Abstract
Purpose
We explored the mechanisms leading to the distinct overexpression of EPOR as well as the effects of EPO signaling on ETV6/RUNX1-positive acute lymphoblastic leukemias.
Experimental Design
ETV6/RUNX1-expressing model cell lines and leukemic cells were used for real-time PCR of EPOR expression. Proliferation, viability, and apoptosis were analyzed on cells exposed to EPO, prednisone, or inhibitors of EPOR pathways by [3H]thymidine incorporation, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay, and Annexin V/propidium iodide staining. Western blot analysis was done to detect activation of signaling proteins. Serum EPO levels and sequences of the EPOR (n = 53) as well as hemoglobin levels were taken from children with acute lymphoblastic leukemia enrolled in Austrian protocols.
Results
We show here that ectopic expression of ETV6/RUNX1 induced EPOR up-regulation. Anemia, however, did not appear to influence EPOR expression on leukemic cells, although children with ETV6/RUNX1-positive leukemias had a lower median hemoglobin than controls. Exposure to EPO increased proliferation and survival of ETV6/RUNX1-positive leukemias in vitro, whereas blocking its binding site did not alter cell survival. The latter was not caused by activating mutations in the EPOR but might be triggered by constitutive activation of phosphatidylinositol 3-kinase/Akt, the major signaling pathway of EPOR in these cells. Moreover, prednisone-induced apoptosis was attenuated in the presence of EPO in this genetic subgroup.
Conclusions
Our data suggest that ETV6/RUNX1 leads to EPOR up-regulation and that activation by EPO might be of relevance to the biology of this leukemia subtype. Further studies are, however, needed to assess the clinical implications of its apoptosis-modulating properties.
The t(12;21)(p13;q22) with its molecular counterpart, the ETV6/RUNX1 (also known as TEL/AML1) fusion gene, is present in ~25% of childhood B-cell precursor (BCP) acute lymphoblastic leukemia (ALL) and generally implies a good prognosis (1, 2). Accumulating evidence suggests that the fusion gene formation occurs already in utero based on its presence at birth in the majority of children with ETV6/RUNX1-positive ALL (3). The cell, however, in which the fusion gene originates is not known, but it is presumably at a progenitor stage preceding the transition from pro-B to pre-B (4, 5). Based on studies in healthy newborns and animal models, the fusion gene alone is not sufficient for the clinical manifestation of the leukemia, which requires secondary events (4, 6-10).
Gene expression profiling has been employed to characterize the different genetic subgroups of leukemias (1, 11). By this means, a relatively high expression of EPOR was found to distinguish the ETV6/RUNX1-positive leukemias from the other subgroups of ALL. It was therefore assumed that signaling by EPOR might be important for the pathogenesis of this leukemia subtype (11).
Expression of the EPOR and its activation by EPO has, for a long time, been exclusively associated with the erythroid lineage in which EPOR signaling is pivotal for differentiation, proliferation, and survival of progenitor cells (12, 13). Over the last years, it has become well established that the EPOR is also expressed in many normal and malignant tissues, suggesting a potential influence on cell survival (12, 13). This fact turned out to be an important issue when recombinant human EPO became available for clinical use to alleviate cancer-associated anemia and its side effects. Consequently, many in vivo and in vitro studies have been initiated to explore the effect of EPO on its apoptosis-modulating function in tumor cells. These efforts have led to contradictory results, potentially reflecting distinct biological features and activated pathways of the different malignancies (12, 13).
The signaling cascade triggered by EPO has been widely studied in the erythroid lineage. On engagement of EPO with its receptor, Janus kinase 2 is activated, which, in turn, phosphorylates the EPOR. The ensuing signaling cascade includes the STAT5, mitogen-activated protein kinase, and phosphatidylinositol 3-kinase (PI3K)/Akt pathways conferring proliferative and antiapoptotic function (12, 13). At present, there is only little information on the signaling pathways affected by the EPOR in leukemia cells.
Herein, we provide first evidence that the EPOR is expressed as a function of the ETV6/RUNX1 fusion protein. EPO promotes proliferation of ETV6/RUNX1-positive leukemias and interferes with prednisone-induced apoptosis in vitro. The PI3K/Akt pathway appears to be involved in the observed EPO-mediated survival advantage.
Materials and Methods
Leukemic cells and cell culture
REH, AT-1, and AT-2 (ETV6/RUNX1-positive BCP leukemia cell lines), (AT-1 and AT-2 were kindly provided by J.D. Rowley, University of Chicago), Nalm6 and SEM (both BCP ALL), K562 (erythroid blast crisis of chronic myeloid leukemia), and Jurkat (T-ALL) cells were cultured in RPMI 1640 with Glutamax (Life Technologies/Invitrogen) supplemented with 10% heat-inactivated FCS, 100 IU/mL penicillin, and 100 g/mL streptomycin (all PAA).
Mouse pro-B Ba/F3 cells were grown in RPMI 1640 containing 10% FCS and 5% WEHI-3B conditioned medium as a source for interleukin-3. Stably ETV6/RUNX1-expressing Ba/F3 clones and empty vector controls were established as reported previously (14).
HEK 293 cells, transformed human embryonic kidney cells, were grown in RPMI 1640 containing 10% FCS. ETV6/RUNX1 cDNA was inserted into a pcDNA3.1-myc expression vector (Invitrogen). Stably expressing ETV6/RUNX1 clones were obtained after single-cell dilution and clonal expansion of transfected and G418 (900 μg/mL)-selected cells.
Primary leukemic cells were obtained from bone marrow aspirations from children with ALL. Written informed consent was obtained from the patients or their parents. The study was approved by the ethical committees of the Children’s Cancer Research Institute and the St. Anna Kinderspital. Cells were isolated by density-gradient centrifugation before further processing. For positive selection, mononuclear cells containing >95% of leukemic blasts were incubated with anti-CD10 FITC antibody (DakoCytomation) followed by incubation with anti-FITC magnetic beads and magnetic field separation using MACS separation columns (Miltenyi Biotec) according to the manufacturer’s recommendation and cultured within 4 h after aspiration in IMDM with 20% FCS, 100 IU/mL penicillin, and 100 g/mL streptomycin at 37 °C in 5% CO2 in a humidified incubator.
For stimulation with growth factors and treatment with pathway inhibitors, cells were washed in PBS and serum-deprived overnight in RPMI 1640 containing 0.1% bovine serum albumin (Invitrogen). The PI3K and Jak kinase inhibitors Ly294002 and AG490 (Calbiochem) were used at 25 and 10 μmol/L concentrations, respectively. To assess cell proliferation and viability, cells were plated in triplicates at a density of 1 × 105 to 2 × 105 in flat-bottomed 96-well plates (Iwaki) in 100 μL RPMI 1640 without supplements and stimulated with different concentrations of EPO (10-100 units/mL; Neorecormon; Roche). The monoclonal anti-human EPOR antibody MAB307 (R&D Systems), which binds to the extracellular part of the EPOR and was shown to block specifically EPO-mediated effects, was used as a blocking antibody at a concentration of 30 μg/mL as reported previously (15). Exposure to drugs was done as described above in the presence of 10% FCS with the addition of prednisone (Solu-Dacortin; Merck) in LC50 concentrations (REH, 1 mg/mL; AT-1 and AT-2, 1 μg/mL; Nalm6 and SEM, 0.5 mg/mL; primary leukemia cells, 50 μg/mL).
