Abstract
Background
Epidemiological studies of the association between nonsteroidal anti-inflammatory drug (NSAID) intake and the risk of prostate cancer still remain controversial. Therefore, we conducted a meta-analysis to evaluate the potential association between NSAID intake and prostate cancer risk.
Methods
Eligible studies were retrieved by both computerized searches and reviews of references. Subgroup analyses on country and design of study were also performed. Random or fixed-effect models were used to pool estimates of odds ratios (ORs) with 95% confidence intervals (CIs).
Results
We observed that the intake of aspirin was associated with a marginally decreased risk of prostate cancer (OR =0.95, 95% CI =0.93 to 0.98). A similar result was found between nonaspirin NSAIDs and prostate cancer risk (OR =0.94, 95% CI =0.90 to 0.98). However, a positive relation between all-NSAID intake and prostate cancer risk was observed (OR =1.18, 95% CI =1.15 to 1.22).
Conclusions
We observed a marginally inverse correlation between the intake of aspirin and prostate cancer risk. On the contrary, a positive relationship between all-NSAID intake and prostate cancer was detected. Further research needs to be conducted to better clarify potential biological mechanisms.
Keywords: Etiology, Meta-analysis, NSAIDs, Prostate cancer
Background
Prostate cancer is generally accepted as the most common type of cancer among men and ranks as the second cause of cancer-specific death in the whole world [1]. In the USA, approximately 238,590 men were diagnosed with prostate cancer, of which 12% were expected to die in 2013 [1]. Epidemiologic studies illustrated that both environmental and genetic alterations are well-established risk factors of prostate cancer [2, 3]. However, this could hardly explain the different incidences of prostate cancer in different countries [1, 4]. Therefore, daily lifestyle could play an important role in carcinogenesis.
Previous research demonstrated that nonsteroidal anti-inflammatory drugs (NSAIDs) could reduce the risk of several types of cancer, including colorectal cancer, esophageal cancer, and breast cancer [5–7]. Laboratory research has consistently illustrated that inflammation plays an essential role in prostate carcinogenesis [8]. It is generally accepted that cyclooxygenase enzymes, which are the targets of most NSAIDs, are involved in prostate carcinogenesis. A previous review, focusing on the potential relationship between NSAID intake and prostate cancer risk, showed that NSAIDs could prevent prostate cancer from developing into advanced disease, suggesting that NSAIDs could have a potentially protective effect on prostate cancer [9].
Nevertheless, epidemiological studies of the association between NSAID intake and the risk of prostate cancer still remained controversial. A previous meta-analysis in 2010 (including articles published before 2008) indicated that aspirin and other types of NSAID had a marginally, but not conclusively, protective effect on prostate cancer [10]. In addition, multicenter and large-sample studies were performed in the last 5 years, which provided us with more reliable statistics.
Therefore, we conducted a meta-analysis to evaluate both the strength and consistency of the potential association between NSAID intake and prostate cancer risk. Stratified analyses were also conducted on possible variables.
Methods
Literature search
To obtain an overall view of NSAID intake and prostate cancer risk, we performed a comprehensive and systematic searching strategy. We searched for publications updated to October 2013 using PubMed, the Web of Science, and Cochrane Library. We selected the keyword search ‘(“Anti-Inflammatory Agents, Non-Steroidal” [Mesh] OR “Aspirin” [Mesh]) AND “Prostatic Neoplasms” [Mesh]’ to identify publications. Additional publications were assessed either by cited references in the recruited articles or reported meta-analyses on NSAID intake and prostate cancer risk. Each retrieved publication was evaluated for the following criteria: (1) case-control or cohort study assessing the potential correlation between NSAIDs intake and prostate cancer risk; (2) exact data in both case and control groups should be identified; (3) articles published before October 2013 written in English; (4) results including odds ratios (ORs) or relative risk and 95% confidence intervals (95% CIs), or supplying sufficient information to calculate them. Studies with insufficient or overlapping data were excluded. Figure 1 illustrates the process of determining and selecting articles.
Figure 1.
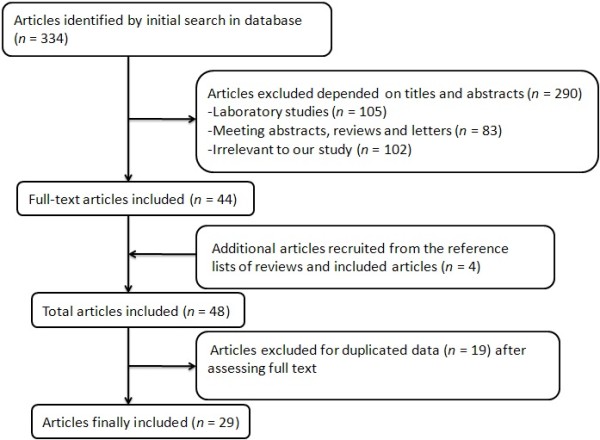
Article selection.
