Abstract
This study examined whether a novel imidazoline I2 receptor ligand CR4056 could serve as a discriminative stimulus and whether it shares similar discriminative stimulus effects with other reported I2 receptor ligands. Eight male Sprague-Dawley rats were trained to discriminate 10.0 mg/kg CR4056 (i.p.) from vehicle in a two-lever food-reinforced drug discrimination procedure. Once rats acquired the discrimination, substitution and combination studies were conducted to elucidate the underlying receptor mechanisms. All rats acquired CR4056 discrimination after an average of 26 training sessions. Several I2 receptor ligands (phenyzoline, tracizoline, RS45041, and idazoxan, 3.2–75 mg/kg, i.p.) all occasioned > 80% CR4056-associated lever responding. Other drugs that occasioned partial or no CR4056-associated lever responding included methamphetamine, ketamine, the endogenous imidazoline ligand agmatine, the monoamine oxidase (MAO) inhibitor harmane, the α2-adrenoceptor agonist clonidine, the μ-opioid receptor agonists morphine and methadone, and the selective I2 receptor ligands BU224 and 2-BFI. The α1 adrenoceptor antagonist WB4101, α2 adrenoceptor antagonist yohimbine and μ-opioid receptor antagonist naltrexone failed to alter the stimulus effects of CR4056. Together, these results show that CR4056 can serve as a discriminative stimulus in rats, which demonstrates high pharmacological specificity and appears to be mediated by imidazoline I2 receptors.
Imidazoline receptors represent a group of heterogeneous receptors (e.g., I1, I2 and I3 subtypes) that involve diverse physiological and/or pharmacological functions1,2. In particular, I2 receptors have recently been increasingly recognized as a potential target for the development of novel analgesics3,4,5,6,7.
Until now, the molecular identity and downstream signaling transduction mechanism of I2 receptors remain unclear and the understanding of I2 receptors relies primarily on pharmacological tools. In the past two decades, several selective I2 receptor ligands with varying potency and affinity have been described5,8,9,10. However, little is known of the comparative pharmacology of these ligands as few studies have examined the effects of these I2 receptor ligands using functional assays. We previously showed that a group of seven putative I2 receptor ligands all exert hypothermic activity in rats which appears to be mediated through I2 receptors11. Further comparative pharmacology study of the reported I2 receptor ligands remains important because without the knowledge of I2 receptor identity and with the fact that essentially all compounds bind to more than one receptor, future studies using selected I2 receptor ligands to understand I2 receptor pharmacology depend on how well we know the similarities and differences of these valuable ligands as research tools.
Drug discrimination is a widely used behavioral pharmacology procedure to study receptor mechanisms underlying the effects of drugs from various pharmacological classes primarily due to its high pharmacological specificity. Of the reported I2 receptor ligands, only 2-BFI has been studied as a discriminative stimulus. It was found that rats can recognize 2-BFI as a discriminative stimulus, and that the effect is pharmacologically specific and seems to be associated with irreversible inhibition of monoamine oxidase A (MAO-A)12,13,14.
CR4056 is a newly described highly selective I2 receptor ligand5. In a receptor characterization study including 35 common receptors, enzymes and ion channels, CR4056 shows high affinity at I2 receptors and MAO-A but negligible binding activity at all other sites, demonstrating its high receptor selectivity5. In preclinical studies, CR4056 demonstrates potent antinociceptive activity in rodent models of persistent inflammatory and neuropathic pain3,5,7. CR4056 also produces hypothermia in rats to a degree that is similar with other I2 receptor ligands11. Given the well-characterized high I2 receptor selectivity, this offers an opportunity to use drug discrimination procedure to characterize the discriminative stimulus effects of CR4056 and compare it with other purported I2 receptor ligands, which will also assist in understanding the functionality of I2 receptors.
Results
At the training dose of 10 mg/kg (i.p.), all 8 subjects acquired CR4056 discrimination after an average of 26 (range = 11–52) training sessions, including the sessions used for the calculation of criteria performance.
