily conserved migration machinery to undergo chemotaxis
Hemocytes migrate as individual cells tightly confined between tissues when colonizing the embryo (Figure 1a,b) [5, 6]. Dispersal is critical for normal morphogenesis [7, 8, 9, 10, 11], allowing hemocytes to reach distant locations where their developmental functions are necessary and facilitates surveillance against potential pathogens. Consequently, dispersal is a carefully orchestrated and hard-wired process and its stereotyped nature provides numerous opportunities at which to determine the genetic requirements for chemotaxis. After dispersal, hemocytes become responsive to wound stimuli owing to downregulation of developmental cues [12], suggesting a prioritization of developmental cues over wound cues; a large overlap exists in the machinery used to respond to either cue. Migrating hemocytes possess large actin-rich lamellipodia into which microtubules protrude from the cell body. These microtubules are often bundled into an ‘arm-like’ structure (Figure 1c), which facilitates persistent motility [13]. A number of classic cytoskeletal regulators are autonomously required within hemocytes for dispersal or normal motility, including the GTPases Rho, Rac and Cdc42 [14, 15], and actin regulators Ena [6] and fascin [16, 17••]; all these play related roles in vertebrate cells.
Figure 1.
Embryonic migration routes and chemoattractant expression. Schematics showing expression of Pvf2 and Pvf3 chemoattractants (pink shading) in the developing Drosophila embryo at stages 11 (a) and 12 (b). Cartoons below embryos correspond to boxed regions and show RhoL-dependent invasion of the germband (gb) towards a source of Pvfs, some of which is expressed by the developing malphigian tubules (mp) (a) and movement along the developing ventral nerve cord (VNC; grey) (b); arrows indicate hemocyte movements at these stages of development. During progression along the VNC hemocytes are tightly confined between the ventral side of the VNC and epithelium (ep) and as they migrate along the VNC in response to the Pvf ligands that are expressed there, the epithelium and VNC separate, creating a channel for hemocyte progression. Hemocytes also migrate along the developing dorsal vessel at this stage (dv); a = anterior, p = posterior, d = dorsal, v = ventral, lat = lateral. Later in development cell–cell repulsion begins to occur and this depends upon the microtubules, which are frequently bundled into an arm-like structure (arrow) that facilitates persistent migration (c). Microtubules labeled via Clip-GFP expression in hemocytes; white line indicates edges of hemocytes, drawn according to mCherry-moesin localization (not shown). After initial dispersal hemocytes migrate at right angles from the ventral midline to the edges of the VNC (purple arrows) to form three lines (white arrows) on the ventral side of the embryo, immediately beneath the epithelium (d). Maximum projection images show GFP and nls-red stinger localization in hemocytes from the ventral side of the embryo; scale bars represent 50 μm; ant = anterior, post = posterior.
A family of PDGF/Vegf-related ligands called the Pvfs is expressed along the routes hemocytes take through the embryo (Figure 1a,b) [18, 19], suggesting they operate as chemoattractants to drive dispersal. Pvf signaling via the receptor Pvr is indispensible for both hemocyte viability and migration [18, 20, 21]. Importantly, blocking hemocyte apoptosis in pvr mutants rescues hemocyte numbers in the embryo [20], but fails to restore developmental dispersal fully [19, 20], while misexpression of Pvf2 can re-route hemocytes [5, 18], signifying Pvr promotes more than simply hemocyte survival. The route most sensitive to loss of Pvr signaling is penetration of the extended germband: here invasive hemocytes breach an epithelial barrier, involving a hemocyte-dependent disassembly of epithelial adhesions (Figure 1a; [22]). This strongly resembles transepithelial migration of vertebrate immune cells and critically depends on a small GTPase, RhoL [22]. RhoL function during transepithelial migration depends upon Rap1, which itself operates upstream of integrins in both hemocytes [23] and transmigrating vertebrate leukocytes [24]. Recent work has demonstrated that the main β-integrin (encoded by myospheroid) is required for normal hemocyte motility and migration to wounds in both embryos and pupae [25••, 26•]. In myospheroid embryos failed separation of the ventral nerve cord (VNC) and epithelium [25••] contributes to dispersal phenotypes. Loss of ECM (laminin) [27] or integrin complex components (rhea/talin and fermitin 1) also impairs migration [25••, 26•]. Whilst loss of integrin complex components did not interfere with repolarization towards wounds [25••, 26•], microtubule dynamics within hemocytes were affected with rapid and repeated collapse of microtubule arms observed in vivo [25••], presumably explaining the defects in contact inhibition of motility (cell–cell repulsion — a phenomenon that depends on microtubules [13]) observed in myospheroid mutants. Collapse events may occur via uncoupling of the actin and microtubule cytoskeletons or increased actin retrograde flow forcing microtubules rearwards when integrin-mediated anchoring of actin to ECM is absent.
Nucleation of actin filaments in migrating hemocytes
Although Drosophila cell RNAi screens identified numerous regulators of cellular morphology and the actin cytoskeleton [28, 29, 30], in vivo roles for many regulators have not been investigated. Addressing how hemocytes generate actin networks to drive migration has provided novel insights into hemocyte function in vivo.
