Abstract
A pallial-basal-ganglia-thalamic-pallial loop in songbirds is involved in vocal motor learning. Damage to its basal ganglia part, Area X, in adult zebra finches has been noted to have no strong effects on song and its function is unclear. Here we report that neurotoxic damage to adult Area X induced changes in singing tempo and global syllable sequencing in all animals, and considerably increased syllable repetition in birds whose song motifs ended with minor repetitions before lesioning. This stuttering-like behavior started at one month, and improved over six months. Unexpectedly, the lesioned region showed considerable recovery, including immigration of newly generated or repaired neurons that became active during singing. The timing of the recovery and stuttering suggest that immature recovering activity of the circuit might be associated with stuttering. These findings indicate that even after juvenile learning is complete, the adult striatum plays a role in higher level organization of learned vocalizations.
Like humans, songbirds are one of the few groups of animals that possess the ability to learn their vocalizations via imitation. This ability is controlled by specialized brain regions organized into two main forebrain pathways, which in songbirds are the posterior pathway involved in production of the learned vocalizations and the anterior one (including the basal ganglia) involved in song learning (Fig. 1)1,2. Similar to humans, the basal ganglia in adult songbirds are not required for the motor act of producing learned vocalizations but are required for learning how to produce them1,3,4. Lesions to the striatal basal ganglia song nucleus, Area X, disrupt a male juvenile bird's ability to imitate song correctly from an adult tutor bird, causing the song to remain more variable3,5. Lesions or inactivation of one of the inputs of Area X, the cortical-like lateral magnocellular nucleus of anterior nidopallium (LMAN), and the thalamic input into LMAN, the anterior dorsal lateral nucleus of dorsomedial thalamus [aDLM; redefined in6], bring about premature and rapid song stereotypy3,7,8,9. After adult birds have mastered their song, lesions to LMAN lead to a smaller but significant decrease on song variability10 and prevent experimentally induced song plasticity11,12,13. The reduced variability transforms the more variable undirected song, thought to be used for practice, to become more like directed song sung to females, being more stereotyped in pitch and containing more introductory notes14,15,16. Although lesions to adult Area X were originally reported to not notably alter song of adult zebra finches during the course of 4 weeks3 they have been reported to result in transient song syllable repetition in Bengalese finches17, a species that produces more syllable repetitions and variable songs than the zebra finch. Syllable repetitions in zebra finches have been regarded as analogous to part-word repetitions in humans characteristic for developmental or genetically influenced stuttering, and can be imitated by up to 7% of captive animals18,19,20. These and other findings derived from singing-driven immediate early genes, gene-function analysis and neurophysiological recordings6,7,10,21,22,23,24,25 suggest that Area X continues to play a role in modulating adult song production.
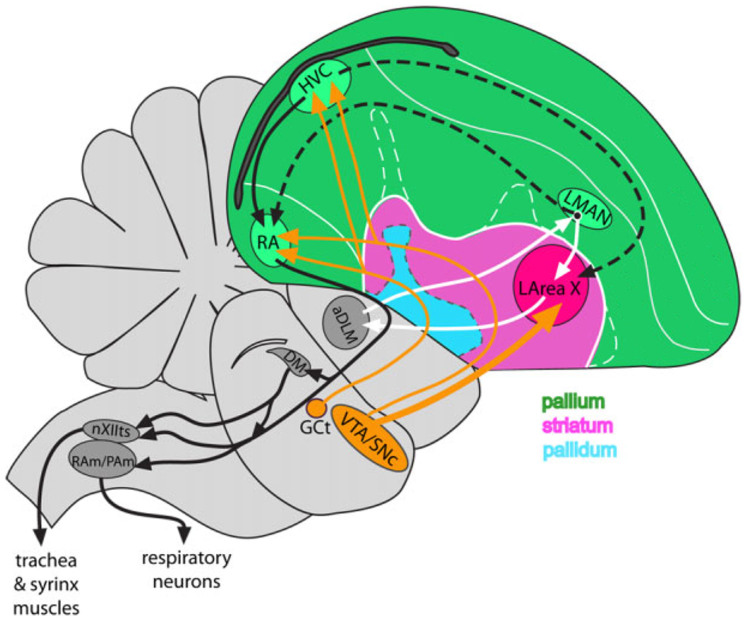
Figure 1. Brain diagram of song nuclei and telencephalic areas relevant for the present study.
The two main pathways, posterior (black solid arrows) and anterior (white arrows), are shown. Black dashed lines show connections between the two pathways; orange arrows show dopaminergic input. Abbreviations: aDLM – anterior nucleus of dorsal lateral nucleus of dorsomedial thalamus, DM – dorsal medial nucleus of the midbrain, GCt- mesencephalic central gray, HVC – nucleus HVC (a letter-based name), LArea X – lateral Area X, LMAN – lateral magnocellular nucleus of anterior nidopallium, nXIIts – tracheosyringeal part of the hypoglossal nerve, RA – robust nucleus of arcopallium, RAm/PAm – nuclei retroambiguus and paraambiguus VTA/SNc – ventral tegmental area/substantia nigra, pars compacta. The brain diagram is based on63 with permission from John Wiley & Sons.
Here we tested this hypothesis in adult male zebra finches and found that neurotoxic injury to Area X caused a long-term increase in song tempo and changes in syllable sequencing, particularly profound repetition. This stuttering occurred in birds that had a predisposition to repeat syllables already before the lesion, and it was worse during directed singing. In contrast, electrolytic lesions only caused the tempo and syllable sequencing to change. Further, stuttering behavioral changes were accompanied with brain tissue recovery. These findings show that Area X modifies song even in adult songbirds and that lesion-induced repetition might be associated with brain recovery.
Results
To determine the role of Area X in adults we created both neurotoxic and electrolytic lesions of this brain region in male zebra finches. First we describe the behavioral and molecular effects found in neurotoxically lesioned birds.
Neurotoxic lesions of LArea X cause tempo, sequence, and repetition changes in adult birds
First we performed bilateral neurotoxic lesions of lateral Area X (LArea X) in a pilot set of birds (n = 4 LArea X lesions, n = 4 MSt lesions and n = 3 sham controls). The birds survived between 3 and 17 months after surgery. We noted that these birds were singing multiple song motif types before the LArea X lesion, but after the lesion they gradually decreased motif type variability and only produced one song motif type by 3–5 months after the lesion (Fig. 2). Further we noted that one of the LArea X lesioned birds started to repeat the last syllable of his motif in a stuttering-like manner (Fig. 2). Before the LArea X lesion he repeated this syllable only twice in a motif. However, after the lesion the syllable was repeated up to 8 times. At 17 months the bird’s song had recovered to his original syllable repetition rate. Based on this pilot experiment, we hypothesized that basal ganglia damage might cause some birds to change the song stereotypy and that the presence of syllable repetitions before basal ganglia damage might predispose an animal to stutter after the injury. We proceeded to test this hypothesis on animals with and without repeated syllables in their songs.
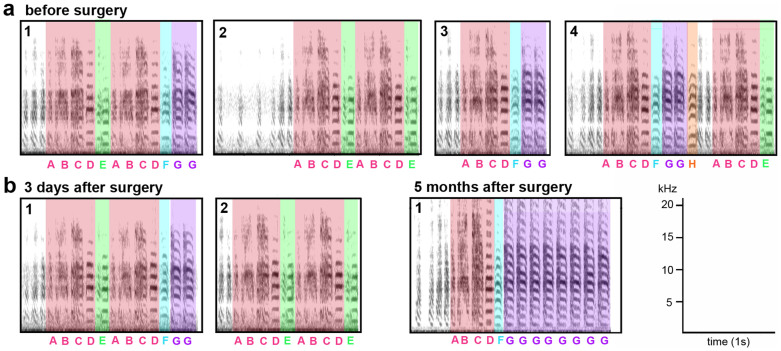
Figure 2. Examples of changes to song behavior in adult zebra finches following LArea X lesions.
(a), Sonograms showing the four (1–4) motif variants of song that male pu267 sang before LArea X lesion. (b), 3 days after lesion the same bird sang only two motif variants (1–2) and 5 months after lesion he sang only one motif variant (1), with added stuttered notes (purple). Pink color designates the first part of the motif and other colors designate the following individual syllables that vary among motifs. Corresponding letters for individual syllables are noted underneath. Non-colored vocalizations are the introductory notes. Sonograms were generated using Avisoft software.
Birds received either bilateral neurotoxic LArea X lesions or sham lesions, where for the later group a glass micropipette was lowered into the brain but no chemical was injected. We assigned birds to groups based on their song sequencing: 1) those that sang pre-op song motifs without any syllable repetition (such as an ABCDE syllable sequence; n = 9 lesioned and 5 control birds); and those that sang pre-op motifs with repetition in either 2) the beginning ([A]nBCDE; n = 3 lesioned and 3 controls), 3) the middle (A[B]nCDE; n = 6 lesioned and 3 controls), or 4) the end (ABCD[E]n; n = 6 lesioned and 4 controls).
Tempo
As song tempo is known to change throughout the day26 we measured the first songs sung during the morning (the first at least 20 motifs per time point found in 10–20 song bouts), using a specific motif variant for each bird (the longest and abundant motif; n = 7 neurotoxically lesioned [n = 2, 2 and 3 birds with no, beginning/middle and end repetition, respectively] and 7 sham controls [n = 3, 2 and 2 birds]). We found that the surgery alone in both sham and LArea X neurotoxically lesioned birds, regardless of the song type, caused a significant increase in motif duration on the next day, but this increase was more profound in LArea X lesioned birds (Fig. 3a). Thereafter the tempo returned to normal levels in the sham control birds, whereas in the LArea X lesioned birds it stayed slower during the first week, then reversed direction and steadily increased in speed for the entire 6 months. The initial increase in song motif duration was due to longer silent intervals between syllables and the subsequent decrease in song motif duration was more due to decreased syllable duration (Fig. 3b). These results were found in all birds, regardless of the syllable repetition group.
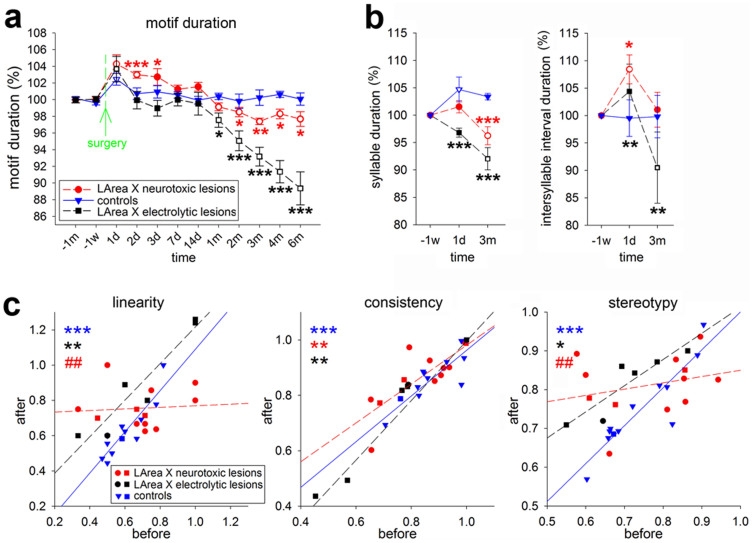
Figure 3. Quantification of song tempo and sequence changes.
