Abstract
The oral microbiota change dramatically with each part of the oral cavity, even within the same mouth. Nevertheless, the microbiota associated with peri-implantitis and periodontitis have been considered the same. To improve our knowledge of the different communities of complex oral microbiota, we compared the microbial features between peri-implantitis and periodontitis in 20 patients with both diseases. Although the clinical symptoms of peri-implantitis were similar to those of periodontitis, the core microbiota of the diseases differed. Correlation analysis revealed the specific microbial co-occurrence patterns and found some of the species were associated with the clinical parameters in a disease-specific manner. The proportion of Prevotella nigrescens was significantly higher in peri-implantitis than in periodontitis, while the proportions of Peptostreptococcaceae sp. and Desulfomicrobium orale were significantly higher in periodontitis than in peri-implantitis. The severity of the peri-implantitis was also species-associated, including with an uncultured Treponema sp. that correlated to 4 clinical parameters. These results indicate that peri-implantitis and periodontitis are both polymicrobial infections with different causative pathogens. Our study provides a framework for the ecologically different bacterial communities between peri-implantitis and periodontitis, and it will be useful for further studies to understand the complex microbiota and pathogenic mechanisms of oral polymicrobial diseases.
The oral microbiota comprises hundreds of prevalent taxa1 that are also associated with oral and systematic diseases (e.g., diabetes and cardiovascular disease)2. Therefore, the oral microbiota are as important to human health as are the gut microbiota. The NIH launched the Human Microbiome Project (HMP), which aims to more fully characterize the human microbiota and to address their role in health and disease3. The HMP classifies oral specimens as coming from the saliva, buccal mucosa, hard palate, palatine tonsil, sub-gingiva, supra-gingiva, throat, or tongue dorsum, and their data indicate that healthy sites are dominated by genera such as Streptococcus, Prevotella, Haemophilus, Fusobacterium, and Veillonella. However, the microbial composition and abundances are different in each of these habitats. Of the body's habitats, the oral habitat has the most stable microbiota with the highest alpha diversity, suggesting that the oral environment is likely to be complex3. The ecology of the microbial communities of dental caries and periodontitis have been studied4,5,6. The oral disease peri-implantitis has become a concern recently because several studies have suggested that patients with a history of periodontitis are susceptible to peri-implant disease and are likely to undergo a bacterial shift from periodontitis to the peri-implant7,8. Although peri-implantitis is an oral microbial infection, information is limited, and the microbiota have been treated as the same as those of periodontitis9.
Implant-borne reconstructions are now preferred over conventional fixed or removable partial dentures, and marketing estimates indicate that over 2 million implants were installed per year in the past decade10. According to the American Academy of Implant Dentistry (AAID), 3 million Americans have implants and that number is growing by 500,000 a year. However, the number of problems associated with the implants has been increasing, and peri-implantitis has been identified in 28–56% of implant recipients and in 12–43% of their implant sites11. Peri-implantitis is considered an inflammatory disease that is strongly associated with the oral microbiota12, and its clinical symptoms are similar to those of periodontitis. However, because pain is infrequent in peri-implantitis, patients do not recognize that a problem exists until implant mobility occurs, which indicates the disease's final stage13. The progression of peri-implantitis can trigger a loss of the implants, maxillary sinusitis, mandibular fracture, or the infection of other implants or natural teeth14. While the progression of periodontitis can be suppressed by appropriate infection controls (e.g., surgical therapy and antibiotic therapy), the same results cannot be obtained for peri-implantitis because of the difficulty in decontaminating the roughened, threaded surfaces of the endosseous implants15.
Oral implants are exposed to the microbiota present in the oral cavity and easily develop a biofilm on the implant surfaces; therefore, a survey of the microbiota involved is necessary to our understanding of the disease. Recently, several studies have used high-throughput sequencing based on the 16S rRNA gene to establish the core microbiota by characterizing the human oral bacteria under healthy or diseased conditions, such as dental caries or periodontitis4,5,6,16,17. These studies have found that even in healthy people, the bacteria comprising the oral microbiota varied considerably between individuals. To further understand the bacterial ecology of peri-implantitis, and to determine the core microbiota for future prevention and treatment, we sought to clarify the microbial differences between peri-implantitis and the clinically similar disease of periodontitis by comparing the individual microbiota of each disease in patients with both conditions.
Results
Patient's clinical characteristics and obtained sequences
Twenty patients with both peri-implantitis and periodontitis were recruited for this study, 7 men (35.0%) and 13 women (65.0%), with an age range of 40–76 (average = 60.1 ± 1.7) years. The clinical characteristics of the patients are shown in Table 1. The probing pocket depth (PPD) and number of sites with pus differed significantly between the peri-implantitis and periodontitis samples. After trimming the disqualified sequences, we obtained a total of 436,320 sequences for the 16S rRNA gene (average-read length = 294.4 bp) that were used for the analyses (Supplementary Table S1). From these sequences, 19 phyla, 188 genera, and 235 species were identified (Supplementary Figure S1).
Table 1. Summary of meta-data on patients.
| Peri-implantitis sites | Periodontitis sites | P valuea | |
|---|---|---|---|
| Age | 60.1 ± 1.7 | ||
| Gender | 7 males, 13 females | ||
| Smoking | 6 | ||
| Maxillary anterior | 7 | 5 | 0.49 |
| Maxillary posterior | 5 | 8 | 0.33 |
| Mandibular anterior | 0 | 2 | 0.16 |
| Mandibular posterior | 8 | 5 | 0.33 |
| Years in function | 5.7 ± 0.7 | ||
| PPD (sampled sites)b | 7.0 ± 0.6 mm | 5.5 ± 0.4 mm | <0.05 |
| CAL (sampled sites)c | 7.5 ± 0.6 mm | 6.4 ± 0.4 mm | 0.09 |
| BOP (sampled sites)d | 100% | 100% | |
| Number of sites with pus | 9 | 0 | <0.05 |
| Radiographic bone loss | 45.2 ± 7.2% | 42.9 ± 5.2% | 0.78 |
Numbers shown are mean ± s.d.
aStatistical differences were calculated using paired t-tests; bProbing pocket depth.
cClinical attachment loss; dBleeding on probing.
