Abstract
The management of gastric cancer continues to evolve. Whilst surgery alone is effective when tumours present early, a large proportion of patients are diagnosed with loco-regionally advanced disease, resulting in high loco-regional and distant relapse rates, with subsequent poor survival. Early attempts at improving outcomes following resection were disappointing; however, randomized trials have now established either post-operative chemoradiotherapy (INT0116) or peri-operative chemotherapy as standard adjuvant therapies in the Western world. There remain, however, significant differences in the approach to management between the West and East. In Asia, where there is the highest incidence of gastric cancer, extended resection followed by adjuvant chemotherapy represents the standard of care. This review discusses current standard adjuvant therapy in gastric adenocarcinoma, as well as recent and ongoing trials investigating novel (neo)adjuvant approaches, which hope to build on the successes of previous studies.
Keywords: Adjuvant, Gastric, Stomach, Cancer, Chemoradiation, Chemoradiotherapy, Chemotherapy, Peri-operative, Neo-adjuvant
Core tip: Surgery remains the cornerstone of curative therapy in gastric cancer. However, a large proportion of patients are diagnosed with locally advanced disease, resulting in poor survival. Randomized trials have now established either post-operative chemoradiotherapy or perioperative chemotherapy as standard adjuvant therapies in the Western world. There remain, however, significant differences in the approach to management between the West and East. In Asia, extended resection followed by adjuvant chemotherapy represents the standard of care. This review discusses the evidence supporting current standard adjuvant therapy in gastric cancer, as well as recent and ongoing trials investigating novel (neo)adjuvant approaches.
INTRODUCTION
Gastric cancer represents the second leading cause of cancer death worldwide and is the fourth most common malignancy in the world, with an estimated 988000 new cases diagnosed in 2008, and accounting for 736000 cancer-related deaths[1].
Surgery provides high rates of cure in the early stages; however less than 25% of patients present with early stage disease. The survival of the remaining patients with potentially curable, non-metastatic disease falls below 50% and 20% when the tumour invades through the muscularis propria and involves regional lymph nodes, respectively[2], prompting much effort to improve patient outcomes following gastrectomy.
The publication of two landmark trials in 2001 and 2006 established both post-operative chemo-radiotherapy (CRT) and perioperative chemotherapy (CT) as effective adjuvant treatment options, and both are currently accepted standards of care in the Western world[3,4]. However, debate continues concerning the applicability of these trials to the Asian population, where there is the highest incidence of gastric cancer.
The staging of gastric cancer has recently undergone revision, with the 7th edition of the American Joint Committee on Cancer classification amending the T-stage definition of serosal and subserosal invasion, as well as the extent of lymph node involvement (Table 1)[2,5]. It also addresses the classification of tumours arising at the gastro-oesophageal junction. Though there continues to be wide variation and imprecise definitions of gastro-oesophageal junction (GOJ) tumours (including gastric cardia), the current staging system classifies tumours that arise in the gastric cardia, or within 5 cm of the GOJ and extending into the oesophagus, as oesophageal cancers.
Table 1.
American Joint Committee on Cancer 6th vs 7th edition staging of gastric cancer
| AJCC 6th | AJCC 7th | ||
| T1 | Tumour invades lamina propria or submucosa | T1a | Invades Lamina propria or muscularis mucosae |
| T1b | Invades Submucosa | ||
| T2a | Invades Muscularis propria | T2 | Invades Muscularis propria |
| T2b | Invades Subserosa | ||
| T3 | Penetrates Serosa (visceral peritoneum), without invasion of adjacent structures | T3 | Penetrates subserosal connective tissue without invasion of visceral peritoneum or adjacent structures |
| T4 | Invades adjacent structures | T4a | Tumor invades serosa (visceral peritoneum) |
| T4b | Tumor invades adjacent structures | ||
| N1 | 1-6 nodes involved | N1 | 1-2 nodes involved |
| N2 | 7-15 nodes involved | N2 | 3-6 nodes involved |
| N3 | > 15 nodes involved | N3a | 7-15 nodes involved |
| N3b | > 15 nodes involved |
AJCC: American Joint Committee on Cancer.
This review will examine current standard adjuvant therapies in resectable gastric adenocarcinoma, and will focus predominantly on tumours arising in the stomach, rather than the GOJ. In addition, newer strategies and trials, which aim to build on the successes of current adjuvant and neoadjuvant approaches will be discussed.
“EAST VS WEST” APPROACH TO GASTRIC CANCERS
There continues to be differences between Western and Asian countries, both in terms of approach to management of gastric cancer, as well as efficacy of therapy. Whereas a D1 or less lymph node dissection has been common practice amongst Western surgeons, Japanese surgeons in particular consider an adequate lymphadenectomy to at least include the D2 echelon of nodes (described below). There are also frequently observed differences in outcomes, where reported survival rates are consistently higher in Asian studies[6-8]. It is not clear whether the extent of surgery can solely be responsible for this. Numerous hypotheses have been suggested to explain this observation, the first of which relates to the issue of stage migration, where a more extensive nodal dissection leading to more accurate prognostic stratification and inevitable upstaging in a proportion of patients may in fact falsely suggest a treatment benefit[9]. Other factors may also contribute, including inherent biological differences in the disease between the two populations[10], as well as differing tolerance and sensitivity to chemotherapeutic agents.
EXTENT OF LYMPH NODE DISSECTION
The extent of lymphadenectomy remains a major area of controversy in gastric cancer management. Though a comprehensive discussion of lymphadenectomy is beyond the scope of the current review, a brief discussion follows, as it puts into context some of the difficulties with interpreting and comparing results of older vs newer studies, as well as Asian vs Western trials.