SCAR encodes the Drosophila homologue of the WAVE proteins, activators of the Arp2/3 complex. SCAR interacts genetically with pvr during hemocyte dispersal along the VNC [5], potentially becoming activated downstream of Pvr via Vav, a Rac GEF downstream of Pvr in border cell migration [31•], or the adapter Pico/Lamellipodin [32••]. Unsurprisingly SCAR is necessary for all hemocyte migrations and drives formation of lamellipodia, revealing that branched Arp2/3-nucleated actin is a key component of these protrusive structures in vivo. However loss of SCAR also leads to hemocytes becoming engorged with undigested apoptotic cells [33••], a phenotype possibly related to SCAR mutant trafficking defects previously only observed in Dictyostelium [34]. Remarkably, blocking apoptosis to remove the source of apoptotic cells rescues hemocyte lamellipodia and dispersal and also partially restores their motility, suggesting that SCAR-independent mechanisms to form lamellipodia exist and that these can be suppressed by contact with apoptotic cells [33••], which may have important implications for regulation of macrophage behaviors following contact with apoptotic cells in disease situations. SCAR was also recently shown to be necessary for the migration of pupal macrophages to wounds [35•].
Formins represent another means to nucleate actin filaments. Drosophila contain examples of seven of the eight human formin families [36] and the homologue of the formin FHOD (encoded by fhos/knittrig) localizes to the rear of migrating pupal hemocytes and is required for spreading of pupal macrophages in vitro and normal migration to wounds [37••]. FHOD formins are thought to be activated by Rho kinase/Rock [36] and this seems to be the case for Fhos [37••]; Fhos may therefore act as a Rock-dependent effector of the RhoA-mediated retraction events necessary during migration to wounds [15]. How the activities of the numerous actin regulators known to operate in hemocytes are integrated to facilitate coordinated cell migration will doubtlessly be an important area to watch. Notably the group of Mark Peifer recently showed that Ena antagonizes Diaphanous (in both hemocytes and epithelial cells), which helps control the nature of filopodial protrusions ultimately produced [38•].
Developmental dispersal after downregulation of the Pvfs
After migrating the length of the ventral midline, hemocytes undergo a characteristic migration to the edges of the VNC ([19]; Figure 1d). These movements correlate with downregulation of the Pvfs at the midline. Overexpression of Pvfs along the midline delays lateral migration [19], suggesting loss of attractive ligands controls the timing of this event. Mathematical modeling of hemocyte movements raises the intriguing possibility that contact inhibition explains this patterning: simulations of hemocytes released from the ventral midline reproduce lateral migration patterns seen in embryos [39••]. Importantly, reducing hemocyte numbers, a key parameter in the model, causes this pattern to break down both in vivo and in simulations [39••]. The underlying molecular basis for repulsion remains to be established, but potentially targets microtubules, since depolymerization or hyperstabilization of microtubules or loss of the microtubule-binding protein Clasp/Orbit inhibits contact inhibition [13]; dynamic microtubules are also needed during contact inhibition between fibroblasts in vitro [40]. As hemocytes cluster together at wound sites and other sites of pathology, mechanisms to override repulsion must exist to enable normal macrophage behavior. Later in development a subpopulation of hemocytes closely associates with the larval peripheral nervous system, establishing a hematopoietic niche [41••]. Physical disturbance of these hemocytes results in their re-homing to this niche [41••], suggesting the presence of attractive signals regulating developmental migrations post-embryogenesis.
Regulation of migration to sites of pathology
As cells of the innate immune system, the primary role of hemocytes is host defense against invading pathogens and altered self. Hemocytes therefore localize to sites of tissue damage, cancerous growth and cell death (epithelial wounds [15, 42, 43], RasV12;scribble−/− clones [44, 45] and loser cells resulting from cell competition [46], respectively). Tissue resident hemocytes also become activated to deal with damage and promote recovery independent of migration (e.g. in UV-irradiated eye discs; [47•]).
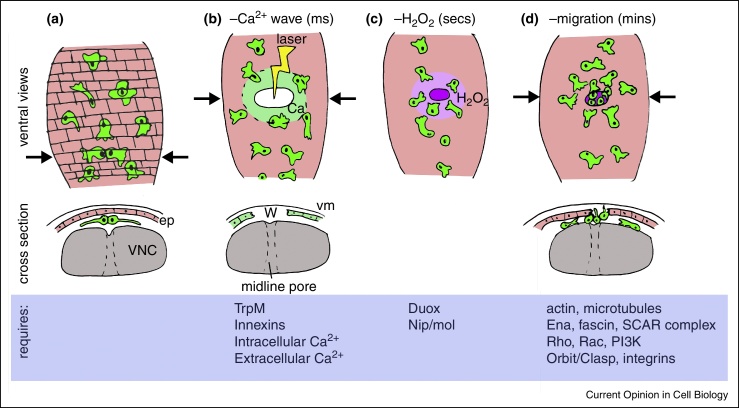
The embryonic wound response is perhaps the best-characterized example of hemocyte chemotaxis; here hemocytes rapidly repolarize and migrate to sites of damage (Figure 2, Figure 3). As is the case following tail fin wounds in zebrafish larvae [48], the NADPH oxidase Duox becomes activated, leading to the production of hydrogen peroxide at wound sites. Duox is both necessary and sufficient for hemocyte recruitment [12, 49••]. In worms, flies and fish wounding induces a rapid calcium flash through the epithelium [49••, 50, 51••], which, in flies at least, leads to Duox activation via a pair of calcium-sensing EF hands in an intracellular loop (Figure 2) [49••]. How hemocytes decode the hydrogen peroxide wound cue is not known, but the zebrafish Src family kinase Lyn contains a conserved cysteine residue, oxidation of which regulates Lyn activity and is necessary for neutrophil chemotaxis to hydrogen peroxide and wounds [52]. This cysteine is conserved in Src42A in flies [52], suggesting this mechanism may be conserved through evolution, although Src42A has an anti-inflammatory role limiting epithelial cell responses to damage in flies [53].