Overall bacterial community composition in peri-implantitis and periodontitis
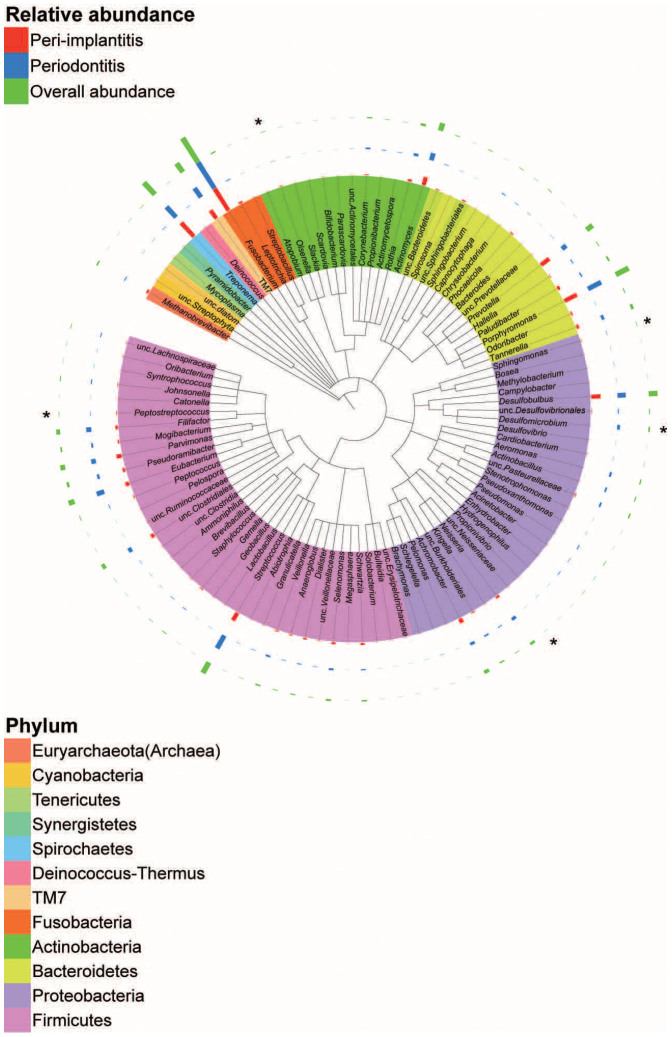
The Ribosomal Database Project (RDP) classifier was initially used to assign each sequence to the taxonomy at the phylum or genus level. At the phylum level, the microbial compositions of the peri-implantitis and periodontitis sites were similar, but the abundance of some genera differed significantly (Figure 1 and Supplementary Figure S2). Compared with periodontitis, peri-implantitis-associated bacterial communities had significantly higher levels of the genera Olsenella, Sphingomonas, Peptostreptococcus, and unclassified Neisseriaceae, and lower levels of the genus Desulfomicrobium. Furthermore, the Methanobrevibacter archaeal genus was detected with 100% RDP confidence using the prokaryotic universal primers; however, we did not detect it at the species level using the Human Oral Microbiome Database (HOMD) 16S rRNA Gene Database. This genus was more abundant in peri-implantitis than periodontitis, but the difference was not significant (Figure 1 and Supplementary Figure S2a).
Figure 1. Circular maximum likelihood phylogenetic tree at the genus level.
The inner band shows the genera coloured by phylum (see key for taxa with multiple members). The outer bands show the relative abundance: red for peri-implantitis, blue for periodontitis, and green for overall relative abundance. The tree was constructed with the Interactive Tree of Life tool and the taxonomic names were based on results from the Ribosomal Database Project classifier. The statistical differences were calculated by Wilcoxon signed rank tests. *P <0.05 and q <0.1.
Biodiversity in peri-implantitis and periodontitis
Next, we estimated the community diversity for all samples to compare the complexity between peri-implantitis and periodontitis. The Shannon index, number of operational taxonomic units (OTUs) based on a 3% genetic difference, and Chao1 estimates were not significantly different between peri-implantitis and periodontitis (Supplementary Figure S3a–c), and the rarefaction curves indicated similar species richness for both diseases (Supplementary Figure S3d). We also compared the characteristics of the constituent species of the peri-implantitis and periodontitis communities based on their oxygen requirements, Gram-staining statuses, and cultivation statuses, and found no significant differences (Supplementary Figure S4a–c, and Supplementary Table S2). The abundances of anaerobic and gram-negative bacteria were statistically higher than the abundances of the aerobic and gram-positive bacteria in both communities (Supplementary Figure S4a–b).
Comparison of the bacterial community structures between peri-implantitis and periodontitis
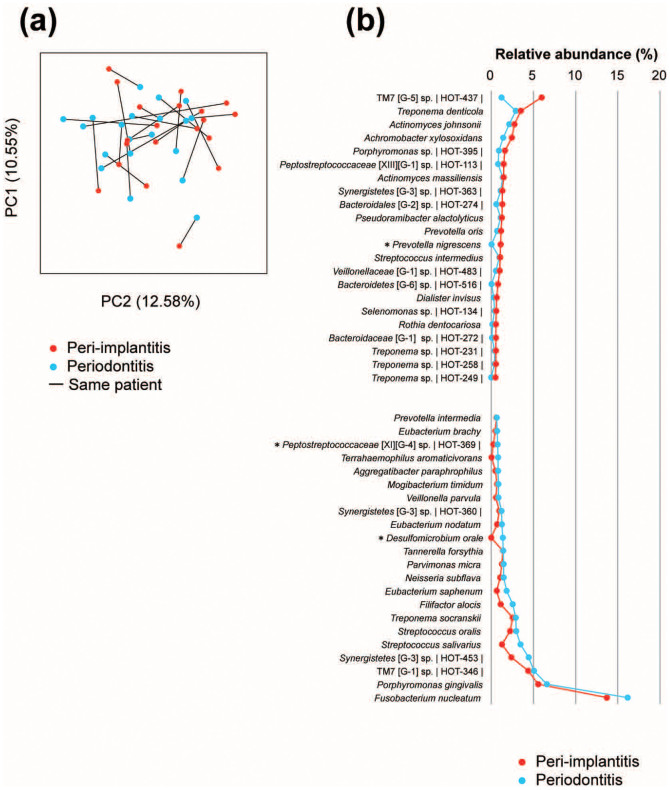
To compare the community structure of the samples, the overall bacterial community composition was calculated based on the unweighted UniFrac distance and visualized with a principal coordinate analysis (PCoA) plot. These plots did not reveal any distinct partitioning of the bacterial communities associated with peri-implantitis or periodontitis, and the similarities of the phylogenetic distances between peri-implantitis and periodontitis varied by patient. However, the analysis of similarities (ANOSIM) tests on the Unifrac distances showed that clustering within the same individual was significant (P = 0.001) relative to the disease status (peri-implantitis versus periodontitis; P = 0.212; Figure 2a).