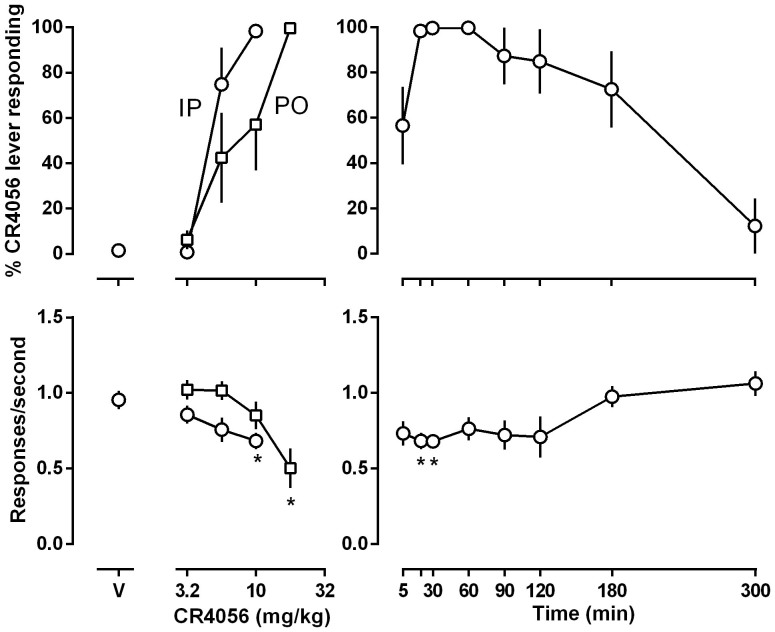
CR4056 dose- and time-dependently produced discriminative stimulus effects, with the highest dose slightly but significantly reducing the rate of responding (Fig. 1). When tested with different doses, CR4056 increased responding on the CR4056-associated lever in a dose-dependent manner, ranging from 1% after vehicle injection to a maximum of 98% after injecting with the training dose of 10 mg/kg CR4056 [ED50 (95% CLs) = 5.0 (4.0, 5.9) mg/kg] (upper left panel, open circles), with the dose of 10 mg/kg slightly but significantly reducing response rate (lower left panel, open circles). Oral administration of CR4056 only slightly reduced its potency for producing CR4056-appropriate lever responding [ED50 (95% CLs) = 9.2 (4.7, 12.5) mg/kg] (upper left panel, open squares) and CR4056 significantly decreased rate of responding at the highest oral dose tested (17.8 mg/kg) (lower left panel, open squares). In tests to examine the duration of action of the training dose of CR4056 (i.e., 10 mg/kg, i.p.), drug was administered with varying pretreatment times. It was found that the discriminative stimulus effects of CR4056 were apparent after 5 min pretreatment, reached the maximal effect after 30 min pretreatment and decreased to 12% 5 hours later (top right panel), with response rate slightly but significantly decreased at 20 and 30 min time points.
Figure 1. Effects of CR4056 in rats trained to discriminate between 10 mg/kg CR4056 (i.p) and vehicle using a two-lever food-reinforced procedure.
The mean (±1 SEM) percentage of responses on the CR4056-appropriate lever (top panels) and the mean (±1 SEM) rate of responding (bottom panels) are plotted as a function of dose in the left panels and as a function of time after i.p. administration of 10 mg/kg CR4056 in the right panels. Points above “V” indicate vehicle. Each point represents the average (±S.E.M.) of eight rats. Asterisks indicate mean rate of responding that were significantly different from control (95% CLs did not overlap).
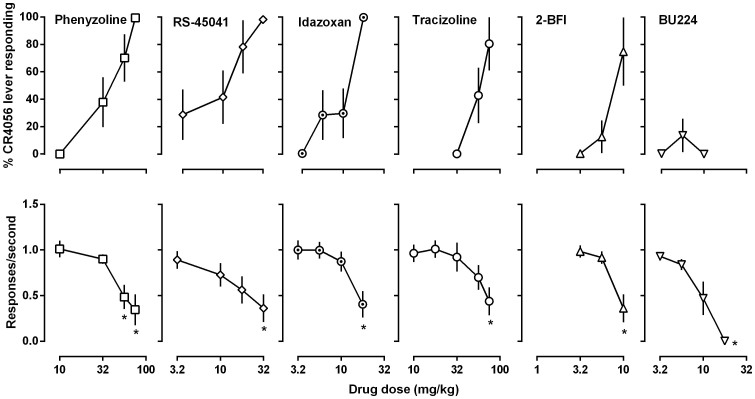
Four reported imidazoline I2 receptor ligands phenyzoline, RS45041, idazoxan and tracizoline produced greater than 80% CR4056-like discriminative stimulus effects and at the highest doses that produced maximal stimulus effects they also significantly reduced response rates (Fig. 2). ED50 (95% CLs) values of these drugs for substituting CR4056 were: phenyzoline 38.7 (23.1, 50.2) mg/kg, RS45041 11.2 (4.8, 15.2) mg/kg, idazoxan 9.6 (4.8, 14.5) mg/kg, and tracizoline 51.3 (40.9, 61.6) mg/kg. The commonly used I2 receptor ligand 2-BFI failed to produce full substitution to CR4056 discrimination, increasing CR4056-appropriate responding to at most 75% at a dose (10 mg/kg) that significantly reduced rate of responding (Fig. 2). Another commonly used I2 receptor ligand BU224 failed to produce greater than 20% CR4056-appropriate responding up to a dose (17.8 mg/kg) that markedly suppressed operant responding (Fig. 2).