Figure 2.
Calcium waves direct inflammatory migration of hemocytes. Ventral and cross-sectional views (anterior-posterior position indicated by arrows) showing immune cell recruitment to sites of tissue damage in Drosophila embryos. Hemocytes (green) sit immediately beneath the epidermis (ep, pink) on the ventral nerve cord (VNC, grey) (a). Laser wounding of the epithelium causes an almost instantaneous calcium wave to flood through the epithelium via cell–cell junctions (b); this depends upon functional cell–cell junctions and TrpM. An increase in intracellular calcium activates the NADPH oxidase Duox via its EF hands driving hydrogen peroxide production (c). Hydrogen peroxide is necessary for the recruitment of hemocytes to this point of tissue damage, which is an active, migratory process requiring the function of the actin and microtubule cytoskeletons (d). The relative timescale is indicated in brackets.
Figure 3.
Comparison of migration to wounds in larval and embryonic stages of Drosophila development with vertebrate inflammatory responses. Cartoon of macrophage migration to wounds in vertebrates (a). Macrophages (green) form transient adhesions with activated endothelial cells (red) and roll, leading to arrest and extravasation and penetration through the basement membrane (brown) before migrating though tissue largely composed of fibroblasts (fb) and ECM to reach wound sites (W). Larval hemocyte responses (b) consist of an adhesive capture that recapitulates rolling and tethering of vertebrate leukocytes; sessile hemocytes do not respond to wounds. Migration of hemocytes to wounds in the embryo occurs in the context of an environment containing ECM deposited between closely opposed tissues (epithelium and VNC) and requires active migration and resembles movement of vertebrate leukocytes post-extravasation.
PI3K signaling is specifically required for hemocyte wound responses in embryos, leading to the hypothesis that inflammatory responses could be regulated via G-protein coupled receptors, similar to other chemoattractants [19]. Alternatively PI3K signaling might be involved in hemocyte activation (i.e. a priming event rendering hemocytes competent to respond to wounds). Curiously PI3Kγ contributes to the wandering migration of neutrophils in zebrafish [54], but appears dispensable for hemocyte developmental migrations [19].
Adhesive capture and hemocyte activation
During late embryogenesis, the primitive fly heart begins to beat and hemocytes are pumped around internal spaces as a constituent of the insect blood for the rest of the lifecycle, although some hemocytes remain attached to the epithelium in sessile patches. From larval stages onwards hemocytes are captured from the circulation via adhesion, with no contribution from the sessile population [42]. This ‘adhesive capture’ superficially resembles the rolling and tethering of vertebrate leukocytes ahead of their extravasation; embryonic migration more closely resembles chemotaxis of macrophages through connective tissue after extravasation (Figure 3). In pupae sessile patch hemocytes recommence motility and become wound responsive [43]. The signals driving inflammatory migration in larvae and pupae remain uncharacterized, but as wounding of the latter triggers integrin-dependent migration and epithelial calcium waves [26•, 55••], a similar mechanism to that of the embryo may be employed.
In larvae and adults activation of adhesion may facilitate capture: the typical blood cell response to damage and infection in Lepidopteran insects (the order containing moths and butterflies) is adhesion, which can be mediated via cytokine-like molecules such as plasmatocyte spreading peptide (PSP) [56, 57]. Injection of PSP into lepidopterans removes immune cells from circulation, presumably via adhesion to internal tissues [56]. Likewise, hemocyte chemotactic peptide (HCP) facilitates recruitment to wounds in moth larvae and directs chemotaxis of their blood cells in vitro [58]. Therefore systemic release of similar molecules may activate Drosophila hemocytes to enable capture at wounds. Recruitment to other sites of pathology (e.g. tumors) post-embryogenesis is also likely to occur via adhesive capture from circulation. Whether local infections can trigger focal recruitment of hemocytes remains unclear — chemotaxis towards pathogens is yet to be demonstrated. Homing of hemocytes to tumors is associated with damage or degradation of the basement membrane [45], which might expose adhesive signals or activate hemocytes to become adherent. Indeed, activation may represent the key step controlling immune responses. The steroid hormone ecdysone has long been associated with control of Drosophila development [59] and two recent studies have confirmed ecdysone to stimulate hemocyte motility, and its crucial role activating clearance of apoptotic cells and immune surveillance during metamorphosis [60••, 61••]. The transition back to a more classical migratory chemotaxis to wounds correlates with the beginning of metamorphosis and is prevented by expression of dominant negative ecdysone receptor in hemocytes [60••]. Ecdysone also turns on immune responses in embryos, since treatment with ecdysone analogues is sufficient to activate immune competence ahead of schedule [62••]. Rac1 and Basket/JNK signaling have also been previously implicated in hemocyte activation [63] and therefore represent potential downstream targets of signaling pathways to trigger recruitment of hemocytes from the circulation.