Figure 2. Principal coordinate analysis (PCoA) and microbial differences at the species level.
(a) PCoA plots of the unweighted UniFrac distances for the samples by disease. (b) The most abundant species (>0.5% abundance) in the peri-implantitis and periodontitis samples. The species name or Human Oral Taxon (HOT) ID in the Human Oral Microbiome is shown. The taxonomy assignments were based on information in the Human Oral Microbiome Database, and the statistical differences were calculated by Wilcoxon signed rank tests. *P <0.05 and q <0.1.
Core microbiota of peri-implantitis
We tried to determine the core microbiota in peri-implantitis based on previous studies4,5. First, we investigated the species-level differences using the HOMD 16S rRNA Gene Database to further assess the differences in the microbial communities for peri-implantitis and periodontitis. Despite inter-individual variability, there were core microbiota representing a baseline oral community for peri-implantitis. We found that some species had significantly different abundance levels between peri-implantitis and periodontitis. Prevotella nigrescens was more abundant in peri-implantitis, while Peptostreptococcaceae [XI][G-4] sp. HOT369 and Desulfomicrobium orale were more abundant in periodontitis (Figure 2b).
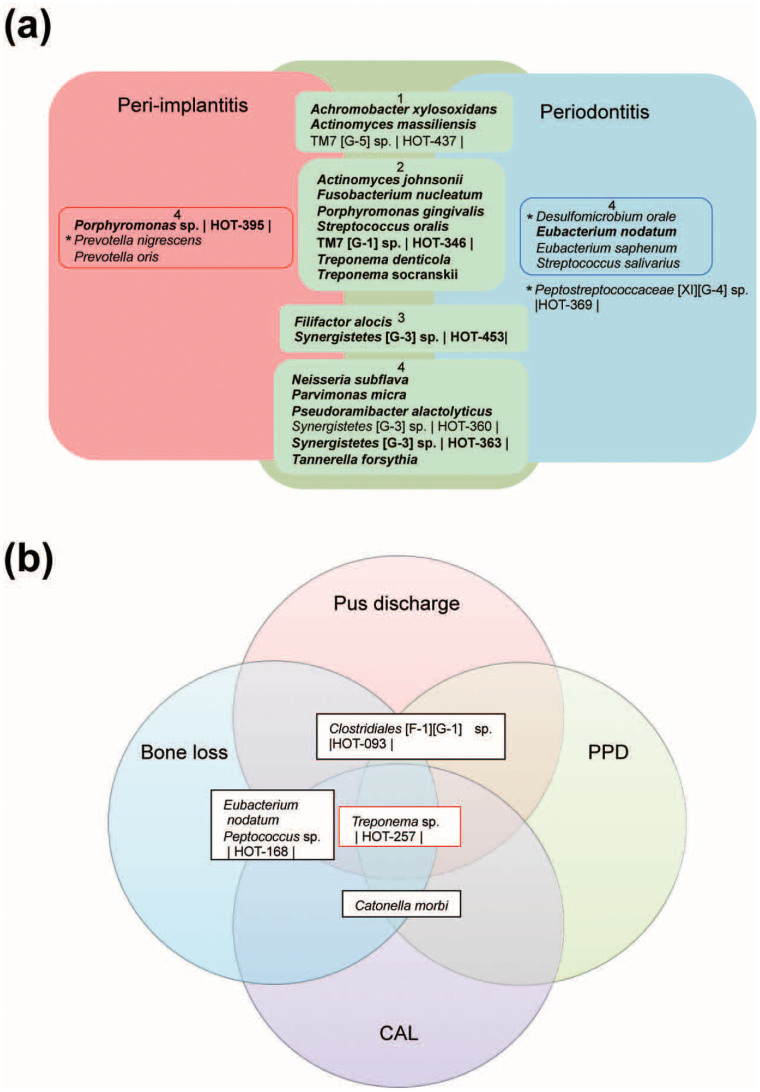
Second, considering that peri-implantitis was caused by intra-individual microbial infections, we considered that there were common species between peri-implantitis and periodontitis. We evaluated the species that were relatively abundant and prevalent in peri-implantitis and periodontitis (Figure 3a). We found that Actinomyces johnsonii, Fusobacterium nucleatum, Porphyromonas gingivalis, Streptococcus oralis, TM7 [G-1] sp. HOT-346, Treponema denticola, and Treponema socranskii were highly abundant and prevalent in both diseases. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia, which are the important periodontal pathogens of the “red complex”18, were abundant and prevalent in most samples in both diseases. However, other species (Achromobacter xylosoxidans, TM7 [G-5] sp. HOT-437, Actinomyces massiliensis, Porphyromonas sp. HOT-395, Prevotella nigrescens, and Prevotella oris) dominated in peri-implantitis samples when compared with periodontitis samples. Although Achromobacter xylosoxidans, Actinomyces massiliensis, and Porphyromonas sp. HOT-395 were prevalent in both diseases, they were more abundant in peri-implantitis. Thus, some species were associated with either peri-implantitis or periodontitis, whereas other species were present in both diseases.
Figure 3. The microbiota associated with peri-implantitis.
(a) The core microbiota of peri-implantitis and periodontitis. The model includes the species detected at peri-implantitis (red), periodontitis (blue), and both sites (green), where the species were found in at least 50% of patients with a mean relative abundance of >1%, or were statistically different (outside of the square boxes; see Figure 2b). The species detected in at least 80% of patients in both sites are indicated in bold. The inner box labelled with 1 indicates a mean relative abundance of ≥2% in periodontitis and <2% in periodontitis. The inner box labelled with 2 indicates a mean relative abundance of ≥2% in both sites. The inner box labelled with 3 indicates a mean relative abundance of <2% in periodontitis and ≥2% in periodontitis. The inner box labelled with 4 indicates a mean relative abundance of <2% in type of site. Peptostreptococcaceae [XI][G-4] sp. HOT369 is statistically abundant, although showed a mean relative abundance of <1% in periodontitis (see Figure 2b). The species name or Human Oral Taxon (HOT) ID in the Human Oral Microbiome Database is shown. The statistical differences were calculated by Wilcoxon signed rank tests. *P <0.05 and q <0.1. (b) Bacterial taxa associated with the progression of peri-implantitis. The model represents all bacterial taxa associated with each of the four clinical parameters of peri-implantitis (P <0.05 and q <0.1). The species name or HOT ID is shown. The taxa correlated to four parameters (red box) and three parameters (black boxes) are shown. PPD, probing pocket depth; CAL, clinical attachment loss.