SDS-PAGE and Western blot analysis
Whole-cell lysates were prepared with radioimmunoprecipitation assay buffer (50 mmol/L Tris, 1% NP-40, 0.25% sodium deoxycholate, 150 mmol/L NaCl, 1 mmol/L EDTA) supplemented with 1 mmol/L NaVO4 and 1% protease inhibitor cocktail (Roche) as described previously (14, 16). For equal loading, the protein concentration of each sample was determined by Bio-Rad protein assay kit. Proteins were resolved by 8% SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Whatman). Nonspecific binding on the membranes was blocked with PBS containing 5% dry milk. The membranes were probed with antibodies specific for phospho-AKT, AKT (Cell Signaling Technology), poly(ADP-ribose) polymerase (PARP; C210; Becton Dickinson), and glyceraldehyde 3-phosphate dehydrogenase (6C5; Santa Cruz Biotechnology) or tubulin (DM1A; Calbiochem) in 1% dry milk in PBS at 4 °C overnight. Using infrared dye-labeled secondary antibodies (LI-COR Biosciences), the membranes were directly scanned with Odyssey Infrared Imaging System (LI-COR Biosciences). Bands were quantified using LI-COR Odyssey Software.
Proliferation, viability, and apoptosis assays
Cells were cultured as described above with different agents for 48 h. [3H]thymidine (1 μCi/well; Hanke Laboratory Products) was added for 24 h. Cells were harvested onto filters and counted in a scintillation counter.
In 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assays, a 5 mg/mL stock solution of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (20 μL; Sigma) were added according to the times indicated. After 3 to 5 h, 100 μL stop solution [10% SDS and 50% formamide (pH 4.7) with acetic acid] was added to dissolve formazan crystals. The absorbance was read at 562 nm in a microplate reader. To assess survival of cells, the mean value of cells cultured with the respective agent was divided by the mean value of control cells × 100%.
The presence of early and late apoptotic cells was determined by flow cytometry (FACSCalibur; BD Biosciences) with anti-Annexin V FITC-labeled antibody (BD Pharmingen) and propidium iodide. Cells were washed in 10 mmol/L HEPES (pH 7.4), 140 mmol/L NaCl, and 2.5 mmol/L CaCl2. Antibody and propidium iodide were added and incubated for 15 min in the dark before analysis. All experiments were done in triplicates and reproduced at least three times in independent experiments.
Sequencing of the EPOR gene
Genomic DNA was isolated with QIAamp DNA Blood Mini Kit (Qiagen). Specific primers for exons 2 to 4 and 6 to 8, located at the respective flanking intronic sequences, were used for amplification.
The nucleotide sequence of the EPOR was determined by sequencing analysis (VBC Genomics) and compared with public database sequences.4
EPO serum levels and EPOR mRNA quantification
The EPO serum levels were determined by an automated chemiluminescent immuno-assay (DPC Immulite; ref. 17).
Random hexamer priming and SuperScript II (Invitrogen) were used to generate cDNA. Quantification of EPOR mRNA abundance in primary leukemias was done as reported previously (11). β2-Microglobulin was used for endogenous control gene amplification. The quantitative PCRs were done in a total volume of 25 μL containing 12.5 μL TaqMan Universal PCR Master Mix (Applied Biosystems), 200 nmol/L forward and reverse primers each, and 100 nmol/L Taqman probe. The reactions were carried out on an ABI Prism 7700 Sequence Detection System (Applied Biosystems).
For amplification of EPOR mRNA expression in cell lines, SYBR Green PCR was done. Primers and conditions were the same as for TaqMan PCR. For murine cell lines, the following EPOR and HPRT (for control amplification) primers were used: EPOR forward 5′-GCCCCCTCTGTCTCCTACT-3′, EPOR reverse 5′-TCCCAGAAACACACCAAGTCTT-3′, HPRT forward 5′-GGGGGCTATAAGTTCTTTGC-3′, and HPRT reverse 5′-TCCAACACTTCGAGAGGTCC-3′.
Blood counts including measurement of hemoglobin (Hb) from patients was analyzed by Advia 120 Hematology System (Bayer).
Statistical analysis
Differences of Hb levels in various leukemia subgroups were assessed by the Wilcoxon two-sample test. The Welch t test was used to analyze the increase in EPOR mRNA expression in cells stably transfected with ETV6/RUNX1, proliferation assays, and cell viability assays. Probability values (P < 0.05) were considered statistically significant.
Results
ETV6/RUNX1 induces up-regulation of EPOR mRNA expression
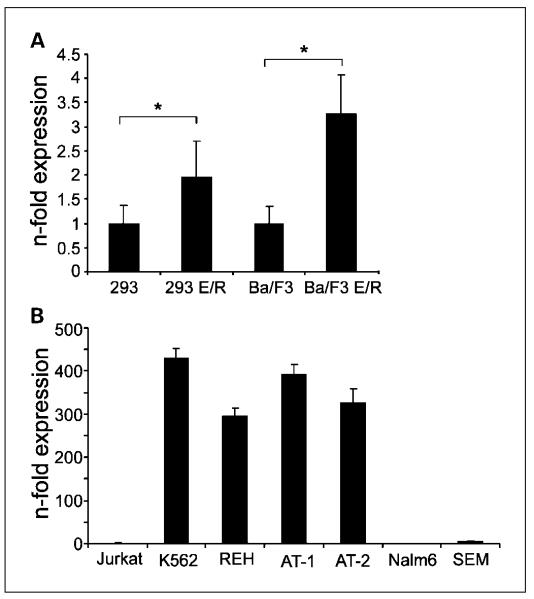
Based on the high expression of the EPOR in ETV6/RUNX1-positive leukemias, we assessed whether EPOR is up-regulated as a result of the fusion gene expression. Stably ETV6/RUNX1-expressing clones of human embryonic kidney HEK 293 cells and murine pro-B Ba/F3 cells showed a 2- to 3.5-fold difference in mRNA expression compared with empty vector controls (Fig. 1A).
Fig. 1.