Conclusions
Hemocytes have long been investigated as part of the innate immune responses to systemic infection [64], but have recently received substantial interest as a model cell type to understand the regulation of cell migration in the context of an intact and immune competent organism. We are now beginning to have a more complete understanding of the molecular mechanisms used by these highly migratory cells to reach the locations necessary for their range of functions and needed for their responses to pathology. As researchers fill in the gaps in our knowledge, we anticipate hemocytes will become a prime cell type to probe regulation of signal integration in vivo and the challenge for Drosophila researchers will be to use the powerful genetics available in the fly to identify novel targets involved in these processes.
Competing interests statement
The authors declare no competing financial interests.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
Will Wood is funded by the Wellcome Trust as a Senior Fellow (090899/Z/09/Z); Iwan Robert Evans is a Wellcome Trust/Royal Society Sir Henry Dale Fellow.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
References
- 1.Barreiro O., Martin P., Gonzalez-Amaro R., Sanchez-Madrid F. Molecular cues guiding inflammatory responses. Cardiovasc Res. 2010;86:174–182. doi: 10.1093/cvr/cvq001. [DOI] [PubMed] [Google Scholar]
- 2.King J.S., Insall R.H. Chemotaxis: finding the way forward with Dictyostelium. Trends Cell Biol. 2009;19:523–530. doi: 10.1016/j.tcb.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Wang L., Kounatidis I., Ligoxygakis P. Drosophila as a model to study the role of blood cells in inflammation, innate immunity and cancer. Front Cell Infect Microbiol. 2013;3:113. doi: 10.3389/fcimb.2013.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wood W., Jacinto A. Drosophila melanogaster embryonic haemocytes: masters of multitasking. Nat Rev Mol Cell Biol. 2007;8:542–551. doi: 10.1038/nrm2202. [DOI] [PubMed] [Google Scholar]
- 5.Evans I.R., Hu N., Skaer H., Wood W. Interdependence of macrophage migration and ventral nerve cord development in Drosophila embryos. Development. 2010;137:1625–1633. doi: 10.1242/dev.046797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tucker P.K., Evans I.R., Wood W. Ena drives invasive macrophage migration in Drosophila embryos. Dis Model Mech. 2011;4:126–134. doi: 10.1242/dmm.005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bunt S., Hooley C., Hu N., Scahill C., Weavers H., Skaer H. Hemocyte-secreted type IV collagen enhances BMP signaling to guide renal tubule morphogenesis in Drosophila. Dev Cell. 2010;19:296–306. doi: 10.1016/j.devcel.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Defaye A., Evans I., Crozatier M., Wood W., Lemaitre B., Leulier F. Genetic ablation of Drosophila phagocytes reveals their contribution to both development and resistance to bacterial infection. J Innate Immun. 2009;1:322–334. doi: 10.1159/000210264. [DOI] [PubMed] [Google Scholar]
- 9.Olofsson B., Page D.T. Condensation of the central nervous system in embryonic Drosophila is inhibited by blocking hemocyte migration or neural activity. Dev Biol. 2005;279:233–243. doi: 10.1016/j.ydbio.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 10.Page D.T., Olofsson B. Multiple roles for apoptosis facilitating condensation of the Drosophila ventral nerve cord. Genesis. 2008;46:61–68. doi: 10.1002/dvg.20365. [DOI] [PubMed] [Google Scholar]
- 11.Sears H.C., Kennedy C.J., Garrity P.A. Macrophage-mediated corpse engulfment is required for normal Drosophila CNS morphogenesis. Development. 2003;130:3557–3565. doi: 10.1242/dev.00586. [DOI] [PubMed] [Google Scholar]
- 12.Moreira S., Stramer B., Evans I., Wood W., Martin P. Prioritization of competing damage and developmental signals by migrating macrophages in the Drosophila embryo. Curr Biol. 2010;20:464–470. doi: 10.1016/j.cub.2010.01.047. [DOI] [PubMed] [Google Scholar]
- 13.Stramer B., Moreira S., Millard T., Evans I., Huang C.Y., Sabet O., Milner M., Dunn G., Martin P., Wood W. Clasp-mediated microtubule bundling regulates persistent motility and contact repulsion in Drosophila macrophages in vivo. J Cell Biol. 2010;189:681–689. doi: 10.1083/jcb.200912134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paladi M., Tepass U. Function of Rho GTPases in embryonic blood cell migration in Drosophila. J Cell Sci. 2004;117:6313–6326. doi: 10.1242/jcs.01552. [DOI] [PubMed] [Google Scholar]
- 15.Stramer B., Wood W., Galko M.J., Redd M.J., Jacinto A., Parkhurst S.M., Martin P. Live imaging of wound inflammation in Drosophila embryos reveals key roles for small GTPases during in vivo cell migration. J Cell Biol. 2005;168:567–573. doi: 10.1083/jcb.200405120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zanet J., Stramer B., Millard T., Martin P., Payre F., Plaza S. Fascin is required for blood cell migration during Drosophila embryogenesis. Development. 2009;136:2557–2565. doi: 10.1242/dev.036517. [DOI] [PubMed] [Google Scholar]
- 17••.Zanet J., Jayo A., Plaza S., Millard T., Parsons M., Stramer B. Fascin promotes filopodia formation independent of its role in actin bundling. J Cell Biol. 2012;197:477–486. doi: 10.1083/jcb.201110135. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work revealed that phosphomimetic mutants of an evolutionarily conserved serine residue prevent both actin bundling and filopodia formation in Drosophila hemocytes, but non-phosphorylatable mutants can rescue filopodia and migration (but not bundling), suggesting that actin filament bundling by fascin might be less important for hemocyte migration than filopodia formation. The role of fascin in nurse cells was also addressed and the authors showed similar roles for fascin in carcinoma cells.