Although there are slight variations in definition, in general, a D1 lymphadenectomy entails removal of perigastric lymph nodes as well as those around the left gastric artery (stations 1-7), whereas a D2 dissection removes additional lymph nodes around the hepatic, celiac, splenic, as well as splenic hilar and hepatoduodenal (stations 8-12) nodes[11]. Comprehensive guidelines describing the extent of lymphadenectomy according to primary tumour location are detailed by the Japanese Research Society for Gastric Cancer (JRSGC)[12]. Extended dissections beyond D2 are not routinely performed, as these lymph nodes are often regarded as distant metastases[2]. Japanese surgeons consider a D2 dissection the standard of care, whilst less extensive dissections are still commonplace amongst Western surgeons.
There have been at least five randomised controlled trials (RCT’s) comparing D1 vs D2 dissection, of which the Dutch and United Kingdom Medical Research Council (MRC) trials were the largest[13-17]. In addition, multiple reviews of these trials have been reported. In summary, most of these trials suggested higher rates of mortality and morbidity with D2 dissection, with no convincing overall survival benefit[18-20]. This was confirmed in a recent meta-analysis published in 2012, of five RCT’s where overall hospital mortality was higher in D2 patients (RR = 2.02; 95%CI: 1.30-3.14, P = 0.002); however, overall survival at 5 years reported in three trials was similar at 43.5% and 44.9% for D1 and D2 patients, respectively (RR = 1.06; 95%CI: 0.85-1.33, P = 0.58)[21]. It is interesting to note that in a recent 15-year update of the Dutch RCT, the authors reported a reduction in gastric cancer-related deaths, although there was still no difference in overall survival (OS)[16].
There is evidence that at least some of the morbidity associated with D2 dissection may relate to the requirement for spleen and pancreas resection. The most recent results from the Italian RCT, which show no difference in morbidity or mortality between D1 and D2 certainly suggests there may be a contribution and benefit from advances in modern surgical technique and perioperative care[17]. Despite a lack of clear evidence of benefit, there is growing consensus amongst Western surgeons that D2 dissection should be performed whenever possible.
STANDARD OF CARE IN THE WEST-POSTOPERATIVE CRT AND PERIOPERATIVE CT
The role of locoregional radiation is based on the fact that a significant proportion of relapses following curative gastrectomy occur in the upper abdomen[22]. In fact, early evidence suggested that these recurrences are likely occult and exceeded clinically detected events, thereby providing further rationale for adjuvant radiotherapy[23,24].
Initial efforts to improve outcomes after surgery alone, however, were disappointing. Earlier trials of pre- and post-operative radiotherapy, which individually did not demonstrate any benefit, were hampered by small numbers, toxicity, and heterogeneous dose-fractionation schedules[25-28].
In 2001, the INT0116 trial was reported by MacDonald et al[4] and demonstrated a major survival advantage for the use of postoperative adjuvant chemoradiotherapy. This trial randomly assigned 556 patients with stage IB-IV gastric cancer following surgery to either observation or adjuvant therapy with 4 monthly cycles of bolus 5-fluorouracil (5-FU) and leucovorin combined with radiation to 45 Gray in 25 fractions. With a median follow-up period of 5 years, the 3-year survival rate was 50% in the CRT group vs 41% in the surgery alone group (P = 0.005). The 10-year update demonstrated persistent median OS benefits of 35 mo vs 27 mo, with a HR of 1.32 (95%CI: 1.10-1.60; P = 0.046)[29].
The criticisms of adjuvant CRT in the MacDonald study included the substantial rates of acute toxicity (33% had ≥ grade 3 gastro-intestinal toxicity), lower rates and extent of nodal dissection (54% had a D0 dissection and only 10% had a formal D2 dissection), the relatively simple (and now outdated) radiotherapy techniques used, and the choice of chemotherapy regimen, the latter two of which are further discussed below.
The publication of the MAGIC trial in 2006 provided a new option for the treatment of gastric cancer[3]. This trial randomly assigned 503 patients with resectable stage IB-IV gastric cancer to either perioperative chemotherapy [three preoperative and three postoperative cycles of epirubicin cisplatin, 5-FU (ECF)] and surgery or surgery alone. With a median follow-up of 4 years, the 5-year survival rate was 36% in the perioperative chemotherapy group vs 23% in the surgery alone group (HR = 0.75; 95%CI: 0.6-0.93; P = 0.009).
Therefore, in Western countries there are two standards of care for patients with resectable gastric cancer[30-32], leaving clinicians with the dilemma of which strategy to employ. There are difficulties in delivering post-operative therapy in patients who are deconditioned following surgery, and this was highlighted in both INT0116, where only 64% of patients randomized to the CRT arm completed all protocol treatment, as well as MAGIC, where less than half of patients completed the post-operative component of protocol chemotherapy. In contrast, pre-operative therapy is far better tolerated: in the MAGIC study, 86% of patients completed all three neo-adjuvant cycles of ECF, with haematological toxicity being the most common toxicity (24%-28% grade 3-4 granulocytopaenia), and grade 3-4 nausea and vomiting occurring in only 6.4% and 5.6%, respectively. In addition, the rates of post-operative complications, deaths within 30 d and median hospital stay were similar in both arms of the MAGIC trial.
It is obviously difficult to directly compare absolute outcomes across both trials as the patient cohorts were dissimilar, in that MAGIC included a slightly higher proportion of node-negative patients (28% vs 15%), as well as patients with distal oesophageal primaries (15% vs 0%). In addition, approximately 66% of patients in MAGIC underwent a curative resection (according to the operating surgeon), whereas in INT0116, all patients underwent an R0 resection. This is likely a reflection of the fact that the MAGIC trial recruited patients pre-operatively, whereas patients in INT0116 were randomised 20-40 days following R0 gastrectomy.
Despite the issues with INT0116 described above, there are other lines of evidence to support a benefit for radiotherapy in gastric cancer, including several meta-analyses and a large population-based database, all of which consistently demonstrate a survival benefit for the addition of radiotherapy to surgery. The four meta-analyses of radiotherapy published since 2006 are summarized in Table 2[33-36]. One of the more recent meta-analysis was reported by Ohri et al[33] and included 13 trials with 2811 patients[37-42]. Their results were consistent with those from previous meta-analyses, suggesting that the addition of radiotherapy to surgery (with or without CT) improves OS [HR = 0.78 (0.70-0.86), P < 0.001]. Inclusion of the three more recent Asian trials, which compared adjuvant CT with adjuvant CRT, also showed improvements in DFS [HR = 0.77 (0.91-0.65), P = 0.002], as well as OS [HR = 0.83 (0.67-1.03), P = 0.087], though the latter did not reach statistical significance.
