Abstract
AIM: To describe the clinical characteristics, technical procedures, and outcomes of patients undergoing laparoscopic spleen-preserving distal pancreatectomy (LSPDP) for benign and malignant pancreatic neoplasms.
METHODS: The clinical data of 38 patients who underwent LSPDP in the Sir Run Run Shaw Hospital between January 2003 and August 2013 were analyzed retrospectively. Surgical techniques for LSPDP included preservation of the splenic artery and vein (Kimura’s technique) and ligation of the splenic pedicle with preservation of the short gastric vessels (Warshaw’s technique).
RESULTS: There were no conversions to open surgery in the 38 patients. Splenic vessels were conserved during spleen-preserving pancreatectomy, except in two patients who underwent resection of the splenic vessels and preservation only of the short gastric vessels. The mean operation time was 123.2 ± 52.4 min, the mean intraoperative blood loss was 78.2 ± 39.5 mL, and the mean postoperative hospital stay was 7.6 ± 2.9 d. The overall rate of postoperative complications was 18.4% (7/38), and the rate of clinical pancreatic fistula was 13.2% (5/38). All postoperative complications were treated conservatively. The postoperative pathological diagnoses were 22 cases of benign pancreatic disease and 16 cases of borderline or low-grade malignant lesions. During a median follow-up of 38 mo (range: 5-133 mo), no recurrence was observed.
CONCLUSION: LSPDP is a safe, feasible and effective procedure for the treatment of benign and low-grade malignant tumors of the distal pancreas.
Keywords: Laparoscopic surgery, Splenic preservation, Distal pancreatectomy, Pancreatic tumor, Pancreas
Core tip: Laparoscopic spleen-preserving distal pancreatectomy (LSPDP), a function-preserving minimally invasive pancreatectomy, is an ideal procedure for treating benign and low-grade malignant tumors in the distal pancreas. We report a consecutive series of 38 patients who underwent LSPDP. There were no conversions to open surgery and rate of splenic vessel preservation was high. LSPDP was found to be a safe, feasible and effective procedure for the treatment of benign and low-grade malignant tumors of the distal pancreas.
INTRODUCTION
With recent advances in laparoscopic instruments and techniques, laparoscopic distal pancreatectomy (LDP) has become a widely accepted surgical technique for benign and low-grade tumors of the pancreas[1-4]. The spleen is traditionally removed when performing distal pancreatectomy, simply because of its anatomical intimacy to the distal pancreas and for the sake of technical simplicity. However, growing interest in the immunological role of the spleen, along with a tendency towards healthy organ preservation whenever possible, have led surgeons to avoid splenectomy during pancreatectomy for benign and low-grade malignant tumors. Unlike those with pancreatic cancer, these patients are expected to survive for a long time; therefore, their quality of life should be considered when choosing surgical techniques. Function-preserving minimally-invasive pancreatectomy may be the ideal surgical technique in these patients, and laparoscopic spleen-preserving laparoscopic distal pancreatectomy (LSPDP) has been recommended for the treatment of benign and low-grade malignant tumors in the distal pancreas[5-8].
We aimed to determine the outcome of LSPDP in patients with pancreatic tumors. On the basis of our extensive laparoscopic experience gained from LDP, laparoscopic gastrectomy and other laparoscopic procedures[9-13], we developed LSPDP for the treatment of these neoplasms. The purpose of this study was to outline our institution’s experience, which consisted of 38 patients who underwent LSPDP performed by the same surgical team.
MATERIALS AND METHODS
Patients
This was a retrospective review of a pancreatic surgery database and was approved by institutional review board. All patients undergoing attempted LSPDP were identified. Thirty-eight patients who underwent LSPDP in the Sir Run Run Shaw Hospital between January 2003 and August 2013, and who gave informed consent for surgical management, were selected. Medical records were reviewed retrospectively, and perioperative clinicopathological variables, such as gender, age, body mass index (BMI), symptoms, presence of preoperative diabetes mellitus, preoperative physical classification defined by the American Society of Anesthesiologists (ASA) score, pathological diagnosis, tumor size, surgical records, and postoperative morbidity and mortality were evaluated. The severity of complications was determined based on the grading system defined by Clavien et al[14]. Pancreatic leakage was defined according to the guidelines of the International Study Group on Pancreatic Fistulas (ISGPF)[15].