- 18.Cho N.K., Keyes L., Johnson E., Heller J., Ryner L., Karim F., Krasnow M.A. Developmental control of blood cell migration by the Drosophila VEGF pathway. Cell. 2002;108:865–876. doi: 10.1016/s0092-8674(02)00676-1. [DOI] [PubMed] [Google Scholar]
- 19.Wood W., Faria C., Jacinto A. Distinct mechanisms regulate hemocyte chemotaxis during development and wound healing in Drosophila melanogaster. J Cell Biol. 2006;173:405–416. doi: 10.1083/jcb.200508161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruckner K., Kockel L., Duchek P., Luque C.M., Rorth P., Perrimon N. The PDGF/VEGF receptor controls blood cell survival in Drosophila. Dev Cell. 2004;7:73–84. doi: 10.1016/j.devcel.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Heino T.I., Karpanen T., Wahlstrom G., Pulkkinen M., Eriksson U., Alitalo K., Roos C. The Drosophila VEGF receptor homolog is expressed in hemocytes. Mech Dev. 2001;109:69–77. doi: 10.1016/s0925-4773(01)00510-x. [DOI] [PubMed] [Google Scholar]
- 22.Siekhaus D., Haesemeyer M., Moffitt O., Lehmann R. RhoL controls invasion and Rap1 localization during immune cell transmigration in Drosophila. Nat Cell Biol. 2010;12:605–610. doi: 10.1038/ncb2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huelsmann S., Hepper C., Marchese D., Knoll C., Reuter R. The PDZ-GEF dizzy regulates cell shape of migrating macrophages via Rap1 and integrins in the Drosophila embryo. Development. 2006;133:2915–2924. doi: 10.1242/dev.02449. [DOI] [PubMed] [Google Scholar]
- 24.Abram C.L., Lowell C.A. The ins and outs of leukocyte integrin signaling. Annu Rev Immunol. 2009;27:339–362. doi: 10.1146/annurev.immunol.021908.132554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25••.Comber K., Huelsmann S., Evans I., Sanchez-Sanchez B.J., Chalmers A., Reuter R., Wood W., Martin-Bermudo M.D. A dual role for the betaPS integrin myospheroid in mediating Drosophila embryonic macrophage migration. J Cell Sci. 2013;126:3475–3484. doi: 10.1242/jcs.129700. [DOI] [PMC free article] [PubMed] [Google Scholar]; Loss of myospheroid gene function plays two roles in the developing embryo — one in enabling separation of the VNC from epithelium, creating a channel for hemocytes to move into as they progress down the midline and another autonomous role in hemocytes in which they are needed for efficient migration in vivo. Live imaging studies using hemocytes with labeled microtubules showed that the β-integrin Myospheroid is also necessary to maintain the bundles of microtubules that enable persistent migration and contact inhibition.
- 26•.Moreira C.G., Jacinto A., Prag S. Drosophila integrin adhesion complexes are essential for hemocyte migration in vivo. Biol Open. 2013;2:795–801. doi: 10.1242/bio.20134564. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work describes the requirements of key components of integrin complexes for hemocyte migration in the pupae. RNAi of zyxin actually increases migration rates, whereas other components hinder motility on their knockdown in pupal hemocytes.
- 27.Urbano J.M., Torgler C.N., Molnar C., Tepass U., Lopez-Varea A., Brown N.H., de Celis J.F., Martin-Bermudo M.D. Drosophila laminins act as key regulators of basement membrane assembly and morphogenesis. Development. 2009;136:4165–4176. doi: 10.1242/dev.044263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiger A.A., Baum B., Jones S., Jones M.R., Coulson A., Echeverri C., Perrimon N. A functional genomic analysis of cell morphology using RNA interference. J Biol. 2003;2:27. doi: 10.1186/1475-4924-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunda P., Craig G., Dominguez V., Baum B. Abi, Sra1, and Kette control the stability and localization of SCAR/WAVE to regulate the formation of actin-based protrusions. Curr Biol. 2003;13:1867–1875. doi: 10.1016/j.cub.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Rogers S.L., Wiedemann U., Stuurman N., Vale R.D. Molecular requirements for actin-based lamella formation in Drosophila S2 cells. J Cell Biol. 2003;162:1079–1088. doi: 10.1083/jcb.200303023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31•.Fernandez-Espartero C.H., Ramel D., Farago M., Malartre M., Luque C.M., Limanovich S., Katzav S., Emery G., Martin-Bermudo M.D. GTP. exchange factor Vav regulates guided cell migration by coupling guidance receptor signalling to local Rac activation. J Cell Sci. 2013;126:2285–2293. doi: 10.1242/jcs.124438. [DOI] [PubMed] [Google Scholar]; In border cells, a model for collective cell migration in which a cluster of cells delaminates from an epithelium and migrates towards the oocyte during oogenesis, the GEF Vav operates downstream of Pvf1/Pvr to activate Rac and generate protrusive actin structures. The authors showed Vav to interact directly with Pvr and using FRET revealed Vav drives polarized recruitment of Rac at the leading edge.