Figure 2. Effects of phenyzoline, RS45041, idazoxan, tracizoline, 2-BFI and BU224 in rats trained to discriminate between 10 mg/kg CR4056 (i.p) and vehicle using a two-lever food-reinforced procedure.
See Figure 1 for other details.
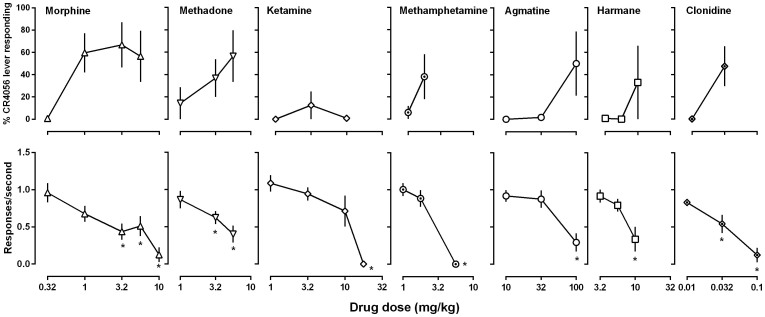
Drugs that have no known I2 receptor binding activities generally produced partial or no CR4056-like discriminative stimulus effects. The opioids morphine and methadone increased CR4056-appropriate responding to at most 67% (morphine) and 56% (methadone) (open triangles, upper panels, Fig. 3) when tested up to doses that significantly reduced rate of responding (lower panels, Fig. 3). The NMDA receptor antagonist ketamine produced a maximum of 13% CR4056-appropriate responding up to a dose (17.8 mg/kg) that eliminated operant responding (open diamonds, Fig. 3). The monoamine releaser methamphetamine produced a maximum of 38% CR4056-associated lever-responding up to a dose (5.6 mg/kg) that eliminated operant responding (filled circles, Fig. 3). The putative endogenous imidazoline receptor ligand agmatine produced a maximum of 50% CR4056-associated responding up to a dose (100 mg/kg) that significantly decreased rate of responding (open circles, Fig. 3). A β-carboline harmane produced a maximum of 33% CR4056-associated responding up to a dose (10 mg/kg) that significantly suppressed rate of responding (open squares, Fig. 3). The α2 adrenoceptor agonist clonidine produced a maximum of 47% CR4056-appropriate responding up to a dose (0.1 mg/kg) that significantly reduced response rate (filled diamonds, Fig. 3).
Figure 3. Effects of morphine, methadone, ketamine, methamphetamine, agmatine, harmane and clonidine in rats trained to discriminate between 10 mg/kg CR4056 (i.p) and vehicle using a two-lever food-reinforced procedure.
See Figure 1 for other details.
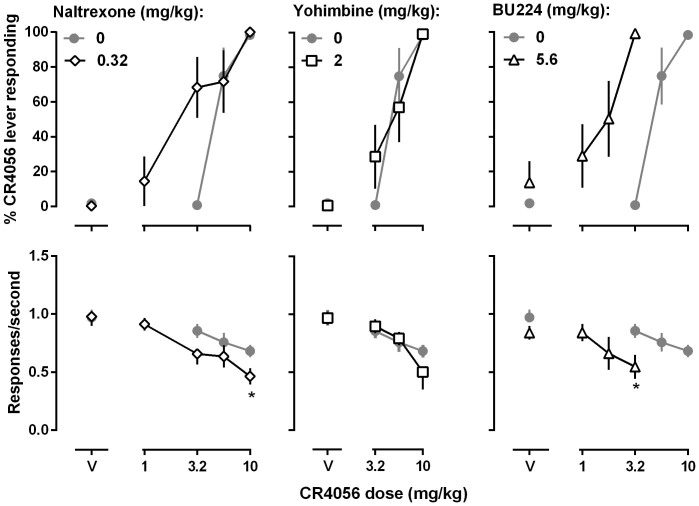
The opioid receptor antagonist naltrexone and the α2 adrenoceptor antagonist yohimbine failed to attenuate the discriminative stimulus effects of CR4056 (open diamonds and open squares, respectively, Fig. 4). The dose ratios (95% CLs) of CR4056 in the absence and presence of 0.32 mg/kg naltrexone or 2 mg/kg yohimbine were 2.7 (0.7, 4.8) and 1.1 (0.8, 1.3), respectively. Because the 95% CLs of the dose ratios included 1, the potency changes of CR4056 in the presence of naltrexone or yohimbine were considered insignificant. Because the commonly used I2 receptor ligand BU224 failed to produce significant CR4056-like discriminative stimulus effects, it was studied in combination with CR4056. Pretreatment with 5.6 mg/kg BU224 significantly decreased the potency of CR4056 (open upper triangles, Fig. 4). The dose ratio (95% CLs) of CR4056 in the absence and presence of 5.6 mg/kg BU224 was 4.0 (1.9, 6.1), representing a 4-fold leftward shift of the CR4056 dose response curve.
