Abstract
Osteoarthritis is a degenerative joint disease, which affects millions of people around the world. It occurs when the protective cartilage at the end of bones wears over time, leading to loss of flexibility of the joint, pain and stiffness. The cause of osteoarthritis is unknown, but its development is associated with different factors, such as metabolic, genetic, mechanical and inflammatory ones. In recent years the biological role of chitinases has been studied in relation to different inflammatory diseases and more in particular the elevated levels of human cartilage glycoprotein 39 (CHI3L1) and chitotriosidase (CHIT1) have been reported in a variety of diseases including chronic inflammation and degenerative disorders. The aim of this study was to investigate, by immunohistochemistry, the distribution of CHI3L1 and CHIT1 in osteoarthritic and normal rat articular cartilage, to discover their potential role in the development of this disease. The hypothesis was that the expression of chitinases could increase in OA disease. Immunohistochemical analysis showed that CHI3L1 and CHIT1 staining was very strong in osteoarthritic cartilage, especially in the superficial areas of the cartilage most exposed to mechanical load, while it was weak or absent in normal cartilage. These findings suggest that these two chitinases could be functionally associated with the development of osteoarthritis and could be used as markers, so in the future they could have a role in the daily clinical practice to stage the severity of the disease. However, the longer-term in vivoand in vitro studies are needed to understand the exact mechanism of these molecules, their receptors and activities on cartilage tissue.
Key words: CHI3L1, chitotriosidase, osteoarthritis, ACLT, immunohistochemistry
Introduction
Osteoarthritis (OA) also known as degenerative arthritis or degenerative joint disease or osteoarthrosis is the most common form of arthritis, affecting millions of people around the world. It affects about 8 million people in the United Kingdom and nearly 27 million people in the United States.1 Often called wear-and-tear arthritis, OA occurs when the protective cartilage at the ends of bones wears down over time.2 Although OA can damage any joint in the body, the disorder most commonly affects joints in the hands, neck, lower back, knees and hips. OA is a group of mechanical abnormalities involving degradation of joints including not only the articular cartilage but also the subchondral bone.3 OA gradually worsens with time, and no cure exists. Treatments can slow the progression of the disease, relieve pain and improve joint function.4 Symptoms of OA include loss of flexibility, limited movement, pain and swelling within the joint.5 The condition results from injury to the cartilage, which normally absorbs stress and covers the bones, so they can move smoothly. A variety of causes, hereditary, developmental, metabolic, and mechanical deficits, may initiate processes leading to loss of cartilage. When bone surfaces become less protected by cartilage, bone may be exposed and damaged. As a result of decreased movement secondary to pain, regional muscles may atrophy, and ligaments may become more lax. The main symptom is pain, causing loss of ability and often stiffness. Pain is generally described as a sharp ache, or a burning sensation in the associate muscles and tendons. Treatment generally involves a combination of exercise, lifestyle modification, and analgesics;5 if pain becomes debilitating, joint replacement surgery may be used to improve the quality of life.6 For most people, the cause of OA is unknown, but metabolic, genetic, chemical, inflammation and mechanical factors play a role in its development.7 For this reason, in the present study, we investigated, in an in vivo osteoarthritic rat model, the human cartilage glycoprotein 39 (GP-39,YKL-40), also known as CHI3L1 and the chitotriosidase (CHIT1). Mammalian chitinases belong to the glycohydrolase family 18, which have evolved to hydrolyze chitin, a polymer of N-acetylglucosamine.8,9 The family of chitinases includes members both with and without glycohydrolase enzymatic activity against chitin. CHIT1 is a true chitinase possessing chitinolytic (glycohydrolase) activities.10 In contrast, chitinase-like-lectins (Chi-lectins) or chitinase-like proteins (C/CLPs), including chitinase 3-like-1 (CHI3L1, YKL40, HC-gp39), show enzymatic activity despite the retention and conservation of the substrate-binding cleft of the chitinases.11 For the majority of the mammalian chitinases important biological roles in chronic inflammatory diseases have been identified.12-14 So far, CHIT1 is the best-characterized true chitinase from a clinical and biological perspective. Elevated levels have been reported in a variety of diseases including infections, chronic inflammation and degenerative disorders.15,16 The sources of secreted CHIT1 are abnormal lipid-laden macrophages formed in tissues of patients with Gaucher’s disease.17 This molecule correlated strongly with disease symptoms and is used to monitor the efficacy of therapy.18 Recently, it was hypothesized that cellular alteration in Gaucher’s disease produced a proinflammatory milieu leading to bone destruction through enhancement of monocyte differentiation to osteoclasts and improvement of osteoclasts resorption activity.19 To confirm this data it was demonstrated that the chitinases, CHIT1 and CH3L1, are closely related to the process of osteoclastogenesis and the digestion of bone matrix via MMP9.20 Despite various theories having been proposed to explain the disruption of bone homeostatic balance in Gaucher’s disease, implying dysfunction of osteoclasts, osteoblasts and mesenchymal cells,21-23 to date the effect of CHIT1 remains nearly unexplored. Only one study has shown that, in periprosthetic soft tissue from patients with osteolysis the expression of alternative macrophage activation markers (CHIT1, CCL18) was increased in comparison to OA controls.24 Interestingly this finding indicated that the activation of alternative macrophage is involved in osteolysis and suggested a correlation between CHIT1 and osteolytic lesions.22 In contrast to CHIT1, some evidence reports increased levels of CHI3L1 protein and/or mRNA in patients with a wide spectrum of pathologies.25 The CHI3L1 is a glycoprotein secreted by articular chondrocytes, synoviocytes and macrophages. Serum and synovial fluid CHI3L1 levels are elevated in inflammatory diseases and correlate with the degree of joint destruction in rheumatoid arthritis. CHI3L1 is a candidate auto antigen in rheumatoid arthritis and is important in the capacity of cells to respond to and cope with changes in their environment.26 Recently Einarsson and coauthors stated that chondrocytes of human osteoarthritic cartilage secrete the inflammation associated chitolectin CHI3L1.27 CHI3L1, is a major secretory protein of human chondrocytes in cell culture. CHI3L1 mRNA is expressed by cartilage from patients with rheumatoid arthritis, but is not detectable in normal human cartilage.28 Moreover, it was observed that in patients with myeloma elevated serum concentrations of CHI3L1 aggravated bone destruction and were associated with an increase of bone resorption activity hastening the progression of bone disease.29 The aim of this study was to investigate, by immunohisto-chemistry the distribution of CHI3L1 and CHIT1 in osteoarthritic (n=20) and normal (n=10) rat articular cartilage, collected from femoral condyles after anterior cruciate ligament transection (ACLT), to discover a potential role for CHI3L1 and CHIT1 in osteoarthritic cartilage and to improve a new possible therapeutic concept for treating inflammatory joint diseases. The hypothesis is that the expression of chitinases could increase in OA disease.