Surgical procedure
Surgical techniques for LSPDP included preservation of the splenic artery and vein (Kimura’s technique)[16] and ligation of the splenic pedicle with preservation of the short gastric vessels (Warshaw’s technique)[17].
Kimura’s technique
The patient was placed in the supine position on the surgical table and then shifted into the reverse Trendelenburg position with the left side up. We used five trocars. The first one was 10 mm and was inserted into the umbilicus for location of a 30° telescope, one 12-mm and one 5-mm trocar in the right upper quadrant for the surgeon, and two 5-mm trocars in the left upper quadrant for the assistant. Port placement is depicted in Figure 1. The surgical procedure included: (1) exploration: we explored the abdominal cavity to exclude metastasis in the abdominal organs, such as the liver surface or the peritoneum. Using an ultrasonic knife, the gastrocolic and gastrosplenic ligaments were dissected, revealing the pancreatic lesion, its size and adjacent tissue. If necessary, intraoperative laparoscopic ultrasound was used to assist the positioning of the lesion; (2) dissection of the splenic artery: the upper border of the pancreas was separated to expose the splenic artery on the superior edge of the pancreas. The artery was dissected from the pancreatic border and was tied with a rubber band (Figure 1B); (3) dissection of the splenic vein: the lower pancreatic border was freed from the transverse colon by blunt dissection, thus revealing the superior mesenteric vein, splenic vein and portal vein (Figure 1C); (4) dividing the pancreas: using the endoscopic stapler (ENDO-GIA) or ultrasonic knife, the pancreas was cut at the level of the neck of the pancreas (Figure 1D); (5) resecting the pancreatic body and tail: the distal pancreas was lifted gently and the loose tissue between the pancreas and splenic vessels was separated using the ultrasound knife, thus freeing the splenic artery and vein from the pancreatic parenchyma. Small blood vessel branches were occluded using the ultrasonic scalpel directly or using titanium clips (Figure 1E); and (6) removing the specimen: all bleeding points were stopped; the umbilical incision was extended by approximately 1.2-3 cm and the specimen was removed. A rubber drainage tube was placed at the remnant pancreas (Figure 1F).
Figure 1.
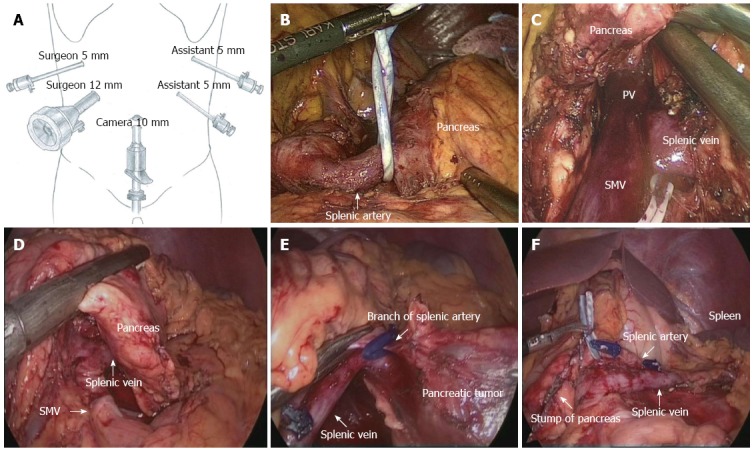
Surgical procedure of laparoscopic spleen-preserving distal pancreatectomy (Kimura’s technique). A: Trocar placement for LSPDP; B: The splenic artery was temporarily tied with a rubber band at its root in LSPDP; C: Mobilizing the inferior border of the pancreas and revealing the splenic vein; D: Transection of the pancreas with an endoscopic stapler; E: Branches of the splenic artery underwent ligation using a harmonic scalpel; F: Check the proximal pancreatic stump and splenic vessels after LSPDP. LSPDP: Laparoscopic spleen-preserving distal pancreatectomy; PV: Portal vein; SMV: Superior mesenteric vein.
Warshaw’s technique
The patient’s position and port placement were as in Kimura’s technique. Up to the division of the retroperitoneum along the inferior margin on the pancreas, the procedure was performed in the same way as Kimura’s technique. After the upper border of the pancreas was separated, the splenic artery was dissected and sectioned between clips. The splenic vein was sectioned after the lower pancreatic border was freed, or the pancreatic parenchyma and the splenic vein were both cut with an endoscopic stapler (Figure 2A). Finally, the distal side of the splenic vein and artery were sectioned between clips or by an endoscopic stapler (Figure 2B).
