Abstract
The Chinese brown frog (Rana dybowskii) is a special amphibian with one unique physiological phenomenon, which is that its oviduct expands prior to hibernation, instead of during the breeding period. In this study, we investigate the localization and expression level of PPARγ2, leptin and leptin receptor proteins in oviduct of Rana dybowskii during breeding period and pre-hibernation. There were significant variations in oviductal weight and size, with values much lower in the breeding period than in pre-hibernation. PPARγ2 was observed in stromal and epithelial cells in both periods. Leptin was immunolocalized in epithelial cells in both periods, whereas leptin receptor was detected only in stromal cells. Consistently, the protein levels of PPARγ2, leptin and leptin receptor were higher in pre-hibernation as compared to the breeding period. These results suggested that oviduct was the target organ of leptin, which may play an important paracrine role in regulating the oviductal hypertrophy during prehibernation.
Key words: Leptin, leptin receptor, oviduct, PPARγ2, Rana dybowskii.
Introduction
Oviduct is a dynamic organ that goes through significant morphological, biochemical, and physiological modifications throughout the reproductive cycle. It is not only a passive channel for gamete and embryo transport, but also encompasses a highly active secretory organ involved in several critical reproductive events, such as estrous cycle and ovulation.1 Oviduct is regulated by a wide variety of factors, including locally synthesized molecules working in an collaborative, synergistic, or antagonistic manner to regulate different oviductal functions, such as gene expression, protein synthesis, morphology with sexual maturation and reproductive activity.2 Oviductal fluid is essential for the oviduct to perform its reproductive functions,3 which offers an optimal microenvironment for biological functions that contribute to sperm capacitation, final oocyte maturation, fertilization and early embryo development.4,5 Oviductal fluid is consisted of hundreds of macromolecules which can be secreted from oviduct epithelium or serum transudate.6,7
The Chinese brown frog (Rana dybowskii) is a unique amphibian species in northeastern China. It is a seasonal breeder with the habit of seasonal migration between mountain and wetland. Rana dybowskii is a famous economic species which has been used widely in the Traditional Chinese Medicine.8 The hibernation for Rana dybowskii takes place from October to February, which is followed by the breeding period ranging from February to June depending on the latitude and altitude. Interestingly, one unique physiological phenomenon of Rana dybowskii is that its oviduct expands during prehibernation but not during the breeding period. Besides, dried oviduct of the female Rana dybowskii, Oviductus Ranae, is one of the best-known and highly valued oriental foods and Chinese crude drugs, which is recorded in the Pharmacopoeia of the People’s Republic of China.9 Traditional Chinese medicine holds that Oviductus Ranae can nourish the yin, moisten the lung and replenish the kidney essence.10 Meanwhile, Oviductus Ranae is mainly composed of proteins and lipid, which are up to 50% or more.11
Vast published literature has been made in our understanding of adipocyte differentiation and adipocyte-specific gene expression. Peroxisome proliferator-activated receptor (PPAR)-γ is a ligand-activated transcription factor and a member of the nuclear hormone receptor superfamily. It has been proven to be involved in directing expression of fat-specific genes and in activating the program of adipocyte differentiation.12 PPARγ can also induce trans-differentiation from fibroblasts and myoblasts to adipocytes.13,14 Besides, PPAR has two main iso-forms: PPAR 1 and PPARγ2.15 Adipose tissue is the most abundant site for both isoforms.16 The difference is that PPARγ2 is restricted in fat,17 whereas PPAR 1 is predominant in other tissues.18 Adipose tissue contributes to the regulation of energy homeostasis, secreting a large number of active adipokines.19 Leptin is one of the best-characterized adipokines, which affect carbohydrate and lipid metabolism and energy.20 Leptin is a 16-kDa polypeptide hormone coded by the obese (ob) gene.21 In addition to regulating energy homeostasis, leptin is also a vital hormone/cytokine for a number of diverse physiological processes such as reproduction, angio-genesis, inflammation, and immune function.22 The leptin receptor (Ob-R, also known as LEP-R, LR) was first isolated from mouse choroid plexus by expression cloning and is a member of the interleukin-6 receptor family of class I cytokine receptors.23 Ob-Rb is the long form of the leptin receptor and has a long cytoplasmic region containing several motifs required for signal transduction.24 Leptin binding to this receptor induces conformational changes in the intracellular receptor domain.25 Many studies have shown that dietary intake and fat stores regulate the production of leptin, which then enters the circulatory system and binds to leptin receptor in multiple tissues, modulating numerous other physiological processes, including body temperature, energy regulation, immune reaction, reproduction and development.26,27 Leptin and its receptor have also been shown to express in ovary and testis,28,29 as well as in several other tissues of the reproductive tract.30
Our previous study has shown that c-kit and proliferating cell nuclear antigen (PCNA) had higher expressions in the pre-hibernation oviduct, which suggested that as the intrinsic regulator including the c-kit receptor might play a regulatory role in oviductal cell proliferation.31 In order to elucidate the relationship between leptin and oviductal hypertrophy during prehibernation, this study investigated the expression of PPARγ2, leptin and leptin receptor proteins in the oviduct of Rana dybowskii during the breeding and pre-hibernation periods.