Species associated with the clinical parameters
We hypothesized that some bacteria might be associated with the clinical data and therefore examined the relationship between the bacterial taxa and clinical parameters by calculating Spearman's ranked correlations. Although we did not find a significant correlation between clinical parameters and microbial characteristics (Supplementary Table S3), smoking was positively correlated with Lachnospiraceae [G-1] sp. HOT-496 in peri-implantitis and Actinomyces sp. HOT848 in periodontitis (Supplementary Table S4). Next, we tried to identify the species associated with the severity of peri-implantitis, as evidenced by the clinical examination parameters; PPD, clinical attachment loss (CAL), bleeding on probing (BOP), bone loss, and pus discharge, but no species could be associated with BOP because it was seen at all of the sampled sites. However, the significantly different aerobic/facultative bacteria were negatively correlated with the other parameters, indicating increased inflammation. Not all of the anaerobic bacteria correlated positively with the clinical parameters, and some were negatively correlated (Supplementary Table S5 and S6). We also found that more species were associated with the severity of the clinical parameters for peri-implantitis than were for those for periodontitis (Supplementary Table S5), and that some species were associated with ≥3 parameters. The bacterial species Treponema sp. HOT257, Eubacterium nodatum, Peptococcus sp. HOT168, Clostridiales [F-1][G-1] sp. HOT093, and Catonella morbi correlated positively with the clinical parameters (Figure 3b and Supplementary Table S5), while some species such as Eubacterium saburreum and Selenomonas noxia correlated negatively (Supplementary Table S6). Among the species associated with the severity of peri-implantitis, some were uncultured bacteria, including Treponema sp. HOT257, which correlated with all 4 parameters.
Co-occurrence of the bacterial taxa
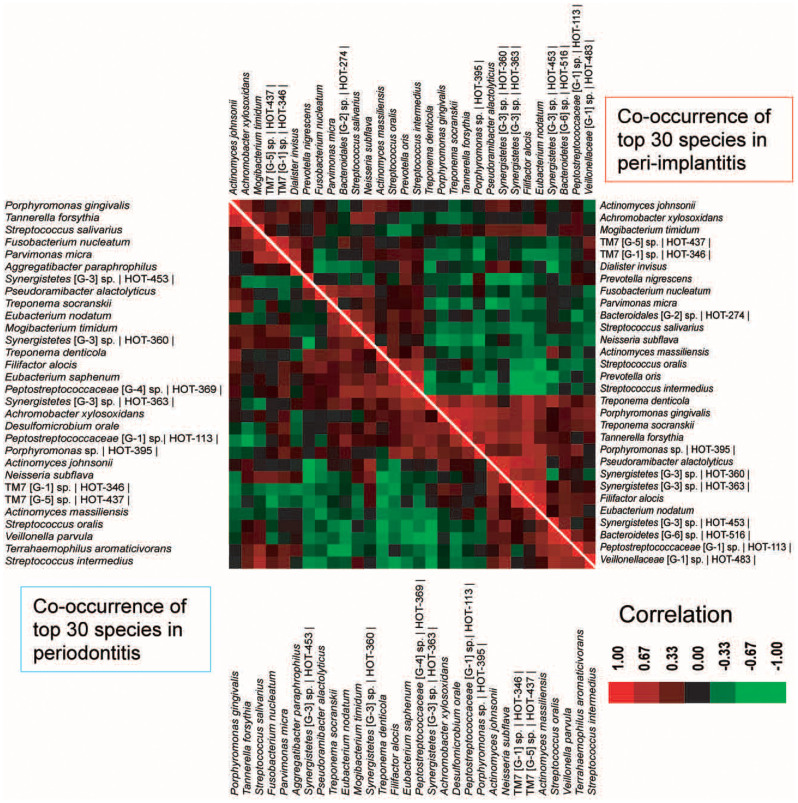
To compare the ecologically significant competitive interactions of the bacteria in peri-implantitis with those in periodontitis, we performed a co-occurrence analysis of the abundant species (Figure 4) and found several interesting microbial relationships. First, although 24 of the top 30 abundant species were shared between peri-implantitis and periodontitis, their correlations were not the same. For peri-implantitis, Synergistetes [G-3] sp. HOT-360 and Pseudoramibacter alactolyticus were the most positively correlated (ρ = 0.849), whereas Eubacterium nodatum and Streptococcus intermedius were the most negatively correlated (ρ = −0.692). For periodontitis, the most positively correlated species were Streptococcus oralis and Veillonella parvula (ρ = 0.826), whereas Synergistetes [G-3] sp. HOT-453 and Neisseria subflava were the most negatively correlated (ρ = −0.794). Second, associations between red complex bacteria were common in peri-implantitis and periodontitis. The abundance of Porphyromonas gingivalis was significantly and positively correlated with that of Tannerella forsythia in both diseases (ρ = 0.768 and 0.752).
Figure 4. Co-occurrence and co-exclusion analysis of the bacterial taxa.
Co-occurrence and co-exclusion were calculated by Spearman rank correlations between the abundant bacterial taxa. The co-occurrence of the top 30 species in the peri-implantitis and periodontitis samples is shown on the right and left, respectively. The species name or Human Oral Taxon ID in the Human Oral Microbiome Database is shown. The correlation values range from -1.00 (green) to 1.00 (red).
Finally, the microbial correlations within the same genera changed in a manner dependent on the species. All of the species in the same genera of Streptococcus and TM7 were positively correlated in both diseases (Figure 4). By contrast, the abundance of some Synergistetes sp. was correlated negatively even within the same genus (e.g., Synergistetes [G-3] sp. HOT360 and Synergistetes [G-3] sp. HOT453 in periodontitis), although the former correlated positively with Synergistetes [G-3] sp. HOT363 in peri-implantitis.
Discussion
Peri-implantitis is caused by a microbial infection that shifts from other oral sites to the peri-implant, affecting the tissues around and function of an osseointegrated implant. We recently reported that the microbiota of peri-implantitis were more complex than those of periodontitis using a 16S rRNA gene clone library technique19,20. However, the oral microbiome is comprised of hundreds of prevalent taxa, and we therefore believed it important to investigate the microbiota associated with peri-implantitis more precisely. Using high-throughput sequencing, we obtained enough sequence information to clarify the ecology of the microbial communities of peri-implantitis.