Figure 2.
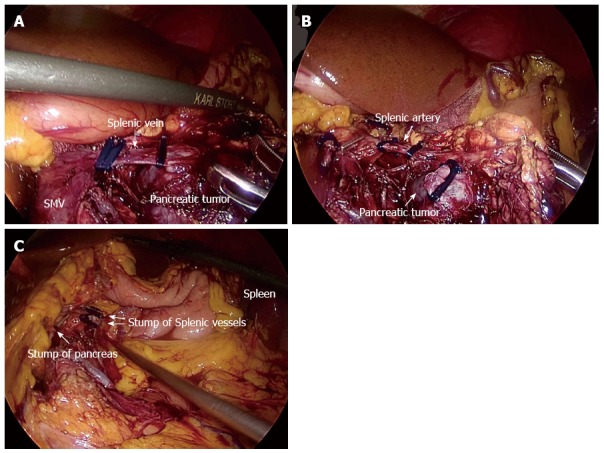
Surgical procedure of laparoscopic spleen-preserving distal pancreatectomy (Warshaw’s technique). A: Dissect the splenic vein at its root, which was surrounded by the pancreatic mass; B: Dissect the splenic artery; C: Check the proximal pancreatic stump and spleen after LSPDP with ligation of splenic vessels. LSPDP: Laparoscopic spleen-preserving distal pancreatectomy; SMV: Superior mesenteric vein.
Statistical analysis
Quantitative data are presented as the mean ± SD. All statistical analyses were performed using SPSS software, version 18.0 (SPSS Inc, Chicago, United States)
RESULTS
Patient characteristics
Thirty-eight patients (29 female and 9 male), with a mean age of 53.2 ± 13.6 years (range: 31-79 years) successfully underwent LSPDP for a benign or borderline malignant tumor of the distal pancreas during the study period. Their mean BMI was 24.4 ± 2.7 kg/m2 (range: 20.8-32.1 kg/m2). Less than one-third (12/38; 31.6%) of the patients had comorbidities, the most common being diabetes mellitus. Table 1 shows the patient characteristics. The mean neoplasm size was 4.5 cm (range: 1-8.5 cm) and the neoplasms were located in the body (14 cases, 36.8%) and tail (24 cases, 63.2%) of the pancreas. The pathology of the resected pancreas specimens and the tumor sizes are listed in Table 2.
Table 1.
Patient demographics n (%)
| Variable | Data |
| No. of patients | 38 |
| Gender (Male/Female) | 9 (23.7)/29 (76.3) |
| Mean age (yr) | 53.2 ± 13.6 |
| ASA classification (I/II) | 26 (68.4)/12 (31.6) |
| BMI (kg/m2) | 24.4 ± 2.7 |
| Comorbidities | 12 (31.6) |
| Diabetes mellitus | 6 (15.7) |
| Hypertension | 3 (7.9) |
| Cardiovascular | 2 (5.3) |
| Pulmonary | 2 (5.3) |
| Liver | 1 (2.6) |
| Others | 1 (2.6) |
ASA: American Society of Anesthesiologists classification; BMI: Body mass index.
Table 2.
Histological analysis
| Histological diagnosis | n | Mean size (cm) |
| Pancreatic retention cyst | 2 | 5.0 |
| Pancreatic epithelial cyst | 5 | 5.2 |
| Congenital cyst of pancreas | 1 | 4.8 |
| Insulinoma | 1 | 3.8 |
| Serous cystadenoma | 13 | 3.9 |
| Mucinous cystadenoma | 10 | 3.1 |
| Pancreatic solid-pseudopapillary tumors | 4 | 3.0 |
| Well-differentiated neuroendocrine tumor | 1 | 2.1 |
| Intraductal papillary mucinous tumor | 1 | 2.3 |
| Total | 38 | 4.5 |
Intraoperative outcomes
None of the 38 patients underwent conversion to open procedures. Thirty-six patients underwent Kimura’s technique and two patients underwent Warshaw’s technique because of the close proximity of the lesion to the vessels. This series included seven patients who underwent concomitant surgery as follows: four cases underwent cholecystectomy, one underwent case resection of a right adrenal tumor, one case underwent myomectomy and left ovarian teratoma resection, and one case underwent resection of the left lateral liver lobe and choledocholithotomy. The mean operation time was 123.2 ± 52.4 min (range: 70-320 min), the mean intraoperative blood loss was 78.2 ± 39.5 mL (range: 50-300 mL), and only one patient needed a transfusion. The intraoperative outcomes are shown in Table 3.