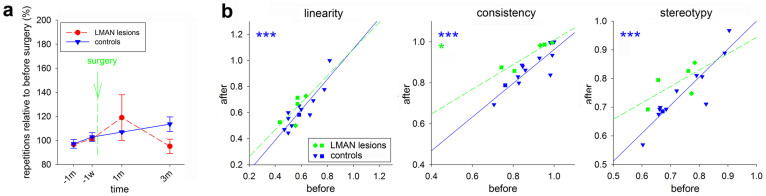
(a), Motif duration of LArea X lesioned and control birds (n = 7 neurotoxically lesioned/n = 8 electrolytically lesioned/7 sham controls), shown with respect to the duration preoperatively. All three groups were composed of comparable numbers of birds singing the various types of song motifs, including the end syllable repetition. (b), Syllable and intersyllable interval durations measured in the same birds as shown in (a). The color matched stars represent the statistical significance for comparison of lesioned and control birds at the given time point; the empty symbols represent significance at each time point post surgery compared to the levels before surgery (ANOVA followed by Fisher’s PLSD post hoc test). Abbreviations for time points on x-axis are d-day, w-week, and m-month; minus signs indicate recording times before surgery. Values are means ± SEM. (c), Song sequence changes expressed as linearity, consistency, and stereotypy of neurotoxically lesioned (n = 12), electrolytically lesioned (n = 7), and control (n = 12) birds before and 3 months after surgery. All groups included a mixture of birds with song variants that contained no syllable repetition or repetition at the beginning or middle of the song (circles for lesioned and triangles for control birds), or at the end (squares). The color matched stars represent statistically significant simple regressions, and the color matched # symbols represent statistically significant differences of slope of the given group in comparison to controls using ANCOVA. * p<0.05, ** and ## p<0.01, *** p<0.001.
Sequence
We confirmed our initial finding that the neurotoxic damage to LArea X also led to changes in song bout sequencing. In sham control birds, although different birds had different levels of syllable sequence linearity, consistency, and stereotypy before surgery, they did not change them after surgery. Therefore, there was a strong correlation among the control group before and after surgery (Fig. 3c, blue * symbols, r = 0.89, 0.84, and 0.91 for linearity, consistency, and stereotypy, respectively). However, in the birds with LArea X neurotoxic lesions, the correlation before and after surgery was eliminated for song linearity and stereotypy (Fig. 3c, r = 0.08 and 0.26, respectively; r = 0.79 for consistency). ANCOVA analyses revealed that the relationship in the LArea X neurotoxically lesioned group was significantly different from the sham controls (Fig. 3c, red # symbols). The overall changes for linearity and stereotypy resulted in relatively flat trend lines. The changes occurred whether or not birds produced repeated syllables before the lesion (Fig. 3c; squares for end syllable repetition birds). These findings demonstrate that neurotoxic lesions of LArea X in adults affect song sequencing.
Repetitions
Before surgery, the mean number of syllable repetitions per motif across syllable repetition groups 2–4 ranged from 2.43 to 4.33 and it did not differ between birds selected for the neurotoxically lesioned and sham control groups (p = 0.19–0.55, t-test). The number of syllables within the repetitions between the 2 analyzed days before surgery was stable for all groups (Fig. 4a,b). After LArea X lesions, syllable repetitions did not change significantly in the groups without pre-op syllable repetitions, or with syllable repetition at the beginning or middle of the motif (Fig. 4a). Assessing individuals, 1 of the 3 lesioned birds with syllable repetition at the beginning slightly increased the repetitions by less than 2-fold. One of 6 birds with repetition in the middle of the song motif increased the repetitions up to 2.8-fold between 1–3 months after the LArea X injury. However, all 6 birds that repeated syllables at the end of the motif before surgery significantly increased the number of repetitions of that syllable per motif by 1 month after surgery, with a further increase between 2 and 5 months (Fig. 4b, d–e; Movies 1–2). The peak in repetitions per motif occurred between 3–5 months, depending on the bird. At the peak, the mean number of repetitions was 2.54 ± 0.22 (mean ± SEM) fold higher than before the lesions; the mean maximal occurrences of the syllable increased from 4.3 before the lesion to 10.2 repetitions after the lesions. There was a decrease in repetition between 5 and 6 months post lesion (Fig. 4b; p<0.05, paired t-test). When listening to these songs, it sounded as if the birds often got stuck stuttering at the end of the 1st or 2nd motif (compare Movies 1–2 for lesioned with Movies 3–4 for control birds). These findings suggest that birds with syllable repetitions are predisposed to produce a significant amount of repetition after neurotoxic basal ganglia damage, and the effect is more robust with end motif repetitions.
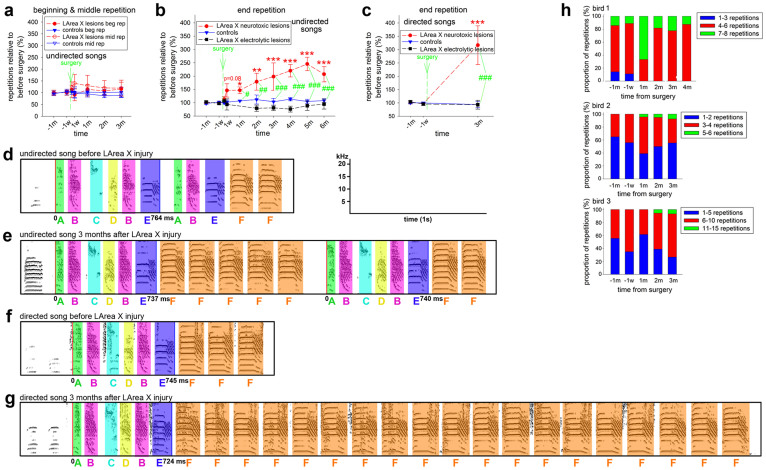
Figure 4. Quantitative behavioral analysis of LArea X lesioned and control birds.
Syllable repetition of birds whose songs contained syllable repetitions before the surgery (a), at the beginning (n = 3 neurotoxically lesioned/3 sham controls) and in the middle (n = 6 neurotoxically lesioned/3 sham controls), and (b), at the end of their undirected song motifs (n = 6 neurotoxically lesioned/n = 6 electrolytically lesioned/4 sham controls). (c), Syllable repetition of some of the same birds in (B) with a repetition at the end of their directed song motifs (n = 3 neurotoxically lesioned/n = 6 electrolytically lesioned/4 sham controls). Abbreviations for time points on the x-axis are d-day, w-week, and m-month; minus signs indicate recording times before surgery. Values are means ± SEM. In (a–c), the color matched stars * represent the statistical significance for each group at each time point post surgery compared to the levels before surgery; the green # symbols represent significance for comparison of lesioned and control birds at the given time point (ANOVA and Fisher’s PLSD test). (d–g), Sonograms of songs several days before and 3 months after bilateral neurotoxic lesions to LArea X. In the bird shown, before lesioning, syllable repetition at the end of the motif varied from 1 to 4 in both undirected and directed singing (averages 1.62 and 2.5, respectively), and after lesioning it varied from 1 to 12 in undirected and from 1 to 19 in directed singing (averages 3.38 and 8.22, respectively). Colors designate individual syllables, with corresponding letters underneath. Non-colored vocalizations are the introductory notes. Not all introductory notes are shown and only the first motif is shown in G due to the space limitations (full song in the Movies). The red and blue vertical lines mark the beginning and the end of motif, respectively, and motif length is shown below the line indicating the motif end. (h), Proportion graphs of three birds with unusually high numbers of syllable repetitions at the end of their song motifs showing the occurrence of repetitions before and after LArea X injury. The data for each bird are grouped into three bins (color-coded). The larger size bins of repetitions increase after the lesions.
The above experiments were conducted on birds that produced a few repetitions before surgery (mean 1–2.07/motif for the end syllable repetitions, i.e. the group 4). However, we identified three birds that produced many more repetitions at the end of the song motif (mean 2.30–6.29/motif), up to 10 times, and thus naturally stuttered at a rate comparable to that found in the human population27. We performed bilateral neurotoxic LArea X lesions in these birds and found that after the injury, they did not show a further increase in the mean number of repetitions but a noticeable increase in the distribution of the syllable repetition and maximum occurrences (e.g. in bird 1: 1–8 syllables per repetition before the lesion to 4–8 after the lesion; bird 2: 1–4 before to 1–6 after; and bird 3: 3–10 before to 2–15 after; Fig. 4h).
The stuttering effect is more dramatic during directed singing
Since excessive dopaminergic signaling can contribute to stuttering in humans28 and there are higher dopamine levels in striatal Area X when birds sing courtship song directed towards females than when singing undirected song29, we investigated whether neurotoxic LArea X lesions also affected stuttering during directed singing. Before LArea X lesions, syllable repetition in birds with end repetitions did not differ between directed and undirected singing (p = 0.20, paired t-test). However, after LArea X lesions, there was also a significant increase in the mean syllable repetitions per motif for directed singing (Fig. 4c; 1.77 to 5.89-fold at 3 months relative to before the LArea X lesion) and this increase was more dramatic than for undirected singing (Fig. 4b vs 4c; p<0.05, paired t-test for directed and undirected songs within the same individuals). The maximal syllable repetition recorded at the end of the motifs in these males was 7–8 for undirected singing and 6–19 for directed singing (Fig. 4f–g, Movies 5–6). We have never observed such high syllable repetition in any intact birds in our colony. These findings suggest that the magnitude of the effect of stuttering following neurotoxic basal ganglia damage is context dependent.
Stuttering does not occur with pallial (cortical) neurotoxic lesions of the pathway
Next we asked if the effect on stuttering was a general property of neurotoxic damage to the pallial-basal-ganglia-thalamic-pallial pathway, or if it was specific to the striatum. To address this question, we performed neurotoxic lesions of the pallial (cortical-like) part of the pathway, the song nucleus LMAN (n = 3 pre-op end repetition birds and n = 2 non-repetition controls). Unlike injury to LArea X, lesions in LMAN did not result in increased repetition in birds repeating their end syllable of the motif (n = 3), for either undirected (Fig. 5a) or directed song. The maximal increase in repetition was 1.4-fold, which was within the range of sham control birds. Although the sample size was small, it was clear that none of the end repetition LMAN lesioned birds recapitulated any of the increased magnitude in stuttering found in all of the LArea X neurotoxically lesioned birds with end repetitions. These findings imply that the effect is specific to the neurotoxic injury of the striatal song nucleus, LArea X.
Figure 5. Quantitative behavioral analysis of LMAN lesioned and control birds.
(a), Syllable repetitions in LMAN lesioned and control birds that repeated a syllable at the end of their undirected motif before lesion (n = 3 lesioned/4 sham control birds). Abbreviations for time points on x-axis are w-week and m-month; minus signs indicate recording times before surgery. Values are means ± SEM. Statistical differences were assessed by ANOVA followed by Fisher’s PLSD post hoc test. (b), Song sequence changes expressed as linearity, consistency, and stereotypy of LMAN lesioned (n = 5) and control (n = 12) birds before and 3 months after surgery. Both groups included a mixture of birds with song variants that contained no syllable repetition or repetition at the beginning or middle of the song (circles), or at the the end (squares). The color matched stars represent statistical significant simple regressions; slopes of LMAN and control curves were compared using ANCOVA (no significant differences were found). * p<0.05, *** p<0.001.