Materials and Methods
Animals
Totally, 40 adult female Chinese brown frogs were obtained in April (n=20), and October (n=20), 2012 from Jilin Paektu Mountain Chinese Brown Frog Breeding Farm, Jilin Province (125°40 E-127°56 E, 42°31 N-44°40 N), China. All these animals were treated in accordance with the National Animal Welfare Legislation. All experimental procedures were carried out in accordance with the guidelines established by the Beijing Forestry University. The animals were euthanized by 4% isoflurane before tissue removal. The weight of each female frog was measured using scales. Each pair of oviducts was obtained from each body, and the weight of oviduct was measured after necropsy. One side of oviduct was immediately fixed for 24 h in 4% paraformaldehyde (Sigma Chemical Co., St. Louis, MO, USA) in 0.05 M PBS, pH 7.4 for histological and immunohistochemical observations; the other side of oviduct was immediately frozen and stored at -20°C for Western blotting detection.
Histology
The oviduct samples were dehydrated in ethanol series and embedded in paraffin wax. Serial sections (4 µm) were mounted on slides coated with poly-L-lysine (Sigma). Sections were stained with hematoxylin-eosin (HE) for observations of general histology.
Immunohistochemistry
The serial sections of the oviduct tissues were incubated with 10% normal goat serum to reduce background staining caused by the second antibody. The sections were then incubated with primary polyclonal antibody (1:500) against leptin (Y-20) (Santa Cruz Biotechno logy, Santa Cruz, CA, USA), leptin receptor (H-300) (Santa Cruz Biotechnology) and PPARγ2 (bs-7114R) (Beijing Biosynthesis Biotechno logy Co., Beijing, China) for 12 h at 4°C. The control sections were treated with normal rabbit serum (Sigma) instead of the primary antisera. The sections were then incubated with a second antibody for 0.5 h at room temperature, goat anti-rabbit lgG conjugated with biotin and peroxidase with avidin, The sections were visualized using a rabbit ExtrAvidin™ staining kit (Sigma) in 150 mL of 0.05 M Tris-HCl buffer, pH 7.6 containing 30 mg 3, 3-diaminobenzidine (Wako, Tokyo, Japan) plus 30 μL H2O2. Finally, the reacted sections for leptin and leptin receptor were counterstained with hematoxylin solution (Merck, Tokyo, Japan).
Western blot
The oviducts were weighed and diced into small pieces using a sterilized razor blade, respectively. The tissue was homogenized in a homogenizer containing 300 µL of 10 mg/mL PMSF stock and incubated on ice for 30 min while maintaining the temperature at 4°C throughout all the procedures. Homogenates were centrifuged at 12,000 x g for 10 min at 4°C. Protein extracts (25 g) were mixed with an equal volume of 2× Laemmli sample buffer. Equal amounts of each sample were loaded and run on an 8% and 13% SDS-PAGE gel at 18V/cm and transferred to nitrocellulose membranes using a wet transblotting apparatus (Bio-Rad, Richmond, CA, USA). The concentration of SDS used for protein gel and protein sample buffer was 10%. The membranes were blocked in 3% BSA for 1 h at room temperature. Primary incubation of the membranes was carried out using a 1:500 dilution of rabbit anti-rat leptin antibody, rabbit anti-rat leptin receptor and rabbit anti-rat PPARγ2 for 1 h. Secondary incubation of the membrane was then carried out using a 1:1000 dilution of goat anti-rabbit IgG tagged with horseradish peroxidase for 1 h. Finally, the membrane was colored with 10 mg 3,3-diaminobenzidine (Wako) solution in 50 mL phosphate buffer (0.03 M) plus 3 µL H2O2. -actin was used for the endogenous control. Preabsorptions of the antibodies were performed with an excess of relative antigens (Sigma) for the negative control.