Table 3.
Intra-operative features of patients undergoing laparoscopic spleen-preserving distal pancreatectomy n (%)
| LSPDP (n = 38) | |
| Surgical technique | |
| Splenic vessels preservation (Kimura’s method) | 36 (94.7) |
| Splenic vessels resection (Warshaw’s method) | 2 (5.3) |
| Operative time (min) | 123.2 ± 52.4 |
| Estimated blood loss (mL) | 78.2 ± 39.5 |
| Transfusion (cases) | 1 (2.6) |
LSPDP: Laparoscopic spleen-preserving distal pancreatectomy.
Postoperative outcomes
The mean time to resuming daily activities after surgery was 1.5 ± 0.6 d (range: 1-5 d); the mean time to first flatus was 2.2 ± 1.0 d (range: 1-4 d); the mean time to starting liquid and soft diets was 2.8 ± 0.9 d (range: 1-4 d) and 4.0 ± 1.2 d (range: 3-8 d); and the mean postoperative hospital stay was 7.6 ± 2.9 d (range: 5-19 d). The overall rate of postoperative complications was 18.4% (7/38), and the rate of clinical pancreatic fistula was 13.2% (5/38). All postoperative complications (three grade A and two grade B postoperative pancreatic fistula; one pulmonary infection; one intra-abdominal abscess) were treated conservatively. The postoperative outcomes are listed in Table 4. During a median follow-up of 38 mo (range: 5-133 mo), no recurrence was observed.
Table 4.
Post-operative features of patients undergoing laparoscopic spleen-preserving distal pancreatectomy n (%)
| LSPDP (n = 38) | |
| Total complications | 7 (18.4) |
| Grade I | 5 (13.2) |
| Grade II | 2 (5.3) |
| Grade IIIa/IIIb | 0 (0)/0 (0) |
| Details of complications | |
| Pancreatic fistula | 5 (13.2) |
| Grade A | 3 (7.9) |
| Grade B | 2 (5.3) |
| Grade C | 0 (0.0) |
| Pneumonia | 1 (2.6) |
| Intra-abdominal abscess | 1 (2.6) |
| Time to activities (d) | 1.5 ± 0.6 |
| Time to first flatus (d) | 2.2 ± 1.0 |
| Time to starting liquid (d) | 2.8 ± 0.9 |
| Time to starting soft diets (d) | 4.0 ± 1.2 |
| Postoperative hospital stay (d) | 7.6 ± 2.9 |
| 30-d mortality | 0 (0.0) |
LSPDP: Laparoscopic spleen-preserving distal pancreatectomy.
DISCUSSION
In 1994, Soper et al[18] first performed laparoscopic distal pancreatectomy in a pig model to document its safety and feasibility, and since then the use of this technique has been reported in large series and comparative studies. The body and tail of the pancreas, and the spleen, are generally regarded as one anatomical unit, as these parts of the pancreas are closely associated with the spleen. In addition, the splenic artery and vein have many branches in the pancreatic parenchyma. In the past, surgeons preferred to remove the spleen simultaneously as the intimate relationship between the splenic vessels and the pancreas made separation difficult. However, splenectomy combined with other major abdominal organ resection was found to be associated with increased postoperative morbidity, especially the complications of infection[19]. In addition, there is concern about the increased risk of subsequent hematologic complications, myocardial infarction, and even cancer in patients with elective splenectomy in later years[20,21]. With regard to the many adverse consequences reported after splenectomy, patients with benign and low-grade malignant tumors are expected to have long-term survival, thus their quality of life needs to be fully considered. Therefore, spleen preservation is desirable.