Figure 4. Effects of naltrexone, yohimbine and BU224 on the discriminative stimulus effects of CR4056 in rats trained to discriminate between 10 mg/kg CR4056 (i.p) and vehicle using a two-lever food-reinforced procedure.
Pretreatment drugs were administered 10 min prior to CR4056. See Figure 1 for other details.
The α1 adrenoceptor antagonist WB4101 failed to attenuate the discriminative stimulus effects of the training dose of CR4056 (i.e., 10 mg/kg) up to a dose that markedly reduced the operant responding (Fig. 5).
Figure 5. Effects of WB4101 on the discriminative stimulus effects of the training dose of CR4056 (10 mg/kg) in rats.
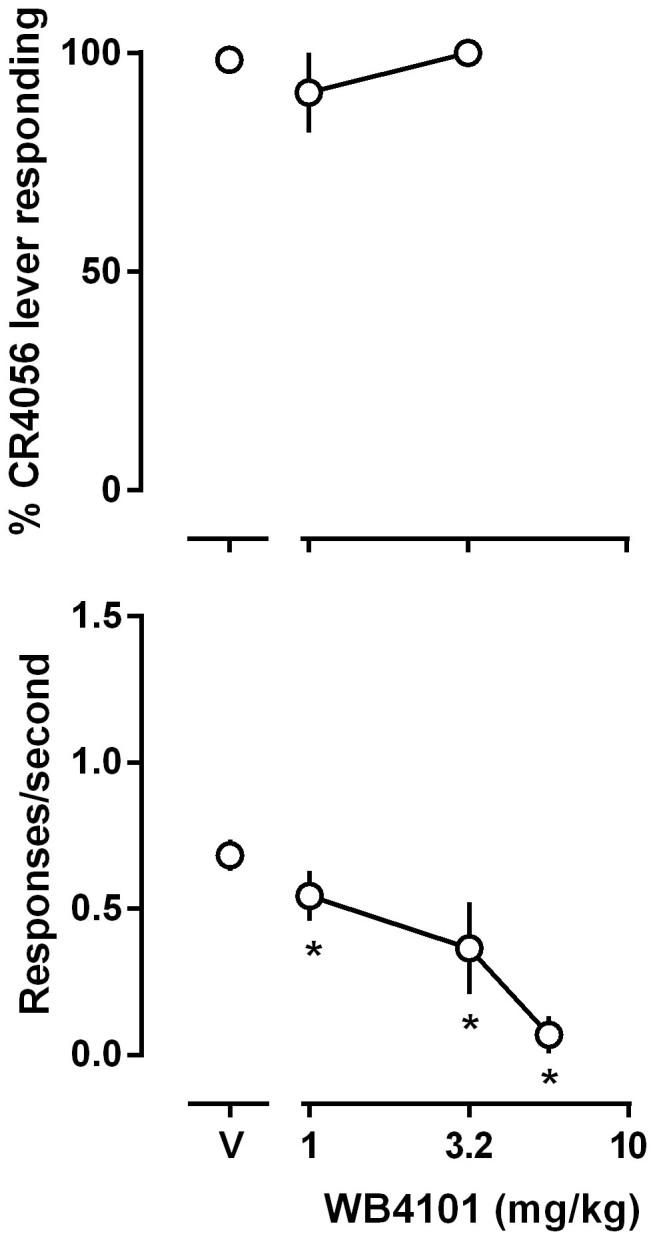
See Figure 1 for other details.