Table 2.
Meta-analyses of adjuvant chemo-radiotherapy trials after 2006
An analysis of the Surveillance, Epidemiology, and End Results database, which includes 11630 patients, suggested a benefit for adjuvant radiotherapy predominantly in node-positive patients (5-year OS 30.4% vs 21.4%, P < 0.0001)[43]. The survival benefit of CRT was consistently demonstrated when > 15 or > 30 lymph nodes were removed in N1/2 and N3 patients, respectively.
Current consensus and practice guidelines from the National Comprehensive Cancer Network, European Society for Medical Oncology and Canada recommend either perioperative CT or post-operative CRT as standard treatment options for patients with resectable gastric cancer[30-32].
STANDARD OF CARE IN THE EAST-ADJUVANT CHEMOTHERAPY
Adjuvant CT has been a widely explored approach both in Western and Asian countries since the 1960’s, given the propensity for distant and metastatic relapse following curative resection. Various agents have been tested including 5-FU, doxorubicin, mitomycin C, epirubicin, cisplatin and various combinations of these.
Multiple meta-analyses of adjuvant CT have been reported, the most recent of which are summarised in Table 3[44-48]. These show a modest survival benefit for adjuvant CT, particularly in Asian patients. The GASTRIC meta-analysis was an individual patient-level analysis of 17 trials including 3838 patients[49]. At a median follow-up of 7 years, adjuvant CT significantly increased OS from 49.6% to 55.3% [HR = 0.82 (0.76-0.90); P < 0.001]. Similar statistically significant effects were seen with DFS.
Table 3.
Meta-analyses of adjuvant chemotherapy after 2006
| Author | Year | Number of trials included | Number of patients | HR for OS | P value |
| GASTRIC | 2010 | 17 | 3838 | HR = 0.82 [0.76-0.90] | < 0.001 |
| Sun et al | 2009 | 12 | 3809 | HR = 0.78 [0.71-0.85] | < 0.001 |
| Zhao et al | 2008 | 15 | 3212 | RR = 0.88 [0.77-0.99] | 0.001 |
| Liu et al | 2008 | 19 | 4599 | RR = 0.85 [0.80-0.90] | < 0.00001 |
GASTRIC: Global Advanced/Adjuvant Stomach Tumor Research International Collaboration; OS: Overall survival.
Although adjuvant CT is not used routinely in the West, it represents standard practice in the East, following the reporting of two large RCT’s in Asian patients, namely the CLASSIC trial from East Asia and the Japanese ACTS-GC trial, both of which used different CT regimens[50-52].
The ACTS-GC trial randomized 1059 patients with stage II or III gastric cancer following D2 gastrectomy to observation or S-1, an oral fluoropyrimidine CT preparation containing tegafur, gimeracil and oteracil, for 1 year after surgery. Initial results were published in 2007[50], and 5-year follow-up data have recently been reported[51]. There is a persistent OS benefit of S-1 from 61% to 71% [HR = 0.669 (0.54-0.828)]. S-1 appears well tolerated with < 5% of patients reporting grade 3 or higher toxicities. S-1, however, is not widely available in Western countries.
The CLASSIC trial randomized 1035 patients with stage II or III gastric cancer following D2 gastrectomy to observation or eight cycles of capecitabine and oxaliplatin[52]. Patients in the CT arm had improved 3-year disease-free survival, from 59% to 74% (P < 0.0001). Overall survival was also improved (78% vs 83%, P = 0.0493). However, more than half the patients in the CT arm experienced grade 3 or 4 toxicities.
As a result of these two trials, the current standard of care for adjuvant therapy of gastric cancer in the East is postoperative CT with either S-1 or capecitabine/oxaliplatin.
BEYOND MAGIC AND INT0116
Since the reporting of the MAGIC trial in 2006, a new generation of adjuvant gastric studies have been reported that include more recent RCT’s, and studies of novel neoadjuvant approaches.
Perioperative chemotherapy
The MAGIC study reported that only 49.5% of patients in the perioperative CT arm completed all post-operative treatment, prompting some investigators to question the relative contribution of the post-operative cycles of CT.
Two European RCT’s have evaluated pre-operative CT vs surgery alone. The ACCORD-07/FFCD 9703 trial reported by Ychou et al[53] which closed early because of poor accrual, randomized 224 patients to surgery alone vs two to three cycles of pre-operative cisplatin and 5-FU CT. This study included predominantly patients with gastro-oesophageal junction adenocarcinomas, with stomach primaries allowed later in the study and comprising 25% of the patients enrolled. At a median follow-up of 5.7 years, pre-operative CT improved the R0 resection rate from 74% to 84% (P = 0.004), as well as increasing 5-year OS and DFS from 24% to 38% (P = 0.02), and 19% to 34% (P = 0.003), respectively. The EORTC 40954 trial, which also closed early because of poor accrual, randomized 144 patients (of a planned 360) to surgery alone vs two cycles of pre-operative cisplatin and 5-FU CT. Although this trial allowed the inclusion of Siewert I and II GOJ tumours, 47.2% of patients had primary tumours in the middle and lower third of the stomach. At a median follow up of 4.4 years, despite an improvement in RO resection rate from 66.7% to 81.9%, there was no difference in overall survival between the two arms[54].
The reasons for the negative result in the EORTC trial are not clear. Possible explanations include poor accrual, higher reported rates of postoperative complications and lower proportion of patients completing the full protocol of pre-operative CT. In addition, the trial has also highlighted some of the difficulties with accurate pre-operative staging, where 50% of patients in the surgery alone arm had pT1-2 tumours, despite inclusion criteria requiring endoscopic ultrasound (EUS) stage T3-4 tumours only, suggesting a degree of over-staging with EUS.