Data extraction
Data extracted from the articles included the name of the first author, year of publication, study period, country, type of NSAID, design of study, information resource, and odds ratio comparing the highest level of NSAID intake with the lowest. Given that prostate cancer is a relatively rare disease, the relative risk was considered to be the same as the OR. Therefore, we chose OR as the result to assess the potential relationship between NSAID intake and prostate cancer risk. Investigators were divided into two groups, and extracted data from all the potentially qualifying publications simultaneously, to avoid omissions or mistakes. Discussions were conducted whenever necessary.
Statistical analysis
A fixed-effects model using the method of Mantel and Haenszel [11] was selected to provide pooled estimation of the correlation between NSAID intake and prostate cancer risk when no heterogeneity was detected; otherwise, the random-effect model of the method of DerSiomonian and Laird [12] was selected for summarization.
We chose OR and 95% CI to evaluate the strength of the relationship between NSAID intake (all NSAIDs, aspirin, and nonaspirin NSAIDs, respectively) and prostate cancer risk (prostate cancer and advanced prostate cancer, respectively). Advanced prostate cancer was defined as Gleason score ≥8, clinical stage ≥ T2C, or prostate-specific antigen ≥10 ng/ml. Stratified analyses were conducted by country (USA or other countries) and study design (cohort study or case-control).
We used the quantified Q test [12] and I2 test [13] to assess the extent of heterogeneity across eligible studies. Publication bias was evaluated by Egger’s [14] and Begg’s [15] tests. The statistical significance level was set at 0.05.
Sensitivity analyses were also conducted to evaluate the effect of each study on the overall estimate.
All statistical analyses were conducted using STATA version 11 (StataCorp, College Station, Texas, USA).
Results
Description of the meta-analysis
A total of 334 articles were found when the mentioned keywords were used. After a closer examination, 309 articles were excluded based on titles and abstracts, while 4 articles were recruited from the reference lists of reviews and included articles [16–19]. Figure 1 illustrates the detailed process of identifying and selecting articles. Finally, we identified a total of 29 articles on NSAID intake and prostate cancer risk that were published between 1989 and 2013 [16–44].
Among the included articles, 15 were cohort studies [16–20, 22, 24, 28–30, 33, 36, 37, 39, 40], and 14 were case-control ones [21, 23, 25–27, 31, 32, 34, 35, 38, 41–44]. Sixteen studies were conducted in the USA [16–18, 20, 22, 24, 25, 28, 30, 31, 37, 39–43], while the remaining 13 were performed in other countries [19, 21, 23, 26, 27, 29, 32–36, 38, 44]. Eleven studies were associated with all-NSAID intake [19, 20, 26–28, 32–34, 38, 42, 44], 24 with aspirin [16–18, 21–33, 36–41, 43, 44], and 14 with nonaspirin NSAIDs [23, 25–28, 30, 32, 35, 36, 38–41, 44]. Eleven studies were concerned about advanced prostate cancer [18, 24, 26–28, 30, 37–39, 41, 43]. Information was collected from either databases or questionnaires. Detailed characteristics of the eligible studies are listed in Table 1.
Table 1.
Characteristics of the included studies on nonsteroidal anti-inflammatory drugs intake and prostate cancer
| First author | Published year | Study design | Country | Drugs | Study period | Information resource |
|---|---|---|---|---|---|---|
| Paganini-Hill [16] | 1989 | Cohort | USA | Aspirin | 1981 to 1988 | Questionnaire |