We also performed sequence analyses of the LMAN lesioned birds. We found that song consistency before and 3 months after lesion significantly and positively correlated (Fig. 5b, green * symbol, r = 0.94) and this correlation was not different from the correlation of sham control birds (Fig. 5b). However, song linearity and stereotypy did not significantly correlate before and after the LMAN lesion (r = 0.71 and 0.66, respectively), but they were not different from control birds either (Fig. 5b; ANCOVA) indicating that the change was smaller than for the Area X lesions. These results show that neurotoxic lesion of LMAN does not induce stuttering but affects the global bout sequence so that the measures before LMAN lesion are not as predictive of the measures after the lesion.
Stuttering occurs at the same time as basal ganglia recovery
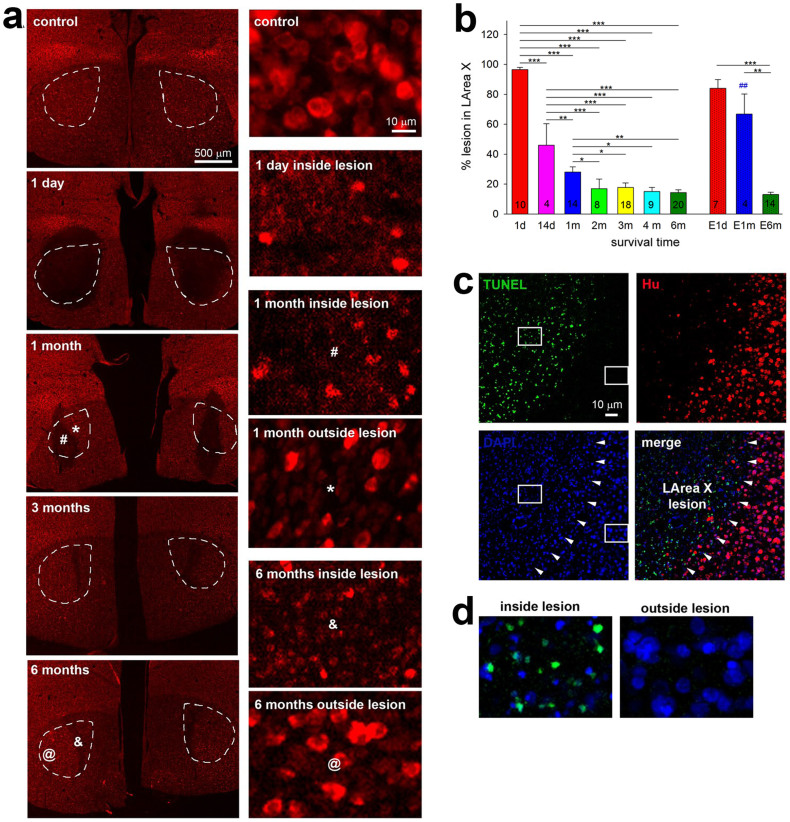
When assessing the lesion location and effectiveness of the above-described birds using the neuronal marker Hu in a time course study, we found a surprising result. After several months, LArea X greatly recovered from the lesion in adult birds. Specifically, one day after the neurotoxic lesion, in all birds examined, most (96.50 ± 1.47%; mean ± SEM) of LArea X was bilaterally damaged as defined by the Hu staining area relative to control animals (Fig. 6a,b), similar to our previous study on unilateral LArea X lesions21. However, between 2 weeks and 1 month afterwards, the lesioned area was significantly smaller, amounting to about half the normal size of LArea X (Fig. 6a,b). By 2 months, the lesioned area of LArea X was even more reduced, up to less than 20% on average, and did not change further from 2 to 6 months (Fig. 6a,b). We do not believe that the large differences in lesion size between time points are due to technical surgical differences at the time the lesions were conducted, as animals that received lesions on the same day were staggered among survival time points, and there were no birds that showed complete lesions sizes after 1 month. Further, although inside of the lesioned area we could still find isolated surviving neurons expressing Hu from 1 day onward, these neurons appeared to be smaller than the neurons in the adjacent, apparently recovered LArea X or in LArea X of control birds, and their density increased over time (Fig. 6a, right panels).
Figure 6. Lesion in LArea X.
(a), Example photomicrographs of coronal sections showing both hemispheres in a control bird and in birds 1 day and 1, 3 and 6 months after bilateral LArea X neurotoxic lesions. Neurons are visualized by red Hu-immunofluorescence. Left panels, low magnification images with LArea X indicated by dashed lines; note recovery as well as presence of lesioned tissue even by 6 months. Right panels, higher magnification images of control, lesioned, or recovered parts of LArea X; the symbols inside LArea X depict the locations in the right panels. Note the difference in size of the neurons and quality of tissue inside and outside the lesion area. (b), Quantification of lesion size in LArea X measured as % lesioned relative to the average LArea X size in sham control birds; n represents the number of hemispheres. First 7 bars, neurotoxic lesions; last 3 bars, electrolytic lesions. Values are means ± SEM. * p < 0.05; ** p < 0.01; *** p < 0.001 (ANOVA followed by Fisher’s PLSD post hoc test). ## is p<0.01 for comparison 1m neurotoxic and electrolytic lesion (t-test). (c), Apoptotic cells in lesioned LArea X 1 day after the injury. Green, apoptotic cells stained by the TUNEL method; red, Hu labeled neurons; blue, DAPI stained nuclei. The merged panel combines the three labels into one image. Arrowheads point to the lesion boundary. (d), Higher magnification of the boxed regions in c inside and outside lesion showing double label of TUNEL and DAPI.
To test the possibility that the neurons in LArea X were still alive but temporally stopped expressing Hu as a result of the neurotoxic lesion and later started to express Hu again, we used the TUNEL method that stains apoptotic, dead cells. We found that at 1 day after the lesions, the LArea X lesioned region was filled with apoptotic cells (Fig. 6c). Moreover, the DAPI stained cell nuclei inside the lesion area shrunk in comparison to the cells in lateral striatum adjacent to the lesion (Fig. 6d), which is indicative of dead cells.
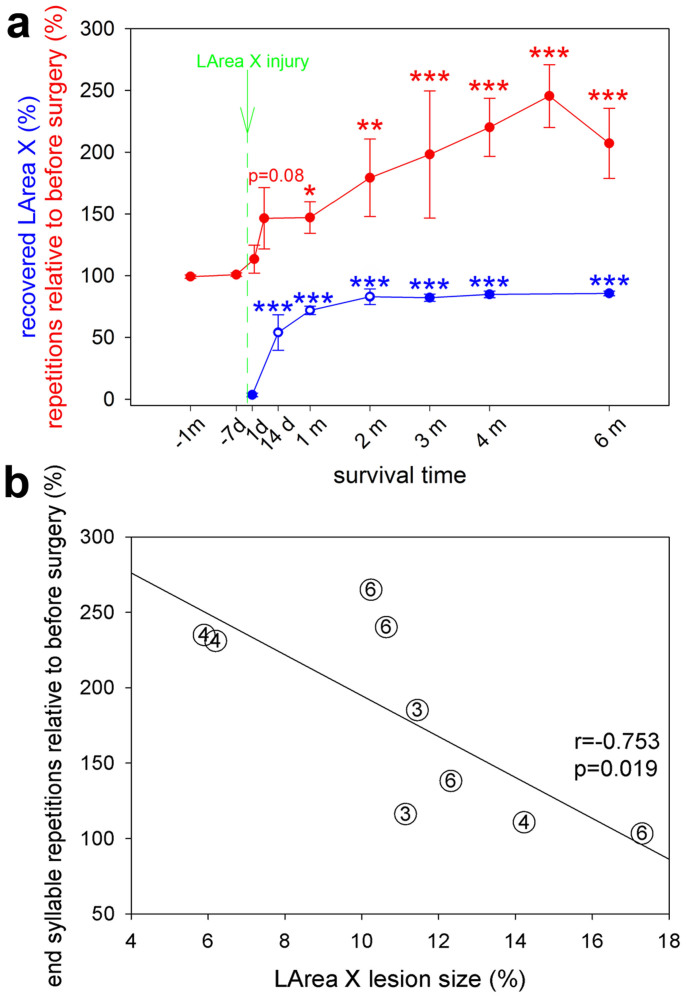
We examined the time courses of the neural changes and song behavior and found that the significant changes in LArea X recovery based on Hu staining (from 14 days) occurred at the time of significant increases in end syllable repetition (14 days or 1 month; Fig. 7a). Further, the shrinking lesion size at the time of sacrifice correlated with increased end syllable repetition rate (Fig. 7b). These results suggest that the recovering lesion might be associated with increasing stuttering.
Figure 7. Striatal recovery and end syllable repetitions.
(a), The relationship of Area X tissue recovery (blue) and syllable stuttering (red) in LArea X neurotoxically lesioned birds with motif end syllable repetitions. Blue stars, statistical differences of brain recovery at individual time points relative to before the injury; blue open circles, statistical differences of brain recovery at individual time points relative to the previous time point; red stars, statistical differences of repetition at the end of the song motif at individual time points relative to the repetition before the injury. * p < 0.05; ** p < 0.01; *** p < 0.001 (ANOVA followed by Fisher’s PLSD post hoc test). (b), Correlation of lesion size at sacrifice (number of months in circle) with end syllable repetitions after neurotoxic lesions of LArea X (linear regression).
Neurons in the recovered LArea X are functional
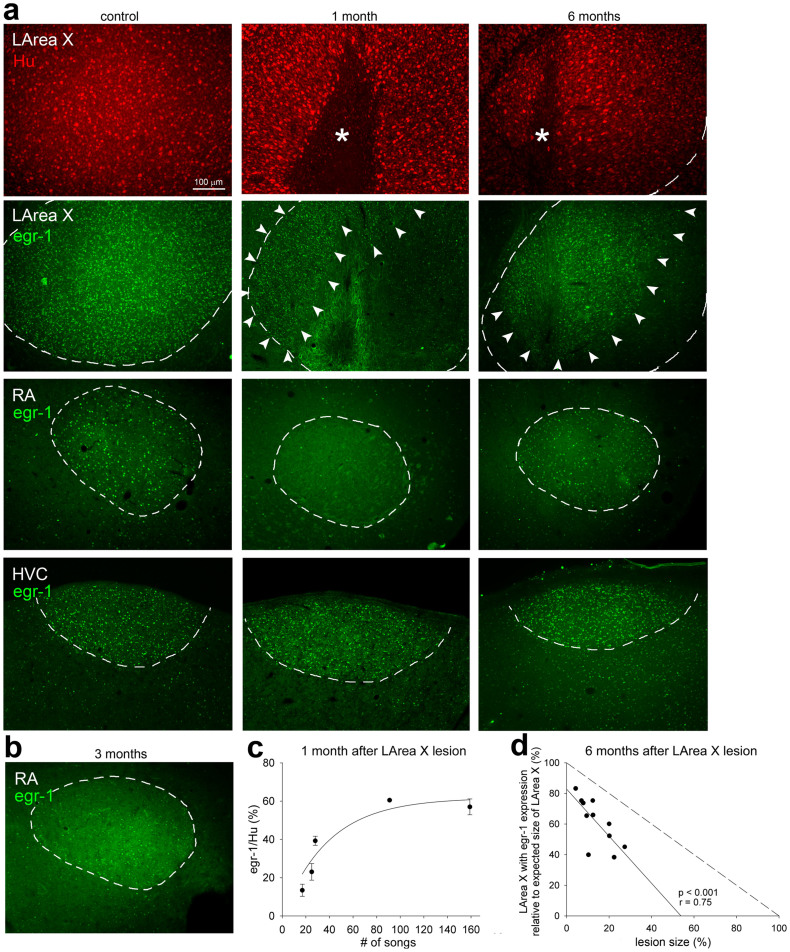
Next we wanted to know if the neurons in the recovered part of LArea X were functional during singing. We assessed undirected singing-driven IEG egr-1 expression, which normally results in high levels of egr-1 throughout Area X21,24. Our previous work showed that within 1 week post neurotoxic lesion, there were barely any detectable cells in the lesioned LArea X area that show singing-driven egr-1 expression21. Here we found that by 1 month after the neurotoxic lesions, some sparsely spaced neurons in the recovered part of LArea X showed singing-driven gene expression (Fig. 8a). The presence of egr-1 was not due to a long-term injury response, as the number of neurons and thus amount of expression correlated with the amount of songs produced, labeling about 60% of the Hu+ neurons at high levels of singing (Fig. 8c). This singing-driven gene expression occurred at all time points up to 6 months, the latest time point we examined. However, by 6 months, the LArea X region that showed singing-driven gene expression was only 61.76 ± 4.77% (mean ± SEM) of its normal size (Fig. 8a,d), and was a part of a larger Hu+ region with defined boundaries that appeared like LArea X (boundaries visible as a cell density difference with the surrounding striatum), but without singing-induced egr-1 cells at its borders.