Statistical analysis
Statistical comparisons were made with the Students t-test. A value of P<0.01 was considered indication of statistical significance.
Results
Seasonal changes in oviductal morphology and histology between breeding period and pre-hibernation
Morphological differences were observed in regard to the oviduct of Rana dybowskii during breeding period and pre-hibernation (Figure 1 A-D). The weight of the oviduct in pre-hibernation (3.37±0.14) was significantly larger than that of the breeding period (0.34±0.02) (Figure 1E). Similar differences between pre-hibernation (2.49±0.01) and breeding period (0.61±0.09) were also seen for relative circumference values (Figure 1F). The HE staining also revealed a significant histological variance. As shown in Figure 1 G,H, the oviduct of Rana dybowskii mainly comprises epithelial cells and lobules that consisted of stromal cells.
Figure 1.
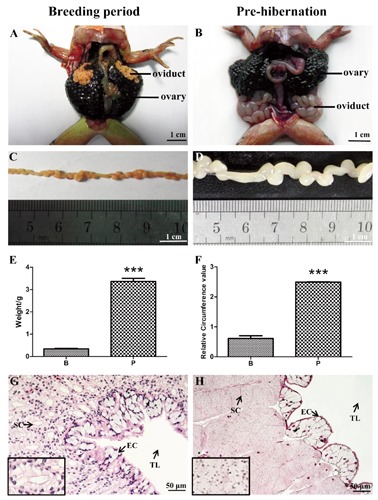
Seasonal changes in oviductal morphology of Rana dybowskii., Topography and morphology of the oviduct during the breeding period (A,C) and pre-hibernation (B,D). Changes in weight (E) and relative circumference (F) of oviduct between the breeding period and pre-hibernation. Histological structure of the oviducts in the breeding period (G) and pre-hibernation (H). EC, epithelial cell; SC, stromal cell; TL, tubule lumen.
Immunohistochemical localization of PPARγ2, leptin and leptin receptor in Rana dybowskii oviduct during breeding period and pre-hibernation
PPARγ2, leptin and leptin receptor were detected in oviduct of Rana dybowskii during breeding period and pre-hibernation (Figure 2). Positive signal of PPARγ2 was localized in the nucleoli in both epithelial and stromal cells of breeding period and pre-hibernation (Figure 2 A,B). Immunohisto chemical reaction for leptin was observed in the cytoplasm of epithelial cells of breeding period and pre-hibernation (Figure 2 C,D), whereas leptin receptor was immunostained only in stromal cells (Figure 2 E,F). The intensities of the immunohistochemical signals for PPARγ2, leptin and leptin receptor were all higher in the pre-hibernation than those in the breeding period (Table 1).
Figure 2.
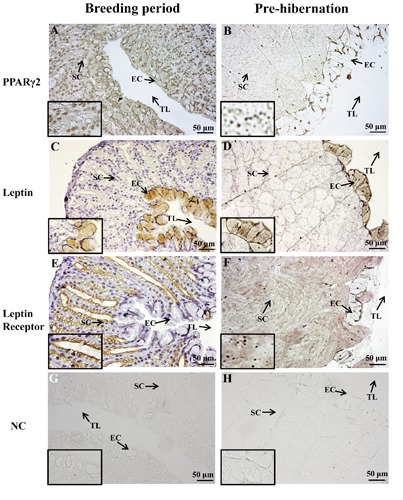
Immunohistochemical localization of PPARγ2, leptin and leptin receptor inRana dybowskii, oviduct during breeding period and pre-hibernation. Positive signal of PPARγ2 was localized in the nucleoli of both epithelial and stromal cells in breeding period and pre-hibernation (A,B), and stronger positive-staining was shown in pre-hibernation (B). Immunohistochemical reaction for leptin was observed in the cytoplasm of epithelial cells of breeding period and pre-hibernation (C,D), whereas leptin receptor was immunostained only in stromal cells (E,F). G,H) Negative controls. EC, epithelial cell; SC, stromal cell; TL, tubule lumen; NC, negative control.