| Schreinemachers [22] | 1994 | Cohort | USA | Aspirin | 1971 to 1987 | Questionnaire |
| Neugut [31] | 1998 | Case-control | USA | Aspirin | 1984 to 1986 | Questionnaire |
| Norrish [38] | 1998 | Case-control | New Zealand | Aspirin, NA-NSAIDs, NSAIDs | 1996 | Questionnaire |
| Nelson [25] | 2000 | Case-control | USA | Aspirin, NA-NSAIDs | 1992 to 1995 | Questionnaire |
| Langman [34] | 2000 | Case-control | UK | NSAIDs | 1993 to 1995 | Database |
| Leitzmann [18] | 2002 | Cohort | USA | Aspirin | 1986 to 1998 | Questionnaire |
| Roberts [20] | 2002 | Cohort | USA | NSAIDs | 1990 to 1996 | Questionnaire |
| Habel [24] | 2002 | Cohort | USA | Aspirin | 1964 to 1996 | Questionnaire |
| Irani [35] | 2002 | Case-control | France | NA-NSAIDs | 1999 to 2000 | Questionnaire |
| Sorensen [19] | 2003 | Cohort | Denmark | NSAIDs | 1989 to 1995 | Database |
| Friis [29] | 2003 | Cohort | Denmark | Aspirin | 1989 to 1997 | Database |
| Perron [33] | 2003 | Cohort | Canada | Aspirin, NSAIDs | 1993 to 1996 | Database |
| Ratnasinghe [17] | 2004 | Cohort | USA | Aspirin | 1976 to 1992 | Questionnaire |
| García Rodríguez [36] | 2004 | Cohort | UK | Aspirin, NA-NSAIDs | 1995 to 2001 | Database |
| Jacobs [28] | 2005 | Cohort | USA | Aspirin, NA-NSAIDs, NSAIDs | 1992 to 2001 | Questionnaire |
| Platz [40] | 2005 | Cohort | USA | Aspirin, NA-NSAIDs | 1980 to 2004 | Questionnaire |
| Bosetti [21] | 2006 | Case-control | Italy | Aspirin | 1991 to 2002 | Questionnaire |
| Dasgupta [23] | 2006 | Case-control | Canada | Aspirin, NA-NSAIDs | 1999 to 2002 | Database |
| Mahmud [26] | 2006 | Case-control | Canada | Aspirin, NA-NSAIDs, NSAIDs | 1999 to 2003 | Questionnaire |
| Menezes [41] | 2006 | Case-control | USA | Aspirin, NA-NSAIDs | 1982 to 1998 | Questionnaire |
| Murad [32] | 2010 | Case-control | UK | Aspirin, NA-NSAIDs, NSAIDs | 2001 to 2008 | Database |
| Brasky [39] | 2010 | Cohort | USA | Aspirin, NA-NSAIDs | 2000 to 2007 | Questionnaire |
| Coogan [42] | 2010 | Case-control | USA | NSAIDs | 1992 to 2008 | Questionnaire |
| Salinas [43] | 2010 | Case-control | USA | Aspirin | 2002 to 2005 | Questionnaire |
| Dhillon [37] | 2011 | Cohort | USA | Aspirin | 1988 to 2006 | Questionnaire |
| Mahmud [44] | 2011 | Case-control | Canada | Aspirin, NA-NSAIDs, NSAIDs | 1985 to 2000 | Questionnaire |
| Shebl [30] | 2012 | Cohort | USA | Aspirin, NA-NSAIDs | 1993 to 2009 | Questionnaire |
| Veitonmaki [27] | 2013 | Case-control | Finland | Aspirin, NA-NSAIDs, NSAIDs | 1995 to 2002 | Database |
NA-NSAIDs, nonaspirin nonsteroidal anti-inflammatory drugs; NSAIDs, total nonsteroidal anti-inflammatory drugs.
Intake of aspirin and prostate cancer
Of the 24 studies concerned with aspirin intake, we observed that the intake of aspirin was associated with a marginally decreased risk of prostate cancer (OR =0.95, 95% CI =0.93 to 0.98) (Figure 2). A statistical heterogeneity was detected, therefore, random-effect analysis was performed (I2 = 60.7%, P >0.001). The risk of advanced prostate cancer was lower than that of prostate cancer (OR =0.89, 95% CI =0.82 to 0.96). The fixed-effect analysis was conducted and no significant heterogeneity was found (I2 = 4.5%, P =0.4).
Figure 2.
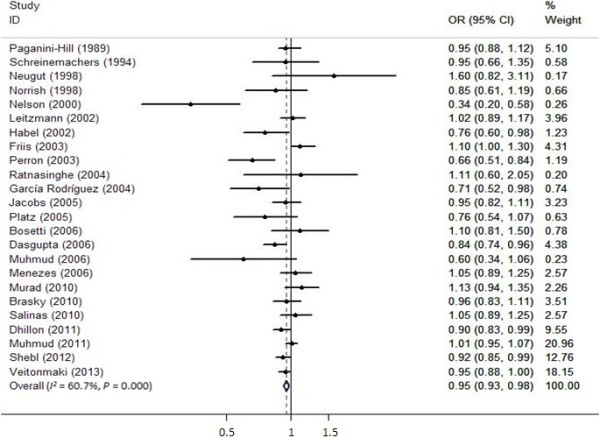
Forest plots depicting the risk estimates from included studies on the association between the intake of aspirin and prostate cancer risk. CI, confidence interval; OR, odds ratio.
Begg’s and Egger’s tests were performed to evaluate publication bias among the included studies; no such bias was found by either Begg’s or Egger’s test (P =0.503, P =0.160, respectively).