Postoperative chemoradiotherapy
An important question, particularly for Asian surgeons, is the relative and incremental benefit of postoperative radiation above and beyond that of CT, in the context of extended lymphadenectomy.
There have been at least six recent trials examining the addition of postoperative CRT to CT, five of which have been conducted in Asian patients. These are outlined in Table 4, with the ARTIST trial from South Korea being the largest[37-42]. This Phase III RCT randomized 458 patients following D2 gastrectomy to adjuvant CT alone with six cycles of capecitabine and cisplatin (XP) vs adjuvant CRT comprising 2 cycles of XP, then 45Gy CRT with Capecitabine, followed by two further cycles of XP. At a median follow up of 53 mo, the primary endpoint was not met, with no difference in 3-year DFS. However, an unplanned subgroup analysis of node-positive patients (comprising 86% of patients), showed significant improvement in 3-year DFS (77.5% vs 72.3%; P = 0.0365). It is worth noting that the final analysis of the study was performed earlier than initially planned, as there were fewer events than expected. This was likely related to the fact that almost 60% of patients had early stage disease (i.e., IB/IIA), of which more than 20% had T1 or T2 primaries.
Table 4.
Trials of adjuvant chemo-radiotherapy vs adjuvant chemotherapy in gastric cancer after 2006
| Ref. | Year | Postop CRT arms | No. of patients | % having D2 dissection | Survival Outcome | P value | Outcome | P value | |
| Lee et al[37] | 2012 | Surg | Cape/Cis x2 - 45Gy/Cape - Cape/Cis x2 | 458 | 100% | 3-yr DFS 78.2% vs 74.2% | 0.862 | ||
| Surg | Cape/Cis - x6 | ||||||||
| Yu et al[42] | 2012 | Surg | FU/LV - 45Gy/FU/LV - FU/LV | 404 | 100% | 5-yr OS 48% vs 41.8 | 0.122 (for median OS) | 5-yr RFS 45.2% vs 35.8% | 0.029 (for median OS) |
| Surg | FU/LV | ||||||||
| Kim et al[39] | 2012 | Surg | 5-FU/LV x5 | 90 | 100% | 54.6% vs 65.2% | 0.67 | ||
| Surg | x1 5-FU/LV - 45Gy/5-FU - x2 5-FU/LV | ||||||||
| Bamias et al[40] | 2010 | Surg | Docetaxel/Cisplatin x6 | 147 | 44% D1-2 | OS/DFS no difference | |||
| Surg | Docetaxel/Carboplatin + 45Gy | ||||||||
| Yu et al[42] | 2012 | Surg | 5-FU/LV x5 | 68 | 69% | 3yr OS 68% vs 44% | < 0.05 | 3-yr DFS 56% vs 29% | < 0.05 |
| Surg | INT-0116 | ||||||||
| Kwon et al[41] | 2010 | Surg | 5-FU/Cis x6 | 61 | 100% | 5-yr OS 70.1 vs 70% | 0.814 | 5-yr DFS 80% vs 75% | 0.887 |
| Surg | FPx1 - 45Gy/Cape - FP x3 |
Cape: Capecitabine; Cis: Cisplatin; FU/LV: Fluorouracil/leucovorin; FP: Fluorouracil/cisplatin; OS: Overall survival; DFS: Disease-free survival; RFS: Relapse-free survival.
In terms of treatment tolerability, only three of 203 patients that started CRT did not complete radiotherapy, and the majority completed two further protocol cycles of chemotherapy. In contrast to the INT0116 study, where 33% of patients experienced grade 3 or higher GI toxicity, the incidence of grade 3 or higher nausea, vomiting, diarrhoea, stomatitis or constipation was approximately 19%, compared with 19.9% in the chemotherapy only arm. It is also interesting to note the incidence of Grade 2 nausea and vomiting seemed lower in the CRT arm (18.9% and 4.8%) compared with the chemotherapy alone arm (27.9% and 8.4% respectively).
An analysis of failure pattern data from INT0116 showed minimal effect of 5-FU/LV on distant failure, suggesting that the improvement noted in the study entirely reflected an improvement in local control, with little effect on distant metastases. This strongly suggests that the 5-FU/LV combination delivered in this study produced its effect through radiosensitisation to assist radiation therapy in obtaining local control. With the aim of improving distant disease control, the US Intergroup have recently completed a phase III RCT, which attempted to build on the results from INT0116 by incorporating a potentially more active chemotherapy regimen using ECF[55]. This study randomised 546 patients to post-operative CRT using the INT0116 regimen with 5-FU/LV vs post-operative CRT sandwiched between cycles of ECF (Figure 1). Preliminary results, which have only been reported in abstract form, suggest no difference in survival outcomes, although the toxicity profile favoured the ECF arm.
Figure 1.
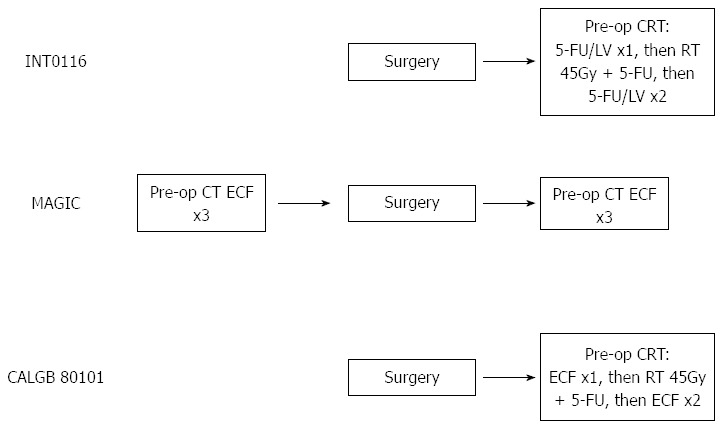
Experimental arms for INT0116, MAGIC and CALGB 80101 study. ECF: Epirubicin, cisplatin, 5-fluoro-uracil; LV: Leucovorin; FU/LV: Fluorouracil/leucovorin; CT: Chemotherapy; CRT: Chemo-radiotherapy.