Materials and Methods
Breeding and housing of animals
In our study, we used thirty 6-months-old healthy male Sprague Dawley rats (Charles River Laboratories, Milan, Italy), with an average body weight of 200±40 g. Rats were individually housed in polycarbonate cages (cage dimensions: 10.25”W x 18.75”D x 8”H) during the entire period of the study and were housed at controlled temperature (20-23°C) and humidity, with free access to water and food and photoperiod of 12 hours light/dark. All surgical procedures for anterior cruciate ligament transection were performed in accordance with the method previously described.30,31 The ACLT procedure was made under total anesthesia, 30 mg/Kg Zoletil 100 + altadol 5 mg/kg + maintenance mixture of O2 and isoflurane 2-2,5%, (Vibrac, Milan, Italy). Postoperatively, the animals were permitted free cage activity without joint immobilization and treated with the administration of an antibiotic, Convenia® 0.1 mL/kg, (Vibrac). The 30 animals were distributed in two different groups: 10 rats for control group without ACLT, and 20 rats for OA group with ACLT. The control group consists of two subgroups: control normal group (5 rats) without surgical treatment and sham-operated control group (5 rats), rats that have received exactly the same surgical procedure as the experimental group, without ACLT. The OA group instead was made up of 20 rats with surgical treatment submitted to ACLT to induce OA model. During the experiment the possible suffering of the animals was evaluated through the clinical appearance of the animal (fur, weight, lameness, consumption of food and water) and their possible elimination from the trial was evaluated once a day. The animals from all groups at 3 months after the surgical procedures were sacrificed by intracardial Pentothal® injection 30-40 mg/kg (Biochemie, Kundl, Austria); under Furane 2%®-narcosis (Abbott Lab., Maidenhead, UK). Both femurs were explanted, cleaned of soft tissues and the samples were used to perform histomorphometric evaluations. Cartilage tissue were used to perform histological and immunohistochemical analyses. All procedures conformed to the guidelines of the Institutional Animal Care and Use Committee of the University of Catania. The experiments were conducted in accordance with the European Community Council Directive (86/609/EEC) and the Italian Animal Protection Law (116/1992).
Histomorphometric analysis
Femurs were explanted and cleaned of soft tissues as previously described.32 Histomor-phometric analysis was performed on the total number of rats used and specifically on both medial and lateral femoral condyles from all groups (untreated and treated animals). Histomorphometry was performed with image analysis, Kontron KS 300 software (Kontron Electronics, Eching bei Munchen, Germany). Two blinded investigators (2 anatomical morphologists) made the analyses. We assumed that the evaluations were correct if there were no statistically different values between the investigators. Fifteen fields randomly selected from each section were analyzed. The semi-quantitative histological grading criteria of Kraus’ modified Mankin score33,34 and histopathology OARSI system35 were used.
Histology
Samples were fixed in 10% neutral buffered-formalin (Bio-Optica, Milan, Italy), following overnight washing and routinely embedded in paraffin as previously described.36 After wax infiltration the tissue samples were orientated in the cassettes in the same direction. Sections 4-5 µm thick were cut from paraffin blocks using a rotary manual microtome (Leica RM2235, Milan, Italy) and mounted on silane-coated slides (Menzel-Gläser, Braunschweig, Germany) and stored at room temperature. Slides were dewaxed in xylene, hydrated using graded ethanol, and stained as previously described37 for routine histological evaluation by hematoxylin and eosin (H&E) staining for the morphological structure. The sections were examined with a Zeiss Axioplan light microscope (Carl Zeiss, Oberkochen, Germany) and photographed with a digital camera (AxioCam MRc5, Carl Zeiss).
Immunohistochemistry
For immunohistochemical analysis slides were processed as previously described.38 Briefly, the slides were dewaxed in xylene, hydrated using graded ethanol and were incubated for 30 min in 0.3% H2O2/methanol to quench endogenous peroxidase activity and then rinsed for 20 min with phosphate-buffered saline (PBS; Bio-Optica). The sections were heated (5 min x 3) in capped polypropylene slide-holders with citrate buffer (10 mM citric acid, 0.05% Tween 20, pH 6.0; Bio-Optica), using a microwave oven (750 W) to unmask antigenic sites. The blocking step was performed before application of the primary antibody with 5% bovine serum albumin (BSA, Sigma-Aldrich, Milan, Italy) in PBS for 1 h in a moist chamber. BSA was used as a blocking agent to prevent non-specific binding of the antibody. Following blocking, the sections were incubated overnight at 4°C with goat polyclonal GP-39 antibody (CHI3L1), work dilution in PBS 1:100 (sc-30465, Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and rabbit polyclonal Chitotriosidase (CHIT1) antibody, work dilution in PBS 1:100 (sc-99033, Santa Cruz Biotechnology, Inc.). Immune complexes were then treated with a biotinylated link antibody (HRP-conjugated anti-goat and anti-rabbit were used as secondary antibodies) and then detected with peroxidase labeled streptavin, both incubated for 10 min at room temperature (LSAB+ System-HRP, K0690, Dako, Glostrup, Denmark). The immunoreaction was visualized by incubating the sections for 2 min in a 0.1% 3,3’-diaminobenzidine and 0.02% hydrogen peroxide solution (DAB substrate Chromogen System; Dako). The sections were lightly counterstained with Mayer’s Hematoxylin (Histolab Products AB, Goteborg, Sweden) mounted in GVA mount (Zymed Laboratories Inc., San Francisco, CA, USA) and observed with an Axioplan Zeiss light microscope (Carl Zeiss) and photographed with a digital camera (AxioCam MRc5, Carl Zeiss).