- 32••.Law A.L., Vehlow A., Kotini M., Dodgson L., Soong D., Theveneau E., Bodo C., Taylor E., Navarro C., Perera U. Lamellipodin and the Scar/WAVE complex cooperate to promote cell migration in vivo. J Cell Biol. 2013;203:673–689. doi: 10.1083/jcb.201304051. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper indicates that Pico/Lamellipodin and SCAR/WAVE work together to drive cell migration in Xenopus neural crest cells and Drosophila border cells, while melanoblast dispersal is affected in Lamellipodin knock out mice, suggestive of neural crest migration defects. The authors show a direct interaction between Lamellipodin and Rac-GTP and that this association helps promote Lamellipodin–SCAR/WAVE interactions via Abi. This coupling helps to drive lamellipodia formation and enhances migration in vitro.
- 33••.Evans I.R., Ghai P.A., Urbancic V., Tan K.L., Wood W. SCAR/WAVE-mediated processing of engulfed apoptotic corpses is essential for effective macrophage migration in Drosophila. Cell Death Differ. 2013;20:709–720. doi: 10.1038/cdd.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]; The work was the first to show a genetic and cell autonomous requirement for SCAR/WAVE in hemocytes in vivo, where it is vital for normal hemocyte migration. In addition to driving lamellipodial protrusion SCAR seems important in normal degradation of apoptotic cells. Surprisingly removing apoptosis from the developing embryo rescued lamellipodia and partly restored migration in a SCAR mutant background.
- 34.Seastone D.J., Harris E., Temesvari L.A., Bear J.E., Saxe C.L., Cardelli J. The WASp-like protein scar regulates macropinocytosis, phagocytosis and endosomal membrane flow in Dictyostelium. J Cell Sci. 2001;114:2673–2683. doi: 10.1242/jcs.114.14.2673. [DOI] [PubMed] [Google Scholar]
- 35•.Sander M., Squarr A.J., Risse B., Jiang X., Bogdan S. Drosophila pupal macrophages — a versatile tool for combined ex vivo and in vivo imaging of actin dynamics at high resolution. Eur J Cell Biol. 2013;92:349–354. doi: 10.1016/j.ejcb.2013.09.003. [DOI] [PubMed] [Google Scholar]; This work demonstrates a role for SCAR in pupal macrophage migration to wounds. The authors developed an interesting and useful way to analyze wound responses based upon movement of hemocytes towards wounds causing an increase in intensity of fluorescent reporters expressed in hemocytes nearer the point of ablation.
- 36.Schonichen A., Geyer M. Fifteen formins for an actin filament: a molecular view on the regulation of human formins. Biochim Biophys Acta. 2010;1803:152–163. doi: 10.1016/j.bbamcr.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 37••.Lammel U., Bechtold M., Risse B., Berh D., Fleige A., Bunse I., Jiang X., Klambt C., Bogdan S. The Drosophila FHOD1-like formin Knittrig acts through Rok to promote stress fiber formation and directed macrophage migration during the cellular immune response. Development. 2014;141:1366–1380. doi: 10.1242/dev.101352. [DOI] [PubMed] [Google Scholar]; Investigating the role of the Drosophila homologue of mammalian FHOD1 (Knittrig/Fhos), this work shows a role in the tracheal system of the developing embryo and identifies a role in the migration of pupal macrophages. Live imaging shows recruitment of Fhos to the rear of the cell where it may be involved in contraction processes needed for normal migration.
- 38•.Bilancia C.G., Winkelman J.D., Tsygankov D., Nowotarski S.H., Sees J.A., Comber K., Evans I., Lakhani V., Wood W., Elston T.C. Enabled negatively regulates diaphanous-driven actin dynamics in vitro and in vivo. Dev Cell. 2014;28:394–408. doi: 10.1016/j.devcel.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ena and Diaphanous elicit distinct forms of filopodia. Using biochemistry and analyzing Drosophila epithelial cells and hemocytes in vivo this work shows Ena to suppress Diaphanous-mediated filopodia, which tend to be longer and less dynamic than their Ena-driven counterparts. Overexpression of Ena in hemocytes rescues the migratory defects caused by overexpression of active Diaphanous in hemocytes.
- 39••.Davis J.R., Huang C.Y., Zanet J., Harrison S., Rosten E., Cox S., Soong D.Y., Dunn G.A., Stramer B.M. Emergence of embryonic pattern through contact inhibition of locomotion. Development. 2012;139:4555–4560. doi: 10.1242/dev.082248. [DOI] [PMC free article] [PubMed] [Google Scholar]; Davis et al. performed simulations of hemocyte movements based upon speeds and trajectories of hemocytes before and after cell–cell contact. Hemocytes were lined up along the ventral midline as per stage 13 of embryonic development. Remarkably their simulations reproduced the patterned tracks exhibited by hemocytes undergoing lateral migration in vivo, suggesting contact inhibition may explain this phase of dispersal.
- 40.Kadir S., Astin J.W., Tahtamouni L., Martin P., Nobes C.D. Microtubule remodelling is required for the front-rear polarity switch during contact inhibition of locomotion. J Cell Sci. 2011;124:2642–2653. doi: 10.1242/jcs.087965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41••.Makhijani K., Alexander B., Tanaka T., Rulifson E., Bruckner K. The peripheral nervous system supports blood cell homing and survival in the Drosophila larva. Development. 2011;138:5379–5391. doi: 10.1242/dev.067322. [DOI] [PMC free article] [PubMed] [Google Scholar]; An exciting paper showing the presence of post-embryonic hematopoietic niches around the glia and neurons of the PNS. When hemocytes are physically dislodged, they return to these positions, suggesting the presence of attractive cues.