Table 1.
Relative abundance of PPARγ 2, leptin and leptin receptor in Rana dybowskii oviduct during breeding period and pre-hibernation.
| PPARγ2 | Leptin | Leptin receptor | ||||
|---|---|---|---|---|---|---|
| B | P | B | P | B | P | |
| Epithelial cell | + | + | ++ | ++/+ | - | - |
| Stromal cell | ++ | ++/+ | - | - | ++ | +++ |
B, Breeding period; P, pre-hibernation. The immunohistochemical staining was determined as positive (+), strong positive (++), very strong positive (+++), and negative (-). Staining that was weak but higher than control was set as positive (+); the highest intensity staining was set as very strong positive (+++); staining intensity between + and +++ was set as strong positive (++).
Expression level of PPARγ2, leptin and leptin receptor proteins in Rana dybowskii oviduct of the breeding period and pre-hibernation
The results of Western blot analysis for PPARγ2, leptin and leptin receptor in Rana dybowskii oviduct of the breeding period and pre-hibernation are shown in Figure 3. Bands of approximately 52kDa for PPARγ2 (Figure 3A), 16kDa for leptin (Figure 3B) and 132kDa for leptin receptor (Figure 3C) were identified in lysates, in consistent with previously reported in rat.12,32 The results were normalized to the expression level of ß-actin. The expression of PPARγ2, leptin and leptin receptor was significantly high in pre-hibernation as compared to the breeding period. Protein extracted from the fat tissue of rat was used for the positive control (Figure 3, lane PC). The primary antibodies preabsorbed with an excess amount of the antigens were used for the negative control (Figure 3, lane NC).
Figure 3.
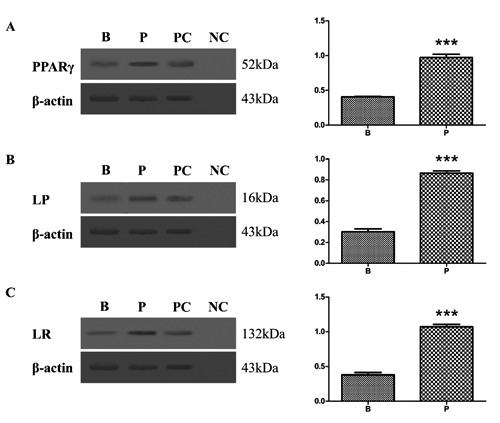
Western blot of of PPAR 2, leptin and leptin receptor in the oviduct tissues of Rana dybowskii, during breeding period (B) and pre-hibernation (P). Positive bands of PPAR 2, leptin and leptin receptor were observed in the position of about 52kDa (A), 16kDa (B) and 132kDa (C) respectively. ß-actin was used as controls to correct for loading in each lane. PC, positive control; NC, negative control. The expression levels were determined by densitometric analysis. Bars represent means ±SD for three independent experiments; asterisks indicate significant difference (P<0.01).
Discussion
The present study indicated for the first time that PPARγ2, leptin and its receptor proteins were expressed in oviduct of Rana dybowskii and revealed different expression patterns of these proteins during breeding period and pre-hibernation. Moreover, leptin was immunolocalized in epithelial cells of oviduct in both periods, whereas leptin receptor was immunostained only in stromal cells. These findings suggested that leptin was produced in oviduct of Rana dybowskii and may play an important paracrine regulatory role in oviductal hypertrophy during prehibernation. The present results provided a basis for future investigation of other regulatory factors in oviductal hypertrophy of Rana dybowskii.