Carrère et al[22] compared the results of 38 patients who underwent open spleen-preserving distal pancreatectomy with a matched cohort of patients undergoing open distal pancreatectomy with splenectomy, and showed that the conservative group had less postoperative morbidity. Shoup et al[23] demonstrated that distal pancreatectomy with spleen preservation was associated with a reduction in perioperative infectious complications, severe complications, and the length of hospital stay, suggesting the value of spleen preservation in distal pancreatectomy.
At our hospital, we have performed laparoscopic distal pancreatectomy since 2003[9]. When the tumor is distant from the splenic artery and vein, and is either benign or low-grade malignant, LSPDP is recommended. This retrospective study shows a conversion rate of 0% and a high percentage (94.7%) of splenic vessel preservation; mean operative time of 123.2 min and mean operative blood loss of 78.2 mL; low overall rate of postoperative complications (18.4%), and low rate of clinical pancreatic fistula (13.2%). These findings are consistent with the best case series published to date[24-26], and demonstrated that LSPDP is a feasible, safe and efficient approach for benign or low-grade malignant pancreatic neoplasms.
Surgical techniques in LSPDP include preservation of the splenic artery and vein, as well as ligation of the splenic pedicle with preservation of the short gastric vessels. The use of LSPDP has been reported from several institutes in a relatively large number of patients. However, there are relatively few reported studies of laparoscopic vessel-preserving SPDP. The highlight of this study is the high rate of splenic vessel preservation (94.7%). Whether one approach is superior to another is still a matter of debate. Although the perioperative and functional results of spleen-preserving distal pancreatectomy with splenic vessel resection seem acceptable in the short-term, concern has been raised regarding potential long-term complications, including the high incidence of left-sided portal hypertension and perigastric varices during follow-up, with a theoretical risk of gastrointestinal bleeding. Fernández-Cruz et al[5] compared the outcomes of laparoscopic spleen-preserving distal pancreatectomy with either splenic vessels preservation or resection. Splenic vessels resection was faster and associated with reduced blood loss. Miura et al[21] analyzed the long-term hemodynamic changes in the splenogastric circulation retrospectively in 10 patients after open spleen-preserving pancreatectomy with excision of splenic vessels (with a minimum follow-up of 52 mo). The incidence of perigastric and submucosal varices was 70% and 20%, respectively, and one patient experienced gastrointestinal bleeding from gastric varices 6.5 years after middle segment pancreatectomy. On the other hand, a recent study by Yoon et al[27] evaluated the short- and long-term patency of the splenic vessel in 22 patients after LSPDP with splenic vessel preservation. Vascular obliteration in the preserved artery and vein was found in 6 (27.3%) and 17 patients (77.3%), respectively, within 1 mo of surgery, and in 3 (13.6%) and 13 patients (59.1%) 6 mo or more after surgery. Nine (90%) of ten patients with complete splenic vein occlusion developed a collateral circulation during the late postoperative phase.
In our study, some patients received computed tomography (CT) scanning during the follow-up period to evaluate the patency of the splenic vessel, while the remaining patients only received B ultrasound examination because of economic reasons. This, and the relatively short follow-up period, meant that we did not observe patients with splenic vessel occlusion after preservation of the splenic vessels. We think that splenic vessels should be preserved as far as possible during spleen-preserving pancreatectomy. For LSPDP, the key point and difficulty lie in the handling of splenic vessels and special attention should be given to the followings: (1) Gentle manipulation. The lack of direct touch and enlarged view of tissues on laparoscopy might lead to a wrong impression for the need of more strength when manipulating the vessels, which in turn leads to vascular rupture due to excessive traction. Therefore, gentle actions are needed and when required, small gauze should be used to gently move the blood vessels; (2) Pre-exposure of vessels. Pre-exposed large blood vessels will help to quickly control bleeding during vascular rupture; (3) Bleeding. For the splenic artery and vein bleeding, when the bleeding point is clear, bleeding is first controlled with a clamp. After suction, temporary occlusion is performed with titanium clips, and then the bleeding points are sutured with 5-0 Prolene under direct vision and the titanium clips are then removed. If the bleeding point is not clear, gauze can be applied for small blood vessel bleeding, and after removal of the specimen, the bleeding point can be detected; in the setting of massive bleeding without a clear bleeding point or severe vascular rupture, timely conversion to laparotomy is mandatory; and (4) Surgical team. Highly precise laparoscopic surgery requires an understanding between the main surgeon and the assistant such that in the event of bleeding, adept, timely and accurate exposure of the bleeding point can help control the bleeding within the shortest possible time.