- 42.Babcock D.T., Brock A.R., Fish G.S., Wang Y., Perrin L., Krasnow M.A., Galko M.J. Circulating blood cells function as a surveillance system for damaged tissue in Drosophila larvae. Proc Natl Acad Sci U S A. 2008;105:10017–10022. doi: 10.1073/pnas.0709951105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moreira C.G., Regan J.C., Zaidman-Remy A., Jacinto A., Prag S. Drosophila hemocyte migration: an in vivo assay for directional cell migration. Methods Mol Biol. 2011;769:249–260. doi: 10.1007/978-1-61779-207-6_17. [DOI] [PubMed] [Google Scholar]
- 44.Cordero J.B., Macagno J.P., Stefanatos R.K., Strathdee K.E., Cagan R.L., Vidal M. Oncogenic Ras diverts a host TNF tumor suppressor activity into tumor promoter. Dev Cell. 2010;18:999–1011. doi: 10.1016/j.devcel.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pastor-Pareja J.C., Wu M., Xu T. An innate immune response of blood cells to tumors and tissue damage in Drosophila. Dis Model Mech. 2008;1:144–154. doi: 10.1242/dmm.000950. discussion 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lolo F.N., Casas-Tinto S., Moreno E. Cell competition time line: winners kill losers, which are extruded and engulfed by hemocytes. Cell Rep. 2012;2:526–539. doi: 10.1016/j.celrep.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 47•.Kelsey E.M., Luo X., Bruckner K., Jasper H. Schnurri regulates hemocyte function to promote tissue recovery after DNA damage. J Cell Sci. 2012;125:1393–1400. doi: 10.1242/jcs.095323. [DOI] [PMC free article] [PubMed] [Google Scholar]; A transcriptional regulator called Schnurri helps promote regeneration after UV irradiation of Drosophila eye discs. Schnurri drives retinal expression of Pvf1, which activates hemocytes to become phagocytic. Removal of hemocytes or attenuation of this signaling network hampers regeneration.
- 48.Niethammer P., Grabher C., Look A.T., Mitchison T.J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49••.Razzell W., Evans I.R., Martin P., Wood W. Calcium flashes orchestrate the wound inflammatory response through DUOX activation and hydrogen peroxide release. Curr Biol. 2013;23:424–429. doi: 10.1016/j.cub.2013.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper was the first to show a calcium wave through a multicellular epithelium in vivo and demonstrates that this calcium response is coupled to hemocyte inflammatory responses. The TRP channel TrpM and gap junctions are necessary for the spread of the calcium wave and dampening the wave using mutants in these genes or pharmacological means reduces inflammatory responses of hemocytes to laser-induced wounds in the embryo. The paper also shows that the maturation factor Nip/Mol is necessary for the role of Duox in triggering inflammatory migration.
- 50.Xu S., Chisholm A.D. A Galphaq-Ca2+ signaling pathway promotes actin-mediated epidermal wound closure in C. elegans. Curr Biol. 2011;21:1960–1967. doi: 10.1016/j.cub.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51••.Yoo S.K., Freisinger C.M., LeBert D.C., Huttenlocher A. Early redox, Src family kinase, and calcium signaling integrate wound responses and tissue regeneration in zebrafish. J Cell Biol. 2012;199:225–234. doi: 10.1083/jcb.201203154. [DOI] [PMC free article] [PubMed] [Google Scholar]; In contrast to Razzell et al. this study failed to find a link between the calcium wave induced at sites of injury in zebrafish larvae and neutrophil responses (using the drug thapsigargin). However the calcium response was important (in concert with Src family kinases and reactive oxygen species) for normal regeneration after injury.
- 52.Yoo S.K., Starnes T.W., Deng Q., Huttenlocher A. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature. 2011;480:109–112. doi: 10.1038/nature10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Juarez M.T., Patterson R.A., Sandoval-Guillen E., McGinnis W. Duox, Flotillin-2, and Src42A are required to activate or delimit the spread of the transcriptional response to epidermal wounds in Drosophila. PLoS Genet. 2011;7:e1002424. doi: 10.1371/journal.pgen.1002424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoo S.K., Deng Q., Cavnar P.J., Wu Y.I., Hahn K.M., Huttenlocher A. Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish. Dev Cell. 2010;18:226–236. doi: 10.1016/j.devcel.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55••.Antunes M., Pereira T., Cordeiro J.V., Almeida L., Jacinto A. Coordinated waves of actomyosin flow and apical cell constriction immediately after wounding. J Cell Biol. 2013;202:365–379. doi: 10.1083/jcb.201211039. [DOI] [PMC free article] [PubMed] [Google Scholar]; Antunes et al. show the existence of a calcium response in the pupal epithelium (the notum) of Drosophila. Taking a genetic approach they show that a Diaphanous and Rock-dependent actomyosin flow helps set up the actin cable that is involved in wound closure and that these processes depend upon calcium fluxes in the epithelium, which TrpM helps to generate.