FUTURE DIRECTIONS/ONGOING TRIALS
A number of ongoing RCT’s are examining various neo-adjuvant and adjuvant strategies.
In Asian countries, ongoing trials aim to define the optimum postoperative strategy. The SAMIT trial from Japan has recruited 1495 patients and will examine the addition of paclitaxel to fluoropyrimidine adjuvant CT following gastrectomy[56]. The addition of post-operative CRT to CT is being further evaluated in the ARTIST2 trial from South Korea. This trial aims to build on the first ARTIST trial by limiting eligibility to patients with node-positive tumours only, thus randomizing patients to adjuvant CT vs adjuvant CRT. It will incorporate S1 and oxaliplatin CT regimens in a 4-arm design with radiotherapy.
In Western countries, ongoing trials aim to build on the results of perioperative ECF as demonstrated in MAGIC, either by adding novel agents or radiotherapy. The MRC ST03 trial from the United Kingdom is a phase III RCT that will examine the addition of bevacizumab (a humanized monoclonal antibody against vascular endothelial growth factor) to perioperative ECF. The Dutch CRITICS trial is a phase III RCT of preoperative chemotherapy using epirubicin, cisplatin and capecitabine (ECC) followed by surgery and further ECC (i.e., MAGIC), or by surgery and CRT (Figure 2)[57].
Figure 2.
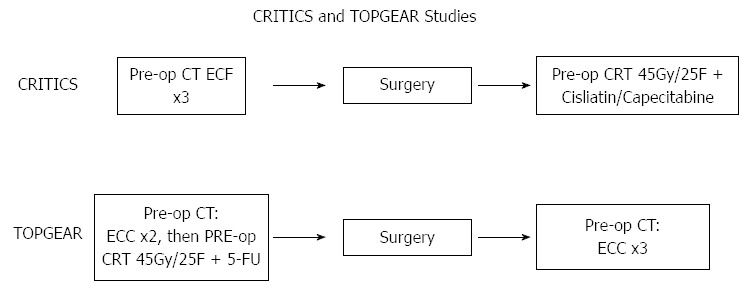
Experimental arms of CRITICS and TOPGEAR trials. ECC: Epirubicin, cisplatin and capecitabine; CRT: Chemo-radiotherapy; FU: Fluorouracil; ECF: Epirubicin, cisplatin, 5-FU; LV: Leucovorin; CT: Chemotherapy.
Preoperative radiotherapy
The benefit of using radiotherapy in the pre-operative setting has been conclusively demonstrated in a number of other cancer sites[58-60]. However, there are concerns when adopting a neoadjuvant approach, including potential delay before definitive resection, the possibility of disease progression, and peri- and post-operative morbidity. There are sound radiobiological and practical reasons to support a neoadjuvant RT approach, including a theoretical reduction in hypoxia and, therefore, radioresistance, potential for tumour down-staging and increased R0 resection rate, improved tolerability, as well as improved target and tumour delineation.
A previous RCT in 370 patients with tumours of the gastric cardia demonstrated a survival benefit for preoperative radiotherapy alone[61]. There has been increasing interest in incorporating concurrent radio-sensitising CT in this preoperative setting. Although no RCT’s have yet been reported, several prospective Phase II studies have reported promising results (Table 5), with > 70% of patients undergoing R0 resections, and complete pathological response rates of up to 30%[62-64]. An ongoing international RCT (TOPGEAR), is examining the addition of this neo-adjuvant CRT strategy to perioperative CT (Figure 2)[65].
Table 5.
Prospective phase 2 trials of preoperative chemo-radiotherapy
| Ref. | Year | No. of patients | Induction CT | CRT | Proceeded to surgery | R0 resection | pCR |
| Ajani et al[62] | 2004 | 34 | x2 FU/Cis/LV | 45Gy/FU | 85% | 70% | 30% |
| Ajani et al[63] | 2005 | 41 | x2 FU/Cis/Paclitaxel | 45Gy/FU/Paclitaxel | 98% | 78% | 20% |
| Ajani et al[64] | 2006 | 49 | x2 FU/Cis/LV | 45Gy/FU/Paclitaxel | 83% | 77% | 26% |
pCR: Pathological complete response; Cis: Cisplatin; CRT: Chemo-radiotherapy; FU: Fluorouracil; CT: Chemotherapy; LV: Leucovorin.
CONCLUSION
The treatment of locally advanced gastric cancer remains a challenge. Whilst there are promising approaches, incorporating novel targeted agents, as well as neo-adjuvant CRT strategies, current evidence suggests postoperative CRT and perioperative CT remain appropriate standard adjuvant treatments in the Western world. Large randomized trials have also established adjuvant CT alone, either with S-1 or Capecitabine/Oxaliplatin, as standards of care in Asian countries, where patients routinely undergo D2 gastrectomy.
The role of postoperative CRT continues to be debated, especially in the setting of D2 gastrectomy. However, based on the current evidence, post-operative chemoradiotherapy should be considered in high-risk gastric cancer patients who undergo less than a D2 dissection. The ongoing ARTIST II (in the Asian population) and CRITICS (in the Western population) trials will help to clarify the role of postoperative CRT in these settings.