Figure 8. Singing-driven egr-1 expression in the recovered part of LArea X and other song nuclei after neurotoxic lesion.
(a), Representative photomicrographs of LArea X, RA, and HVC in a control and LArea X lesion birds at 1 and 6 months after the injury, singing 72, 159, and 32 undirected songs in 1 h, respectively. Red, Hu staining showing neurons in the control and regenerated LArea X as well as the lesion (*). Green, egr-1 protein signal. Dashed lines designate boundaries of song nuclei based on the Hu label. The arrowheads point to the region within LArea X with higher singing-driven egr-1 expression in the lesioned animals. (b), Representative photomicrograph of singing-driven egr-1 expression in RA of a bird 3 months after LArea X lesion that sung 56 undirected songs, the first time point we noted recovery of singing-driven gene expression in RA. (c), Quantification showing that the proportion of neurons (Hu+) that express egr-1 at 1 month after the lesion increases with the amount of songs produced, indicating that the expression is driven by singing in the lesioned animals. Each point represents mean ± SEM for both hemispheres of one bird. (d), Correlation of lesion size based on Hu staining (non-Hu area divided by the average size of LArea X in control birds; x-axis) with the proportion of LArea X that shows singing-driven egr-1 after the lesion to the average size of LArea X in control birds (y-axis) at 6 months. The values of the two hemispheres for each brain are averaged into one value. The solid line is a linear regression for the lesioned birds. The dashed line is the theoretical expectation on the 45° angle if all of the recovered LArea X showed egr-1 expression.
A prior study of ours showed that unilateral neurotoxic lesions of LArea X eliminate singing-driven gene expression in the ipsilateral RA but not HVC, indicating that the synaptic effects that trigger activity-driven gene expression in LArea X propagate through the anterior pathway via DLM and LMAN to RA, and are required for singing-driven gene expression in RA21 (Fig. 1). Those studies were conducted within a week after LArea X lesions. Here we found that bilateral neurotoxic LAreaX lesions still prevented singing-driven egr-1 expression in RA bilaterally by 1 month after the lesions (Fig. 8a), despite finding some singing-driven egr-1 expression in LArea X (Fig. 8a,c). However, from 3 months onward, the singing-driven gene expression in RA began to recover (Fig. 8a,b). In contrast, the singing-driven gene expression in the nucleus presynaptic to LArea X, HVC, was persistent at all times after the LArea X lesions (Fig. 8a). In summary, the singing-dependent expression in the recovered part of LArea X and in RA suggest that at least some neurons in the recovered LArea X are functional and reconnect into the song circuit.
New or repaired neurons are associated with basal ganglia recovery
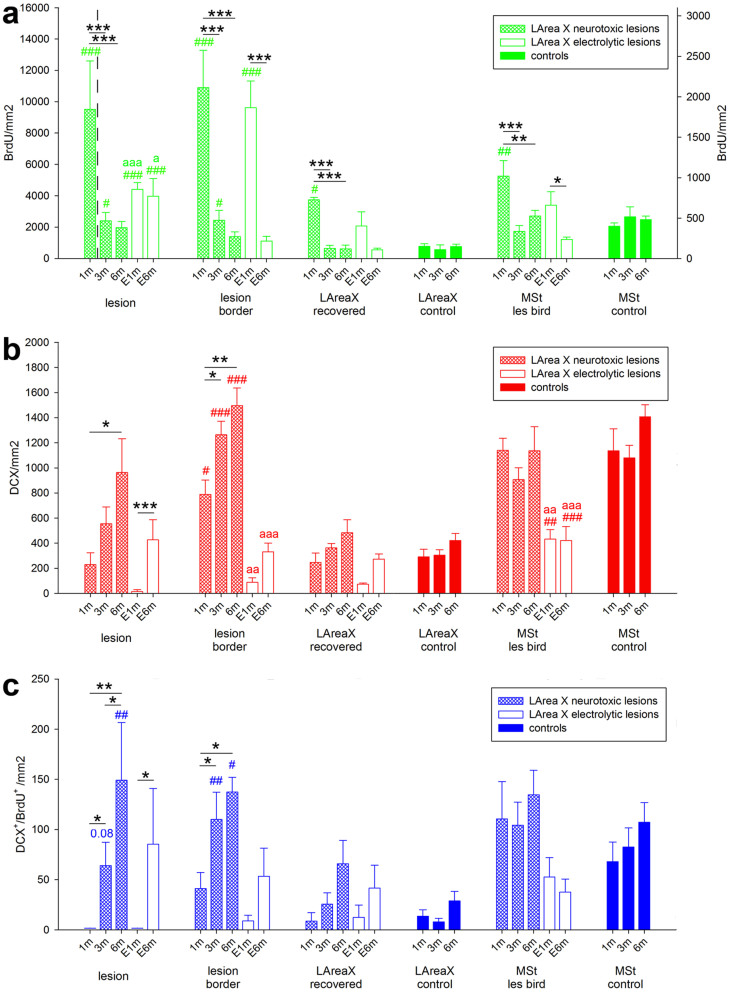
We wondered what was the source and thus mechanism of neuron recovery in LArea X. We hypothesized two alternatives: 1) old neurons migrated in from the surrounding non-song regions and became incorporated into the song circuit; or 2) new neurons generated soon before or after the lesions entered the song nucleus and became functional within it. The later is possible given that in songbirds Area X receives new neurons throughout life30,31. To address these hypotheses, in some of the lesioned birds described above and in additional birds, we injected the cell division marker 5-bromo-2’-deoxyuridine (BrdU) for 7 days at different time points after the surgery, and then waited 24 days (30 day survival period counted after the first day of the BrdU injection) before sacrificing the birds at 1, 3, and 6 months after the surgery (Fig. 9a); BrdU is a thymidine analog that is incorporated to the DNA of dividing cells or of cells undergoing repair from DNA damage. This experimental design allowed us to tag the state of cells at different time points after the lesion. Relative to the rest of LArea X and to sham control animals, we found much higher numbers of BrdU labeled cells inside the neurotoxically lesioned Area X at 1 month (Fig. 9b; Fig. 10a, green # symbols for comparison with control; left y-axis for 1 month, and right y-axis for all other time points), which means that these cells were born immediately after the injury. There were fewer BrdU labeled cells inside a 60–70 μm wide lesion border, but still significantly more than those seen in sham controls (Fig. 9b, 10a). By 3 and 6 months, the number of BrdU labeled cells added to the lesioned area was significantly lower, but still higher than that normally seen in control animals, whereas the number of new cells added in the recovered area returned back to the normal rate (Fig. 10a). Interestingly, it is known that in the avian brain new neurons migrate into the striatum from the lateral ventricle on the medial edge of the striatum31,32. Consistent with this finding, we found that in control animals there were more BrdU labeled cells in the medial striatum (MSt) than in LArea X. However, in the LArea X neurotoxically lesioned animals, there was a significant increase in BrdU labeled cells in the MSt at 1 month, even though the MSt was not lesioned, and the levels returned to normal by 3 months (Fig. 10a). These findings suggest that the many of the BrdU labeled cells were new cells and not repaired cells damaged from the neurotoxic chemical.
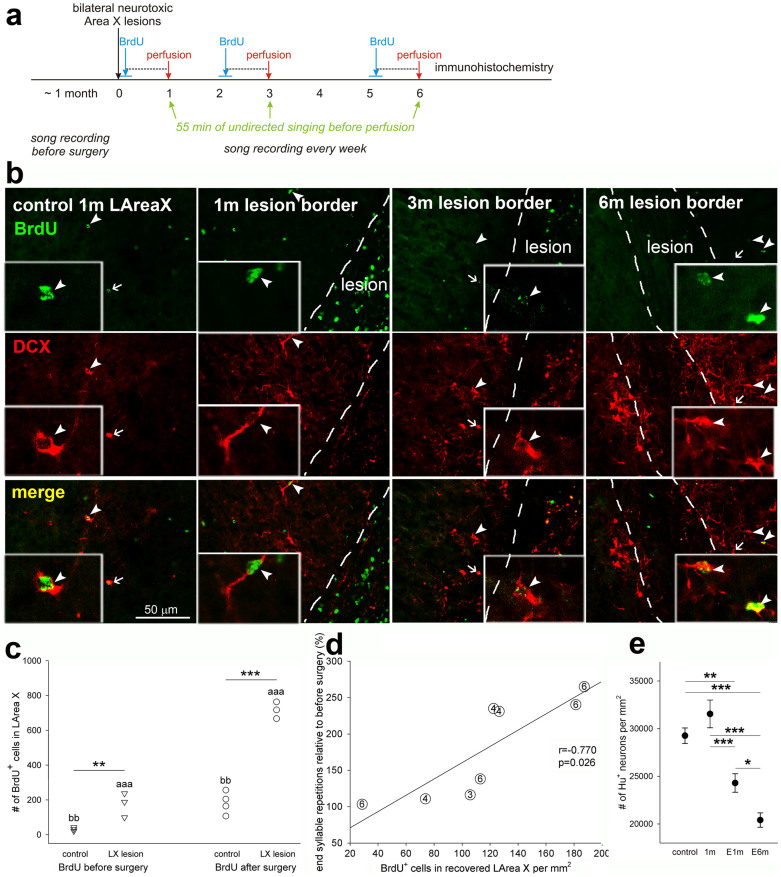
Figure 9. Timeline and Area X tissue recovery.