EPOR expression in ectopic ETV6/RUNX1 transfectants and leukemic cell lines. Quantification of EPOR mRNA expression by real-time quantitative PCR. A, EPOR expression in ectopically ETV6/RUNX1 (E/R)-expressing HEK 293 and Ba/F3 cells. B, EPOR expression in ETV6/RUNX1-positive (REH, AT-1, and AT-2) and ETV6/RUNX1-negative (SEM, Nalm6, Jurkat, and K562) leukemic cell lines. EPOR mRNA levels were determined at least in triplicates and normalized to either HPRT in Ba/F3 cells or h2-microglobulin expression in the remaining cell lines.The n-fold expression was calculated from ETV6/RUNX1-expressing cells relative to control vector-containing cells in A or to Jurkat cells in B. Bars, SD. *, P < 0.01.
Next, we determined the expression levels of EPOR in our model cell lines. In line with data by Fine et al. (11), quantification of mRNA revealed that the EPOR is highly expressed in all ETV6/RUNX1-positive BCP ALL cell lines but barely detectable in the ETV6/RUNX1-negative BCP and T-ALL cell lines (Fig. 1B). Jurkat was used for normalization and K562 was used as a positive control for EPOR overexpression. We further evaluated whether these transcriptional differences between ETV6/RUNX1-positive and ETV6/RUNX1-negative leukemias would also be detectable at the protein level. Because lack of specificity of several EPOR antibodies cautions its usage for Western blotting (18, 19), the directly labeled EPOR antibody (MAB307-PE), which competes with EPO for the binding to its receptor (20), was used for surface protein detection by flow cytometry. There was, however, no clear difference between ETV6/RUNX1-positive and ETV6/RUNX1-negative leukemias (data not shown) possibly due to generally low abundance of EPOR expression on the cell surface (19).
Low Hb is associated with the ETV6/RUNX1 genetic subtype of ALL
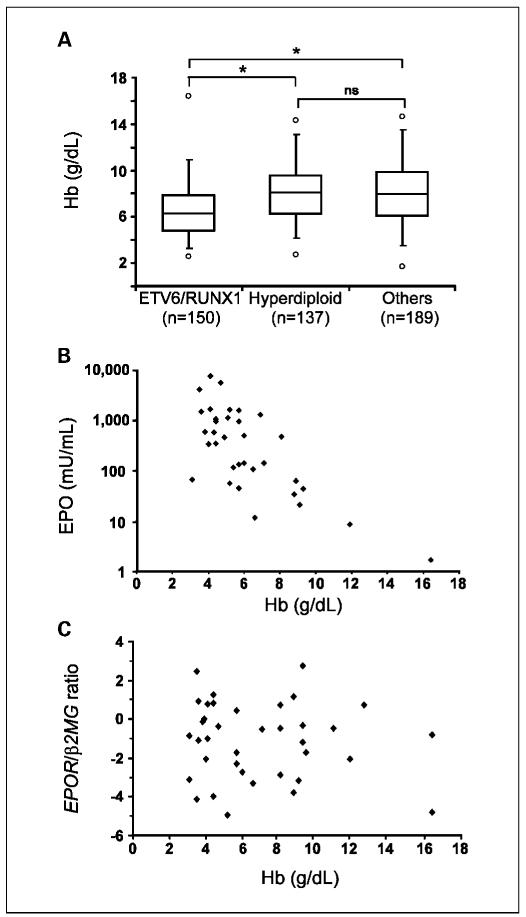
We also considered the possibility that, in ETV6/RUNX1-positive leukemias, EPOR expression is, to some extent, further influenced by other factors known to up-regulate the receptor (21), as, for example, hypoxemia, the fundamental physiologic stimulus that causes a rapid increase in renal production of EPO (22). It was thus assessed whether children with ETV6/RUNX1-positive ALL have lower Hb levels than those with other subgroups of BCP ALL. For this purpose, 476 patients with BCP ALL, who were consecutively registered in the Austrian BFM 95 and 2000 studies (23, 24), were included. BCP leukemias were grouped in ETV6/RUNX1-positive cases, leukemias with a high hyperdiploid chromosome number (subsequently designated as hyperdiploid leukemias), and “others” (n = 150, 137, and 189, respectively). The latter are characterized by the lack of the previous group characteristics. As shown in Fig. 2A, the median Hb level of children with ETV6/RUNX1-positive ALL is significantly lower (P < 0.001) compared with those with hyperdiploid and “other” leukemias, whereas the difference between the latter two groups is not significant (P = 0.77). Next, we confirmed that for this particular genetic subgroup of leukemias, Hb and EPO are inversely correlated (Fig. 2B), indicating that EPO production is adequate for the Hb level, a finding that has been reported previously for genetically undefined cases of childhood ALL (25). This allowed us to use Hb levels as a readily available correlate for the amount of EPO in the serum. Hence, to evaluate an influence of EPO on its receptor expression, patients were selected according to their Hb level to fit into one of the three arbitrarily chosen groups (Hb levels 3-5, >5 to 8, and >8 to 15 g/dL). This approach should enable the analysis of a wide range of physiologic EPO concentrations in the serum (0.01-10 units/mL). As illustrated in Fig. 2C, the amount of EPOR mRNA did not change as a function of Hb, supporting our view that EPO does not play a major role in EPOR mRNA up-regulation in ETV6/RUNX1-positive leukemia.
Fig. 2.
Low Hb levels inversely correlate with serum EPO levels but not with EPOR mRNA expression in ETV6/RUNX1-positive leukemias. A, Hb levels from patients with various subtypes of BCP leukemia.The box shows the median and the interquartile range (25-75th percentiles). Ninety-five percent of the values are within the range of a whisker and the circles indicate the minimum and maximum values in each group. *, P < 0.001 (Wilcoxon two-sample test). B, correlation between EPO and Hb levels in patients with ETV6/RUNX1-positive leukemia. C, comparison of individual patients’ Hb level, as a surrogate parameter of EPO, with relative expression levels of EPOR, normalized to β2-microglobulin (β2MG) and shown on a log2 scale, in matched leukemic cells.
Lack of activating mutations of the EPOR in ETV6/RUNX1-positive leukemias
Based on the observation that overexpressed receptor kinase genes (e.g., FLT3, C-KIT, and NOTCH1) are frequently constitutively activated by mutations in acute leukemia (26-28) and that activating mutations of the EPOR are present in polycythemia vera (29), we evaluated whether such mutations would also occur in ETV6/RUNX1-positive ALL. Exons 2, 3, and 6 to 8 of the EPOR were chosen for sequencing based on their functional importance and the presence of previously identified mutations in these regions (29, 30). As no genomic variations in the EPOR sequence were, at least in the dominant clone of the leukemic population, detected in ETV6/RUNX1-positive leukemias (primary leukemias, n = 53; leukemic cell lines, n = 3), we assume that activating mutations are rarely present in these leukemias, if they occur at all.