Footnotes
P- Reviewer: Contini S, Greenberger JS, Marrelli D, Wu AW S- Editor: Qi Y L- Editor: Stewart GJ E- Editor: Ma S
References
- 1.Ferlay J, Shin H, Bray F, Forman D, Mathers C, Parkin D. GLOBOCAN 2008 v2.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet] Lyon: Int. Agency Res. Cancer; 2010. [Google Scholar]
- 2.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. American Joint Committee on Cancer Staging Manual. 7th ed. New York: Springer; 2010. [Google Scholar]
- 3.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 4.Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 5.Greene FL, Page D, Fleming I. Fritz A, Balch CM, Haller DG, Morrow M. American Joint Committee on Cancer Staging Manual. 6th ed. New York: Springer; 2002. [Google Scholar]
- 6.Sasako M. Principles of surgical treatment for curable gastric cancer. J Clin Oncol. 2003;21:274s–275s. doi: 10.1200/JCO.2003.09.172. [DOI] [PubMed] [Google Scholar]
- 7.Sasako M, Saka M, Fukagawa T, Katai H, Sano T. Modern surgery for gastric cancer--Japanese perspective. Scand J Surg. 2006;95:232–235. doi: 10.1177/145749690609500404. [DOI] [PubMed] [Google Scholar]
- 8.Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453–462. doi: 10.1056/NEJMoa0707035. [DOI] [PubMed] [Google Scholar]
- 9.Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med. 1985;312:1604–1608. doi: 10.1056/NEJM198506203122504. [DOI] [PubMed] [Google Scholar]
- 10.Shim JH, Song KY, Jeon HM, Park CH, Jacks LM, Gonen M, Shah MA, Brennan MF, Coit DG, Strong VE. Is gastric cancer different in Korea and the United States? Impact of tumor location on prognosis. Ann Surg Oncol. 2014;21:2332–2339. doi: 10.1245/s10434-014-3608-7. [DOI] [PubMed] [Google Scholar]
- 11.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 12.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 13.Wu CW, Hsiung CA, Lo SS, Hsieh MC, Shia LT, Whang-Peng J. Randomized clinical trial of morbidity after D1 and D3 surgery for gastric cancer. Br J Surg. 2004;91:283–287. doi: 10.1002/bjs.4433. [DOI] [PubMed] [Google Scholar]
- 14.Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, Sydes M, Fayers P. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999;79:1522–1530. doi: 10.1038/sj.bjc.6690243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dent DM, Madden MV, Price SK. Randomized comparison of R1 and R2 gastrectomy for gastric carcinoma. Br J Surg. 1988;75:110–112. doi: 10.1002/bjs.1800750206. [DOI] [PubMed] [Google Scholar]
- 16.Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439–449. doi: 10.1016/S1470-2045(10)70070-X. [DOI] [PubMed] [Google Scholar]
- 17.Degiuli M, Sasako M, Ponti A. Morbidity and mortality in the Italian Gastric Cancer Study Group randomized clinical trial of D1 versus D2 resection for gastric cancer. Br J Surg. 2010;97:643–649. doi: 10.1002/bjs.6936. [DOI] [PubMed] [Google Scholar]
- 18.Lustosa SA, Saconato H, Atallah AN, Lopes Filho Gde J, Matos D. Impact of extended lymphadenectomy on morbidity, mortality, recurrence and 5-year survival after gastrectomy for cancer. Meta-analysis of randomized clinical trials. Acta Cir Bras. 2008;23:520–530. doi: 10.1590/s0102-86502008000600009. [DOI] [PubMed] [Google Scholar]
- 19.Tanizawa Y, Terashima M. Lymph node dissection in the resection of gastric cancer: review of existing evidence. Gastric Cancer. 2010;13:137–148. doi: 10.1007/s10120-010-0560-5. [DOI] [PubMed] [Google Scholar]
- 20.McCulloch P, Nita ME, Kazi H, Gama-Rodrigues J. Extended versus limited lymph nodes dissection technique for adenocarcinoma of the stomach. Cochrane Database Syst Rev. 2003;(4):CD001964. doi: 10.1002/14651858.CD001964. [DOI] [PubMed] [Google Scholar]
- 21.Seevaratnam R, Bocicariu A, Cardoso R, Mahar A, Kiss A, Helyer L, Law C, Coburn N. A meta-analysis of D1 versus D2 lymph node dissection. Gastric Cancer. 2012;15 Suppl 1:S60–S69. doi: 10.1007/s10120-011-0110-9. [DOI] [PubMed] [Google Scholar]
- 22.Wisbeck WM, Becher EM, Russell AH. Adenocarcinoma of the stomach: autopsy observations with therapeutic implications for the radiation oncologist. Radiother Oncol. 1986;7:13–18. doi: 10.1016/s0167-8140(86)80120-7. [DOI] [PubMed] [Google Scholar]
- 23.Gunderson LL, Sosin H. Adenocarcinoma of the stomach: areas of failure in a re-operation series (second or symptomatic look) clinicopathologic correlation and implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 1982;8:1–11. doi: 10.1016/0360-3016(82)90377-7. [DOI] [PubMed] [Google Scholar]
- 24.Gunderson LL. Gastric cancer--patterns of relapse after surgical resection. Semin Radiat Oncol. 2002;12:150–161. doi: 10.1053/srao.2002.30817. [DOI] [PubMed] [Google Scholar]
- 25.Skoropad VY, Berdov BA, Mardynski YS, Titova LN. A prospective, randomized trial of pre-operative and intraoperative radiotherapy versus surgery alone in resectable gastric cancer. Eur J Surg Oncol. 2000;26:773–779. doi: 10.1053/ejso.2000.1002. [DOI] [PubMed] [Google Scholar]