(a), Timeline. In different groups of birds, songs were recorded from about 1 month before and up to 6 months after the surgery. The cell division marker BrdU was injected for 7 days at 0, 2, or 5 months after the surgery and birds sacrificed 30 days after the first injection, after performing 55 min of undirected singing. (b), Representative photomicrographs of control LArea X and lesion border 1, 3 and 6 months after the neurotoxic injury showing higher numbers of new (BrdU+) young neurons (DCX+) with increasing age. The dashed lines highlight the visible boundary between the lesion and nonlesioned or recovering parts of LArea X. Arrows and arrowheads point to double labeled DCX+/BrdU+ neurons. Inserts show double-labeled neurons magnified 6x relative to the main panels. Note the high number of BrdU+ cells inside the lesion at 1 month and higher number of DCX+ neurons inside the lesion at 3 and 6 months in comparison to 1 month. The DCX+ neurons at 1 and 3 months are rounded and with few dendritic processes while at 6 months their expression was less and the cells appeared to have more dendritic processes. (c), New cells (BrdU+) incorporated in the recovered part of LArea X with BrdU injected during 7 consecutive days either before or immediately after the surgery. ** p < 0.01, *** p < 0.001, comparison between control and lesioned birds; ‘aaa’ p < 0.001 for the comparison between lesioned birds with BrdU injections before and after surgery; ‘bb’ p < 0.01 for the comparison between controls (ANOVA followed by Fisher’s PLSD post hoc test). (d), Correlation of the number of new (BrdU+) cells in the recovered part of LArea X and number of end syllable repetitions after neurotoxic lesion (linear regression). The numbers in circles indicate survival of the bird (in months). (e), Neuronal density in MSt adjacent to the lesion 1 month after neurotoxic lesion (1m) and 1 and 6 months after electrolytic lesion (E1m and E6m). * p < 0.05, ** p < 0.01, *** p < 0.001 (ANOVA followed by Fisher’s PLSD post hoc test).
Figure 10. Quantification of lesion induced and spontaneous neurogenesis in LArea X lesioned and control birds, respectively, at 1, 3 and 6 months after surgery.
Graphs show on the y-axis the number of (a), new cells (BrdU+), (b), young neurons (DCX+), and (c), new young neurons (DCX+/BrdU+) that arose 1 month before sacrifice. There are two y-axes in a; the left one is for the first bar and the right one is for all the other bars. The two parts of the graph are also divided by the black vertical dashed line. The x-axis in all graphs shows designated brain regions for cell counts and survival times in neurotoxically (1, 3, and 6 months) and electrolytically (E 1 and 6 months) lesioned and sham control birds. Values are means ± SEM. * statistical significance for comparison among the time points for a given area; # statistical significance for comparison of lesioned and control birds at the given time point; “a” statistical significance for comparison of neurotoxically and electrolytically lesioned birds at the given time point. * and # p < 0.05; **, ## and aa p < 0.01; ***, ### and aaa p < 0.001 (ANOVA followed by Fisher’s PLSD post hoc test).
To test for the possibility that new cells generated before the neurotoxic lesion could be recruited to repair the lesion, we injected BrdU into another set of neurotoxically lesioned and sham control animals (n = 3 each) 1 week before the surgical damage and then examined BrdU label 1 month after the surgeries. Relative to sham control, we again found more BrdU+ cells in the lesioned Area X (Fig. 9c) suggesting that some cells born before the surgery have increased incorporation into the recovering LArea X. However, the number of newly incorporated cells in the lesioned LArea X born before surgery was much lower than the number of cells born immediately after surgery (Fig. 9c; aaa symbols for p<0.001). The sham lesion with a glass micropipette lowered into LArea X itself also seems to have a small effect on BrdU+ cell incorporation relative to the surrounding striatum, but this was lower than in the LArea X lesioned animals (Fig. 9c; controls comparison shown by bb symbols for p<0.01). These findings indicate that new cells born both before and after the lesion were used for the LArea X recovery, but incorporation of new cells generated after the injury was much greater.
To further test if the recovered neurons in LArea X represent newly generated neurons or old neurons that were repaired through a DNA-repair mechanism which also incorporates BrdU33, we performed single and double labeling with BrdU and doublecortin (DCX), the latter a marker of young migrating and differentiating neurons34. Inversely related to the BrdU labeling, we found a steady increase from 1 to 6 months in DCX labeling inside the neurotoxic lesion and the lesion border (Fig. 9b, 10b). Moreover, the highest numbers of DCX+ neurons were always found in the lesion border, and this was significantly different from LArea X of control animals (Fig. 9b, 10b). Similar to BrdU labeling, there were more DCX labeled cells in the MSt even of control animals, but unlike BrdU, there was no peak in the number of DCX labeled neurons in the MSt of lesioned animals (Fig. 10b). We also found double labeled DCX+/BrdU+ neurons (Fig. 9b, 10c), and these followed the recovery pattern of the DCX+ neurons (Fig. 10b). The double labeled DCX+/BrdU+ neurons were about 10% of the amount of DCX+ neurons in both lesioned and control animals implying that the amount of BrdU injected in 7 consecutive days labels about 10% of newly generated neurons and/or that a portion of the DCX+ neurons was born after the BrdU injections and thus these neurons were younger35. These findings suggest that after neurotoxic lesion, thousands of the BrdU+ cells in the lesion are new neurons and there is still ongoing recovery at least up to 6 months after the injury. However, the considerable amount of DCX−/BrdU+ labeled cells at the first month could also include new glia and/or repaired old cells.
We examined whether the number of new cells in the regenerated LArea X was correlated with the behavioral changes in song. We found a significant positive correlation between the density of BrdU+ labeled cells inside the recovered LArea X and the percent increase in the end syllable repetition at the time of sacrifice (Fig. 9d). Together with the negative correlation of end syllable repetition with lesion size at the time of sacrifice (Fig. 7b), these findings indicate that the stuttering is associated with striatal regeneration after neurotoxic injury.
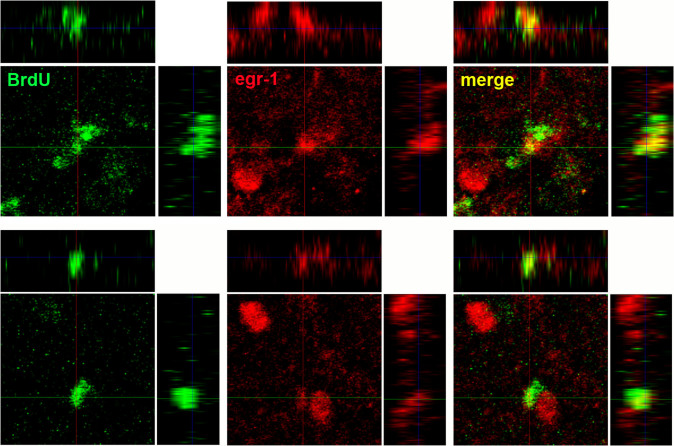
We next asked if the newly generated neurons were functional, by performing double labeling experiments with BrdU and egr-1 in neurotoxically lesioned birds that sang undirected song (egr-1 is only expressed in neurons). We found that some of the BrdU neurons in the recovered LArea X expressed egr-1 already 1 month after the injury (Fig. 11). At later time points of BrdU injection, we also found double labeled egr-1 cells (not shown), although there were fewer BrdU cells to identify since the injections were done later with respect to the time of the lesion. Confocal imaging revealed that these BrdU labeled cells clearly expressed egr-1 (Fig. 11). These findings indicate that newly generated neurons post-injury are functional.
Figure 11. Two examples of confocal images showing newborn cells (BrdU+) 1 month post lesion that expressed singing-induced egr-1 in the recovered part of LArea X.
To confirm that the cells are double labeled, three planes of the confocal image are shown: dorsal y view (largest panel), lateral x view (upper panels), and lateral z view (side panels). Crossing of the perpendicular lines are where the centers reference for each panel.
To address whether old neurons surrounding the lesion site migrated into the lesion site, we counted Hu+ neurons and DAPI+ cells 1 month after surgery in MSt 100–200 μm distant from the lesion. We found that there was a significantly higher number of DAPI+ (p<0.001; not shown) but not Hu+ cells (Fig. 9e) in the adjacent MSt after the neurotoxic lesion in comparison to control suggesting that these additional DAPI+/Hu− cells are either immature neurons and/or likely glia. This shows that at a time of the highest recovery there might be an increased migration of newborn cells into the neurotoxic lesion but the cell and/or neuron density just adjacent to the lesion is not lower due to cell migration into the lesion.
Medium spiny neurons are recovered
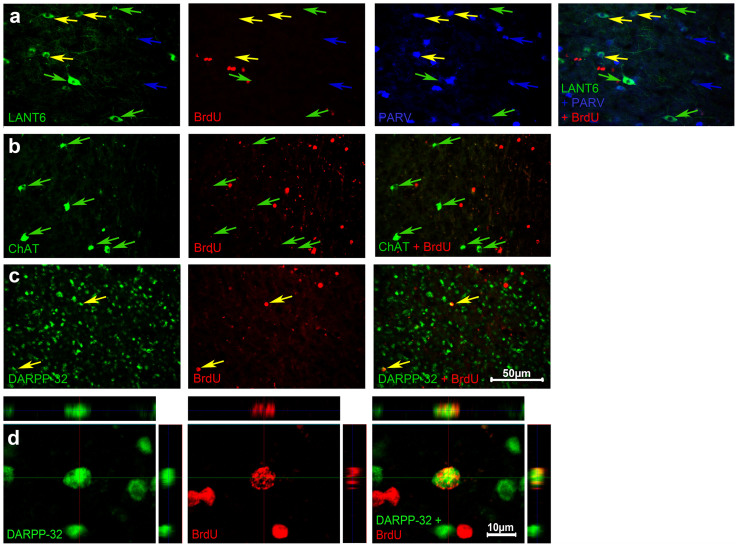
We determined what types of neurons were renewed in the recovered LArea X, using double or triple fluorescent immunohistochemical combinations for known neuron types in normal Area X36. We found BrdU only in the DARPP-32-containing medium spiny neurons (Fig. 12c,d), but we could not find any BrdU labeled parvalbumin-containing interneurons (Fig. 12a), LANT6-containing neurons, known to be the DLM projecting neurons (Fig. 12a), or cholinacetyltransferase-containing interneurons (Fig. 12b) in the recovered part of LArea X. These results, however, do not rule out generation of these other types of neurons in the lesioned area, since relative to the medium spiny neuron, they are present in small numbers (<5% total) and we had to limit the amount of BrdU injections to avoid neurotoxicity. In fact, in a close vicinity to the lesion, we did find all of the above mentioned neuronal types, including the LANT6-containing neurons projecting to DLM, but their origin is unknown. The results demonstrate that the regeneration of the most numerous neuron type in Area X, the medium spiny neurons, are replenished after neurotoxic lesion.
Figure 12. Examples of various neuronal types in LArea X of neurotoxically lesioned birds.
(a), BrdU and labels for DLM projecting neurons (LANT6+/PARV−) and two types of interneurons (LANT6−/PARV+, LANT6+/PARV+). (b), BrdU and cholinergic interneurons (ChAT+) labels. (c), Double labeled BrdU+/DARPP-32+ medium spiny neurons. Only the medium spiny neurons were found colocalized with BrdU. (d), Three planes of the confocal images showing colocalization of DARPP-32 with BrdU. The sections are from animals with BrdU injected immediately after the LArea X lesion and sacrificed 3 months afterwards.