EPO enhances proliferation of ETV6/RUNX1-positive leukemias and attenuates the sensitivity to prednisone-induced apoptosis in vitro
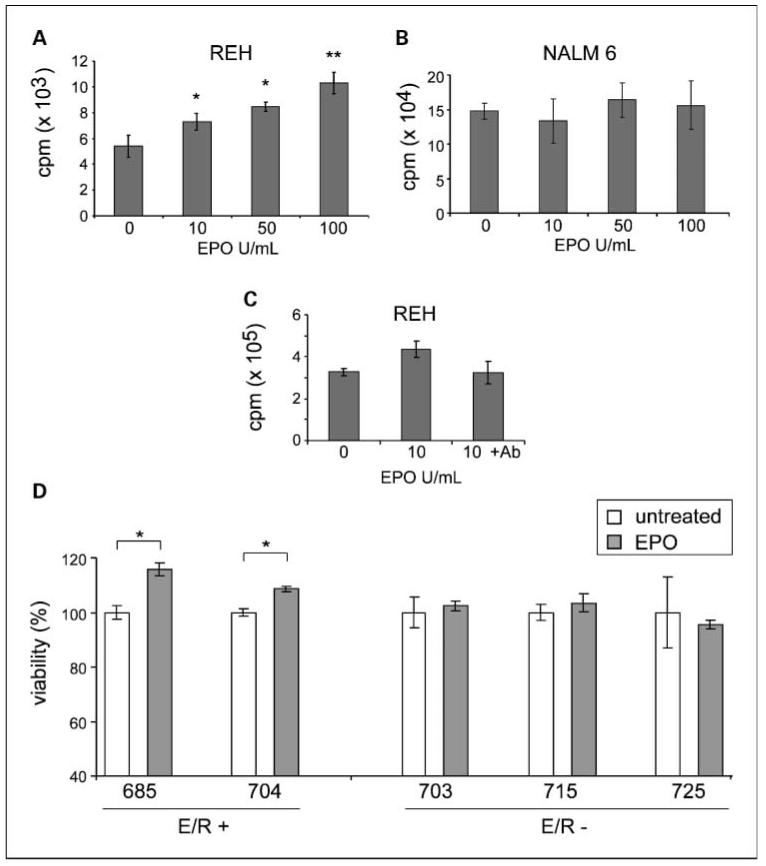
After that, we evaluated whether the overexpression of EPOR provides a proliferative or survival advantage to ETV6/RUNX1-positive leukemias. REH was compared with Nalm6, an ETV6/RUNX1-negative BCP leukemia cell line. Cells were cultured under reduced serum conditions in the presence of different doses of EPO for 72 h. REH cells exhibited a dose-dependent increase in proliferation on addition of EPO (Fig. 3A), whereas Nalm6 did not (Fig. 3B). Specificity of these effects was confirmed by an anti-EPOR blocking antibody, which abrogates EPO-induced effects, as shown in Fig. 3C. Enhanced viability by EPO was also observed in two ETV6/RUNX1-positive primary leukemias but in none of the three ETV6/RUNX1-negative cases (Fig. 3D). Collectively, these data suggest that the EPOR conveys a survival advantage in ETV6/RUNX1-expressing leukemic cells.
Fig. 3.
EPO promotes proliferation and survival of ETV6/RUNX1-positive leukemia cells. Cell proliferation was determined by [3H]thymidine incorporation in REH (A) and Nalm6 (B) cells cultured in the presence of increasing concentrations of EPO and in REH cells grown with EPO and anti-EPOR antibody (C). One representative example of at least three independent experiments is shown. (The difference in counts between A and C results from different cell numbers used for these experiments). D, differences in the metabolic status of cells used to assess cell viability (%) was determined in ETV6/RUNX1-positive (E/R+) and ETV6/RUNX1-negative (E/R−) primary leukemias by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay after exposure to EPO (50 units/mL) for 72 h. Leukemic cells were isolated and cultured within 4 h after bone marrow aspiration. *, P < 0.05; **, P < 0.01.
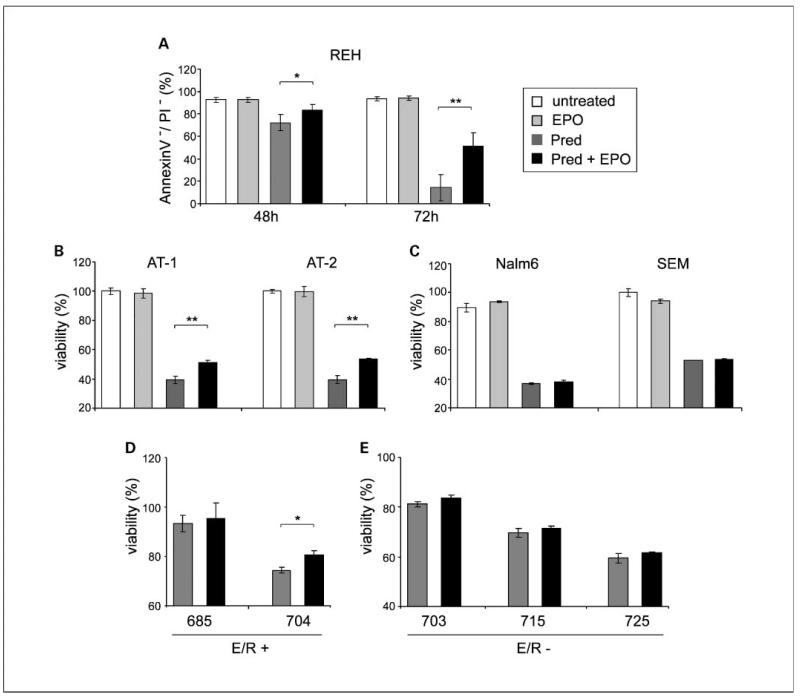
It has been reported previously that EPO may modulate the response of malignant cells to drugs (12, 13). We therefore explored the effects of EPO on drug-induced apoptosis in REH cells as a model system. Different drugs (prednisone, vincristine, daunorubicine, methotrexate, and etoposide), which are currently used in treatment protocols for children with ALL, were tested at published LC50 concentrations (ref. 31; data not shown). EPO (50 units/mL) reduced only prednisone-induced apoptosis by 10% to 16% (Fig. 4A). This effect was Jak dependent, because AG490, a Jak kinase inhibitor, completely abolished the effects of EPO by blocking the direct downstream signaling proteins of EPOR (data not shown). A survival advantage by EPO was also reproduced in two other ETV6/RUNX1-positive leukemic cell lines, AT-1 and AT-2, by 12% to 14% (Fig. 4B). AT cell lines are, similar to the majority of ETV6/RUNX1-positive primary leukemias (32) but unlike REH cells, sensitive to glucocorticoids. In the ETV6/RUNX1-negative BCP cell lines Nalm6 and SEM, EPO had no effects on prednisone-induced apoptosis (Fig. 4C). The alleviation of glucocorticoid-induced apoptosis by EPO was also confirmed in primary ETV6/RUNX1-positive leukemias. To exclude a possible effect of normal EPO-responsive erythroid progenitor cells, which might be present in density-gradient separated mononuclear cells, highly purified (CD10+) primary leukemic blast cells were prepared for these experiments. One of the two ETV6/RUNX1-positive leukemias (patient ID 704) was responsive to glucocorticoid concentrations used for in vitro experiments and displayed a reduced prednisone-induced apoptosis in the presence of EPO (Fig. 4D), whereas the other (patient ID 685) did not respond at all. There was no EPO-mediated influence on ETV6/RUNX1 -negative leukemias (n = 3) detectable (Fig. 4E). Of note, all leukemias used for these experiments had a good prednisone response in vivo. It is therefore concluded that EPOR signaling may lead to an increased survival of ETV6/RUNX1-positive leukemia cells when exposed to prednisone in vitro.