For stratified analysis, the summarized OR estimates indicated that the intake of aspirin was associated with a decreased risk of prostate cancer in the USA (OR =0.937, 95% CI =0.900 to 0.975). However, no such association was detected in other countries (OR =0.969, 95% CI =0.934 to 1.006). When subgroup analysis was performed by study design, we observed a marginally protective effect of aspirin on prostate cancer in the cohort group (OR =0.930, 95% CI =0.894 to 0.967). Nevertheless, no such effect was found in the case-control group (OR =0.976, 95% CI =0.940 to 1.013). Detailed data are given in Table 2.
Table 2.
Stratified pooled odds ratios and 95% confidence intervals of NSAIDs intake and prostate cancer
| Subgroup | Number of studies | Odds ratio (95% confidence interval) | Heterogeneity | |
|---|---|---|---|---|
| P | I 2(%) | |||
| Aspirin | ||||
| USA | 14 | 0.937 (0.900, 0.975) | 0.012 | 52.0 |
| Other countries | 10 | 0.969 (0.934, 1.006) | 0.000 | 70.0 |
| Cohort studies | 13 | 0.930 (0.894, 0.967) | 0.025 | 48.6 |
| Case-control studies | 11 | 0.976 (0.940, 1.013) | 0.000 | 69.0 |
| NSAIDs | ||||
| USA | 3 | 0.922 (0.836, 1.016) | 0.011 | 77.7 |
| Other countries | 8 | 1.212 (1.174, 1.251) | 0.000 | 92.7 |
| Cohort studies | 4 | 1.022 (0.938, 1.113) | 0.000 | 90.2 |
| Case-control studies | 7 | 1.205 (1.167, 1.245) | 0.000 | 93.3 |
| NA-NSAIDs | ||||
| USA | 6 | 1.009 (0.927, 1.099) | 0.807 | 0.0 |
| Other countries | 8 | 0.915 (0.869, 0.964) | 0.001 | 70.3 |
| Cohort studies | 5 | 0.992 (0.909, 1.083) | 0.629 | 0.0 |
| Case-control studies | 9 | 0.922 (0.876, 0.971) | 0.002 | 67.9 |
All-NSAID intake and prostate cancer
A total of 11 studies were associated with all-NSAID intake and prostate cancer. Compared with previous meta-analyses, we observed a positive relationship between all-NSAID intake and prostate cancer risk, owing to new research (OR =1.18, 95% CI =1.15 to 1.22). A random-effect analysis was conducted, owing to significantly statistical heterogeneity (I2 = 92.4%, P >0.001). Similar results were found between all-NSAID intake and advanced prostate cancer (OR =1.43, 95% CI =1.32 to 1.56, I2 = 92.8%, P >0.001). No publication bias was detected when Begg’s and Egger’s tests were conducted (P =0.312, P =0.190, respectively).
In the subgroup analysis, the pooled OR estimates suggested that no direct relationship between all-NSAID intake and prostate cancer risk was observed in the USA (OR =0.922, 95% CI =0.836 to 1.016). Notably, we detected a positive correlation between all-NSAID intake and prostate cancer risk (OR =1.212, 95% CI =1.174 to 1.251). When stratified analysis was conducted by study design, we did not observe a protective effect on prostate cancer in the cohort group (OR =1.022, 95% CI =0.938 to 1.113). However, in case-control groups, all-NSAID intake could be a potential risk factor in prostate carcinogenesis (OR =1.205, 95% CI =1.167 to 1.245). Detailed data are illustrated in Table 2.
Nonaspirin NSAID intake and prostate cancer
Among the 14 articles related to nonaspirin NSAIDs and prostate cancer risk, we observed a marginally inverse relation (OR =0.94, 95% CI =0.90 to 0.98) (Figure 3). A significantly statistical heterogeneity was observed, thus random-effect analysis was performed (I2 = 55.9%, P >0.01). Nevertheless, an opposite relation was detected between nonaspirin NSAID intake and advanced prostate cancer (OR =1.25, 95% CI =1.13 to 1.38, I2 = 65.8%, P >0.01).
Figure 3.
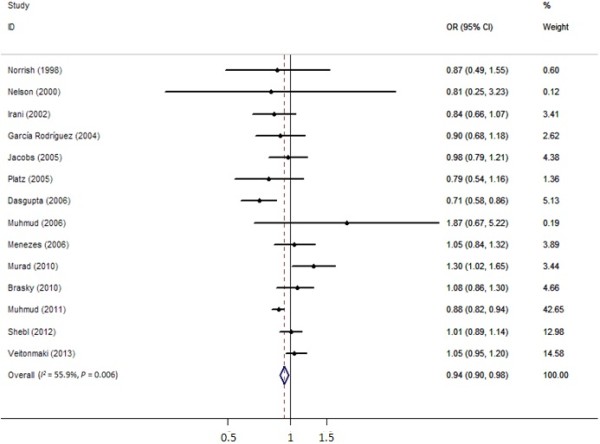
Forest plots depicting the risk estimates from included studies on the association between nonaspirin NSAID intake and prostate cancer risk. CI, confidence interval; OR, odds ratio.