In this study, we showed that the unweighted Unifrac distances for the same individual were significantly similar in comparison to the disease status (peri-implantitis or periodontitis), and the PCoA revealed no distinct partitioning of the bacterial communities associated with peri-implantitis or periodontitis. These results differed from a previous study20,21, probably because we enrolled more patients and compared the microbiota in patients that had both diseases. The bacteria colonized around the implants were transmitted from the teeth12 and the results indicated that the inflammatory peri-implantitis was caused by intra-individual microbial infections. However, the abundant bacteria in peri-implantitis differed from those in periodontitis in the same patients at the species level, which may suggest that the two environments are different. The soft and hard tissues surrounding the implant and the periodontium have several differences22. There are no periodontal ligaments in the peri-implant region because of osseointegration, and the collagen fibres surrounding the implant are arranged circumferentially with minimal vasculature. In addition, the connective tissue attachment of the peri-implant tissue is weaker than that of the periodontium. Severe inflammation with pus is therefore more likely to occur in the peri-implant tissue than in the periodontium, which may then result in a difference in core microbiota between peri-implantitis and periodontitis.
The species Prevotella nigrescens, a member of the orange complex of periodontal pathogens that are thought to be most important for the progression of periodontitis after those of the red complex18, was significantly abundant in peri-implantitis, which is consistent with a previous study23. In contrast, this result did not coincide with those of recent studies21,24 (Supplementary Table S7). We considered that this apparent discrepancy was due to differences in the study population; the previous studies also investigated peri-implant biofilm using pyrotag sequencing, while the samples were collected from not only peri-implantitis but also gingivitis/peri-mucotitis24 or from different individuals21. Although inter-individual variability was observed, some species were abundant in all of the patients. Some abundant species common to peri-implantitis and periodontitis, i.e., Fusobacterium nucleatum, Neisseria subflava, and Streptococcus oralis, and red complex play important roles in biofilm formation25. In previous studies using high-throughput sequencing, Fusobacterium nucleatum, Treponema denticola, and TM7 sp. HOT346 were found to be core microbiota that were highly abundant and highly prevalent in periodontitis4. Porphyromonas gingivalis and Treponema denticola were two of the top three periodontitis-associated species5. We suggested microbial shifts in community structure from periodontitis to peri-implantitis, and some abundant species were common in both diseases as well as from the healthy condition to periodontitis4. Furthermore, we found other abundant species in peri-implantitis or periodontitis, suggesting that these species may be key players in these refractory pathogenic conditions. A previous study21 demonstrated lower levels of the genus Prevotella in peri-implantitis than in periodontitis-associated biofilms (Supplementary Table S7). In this study, Prevotella nigrescens and Prevotella oris were more abundant in peri-implantitis than in periodontitis, and the difference was significant for the former. They may therefore play important roles in the microbial community in peri-implantitis at the species level. Porphyromonas gingivalis was abundant in both diseases. In contrast, Porphyromonas sp. HOT395 was more abundant in peri-implantitis. Porphyromonas endodontitis is considered to be the core species in periodontitis4,5, but was not abundant in our study. We considered that the rules of species in the same genus vary in different environments.
Although the biofilm formed at peri-implantitis and periodontitis sites appears to contain similar species until late colonizers such as the red complex are established, the colonization of the bacteria constituting the biofilm differs between the diseases. Thus, a species-level analysis may be important for understanding the prognosis of these diseases.
We also detected Methanobrevibacter, which belongs to the Archaea (Figure 1), by using prokaryotic universal primers targeting 16S rRNA genes because the study aim was to further elucidate the community associated with peri-implantitis. Although Methanobrevibacter oralis is included in the HOMD database, this species was not identified with the representative sequence by our method in this study. In previous studies, the Methanobrevibacter oralis-like phylotype increased in periodontitis26, and Methanobrevibacter oralis has been detected in peri-implantitis27. Although the role for the archaeal species in these diseases is not clear, further investigation of the association between oral diseases, including peri-implantitis, and archaeons is needed to better understand the ecology of the oral cavity.
We hypothesized that the microbiota in these habitats may be associated with the severity of disease. Some anaerobic species showed a positive correlation to the clinical parameters of peri-implantitis (Supplementary Table S5), although the total number of anaerobic or aerobic bacteria was not significantly correlated with either disease (Supplementary Table S3). In contrast, some aerobic/facultative bacteria such as Streptococcus oralis were negatively correlated to the clinical parameters, suggesting the proportion of these species might indicate the degree of severity of the disease. A higher number of species were significantly correlated with the peri-implant destruction than with the destruction from periodontitis. This suggests that for the samples used in this study, the diseased state of the peri-implant sites was greater than the sites of periodontitis, and the progression of disease was faster for peri-implantitis in comparison to periodontitis in terms of the structural differences, at least as measured by the same clinical parameters.
Regarding the clinical parameters, Treponema sp. HOT257, Eubacterium nodatum, Peptococcus sp. HOT-168, Clostridiales [F-1][G-1] sp. HOT-093, and Catonella morbi were positively correlated with ≥3 parameters in peri-implantitis, and are probably more associated with the increased inflammation of the disease than are other species, even though Eubacterium nodatum was more abundant in periodontitis than in peri-implantitis (Figure 3a). These species were thought to be associated with the progression of disease as a group rather than individually because they have only been detected in oral disease. For example, Catonella morbi and Eubacterium spp. increased or persisted at a high frequency in refractory periodontitis, but were significantly reduced in treatable periodontitis, allowing good responders for periodontal therapy to be identified28. In our study, however, Eubacterium saburreum was negatively correlated to the increased inflammation of peri-implantitis (Supplementary Table S6); thus, the correlation was different even within the same genus. Another species associated with peri-implantitis, Clostridiales sp. has also been detected in dental caries and periodontitis29,30, suggesting an association with oral disease. Smoking is a risk factor for both peri-implantitis and periodontitis12, but the disease severity was not associated with smoking in this study. Although 30% of patients were smokers, the species correlated with smoking were different in both diseases, which may reflect different environments. A previous study mentioned that levels of uncultivated Peptostreptococci, Parvimonas (e.g., P. micra), Fusobacterium, Campylobacter (e.g., C. gracilis), Bacteroides, and Treponema (e.g., T. socranskii) were elevated in smokers31. We included 6 smokers in this study; therefore we could not evaluate correlations between these taxa and smoking. As smoking influences the sub-gingival microbial composition31, further investigation of bacteria, including unclassified species, associated with smoking in peri-implantitis and periodontitis is necessary.