Fig. 4.
EPO attenuates prednisone-induced apoptosis in ETV6/RUNX1-positive leukemias. A, apoptosis rates were determined byAnnexinV/propidium iodide staining in REH cells cultured for 48 and 72 h in the presence of prednisone (Pred; 1mg/mL) and EPO (50 units/mL). The percentage of viable, AnnexinV-positive/propidium iodide-positive cells is depicted. Mean ± SD of four experiments. Evasion from prednisone-induced apoptosis by EPO in AT-1and AT-2 cell lines (B), Nalm6 and SEM (C), and ETV6/RUNX1-positive and ETV6/RUNX1-negative primary leukemias (D and E). Cells were exposed to prednisone (50 μmol/L) in the presence and absence of EPO (50 units/mL) and viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay after 72 h. The increase in cell viability by EPO is indicated in percentage. Mean ± SD from triplicates (in cell lines, one of three independent experiments, in primary leukemic cells from one experiment). *, P < 0.05;**, P < 0.01.
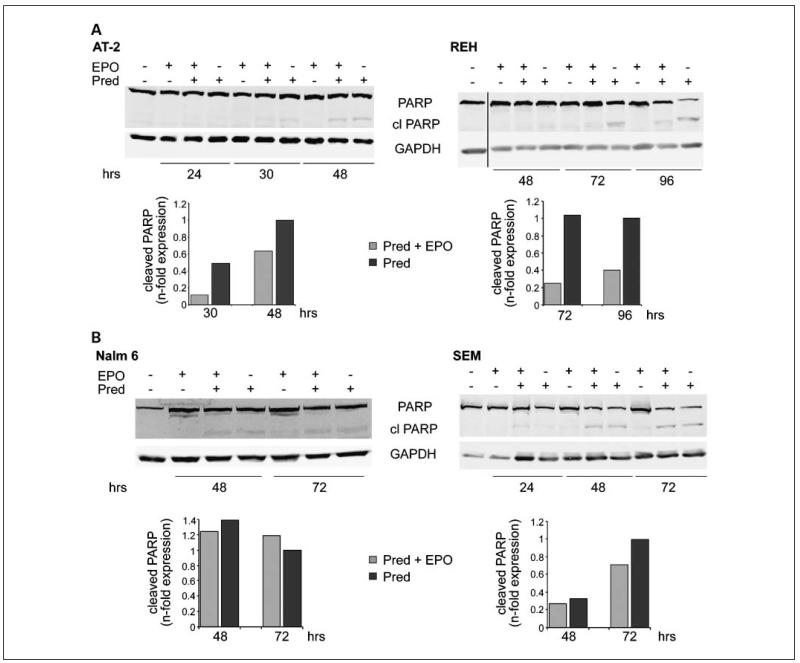
For glucocorticoid-induced apoptosis, the engagement of caspases that cleave substrates at aspartate residues is required (33). We consequently tested whether the EPO-mediated rescue from glucocorticoid-induced apoptosis can be visualized by cleavage of caspase-3 or its downstream target PARP. As shown in Fig. 5, PARP cleavage was reduced by 30% to 50% in AT-2 and REH cells, but not in Nalm6 and SEM cell lines, when EPO was added to the culture medium. We did, however, not detect caspase-3 activation (data not shown), which suggests that probably another effector caspase mediated apoptosis in these cells, as has been reported recently (33).
Fig. 5.
EPO reduces PARP cleavage in ETV6/RUNX1-positive leukemias exposed to prednisone. PARP cleavage was determined byWestern blot analysis in AT-2, REH, Nalm6, and SEM cells after exposure to prednisone and EPO for the indicated times. Glyceraldehyde 3-phosphate dehydrogenase was used as loading control. PARP cleavage was normalized to glyceraldehyde 3-phosphate dehydrogenase and values are shown as n-fold expression at the bottom of the graph.
EPO signals via the PI3K/Akt pathway in ETV6/RUNX1-positive cell lines
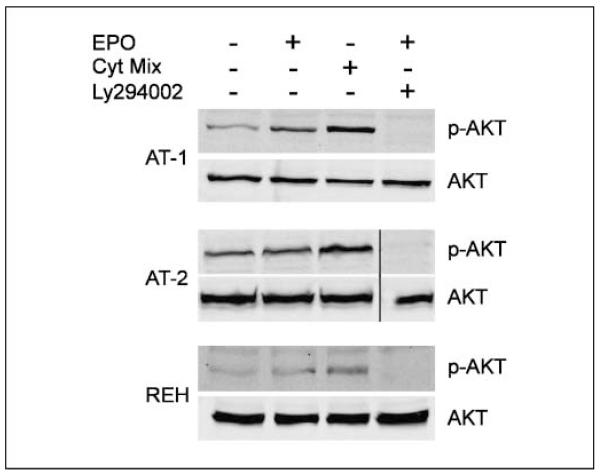
In a next step, we set out to identify the pathways that are involved in the EPOR-mediated survival advantage. Despite the fact that extracellular signal-regulated kinase 1/2 and STAT5 are commonly activated in erythroid progenitor cells on stimulation with EPO but also in many other cells (13), neither of the two pathways was activated in REH cells (data not shown). Further, PI3K and nuclear factor-nB signaling, implicated in glucocorticoid-mediated apoptosis (33), were evaluated. On addition of EPO, the PI3K pathway was activated in all three ETV6/RUNX1-positive cell lines (Fig. 6), whereas a strong EPO-independent phosphorylation of the p65 nuclear factor-κB subunit, a prerequisite for the activation of the pathway, indicates that the nuclear factor-κB pathway was constitutively activated (data not shown). These results imply that activation of the PI3K/Akt pathway is most likely involved in the proliferative and antiapoptotic effects exerted by EPO.
Fig. 6.
EPO triggers signaling via PI3K/Akt pathway in ETV6/RUNX1-positive leukemic cell lines. AT-1, AT-2, and REH cells were stimulated with 50 units/mL EPO or a cytokine mix (Cyt.mix) for 15 min.The PI3K inhibitor Ly294002 was used to show specificity of signaling.Whole-cell extracts were analyzed byWestern blotting and assessed for phosphorylation of AKT by a phospho-AKTantibody.Total AKT was used as loading control. A vertical line has been inserted to indicate where the membrane was cut. These membranes came from the same experiment.