Pancreatic fistula, the most common complication after distal pancreatectomy, is still a major challenge in laparoscopic pancreatic surgery, as well as in LSPDP[28,29]. Encompassing all grades of fistula, we observed a fistula rate of 13.2% in our study, which is comparable to that reported by others. In our experience, the best technique to cut the pancreas is to use the linear stapler: an appropriate ENDO-GIA is selected according to the size and thickness of the pancreas. Usually, a 3.5 mm staple is used. For thickening pancreas and chronic pancreatitis, a 3.8 mm staple is selected.
In conclusion, this study demonstrated the detailed procedure for LSPDP used in our department. It is worth attempting LSPDP in patients with a presumed benign or low-grade malignant tumor of the pancreatic body and tail, and preserving both splenic vessels. This was a retrospective study based on a relatively small population. The surgical approach for spleen preservation or splenic vessel preservation was not chosen on an intention-to-treat basis. Therefore, prospective comparative studies are warranted to better elucidate the short- and long-term outcomes of LSPDP with or without splenic vessel resection.
COMMENTS
Background
Laparoscopic distal pancreatectomy has become a widely accepted surgical technique for benign and low-grade tumors of the pancreas. The spleen is traditionally removed when performing distal pancreatectomy, mainly because of its anatomical intimacy to the distal pancreas and for the sake of technical simplicity. However, growing interest in the immunological role of the spleen, along with a tendency towards healthy organ preservation whenever possible, have led surgeons to avoid splenectomy during pancreatectomy for benign and low-grade malignant tumors. Unlike those with pancreatic cancer, these patients are expected to survive for a long time, thus, their quality of life should be considered when choosing surgical techniques. Function-preserving minimally-invasive pancreatectomy is an ideal surgical technique for these patients. Therefore, laparosopic spleen-preserving distal pancreatectomy (LSPDP) should be recommended for the treatment of benign and low-grade malignant tumors in the distal pancreas.
Research frontiers
LSPDP is a desirable treatment for benign and low-grade malignant tumors in the distal pancreas. LSPDP includes two techniques: preservation of the splenic artery and vein (Kimura’s technique) and ligation of the splenic pedicle with preservation of the short gastric vessels (Warshaw’s technique). A research hotspot is how to modify the surgical procedure of LSPDP and compare the outcomes between the two techniques. Whether one approach is superior to another is still a matter of debate.
Innovations and breakthroughs
Surgical techniques for LSPDP include Kimura’s technique and Warshaw’s technique). The use of LSPDP has been reported from several institutes in a relatively large number of patients. However, there are relatively few studies of laparoscopic vessel-preserving SPDP. The highlight of this study is the high rate of splenic vessel preservation (94.7%). Although the perioperative and functional results of spleen-preserving distal pancreatectomy with splenic vessel resection seem acceptable in the short-term, concern has been raised regarding potential long-term complications, including the high incidence of left-sided portal hypertension and perigastric varices, with a theoretical risk of gastrointestinal bleeding. The authors suggested that splenic vessels should be preserved as far as possible during spleen-preserving pancreatectomy.
Applications
LSPDP is a safe, feasible and effective procedure for the treatment of benign and low-grade malignant tumors of the distal pancreas.
Peer review
This manuscript describes a complex laparoscopic procedure. This is a good descriptive study in which the authors outline one institution’s experience with 38 LSPDPs performed by the same surgical team. The results are interesting and suggest that LSPDP is a safe, feasible and effective procedure for the treatment of benign and low-grade malignant tumors of the distal pancreas.