Discussion
This study firstly demonstrated that rats can be trained to discriminate the novel imidazoline I2 receptor ligand CR4056 from vehicle. All the eight animals met the test criteria after an average of 26 (range: 11–52) training sessions, suggesting that CR4056 is at least as discriminable as 2-BFI (an average of 57 ± 9 training sessions)13. In addition, the discriminative stimulus effects of CR4056 is pharmacologically highly specific such that only drugs that have known binding affinities at I2 receptors (except BU224) produced CR4056-like discriminative stimulus effects. Findings from antagonism studies suggest that opioid receptors and α1/α2 adrenoceptors do not play a prominent role in the discriminative stimulus effects of CR4056.
CR4056 is a recently characterized imidazoline I2 receptor ligand that demonstrates high selectivity at I2 receptors over a panel of more than 35 other common receptors, enzymes and binding sites5. CR4056 can readily cross the blood brain barrier and demonstrates strong antinociceptive activity in several rodent models of persistent inflammatory and neuropathic pain3,5,7. In the current study, the discriminative stimulus effects of the training dose of CR4056 lasted about 5 hours, which is consistent with the duration of its antinociceptive effects (around 6 hours at an oral dose of 20 mg/kg)5. Phenyzoline, RS45041 and tracizoline all have high affinity and selectivity at I2 receptors9,15,16 and they all fully substituted for the discriminative stimulus effects of CR4056. Importantly, drugs that do not bind to I2 receptors do not mimic the discriminative stimulus effects of CR4056. For example, ketamine and methamphetamine did not occasion significant CR4056-like discriminative cueing effects. These results suggest that the discriminative stimulus effects of CR4056 are likely mediated through I2 receptors. Both morphine and methadone produced dose-dependent and partial substitution for CR4056 and the effects were antagonized by naltrexone (data not shown), suggesting that µ opioid receptors might be involved in the discriminative stimulus effects of CR4056. However, subsequent antagonist studies with naltrexone found that naltrexone did not alter the effects of CR4056, which suggests that although a small secondary role cannot be ruled out, it is unlikely that interactions of CR4056 with µ opioid receptors play a major role in its discriminative stimulus effects.
Idazoxan is traditionally used to identify and characterize I2 receptors17 and has been shown to block the antinociceptive effects of CR4056, 2-BFI and phenyzoline3,5,16. In rats discriminating 2-BFI, idazoxan produces full 2-BFI-like discriminative stimulus effects14. In the current study, idazoxan produced full CR4056-like discriminative stimulus effects. It is possible that idazoxan has lower efficacy at I2 receptors than 2-BFI and CR4056 and functions as a partial agonist. Thus, in assays that have higher efficacy demand (e.g., antinociception) idazoxan serves as an antagonist while in assays that have lower efficacy demand (e.g., drug discrimination) idazoxan is fully effective. However, there are cases that idazoxan does not enhance or block the behavioral effects of 2-BFI and BU22418.
2-BFI and BU224 are two most widely studied I2 receptor ligands. In rats discriminating 2-BFI, BU224 fully substitutes for 2-BFI13. All three compounds (2-BFI, BU224 and CR4056) produce hypothermia in rats11 and produce antinociceptive effects through their actions on I2 receptors3,5,19. Thus, it is expected that these compounds share similar pharmacological mechanisms (i.e., I2 receptors). However, the current data suggest that there are important differences among these compounds. First, 2-BFI only partially substituted for while BU224 did not substitute for CR4056. Second, the stimulus effects of 2-BFI is significantly antagonized by the α1 adrenoceptor antagonist WB410112 but WB4101 did not block the discriminative stimulus effects of CR4056 (Fig. 5). Third, the MAO-A inhibitor harmane fully substitutes for 2-BFI in rats discriminating 2-BFI13 but it did not substitute for CR4056 (Fig. 3). There is evidence that BU224 has lower efficacy than 2-BFI because it blocks the behavioral effects of 2-BFI under certain conditions20,21 and is sometimes used as an I2 receptor antagonist22,23. However, the lack of substitution of BU224 for CR4056 in the current study does not seem to be due to its limited efficacy at I2 receptors, because if this were the case then it would be expected that BU224 should be able to antagonize the effects of CR4056. In contrast, BU224 markedly enhanced the discriminative stimulus effects of CR4056. Both 2-BFI and CR4056 are highly selective I2 receptor ligands and counter selectivity binding studies fail to identify any receptor that these compounds bind with high affinity other than I2 receptors5,24. One parsimonious explanation is that the pharmacological mechanisms of these compounds are overlapping but have important (yet unknown) differences.