Discussion
The overexpression of EPOR in ETV6/RUNX1-positive leukemias has become a well-recognized aspect of this leukemia subtype since its description by Fine et al. (11). The underlying mechanisms, however, as well as the biological consequences for pathogenesis and implications for the clinic have not been investigated thus far. In this study, we provide first evidence that EPOR expression is up-regulated by ETV6/RUNX1. On engagement with EPO, signaling by the receptor exerts a proliferative stimulus and evasion from glucocorticoid-induced apoptosis in ETV6/RUNX1-positive leukemias. Low Hb levels of children at the time of diagnosis emerged as a new clinical feature for ETV6/RUNX1-positive leukemia. It did not result from lack of EPO production, which was appropriate for the degree of anemia. High EPO levels, however, did not translate into higher EPOR mRNA expression in leukemic cells.
The data presented here strongly suggest a fusion gene-dependent up-regulation of EPOR expression in model systems. Although a RUNX1 consensus binding site (TCTGGT) was found 300 bp upstream of the EPOR promoter region, we rather assume that EPOR up-regulation is an indirect effect of the fusion protein based on its proposed repressor function on RUNX1 target genes (34). Support for the EPOR overexpression as a result of ETV6/RUNX1 in leukemias also results from siRNA-mediated silencing of the fusion gene in REH cells, which leads to down-regulation of the receptor.5 Such a scenario would be consistent with the distinct overexpression of this receptor in the ETV6/RUNX1-positive subgroup of leukemias, although it does not preclude other mechanisms being operative as well.
We therefore considered the possibility that the cell in which the ETV6/RUNX1 fusion gene occurs first might be a progenitor cell with erythroid potential. Based on the proposed existence of a lymphoid-primed multipotent progenitor, which retains the potential for erythroid gene expression (35), the overexpression of GATA2 and the transferrin receptor together with EPOR in ETV6/RUNX1-expressing leukemias would be compatible with such a model.6 GATA2 is also highly expressed at the stem and progenitor cell stage (36) and might thus, alternatively, reflect the activation of a stem cell self-renewal program. The issue of an erythroid component in ETV6/RUNX1-positive leukemias has been addressed already before by Andersson et al. comparing leukemia signatures with those of normal hematopoietic cells (37). These authors did, however, not detect overlapping gene expression with the erythroid lineage. A possible explanation for missing the erythroid lineage-associated genes that have emerged from our analysis may lie in the selection of the top 200 differentially regulated genes from the ETV6/RUNX1-positive leukemias for their analysis.
The finding of a significantly lower median Hb level in children with an ETV6/RUNX1-positive leukemia compared with other BCP ALL in this study is of particular interest for two reasons. First, it may mean that the erythroid lineage is affected by the fusion gene, as is the case for the structurally and functionally related fusion gene, RUNX1/RUNX1T1 (also known as AML1/ETO), resulting from the t(8;21) in acute myeloid leukemias (38). In these leukemias, RUNX1/RUNX1T1 leads to a differentiation arrest not only of the myeloid lineage but also of erythroid precursor cells, suggesting the transformation of a multipotent myeloid progenitor cell. These fusion gene-dependent changes are clinically apparent both in the fully leukemic and the myelodysplastic stage in patients as well as in mouse models (38). In contrast, similar alterations of normal hematopoiesis may not be detectable in children with ETV6/RUNX1-positive ALL because normal hematopoiesis is virtually completely replaced by leukemic cells at the time of diagnosis. In mouse and zebra fish models for ETV6/RUNX1-positive leukemia, however, no evidence for a similar phenomenon has been described (8-10, 39), implying that, even if in humans the ETV6/RUNX1 fusion originated in a stem cell, differentiation of the erythroid lineage may not be impaired. Second, a low Hb in children with ETV6/RUNX1-positive leukemia might also influence EPOR expression in leukemic cells, in keeping with the up-regulation of the EPOR by its hormone (21). As shown here, an increased serum EPO in children with low Hb did not result in a higher EPOR expression in leukemic cells compared with patients with normal Hb levels. These data exclude a major effect of the actual EPO levels on the expression of its receptor in these leukemias.
We further evaluated the influence of EPO on the fully leukemic cells. Although EPO led to a dose-dependent proliferation, cell growth and survival were not altered when the EPO-binding site of the receptor was specifically blocked. Constitutive activation of EPOR and its downstream kinase, Jak2, seems an unlikely cause for abrogating these effects because we and others did not detect mutations of these two genes in ETV6/RUNX1-positive leukemias (40, 41). Instead, the PI3K/Akt pathway is constitutively activated in these leukemias, which could explain the lack of downstream effects on EPOR blockage (37, 42). If the compensation for receptor signaling by a downstream molecule was also a potential scenario in vivo, the overexpression of EPOR may provide a survival advantage during leukemia development before the occurrence of this modification.
Recent developments in the treatment of cancer-related anemia by recombinant erythropoietin and its consequences have raised concerns about the safety and potential adverse effects of the drug, such as the promotion of tumor growth (12, 13). The data provided here suggest that, although signaling via EPOR is not pivotal for the overt leukemia, as exemplified in cell lines and a limited number of primary leukemic cells, it may enhance proliferation and survival of ETV6/RUNX1-positive leukemic cells. Of particular interest, EPO mitigated the apoptosis rate induced by prednisone, an essential drug in virtually all treatment protocols for ALL (33), in ETV6/RUNX1-positive leukemias in vitro. Importantly, this effect was not restricted to glucocorticoid-resistant REH cells but was also reproduced in glucocorticoid-sensitive cell lines and leukemias. A potential limitation of our findings lies in the concentrations of EPO used in our experiments, which exceeds values reached under physiologic conditions (43). These concerns may not be relevant in situations when erythropoiesis-stimulating agents are applied to the patients given the chance that EPO levels might differ considerably from normal. Such analyses are currently still missing.
Collectively, our data suggest that the EPOR is overexpressed as a function of ETV6/RUNX1. It promotes survival and evasion from prednisone-induced apoptosis in ETV6/RUNX1-positive leukemias in vitro. Although we are tempted to speculate that EPOR signaling may provide an evolutional advantage during leukemia development, it is too early to judge whether our findings are of clinical relevance. In particular, we do not know whether EPO levels that may be reached during treatment with erythropoiesis-stimulating drugs would support the survival of the fully malignant clone or the sustained growth of preleukemic cells.
Translational Relevance.
The data presented here suggest that the EPOR is overexpressed as a function of ETV6/RUNX1in BCPALL with a chromosomal translocation t(12;21). EPOR signaling promotes survival and evasion from prednisone-induced apoptosis in ETV6/RUNX1-positive leukemias in vitro.