Evaluation of immunohistochemistry
The CHI3L1 and CHIT1-staining status was identified as either negative or positive. Immunohistochemical positive staining was defined as the detection of brown chromogen on the edge of the hematoxylin-stained cell nucleus, distributed within the cytoplasm or in the membrane and evaluated as previously described.38 Stain intensity and the proportion of immunopositive cells were also assessed by light microscopy. Intensity of staining (IS) was graded on a scale of 0-4, according to the following assessment: no detectable staining = 0, weak staining = 1, moderate staining = 2, strong staining = 3, very strong staining = 4. The percentage of antibodies immunopositive cells (Extent Score=ES) was independently evaluated by 2 investigators (2 anatomical morphologists) and scored as a percentage of the final number of 100 cells in five categories: <5% (0); 5-30% (+); 31-50% (++); 51-75% (+++), and >75% (++++). Counting was performed under Zeiss Axioplan light microscope at x200 magnification. In case of disputes concerning the interpretation, the case has been revised to reach a unanimous agreement, as previously described.39 Digital pictures were photographed with a digital camera (Canon, Tokyo, Japan) at 20x, 40x and 60x magnification. Positive and negative controls were performed to test the specific reaction of primary antibodies used in this study at a protein level. Positive control for both antibodies consisted of rat liver sections. Sections treated with PBS without the primary antibodies served as negative controls. Positive immunolabeling for antibodies were nuclear/cytoplasmic.
Computerized morphometric measurements and image analysis
Fifteen fields, randomly selected from each section, were analyzed and the percentage area stained with CHI3L1 and CHIT1 antibodies was calculated using image analysis software (AxioVision Release 4.8.2 - SP2 Software, Carl Zeiss Microscopy GmbH, Jena, Germany), which quantifies the level of staining intensity of positive immunolabelling in each field. Digital micro-graphs were taken using the Zeiss Axioplan light microscope (Carl Zeiss) using objective lens of magnification x20, i.e., total magnification 400) fitted with a digital camera (AxioCam MRc5, Carl Zeiss); evaluations were made by two blinded investigators, whose evaluations were assumed to be correct if values were not significantly different. In case of dispute concerning interpretation, the samples were re-evaluated in order to reach a unanimous agreement.
Statistical analysis
Statistical analysis was performed using SPSS software (SPSS® release 16.0, IBM, Chicago, IL, USA). Data were tested for normality with the Kolmogorov-Smirnov test. All variables were normally distributed. Comparisons between two means were tested with the Student’s t-test, whilst comparison between more than two groups was tested using analysis of variance (ANOVA) and Bonferroni’s test. P-values of less than 0.05 were considered statistically significant; p-values of less than 0.01 were considered highly statistically significant. Data are presented as the mean ±SEM as previously described.38 Cohen’s kappa was applied to measure the agreement between the two observers and averaged to evaluate overall agreement as previously described.38
Results
Histomorphometric analyses
The histomorphometric parameters performed in group 1 (without ACLT), confirmed that the animals demonstrated no sign of cartilage degeneration with an intact and normal cartilage structure, whilst in group 2 (with ACLT) the animals demonstrated more serious pathological changes to the cartilage, OA moderate and severe, in fact horizontal cleavage tears or flaps and deep lesions were present. Group 2 confirmed the development of articular degenerative processes, which were significantly different from the control group, as confirmed by Kraus’ modified Mankin score (Figure 1A), and histopathology OARSI system (Figure 1B). The inter-observer variability among three observers for the MANKIN system showed a similar good intra-class correlation coefficient (ICC>0.82) as for the OARSI system (ICC>0.72). Repeat scoring by investigators showed very good agreement (ICC>0.96). The surface represented by lesion depth was the parameter where all investigators showed an excellent agreement. Other parameters such as cellularity and tidemark had greater inter-reader disagreement.
Figure 1.
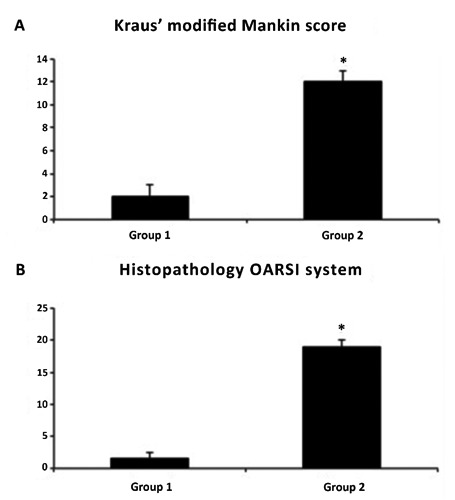
A) Kraus’ modified Mankin score between groups; results are presented as the mean ±SEM; Student’s ttest was used to evaluate the significance of the results; *P<0.01, when compared to the control group. B) Histopathology OARSI system between groups; results are presented as the mean ± SEM; Student’s ttest was used to evaluate the significance of the results; *P<0.01, when compared to the control group.
Histology
The histological (H&E staining) analysis of cartilage from group 1 (without ACLT), showed a preserved morphological structure (Figure 2A). This contrasts with group 2 (with ACLT), moderate OA cartilage where structural alterations included a reduction of cartilage thickness of the superficial and the middle zones (Figure 2B). The structure of the collagen network is damaged, which leads to reduced thickness of the cartilage. The chondrocytes are unable to maintain their repair activity with subsequent loss of the cartilage tissue. In severe OA, group 2 (with ACLT), the cartilage demonstrated deep surface clefts, disappearance of cells from the tangential zone, cloning, and a lack of cells in the intermediate and radial zone, which are not arranged in columns. The tidemark is no longer intact and the subchondral bone shows fibrillation (Figure 2C). Moreover, while the surface of healthy hyaline cartilage appears white, shiny, elastic and firm, in OA the surface becomes dull and irregular.
Figure 2.