- 26.Allum WH, Hallissey MT, Ward LC, Hockey MS. A controlled, prospective, randomised trial of adjuvant chemotherapy or radiotherapy in resectable gastric cancer: interim report. British Stomach Cancer Group. Br J Cancer. 1989;60:739–744. doi: 10.1038/bjc.1989.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dent DM, Werner ID, Novis B, Cheverton P, Brice P. Prospective randomized trial of combined oncological therapy for gastric carcinoma. Cancer. 1979;44:385–391. doi: 10.1002/1097-0142(197908)44:2<385::aid-cncr2820440203>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 28.Moertel CG, Childs DS, O’Fallon JR, Holbrook MA, Schutt AJ, Reitemeier RJ. Combined 5-fluorouracil and radiation therapy as a surgical adjuvant for poor prognosis gastric carcinoma. J Clin Oncol. 1984;2:1249–1254. doi: 10.1200/JCO.1984.2.11.1249. [DOI] [PubMed] [Google Scholar]
- 29.Smalley SR, Benedetti JK, Haller DG, Hundahl SA, Estes NC, Ajani JA, Gunderson LL, Goldman B, Martenson JA, Jessup JM, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012;30:2327–2333. doi: 10.1200/JCO.2011.36.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ajani JA, Bentrem DJ, Besh S, D’Amico TA, Das P, Denlinger C, Fakih MG, Fuchs CS, Gerdes H, Glasgow RE, et al. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11:531–546. doi: 10.6004/jnccn.2013.0070. [DOI] [PubMed] [Google Scholar]
- 31.Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D. Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Eur J Surg Oncol. 2014;40:584–591. doi: 10.1016/j.ejso.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 32.Knight G, Earle CC, Cosby R, Coburn N, Youssef Y, Malthaner R, Wong RK. Neoadjuvant or adjuvant therapy for resectable gastric cancer: a systematic review and practice guideline for North America. Gastric Cancer. 2013;16:28–40. doi: 10.1007/s10120-012-0148-3. [DOI] [PubMed] [Google Scholar]
- 33.Ohri N, Garg MK, Aparo S, Kaubisch A, Tome W, Kennedy TJ, Kalnicki S, Guha C. Who benefits from adjuvant radiation therapy for gastric cancer? A meta-analysis. Int J Radiat Oncol Biol Phys. 2013;86:330–335. doi: 10.1016/j.ijrobp.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fiorica F, Cartei F, Enea M, Licata A, Cabibbo G, Carau B, Liboni A, Ursino S, Cammà C. The impact of radiotherapy on survival in resectable gastric carcinoma: a meta-analysis of literature data. Cancer Treat Rev. 2007;33:729–740. doi: 10.1016/j.ctrv.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Valentini V, Cellini F, Minsky BD, Mattiucci GC, Balducci M, D’Agostino G, D’Angelo E, Dinapoli N, Nicolotti N, Valentini C, et al. Survival after radiotherapy in gastric cancer: systematic review and meta-analysis. Radiother Oncol. 2009;92:176–183. doi: 10.1016/j.radonc.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Guo L, Wang X, Ma B, Yang K, Zhang Q, Ye X, Luo H, Liu R. Radiotherapy combined with surgical treatment for gastric cancer: a meta-analysis. Chinese-German J Clin Oncol. 2011;10:442–429. [Google Scholar]
- 37.Lee J, Lim do H, Kim S, Park SH, Park JO, Park YS, Lim HY, Choi MG, Sohn TS, Noh JH, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol. 2012;30:268–273. doi: 10.1200/JCO.2011.39.1953. [DOI] [PubMed] [Google Scholar]
- 38.Zhu WG, Xua DF, Pu J, Zong CD, Li T, Tao GZ, Ji FZ, Zhou XL, Han JH, Wang CS, et al. A randomized, controlled, multicenter study comparing intensity-modulated radiotherapy plus concurrent chemotherapy with chemotherapy alone in gastric cancer patients with D2 resection. Radiother Oncol. 2012;104:361–366. doi: 10.1016/j.radonc.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 39.Kim TH, Park SR, Ryu KW, Kim YW, Bae JM, Lee JH, Choi IJ, Kim YJ, Kim DY. Phase 3 trial of postoperative chemotherapy alone versus chemoradiation therapy in stage III-IV gastric cancer treated with R0 gastrectomy and D2 lymph node dissection. Int J Radiat Oncol Biol Phys. 2012;84:e585–e592. doi: 10.1016/j.ijrobp.2012.07.2378. [DOI] [PubMed] [Google Scholar]
- 40.Bamias A, Karina M, Papakostas P, Kostopoulos I, Bobos M, Vourli G, Samantas E, Christodoulou Ch, Pentheroudakis G, Pectasides D, et al. A randomized phase III study of adjuvant platinum/docetaxel chemotherapy with or without radiation therapy in patients with gastric cancer. Cancer Chemother Pharmacol. 2010;65:1009–1021. doi: 10.1007/s00280-010-1256-6. [DOI] [PubMed] [Google Scholar]
- 41.Kwon HC, Kim MC, Kim KH, Jang JS, Oh SY, Kim SH, Kwon KA, Lee S, Lee HS, Kim HJ. Adjuvant chemoradiation versus chemotherapy in completely resected advanced gastric cancer with D2 nodal dissection. Asia Pac J Clin Oncol. 2010;6:278–285. doi: 10.1111/j.1743-7563.2010.01331.x. [DOI] [PubMed] [Google Scholar]
- 42.Yu C, Yu R, Zhu W, Song Y, Li T. Intensity-modulated radiotherapy combined with chemotherapy for the treatment of gastric cancer patients after standard D1/D2 surgery. J Cancer Res Clin Oncol. 2012;138:255–259. doi: 10.1007/s00432-011-1085-y. [DOI] [PubMed] [Google Scholar]
- 43.Shridhar R, Dombi GW, Weber J, Hoffe SE, Meredith K, Konski A. Adjuvant radiation therapy increases overall survival in node-positive gastric cancer patients with aggressive surgical resection and lymph node dissection: a SEER database analysis. Am J Clin Oncol. 2012;35:216–221. doi: 10.1097/COC.0b013e31820dbf08. [DOI] [PubMed] [Google Scholar]
- 44.Hermans J, Bonenkamp JJ, Boon MC, Bunt AM, Ohyama S, Sasako M, Van de Velde CJ. Adjuvant therapy after curative resection for gastric cancer: meta-analysis of randomized trials. J Clin Oncol. 1993;11:1441–1447. doi: 10.1200/JCO.1993.11.8.1441. [DOI] [PubMed] [Google Scholar]
- 45.Earle CC, Maroun JA. Adjuvant chemotherapy after curative resection for gastric cancer in non-Asian patients: revisiting a meta-analysis of randomised trials. Eur J Cancer. 1999;35:1059–1064. doi: 10.1016/s0959-8049(99)00076-3. [DOI] [PubMed] [Google Scholar]