Recent developments in the treatment of cancer-related anemia by recombinant erythropoietin and its consequences have raised concerns about the safety and potential adverse effects of the drug, such as the promotion of tumor growth. The data provided here suggest that although signaling via EPOR is not pivotal for the overt leukemia, as exemplified in cell lines and a limited number of primary leukemic cells, it may enhance proliferation and survival of ETV6/RUNX1-positive leukemic cells. Moreover, EPO mitigated the apoptosis rate induced by prednisone, an essential drug in virtually all treatment protocols forALL, in ETV6/RUNX1-positive leukemias in vitro. Of note, the concentrations of EPO used in our experiments exceed those reached under physiologic conditions. Such considerations may, however, not be relevant in situations when erythropoiesis-stimulating agents are applied to the patients given the chance that EPO levels might differ considerably from normal. EPO analyses in patients receiving erythropoiesis-stimulating agents are currently still missing.
Acknowledgments
We thank Prof. Christian Bieglmayer (Department of Laboratory Medicine, Medical University of Vienna) for EPO analysis, Uli Pötschger for support in statistical evaluations, Andreas Heitger and Idriss Benanni-Baiti for fruitful discussions, and Marion Zavadil for proofreading the article.
Grant support: ÖNB Jubiläumsfond 10720 and 12213, FWF P17551B14, and GENAU-CHILD Projekt GZ200.136/1-VI/1/2005 (E.R. Panzer-Grümayer) and St. Anna Kinderkrebsforschung.
Footnotes
Sanger Vega Gene ID: http://vega.sanger.ac.uk/index.html and ENSG00000187266.
C. Diakos and E.R. Panzer-Grümayer, unpublished data.
A. Inthal et al., unpublished observation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–78. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 2.Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med. 2004;350:1535–48. doi: 10.1056/NEJMra023001. [DOI] [PubMed] [Google Scholar]
- 3.Wiemels JL, Cazzaniga G, Daniotti M, et al. Prenatal origin of acute lymphoblastic leukaemia in children. Lancet. 1999;354:1499–503. doi: 10.1016/s0140-6736(99)09403-9. [DOI] [PubMed] [Google Scholar]
- 4.Mori H, Colman SM, Xiao Z, et al. Chromosome translocations and covert leukemic clones are generated during normal fetal development. Proc Natl Acad Sci U S A. 2002;99:8242–7. doi: 10.1073/pnas.112218799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong D, Gupta R, Ancliff P, et al. Initiating and cancer-propagating cells in TEL-AML1-associated childhood leukemia. Science (NY) 2008;319:336–9. doi: 10.1126/science.1150648. [DOI] [PubMed] [Google Scholar]
- 6.Andreasson P, Schwaller J, Anastasiadou E, Aster J, Gilliland DG. The expression of ETV6/CBFA2 (TEL/AML1) is not sufficient for the transformation of hematopoietic cell lines in vitro or the induction of hematologic disease in vivo. Cancer Genet Cytogenet. 2001;130:93–104. doi: 10.1016/s0165-4608(01)00518-0. [DOI] [PubMed] [Google Scholar]
- 7.Bernardin F, Yang Y, Cleaves R, et al. TEL-AML1, expressed from t(12;21) in human acute lymphocytic leukemia, induces acute leukemia in mice. Cancer Res. 2002;62:3904–8. [PubMed] [Google Scholar]
- 8.Fischer M, Schwieger M, Horn S, et al. Defining the oncogenic function of theTEL/AML1 (ETV6/RUNX1) fusion protein in a mouse model. Oncogene. 2005;24:7579–91. doi: 10.1038/sj.onc.1208931. [DOI] [PubMed] [Google Scholar]
- 9.Morrow M, Horton S, Kioussis D, Brady HJ, Williams O. TEL-AML1 promotes development of specific hematopoietic lineages consistent with pre-leukemic activity. Blood. 2004;103:3890–6. doi: 10.1182/blood-2003-10-3695. [DOI] [PubMed] [Google Scholar]
- 10.Tsuzuki S, Seto M, Greaves M, Enver T. Modeling first-hit functions of the t(12;21) TEL-AML1 translocation in mice. Proc Natl Acad Sci U S A. 2004;101:8443–8. doi: 10.1073/pnas.0402063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fine BM, Stanulla M, Schrappe M, et al. Gene expression patterns associated with recurrent chromosomal translocations in acute lymphoblastic leukemia. Blood. 2004;103:1043–9. doi: 10.1182/blood-2003-05-1518. [DOI] [PubMed] [Google Scholar]
- 12.Hardee ME, Arcasoy MO, Blackwell KL, Kirkpatrick JP, Dewhirst MW. Erythropoietin biology in cancer. Clin Cancer Res. 2006;12:332–9. doi: 10.1158/1078-0432.CCR-05-1771. [DOI] [PubMed] [Google Scholar]
- 13.Spivak JL. The anaemia of cancer: death by a thousand cuts. Nat Rev Cancer. 2005;5:543–55. doi: 10.1038/nrc1648. [DOI] [PubMed] [Google Scholar]
- 14.Diakos C, Krapf G, Gerner C, et al. RNAi-mediated silencing of TEL/AML1 reveals a heat-shock protein- and survivin-dependent mechanism for survival. Blood. 2007;109:2607–10. doi: 10.1182/blood-2006-04-019612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akimoto T, Kusano E, Inaba T, et al. Erythropoietin regulates vascular smooth muscle cell apoptosis by a phosphatidylinositol 3 kinase-dependent pathway. Kidney Int. 2000;58:269–82. doi: 10.1046/j.1523-1755.2000.00162.x. [DOI] [PubMed] [Google Scholar]
- 16.Dohnal AM, Inthal A, Felzmann T, et al. Leukemia-associated antigenic isoforms induce a specific immune response in children withT-ALL. Int J Cancer. 2006;119:2870–7. doi: 10.1002/ijc.22224. [DOI] [PubMed] [Google Scholar]
- 17.Benson EW, Hardy R, Chaffin C, Robinson CA, Konrad RJ. New automated chemiluminescent assay for erythropoietin. J Clin Lab Anal. 2000;14:271–3. doi: 10.1002/1098-2825(20001212)14:6<271::AID-JCLA4>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elliott S, Busse L, Bass MB, et al. Anti-Epo receptor antibodies do not predict Epo receptor expression. Blood. 2006;107:1892–5. doi: 10.1182/blood-2005-10-4066. [DOI] [PubMed] [Google Scholar]
- 19.Sinclair AM, Todd MD, Forsythe K, Knox SJ, Elliott S, Begley CG. Expression and function of erythropoietin receptors in tumors: implications for the use of erythropoiesis-stimulating agents in cancer patients. Cancer. 2007;110:477–88. doi: 10.1002/cncr.22832. [DOI] [PubMed] [Google Scholar]