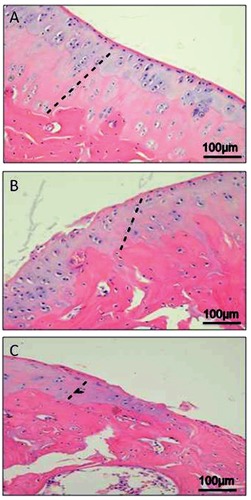
A) H&E staining demonstrated absence of structural alterations in control cartilage, group 1 without anterior cruciate ligament transection (ACLT); the dashed line represents the layers (thickness) of hyaline healthy cartilage; in the superficial zone, cells are flat and small; in the middle and deep zone, cells are organized in columns; the tidemark is evident. B) H&E staining demonstrated signs of structural alterations in moderate OA cartilage (group 2 with ACLT); the dashed line represents the layers (thickness) of hyaline cartilage; the structural alterations included a reduction of cartilage thickness of the superficial and the middle zones; the tidemark is almost intact. C) H&E staining demonstrated signs of structural alterations in severe OA (group 2 with ACLT); the dashed line represents the layers (thickness) of hyaline cartilage; severe OA cartilage demonstrated deep surface clefts, disappearance of cells from the superficial zone, cloning, and a lack of cells in the intermediate and deep zone, which are not arranged in columns; the tidemark is no longer intact and the subchondral bone shows fibrillation; cartilage is completely replaced by fibrocartilaginous, scar-like tissue with fibroblast like cells.
Immunohistochemical observations
CHI3L1 and CHIT1 were evaluated by immunohistochemical staining in cartilage of all groups (Table 1). Different patterns of immunopositive cells in the sets of specimens were seen. This immunohistochemical staining was found in chondrocytes of osteoarthritic cartilage mainly in the superficial and middle zone of the cartilage rather than the deep zone, while it was weak/absent in normal cartilage. CHI3L1 immunolabeling was weak/absent in cartilage tissue samples from group 1, without ACLT, [(Figure 3A); (ES=+; IS=1)], and very strong in group 2, with ACLT, moderate/severe OA [(Figure 3 B,C); (ES=++++; IS=4)]. No immunostaining was observed in the negative control (ES=0; IS=0) treated with PBS without the primary antibody (data not shown). The percentage of CHI3L1-positive cells was observed among groups (P<0.01 vs others) (Figure 4A). Interobserver agreement, measured as Kappa coefficient, was 0.95 (almost perfect). CHIT1 immunolabeling was weak/absent in cartilage tissue samples from group 1, without ACLT, [(Figure 5A); (ES=+; IS=1)], and very strong in group 2, with ACLT, moderate/severe OA [(Figure 5 B,C); (ES=++++; IS=4)]. No immunostaining was observed in the negative control (ES=0; IS=0) treated with PBS without the primary antibody (data not shown). The percentage of CHIT1-positive cells observed among groups (P<0.01 vs others) (Figure 4B). Interobserver agreement, measured as Kappa coefficient, was 0.92 (almost perfect).
Table 1.
Evaluation of CHI3L1 and CHIT1 immunostaining.
| Groups | Intensity of CHI3L1 immunostaining and percentage of CHI3L1 immunopositive cells |
Intensity of CHIT1 immunostaining and percentage of CHIT1 immunopositive cells |
|---|---|---|
| Control rats without ACLT | Weak/absent immunostaining (ES=+; IS=1) |
Weak/absent immunostaining (ES=+; IS=1) |
| Experimental rats with ACLT | Very strong immunostaining (ES=++++; IS=4) |
Very strong immunostaining (ES=++++; IS=4) |
ACLT, Anterior cruciate ligament transection; IS, intensity of staining; ES, extent score; IS was graded on a scale of 0-4, according to the following assessment: no detectable staining (0), weak staining (1), moderate staining (2), strong staining (3), very strong staining (4). The percentage of lubricin immunopositive cells was independently evaluated by 3 investigators (2 anatomical morphologists and one histologist) and scored as a percentage of the final number of 100 cells in five categories: <5% (0); 5–30% (+); 31–50% (++); 51-75% (+++), and >75% (++++).
Figure 3.
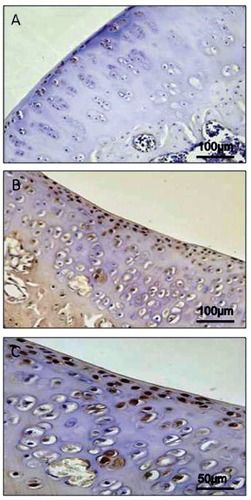
A) CHI3L1 immunohistochemistry specimen from control cartilage, group 1 without anterior cruciate ligament transection (ACLT), exhibited a weak/absent (ES=+; IS=1) immunostaining in chondrocytes from rat femoral articular cartilage. B) CHI3L1 immunohistochemistry specimen from moderate/severe OA cartilage (group 2, with ACLT) exhibited a very strong (ES=++++; IS=4) immunostaining in chondrocytes from rat femoral articular cartilage (superficial and middle zone). C) Magnification of panel B.
Figure 4.
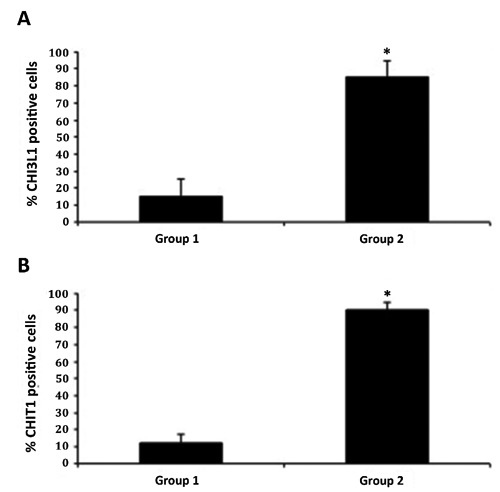
A) Immunohistochemistry: percentage of CHI3L1 positive cells out of the total number of cells counted in control group and in treated group; results are presented as the mean ±SEM; Student’s t-test, was used to evaluate the significance of the results; *P<0.01, when compared to the control group. B) Immunohistochemistry: percentage of CHIT1 positive cells out of the total number of cells counted in control group and in treated group; results are presented as the mean ±SEM; Student’s t-test, was used to evaluate the significance of the results; *P<0.01, when compared to the control group.
Figure 5.