Begg’s and Egger’s tests were conducted to assess for publication bias among the included articles; no such bias was detected by either Begg’s or Egger’s test (P =0.956, P =0.500, respectively).
In the stratified analysis, the summarized OR estimates showed that nonaspirin NSAIDs were associated with a decreased risk of prostate cancer in other countries except the USA (OR =0.915, 95% CI =0.869 to 0.964). However, we failed to find a similar relation in the USA (OR =1.009, 95% CI =0.927 to 1.099). When stratified analysis was performed on study design, we observed a marginally protective effect on prostate cancer in the case-control group (OR =0.922, 95% CI =0.876 to 0.971). Nevertheless, no such result was detected in the cohort group (OR =0.992, 95% CI =0.909 to 1.083). Detailed data are illustrated in Table 2.
Sensitivity analysis
Simultaneously, sensitivity analyses were conducted to evaluate the effect of each study on the overall estimate by sequentially excluding each study in turn. In our meta-analysis, we found that probably no study could affect the summary of risk estimate (data not shown).
Discussion
Nonsteroidal anti-inflammatory drugs are one of the most commonly used medicine types in the whole world. Studies showed that daily nonsteroidal anti-inflammatory drug intake could be a non-invasive, economical, and valuable method of preventing cancer. Previous research illustrated that inflammation was involved in the process of prostate carcinogenesis [45]. Evidence demonstrated that cyclooxygenase enzymes, the targets of most NSAIDs, played a critical role in the development of prostate cancer [8]. Further studies verified that NSAIDs could inhibit the development and progression of prostate cancer, which meant that NSAIDs might be effective in the prevention of prostate cancer [9]. Nevertheless, conflicting results were obtained by different research centers. Therefore, we performed a meta-analysis to evaluate both the strength and consistency of the potential association between NSAID intake and prostate cancer risk.
We observed that the intake of aspirin was associated with a marginally decreased risk of prostate cancer (OR =0.95, 95% CI =0.93 to 0.98). The risk of advanced prostate cancer was less than that of prostate cancer (OR =0.89, 95% CI =0.82 to 0.96). This indicated that the intake of aspirin showed a marginally inverse correlation with prostate cancer risk. The results of the 24 studies included in our analysis were heterogeneous, probably resulting from differences in country and study design. To further illustrate the relationship between the intake of aspirin and prostate cancer risk, subgroup analyses were conducted. The summarized OR estimates illustrated that the intake of aspirin was associated with a decreased risk of prostate cancer while separately analyzed by country and design of study. In the stratified analysis by country, we found that an inversed correlation between the intake of aspirin and prostate cancer risk in the USA (OR =0.937, 95% CI =0.900 to 0.975). Conversely, no such association was observed in other countries (OR =0.969, 95% CI =0.934 to 1.006). Additionally, we found that none of the studies analyzing the association between the intake of aspirin and prostate cancer risk was conducted in Asian countries, probably because of different daily habits in those countries; this aspect requires further research.
A total of 11 studies were associated with all-NSAID intake and prostate cancer risk. We found a positive relation between all-NSAID intake and prostate cancer risk, owing to more recent research, compared with previous meta-analyses (OR =1.18, 95% CI =1.15 to 1.22). Similar results were found between all-NSAID intake and advanced prostate cancer (OR =1.43, 95% CI =1.32 to 1.56). To explain the phenomenon, we took a deeper look at the included studies. One study conducted in Finland illustrated a significantly elevated prostate cancer risk of total and advanced prostate cancer among all-NSAID users [27]. The association was not dose-dependent in the study, indicating that it could result from systematic differences between users of prescription NSAIDs and users of non-prescription NSAIDs. Furthermore, people who take prescribed NSAIDs are possibly frequent users of other health services, such as cancer screening, including testing for prostate-specific antigen. This could bring about a positive detection bias.
Meanwhile, among the 14 articles associated with nonaspirin NSAIDs and prostate cancer risk, we observed a marginally inverse correlation between them (OR =0.94, 95% CI =0.90 to 0.98). Nevertheless, an opposite correlation was detected between nonaspirin NSAIDs intake and advanced prostate cancer (OR =1.25, 95% CI =1.13 to 1.38).
Our study could have limitations in several ways
Firstly, although no publication bias was detected in our analysis, using both Begg’s and Egger’s tests, the strategy of selection of published studies in English only and the exclusion of studies without sufficient information could bring about possible publication bias, which remains an alternative explanation of our results. Furthermore, our search was restricted to published articles, which could also cause potential bias to affect our findings.