- 20.Carvalho G, Lefaucheur C, Cherbonnier C, et al. Chemosensitization by erythropoietin through inhibition of the NF-nB rescue pathway. Oncogene. 2005;24:737–45. doi: 10.1038/sj.onc.1208205. [DOI] [PubMed] [Google Scholar]
- 21.Grossi A, Vannucchi AM, Bacci P, et al. Erythropoietin upregulates the expression of its own receptor in TF-1cell line. Leuk Res. 1998;22:145–51. doi: 10.1016/s0145-2126(97)00134-3. [DOI] [PubMed] [Google Scholar]
- 22.Koury ST, Koury MJ, Bondurant MC, Caro J, Graber SE. Quantitation of erythropoietin-producing cells in kidneys of mice by in situ hybridization: correlation with hematocrit, renal erythropoietin mRNA, and serum erythropoietin concentration. Blood. 1989;74:645–51. [PubMed] [Google Scholar]
- 23.Attarbaschi A, Mann G, Dworzak M, et al. Treatment results of childhood acute lymphoblastic leukemia in AustriaPa report of 20 years’ experience. Wien Klin Wochenschr. 2002;114:148–57. [PubMed] [Google Scholar]
- 24.Schrappe M, Reiter A, Zimmermann M, et al. Longterm results of four consecutive trials in childhood ALL performed by the ALL-BFM study group from 1981to 1995. Berlin-Frankfurt-Munster. Leukemia. 2000;14:2205–22. doi: 10.1038/sj.leu.2401973. [DOI] [PubMed] [Google Scholar]
- 25.Corazza F, Beguin Y, Bergmann P, et al. Anemia in children with cancer is associated with decreased erythropoietic activity and not with inadequate erythropoietin production. Blood. 1998;92:1793–8. [PubMed] [Google Scholar]
- 26.Stubbs MC, Armstrong SA. FLT3 as a therapeutic target in childhood acute leukemia. Curr Drug Targets. 2007;8:703–14. doi: 10.2174/138945007780830782. [DOI] [PubMed] [Google Scholar]
- 27.Wang YY, Zhou GB, Yin T, et al. AML1-ETO and C-KIT mutation/overexpression in t(8;21) leukemia: implication in stepwise leukemogenesis and response to Gleevec. Proc Natl Acad Sci U S A. 2005;102:1104–9. doi: 10.1073/pnas.0408831102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weng AP, Ferrando AA, Lee W, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306:269–71. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 29.Arcasoy MO, Karayal AF. Erythropoietin hypersensitivity in primary familial and congenital polycythemia: role of tyrosines Y285 and Y344 in erythropoietin receptor cytoplasmic domain. Biochim Biophys Acta. 2005;1740:17–28. doi: 10.1016/j.bbadis.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Arcasoy MO, Karayal AF, Segal HM, Sinning JG, Forget BG. A novel mutation in the erythropoietin receptor gene is associated with familial erythrocytosis. Blood. 2002;99:3066–9. doi: 10.1182/blood.v99.8.3066. [DOI] [PubMed] [Google Scholar]
- 31.Frost BM, Forestier E, Gustafsson G, et al. Translocation t(12;21) is related to in vitro cellular drug sensitivity to doxorubicin and etoposide in childhood acute lymphoblastic leukemia. Blood. 2004;104:2452–7. doi: 10.1182/blood-2003-12-4426. [DOI] [PubMed] [Google Scholar]
- 32.Schrappe M, Camitta B, Pui CH, et al. Long-term results of large prospective trials in childhood acute lymphoblastic leukemia. Leukemia. 2000;14:2193–4. doi: 10.1038/sj.leu.2401977. [DOI] [PubMed] [Google Scholar]
- 33.Frankfurt O, Rosen ST. Mechanisms of glucocorticoid-induced apoptosis in hematologic malignancies: updates. Curr Opin Oncol. 2004;16:553–63. doi: 10.1097/01.cco.0000142072.22226.09. [DOI] [PubMed] [Google Scholar]
- 34.Zelent A, Greaves M, Enver T. Role of the TEL-AML1 fusion gene in the molecular pathogenesis of childhood acute lymphoblastic leukaemia. Oncogene. 2004;23:4275–83. doi: 10.1038/sj.onc.1207672. [DOI] [PubMed] [Google Scholar]
- 35.Mansson R, Hultquist A, Luc S, et al. Molecular evidence for hierarchical transcriptional lineage priming in fetal and adult stem cells and multipotent progenitors. Immunity. 2007;26:407–19. doi: 10.1016/j.immuni.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Ling KW, Ottersbach K, van Hamburg JP, et al. GATA-2 plays two functionally distinct roles during the ontogeny of hematopoietic stem cells. J Exp Med. 2004;200:871–82. doi: 10.1084/jem.20031556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andersson A, Olofsson T, Lindgren D, et al. Molecular signatures in childhood acute leukemia and their correlations to expression patterns in normal hematopoietic subpopulations. Proc Natl Acad Sci U S A. 2005;102:19069–74. doi: 10.1073/pnas.0506637102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi Y, Elagib KE, Goldfarb AN. AML-1-ETO-mediated erythroid inhibition: new paradigms for differentiation blockade by a leukemic fusion protein. Crit Rev Eukaryot Gene Expr. 2005;15:207–16. doi: 10.1615/critreveukargeneexpr.v15.i3.30. [DOI] [PubMed] [Google Scholar]
- 39.Sabaawy HE, Azuma M, Embree LJ, Tsai HJ, Starost MF, Hickstein DD. TEL-AML1 transgenic zebrafish model of precursor B cell acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 2006;103:15166–71. doi: 10.1073/pnas.0603349103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kratz CP, Boll S, Kontny U, Schrappe M, Niemeyer CM, Stanulla M. Mutational screen reveals a novel JAK2 mutation, L611S, in a child with acute lymphoblastic leukemia. Leukemia. 2006;20:381–3. doi: 10.1038/sj.leu.2404060. [DOI] [PubMed] [Google Scholar]
- 41.Sulong S, Case M, Minto L, Wilkins B, Hall A, Irving J. The V617F mutation in Jak2 is not found in childhood acute lymphoblastic leukaemia. Br J Haematol. 2005;130:964–5. doi: 10.1111/j.1365-2141.2005.05697.x. [DOI] [PubMed] [Google Scholar]
- 42.Roman-Gomez J, Jimenez-Velasco A, Agirre X, et al. CpG island methylator phenotype redefines the prognostic effect of t(12;21) in childhood acute lymphoblastic leukemia. Clin Cancer Res. 2006;12:4845–50. doi: 10.1158/1078-0432.CCR-05-2592. [DOI] [PubMed] [Google Scholar]
- 43.Cazzola M, Mercuriali F, Brugnara C. Use of recombinant human erythropoietin outside the setting of uremia. Blood. 1997;89:4248–67. [PubMed] [Google Scholar]