It has recently been reported that Treponema spp., including Treponema denticola, were core microbiota in periodontitis4. In our study, Treponema sp. was more associated with the increased inflammation of peri-implantitis. A previous study21 demonstrated higher levels of the genera Eubacterium, Peptococcus, and Treponema associated with peri-implantitis in comparison to periodontitis-associated biofilms (Supplementary Table S7). Although the abundance of these genera were not significantly different between the diseases, they might be important for the progression of peri-implantitis at the species level.
Our data confirmed that peri-implantitis was a polymicrobial infection and not associated with a specific pathogen, so we need to regard the microbiota of this disease as a complex microbial community. The co-occurrence analysis revealed that the strength of the bacterial correlations in peri-implantitis and periodontitis were different, and we believe that this would reflect environmental differences. Interesting bacterial interactions were also observed in both diseases. First, the associations between anaerobic bacteria and aerobic/facultative bacteria were different in the situation of biofilm formation. The early colonizers25 were correlated positively with each other (e.g., Fusobacterium nucleatum and Streptococcus salivarius in both diseases). The early colonizers and late colonizers25 were negatively correlated (e.g., Streptococcus intermedius and Eubacterium nodatum in peri-implantitis or Streptococcus oralis and Treponema denticola in periodontitis). Second, associations among the red-complex species were common. Porphyromonas gingivalis was correlated significantly with Tannerella forsythia at both sites.
Our data also corresponded with a previous study32 that reported a positive relationship between members of the phyla Synergistetes and Spirochaetes in the sub-gingival biofilm; and we showed this correlation at the species level (Synergistetes sp. HOT453 and Treponema denticola) for peri-implantitis (ρ = 0.615).
In conclusion, this initial characterization furthers our understanding of the microbial community in the oral cavity by defining the microbiota associated with peri-implantitis. We have shown, by comparing the 2 diseases within the same patients using high-throughout sequencing and correlation analysis, that similar to other oral diseases, peri-implantitis is a polymicrobial disease. We showed that the core microbiota of peri-implantitis was different from that of periodontitis, even though the sources of bacteria around the implant were present elsewhere in the oral cavity, such as the remaining teeth. Although the red-complex species co-occurred as in past studies, other species were significantly abundant or associated with the progression of disease in peri-implantitis sites, suggesting that other periodontal pathogens and polymicrobial infections are associated with peri-implantitis, and therefore the target pathogens for its treatment or prevention may be different from those for periodontitis. Thus, our data could provide the basis for future molecular analyses of the bacteria suspected of being associated with peri-implantitis, including the uncultured species In addition, our finding that peri-implantitis results from the complexity of the oral microbiota suggests that understanding of the healthy oral microbiota will require more studies of the microbial ecology of other oral infectious diseases.
Methods
Ethical statement
This study was carried out in accordance with the Ethical Guidelines for Clinical Studies (2008 Notification number 415 of the Ministry of Health, Labor, and Welfare) and approved by the Tokyo Medical and Dental University Institutional Review Board (No.661). Written informed consent was obtained from all patients.
Patients and clinical examinations
Twenty patients with both peri-implantitis and periodontitis were recruited from the clinics of the Department of Periodontology, Tokyo Medical and Dental University Hospital. The patients were systemically healthy adults and had not received anti-inflammatory drugs, oral anti-microbial agents, or systemic antibiotics within the previous 3 months.
The following clinical examinations of each implant or tooth were performed at the mesio-buccal, buccal, disto-buccal, mesio-lingual, lingual, and disto-lingual sites: PPD, CAL, BOP, and noting the presence of pus. Intraoral periapical radiographs (Insight Dental Film; Eastman Kodak Company, SP, Tokyo, Japan) were obtained using the parallel technique, and the same examiner analysed the radiographs for bone loss from peri-implantitis at implants functioning at least 1 year and from periodontitis33,34. Based on the clinical and radiographic data, peri-implantitis and periodontitis sites were selected that exhibited PPD ≥ 4 mm, BOP and/or pus-discharge presence, and concomitant radiographic bone-loss presence.
Sample collection and DNA extraction
Sub-mucosal and sub-gingival plaque samples were obtained from the deepest pockets at the peri-implantitis and periodontitis sites, respectively. The sampling sites were isolated with sterile cotton rolls and the supra-mucosal or supra-gingival plaque was removed. After drying the target sites, 3 paper points were inserted into the pocket for 30 s and then placed in a sterile tube for storage at −80°C until further analysis. To separate the bacteria from the paper points, 1 mL of sterile distilled water was added and they were vortexed for 1 min before removing the points. The tubes were then centrifuged at 12,000 × g for 5 min to pellet the bacterial samples, and the DNA was extracted and purified with the MORA-EXTRACT DNA extraction kit (Kyokuto Pharmaceuticals, Tokyo, Japan) in accordance with the manufacturer's instructions35. The total bacterial DNA was eluted with 200 μL of Tris-EDTA buffer and was stored at −20°C.
Preparation of the bacterial 16S rRNA gene amplicons
The 16S rRNA genes from each sample were amplified with the following PCR primers36: 806R with the adaptor B sequences from 454 Life Sciences (Roche, Basel, Switzerland) (5′-CCTATCCCCTGTGTGCCTTGGCAGTCGGACTACVSGGGTATCTAAT-3′) and 515F with adaptor A and subject-specific ten-base barcode sequences (5′-CCATCTCATCCCTGCGTGTCTCCGACTCAG -10-bp-barcode- GTGCCAGCMGCCGCGGT-3′); the small subunit ribosomal rRNA sequence is in bold.
The amplification of the V3–4 region of the 16S rRNA gene was performed in 50-μL reaction mixtures composed of 10× polymerase buffer, 2.5 mM dNTPs, 0.2 μM of each primer, 1.25 U Takara Ex Taq Hot Start (TaKaRa Biomedicals, Tokyo, Japan), and 1–2 μL of template DNA. After a denaturation step at 94°C for 1 min, the PCR cycling parameters were 35 cycles of 98°C for 10 s, 55°C for 30 s, and 72°C for 2 min, followed by a final extension at 72°C for 1 min. The amplicons were visualized by electrophoresis on 2% agarose gels, and the bands were purified using the NucleoSpin Extract II kit (Macherey-Nagel, Düren, Germany) followed by AMPure paramagnetic beads (Agencourt Bioscience Corporation, Beverly, MA, USA) according to the manufacturers' protocols.