- 46.Mari E, Floriani I, Tinazzi A, Buda A, Belfiglio M, Valentini M, Cascinu S, Barni S, Labianca R, Torri V. Efficacy of adjuvant chemotherapy after curative resection for gastric cancer: a meta-analysis of published randomised trials. A study of the GISCAD (Gruppo Italiano per lo Studio dei Carcinomi dell’Apparato Digerente) Ann Oncol. 2000;11:837–843. doi: 10.1023/a:1008377101672. [DOI] [PubMed] [Google Scholar]
- 47.Janunger KG, Hafström L, Nygren P, Glimelius B. A systematic overview of chemotherapy effects in gastric cancer. Acta Oncol. 2001;40:309–326. doi: 10.1080/02841860151116385. [DOI] [PubMed] [Google Scholar]
- 48.Sun P, Xiang JB, Chen ZY. Meta-analysis of adjuvant chemotherapy after radical surgery for advanced gastric cancer. Br J Surg. 2009;96:26–33. doi: 10.1002/bjs.6408. [DOI] [PubMed] [Google Scholar]
- 49.Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, Rougier P, Sakamoto J, Sargent D, Sasako M, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303:1729–1737. doi: 10.1001/jama.2010.534. [DOI] [PubMed] [Google Scholar]
- 50.Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–1820. doi: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 51.Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387–4393. doi: 10.1200/JCO.2011.36.5908. [DOI] [PubMed] [Google Scholar]
- 52.Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–321. doi: 10.1016/S0140-6736(11)61873-4. [DOI] [PubMed] [Google Scholar]
- 53.Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715–1721. doi: 10.1200/JCO.2010.33.0597. [DOI] [PubMed] [Google Scholar]
- 54.Schuhmacher C, Gretschel S, Lordick F, Reichardt P, Hohenberger W, Eisenberger CF, Haag C, Mauer ME, Hasan B, Welch J, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010;28:5210–5218. doi: 10.1200/JCO.2009.26.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fuchs CS, Tepper JE, Niedzwiecki D, Hollis D, Mamon HJ, Swanson R, Haller DG, Dragovich T, Alberts SR, Bjarnason GA, et al. Postoperative adjuvant chemoradiation for gastric or gastroesophageal junction (GEJ) adenocarcinoma using epirubicin, cisplatin, and infusional (CI) 5-FU (ECF) before and after CI 5-FU and radiotherapy (CRT) compared with bolus 5-FU/LV before and after CR. J Clin Oncol. 2011;29:4003. [Google Scholar]
- 56.Tsuburaya A, Yoshida K, Kobayashi M, Yoshino S, Miyashita Y, Morita S, Oba K, Buyse ME, Macdonald JS, Sakamoto J. SAMIT: Preliminary safety data from a 2x2 factorial randomized phase III trial to investigate weekly paclitaxel (PTX) followed by oral fluoropyrimidines (FPs) versus FPs alone as adjuvant chemotherapy in patients (pts) with gastric cancer. J Clin Oncol. 2011;29(Suppl):4017. [Google Scholar]
- 57.Dikken JL, van Sandick JW, Maurits Swellengrebel HA, Lind PA, Putter H, Jansen EP, Boot H, van Grieken NC, van de Velde CJ, Verheij M, et al. Neo-adjuvant chemotherapy followed by surgery and chemotherapy or by surgery and chemoradiotherapy for patients with resectable gastric cancer (CRITICS) BMC Cancer. 2011;11:329. doi: 10.1186/1471-2407-11-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O'Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, Wunder J, Kandel R, Goddard K, Sadura A, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359:2235–2241. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 59.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 60.Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, Quirke P, Couture J, de Metz C, Myint AS, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811–820. doi: 10.1016/S0140-6736(09)60484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang ZX, Gu XZ, Yin WB, Huang GJ, Zhang DW, Zhang RG. Randomized clinical trial on the combination of preoperative irradiation and surgery in the treatment of adenocarcinoma of gastric cardia (AGC)--report on 370 patients. Int J Radiat Oncol Biol Phys. 1998;42:929–934. doi: 10.1016/s0360-3016(98)00280-6. [DOI] [PubMed] [Google Scholar]
- 62.Ajani JA, Mansfield PF, Janjan N, Morris J, Pisters PW, Lynch PM, Feig B, Myerson R, Nivers R, Cohen DS, et al. Multi-institutional trial of preoperative chemoradiotherapy in patients with potentially resectable gastric carcinoma. J Clin Oncol. 2004;22:2774–2780. doi: 10.1200/JCO.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 63.Ajani JA, Mansfield PF, Crane CH, Wu TT, Lunagomez S, Lynch PM, Janjan N, Feig B, Faust J, Yao JC, et al. Paclitaxel-based chemoradiotherapy in localized gastric carcinoma: degree of pathologic response and not clinical parameters dictated patient outcome. J Clin Oncol. 2005;23:1237–1244. doi: 10.1200/JCO.2005.01.305. [DOI] [PubMed] [Google Scholar]
- 64.Ajani JA, Winter K, Okawara GS, Donohue JH, Pisters PW, Crane CH, Greskovich JF, Anne PR, Bradley JD, Willett C, et al. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol. 2006;24:3953–3958. doi: 10.1200/JCO.2006.06.4840. [DOI] [PubMed] [Google Scholar]
- 65.Leong T, Smithers M, Michael M, Gebski V, Boussioutas A, Miller D, Zalcberg JR, Wong R, Haustermans K. TOPGEAR: An international randomized phase III trial of preoperative chemoradiotherapy versus preoperative chemotherapy for resectable gastric cancer (AGITG/TROG/EORTC/NCIC CTG) J Clin Oncol. 2012;30:TPS4141. [Google Scholar]