Secondly, both case-control and cohort studies with different populations, drug intakes, and outcomes were included in our analysis. Considering the existing heterogeneity, it could be inappropriate to choose a single global effect estimate to pool the data, and the summarized estimates in our study should be treated with caution [46]. Therefore, we performed stratified analyses to explain the possible sources of heterogeneity [47]. Additionally, some studies were case-control ones; these could lead to selection and recall bias.
Thirdly, there are several types of NSAID, which could have different effects on prostate cancer. However, most studies recruited in our study merely provided general data on NSAID intake rather than detailed information on specific dose and duration of use, which might bring about inaccurate estimates.
Conclusions
We conducted a detailed meta-analysis for summarized OR estimates from researches focused on the association between NSAIDs intake and prostate cancer risk. Although we could not deny possible confounding factors, the results indicated a marginally inverse correlation between the intake of aspirin and prostate cancer risk. On the contrary, a positive relation between all-NSAID intake and prostate cancer was observed. Further research needs to be conducted to better clarify potential biological mechanisms.
Acknowledgements
The work was supported by grants from the National Key Clinical Specialty Construction Project of China, key medical disciplines of Zhejiang province, Combination of traditional Chinese and Western medicine key disciplines of Zhejiang province (2012-XK-A23), and a health sector scientific research special project (201002010).
Abbreviations
- CI
confidence interval
- NSAID
nonsteroidal anti-inflammatory drug
- OR
odds ratio.
Footnotes
Competing interests
The authors declare that they had no competing interest.
Authors’ contributions
XW and YWL drafted the manuscript. JW, YZ, and XYZ assisted with manuscript preparation. XLX, ZHH, and XX extracted the data from the articles. ZL, SQL, and LPX revised the manuscript. All authors have read and approved the final manuscript.
Contributor Information
Xiao Wang, Email: zjuwangxiao@126.com.
Yi-wei Lin, Email: 31918102@qq.com.
Jian Wu, Email: 11318142@zju.edu.cn.
Yi Zhu, Email: 21018052@zju.edu.cn.
Xiang-lai Xu, Email: oct24wangxiao@gmail.com.
Xin Xu, Email: 21018037@zju.edu.cn.
Zhen Liang, Email: zjuwangxiao@zju.edu.cn.
Zheng-hui Hu, Email: zjuwangxiao2010@gmail.com.
Shi-qi Li, Email: sherwinbayern@163.com.
Xiang-yi Zheng, Email: 524615925@qq.com.
Li-ping Xie, Email: xielp@zjuem.zju.edu.cn.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Belpomme D, Irigaray P, Ossondo M, Vacque D, Martin M. Prostate cancer as an environmental disease: an ecological study in the French Caribbean islands, Martinique and Guadeloupe. Int J Oncol. 2009;34(4):1037–1044. doi: 10.3892/ijo_00000229. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Wang S, Lin YW, Wu J, Chen H, Mao YQ, Zheng XY, Zhou C, Xie LP. Angiotensin-converting enzyme insertion/deletion polymorphism and the risk of prostate cancer in the Han population of China. Med Oncol. 2012;29(3):1964–1971. doi: 10.1007/s12032-011-0051-5. [DOI] [PubMed] [Google Scholar]
- 4.Quinn M, Babb P. Patterns and trends in prostate cancer incidence, survival, prevalence and mortality. Part I: international comparisons. BJU Int. 2002;90(2):162–173. doi: 10.1046/j.1464-410X.2002.2822.x. [DOI] [PubMed] [Google Scholar]
- 5.Rostom A, Dube C, Lewin G, Tsertsvadze A, Barrowman N, Code C, Sampson M, Moher D. NSAIDs and cyclooxygenase-2 inhibitors for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force. Ann Intern Med. 2007;146(5):376–389. doi: 10.7326/0003-4819-146-5-200703060-00010. [DOI] [PubMed] [Google Scholar]
- 6.Corley DA, Kerlikowske K, Verma R, Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology. 2003;124(1):47–56. doi: 10.1053/gast.2003.50008. [DOI] [PubMed] [Google Scholar]