Stuttering does not occur after electrolytic lesion of LArea X
All of the above experiments were done with neurotoxic lesions that leave fibers and the extracellular matrix intact. To test whether the increased stuttering occurs also when fibers are eliminated, we performed bilateral electrolytic lesions that damage also axons and dendrites37. Unlike the neurotoxic lesions, electrolytic lesions of LArea X did not cause any significant differences in the production of either beginning (n = 5; not shown) or end syllable repetitions with undirected or directed singing (n = 6; Fig. 4b,c). In contrast, similar to the neurotoxic lesions, electrolytic lesions did cause a transient increase followed by an even more dramatic and systematic decrease in the song motif duration over 6 months (Fig. 3a). The decrease was due to shortening both the syllables and inter-syllable intervals (Fig. 3b). Further, unlike the neurotoxic lesions, there was still a correlation in song linearity, consistency and stereotypy before and after LArea X electrolytic lesions (Fig. 3c, black * symbols, r = 0.90–0.95). However, the slope of the linearity regression after electrolytic lesion differed from neurotoxic lesion (p = 0.002; ANCOVA). After LArea X electrolytic lesions, song linearity and stereotypy increased in nearly all animals and were higher as a group than those in control birds (Fig. 3c, slope was not different [p = 0.61 and 0.21 for linearity and stereotypy, respectively] but the intercept differed p<0.0001 for both, ANCOVA).
Within the brain, similarly to neurotoxic lesions, most (84.05 ± 5.84%; mean ± SEM) of LArea X was damaged 1 day after electrolytic lesions as determined by Hu+ staining (Fig. 6b). However, unlike the neurotoxic lesions, no significant recovery occurred 1 month after the surgery (Fig. 6b); but at 6 months LArea X size had recovered to the level similar as seen after neurotoxic lesions (Fig. 6b). Interestingly, there was a lower number of BrdU+ cells inside the electrolytic relative to the neurotoxic lesions at 1 month, but a higher number at 6 months and these were higher than in control LArea X (Fig. 10a). Although there were some DCX+ and DCX+/BrdU+ labeled neurons inside the electrolytic lesion, especially at 6 months (Fig. 10b,c), these were only in very close vicinity to the lesion border (<100 μm). Further, there was no intensely labeled region of DCX+ cells around the lesion border (compare with LArea X recovered; Fig. 10b). All other characteristics of BrdU+ and DCX+ labeled cells outside the lesion area were similar to the neurotoxically lesioned animals. In MSt 100–200 μm adjacent to the lesion, we found significantly lower number of both Hu+ neurons (Fig. 9e) and DAPI+ cells (p<0.001; not shown) 1 and 6 months after the electrolytic lesion in comparison to control, and that the number of Hu+ neurons was lower at 6 months than at 1 month after the injury (Fig. 9e). These results suggests that with electrolytic lesions, there is less new neuron incorporation but possibly still a high number of glia and that the recovery of the injured area might be mediated not by neurogenesis but by migration of old neurons from the neighboring region into the damaged area.
In summary, these findings suggest that an intact axonal and dendritic scaffold and/or a higher rate in incoming new neurons in the recovering LArea X is a prerequisite for stuttering to be induced.
Discussion
In this study, we tested the hypothesis that the striatal vocal nucleus Area X modulates song in the adult songbird. We discovered that neurotoxic and electrolytic striatal injury causes a long-term increase in song tempo and alters syllable sequencing. In addition, the neurotoxic striatal injury causes an increase in syllable repetition, particularly in animals that had minor syllable repetitions at the end of their song motifs before the lesion and the repetition increase is even more dramatic when singing to a female. An increased rate of the repetition started at the time of intense tissue recovery and increased even more at the time when new neurons became active during singing. We do not know if the new or repaired neurons are causal for the repetition, but it is suggested by the correlations between both the number of BrdU+ cells and the shrinking lesion size with song behavior. However, the mechanism of induced stuttering might be more complex.
The increased syllable repetition we observed has many of the hallmarks of neurogenic stuttering seen in humans that suffer brain injury, particularly to the basal ganglia38. Stuttering in humans is also often accompanied by abnormal basal ganglia activation38,39,40,41. Because the definition of stuttering varies and because there are different types of stuttering affecting human speech, there is no simple one-to-one relationship of the type of stuttering we found in zebra finches and that found in humans. Helekar et al.20 noted that stuttering in zebra finches can be learned from tutors, and thus this could be considered a developmentally learned disorder in them. The type of stuttering that zebra finches manifested in this study is developmental and neurogenic, as it was the result of both a developmentally learned minor repetition followed by neural injury. Neurogenic stuttering in humans is dominated by increased syllable repetitions38, but unlike in zebra finch song, the repetitions usually occur more at the beginning of an utterance, than in the middle or end42. We noted from observing the examples of song before and after Area X lesions in the Bengalese finches in a previous study17, that the example birds shown stuttered on syllables that were also repeated more often before the lesions. Unlike zebra finches, but like humans, the stuttered syllables occurred either towards the beginning or end of the song motifs. Song of Bengalese finches contains more syllable repetitions and is more variable than song of zebra finches. That study, however, did not note whether there was a predisposition for the repetition, did not examine brain mechanisms, and many of the lesions were said to be partial by the time the brains were examined. We suggest based on our findings that Area X could have been recovering by the time of sacrifice. Taken together, we hypothesize that the mechanism for striatal lesion-induced stuttering might be more ubiquitous among vocal learning species, but with species differences concerning the particular song features that are affected.
The neurogenic stuttering we found might underlie a more general principle of striatal function in singing. Neurons innervating LArea X from HVC of the posterior song motor pathway have been proposed to code for the first or last syllable in a repetition of syllables as well as progress in the repetition43. Further, the syllables produced between or at the end of motifs are less crystallized (in terms of stable production with the motif) than within motifs of adult zebra finches13. Likewise, the repetitions we find are the least “crystallized” part of the zebra finch song in terms of number of repeats. Taken together with our finding that LArea X neurotoxic lesions have the greatest impact on the end syllables, we propose that LArea X may be involved in providing cues for the initiation and selection of the next motor segment in a song sequence in adult animals, and the selection is more critical in individuals with learned repetitions at the end of their motifs. This hypothesis could be tested by stimulating LArea X during singing at different times in a song sequence, and determining if there is a part of the song sequence where stimulation causes more disruption and possible stuttering during singing.
The finding that there is increased repetition during directed singing could be analogous to findings in humans where stuttering increases in higher anxiety situations44. When a male directs his song to a female, the male usually shows more ‘excitatory’ behavior, such as hopping, chasing, and a courtship dance that accompanies singing15. This is associated with longer lasting dopamine release into Area X during directed singing29. Blocking dopamine D2 receptors with haloperidol in humans has been shown to reduce stuttering, supporting the hypothesis that increased dopaminergic activity may contribute to stuttering28. In this regard, it is possible that there is a link between increased excitement, dopamine release, and courtship song that leads to increased stuttering in the birds recovering from striatal damage.
In terms of brain recovery, neurogenesis occurs throughout life in the avian brain, including in the striatum, but new neurons are not thought to be recruited to adult LMAN45. The targeted neuronal death of HVC neurons projecting to RA leads to song deterioration which improves over time, concomitant with neuronal replacement46. In mammals, new striatal neurons can be induced to form following neurotoxic damage47,48. The newly generated neurons move into the striatum via a chain migration, generated from the stem cells in the ventricular zone, at a relatively rapid rate of 28 micrometers per hour49,50,51, which - extrapolated to birds - would translate to about 2–3 days to reach LArea X. However, the proportion of the newly generated neurons that survive in the mammalian striatum is low, representing 0.2% of the neurons lost47, whereas we find that at least 80% of LArea X can be recovered. It is likely that the new neurons we identified with singing-induced egr-1 expression were the most common neuron type, the medium spiny neurons36. Such neurons in the avian striatum, unlike mammals, do not make long distance projections to pallidal neurons outside of the striatum, but instead project to pallidal-like neurons within Area X; in turn, the pallidal-like LArea X neurons make the long projections to the thalamus (aDLM), akin to the internal globus pallidus (GPi) neurons of mammals52. We do not know if the GPi neuron type in LArea X also shows recovery but it was present in the recovered part of LArea X. This long projection neuron type is not replaced in intact unlesioned striatum of birds30. Our findings that birds with electrolytic lesions lacked stuttering and did not show the same type of syllable sequence changes as birds with neurotoxic lesions suggest that a combination of increased incorporation of new neurons and a pre-existing axon pathway scaffold could contribute to the stuttering and the associated sequence changes, but that new neurons alone do not. Future investigation on lesions and neural connectivity will lend insight into the mechanisms of recovery.
Also like in humans, in zebra finches it has been shown that stuttering can be induced by behavioral manipulations of deafening53,54 and perturbation of auditory feedback55. In light of our findings, it is possible that in intact animals LArea X within the anterior forebrain loop could be involved in monitoring auditory feedback during singing and after LArea X lesions the lack of auditory feedback in LArea X could cause stuttering. This hypothesis can be tested by reversibly inactivating LArea X during singing with altered auditory feedback, before new neurons have a chance to have an effect, and determine whether stuttering still occurs.
The other effect we found in all LArea X lesioned birds was a long-term increase in song tempo. It is probable that tempo conveys important information for mates, as female zebra finches prefer the faster directed song over the slower undirected song56. Lesions to LMAN also cause an increase in song tempo11,12. Thus, the faster song is not specific to damage to Area X, but to absence of proper input of the anterior song pathway into the vocal motor pathway. A recent study showed that an increased brain temperature can result in faster song tempo57, and it is possible this could have caused a faster song in the lesioned animals. However, although neurotoxic damage can lead to overheating58 and an inflammatory reaction is seen during the first week after lesion59, the song tempo started to increase only after 1 month and continued to 6 months.
While this study was in preparation, one study demonstrated that like LMAN lesions, Area X neurotoxic lesions prevented deafened-induced song syllable acoustic and sequence plasticity60. Further, in the Area X lesioned animals, LMAN showed increased neural firing during singing, but the firing rate was no longer time locked with the song syllables, indicating that a weaker feedback from RA>aDLM>LMAN was driving a general increase in activity in the absence of Area X. In the context of our results we suggest that Area X in the adult animal may be controlling some of the online syllable sequencing, syllable selection, tempo, and acoustic structure of the song.
In summary, our findings support the hypothesis that the striatal song nucleus Area X plays a role in modulating online song production in adult songbirds. However, as different songbird species sing different types of song, some functions of Area X may not be manifested in all species. Further, our findings show that the songbird striatum can recover extensively after neurotoxic damage and this recovery is associated with neurogenic-like stuttering. This neurogenic-like stuttering has some parallels to that seen for stuttering in human speech.
Methods
Animals
We used 99 male zebra finches (Taeniopygia guttata; 54 lesioned using ibotenic acid [46 lateral Area X {LArea X}, 3 medial striatum {MSt}, 5 LMAN], 14 LArea X electrolytically lesioned, and 31 controls). Animals were from breeding colonies at The Rockefeller University for the first experiment, followed by Duke University and The Institute of Animal Biochemistry and Genetics in Slovakia for all other experiments. All animals were young adults 6–12 months old. Adult females were used as stimuli for directed singing. All treatments and procedures were approved by the Animal care and use committees at Duke University and The Rockefeller University, and The State Veterinary and Food Administration of the Slovak Republic. All experiments were performed in accordance with relevant guidelines and regulations.