Imidazoline I2 receptors as identified by idazoxan or 2-BFI binding are highly heterogeneous and include several proteins17,25,26, including a ~ 45 kDa protein that was recently identified as brain creatine kinase26. However, little is known of the molecular identity and functional activity of these heterogeneous proteins. In most functional studies, I2 receptors are treated as one homogenous receptor for the sake of convenience. However, emerging functional data including the current study increasingly suggest that these proteins that 2-BFI and idazoxan bind to may have different functionalities, although it is too early to link specific functions with particular proteins. For example, it is plausible that CR4056, 2-BFI and BU224 binding profiles on the idazoxan-recognized proteins are overlapping but have important differences. It is possible that the protein(s) underlying the antinociceptive effects of these I2 receptor ligands is different from that underlying the discriminative stimulus effects or hypothermic effects11. Thus, BU224 may only bind to one component of the CR4056 binding sites which may not be sufficient to produce CR4056-like discriminative stimulus effects but adequate to enhance the effects of CR4056 by increasing the activity on the same component that CR4056 also binds to. These speculations need to be tested in future studies that utilize multiple I2 receptor ligands and different functional assays to categorize the potential effects of the different I2 receptor components.
In summary, this study for the first time demonstrates that CR4056 can serve as a discriminative stimulus in rats and the effects are pharmacologically specific that seem to be mediated through imidazoline I2 receptors but not through opioid or α1/α2 adrenoceptors. The discriminative stimulus effects of CR4056 have important differences from those of 2-BFI, which is reminiscent of the biochemical heterogeneity of I2 receptors and may indicate functional diversity of I2 receptors. Drug discrimination is a valuable tool to investigate the in vivo pharmacological properties of putative I2 receptor ligands and provides another dimension toward fuller understanding of I2 receptor ligands and I2 receptor functionality.
Methods
Subjects
Eight adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) were housed individually on a 12/12-h cycle (behavioral experiments were conducted during the light period) with free access to water and restricted access to food. The body weights of the rats were maintained at 85% of their free-feeding body weights by adjusting the amount of standard rodent chow that was provided in the home cages after daily sessions. All animal maintenance and experimental protocols were approved by the Institutional Animal Care and Use Committee, University at Buffalo, and conducted in accordance to the 2011 Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences).
Apparatus
Experiments were conducted in commercially available chambers within sound-attenuating, ventilated enclosures (Coulbourn Instruments Inc., Allentown, PA, USA). Each chamber contained a working area of 30.5 cm by 24.5 cm by 21.0 cm, a grid floor, and a 45 mg pellet dispenser with a pellet receptacle that was centered between responses levers, above which were stimulus lights. A 28 V house light was mounted on the rear aluminum wall of the chamber. Chambers were connected to a computer running Graphic State 3.03 software and an interface (Coulbourn Instruments Inc.) to control experimental events and record data.
Drug Discrimination Procedure
Rats were trained to discriminate 10.0 mg/kg CR4056 (i.p.) from vehicle while responding under a fixed ratio (FR) 10 schedule of food presentation. The experimental procedure described by Li was followed27.
Daily sessions began with a 10-min timeout period, during which the chamber was dark and lever presses had no programmed consequence, followed by a response period, during which stimulus lights above both levers were illuminated and an FR 10 schedule of food presentation was active. Either saline or CR4056 was injected intraperitoneally 10 min before the start of daily sessions. When rats received saline, only responding on the saline-associated lever resulted in food delivery (45 mg; BioServ Inc., Frenchtown, New Jersey, USA), and when they received CR4056 only responding on the other (drug-associated) lever resulted in food delivery. Sessions were conducted 7 days per week according to a double alternation schedule (e.g., saline, saline, drug, drug).
Rats had to satisfy the following criteria for five consecutive or six of seven consecutive sessions: at least 90% of the total responses on the correct lever and fewer than ten responses on the incorrect lever before completion of the FR on the correct lever. Thereafter, tests were conducted every 3rd day provided that the testing criteria were satisfied during intervening training sessions. If rats failed to satisfy these criteria, training continued until the criteria were satisfied for two consecutive sessions.