Ovarian estrogens control development of sex accessory structures such as the hypertrophy of oviduct prior to sexual maturation and during each season prior to ovulation.33 The oviducts regress when estrogen synthesis declines after breeding.33 In Rana dybowskii, the oviductal hypertrophy took place during prehibernation, instead of the breeding period. Histological observation showed that epithelial cells and a large number of stromal cells were observed in the expanded oviductal tissues of pre-hibernation. Moreover, significantly higher values of oviductal weight and size (relative circumference value) were also found in pre-hibernation when compared to those of the breeding period. These findings implied that the hypertrophy of oviduct was independent of ovarian steroid hormones, which might be speculated that locally produced intrinsic regulators might play a regulatory role in the oviductal hypertrophy of Rana dybowskii. PPAR is a ligand-dependent transcription factor that is predominantly expressed in adipose tissue, both white and brown.34 PPAR plays an important promoting role in the differentiation of adipocytes.35 Recent reports have showed that PPAR was also involved in processes that were critical to normal female reproductive function, for example, PPAR was expressed strongly in the granulosa cells of rat, mouse, and sheep, as well as in oocytes from cattle, zebrafish, Xenopus, and human,36 indicating that PPAR may be an important player regulating ovarian gene expression. Since PPAR 1 distributed widely in many tissues, and only PPARγ2 can express in fat,17,18 we focus on PPARγ2 to investigate oviductal hypertrophy. In the present study, PPARγ2 was immunolocalized in stromal and epithelial cells of oviduct during the breeding period and pre-hibernation. The expression level of PPARγ2 protein was significantly higher in pre-hibernation as compared to the breeding period. In addition, our previous studies have shown that higher contents of c-kit receptor and PCNA proteins were present in pre-hibernation oviductal tissues.31 These results implied that as an intrinsic regulator, PPARγ2 might play a regulative role in oviductal hypertrophy of Rana dybowskii during prehibernation.
Previous studies showed that changes in the level of PPAR expression could cause or contribute to the regulation of leptin expression, or that both genes are responding to a common pathway or transacting factor(s).12 More recently, leptin has been shown to play a role in other target reproductive organs, such as the endometrium, placenta, and mammary gland, with corresponding influences on important physiologic processes such as menstruation, pregnancy, and lactation.37 In this study, leptin was immunolocalized in epithelial cells of oviducts during the breeding period and pre-hibernation, whereas leptin receptor was observed only in stromal cells. The presence of leptin and leptin receptor proteins were further confirmed by Western blot, suggesting in situ synthesis and secretion of leptin and leptin receptor in this organ, as well as the possibility of a paracrine regulatory role of leptin in the Rana dybowskii oviducts. These findings are not limited to this amphibian though, as similar observations have also been reported in other species. By using an in vitro model, leptin was produced in the porcine oviduct, making it spatially available to interact with its receptor during pre-implantation development.38 In mice, leptin is expressed in both the oviduct and uterus during early pregnancy, and is thus temporally and spatially available for early embryo development.39,40 Moreover, in the human oviduct, expression of leptin and its receptor has been detected by in situ hybridization and immunohistochemistry, suggesting that leptin could act in a paracrine manner to regulate biological functions of the oviduct.41 Studies in domestic hen also suggested that oviduct may be a target tissue for leptin, where it may participate in egg formation and/or its transport through the oviduct.42 Taken together, the present results were in accordance with the views that leptin may be produced by maternal organs such as oviduct and/or these embryos themselves, and act in a paracrine manner to regulate biological functions.39
Leptin can not only act directly on adipose tissue to increase preadipocyte proliferation,43,44 but also induce an increase in body weight and the relative weights of the liver, spleen, pancreas, kidneys, and small intestine without any changes in triglycerides, glucose and cholesterol levels.45,46 Previous studies have shown that oviduct and endometrial epithelium may produce and secrete leptin to the reproductive tract, and leptin can promote the development of mouse oviduct and pre-implantation embryos through its receptor.39,40 This study revealed that the mass change of oviducts and oviductal histological appearance were parallel to those in the expression patterns of oviductal leptin and its receptor proteins during the breeding period and pre-hibernation, implied that leptin might play a regulatory role in oviductal hypertrophy during prehibernation. Similar results were obtained in pigs and mice, the up-regulation of leptin and its receptor expression in the oviduct during early pregnancy compared to non-pregnant pigs and mice suggests that leptin expression was regulated in stage specific manner.38,39 In humans, Leptin signaling appeared to be essential for endometrial cell adhesion, proliferation, survival and migration,47,48 enhanced the proliferative activity of both the normal myometrium and myoma cells in primary culture.49 In addition, the in vitro effects of leptin on the rabbit oviduct suggested that the oviduct could be a potential target for endocrine regulation by leptin, and leptin was involved in a large array of regulatory actions required for normal reproductive functions.50 Thus, these observations suggested that given the wide distribution of leptin and its receptor in different sites of the reproductive organs, leptin may act as the critical link between adipose tissue and the reproductive system, indicating whether adequate energy reserves are present for normal reproductive function.37 The present findings provided further evidence in support of these views.