A) CHIT1 immunohistochemistry specimen from control cartilage, group 1 without anterior cruciate ligament transection (ACLT), exhibited a weak/absent (ES=+; IS=1) immunostaining in chondrocytes from rat femoral articular cartilage. B) CHIT1 immunohistochemistry specimen from moderate/severe OA cartilage (group 2, with ACLT) exhibited a very strong (ES=++++; IS=4) immunostaining in chondrocytes from rat femoral articular cartilage (superficial and middle zone). C) Magnification of panel B.
Discussion
Articular cartilage injuries are one of the most challenging problems in musculoskeletal medicine due to the poor intrinsic regenerative capacity of this tissue.40 OA represents a major clinical and scientific challenge for clinicians and biologists due to the limited repair capacity of articular cartilage,41 rich in matrix proteins but also avascular.42,43 Articular cartilage defects are increasingly common among the elderly population and cause pain, reduced joint function and significant disability.44,45 In this study, we have demonstrated that CHI3L1 and CHIT1 are expressed in osteoarthritic rat cartilage model (Figure 6). CHI3L1 has been linked to tissue remodelling,46,47 joint injury,48 and significantly elevated levels of CHI3L1 protein have been detected in serum and synovial fluid from OA patients.49,50 The plethora of evidence showing that CHI3L1 stimulates proliferation of connective tissue cells and modulates expression levels of chemokines and metalloproteases in inflammatory fibroblasts, and that enhances chemotaxis of endothelial cells51,52 strongly indicate that CHI3L1 plays a crucial role in stromal cells not only in inflammatory conditions. Additionally, in vitro studies demonstrated that CHI3L1 is secreted by osteosarcoma53 and during osteoclast differentiation and bone digestion.54,20 The findings showing a correlation between CHI3L1 expression and the development of primary and metastatic tumours further support the idea that CHI3L1 plays a role in the development and progression of a variety of malignancies.55,56 Considerable experimental evidence reports the central role of CHIT1 in the expanding spectrum of disorders suggesting that overproduction of CHIT1 could exert deleterious effect in many degenerative disorders.57 This concept is also sustained by our previous findings in which we observed that genetic variation within the CHIT1 gene was strongly associated with human longevity and with several phenotypes of healthy aging,58 and that a functional polymorphism in the CHIT-1 gene protects from non alcoholic fatty liver disease (NAFLD) progression.59 Recently, we demonstrated that CHIT1 production is not a macrophages peculiarity and that CHIT1 may play an important role in the process of bone remodeling.20 These findings suggested that, patients with elevated serum levels of CHIT1 and CHI3L1 may have a more increased osteolytic activity and a faster progression of the disease. Indeed, silencing CHIT1 and CHI3L1 with siRNA resulted in a significant decrease in bone resorption activity and transfection with CHIT1 or CHI3L1 siRNA and co-transfection with both decreased the levels of the pro-differentiative marker MMP9.20
Figure 6.
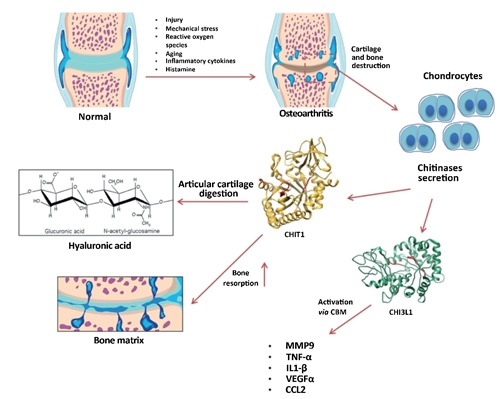
Graphic representation of CHI3L1 and CHIT1 expression in osteoarthritis.
In the present study the histomorphometric parameters performed in group 1 (without ACLT), confirmed that the animals demonstrated no sign of cartilage degeneration with an intact and normal cartilage structure, whilst in group 2 (with ACLT) the animals showed more serious pathological changes to the cartilage, OA moderate and severe, in fact horizontal cleavage tears or flaps and deep lesions were present, as confirmed by Kraus’ modified Mankin score and histopathology OARSI system. These results were corroborated by histological examination. Immunohistochemical analysis showed that CHI3L1 and CHIT1 staining was found in chondrocytes of osteoarthritic cartilage mainly in the superficial and middle zone of the cartilage rather than the deep zone. There was a tendency for a high number of positive chondrocytes in areas of the femoral condyles with a considerable biomechanical load. Our results are in accordance with Connor and co-authors who declared that CHI3L1 is expressed in osteophyte and diseased human osteoarthritic cartilage, but not in non-diseased one, and its distribution within the tissue changes, as disease progresses.60 The number of chondrocytes with a positive staining for both CHI3L1 and CHIT1 was weak/absent in normal cartilage, while the expression for both CHI3L1 and CHIT1 was very strong in osteoarthritic cartilage with ACLT. The two chitinase are typically produced in the lysosomes and subsequently secreted. Their production is closely related to an inflammatory process, and pro-inflammatory cytokines.61 CHIT1 and CH3L1 present Carbohydrate-binding motif (CBM), then the ability to bind carbohydrates, in particular glycosaminoglycans. This ability could explain the altered levels of these two molecules during a chronic inflammatory process such as OA. This observation is consistent with the evidence showing that CHI3L1 inhibition restrains tumour growth and metastasis by its own CBM.62 Furthermore, treatment with both CHIT1 and CHI3L1 siRNAs in osteoclast in vitro model, induced a significant reduction of MMP9.54,20 Recent articles confirm that the gelatinases influence OA onset and progression regulating the subchondral bone remodelling. In particular, a predominant role of MMP-9 emerged during last year. Among various MMPs, the total MMP-9 level is positively correlated with the total MMP-13 level in OA,63,64 and it has been hypothesized that this gelatinase might be involved in the activation of pro-MMP-13 through yet unknown mechanisms. Notably, MMP-13 has long been considered as the major enzyme involved in cartilage erosion during OA, thus MMP-9 might play a role, at least cooperatively, in joint degradation. That being so, it is reasonable that the chitinase may be involved in the resorption of articular cartilage in OA through different pathways. The CHIT1 could be involved in the resorption of cartilage and bone matrix via its catalytic site and the CBM, while the CHI3L1 could activate pro-inflammatory cytokines through its CBM. The present finding indicates that CHI3L1 and CHIT1 could play an important role in cartilage remodeling/degradation of osteoarthritic joints. CHIT1 and CHI3L1 may emerge as useful markers for OA and tissue degeneration. The expression of CHIT1 and of CHI3L1 in chondrocytes of osteoarthritic cartilage has a detrimental role in the cellular remodelling during the OA. These results are preliminary and further, longer-term in vivoand in vitro studies are needed to understand the exact mechanism of CHI3L1 and CHIT1 production and regulation in cartilage tissue and to define in detail these molecules, their receptors and activities on cells involved in OA. Serum and synovial fluid chitinases levels could be a marker of inflammatory cartilage diseases and since they correlate with the degree of joint destruction they could be used in the daily clinical practice to stage the severity of the disease.