- 7.Khuder SA, Mutgi AB. Breast cancer and NSAID use: a meta-analysis. Br J Cancer. 2001;84(9):1188–1192. doi: 10.1054/bjoc.2000.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 9.Sabichi AL, Lippman SM. COX-2 inhibitors and other NSAIDs in genitourinary cancer. Semin Oncol. 2004;31(2 Suppl 7):36–44. doi: 10.1053/j.seminoncol.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 10.Mahmud SM, Franco EL, Aprikian AG. Use of NSAIDs and prostate cancer risk: a meta-analysis. Int J Cancer. 2010;127(7):1680–1691. doi: 10.1002/ijc.25186. [DOI] [PubMed] [Google Scholar]
- 11.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 12.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 16.Paganini-Hill A, Chao A, Ross RK, Henderson BE. Aspirin use and chronic diseases: a cohort study of the elderly. BMJ. 1989;299(6710):1247–1250. doi: 10.1136/bmj.299.6710.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ratnasinghe LD, Graubard BI, Kahle L, Tangrea JA, Taylor PR, Hawk E. Aspirin use and mortality from cancer in a prospective cohort study. Anticancer Res. 2004;24(5B):3177–3184. [PubMed] [Google Scholar]
- 18.Leitzmann MF, Stampfer MJ, Ma J, Chan JM, Colditz GA, Willett WC, Giovannucci E. Aspirin use in relation to risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2002;11(10 Pt 1):1108–1111. [PubMed] [Google Scholar]
- 19.Sorensen HT, Friis S, Norgard B, Mellemkjaer L, Blot WJ, McLaughlin JK, Ekbom A, Baron JA. Risk of cancer in a large cohort of nonaspirin NSAID users: a population-based study. Br J Cancer. 2003;88(11):1687–1692. doi: 10.1038/sj.bjc.6600945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts RO, Jacobson DJ, Girman CJ, Rhodes T, Lieber MM, Jacobsen SJ. A population-based study of daily nonsteroidal anti-inflammatory drug use and prostate cancer. Mayo Clin Proc. 2002;77(3):219–225. doi: 10.4065/77.3.219. [DOI] [PubMed] [Google Scholar]
- 21.Bosetti C, Talamini R, Negri E, Franceschi S, Montella M, La Vecchia C. Aspirin and the risk of prostate cancer. Eur J Cancer Prev. 2006;15(1):43–45. doi: 10.1097/01.cej.0000180665.04335.de. [DOI] [PubMed] [Google Scholar]
- 22.Schreinemachers DM, Everson RB. Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiology. 1994;5(2):138–146. doi: 10.1097/00001648-199403000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Dasgupta K, Di Cesar D, Ghosn J, Rajan R, Mahmud S, Rahme E. Association between NSAIDs and prostate cancer occurrence. Cancer J. 2006;12(2):130–135. [PubMed] [Google Scholar]
- 24.Habel LA, Zhao W, Stanford JL. Daily aspirin use and prostate cancer risk in a large, multiracial cohort in the US. Cancer Causes Control. 2002;13(5):427–434. doi: 10.1023/A:1015788502099. [DOI] [PubMed] [Google Scholar]
- 25.Nelson JE, Harris RE. Inverse association of prostate cancer and non-steroidal anti-inflammatory drugs (NSAIDs): results of a case–control study. Oncol Rep. 2000;7(1):169–170. doi: 10.3892/or.7.1.169. [DOI] [PubMed] [Google Scholar]
- 26.Mahmud SM, Tanguay S, Begin LR, Franco EL, Aprikian AG. Non-steroidal anti-inflammatory drug use and prostate cancer in a high-risk population. Eur J Cancer Prev. 2006;15(2):158–164. doi: 10.1097/01.cej.0000197451.02604.25. [DOI] [PubMed] [Google Scholar]
- 27.Veitonmaki T, Tammela TL, Auvinen A, Murtola TJ. Use of aspirin, but not other non-steroidal anti-inflammatory drugs is associated with decreased prostate cancer risk at the population level. Eur J Cancer. 2013;49(4):938–945. doi: 10.1016/j.ejca.2012.09.030. [DOI] [PubMed] [Google Scholar]
- 28.Jacobs EJ, Rodriguez C, Mondul AM, Connell CJ, Henley SJ, Calle EE, Thun MJ. A large cohort study of aspirin and other NSAIDs and prostate cancer incidence. J Natl Cancer Inst. 2005;97(13):975–980. doi: 10.1093/jnci/dji173. [DOI] [PubMed] [Google Scholar]
- 29.Friis S, Sorensen HT, McLaughlin JK, Johnsen SP, Blot WJ, Olsen JH. A population-based cohort study of the risk of colorectal and other cancers among users of low-dose aspirin. Br J Cancer. 2003;88(5):684–688. doi: 10.1038/sj.bjc.6600760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shebl FM, Sakoda LC, Black A, Koshiol J, Andriole GL, Grubb R, Church TR, Chia D, Zhou C, Chu LW, Huang WY, Peters U, Kirsh VA, Chatterjee N, Leitzmann MF, Hayes RB, Hsing AW. Aspirin but not ibuprofen use is associated with reduced risk of prostate cancer: a PLCO study. Br J Cancer. 2012;107(1):207–214. doi: 10.1038/bjc.2012.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neugut AI, Rosenberg DJ, Ahsan H, Jacobson JS, Wahid N, Hagan M, Rahman MI, Khan ZR, Chen L, Pablos-Mendez A, Shea S. Association between coronary heart disease and cancers of the breast, prostate, and colon. Cancer Epidemiol Biomarkers Prev. 1998;7(10):869–873. [PubMed] [Google Scholar]