This study demonstrated, for the first time, the seasonal expression of PPARγ2, leptin and leptin receptor proteins in oviductal tissues of Rana dybowskii during the breeding period and pre-hibernation. The dynamic regulation of PPARγ2 suggested that active cell differentiation might take place in the pre-hibernation oviduct. Furthermore, the distinct localization and immunoreactivity of leptin and leptin receptor indicate that oviduct might be the target organ of leptin, which possibly play an important paracrine role in regulating the oviductal hypertrophy of Rana dybowskii during prehibernation.
Funding Statement
Fundings: this study is supported by a Grant-in-Aid from National Natural Science Foundation of China (NSFC, No. J1103516) and Beijing Natural Science Foundation (8142029).
References
- 1.Hunter RH.Have the Fallopian tubes a vital role in promoting fertility? Acta Obstet Gynecol Scand 1998;77:475-86 [PubMed] [Google Scholar]
- 2.Fields MJ, Shemesh M.Extragonadal luteinizing hormone receptors in the reproductive tract of domestic animals. Biol Reprod 2004;71:1412-8 [DOI] [PubMed] [Google Scholar]
- 3.Lam PM, Briton-Jones C, Cheung CK, Lok IH, Yuen PM, Cheung LP, et al. Vascular endothelial growth factor in the human oviduct: localization and regulation of messenger RNA expression in vivo. Biol Reprod 2003;68:1870-6 [DOI] [PubMed] [Google Scholar]
- 4.Gabler C, Killian GJ, Einspanier R.Differential expression of extracellular matrix components in the bovine oviduct during the oestrous cycle. Reproduction 2001;122:121-30 [PubMed] [Google Scholar]
- 5.Hunter RH.Fallopian tube fluid: the physiological medium for fertilization and early embryonic development. Hunter RH. (ed.) The Fallopian tubes: their role in fertility and infertility. Berlin, Germany, Springer: 1988 [Google Scholar]
- 6.Buhi WC, Alvarez IM, Kouba AJ.Secreted proteins of the oviduct. Cells Tissues Organs 2000;166:165-79 [DOI] [PubMed] [Google Scholar]
- 7.Leese HJ, Tay JI, Reischl J, Downing SJ.Formation of Fallopian tubal fluid: role of a neglected epithelium. Reproduction 2001;121:339-46 [DOI] [PubMed] [Google Scholar]
- 8.Zhang Z, Zhang B, Nie X, Liu Q, Xie F, Shang D.Transcriptome analysis and identification of genes related to immune function in skin of the Chinese brown frog. Zoolog Sci 2009;26:80-6 [DOI] [PubMed] [Google Scholar]
- 9.The Pharmacopoeia Commission of PRC. Pharmacopoeia of the People’s Republic of China (English Edition 2000). Beijing: People’s Medical Publishing House Co., Ltd.: 2000 [Google Scholar]
- 10.Xuegan Y, Yiquan W, Kaiya Z, Zhongquan L.Authentication of oviductus ranae and its original animals using molecular marker. Biol Pharm Bull 2002;25:1035-9 [DOI] [PubMed] [Google Scholar]
- 11.Zhang M, Li Y, Yao B, Sun M, Wang Z, Zhao Y.Transcriptome sequencing and de novo analysis for Oviductus Ranae of Rana chensinensis using illumina RNA-Seq technology. J Genet Genomics 2013;40: 137-40 [DOI] [PubMed] [Google Scholar]
- 12.Vidal-Puig A, Jimenez-Liñan M, Lowell BB, Hamann A, Hu E, Spiegelman B, et al. Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J Clin Invest 1996;97:2553-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tontonoz P, Hu E, Spiegelman BM.Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 1994;79:1147-56 [DOI] [PubMed] [Google Scholar]
- 14.Hu E, Tontonoz P, Spiegelman BM.Transdifferentiation of myoblasts by the adipogenic transcription factors PPAR gamma and C/EBP alpha. Proc Natl Acad Sci USA 1995;92:9856-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desvergne B, Wahli W.Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev 1999;20:649-88 [DOI] [PubMed] [Google Scholar]