Acknowledgments
This study was supported by grants provided by FIR 2014, University of Catania, Italy. The authors would like to thank Prof. Iain Halliday for commenting and making corrections to the paper.
References
- 1.Musumeci G, Loreto C, Carnazza ML, Strehin I, Elisseeff J.OA cartilage derived chondrocytes encapsulated in poly(ethylene glycol) diacrylate (PEGDA) for the evaluation of cartilage restoration and apoptosis in an in vitro model. Histol Histopathol 2011;26:1265-78 [DOI] [PubMed] [Google Scholar]
- 2.Musumeci G, Loreto C, Carnazza ML, Martinez G.Characterization of apoptosis in articular cartilage derived from the knee joints of patients with osteoarthritis. Knee Surg Sports Traumatol Arthrosc 2011;19: 307-13 [DOI] [PubMed] [Google Scholar]
- 3.Musumeci G, Loreto C, Carnazza ML, Coppolino F, Cardile V, Leonardi R.Lubricin is expressed in chondrocytes derived from osteoarthritic cartilage encapsulated in poly (ethylene glycol) diacrylate scaffold. Eur J Histochem 2011;55:e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Musumeci G, Leonardi R, Carnazza ML, Cardile V, Pichler K, Weinberg AM, et al. Aquaporin 1 (AQP1) expression in experimentally induced osteoarthritic knee menisci: an in vivo and in vitro study. Tissue Cell 2013;45:145-52 [DOI] [PubMed] [Google Scholar]
- 5.Musumeci G, Loreto C, Imbesi R, Trovato FM, Di Giunta A, Lombardo C, et al. Advantages of exercise in rehabilitation, treatment and prevention of altered morphological features in knee osteoarthritis. A narrative review. Histol Histopathol 2014; 29:707-19 [DOI] [PubMed] [Google Scholar]
- 6.Musumeci G, Castrogiovanni P, Leonardi R, Trovato FM, Szychlinska MA, Di Giunta A, et al. Knee osteoarthritis. New perspectives for articular cartilage repair treatment through tissue engineering. A contemporary review. World J Orthop 2014;5: 80-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musumeci G, Castrogiovanni P, Mazzone V, Szychlinska MA, Castorina S, Loreto C.Histochemistry as a unique approach for investigating normal and osteoarthritic cartilage. Eur J Histochem 2014;58:2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henrissat B, Davies G.Structural and sequence-based classification of glycoside hydrolases. Curr Opin Struct Biol 1997;7: 637-44 [DOI] [PubMed] [Google Scholar]
- 9.Herrera-Estrella A, Chet I.Chitinases in biological control. EXS 1999;87:171-84 [DOI] [PubMed] [Google Scholar]
- 10.Boot RG, Blommaart EFC, Swart E, Ghauharali-van der Vlugt K, Bijl N, Moe C, et al. Identification of a novel acidic mammalian chitinase distinct from chitotriosidase. J Biol Chem 2001;276:6770-8 [DOI] [PubMed] [Google Scholar]
- 11.van Aalten DM, Komander D, Synstad B, Gaseidnes S, Peter MG, Eijsink VG.Structural insights into the catalyticmechanism of a family 18 exo-chitinase. Proc Natl Acad Sci USA 2001;98:8979-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Rosa M, Malaguarnera G, De Gregorio C, D'Amico F, Mazzarino MC, Malaguarnera L.Modulation of chitotriosidase during macrophage differentiation. Cell Biochem Biophys 2013;66:239-47 [DOI] [PubMed] [Google Scholar]
- 13.Di Rosa M, De Gregorio C, Malaguarnera G, Tuttobene M, Biazzo F, Malaguarnera L.Evaluation of AMCase and CHIT-1 expression in monocyte macrophages lineage. Mol Cell Biochem 2013;374:73-80 [DOI] [PubMed] [Google Scholar]
- 14.Di Rosa M, Malaguarnera G, De Gregorio C, Drago F, Malaguarnera L.Evaluation of CHI3L-1 and CHIT-1 expression in differentiated and polarized macrophages. Inflammation 2013;36:482-92 [DOI] [PubMed] [Google Scholar]
- 15.Malaguarnera L.Chitotriosidase: the yin and yang. Cell Mol Life Sci 2006;63:3018-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Rosa M, Malaguarnera L.Genetic variants in candidate genes influencing NAFLD progression. J Mol Med (Berl) 2012;90:105-18 [DOI] [PubMed] [Google Scholar]
- 17.Hollak CE, van Weely S, van Oers MH, Aerts JM.Marked elevation of plasma chitotriosidase activity. A novel hallmark of Gaucher disease. J Clin Invest 1994;93:1288-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pacheco N, Uribe A.Enzymatic analysis of biomarkers for the monitoring of Gaucher patients in Colombia. Gene 2013;521:129-35 [DOI] [PubMed] [Google Scholar]
- 19.Mucci JM, Scian R, De Francesco PN, Garcia FS, Ceci R, Fossati CA, et al. Induction of osteoclastogenesis in an in vitro model of Gaucher disease is mediated by T cells via TNF-alpha. Gene 2012;509:51-9 [DOI] [PubMed] [Google Scholar]
- 20.Di Rosa M, Tibullo D, Vecchio M, Nunnari G, Saccone S, Di Raimondo F, et al. Determination of chitinases family during osteoclastogenesis. Bone 2014;61:55-63 [DOI] [PubMed] [Google Scholar]
- 21.Campeau PM, Rafei M, Boivin MN, Sun Y, Grabowski GA, Galipeau J.Characterization of Gaucher disease bone marrow mesenchymal stromal cells reveals an altered inflammatory secretome. Blood 2009;114: 3181-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mistry PK, Liu J, Yang M, Nottoli T, McGrath J, Jain D, et al. Glucocerebrosidase gene-deficient mouse recapitulates Gaucher disease displaying cellular and molecular dys-regulation beyond the macrophage. Proc Natl Acad Sci USA 2010;107:19473-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Dussen L, Lips P, Everts VE, Bravenboer N, Jansen ID, Groener JE, et al. Markers of bone turnover in Gaucher disease: modeling the evolution of bone disease. J Clin Endocrinol Metab 2011;96:2194-205 [DOI] [PubMed] [Google Scholar]