- 56.Clark K.D., Pech L.L., Strand M.R. Isolation and identification of a plasmatocyte-spreading peptide from the hemolymph of the lepidopteran insect Pseudoplusia includens. J Biol Chem. 1997;272:23440–23447. doi: 10.1074/jbc.272.37.23440. [DOI] [PubMed] [Google Scholar]
- 57.Eleftherianos I., Xu M., Yadi H., Ffrench-Constant R.H., Reynolds S.E. Plasmatocyte-spreading peptide (PSP) plays a central role in insect cellular immune defenses against bacterial infection. J Exp Biol. 2009;212:1840–1848. doi: 10.1242/jeb.026278. [DOI] [PubMed] [Google Scholar]
- 58.Nakatogawa S., Oda Y., Kamiya M., Kamijima T., Aizawa T., Clark K.D., Demura M., Kawano K., Strand M.R., Hayakawa Y. A novel peptide mediates aggregation and migration of hemocytes from an insect. Curr Biol. 2009;19:779–785. doi: 10.1016/j.cub.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 59.Thummel C.S. Molecular mechanisms of developmental timing in C. elegans and Drosophila. Dev Cell. 2001;1:453–465. doi: 10.1016/s1534-5807(01)00060-0. [DOI] [PubMed] [Google Scholar]
- 60••.Regan J.C., Brandao A.S., Leitao A.B., Mantas Dias A.R., Sucena E., Jacinto A., Zaidman-Remy A. Steroid hormone signaling is essential to regulate innate immune cells and fight bacterial infection in Drosophila. PLoS Pathog. 2013;9:e1003720. doi: 10.1371/journal.ppat.1003720. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hemocyte-specific expression of dominant negative ecdysone receptor was shown to block hemocyte activation during metamorphosis–pupal hemocytes fail to spread in vitro, disperse from sessile patches or efficiently engulf apoptotic cells during metamorphosis. This ecdysone-dependent activation is necessary to fight off systemic infections and clear pathogens. The authors also conducted microarray analysis to determine hemocyte genes activated by ecdysone during metamorphosis, identifying genes previously implicated in detecting bacteria and phagocytosis.
- 61••.Sampson C.J., Amin U., Couso J.P. Activation of Drosophila hemocyte motility by the ecdysone hormone. Biol Open. 2013;2:1412–1420. doi: 10.1242/bio.20136619. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work follows on from an earlier study in which Sampson and Williams showed pre-pupal hemocytes to migrate in vitro (unlike hemocytes taken from other stages of development). Here Sampson et al., show that ecdysone can activate the motility of otherwise non-motile hemocytes in ex vivo cultures and that this is blocked by hemocyte-specific expression of dominant negative ecdysone receptor.
- 62••.Tan K.L., Vlisidou I., Wood W. Ecdysone mediates the development of immunity in the Drosophila embryo. Curr Biol. 2014 doi: 10.1016/j.cub.2014.03.062. http://dx.doi.org/10.1016/j.cub.2014.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tan et al. show through the injection of a variety of microorganisms that late stage Drosophila embryos are able to mount immune responses (e.g. production of anti-microbial peptides) following infection but early stage embryos are not. The development of immune competence is dependent on the steroid hormone Ecdysone.
- 63.Williams M.J., Wiklund M.L., Wikman S., Hultmark D. Rac1 signalling in the Drosophila larval cellular immune response. J Cell Sci. 2006;119:2015–2024. doi: 10.1242/jcs.02920. [DOI] [PubMed] [Google Scholar]
- 64.Lemaitre B., Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 65.Lanot R., Zachary D., Holder F., Meister M. Postembryonic hematopoiesis in Drosophila. Dev Biol. 2001;230:243–257. doi: 10.1006/dbio.2000.0123. [DOI] [PubMed] [Google Scholar]
- 66.Bidla G., Dushay M.S., Theopold U. Crystal cell rupture after injury in Drosophila requires the JNK pathway, small GTPases and the TNF homolog Eiger. J Cell Sci. 2007;120:1209–1215. doi: 10.1242/jcs.03420. [DOI] [PubMed] [Google Scholar]
- 67.Crozatier M., Meister M. Drosophila haematopoiesis. Cell Microbiol. 2007;9:1117–1126. doi: 10.1111/j.1462-5822.2007.00930.x. [DOI] [PubMed] [Google Scholar]
- 68.Evans C.J., Hartenstein V., Banerjee U. Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev Cell. 2003;5:673–690. doi: 10.1016/s1534-5807(03)00335-6. [DOI] [PubMed] [Google Scholar]
- 69.Rehorn K.P., Thelen H., Michelson A.M., Reuter R. A molecular aspect of hematopoiesis and endoderm development common to vertebrates and Drosophila. Development. 1996;122:4023–4031. doi: 10.1242/dev.122.12.4023. [DOI] [PubMed] [Google Scholar]
- 70.Lebestky T., Chang T., Hartenstein V., Banerjee U. Specification of Drosophila hematopoietic lineage by conserved transcription factors. Science. 2000;288:146–149. doi: 10.1126/science.288.5463.146. [DOI] [PubMed] [Google Scholar]
- 71.Tepass U., Fessler L.I., Aziz A., Hartenstein V. Embryonic origin of hemocytes and their relationship to cell death in Drosophila. Development. 1994;120:1829–1837. doi: 10.1242/dev.120.7.1829. [DOI] [PubMed] [Google Scholar]
- 72.Holz A., Bossinger B., Strasser T., Janning W., Klapper R. The two origins of hemocytes in Drosophila. Development. 2003;130:4955–4962. doi: 10.1242/dev.00702. [DOI] [PubMed] [Google Scholar]