Test sessions were identical to training sessions with the exception that ten consecutive responses on either lever resulted in the delivery of food and different doses of CR4056 and other drugs were administered. In the study that examined the effects of oral administration of CR4056, the drug was administered via gavage 30 min prior to the start of the sessions. In combination studies that involved two drug injections, the pretreatment drug was always administered 10 min prior to the second drug injection. In general, drugs were studied up to doses that occasioned at least 80% responding on the drug-associated lever or to doses that decreased the rate of responding significantly.
Data Analyses
The mean percentage of responses on CR4056-associated lever (±1 S.E.M.) was plotted as a function of dose. If the response rate of an animal was less than 20% of the vehicle control rate, discrimination data from that test were not included for further analysis. Linear regression was used to analyze the dose response curves that attained at least 80% CR4056-appropriate responding and to estimate the dose required to produce 50% responding on the CR4056-appropriate lever [ED50 ± 95% confidence limits (CLs)]. In drug combination studies that determined complete dose response curves, dose ratios (ED50 values of CR4056 before drug treatment divided by those after drug treatment) were calculated to estimate the magnitude of shift in the CR4056 dose response curve produced by other drugs. When the 95% CLs of the mean dose ratio did not encompass 1, the CR4056 dose-response curve was considered to be shifted significantly leftward. Response rates were expressed as the average (±1 S.E.M.) number of responses per second on both levers. If the 95% CLs of the response rates after drug treatment did not overlap with those after vehicle treatment, it was considered that the drug significantly altered the response rate.
Drugs
CR4056, 2-BFI hydrochloride, BU224 hydrochloride, phenyzoline oxalate and tracizoline oxalate were synthesized according to standard procedures at the Research Triangle Institute and fully characterized by NMR and elemental analysis. RS45041 was kindly provided by National Institute of Mental Health's Chemical Synthesis and Drug Supply program (Bethesda, MD, USA). Idazoxan hydrochloride, agmatine hydrochloride, clonidine hydrochloride, yohimbine hydrochloride, naltrexone hydrochloride, harmane hydrochloride and WB4101 hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ketamine hydrochloride was purchased from Patterson Veterinary (Devens, MA, USA). Morphine sulfate, methadone hydrochloride and methamphetamine hydrochloride were provided by Research Technology Branch, National Institute on Drug Abuse, National Institutes of Health (Rockville, MD, USA). All drugs were dissolved in 0.9% physiological saline except CR4056 which was dissolved in 20% DMSO with saline and a drop of hydrochloric acid.
Author Contributions
Y.Q. and J.L. participated in research design; Y.Q. conducted the experiments; Y.Z. provided test compounds; Y.Q. and J.L. conducted data analyses; Y.Q., X.H., Y.Z. and J.L. prepared and approved the final version of the manuscript.
Acknowledgments
This work was supported by the National Institute on Drug Abuse of the National Institutes of Health (Awards no. R01DA034806 and R21DA033426). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Parini A., Moudanos C. G., Pizzinat N. & Lanier S. M. The elusive family of imidazoline binding sites. Trends Pharmacol. Sci. 17, 13–16 (1996). [DOI] [PubMed] [Google Scholar]
- Eglen R. M. et al. ‘Seeing through a glass darkly’: casting light on imidazoline ‘I’ sites. Trends Pharmacol Sci 19, 381–390 (1998). [DOI] [PubMed] [Google Scholar]
- Li J. X., Thorn D. A., Qiu Y., Peng B. W. & Zhang Y. Antihyperalgesic effects of imidazoline I(2) receptor ligands in rat models of inflammatory and neuropathic pain. Br J Pharmacol 171, 1580–1590 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. X. & Zhang Y. Imidazoline I2 receptors: target for new analgesics? Eur J Pharmacol 658, 49–56 (2011). [DOI] [PubMed] [Google Scholar]
- Ferrari F. et al. Analgesic efficacy of CR4056, a novel imidazoline-2 receptor ligand, in rat models of inflammatory and neuropathic pain. J. Pain Research 4, 111–125 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza M., Ferrari F., Menghetti I., Tremolada D. & Caselli G. Modulation of imidazoline I2 binding sites by CR4056 relieves postoperative hyperalgesia in male and female rats. Br. J. Pharmacol. 171, 3693–3701 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meregalli C. et al. CR4056, a new analgesic I2 ligand, is highly effective against bortezomib-induced painful neuropathy in rats. J. Pain Research 5, 151–167 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson A. L. et al. Novel selective compounds for the investigation of imidazoline receptors. Ann. New York Acad. Sci. 881, 81–91 (1999). [DOI] [PubMed] [Google Scholar]