- 24.Koulouvaris P, Ly K, Ivashkiv LB, Bostrom MP, Nestor BJ, Sculco TP, et al. Expression profiling reveals alternative macrophage activation and impaired osteogenesis in periprosthetic osteolysis. J Orthop Res 2008;26:106-16 [DOI] [PubMed] [Google Scholar]
- 25.Kzhyshkowska J, Gratchev A, Goerdt S.Human chitinases and chitinase-like proteins as indicators for inflammation and cancer. Biomark Insights 2007;2:128-46 [PMC free article] [PubMed] [Google Scholar]
- 26.Baeten D, Steenbakkers PG, Rijnders AM, Boots AM, Veys EM, De Keyser F.Detection of major histocompatibility complex/ human cartilage GP-39 complexes in rheumatoid arthritis synovitis as a specific and independent histologic marker. Arthritis Rheum 2004;50:444-51 [DOI] [PubMed] [Google Scholar]
- 27.Einarsson JM, Bahrke S, Sigurdsson BT, Ng CH, Petersen PH, Sigurjonsson OE, et al. Partially acetylated chitooligosaccharides bind to YKL-40 and stimulate growth of human osteoarthritic chondrocytes. Biochem Biophys Res Commun 2013;434: 298-304 [DOI] [PubMed] [Google Scholar]
- 28.Volck B, Ostergaard K, Johansen JS, Garbarsch C, Price PA.The distribution of YKL-40 in osteoarthritic and normal human articular cartilage. Scand J Rheumatol 1999;28:171-9 [DOI] [PubMed] [Google Scholar]
- 29.Mylin AK, Abildgaard N, Johansen JS, Andersen NF, Heickendorff L, Standal T, et al. High serum YKL-40 concentration is associated with severe bone disease in newly diagnosed multiple myeloma patients. Eur J Haematol 2008;80:310-7 [DOI] [PubMed] [Google Scholar]
- 30.Jay GD, Fleming BC, Watkins BA, McHugh KA, Anderson SC, Zhang LX, et al. Prevention of cartilage degeneration and restoration ofchondroprotection by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis Rheum 2010;62:2382-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elsaid KA, Zhang L, Waller K, Tofte J, Teeple E, Fleming BC, et al. The impact of forced joint exercise on lubricin biosynthesis from articular cartilage following ACL transection and intra-articular lubricin's effect in exercised joints following ACL transection. Osteoarthritis Cartilage 2012;20:940-8 [DOI] [PubMed] [Google Scholar]
- 32.Musumeci G, Loreto C, Clementi G, Fiore CE, Martinez G.An in vivo experimental study on osteopenia in diabetic rats. Acta Histochem 2011;113:619-25 [DOI] [PubMed] [Google Scholar]
- 33.Mankin HJ, Dorfman H, Lippiello L, Zarins A.Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. J Bone Joint Surg 1971;53:523-37 [PubMed] [Google Scholar]
- 34.Kraus VB, Huebner JL, Stabler T, Flahiff CM, Setton LA, Fink C, et al. Ascorbic acid increase the severity of spontaneous knee osteoarthritis in a guinea pig model. Arthritis Rheum 2004;50:1822-31 [DOI] [PubMed] [Google Scholar]
- 35.Pauli C, Grogan SP, Patil S, Otsuki S, Hasegawa A, Koziol J, et al. Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthritis Cartilage 2011;19:1132-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Musumeci G, Carnazza ML, Leonardi R, Loreto C.Expression of beta-defensin-4 in "an in vivo and ex vivo model" of human osteoarthritic knee meniscus. Knee Surg Sports Traumatol Arthrosc 2012;20:216-22 [DOI] [PubMed] [Google Scholar]
- 37.Musumeci G, Carnazza ML, Loreto C, Leonardi R, Loreto C.-Defensin-4 (HBD-4) is expressed in chondrocytes derived from normal and osteoarthritic cartilage encapsulated in PEGDA scaffold. Acta Histochem 2012;114:805-12 [DOI] [PubMed] [Google Scholar]
- 38.Musumeci G, Loreto C, Leonardi R, Castorina S, Giunta S, Carnazza ML, et al. The effects of physical activity on apoptosis and lubricin expression in articular cartilage in rats with glucocorticoid-induced osteoporosis. J Bone Miner Metab 2013;31: 274-84 [DOI] [PubMed] [Google Scholar]
- 39.Pichler K, Loreto C, Leonardi R, Reuber T, Weinberg AM, Musumeci G.In rat with glucocorticoid-induced osteoporosis, RANKL is downregulated in bone cells by physical activity (treadmill and vibration stimulation training). Histol Histopathol 2013;28:1185-96 [DOI] [PubMed] [Google Scholar]
- 40.Lehmann M, Martin F, Mannigel K, Kaltschmidt K, Sack U, Anderer U.Three-dimensional scaffold-free fusion culture: the way to enhance chondrogenesis of in vitro propagated human articular chondrocytes. Eur J Histochem 2013;57:e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Musumeci G, Trovato FM, Pichler K, Weinberg AM, Loreto C, Castrogiovanni P.Extra-virgin olive oil diet and mild physical activity prevent cartilage degeneration in an osteoarthritis model. An "in vivo" and "in vitro" study on lubricin expression. J Nutr Biochem 2013;24:2064-2075 [DOI] [PubMed] [Google Scholar]