- 32.Murad AS, Smith GD, Lewis SJ, Cox A, Donovan JL, Neal DE, Hamdy FC, Martin RM. A polymorphism in the glucokinase gene that raises plasma fasting glucose, rs1799884, is associated with diabetes mellitus and prostate cancer: findings from a population-based, case-control study (the ProtecT study) Int J Mol Epidemiol Genet. 2010;1(3):175–183. [PMC free article] [PubMed] [Google Scholar]
- 33.Perron L, Bairati I, Moore L, Meyer F. Dosage, duration and timing of nonsteroidal antiinflammatory drug use and risk of prostate cancer. Int J Cancer. 2003;106(3):409–415. doi: 10.1002/ijc.11250. [DOI] [PubMed] [Google Scholar]
- 34.Langman MJ, Cheng KK, Gilman EA, Lancashire RJ. Effect of anti-inflammatory drugs on overall risk of common cancer: case–control study in general practice research database. BMJ. 2000;320(7250):1642–1646. doi: 10.1136/bmj.320.7250.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Irani J, Ravery V, Pariente JL, Chartier-Kastler E, Lechevallier E, Soulie M, Chautard D, Coloby P, Fontaine E, Bladou F, Desgrandchamps F, Haillot O. Effect of nonsteroidal anti-inflammatory agents and finasteride on prostate cancer risk. J Urol. 2002;168(5):1985–1988. doi: 10.1016/S0022-5347(05)64277-2. [DOI] [PubMed] [Google Scholar]
- 36.García Rodríguez LA, González-Pérez A. Inverse association between nonsteroidal anti-inflammatory drugs and prostate cancer. Cancer Epidemiol Biomarkers Prev. 2004;13(4):649–653. [PubMed] [Google Scholar]
- 37.Dhillon PK, Kenfield SA, Stampfer MJ, Giovannucci EL. Long-term aspirin use and the risk of total, high-grade, regionally advanced and lethal prostate cancer in a prospective cohort of health professionals, 1988-2006. Int J Cancer. 2011;128(10):2444–2452. doi: 10.1002/ijc.25811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norrish AE, Jackson RT, McRae CU. Non-steroidal anti-inflammatory drugs and prostate cancer progression. Int J Cancer. 1998;77(4):511–515. doi: 10.1002/(SICI)1097-0215(19980812)77:4<511::AID-IJC6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 39.Brasky TM, Velicer CM, Kristal AR, Peters U, Potter JD, White E. NSAIDs and prostate cancer risk in the VITamins And Lifestyle (VITAL) cohort. Cancer Epidemiol Biomarkers Prev. 2010;19(12):3185–3188. doi: 10.1158/1055-9965.EPI-10-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Platz EA, Rohrmann S, Pearson JD, Corrada MM, Watson DJ, De Marzo AM, Landis PK, Metter EJ, Carter HB. NSAIDs and risk of prostate cancer in the Baltimore Longitudinal Study of Aging. Cancer Epidemiol Biomarkers Prev. 2005;14(2):390–396. doi: 10.1158/1055-9965.EPI-04-0532. [DOI] [PubMed] [Google Scholar]
- 41.Menezes RJ, Swede H, Niles R, Moysich KB. Regular use of aspirin and prostate cancer risk (United States) Cancer Causes Control. 2006;17(3):251–256. doi: 10.1007/s10552-005-0450-z. [DOI] [PubMed] [Google Scholar]
- 42.Coogan PF, Kelly JP, Strom BL, Rosenberg L. Statin and NSAID use and prostate cancer risk. Pharmacoepidemiol Drug Saf. 2010;19(7):752–755. doi: 10.1002/pds.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salinas CA, Kwon EM, FitzGerald LM, Feng Z, Nelson PS, Ostrander EA, Peters U, Stanford JL. Use of aspirin and other nonsteroidal antiinflammatory medications in relation to prostate cancer risk. Am J Epidemiol. 2010;172(5):578–590. doi: 10.1093/aje/kwq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahmud SM, Franco EL, Turner D, Platt RW, Beck P, Skarsgard D, Tonita J, Sharpe C, Aprikian AG. Use of non-steroidal anti-inflammatory drugs and prostate cancer risk: a population-based nested case-control study. PLoS One. 2011;6(1):e16412. doi: 10.1371/journal.pone.0016412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lieberman R. Chemoprevention of prostate cancer: current status and future directions. Cancer Metastasis Rev. 2002;21(3–4):297–309. doi: 10.1023/A:1021267128567. [DOI] [PubMed] [Google Scholar]
- 46.Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997;315(7121):1533–1537. doi: 10.1136/bmj.315.7121.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]