Behavior
We prescreened birds for syllable repetitions (defined as two syllables with identical acoustic features following each other directly, Fig. 2a, syllables GG) by recording their songs in sound attenuating boxes, one male per box. The percentage of birds with at least one syllable repetition anywhere within the song varied from 10 to 40% depending on whether the birds were bred in our colony (lower%) or purchased as juveniles from breeders (higher%, perhaps due to presence of other species with natural syllable repetitions in their breeding colonies). Repetition syllables at the end of the song motif (5–20% of the birds) usually, but not always, contained harmonic stacks (e.g. Fig. 2a–b syllable G and Fig. 4d–g syllable F). The last repetitive syllable was different than calls in all birds, based upon visual analyses of sonograms. Selected males were then kept in the boxes for about 1 month before surgery and their undirected songs were recorded 2 days each week using an automated song detection set up of Sound Analyses Pro version 2.065 (http://ofer.sci.ccny.cuny.edu/sound_analysis_pro)61. Once per week, a female was added to the box to initiate directed singing, defined as singing while facing the female and accompanied by performing a courtship dance towards the female, containing characteristic beak and tail movements. Video was recorded to ensure quantifying which songs were directed. The birds were assigned to neurotoxically lesioned and sham control groups with planned survivals for 1 day, 14 days, and 1, 2, 3, 4, 6, or 17 months after surgery. We assigned the animals to 4 subgroups according to their songs: 1) typical song motif without syllable repetition (ABCDE), 2) a motif with a syllable repetition at the beginning (AABCDE), 3) a motif with a syllable repetition in the middle ABCCDE, or 4) a motif with a syllable repetition at the end of the motif (ABCDEE). The repetition birds were appointed to the groups surviving at least 3 months after surgery. For testing hypotheses based on the neurotoxic lesion experiments, electrolytically lesioned birds were assigned to three groups with planned survivals for 1 day, 1 month and 6 months. Singing behavior after surgery was recorded the same way as before surgery, except during the whole first week undirected songs were recorded using the Sound Analyses Pro software. Directed songs were recorded on the 6th day. After that, undirected songs were recorded 2 days and directed 1 day each week, similarly as before surgery. On their last day of survival, the birds were allowed to sing undirected songs for 55 min after the first song of that day to induce IEG expression in their song nuclei. Within another 5 min, they were sacrificed by a lethal dose of a ketamin-xylazin mixture (15 mg/ml ketamine; 3 mg/ml xylazine) and their brains were perfused using phosphate buffered saline (PBS) followed by 4% paraformaldehyde.
Surgery
Bilateral neurotoxic, ibotenic acid lesions were made to LArea X of the striatum, MSt, or LMAN in the pallium. Ibotenic acid (Sigma, USA) was dissolved in 0.9% NaCl to a concentration of 1% (pH 7.5–8) and fresh aliquots were used on each surgery day. The birds were anesthetized with isoflurane (1–3%, flow 1 L/min) and fixed in the stereotaxic apparatus. For LArea X, 46 nl of ibotenic acid was pressure injected three times into each hemisphere using a glass micropipette attached to a Nanoject II (Drummond Scientific, USA). The coordinates were 4.2–5.0 mm rostrally, 1.3 mm laterally, and 3.5 mm ventrally from the point where the mid-sagittal sinus bifurcates just rostral to the pineal gland. For MSt, the coordinates were the same except the medio-lateral position was 0.5 mm. For finding and lesioning LMAN, electrophysiological recordings were used following the procedure described in21, and then 3 injections of 32.2 nl of ibotenic acid were made. For bilateral electrolytic lesions of LArea X, an insulated tungsten wire with cut end was lowered down and 3 lesions were made in the rostro-caudal range 1 mm at 3 V each for 5 min. All other conditions were the same as described above. For sham control birds, the micropipette was lowered down but no liquid was injected. The birds after surgeries were kept under a heat lamp for the rest of the day and moved to the sound attenuation boxes in the evening.
Tissue preparation and labeling
The newborn cells were labeled by the cell division marker BrdU (Sigma, USA). BrdU was injected i.m. at a concentration 10 mg/ml and at a dose of 50 mg/kg body weight during 7 consecutive days, either immediately after surgery, or 2 or 5 months after the surgery, and immediately or 5 months after electrolytic lesion. The brains were perfused 30 days after the first injection (Fig. 9a). In another set of birds, BrdU was injected the same way immediately before surgery. After perfusion, the brains were removed, postfixed for 5 h at 4°C, cryoprotected in 20% for 12 h and then 30% sucrose for 24 h, and frozen in Tissue Tek OCT Compound (Sakura, Japan). The brains were kept at −20°C and 30 μm thick coronal sections were cut on a cryostat, collected as free floating sections, stored at 4°C, and used for immunohistochemistry.
Every fourth section was used for Hu/egr-1 double labeling and every eighth section was used for Hu/DCX and DCX/BrdU double labeling. To determine the neuron types renewed, we used sections containing LArea X from birds injected with BrdU immediately after lesion and sacrificed 1, 3, or 6 months later (all n = 3 birds). First, the sections were washed 3x in PBS (pH 7.4). Second, for BrdU staining only, the sections were pre-treated with 2 M HCl at 37°C for 7 min, the reaction was stopped in borate buffer at R.T. for 4 min, and then the sections were washed 3x in PBS. Non-specific binding was blocked in 0.1% bovine serum albumin with the addition of 0.3% Triton X-100 in PBS for 1 h and the sections were then incubated with the primary antibodies diluted in blocking solution at 4°C for 72 h. We used the mouse monoclonal anti-Hu (cat. # A21271; Invitrogen, USA) diluted 1:500, the rabbit polyclonal anti-egr-1 [Egr-1 (C-19): sc-189; Santa Cruz Biotech, USA] diluted 1:200, rat monoclonal anti-BrdU (cat. # OBT0030, Accurate Chemical, USA) diluted 1:500, rabbit polyclonal anti-DCX (cat. # AV41333; Sigma, USA) diluted 1:3000, rabbit polyclonal anti-LANT6 (generously provided by Dr. R. Carraway, University of Massachusetts, Worchester, USA) diluted to 1:300, mouse monoclonal anti-parvalbumin (Sigma, USA) diluted to 1:1000, goat anti-cholinacetyltransferase (cat. # AB144P, Millipore, USA) diluted to 1:500, and rabbit anti-DARPP-32 (cat. # 2302, Cell Signalling Biotechnology, USA) diluted to 1:500. The excess antibodies were washed out 3x in PBS and the sections were incubated with secondary antibodies for 2 h. For secondary antibodies we used donkey anti-mouse IgG conjugated with Alexa 594 for Hu, donkey anti-rabbit IgG conjugated with Alexa 488 for egr-1, LANT6 and DARPP-32, donkey anti-rabbit IgG conjugated with Alexa 594 for DCX, donkey anti-goat IgG conjugated with Alexa 488 for ChAT, donkey anti-rat IgG conjugated with Alexa 594 for BrdU, all diluted 1:500 (all Alexa conjugated antibodies were from Invitrogen, USA), and donkey anti-rat IgG conjugated with FITC diluted 1:400 (Sigma, USA) for BrdU. The sections were then washed 3x in PBS, mounted on silanated slides, rinsed in deionized H2O, and coverslipped with Vectashield mounting medium containing DAPI for Hu/egr-1 and Hu/DCX stainings, and Vectashield without DAPI for stainings containing BrdU (Vector Laboratories, USA).
We used the DeadEnd Fluorometric TUNEL System (Promega, USA) for the TUNEL labeling of apoptotic cells. Sections were washed 2x in PBS both before and after permeabilization in 0.2% Triton X-100 in PBS for 15 min. They were then equilibrated in Equilibration Buffer at R.T. for 10 min. Fresh rTdT incubation buffer was prepared by mixing 45 µl of Equilibration Buffer, 5 µl of Nucleotide Mix containing fluorescein-12-dUTP, and 1 µl of rTdT Enzyme. The sections were incubated in rTdT incubation buffer at 37°C for 1 h in a water bath in the dark. The reaction was stopped in SSC buffer at R.T. for 15 min and then the sections were washed 3x in PBS. After that, the sections were processed for Hu immunolabeling.
Quantification and Statistics
All analyses of songs were performed using the Sound Analysis Pro software. We attempted to use the automated analyses function of Sound Analyses Pro, but syllables were not always reliably identified. Thus for quantitative analyses, we manually analyzed sequences of the first 10–20 song bouts sung in the morning to obtain at least 20 motifs per time point. This was enough to find robust, statistical differences among groups. A song bout typically contained 1–5 song motifs. In addition, for directed singing, the videos were analyzed to unambiguously confirm that the songs were directed. Syllable repetition rate was calculated by dividing the number of repetitions by the number of motifs in which the repetition occurred. Motif duration was measured in motifs with identical sequences from the beginning of the first syllable to the end of the last syllable. Statistical differences were assessed using ANOVAs followed by the Fisher’s protected least significant difference (Fisher’s PLSD) post hoc tests. Song sequences of 2nd order transitions between pairs of syllables were determined either manually or using the “Mouse Vocal Organ” software62. Then song sequence linearity was calculated, as a ratio of the number of different note types divided by the number of transition types in song. Song sequence consistency was calculated as a ratio of the number of common pairwise transitions in the song divided by all transitions. Song sequence stereotypy was calculated as a mean of song linearity and song consistency3.
For analyses of lesion size and labeled cells, images from the stained sections were taken using a Leica DFC340 FX camera attached to Leica DM5500 microscope and Leica microsystem LAS AF6000 software. Lesion size was measured from images of LArea X taken under a 2.5x objective from every fourth section stained for Hu/egr-1/DAPI. Although Hu was the most prominent marker, egr-1 and DAPI were also taken into consideration in determining the lesion size or LArea X dimensions. LArea X was recognized in frontal sections with Hu staining by its distinct boundary with the surrounding striatum and medially at a position where Area X dips ventrally to become larger21. The lesioned part of LArea X in lesioned birds and the whole LArea X in control birds were circled by the lasso tool in Adobe Photoshop software and the number of pixels was measured using the histogram function. To count individual labeled cells, images were taken under 40x objective, and the numbers of DCX+, BrdU+, egr-1+, and Hu+ cells were counted from three sections and in each section from three counting fields measuring 100 × 100 μm. The counting fields were put into the center of the lesion when counting number of the cells in the lesion, about 100 μm from the edge of the lesion when counting in the recovered part of LArea X, and about 100 μm from the lateral ventricle when counting in MSt. Cells in the lesion border were counted not in squares but in an area approximately 60–70 μm wide neighboring with the lesion. Then averages were calculated for each hemisphere. Statistical analyses were performed using ANOVAs followed by the Fisher’s protected least significant difference (Fisher’s PLSD) post hoc tests.
Author Contributions
L.K. designed and performed experiments, analysed data and wrote the paper, E.B. performed experiments and analysed data, M.C. and K.L. analysed data, C.S. designed and performed experiments, analysed data and wrote the paper, E.D.J. designed experiments and wrote the paper.
Supplementary Material
Movie 1
Movie 2
Movie 3
Movie 4
Movie 5
Movie 6
Supplementary information
Acknowledgments
We thank Matt Grossman for assistance in the initial stages of this project, Elena A. Turner and Tereza Vargova for their help with analyzing the behavioral data. We thank Jasmine Grimsley for processing our song recordings in her “Mouse Vocal Organ” program to help out automated quantification of our sequence analyses. We also thank Dr. Robert Carraway from University of Massachusetts for the kind gift of LANT6 antibody. The study was supported by an NIH Fogarty International Research Collaboration Award R03TW007615 to E.D.J. and L.K., NIH R01MH62083 and HHMI to E.D.J., and Slovak Research and Development Agency VVCE-0064-07 and VEGA 2/0177/14 to L.K., as well as Excellence Cluster Neurocure and SFB665 to C.S.