- 16.Kintscher U, Law RE.PPARgamma-mediated insulin sensitization: the importance of fat versus muscle. Am J Physiol Endocrinol Metab 2005;288:E287-91 [DOI] [PubMed] [Google Scholar]
- 17.Tontonoz P, Graves RA, Budavari AI, Erdjument-Bromage H, Lui M, Hu E, et al. Adipocyte-specific transcription factor ARF6 is a heterodimeric complex of two nuclear hormone receptors, PPAR gamma and RXR alpha. Nucleic Acids Res 1994; 22:5628-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L, Jiang J, Wang L, Zhong T, Chen B, Zhan S, et al. Expression patterns of peroxisome proliferator-activated receptor gamma 1 versus gamma 2, and their association with intramuscular fat in goat tissues. Gene 2013;528:195-200 [DOI] [PubMed] [Google Scholar]
- 19.Frühbeck G, Gómez-Ambrosi J, Muruzábal FJ, Burrell MA.The adipocyte: a model for integration of endocrine and metabolic signaling in energy metabolism regulation. Am J Physiol Endocrinol Metab 2001;280:E827-47 [DOI] [PubMed] [Google Scholar]
- 20.Ahima RS.Central actions of adipocyte hormones. Trends Endocrinol Metab 2005; 16:307-13 [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM.Positional cloning of the mouse obese gene and its human homologue. Nature 1994;372:425-32 [DOI] [PubMed] [Google Scholar]
- 22.Trayhurn P, Wood IS.Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 2004;92:347-55 [DOI] [PubMed] [Google Scholar]
- 23.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell 1995;83:1263-71 [DOI] [PubMed] [Google Scholar]
- 24.Tartaglia LA.The leptin receptor. J Biol Chem 1997;272:6093-6 [DOI] [PubMed] [Google Scholar]
- 25.Waelput W, Verhee A, Broekaert D, Eyckerman S, Vandekerckhove J, Beattie JH, et al. Identification and expression analysis of leptin-regulated immediate early response and late target genes. Biochem J. 2000;348Pt 1:55-61 [PMC free article] [PubMed] [Google Scholar]
- 26.Donato J, Jr, Cravo RM, Frazão R, Elias CF.Hypothalamic sites of leptin action linking metabolism and reproduction. Neuroendo -crinology 2011;93:9-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedman JM, Halaas JL.Leptin and the regulation of body weight in mammals. Nature 1998;395:763-70 [DOI] [PubMed] [Google Scholar]
- 28.Archanco M, Muruzábal FJ, Llopiz D, Garayoa M, Gómez-Ambrosi J, Frühbeck G, et al. Leptin expression in the rat ovary depends on estrous cycle. J Histochem Cytochem 2003;51:1269-77 [DOI] [PubMed] [Google Scholar]
- 29.Tena-Sempere M, Pinilla L, González LC, Diéguez C, Casanueva FF, Aguilar E.Leptin inhibits testosterone secretion from adult rat testis in vitro. J Endocrinol 1999;161: 211-8 [DOI] [PubMed] [Google Scholar]
- 30.Löffler S, Aust G, Köhler U, Spanel-Borowski K.Evidence of leptin expression in normal and polycystic human ovaries. Mol Hum Reprod 2001;7:1143-9 [DOI] [PubMed] [Google Scholar]
- 31.Shen Y, Liu Y, Ma J, Ma X, Tian Y, Zhang H, et al. Immunoreactivity of c-kit receptor protein during the prehibernation period in the oviduct of the Chinese brown frog, Rana chensinensis. J Vet Med Sci 2012;74: 209-13 [DOI] [PubMed] [Google Scholar]
- 32.Di Yorio MP, Bilbao MG, Pustovrh MC, Prestifilippo JP, Faletti AG.Leptin modulates the expression of its receptors in the hypothalamic-pituitary-ovarian axis in a differential way. J Endocrinol 2008;198: 355-66 [DOI] [PubMed] [Google Scholar]
- 33.David ON, James AC.Comparative aspects of vertebrate reproduction. David ON. () Vertebrate endocrinology, 4th ed. Academic Press: 2007 [Google Scholar]