- 42.Tsukamoto I, Akagi M, Inoue S, Yamagishi K, Mori S, Asada S.Expressions of local renin-angiotensin system components in chondrocytes. Eur J Histochem 2014;58: 2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shibata S, Sakamoto Y, Baba O, Qin C, Murakami G, Cho BH.An immunohisto-chemical study of matrix proteins in the craniofacial cartilage in midterm human fetuses. Eur J Histochem 2013;57:e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnstone B, Alini M, Cucchiarini, M, Dodge GR, Eglin D, Guilak F, et al. Tissue engineering for articular cartilage repair-the state of the art. Eur Cell Mater 2013;2: 248-267 [DOI] [PubMed] [Google Scholar]
- 45.Mobasheri A, Csaki C, Clutterbuck AL, Rahmanzadeh M, Shakibaei M.Mesenchymal stem cells in connective tissue engineering and regenerative medicine: applications in cartilage repair and osteoarthritis therapy. Histol Histopathol 2009;24:347-366 [DOI] [PubMed] [Google Scholar]
- 46.Mucci JM, Scian R, De Francesco PN, Garcia FS, Ceci R, Fossati CA, et al. Induction of osteoclastogenesis in an in vitro model of Gaucher disease is mediated by T cells via TNF-alpha. Gene 2012;509:51-9 [DOI] [PubMed] [Google Scholar]
- 47.Hartl D, He CH, Koller B, Da Silva CA, Kobayashi Y, Lee CG, et al. Acidic mammalian chitinase regulates epithelial cell apoptosis via a chitinolytic-independent mechanism. J Immunol 2009;182:5098-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chung C, Tallerico T, Seeman P.Schizophrenia hippocampus has elevated expression of chondrex glycoprotein gene. Synapse 2003;50:29-34 [DOI] [PubMed] [Google Scholar]
- 49.Johansen JS, Jensen HS, Price PA.A new biochemical marker for joint injury. Analysis of YKL-40 in serum and synovial fluid. Br J Rheumatol 1993;32: 949-55 [DOI] [PubMed] [Google Scholar]
- 50.Recklies AD, White C, Ling H.The chitinase 3-like protein human cartilage glycoprotein 39 (HC-gp39) stimulates proliferation of human connective-tissue cells and activates both extracellular signal-regulated kinase-and protein kinase B-mediated signalling pathways. Biochem J 2002;365:119-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Recklies AD, Ling H, White C, Bernier SM.Inflammatory cytokines induce production of CHI3L1 by articular chondrocytes. J Biol Chem 2005;280:41213-21 [DOI] [PubMed] [Google Scholar]
- 52.Ling H, Recklies AD.The chitinase 3-like protein human cartilage glycoprotein 39 inhibits cellular responses to the inflammatory cytokines interleukin-1 and tumour necrosis factor-alpha. Biochem J 2004;380: 651-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johansen JS, Jensen HS, Price PA.A new biochemical marker for joint injury. Analysis of YKL-40 in serum and synovial fluid. Br J Rheumatol 1993;32:949-55 [DOI] [PubMed] [Google Scholar]
- 54.Di Rosa M, Tibullo D, Vecchio M, Nunnari G, Saccone S, Di Raimondo F, et al. Determination of chitinases family during osteoclastogenesis. Bone 2014;61:55-63 [DOI] [PubMed] [Google Scholar]
- 55.Chen CC, Pekow J, Llado V, Kanneganti M, Lau CW, Mizoguchi A, et al. Chitinase 3-like-1 expression in colonic epithelial cells as a potentially novel marker for colitis-associated neoplasia. Am J Pathol 2011;179: 1494-503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hanahan D, Weinberg RA.The hallmarks of cancer. Cell 2000;100:57-70 [DOI] [PubMed] [Google Scholar]
- 57.Di Rosa M, Dell’Ombra N, Zambito AM, Malaguarnera M, Nicoletti F, Malaguarnera L.Chito-triosidase and inflammatory mediator levels in Alzheimer’s disease and cerebrovascular dementia. Eur J Neurosci 2006; 23:2648-56 [DOI] [PubMed] [Google Scholar]
- 58.Malaguarnera L, Ohazuruike LN, Tsianaka C, Antic T, Di Rosa M, Malaguarnera M.Human chitotriosidase polymorphism is associated with human longevity in Mediterranean nonagenarians and centenari- ans. J Hum Genet 2010;55:8-12 [DOI] [PubMed] [Google Scholar]
- 59.Di Rosa M, Mangano K, De Gregorio C, Nicoletti F, Malaguarnera L.Association of chitotriosi- dase genotype with the development of nonalcoholic fatty liver disease. Hepatology Research 2013;43:267-75 [DOI] [PubMed] [Google Scholar]
- 60.Kawada M, Hachiya Y, Arihiro A, Mizoguchi E.Role of mammalian chitinases in inflammatory conditions. Keio J Med 2007;56:21-7 [DOI] [PubMed] [Google Scholar]
- 61.Chen CC, Llado V, Eurich K, Tran HT, Mizoguchi E.Carbohydrate-binding motif in chitinase 3-like 1 (CHI3L1/YKL-40) specifically activates Akt signaling pathway in colonic epithelial cells. Clin Immunol Sep 2011;140:268-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim KS, Lee YA, Choi HM, Yoo MC, Yang HI.Implication of MMP-9 and urokinase plasminogen activator (uPA) in the activation of pro-matrix metalloproteinase (MMP)-13. Rheumatol Int 2012;32:3069-75 [DOI] [PubMed] [Google Scholar]
- 63.Loreto C, Leonardi R, Musumeci G, Pannone G, Castorina S.An ex vivo study on immunohistochemical localization of MMP-7 and MMP-9 in temporomandibular joint discs with internal derangement. Eur J Histochem 2013;57:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Connor JR, Dodds RA, Emery JG, Kirkpatrick RB, Rosenberg M, Gowen M.Human cartilage glycoprotein 39 (HC gp-39) mRNA expression in adult and fetal chondrocytes, osteoblasts and osteocytes by in-situ hybridization. Osteoarthritis Cartilage 2000;8:87-95 [DOI] [PubMed] [Google Scholar]