- Brown C. M. et al. RS-45041-190: a selective, high-affinity ligand for I2 imidazoline receptors. Br. J. Pharmacol. 116, 1737–1744 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentili F. et al. Novel ligands rationally designed for characterizing I2-imidazoline binding sites nature and functions. J. Med. Chem. 51, 5130–5134 (2008). [DOI] [PubMed] [Google Scholar]
- Thorn D. A., An X. F., Zhang Y., Pigini M. & Li J. X. Characterization of the hypothermic effects of imidazoline I(2) receptor agonists in rats. Br. J. Pharmacol. 166, 1936–1945 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacInnes N. & Handley S. L. Potential serotonergic and noradrenergic involvement in the discriminative stimulus effects of the selective imidazoline I2-site ligand 2-BFI. Pharmacol. Biochem. Behav. 75, 427–433 (2003). [DOI] [PubMed] [Google Scholar]
- MacInnes N. & Handley S. L. Characterization of the discriminable stimulus produced by 2-BFI: effects of imidazoline I(2)-site ligands, MAOIs, beta-carbolines, agmatine and ibogaine. Br. J. Pharmacol. 135, 1227–1234 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan S., Jackson H. C., Nutt D. J. & Handley S. L. Discriminative stimulus produced by the imidazoline I2 site ligand, 2 -BFI. J. Psychopharmacology 10, 273–278 (1996). [DOI] [PubMed] [Google Scholar]
- Pigini M. et al. Imidazoline receptors: qualitative structure-activity relationships and discovery of tracizoline and benazoline. Two ligands with high affinity and unprecedented selectivity. Bioorg. Med. Chem. 5, 833–841 (1997). [DOI] [PubMed] [Google Scholar]
- Gentili F. et al. Involvement of I2-imidazoline binding sites in positive and negative morphine analgesia modulatory effects. Eur. J. Pharmacol. 553, 73–81 (2006). [DOI] [PubMed] [Google Scholar]
- Regunathan S. & Reis D. J. Imidazoline receptors and their endogenous ligands. Ann. Review Pharmacol. Toxicol. 36, 511–544 (1996). [DOI] [PubMed] [Google Scholar]
- Min J. W., Peng B. W., He X., Zhang Y. & Li J. X. Gender difference in epileptogenic effects of 2-BFI and BU224 in mice. Eur. J. Pharmacol. 718, 81–86 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. X., Zhang Y. & Winter J. C. Morphine-induced antinociception in the rat: supra-additive interactions with imidazoline I(2) receptor ligands. Eur. J. Pharmacol. 669, 59–65 (2011). [DOI] [PubMed] [Google Scholar]
- Thorn D. A., Zhang Y., Peng B. W., Winter J. C. & Li J. X. Effects of imidazoline I(2) receptor ligands on morphine- and tramadol-induced antinociception in rats. Eur. J. Pharmacol. 670, 435–440 (2011). [DOI] [PubMed] [Google Scholar]
- Sanchez-Blazquez P., Boronat M. A., Olmos G., Garcia-Sevilla J. A. & Garzon J. Activation of I(2)-imidazoline receptors enhances supraspinal morphine analgesia in mice: a model to detect agonist and antagonist activities at these receptors. Br. J. Pharmacol. 130, 146–152 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. F. et al. Characterization of imidazoline receptors in blood vessels for the development of antihypertensive agents. BioMed Res. Intl. 2014, 182846 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T. T. et al. Mediation of AMP kinase in the increase of glucose uptake in L6 cells induced by activation of imidazoline I-2 receptors. Hormone Metab. Res. 45, 359–363 (2013). [DOI] [PubMed] [Google Scholar]
- Nutt D. J. et al. Functional studies of specific imidazoline-2 receptor ligands. Ann. New York Acad. Sci. 763, 125–139 (1995). [DOI] [PubMed] [Google Scholar]
- Escriba P. V., Ozaita A. & Garcia-Sevilla J. A. Pharmacologic characterization of imidazoline receptor proteins identified by immunologic techniques and other methods. Ann. New York Acad. Sci. 881, 8–25 (1999). [DOI] [PubMed] [Google Scholar]
- Kimura A. et al. Identification of an imidazoline binding protein: creatine kinase and an imidazoline-2 binding site. Brain Res. 1279, 21–28 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. X., Thorn D. A. & Jin C. The GPR88 receptor agonist 2-PCCA does not alter the behavioral effects of methamphetamine in rats. Eur. J. Pharmacol. 698, 272–277 (2013). [DOI] [PubMed] [Google Scholar]