- 34.Kliewer SA, Forman BM, Blumberg B, Ong ES, Borgmeyer U, Mangelsdorf DJ, et al. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc Natl Acad Sci USA 1994;91:7355-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu Y, Qi C, Korenberg JR, Chen XN, Noya D, Rao MS, et al. Structural organization of mouse peroxisome proliferator-activated receptor gamma (mPPAR gamma) gene: alternative promoter use and different splicing yield two mPPAR gamma iso-forms. Proc Natl Acad Sci USA 1995;92:7921-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dupont J, Reverchon M, Cloix L, Froment P, Ramé C.Involvement of adipokines, AMPK, PI3K and the PPAR signaling pathways in ovarian follicle development and cancer. Int J Dev Biol 2012;56:959-67 [DOI] [PubMed] [Google Scholar]
- 37.Moschos S, Chan JL, Mantzoros CS.Leptin and reproduction: a review. Fertil Steril 2002;77:433-44 [DOI] [PubMed] [Google Scholar]
- 38.Craig JA, Zhu H, Dyce PW, Wen L, Li J.Leptin enhances porcine preimplantation embryo development in vitro. Mol Cell Endocrinol 2005;229:141-7 [DOI] [PubMed] [Google Scholar]
- 39.Kawamura K, Sato N, Fukuda J, Kodama H, Kumagai J, Tanikawa H, et al. Leptin promotes the development of mouse preim-plantation embryos in vitro. Endocrinology 2002;143:1922-31 [DOI] [PubMed] [Google Scholar]
- 40.Kawamura K, Sato N, Fukuda J, Kodama H, Kumagai J, Tanikawa H, et al. The role of leptin during the development of mouse preimplantation embryos. Mol Cell Endocrinol 2003;202:185-9 [DOI] [PubMed] [Google Scholar]
- 41.Liu LL, Qiao J, Wang YZ, Chen YJ, Gao YQ. [Expression of leptin and leptin receptor system in woman reproductive organs].[Article in Chinese] Zhonghua Yi Xue Za Zhi 2003;83:666-8 [PubMed] [Google Scholar]
- 42.Grzegorzewska AK, Paczoska-Eliasiewicz HE, Rzasa J.MRNA expression and immunocytochemical localization of leptin receptor in the oviduct of the laying hen (Gallus domesticus). Folia Biol (Krakow) 2008;56:179-85 [DOI] [PubMed] [Google Scholar]
- 43.Wagoner B, Hausman DB, Harris RB.Direct and indirect effects of leptin on preadipocyte proliferation and differentiation. Am J Physiol Regul Integr Comp Physiol 2006;290:R1557-64 [DOI] [PubMed] [Google Scholar]
- 44.Ramsay TG.Porcine preadipocyte proliferation and differentiation: a role for leptin? J Anim Sci 2005;83:2066-74 [DOI] [PubMed] [Google Scholar]
- 45.Attig L, Brisard D, Larcher T, Mickiewicz M, Guilloteau P, Boukthir S, et al. Postnatal leptin promotes organ maturation and development in IUGR piglets. PLoS One 2013;8(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Attig L, Brisard D, Larcher T, Mickiewicz M, Guilloteau P, Boukthir S, et al. Correction: postnatal leptin promotes organ maturation and development in IUGR piglets. PLoS One 2013;8(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carino C, Olawaiye AB, Cherfils S, Serikawa T, Lynch MP, Rueda BR, et al. Leptin regulation of proangiogenic molecules in benign and cancerous endometrial cells. Int J Cancer 2008;123:2782-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanaka T, Umesaki N.Leptin regulates the proliferation and apoptosis of human endometrial epithelial cells. Int J Mol Med 2008;22:683-9 [PubMed] [Google Scholar]
- 49.Markowska A, Belloni AS, Rucinski M, Parenti AR, Nardelli GB, Drews K, et al. Leptin and leptin receptor expression in the myometrium and uterine myomas: is leptin involved in tumor development? Int J Oncol 2005;27:1505-9 [PubMed] [Google Scholar]
- 50.Zerani M, Boiti C, Dall’Aglio C, Pascucci L, Maranesi M, Brecchia G, et al. Leptin receptor expression and in vitro leptin actions on prostaglandin release and nitric oxide synthase activity in the rabbit oviduct. J Endocrinol 2005;185:319-25 [DOI] [PubMed] [Google Scholar]