Amplicon quantitation, pooling, and pyrosequencing
The amplicons were quantified using a KAPA Library Quantification Kit optimized for the Roche 454 GS Titanium system (KAPA Biosystems, Woburn, MA) and pooled in equal amounts into a single tube, and a final amount of 1 × 107 molecules were analysed via pyrosequencing on a 454 Life Sciences Genome Sequencer Junior (GS-Junior; Roche Diagnostics, Basel, Switzerland). The emulsion PCR, bead enrichment, and 454 GS Junior sequencing were performed as indicated in the manufacturer's protocols, and the resulting flowgram files were used for the downstream analyses.
Sequence analysis
The sequence data were processed and analysed with the software package Quantitative Insights into Microbial Ecology (QIIME) version 1.6.037. The sequences were denoised38 and removed if they had a length <200 bp or >800 bp, average quality score <25, ambiguous bases present, primer mismatches >1.5, homopolymer runs >6 bases, uncorrectable barcodes, or lacked the primer39. The remaining sequences were assigned as samples based on their barcodes, and the software program UCHIME40 was used to identify putative chimeric sequences.
The similar sequences were binned into OTUs using UCLUST41 with a minimum pairwise identity of 97%, and the most abundant sequence in each OTU was chosen to represent its OTU. The taxonomy of representative sequences at the phylum or genus level was determined using RDP classifier version 2.242 against the RDP 16S database in the QIIME with a minimum support threshold of 60%. Because all of the abundant genera were contained in the NCBI database, the phylogenetic tree was generated by PhyloT implemented in the Interactive Tree Of Life (iTOL) tool version 2.2.243, which generates phylogenetic trees based on the NCBI taxonomy, and visualized using the iTOL. Because the pathogenicity often differs even among bacterial species of the same genus, refinement of the taxonomic assignment was performed at the species level based on HOMD database version 13.01, a curated database of comprehensive information for oral 16S rRNA gene sequences. The species were assigned if a sequence had ≥98.5% identity for ≥250 bp of the sequence in a BLASTN search following the methods of previous studies44,45. The bacterial cultivation status of the bacteria was obtained from the HOMD, and the Gram-staining status or oxygen requirement for each taxon was obtained from the NCBI Entrez Genome Project database (http://www.ncbi.nlm.nih.gov/genomes/lproks.cgi).
Alpha-diversity indexes were estimated from the number of observed OTUs, Chao146 species richness estimates, and the Shannon diversity index47 that measures both species richness and evenness. Rarefaction curves were generated to calculate the species richness based on the bacterial OTUs at a 97% identity level and were compared between the peri-implantitis and periodontitis samples. To compare the beta-diversity between peri-implantitis and periodontitis, the representative sequences from OTUs were aligned against the Greengenes database48 (core set aligned February 4, 2011) by PyNAST49, a Python-based implementation of the Nearest Alignment Space Termination (NAST) tool, implemented in the QIIME with a 200-bp minimum length and 75% minimum per cent identity. Subsequently, the hypervariable regions were masked with maskPH39 and we used the unweighted UniFrac distance metrics50 computed using the tree file generated by FastTree51; the resulting distance matrix was then visualized using PCoA. The co-occurrence of taxonomic groups across samples was explored by calculating the Spearman's ranked correlation (ρ), which was >0.6 and statistically significant (P < 0.05 and q <0.1)52, clustering the correlations using Cluster 3.053, and visualizing the results with Java TreeView version 1.1.6r254.
Statistical analysis
Paired t-tests were performed to compare the clinical data and alpha diversity between the peri-implantitis and periodontitis samples4. ANOSIM tests were performed on the unweighted UniFrac distances, and the relative abundances of each bacterial taxon were compared by conducting Wilcoxon signed-rank tests5. Spearman's ranked correlations were used for the analyses of co-occurrence and the bacterial associations with the clinical data52,55. We corrected the obtained P-values for multiple tests using the Benjamini-Hochberg false discovery rate (q-value)56.
Author Contributions
This study was conceived by N.M. and F.M. Samples and clinical data were collected by Y.T. and Y.I. Laboratory work was done by N.M., F.M. and I.N. The manuscript was written by N.M., F.M. and C.A. All authors reviewed the manuscript.
Additional information
Accession codes: The data are available at the DNA Data Bank of Japan (DDBJ) under accession no. DRA000946 (http://www.ddbj.nig.ac.jp/).
Supplementary Material
Supplementary information
Acknowledgments
This research was supported by Grant-in-Aid for Japan Society for the Promotion of Science (JSPS) Fellows; the Japanese Ministry of Education, Global Center of Excellence program, International Research Center for Molecular Science in Tooth and Bone Diseases, Ministry of Education, Culture, Sports, Science and Technology Japan; Grant-in-Aid for Scientific Research 22592032 (F.M.), 21792110 (Y.T.), 24792321(Y.T.), 25670776 (F.M.), and 25713060 (F.M.); a Grant-in-Aid for Scientific Research on Innovative Areas 24117508 (F.M.); and the Funding Program for Next Generation World-Leading Researchers LS041 (I.N.), JSPS.