References
- Jarvis E. D. Learned birdsong and the neurobiology of human language. Ann N Y Acad Sci 1016, 749–777 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe A. J. & Kuhl P. K. Birdsong and human speech: common themes and mechanisms. Annu Rev Neurosci 22, 567–631 (1999). [DOI] [PubMed] [Google Scholar]
- Scharff C. & Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J Neurosci 11, 2896–2913 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K., Horwitz B. & Jarvis E. D. Dopamine regulation of human speech and bird song: a critical review. Brain Lang 122, 142–150 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F., Nordeen E. J. & Nordeen K. W. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav Neural Biol 53, 51–63 (1990). [DOI] [PubMed] [Google Scholar]
- Horita H. et al. Specialized motor-driven dusp1 expression in the song systems of multiple lineages of vocal learning birds. PLoS One 7, e42173 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olveczky B. P., Andalman A. S. & Fee M. S. Vocal experimentation in the juvenile songbird requires a basal ganglia circuit. PLoS Biol 3, e153 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. H. & Fee M. S. Vocal babbling in songbirds requires the basal ganglia-recipient motor thalamus but not the basal ganglia. J Neurophysiol 105, 2729–2739 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottjer S. W., Miesner E. A. & Arnold A. P. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science 224, 901–903 (1984). [DOI] [PubMed] [Google Scholar]
- Kao M. H., Doupe A. J. & Brainard M. S. Contributions of an avian basal ganglia-forebrain circuit to real-time modulation of song. Nature 433, 638–643 (2005). [DOI] [PubMed] [Google Scholar]
- Williams H. & Mehta N. Changes in adult zebra finch song require a forebrain nucleus that is not necessary for song production. J Neurobiol 39, 14–28 (1999). [PubMed] [Google Scholar]
- Brainard M. S. & Doupe A. J. Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations. Nature 404, 762–766 (2000). [DOI] [PubMed] [Google Scholar]
- Horita H., Wada K. & Jarvis E. D. Early onset of deafening-induced song deterioration and differential requirements of the pallial-basal ganglia vocal pathway. Eur J Neurosci 28, 2519–2532 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zann R. A. & Bamford M. The zebra finch: a synthesis of field and laboratory studies. [Vocalizations, 196–247] (Oxford University Press, Oxford, 1996). [Google Scholar]
- Sossinka R. & Böhner J. Song Types in the Zebra Finch Poephila guttata castanotis1. Zeitschrift für Tierpsychologie 53, 123–132 (1980). [Google Scholar]
- Kao M. H. & Brainard M. S. Lesions of an avian basal ganglia circuit prevent context-dependent changes to song variability. J Neurophysiol 96, 1441–1455 (2006). [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Uno H. & Okanoya K. Partial lesions in the anterior forebrain pathway affect song production in adult Bengalese finches. Neuroreport 12, 353–358 (2001). [DOI] [PubMed] [Google Scholar]
- Drayna D. & Kang C. Genetic approaches to understanding the causes of stuttering. J Neurodev Disord 3, 374–380 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent R. D. Research on speech motor control and its disorders: a review and prospective. J Commun Disord 33, 391–427; quiz 428 (2000). [DOI] [PubMed] [Google Scholar]
- Helekar S. A., Espino G. G., Botas A. & Rosenfield D. B. Development and adult phase plasticity of syllable repetitions in the birdsong of captive zebra finches (Taeniopygia guttata). Behav Neurosci 117, 939–951 (2003). [DOI] [PubMed] [Google Scholar]
- Kubikova L., Turner E. A. & Jarvis E. D. The pallial basal ganglia pathway modulates the behaviorally driven gene expression of the motor pathway. Eur J Neurosci 25, 2145–2160 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessler N. A. & Doupe A. J. Social context modulates singing-related neural activity in the songbird forebrain. Nat Neurosci 2, 209–211 (1999). [DOI] [PubMed] [Google Scholar]
- Jarvis E. D. & Nottebohm F. Motor-driven gene expression. Proc Natl Acad Sci U S A 94, 4097–4102 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis E. D., Scharff C., Grossman M. R., Ramos J. A. & Nottebohm F. For whom the bird sings: context-dependent gene expression. Neuron 21, 775–788 (1998). [DOI] [PubMed] [Google Scholar]
- Murugan M., Harward S., Scharff C. & Mooney R. Diminished FoxP2 levels affect dopaminergic modulation of corticostriatal signaling important to song variability. Neuron 80, 1464–1476 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaze C. M. & Troyer T. W. Temporal structure in zebra finch song: implications for motor coding. J Neurosci 26, 991–1005 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchel C. & Sommer M. What causes stuttering? PLoS Biol 2, E46 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J. P. The pharmacology of stuttering: a critical review. Am J Psychiatry 148, 1309–1316 (1991). [DOI] [PubMed] [Google Scholar]
- Sasaki A., Sotnikova T. D., Gainetdinov R. R. & Jarvis E. D. Social context-dependent singing-regulated dopamine. J Neurosci 26, 9010–9014 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort C., He X., Scotto-Lomassese S. & Scharff C. Recruitment of FoxP2-expressing neurons to area X varies during song development. Dev Neurobiol 67, 809–817 (2007). [DOI] [PubMed] [Google Scholar]
- Scott B. B. & Lois C. Developmental origin and identity of song system neurons born during vocal learning in songbirds. J Comp Neurol 502, 202–214 (2007). [DOI] [PubMed] [Google Scholar]
- Schulz S. B., Haesler S., Scharff C. & Rochefort C. Knockdown of FoxP2 alters spine density in Area X of the zebra finch. Genes Brain Behav 9, 732–740 (2010). [DOI] [PubMed] [Google Scholar]
- Duque A. & Rakic P. Different effects of bromodeoxyuridine and [3H]thymidine incorporation into DNA on cell proliferation, position, and fate. J Neurosci 31, 15205–15217 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis F. et al. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron 23, 247–256 (1999). [DOI] [PubMed] [Google Scholar]
- Brown J. P. et al. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol 467, 1–10 (2003). [DOI] [PubMed] [Google Scholar]
- Reiner A., Laverghetta A. V., Meade C. A., Cuthbertson S. L. & Bottjer S. W. An immunohistochemical and pathway tracing study of the striatopallidal organization of area X in the male zebra finch. J Comp Neurol 469, 239–261 (2004). [DOI] [PubMed] [Google Scholar]
- Carlson N. C. Physiology of behavior. [Methods and strategies of research, 134–168] (Allyn & Bacon, Boston, 2010). [Google Scholar]
- Alm P. A. Stuttering and the basal ganglia circuits: a critical review of possible relations. J Commun Disord 37, 325–369 (2004). [DOI] [PubMed] [Google Scholar]
- Alexander M. P., Naeser M. A. & Palumbo C. L. Correlations of subcortical CT lesion sites and aphasia profiles. Brain 110 (Pt 4), 961–991 (1987). [DOI] [PubMed] [Google Scholar]
- Damasio A. R., Damasio H., Rizzo M., Varney N. & Gersh F. Aphasia with nonhemorrhagic lesions in the basal ganglia and internal capsule. Arch Neurol 39, 15–24 (1982). [DOI] [PubMed] [Google Scholar]
- Naeser M. A. et al. Aphasia with predominantly subcortical lesion sites: description of three capsular/putaminal aphasia syndromes. Arch Neurol 39, 2–14 (1982). [DOI] [PubMed] [Google Scholar]
- Tani T. & Sakai Y. Analysis of five cases with neurogenic stuttering following brain injury in the basal ganglia. J Fluency Disord 36, 1–16 (2011). [DOI] [PubMed] [Google Scholar]
- Fujimoto H., Hasegawa T. & Watanabe D. Neural coding of syntactic structure in learned vocalizations in the songbird. J Neurosci 31, 10023–10033 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverach L., Menzies R. G., O'Brian S., Packman A. & Onslow M. Anxiety and stuttering: continuing to explore a complex relationship. Am J Speech Lang Pathol 20, 221–232 (2011). [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A. & Kirn J. R. Birth, migration, incorporation, and death of vocal control neurons in adult songbirds. J Neurobiol 33, 585–601 (1997). [PubMed] [Google Scholar]
- Scharff C., Kirn J. R., Grossman M., Macklis J. D. & Nottebohm F. Targeted neuronal death affects neuronal replacement and vocal behavior in adult songbirds. Neuron 25, 481–492 (2000). [DOI] [PubMed] [Google Scholar]
- Arvidsson A., Collin T., Kirik D., Kokaia Z. & Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 8, 963–970 (2002). [DOI] [PubMed] [Google Scholar]
- Darsalia V., Heldmann U., Lindvall O. & Kokaia Z. Stroke-induced neurogenesis in aged brain. Stroke 36, 1790–1795 (2005). [DOI] [PubMed] [Google Scholar]
- Wichterle H., Garcia-Verdugo J. M. & Alvarez-Buylla A. Direct evidence for homotypic, glia-independent neuronal migration. Neuron 18, 779–791 (1997). [DOI] [PubMed] [Google Scholar]
- Doetsch F., Garcia-Verdugo J. M. & Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci 17, 5046–5061 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R. L. et al. Patterns and dynamics of subventricular zone neuroblast migration in the ischemic striatum of the adult mouse. J Cereb Blood Flow Metab 29, 1240–1250 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farries M. A., Ding L. & Perkel D. J. Evidence for “direct” and “indirect” pathways through the song system basal ganglia. J Comp Neurol 484, 93–104 (2005). [DOI] [PubMed] [Google Scholar]
- Brainard M. S. & Doupe A. J. Postlearning consolidation of birdsong: stabilizing effects of age and anterior forebrain lesions. J Neurosci 21, 2501–2517 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardino A. J. & Nottebohm F. Age at deafening affects the stability of learned song in adult male zebra finches. J Neurosci 20, 5054–5064 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardo A. & Konishi M. Decrystallization of adult birdsong by perturbation of auditory feedback. Nature 399, 466–470 (1999). [DOI] [PubMed] [Google Scholar]
- Woolley S. C. & Doupe A. J. Social context-induced song variation affects female behavior and gene expression. PLoS Biol 6, e62 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronov D. & Fee M. S. Natural changes in brain temperature underlie variations in song tempo during a mating behavior. PLoS One 7, e47856 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin E. A. Brain temperature fluctuations during physiological and pathological conditions. Eur J Appl Physiol 101, 3–17 (2007). [DOI] [PubMed] [Google Scholar]
- Iadecola C., Arneric S. P., Baker H. D., Callaway J. & Reis D. J. Maintenance of local cerebral blood flow after acute neuronal death: possible role of non-neuronal cells. Neuroscience 35, 559–575 (1990). [DOI] [PubMed] [Google Scholar]
- Kojima S., Kao M. H. & Doupe A. J. Task-related “cortical” bursting depends critically on basal ganglia input and is linked to vocal plasticity. Proc Natl Acad Sci U S A 110, 4756–4761 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernichovski O., Lints T. J., Deregnaucourt S., Cimenser A. & Mitra P. P. Studying the song development process: rationale and methods. Ann N Y Acad Sci 1016, 348–363 (2004). [DOI] [PubMed] [Google Scholar]
- Grimsley J. M., Monaghan J. J. & Wenstrup J. J. Development of social vocalizations in mice. PLoS One 6, e17460 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubikova L., Wada K. & Jarvis E. D. Dopamine receptors in a songbird brain. J Comp Neurol 518, 741–769 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie 1
Movie 2
Movie 3
Movie 4
Movie 5
Movie 6
Supplementary information