Footnotes
Supported by Grants from Department of Health of Zhejiang Province, China, No. 2011ZHB003 and No. 2013RCB010
P- Reviewer: Contini S, Ker CG S- Editor: Nan J L- Editor: Stewart GJ E- Editor: Ma S
References
- 1.Melotti G, Butturini G, Piccoli M, Casetti L, Bassi C, Mullineris B, Lazzaretti MG, Pederzoli P. Laparoscopic distal pancreatectomy: results on a consecutive series of 58 patients. Ann Surg. 2007;246:77–82. doi: 10.1097/01.sla.0000258607.17194.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kooby DA, Gillespie T, Bentrem D, Nakeeb A, Schmidt MC, Merchant NB, Parikh AA, Martin RC, Scoggins CR, Ahmad S, et al. Left-sided pancreatectomy: a multicenter comparison of laparoscopic and open approaches. Ann Surg. 2008;248:438–446. doi: 10.1097/SLA.0b013e318185a990. [DOI] [PubMed] [Google Scholar]
- 3.Jayaraman S, Gonen M, Brennan MF, D’Angelica MI, DeMatteo RP, Fong Y, Jarnagin WR, Allen PJ. Laparoscopic distal pancreatectomy: evolution of a technique at a single institution. J Am Coll Surg. 2010;211:503–509. doi: 10.1016/j.jamcollsurg.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Song KB, Kim SC, Park JB, Kim YH, Jung YS, Kim MH, Lee SK, Seo DW, Lee SS, Park do H, et al. Single-center experience of laparoscopic left pancreatic resection in 359 consecutive patients: changing the surgical paradigm of left pancreatic resection. Surg Endosc. 2011;25:3364–3372. doi: 10.1007/s00464-011-1727-9. [DOI] [PubMed] [Google Scholar]
- 5.Fernández-Cruz L, Martínez I, Gilabert R, Cesar-Borges G, Astudillo E, Navarro S. Laparoscopic distal pancreatectomy combined with preservation of the spleen for cystic neoplasms of the pancreas. J Gastrointest Surg. 2004;8:493–501. doi: 10.1016/j.gassur.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Warshaw AL. Distal pancreatectomy with preservation of the spleen. J Hepatobiliary Pancreat Sci. 2010;17:808–812. doi: 10.1007/s00534-009-0226-z. [DOI] [PubMed] [Google Scholar]
- 7.Nau P, Melvin WS, Narula VK, Bloomston PM, Ellison EC, Muscarella P. Laparoscopic distal pancreatectomy with splenic conservation: an operation without increased morbidity. Gastroenterol Res Pract. 2009;2009:846340. doi: 10.1155/2009/846340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakamura M, Nagayoshi Y, Kono H, Mori Y, Ohtsuka T, Takahata S, Shimizu S, Tanaka M. Lateral approach for laparoscopic splenic vessel-preserving distal pancreatectomy. Surgery. 2011;150:326–331. doi: 10.1016/j.surg.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Yan JF, Mou YP, Xu XW, Ni JJ, Chen DW, Zhu YP, Chen QL, Zhou YC, Xie K. [Laparoscopic distal pancreatectomy: the experience of 68 cases in a single centre] Zhonghua Wai Ke Zazhi. 2012;50:802–805. [PubMed] [Google Scholar]
- 10.Xu X, Chen K, Zhou W, Zhang R, Wang J, Wu D, Mou Y. Laparoscopic transgastric resection of gastric submucosal tumors located near the esophagogastric junction. J Gastrointest Surg. 2013;17:1570–1575. doi: 10.1007/s11605-013-2241-2. [DOI] [PubMed] [Google Scholar]
- 11.Chen K, Xu X, Mou Y, Pan Y, Zhang R, Zhou Y, Wu D, Huang C. Totally laparoscopic distal gastrectomy with D2 lymphadenectomy and Billroth II gastrojejunostomy for gastric cancer: short- and medium-term results of 139 consecutive cases from a single institution. Int J Med Sci. 2013;10:1462–1470. doi: 10.7150/ijms.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang RC, Yan JF, Xu XW, Chen K, Ajoodhea H, Mou YP. Laparoscopic vs open distal pancreatectomy for solid pseudopapillary tumor of the pancreas. World J Gastroenterol. 2013;19:6272–6277. doi: 10.3748/wjg.v19.i37.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen K, Mou YP, Xu XW, Cai JQ, Wu D, Pan Y, Zhang RC. Short-term surgical and long-term survival outcomes after laparoscopic distal gastrectomy with D2 lymphadenectomy for gastric cancer. BMC Gastroenterol. 2014;14:41. doi: 10.1186/1471-230X-14-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 15.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Kimura W, Yano M, Sugawara S, Okazaki S, Sato T, Moriya T, Watanabe T, Fujimoto H, Tezuka K, Takeshita A, et al. Spleen-preserving distal pancreatectomy with conservation of the splenic artery and vein: techniques and its significance. J Hepatobiliary Pancreat Sci. 2010;17:813–823. doi: 10.1007/s00534-009-0250-z. [DOI] [PubMed] [Google Scholar]