References
- Dewhirst F. E. et al. The human oral microbiome. J Bacteriol 192, 5002–5017 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour G. J. et al. Relationship between periodontal infections and systemic disease. Clin Microbiol Infect 13, 3–10 (2007). [DOI] [PubMed] [Google Scholar]
- Zhou Y. et al. Biogeography of the ecosystems of the healthy human body. Genome Biol 14, R1. 10.1186/gb-2013-14-1-r1 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abusleme L. et al. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J 7, 1016–1025 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffen A. L. et al. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J 6, 1176–1185 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F. et al. Saliva microbiomes distinguish caries-active from healthy human populations. ISME J 6, 1–10 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. C. Y. et al. Residual periodontal pockets are a risk indicator for peri-implantitis in patients treated for periodontitis. Clin Oral Impl Res 23, 325–333 (2012). [DOI] [PubMed] [Google Scholar]
- Pjetursson B. E. et al. Peri-implantitis susceptibility as it relates to periodontal therapy and supportive care. Clin Oral Impl Res 23, 888–894 (2012). [DOI] [PubMed] [Google Scholar]
- Botero J. E. et al. Subgingival microbiota in peri-implant mucosa lesions and adjacent teeth in partially edentulous patients. J Periodontol 76, 1490–1495 (2005). [DOI] [PubMed] [Google Scholar]
- Karoussis I. K. et al. Long-term implant prognosis in patients with and without a history of chronic periodontitis: a 10-year prospective cohort study of the ITI® Dental Implant System. Clin Oral Impl Res 14, 329–339 (2003). [DOI] [PubMed] [Google Scholar]
- Zitzmann N. U. & Berglundh T. Definition and prevalence of peri-implant diseases. J Clin Periodontol 35, 286–291 (2008). [DOI] [PubMed] [Google Scholar]
- Heitz-Mayfield L. J. A. & Lang N. P. Comparative biology of chronic and aggressive periodontitis vs. peri-implantitis. Periodontol 2000 53, 167–181 (2010). [DOI] [PubMed] [Google Scholar]
- Mombelli A. & Lang N. P. The diagnosis and treatment of peri-implantitis. Periodontol 2000 17, 63–76 (1998). [DOI] [PubMed] [Google Scholar]
- Costa F. et al. Endoscopic surgical treatment of chronic maxillary sinusitis of dental origin. J Oral Maxillofac Surg 65, 223–228 (2007). [DOI] [PubMed] [Google Scholar]
- Claffey N. et al. Surgical treatment of peri-implantitis. J Clin Periodontol 35, 316–332 (2008). [DOI] [PubMed] [Google Scholar]
- Zaura E. et al. Defining the healthy "core microbiome" of oral microbial communities. BMC Microbiol 9, 259 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao W. W. L. et al. Microbial transformation from normal oral microbiota to acute endodontic infections. BMC Genomics 13, 345 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky S. S. et al. Microbial complexes in subgingival plaque. J Clin Periodontol 25, 134–144 (1998). [DOI] [PubMed] [Google Scholar]
- Koyanagi T. et al. Analysis of microbiota associated with peri-implantitis using 16S rRNA gene clone library. J Oral Microbiol 2, 10.3402/jom.v2i0.5104 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi T. et al. Comprehensive microbiological findings in peri-implantitis and periodontitis. J Clin Periodontol 40, 218–226 (2013). [DOI] [PubMed] [Google Scholar]
- Kumar P. S. et al. Pyrosequencing reveals unique microbial signatures associated with healthy and failing dental implants. J Clin Periodontol 39, 425–433 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic S. A. The management of peri-implant breakdown around functioning osseointegrated dental implants. J Periodontol 64, 1176–1183 (1993). [DOI] [PubMed] [Google Scholar]
- do Nascimento C. et al. Bacterial diversity of periodontal and implant-related sites detected by the DNA checkerboard method. Eur J Clin Microbiol Infect Dis 30, 1607–1613 (2011). [DOI] [PubMed] [Google Scholar]
- Dabdoub S. M. et al. Patient-specific analysis of periodontal and peri-implant micobiomes. J Dent Res 92, 168S–75S (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E. et al. Oral multispecies biofilm development and the key role of cell-cell distance. Nat Rev Microbiol 8, 471–480 (2010). [DOI] [PubMed] [Google Scholar]
- Lepp P. W. et al. Methanogenic Archaea and human periodontal disease. Proc Natl Acad Sci U S A 101, 6176–6181 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faveri M. et al. Prevalence and microbiological diversity of Archaea in peri-implantitis subjects by 16S ribosomal RNA clonal analysis. J Periodontal Res 46, 338–344 (2011). [DOI] [PubMed] [Google Scholar]
- Colombo A. P. V. et al. Impact of periodontal therapy on the subgingival microbiota of severe periodontitis: comparison between good responders and individuals with refractory periodontitis using the human oral microbe identification microarray. J Periodontol 83, 1279–1287 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belda-Ferre P. et al. The oral metagenome in health and disease. ISME J 6, 46–56 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson J. C. et al. Use of 16S ribosomal RNA gene analyses to characterize the bacterial signature associated with poor oral health in West Virginia. BMC Oral Health 11, 10.1186/1472-6831-11-7 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shchipkova A. Y. et al. Subgingival microbial profiles of smokers with periodontitis. J Dent Res 89, 1247–53 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust K. et al. Microbial Co-occurrence Relationships in the Human Microbiome. PLoS Comput Biol 8, e1002606 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrami G. et al. Prediction of future marginal bone level: a radiographic study. J Clin Periodontol 38, 933–938 (2011). [DOI] [PubMed] [Google Scholar]
- Fransson C. et al. Severity and pattern of peri-implantitis-associated bone loss. J Clin Periodontol 37, 442–448 (2010). [DOI] [PubMed] [Google Scholar]
- Nakamura A. et al. Diagnostic value of PCR analysis of bacteria and fungi from blood in empiric-therapy-resistant febrile neutropenia. J Clin Microbiol 48, 2030–2036 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate S. et al. Examining the global distribution of dominant archaeal populations in soil. ISME J 5, 908–917 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J. G. et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7, 335–336 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder J. & Knight R. Rapid denoising of pyrosequencing amplicon data: exploiting the rank-abundance distribution. Nat Methods 7, 668–669 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren O. et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci U S A 108, 4592–4598 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. C. et al. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27, 2194–2200 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461 (2010). [DOI] [PubMed] [Google Scholar]
- Cole J. R. et al. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 37, D141–D145 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I. & Bork P. Interactive Tree Of Life v2: online annotation and display of phylogenetic trees made easy. Nucleic Acids Res 39, W475–W478 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita T. et al. Discrimination of the oral microbiota associated with high hydrogen sulfide and methyl mercaptan production. Sci Rep 2, 215 10.1038/srep00215 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler C. J. et al. Sequencing ancient calcified dental plaque shows changes in oral microbiota with dietary shifts of the Neolithic and Industrial revolutions. Nat Genet 45, 450–5, 455e1 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao A. Nonparametric estimation of the number of classes in a population. Scand J Stat 11, 265–270 (1984). [Google Scholar]
- Shannon C. E. A mathematical theory of communication. Bell Syst Tech J 27, 379–423 (1948). [Google Scholar]
- DeSantis T. Z. et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72, 5069–5072 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J. G. et al. PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics 26, 266–267 (2010a). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C. A. et al. Quantitative and qualitative β diversity measures lead to different insights into factors that structure microbial communities. Appl Environ Microbiol 73, 1576–1585 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price M. N. et al. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol 26, 1641–1650 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberán A. et al. Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J 6, 343–351 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoon M. J. L. et al. S. Open source clustering software. Bioinformatics 20, 1453–1454 (2004). [DOI] [PubMed] [Google Scholar]
- Saldanha A. J. Java Treeview—extensible visualization of microarray data. Bioinformatics 20, 3246–3248 (2004). [DOI] [PubMed] [Google Scholar]
- Lauber C. L. et al. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl Environ Microbiol 75, 5111–20 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y. & Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57, 289–300 (1995). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information