- 17.Warshaw AL. Conservation of the spleen with distal pancreatectomy. Arch Surg. 1988;123:550–553. doi: 10.1001/archsurg.1988.01400290032004. [DOI] [PubMed] [Google Scholar]
- 18.Soper NJ, Brunt LM, Dunnegan DL, Meininger TA. Laparoscopic distal pancreatectomy in the porcine model. Surg Endosc. 1994;8:57–60; discussion 60-1. doi: 10.1007/BF02909495. [DOI] [PubMed] [Google Scholar]
- 19.McGory ML, Zingmond DS, Sekeris E, Ko CY. The significance of inadvertent splenectomy during colorectal cancer resection. Arch Surg. 2007;142:668–674. doi: 10.1001/archsurg.142.7.668. [DOI] [PubMed] [Google Scholar]
- 20.Mellemkjoer L, Olsen JH, Linet MS, Gridley G, McLaughlin JK. Cancer risk after splenectomy. Cancer. 1995;75:577–583. doi: 10.1002/1097-0142(19950115)75:2<577::aid-cncr2820750222>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 21.Miura F, Takada T, Asano T, Kenmochi T, Ochiai T, Amano H, Yoshida M. Hemodynamic changes of splenogastric circulation after spleen-preserving pancreatectomy with excision of splenic artery and vein. Surgery. 2005;138:518–522. doi: 10.1016/j.surg.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Carrère N, Abid S, Julio CH, Bloom E, Pradère B. Spleen-preserving distal pancreatectomy with excision of splenic artery and vein: a case-matched comparison with conventional distal pancreatectomy with splenectomy. World J Surg. 2007;31:375–382. doi: 10.1007/s00268-006-0425-6. [DOI] [PubMed] [Google Scholar]
- 23.Shoup M, Brennan MF, McWhite K, Leung DH, Klimstra D, Conlon KC. The value of splenic preservation with distal pancreatectomy. Arch Surg. 2002;137:164–168. doi: 10.1001/archsurg.137.2.164. [DOI] [PubMed] [Google Scholar]
- 24.Jean-Philippe Adam B, Fernández-Cruz L, Sa-Cunha A. Laparoscopic spleen-preserving distal pancreatectomy: splenic vessel preservation compared with the Warshaw technique. JAMA Surg. 2013;148:246–252. doi: 10.1001/jamasurg.2013.768. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda T, Yoshiya S, Toshima T, Harimoto N, Yamashita Y, Ikegami T, Yoshizumi T, Soejima Y, Shirabe K, Maehara Y. Laparoscopic distal pancreatectomy preserving the spleen and splenic vessels for benign and low-grade malignant pancreatic neoplasm. Fukuoka Igaku Zasshi. 2013;104:54–63. [PubMed] [Google Scholar]
- 26.Schlöricke E, Nolde J, Hoffmann M, Roblick U, Bruch HP. Laparoscopic spleen-preserving distal pancreatectomy. Langenbecks Arch Surg. 2011;396:1119–1123. doi: 10.1007/s00423-011-0755-1. [DOI] [PubMed] [Google Scholar]
- 27.Yoon YS, Lee KH, Han HS, Cho JY, Ahn KS. Patency of splenic vessels after laparoscopic spleen and splenic vessel-preserving distal pancreatectomy. Br J Surg. 2009;96:633–640. doi: 10.1002/bjs.6609. [DOI] [PubMed] [Google Scholar]
- 28.Mekeel KL, Moss AA, Reddy KS, Mulligan DC, Harold KL. Laparoscopic distal pancreatectomy: does splenic preservation affect outcomes? Surg Laparosc Endosc Percutan Tech. 2011;21:362–365. doi: 10.1097/SLE.0b013e31822e0ea8. [DOI] [PubMed] [Google Scholar]
- 29.Bruzoni M, Sasson AR. Open and laparoscopic spleen-preserving, splenic vessel-preserving distal pancreatectomy: indications and outcomes. J Gastrointest Surg. 2008;12:1202–1206. doi: 10.1007/s11605-008-0512-0. [DOI] [PubMed] [Google Scholar]


